Morphic forms of complement factor d inhibitors
A form and crystallization technology, applied in anti-inflammatory agents, non-central analgesics, medical preparations containing active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
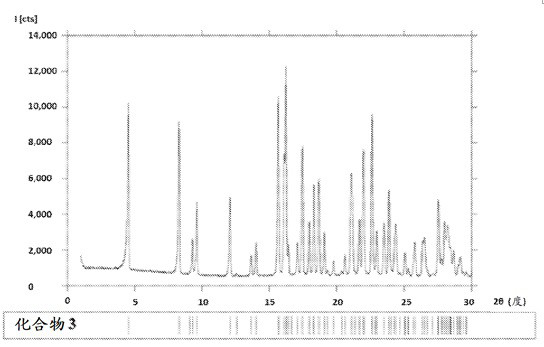
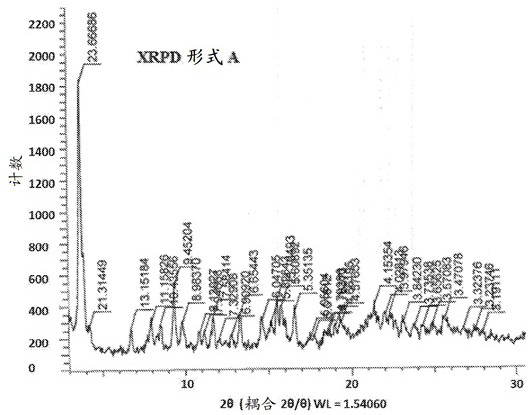
[0106] a) an isolated crystalline form A of compound 3, characterized in that it comprises XRPD patterns of at least three 2θ values of ±0.2°, 5.7±0.2°, 5.6±0.2°, 5.4±0.2° and 4.2±0.2°;
[0107] b) isolated crystalline form A of compound 3 of embodiment (a), characterized in that it comprises XRPD patterns of at least four 2θ values of 0.2°, 6.0±0.2°, 5.7±0.2°, 5.6±0.2°, 5.4±0.2° and 4.2±0.2°;
[0108] c) isolated crystalline Form A of Compound 3 according to embodiment (a) or (b), characterized by an XRPD pattern comprising at least a 2Θ value of 3.7 ± 0.2°;
[0109] d) isolated crystalline Form A of Compound 3 of embodiment (a), (b) or (c), characterized by an XRPD pattern comprising at least a 2Θ value of 9.3±0.2°;
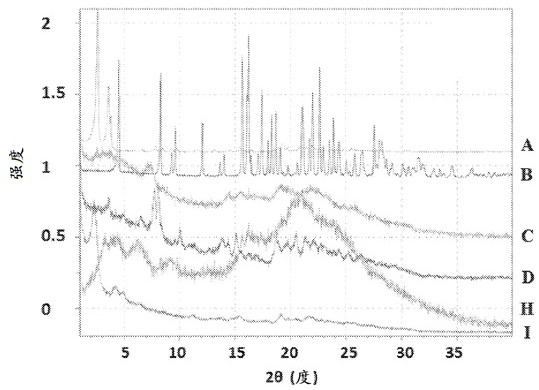
[0110] e) Isolated crystalline Form A of Compound 3 according to any one of embodiments (a)-(d), wherein the XRPD pattern has image 3 The characteristic 2θ value of ;
[0111] f) A pharmaceutical composition comprising an isolated crystalline Form A of...
Embodiment 1
[0423] Scheme 1. Synthesis of compound 3
[0424]
[0425] To a solution of intermediate 10 and intermediate 33 in DMF was added N,N-diisopropylethylamine. TBTU was added while maintaining the reaction temperature. The reaction was warmed to room temperature and stirred for 4-8 hours. The reaction was diluted with water and the resulting solid formed was collected by centrifugation. The solid was washed twice with water, then dissolved in DCM and treated with Siliabondthiol resin and activated carbon to remove Pd-based impurities. The resin and charcoal were removed by filtration and washed with MeOH / DCM. The filtrate was evaporated to dryness and the residue was purified by silica gel chromatography using methanol / DCM. Pure fractions were combined and evaporated to dryness.
Embodiment 2
[0426] Example 2. Polymorph experiment of compound 3
[0427] Table 1. Polymorph studies of compound 3, starting material is disordered compound 3 unless otherwise indicated.
[0428]
[0429]
[0430]
[0431]
[0432] *Graph successfully indexed
[0433] FE: fast evaporation
[0434] ET: rapid evaporation at elevated temperature
[0435] SE: slow evaporation
[0436] VR: Volume reduction
[0437] SC: slow cooling
[0438] RT ppt: ambient temperature precipitation
[0439] CP: sharp precipitation
[0440] SVD: Solid-Vapor Diffusion
[0441] Roto-vap: rotary evaporation
[0442] The procedure for the conditions in Table 1 is discussed below.
[0443] Fast Evaporation (FE)
[0444] Prepare solutions of compound 3 and the solvent / solvent system of interest. Samples were filtered and placed at ambient conditions until dry.
[0445] Slow evaporation (SE)
[0446] Prepare solutions of compound 3 and the solvent / solvent system of interest. Filter the sample. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com