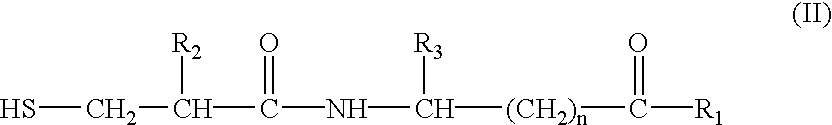
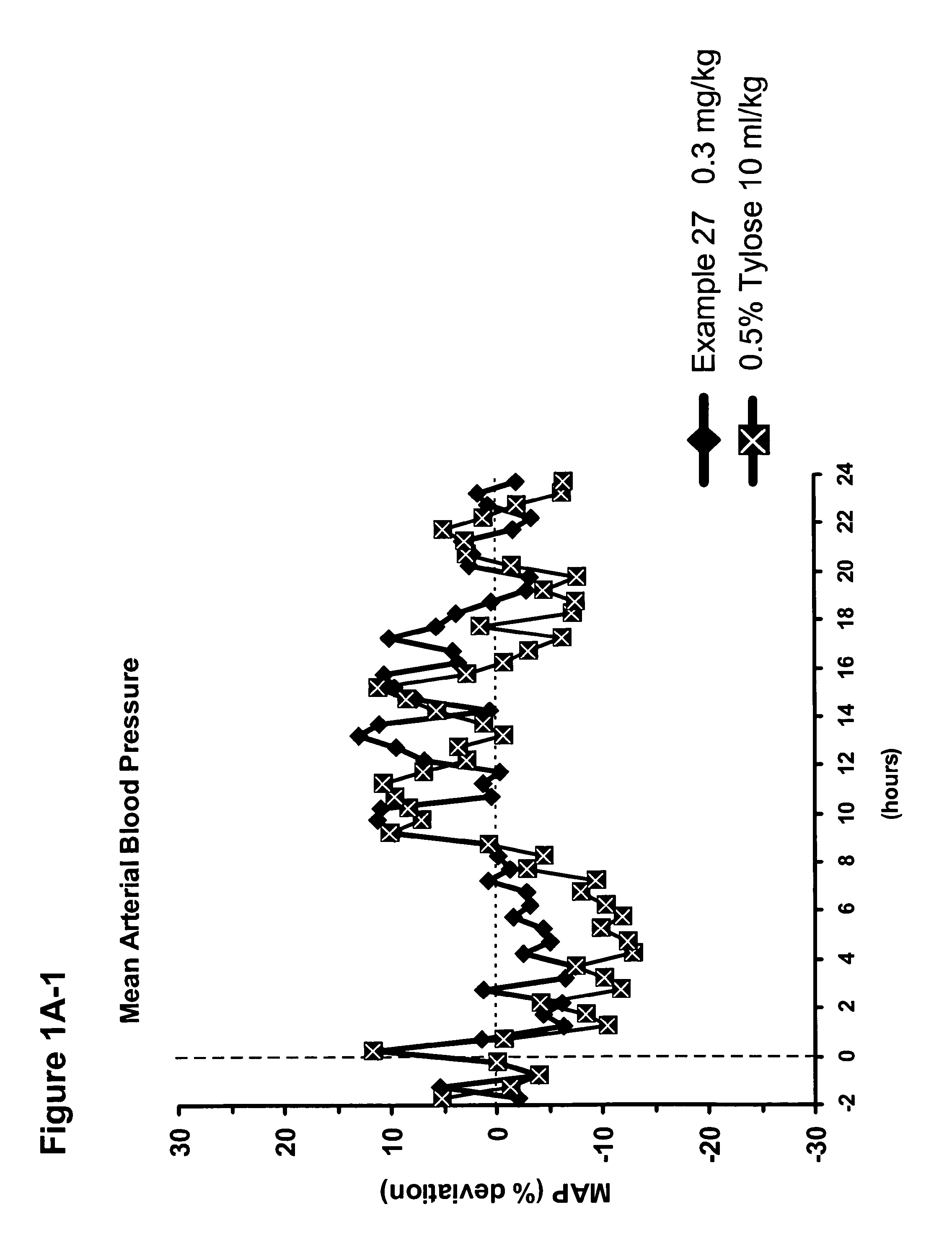
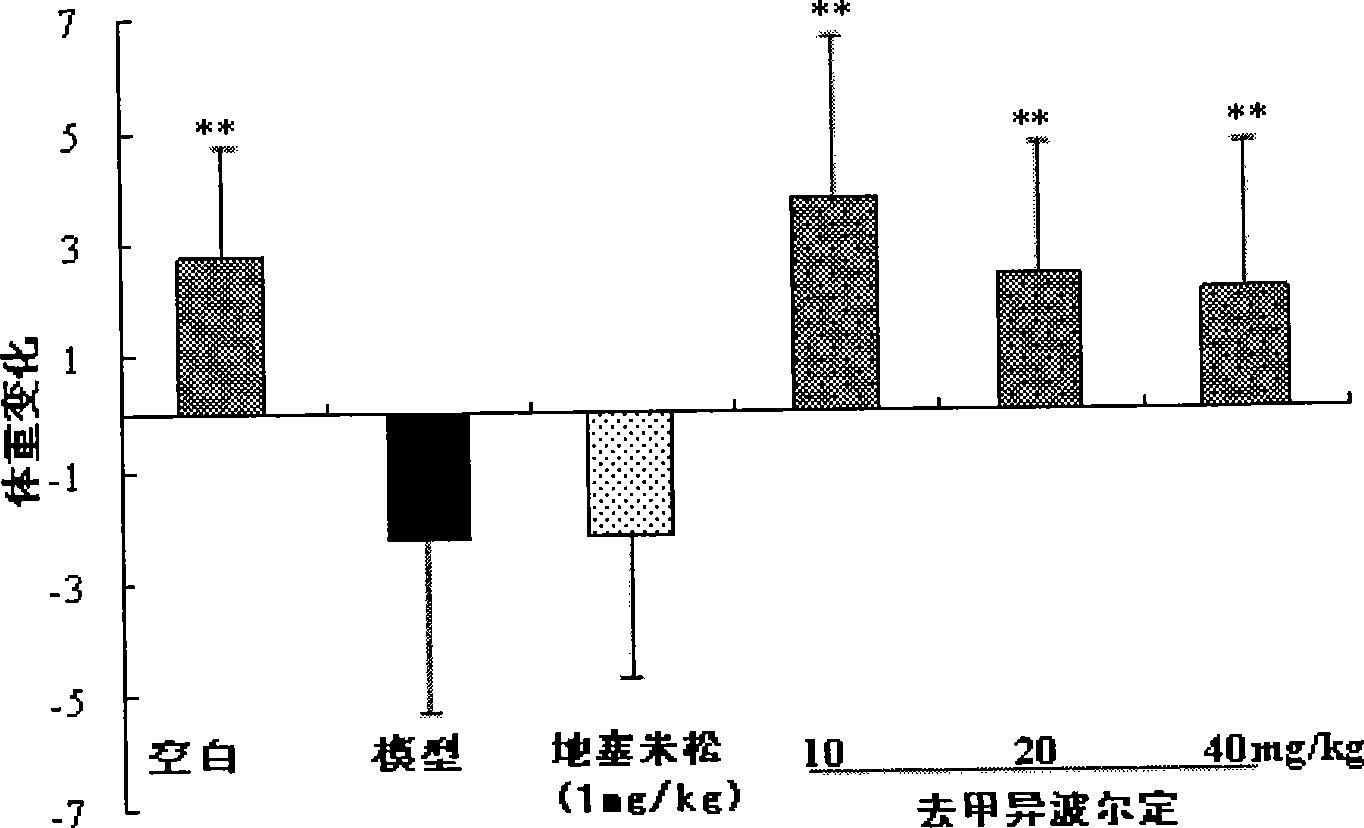
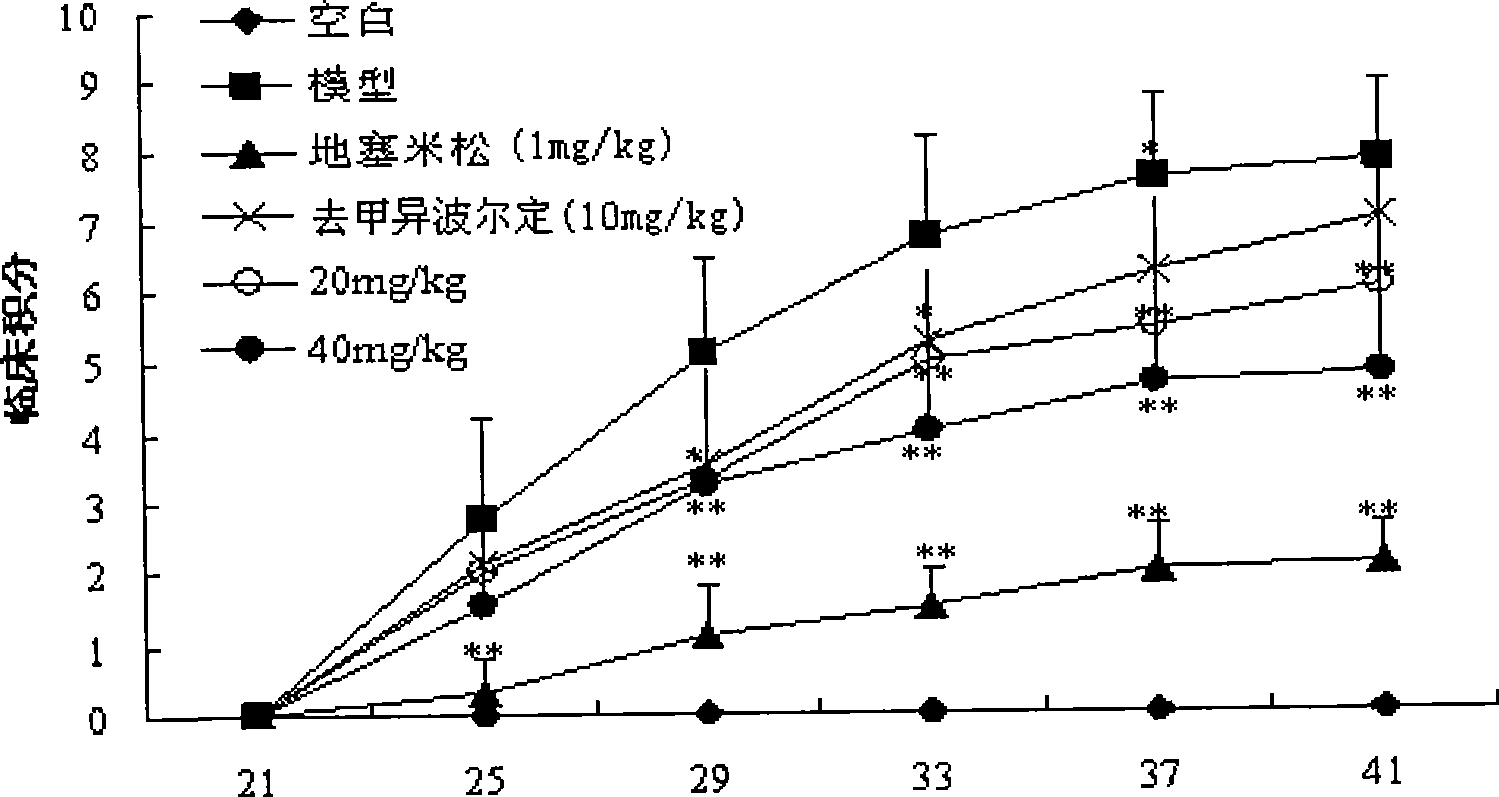
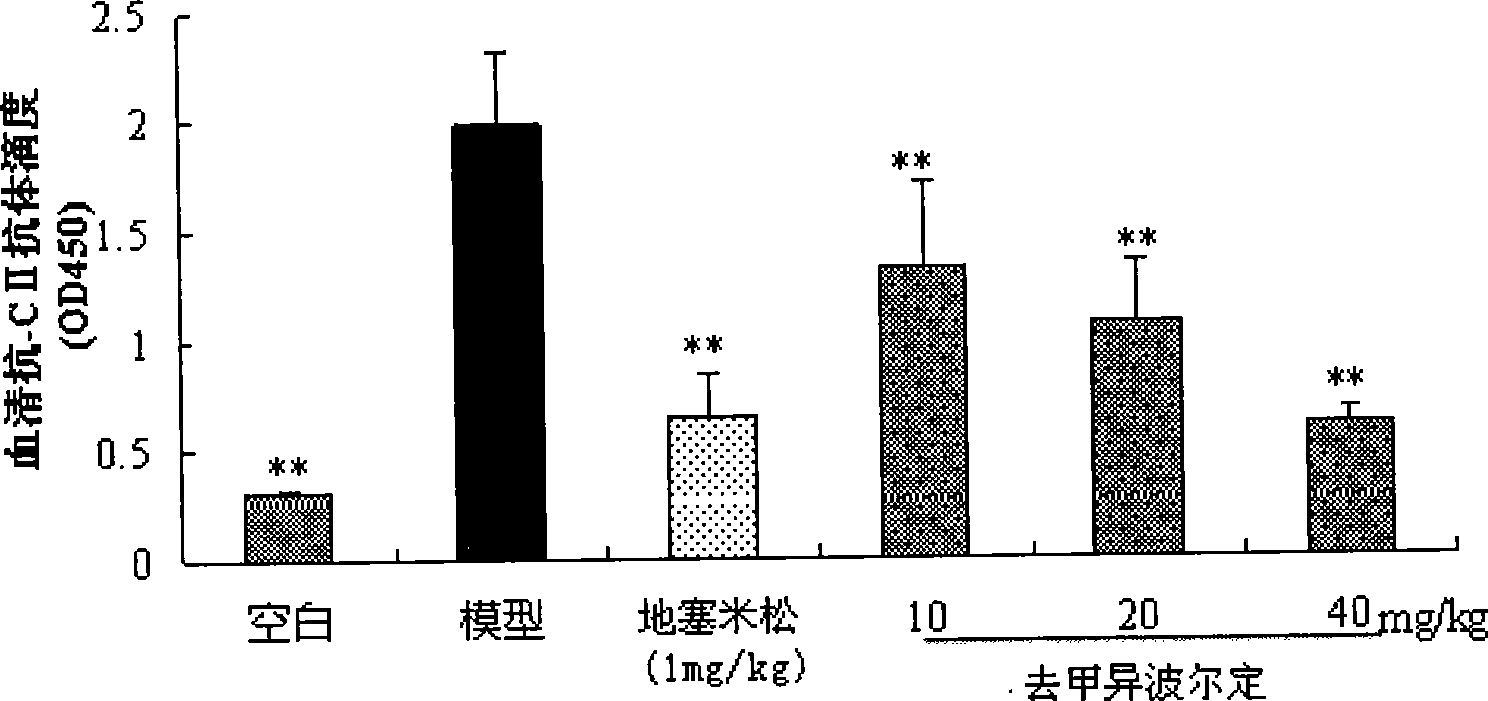
Patents
Literature
239 results about "Sclerosis skin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Scleroderma is a group of autoimmune diseases that may result in changes to the skin, blood vessels, muscles, and internal organs. The disease can be either localized to the skin or involve other organs in addition to the skin.
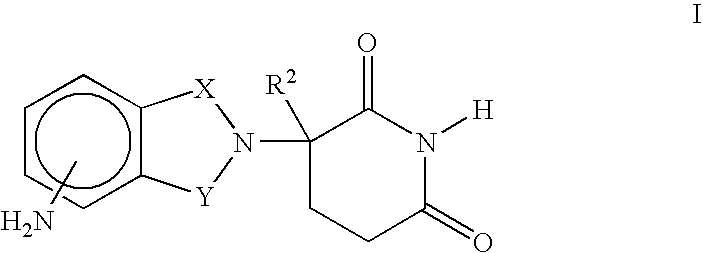
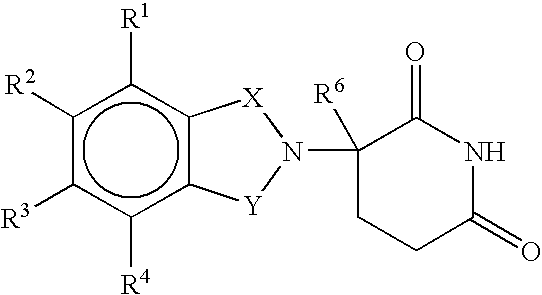
Methods of using and compositions comprising immunomodulatory compounds for the treatment and management of skin diseases or disorders
Methods of treating, preventing, correcting and / or managing skin diseases or disorders characterized by overgrowths of the epidermis, keratoses, scleroderma, cutaneous vasculitis, acne or wrinkles are disclosed. Specific embodiments encompass the administration of an immunomodulatory compound, or a pharmaceutically acceptable salt, solvate, hydrate, stereoisomer, clathrate, or prodrug thereof, alone or in combination with a second active agent. Specific second active ingredients are capable of affecting or inhibiting cell growth or proliferation, removing or improving acne scars, or reducing or correcting wrinkle lines. Pharmaceutical compositions, single unit dosage forms, and kits suitable for use in methods of the invention are also disclosed.
Owner:CELGENE CORP
Novel Composition for a Topical Skin Treatment Base and Medicated Applications Thereof
A novel topical preparation comprised of carboxylic acids, chelating agents, dimethyl Sulfone and magnesium sulfate that forms a functional and versatile base formation for the addition of numerous medications and active ingredients for the purpose of treating certain skin conditions including, but not limited to psoriasis, eczema, dermatitis, acne, rosacea, scleroderma, skin stones, fungal infections, bacterial infections, or other skin disorders and diseases with improved efficacy and penetration
Owner:CALGENEX CORP
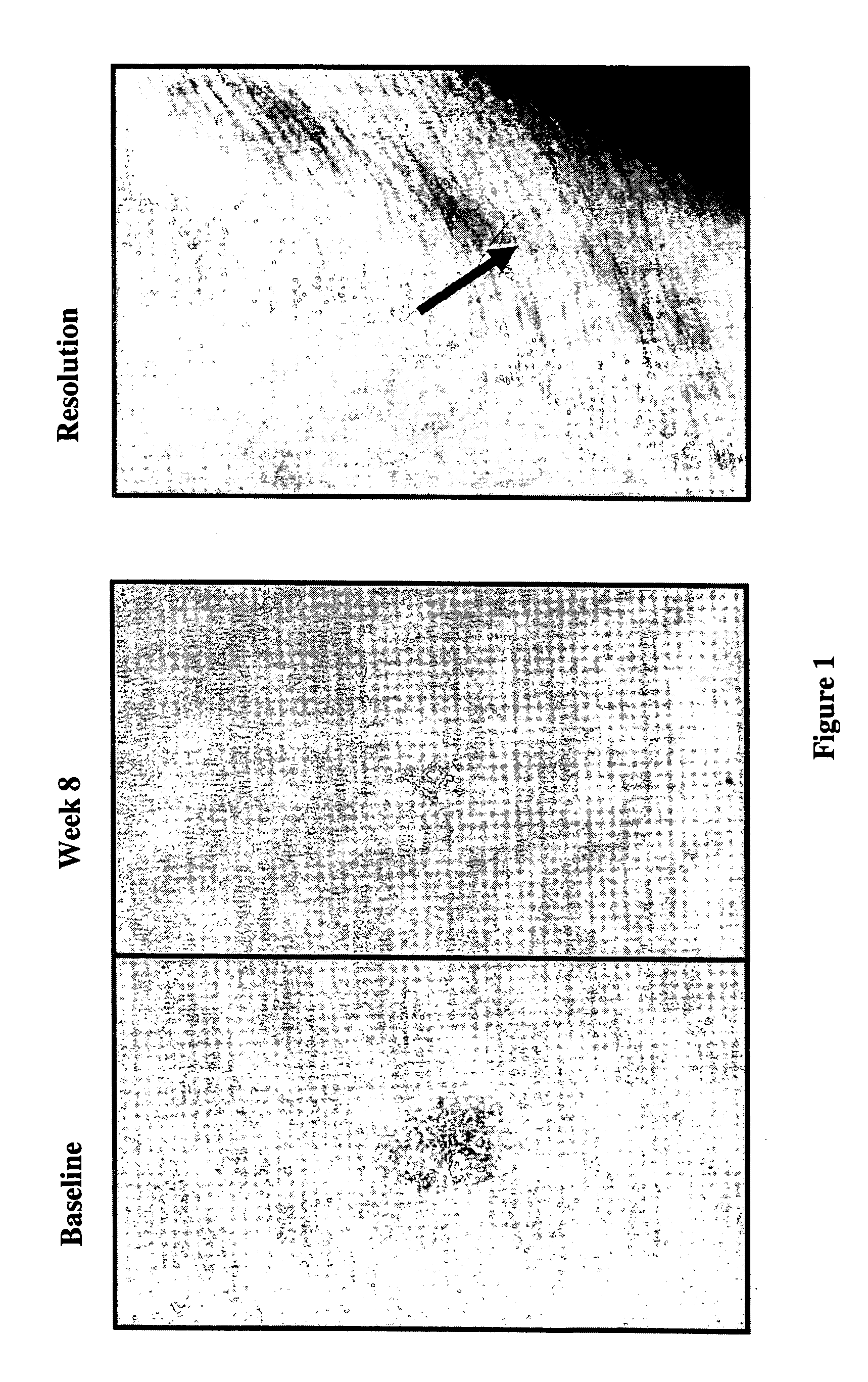
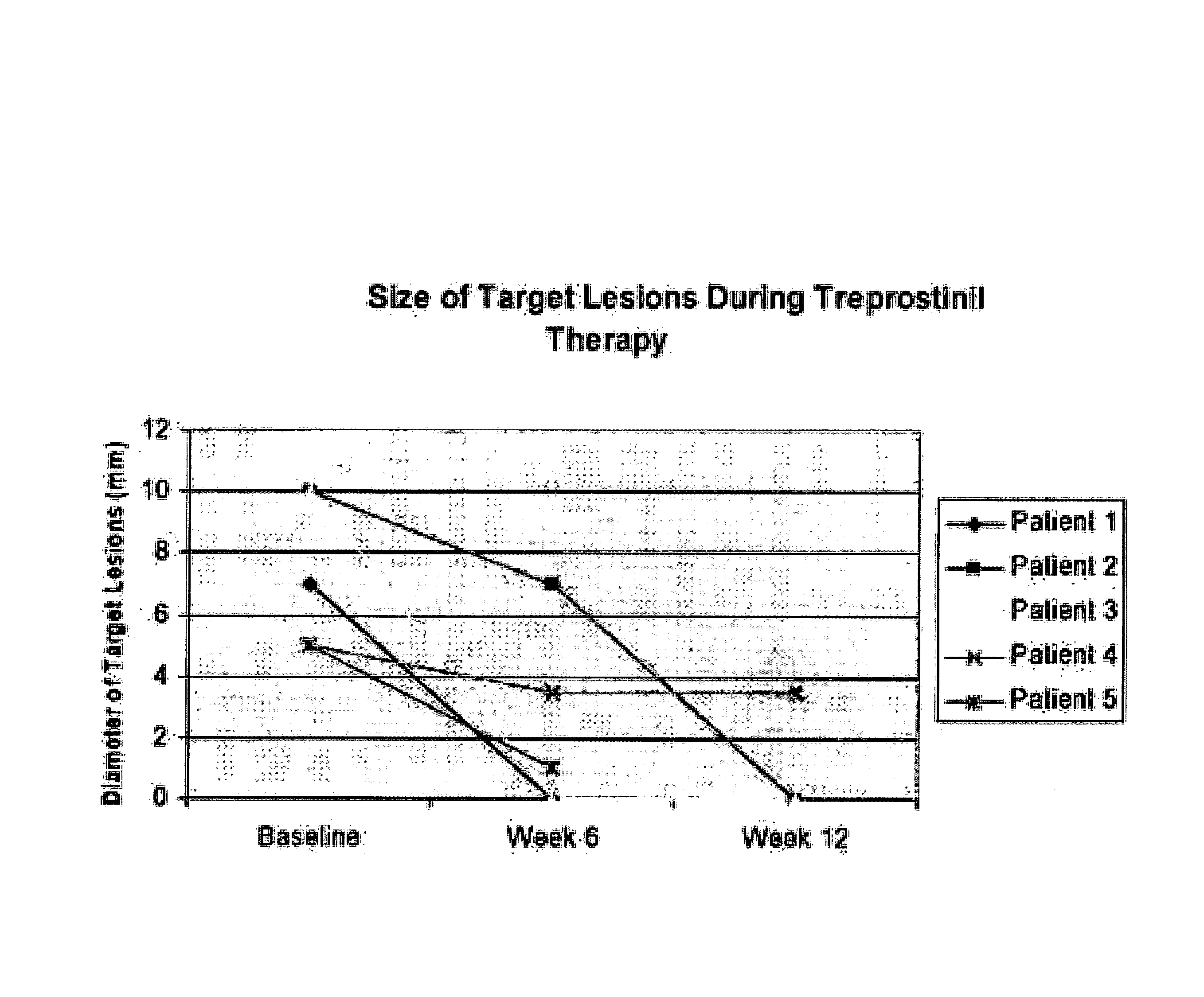
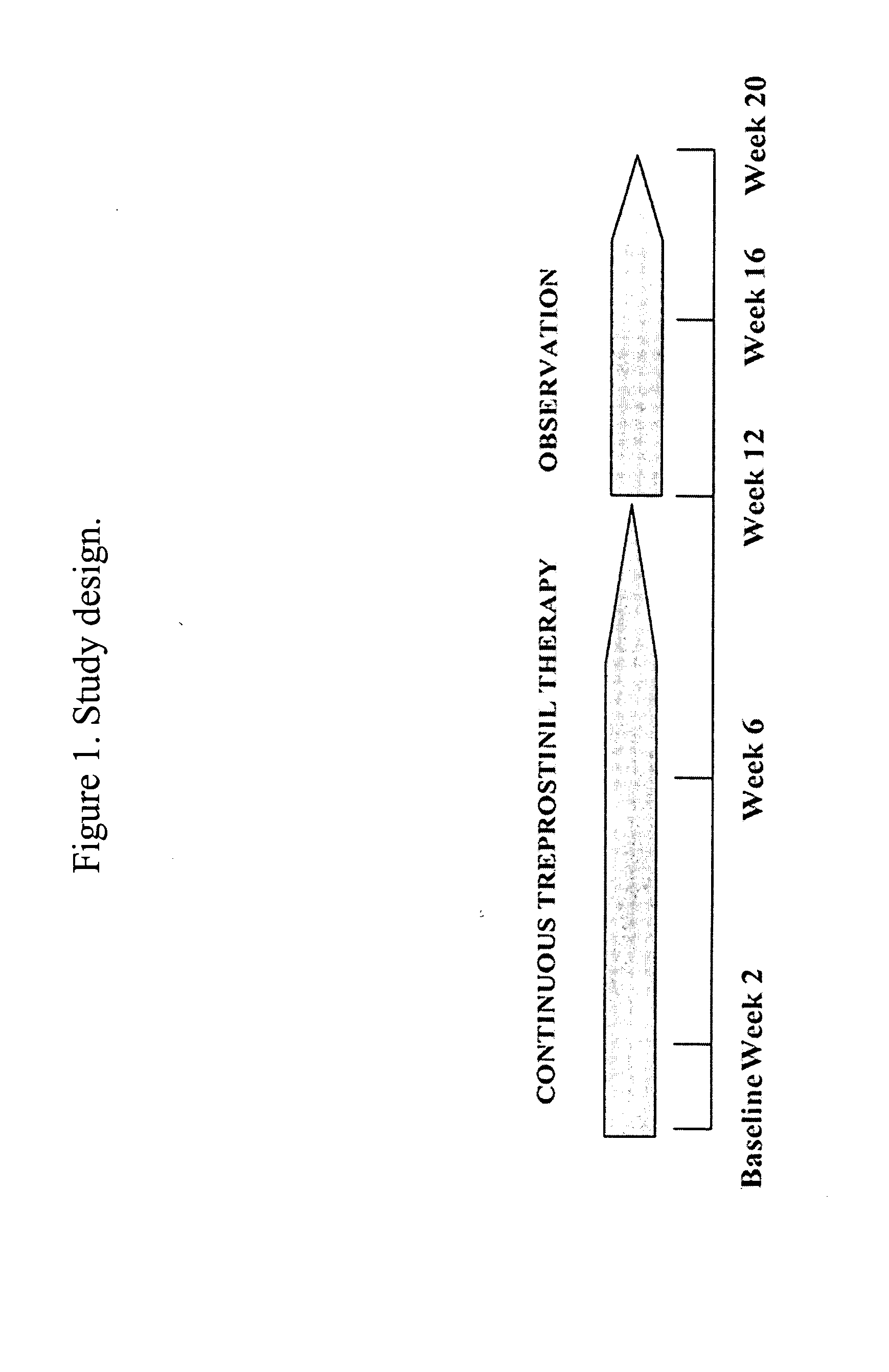
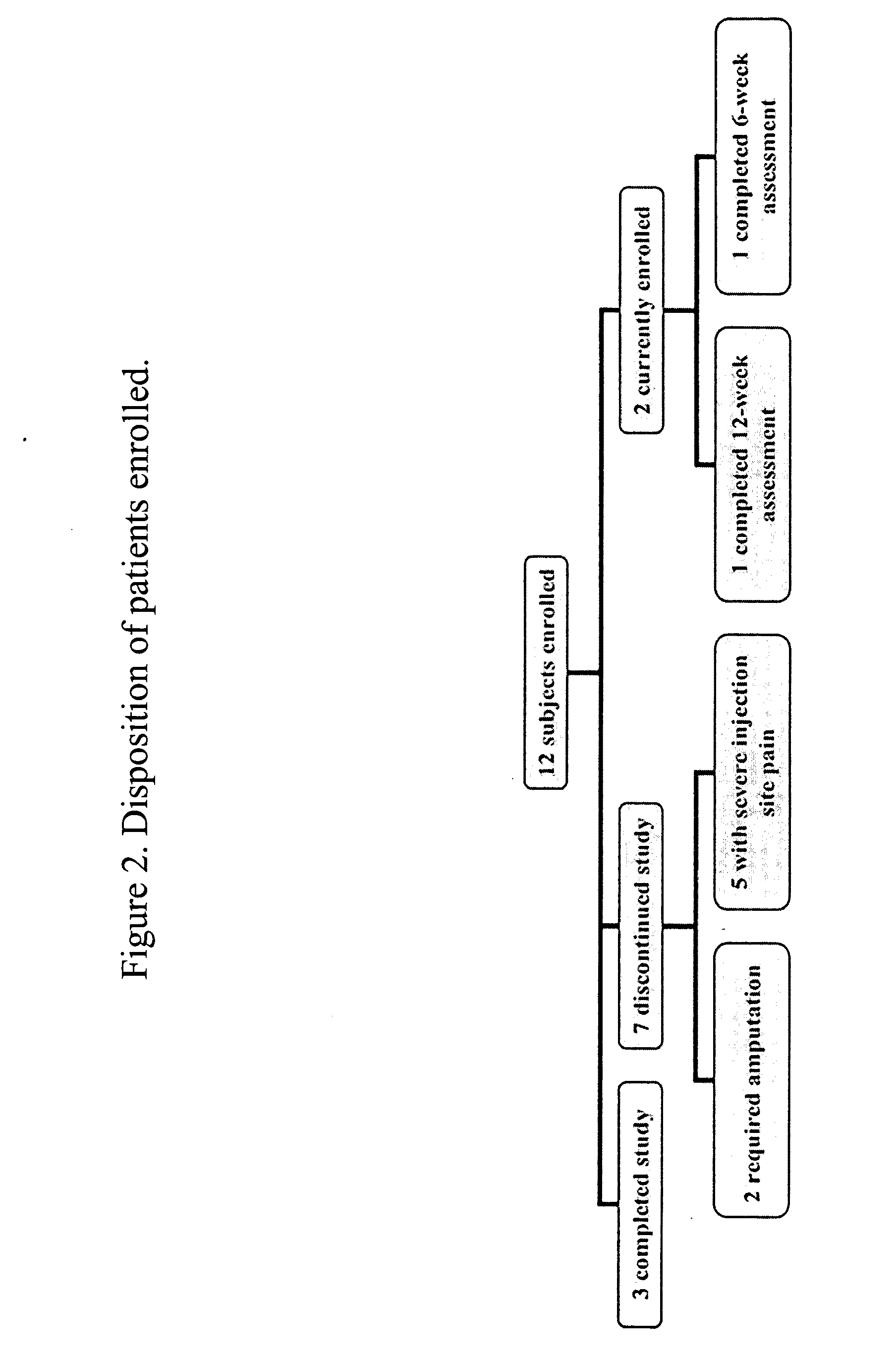
Use of treprostinil to treat and prevent ischemic lesions
ActiveUS20050165111A1Reduce occurrenceReduce the numberBiocideNervous disorderTreprostinilIschemic lesion
The present invention describes novel methods for using Treprostinil or its derivative, or a pharmaceutically acceptable salt thereof, for the treatment and / or prevention of ischemic lesions, such as digital ulcers, in subjects with scleroderma (including systemic sclerosis), Buerger's disease, Raynaud's disease, Raynaud's phenomenon and / or other conditions that cause such lesions. The invention also relates to kits for treatment and / or prevention of ischemic lesions, comprising an effective amount of Treprostinil or its derivative, or a pharmaceutically acceptable salt thereof.
Owner:UNITED THERAPEUTICS CORP
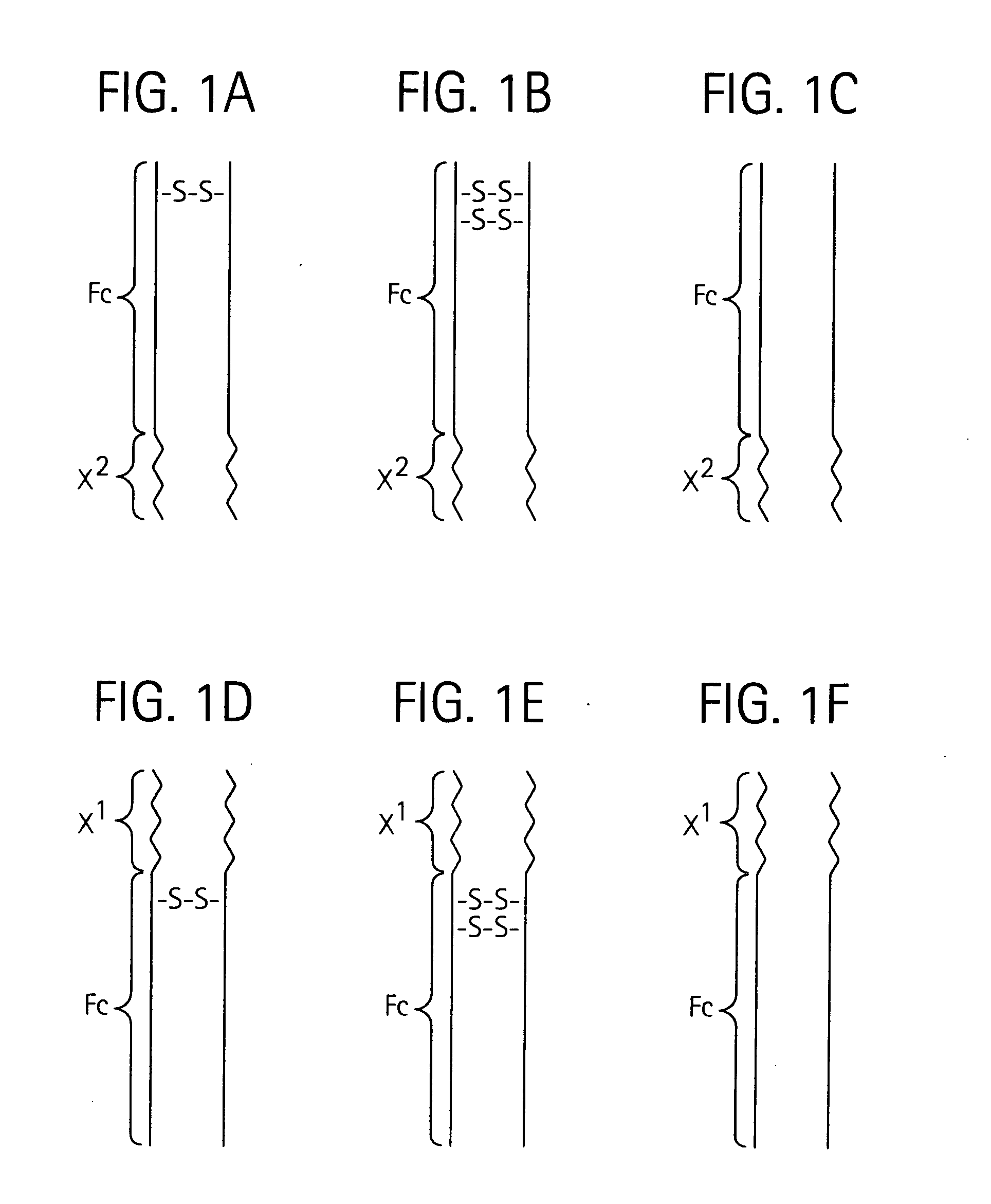
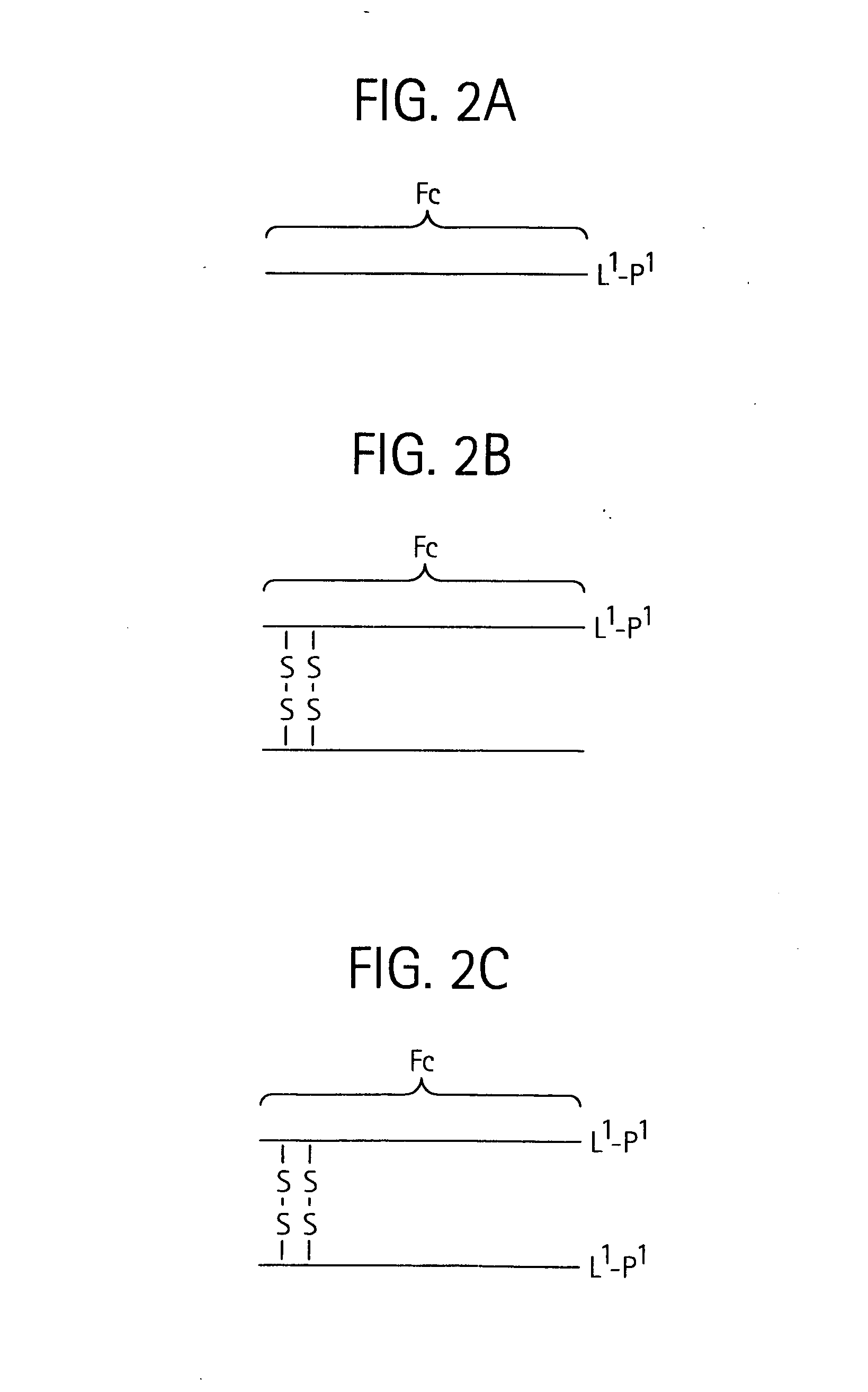
Toxin peptide therapeutic agents
ActiveUS20070071764A1Avoid it happening againRelieve symptomsNervous disorderAntipyreticHalf-lifeSjögren syndrome
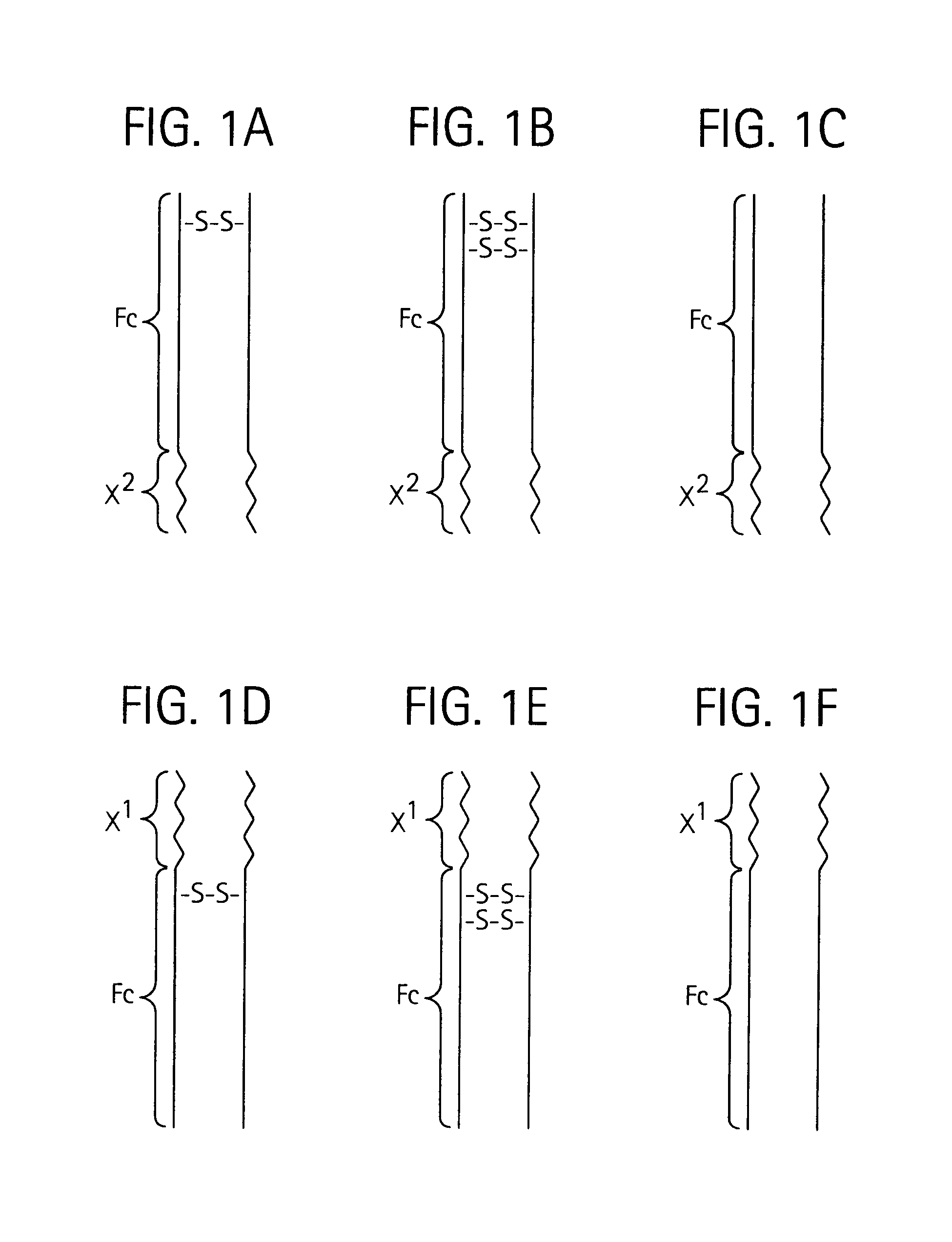
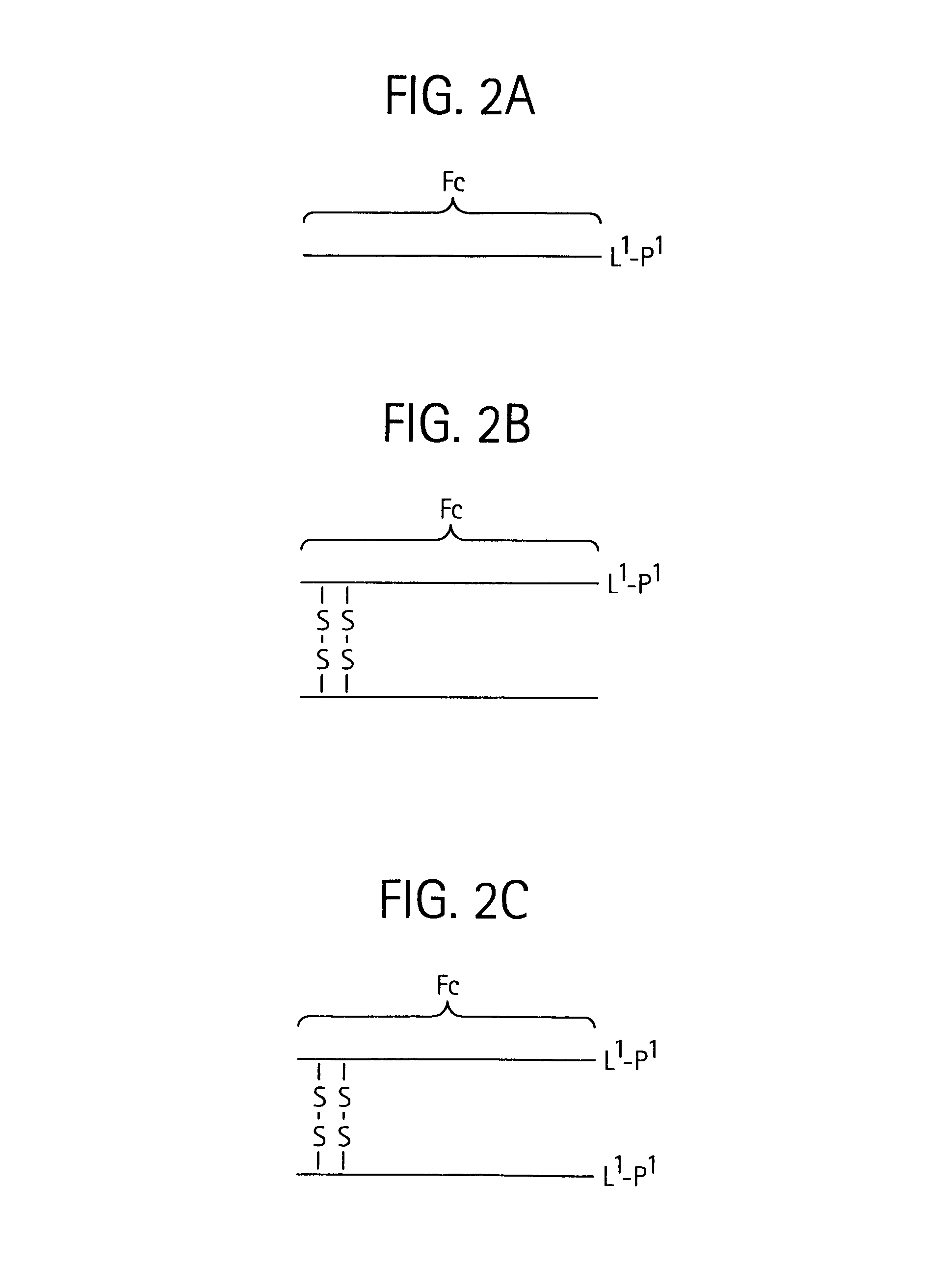
Disclosed is a composition of matter of the formula (X1)a—(F1)d—(X2)b—(F2)e—(X3)c (I) and multimers thereof, in which F1 and F2 are half-life extending moieties, and d and e are each independently 0 or 1, provided that at least one of d and e is 1; X1, X2, and X3 are each independently -(L)f-P-(L)g-, and f and g are each independently 0 or 1; P is a toxin peptide of no more than about 80 amino acid residues in length, comprising at least two intrapeptide disulfide bonds; L is an optional linker; and a, b, and c are each independently 0 or 1, provided that at least one of a, b and c is 1. Linkage to the half-life extending moiety or moieties increases the in vivo half-life of the toxin peptide, which otherwise would be quickly degraded. A pharmaceutical composition comprises the composition and a pharmaceutically acceptable carrier. Also disclosed are a DNA encoding the inventive composition of matter, an expression vector comprising the DNA, and a host cell comprising the expression vector. Methods of treating an autoimmune disorder, such as, but not limited to, multiple sclerosis, type 1 diabetes, psoriasis, inflammatory bowel disease, contact-mediated dermatitis, rheumatoid arthritis, psoriatic arthritis, asthma, allergy, restinosis, systemic sclerosis, fibrosis, scleroderma, glomerulonephritis, Sjogren syndrome, inflammatory bone resorption, transplant rejection, graft-versus-host disease, and lupus and of preventing or mitigating a relapse of a symptom of multiple sclerosis are also disclosed.
Owner:AMGEN INC
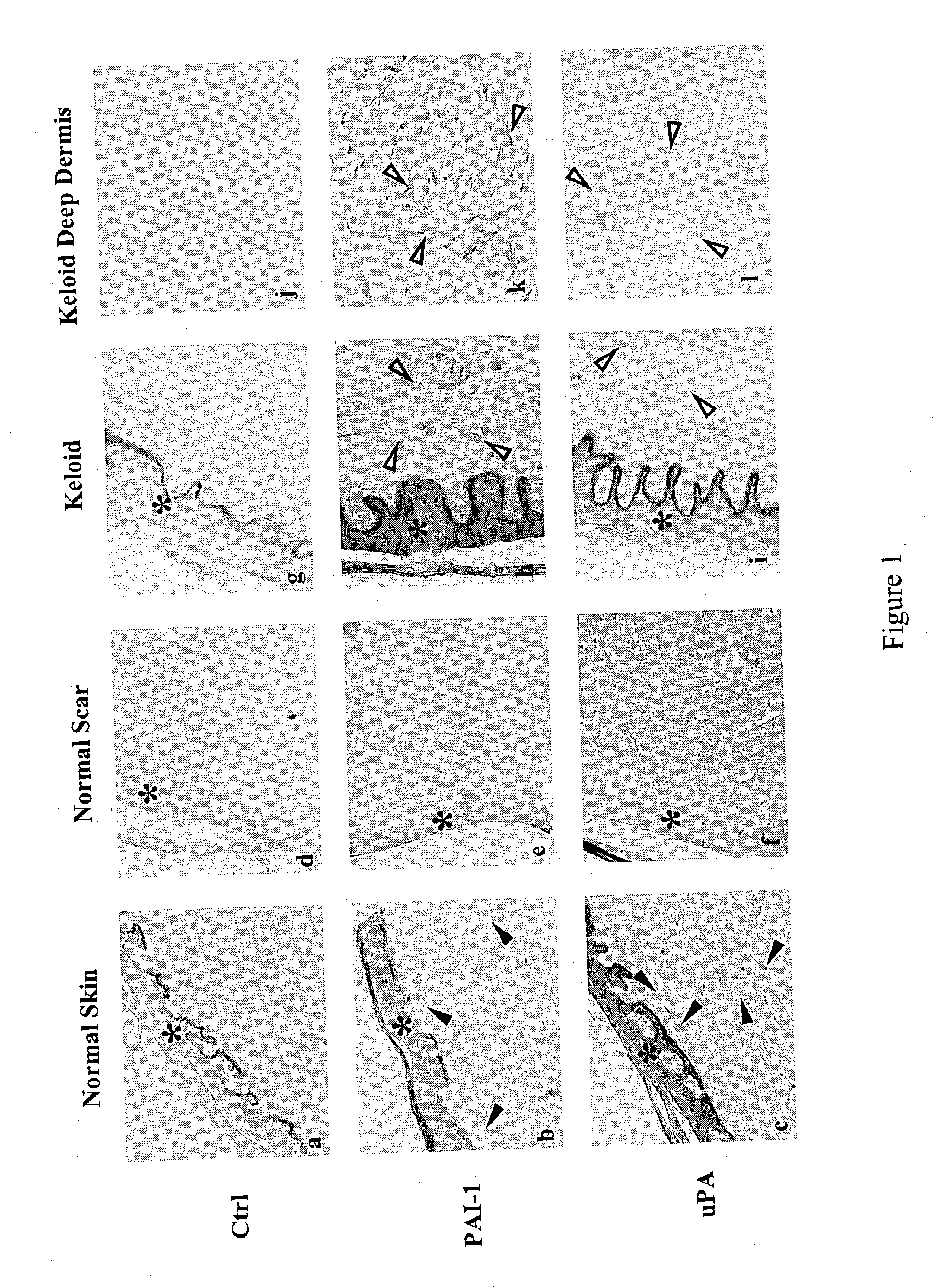
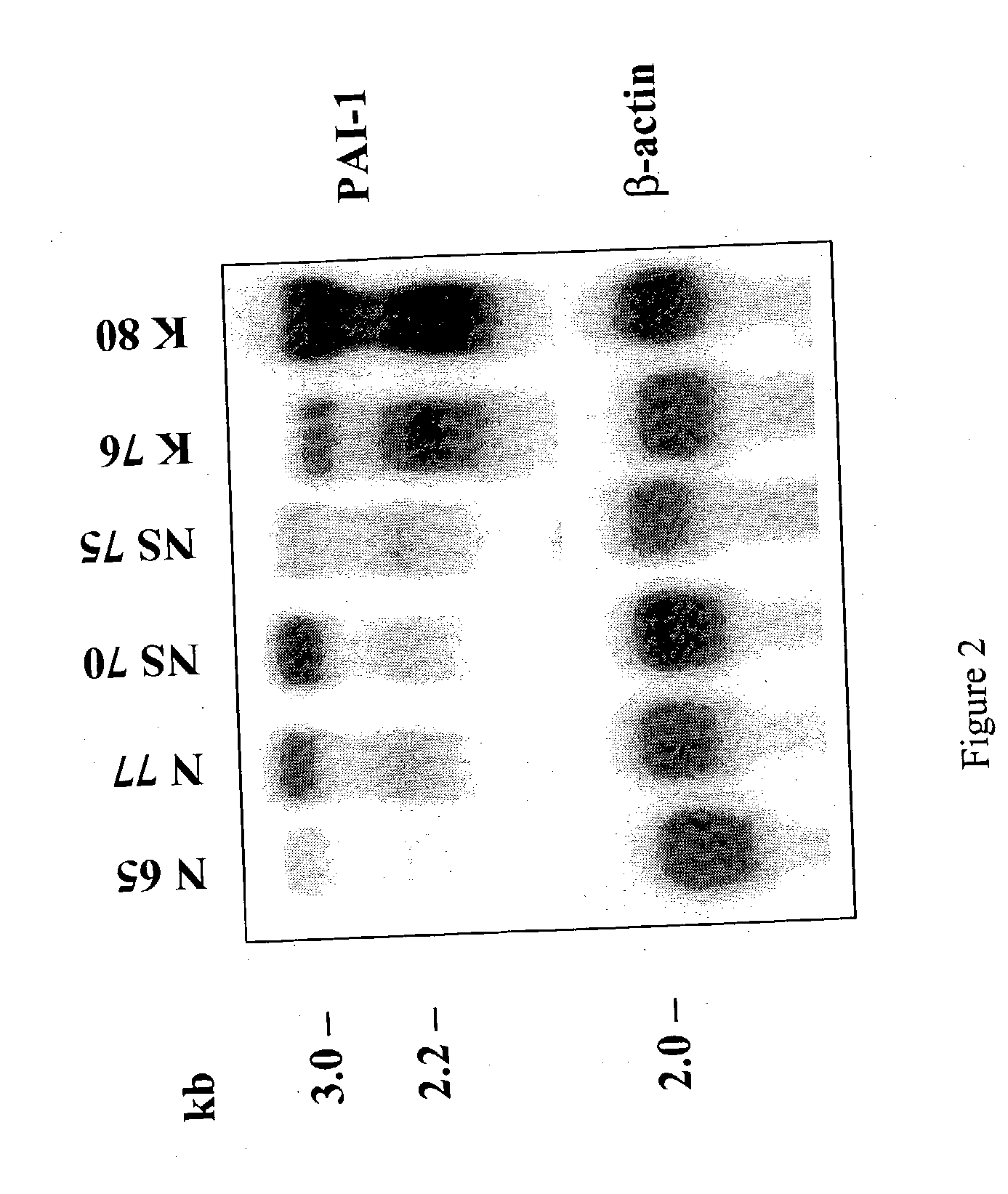
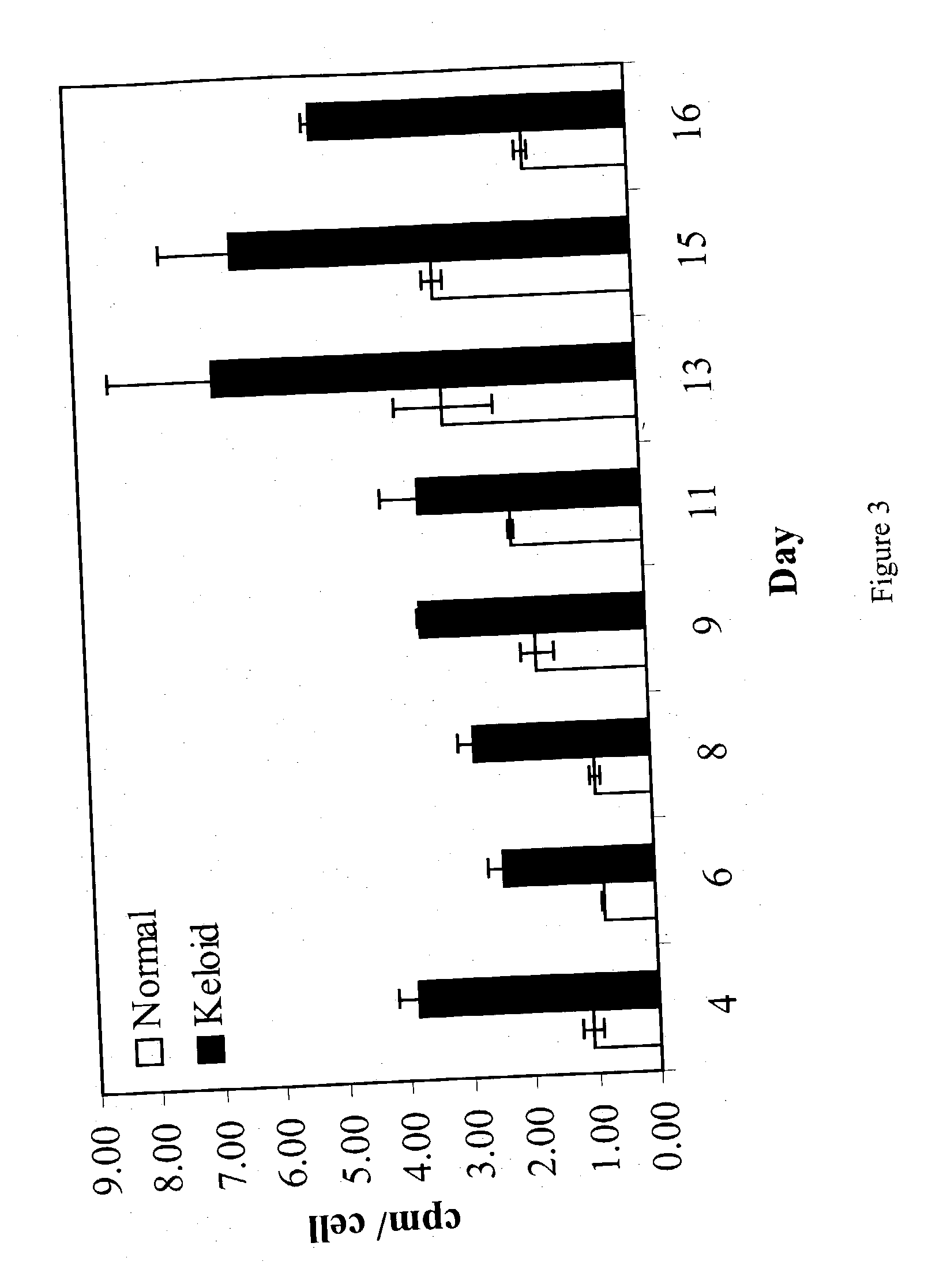
Treatment and prevention of abnormal scar formation in keloids and other cutaneous or internal wounds or lesions
The present invention relates to findings that reducing the activity of Plasminogen Activator Inhibitor-1 (PAI-1) suppresses an excessive deposition of collagen which is known as a cause for the formation of abnormal scars. These abnormal scars include but are not limited to keloids, adhesions, hypertrophic scars, skin disfiguring conditions, fibrosis, fibrocystic conditions, contractures, and scleroderma, all of which are associated with or caused by an excessive deposit of collagen in a wound healing process. Accordingly, aspects of the present invention are directed to the reduction of PAI-1 activity to decrease an excessive accumulation of collagen, prevent the formation of an abnormal scar, and / or treat abnormal scars that result from an excessive accumulation of collagen. The PAI-1 activity can be reduced by PAI-1 inhibitors which include but are not limited to PAI-1 neutralizing antibodies, diketopiperazine based compounds, tetramic acid based compounds, hydroxyquinolinone based compounds, Enalapril, Eprosartan, Troglitazone, Vitamin C, Vitamin E, Mifepristone (RU486), and Spironolactone to name a few. Another aspect of the present invention is directed to methods of measuring PAI-1 activity in a wound healing process and determining the propensity of the formation of an abnormal scar.
Owner:CHILDRENS HOSPITAL OF LOS ANGELES +1
Method of treating mast cell activation-induced diseases with a proteoglycan
InactiveUS6689748B1Decrease in urinaryBiocidePeptide/protein ingredientsInflammatory Bowel DiseasesInterstitial cystitis
The invention provides a method for preventing and treating the harmful biological effects of biochemicals secreted from activated mast cells in the organism of warm blooded animals and more especially human beings, said effects being associated with allergy (including but not limited to allergic conjunctivitis, allergic rhinitis, allergic otitis, asthma, allergic uticaria, food allergy and atopic dermatitis), hyperproliferative diseases such as leukemia and systemic mastocytosis, interstitial cystitis, inflammatory bowel disease, irritable bowel syndrome, osteoporosis and scleroderma. The method consists in administering to said animals and especially to human beings an effective amount of a proteoglycan such as chondroitin sulfate with mast cell secretion inhibitory activity, alone or in combination with one or more synergistic adjuvants such those belonging to the class of flavonoids or compounds with histamine-1 receptor antagonist activity.
Owner:THETA BIOMEDICAL CONSULTING & DEVMENT
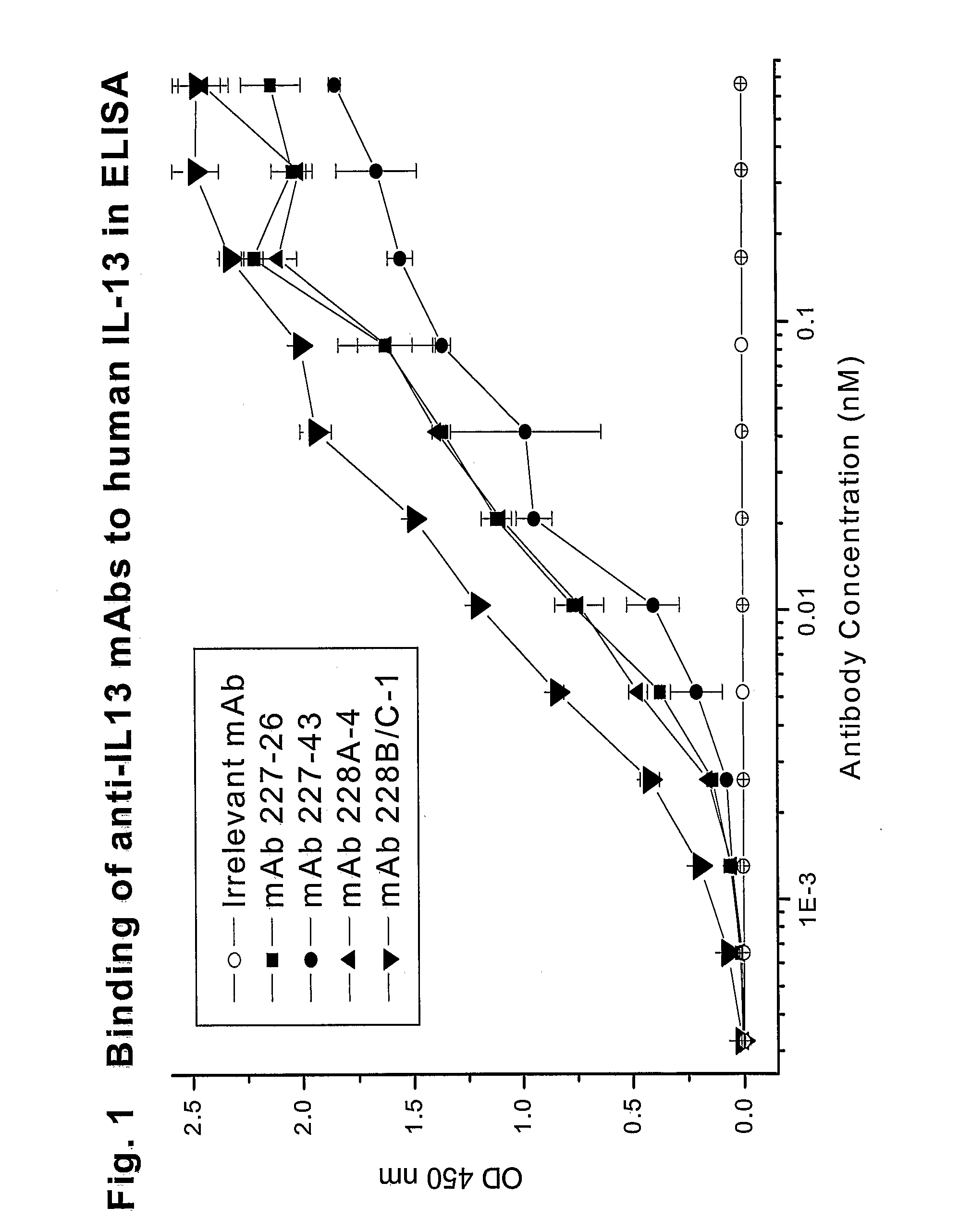
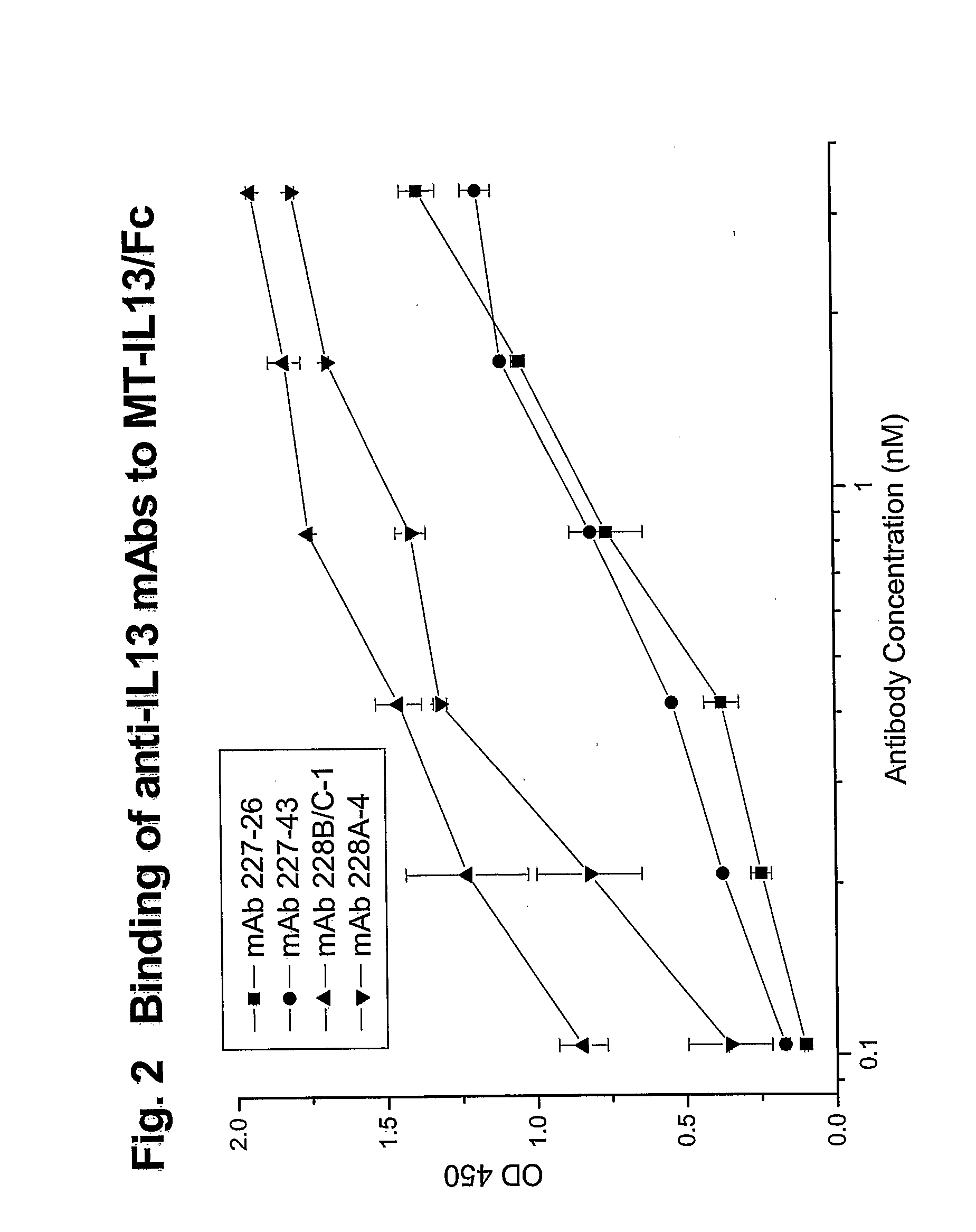
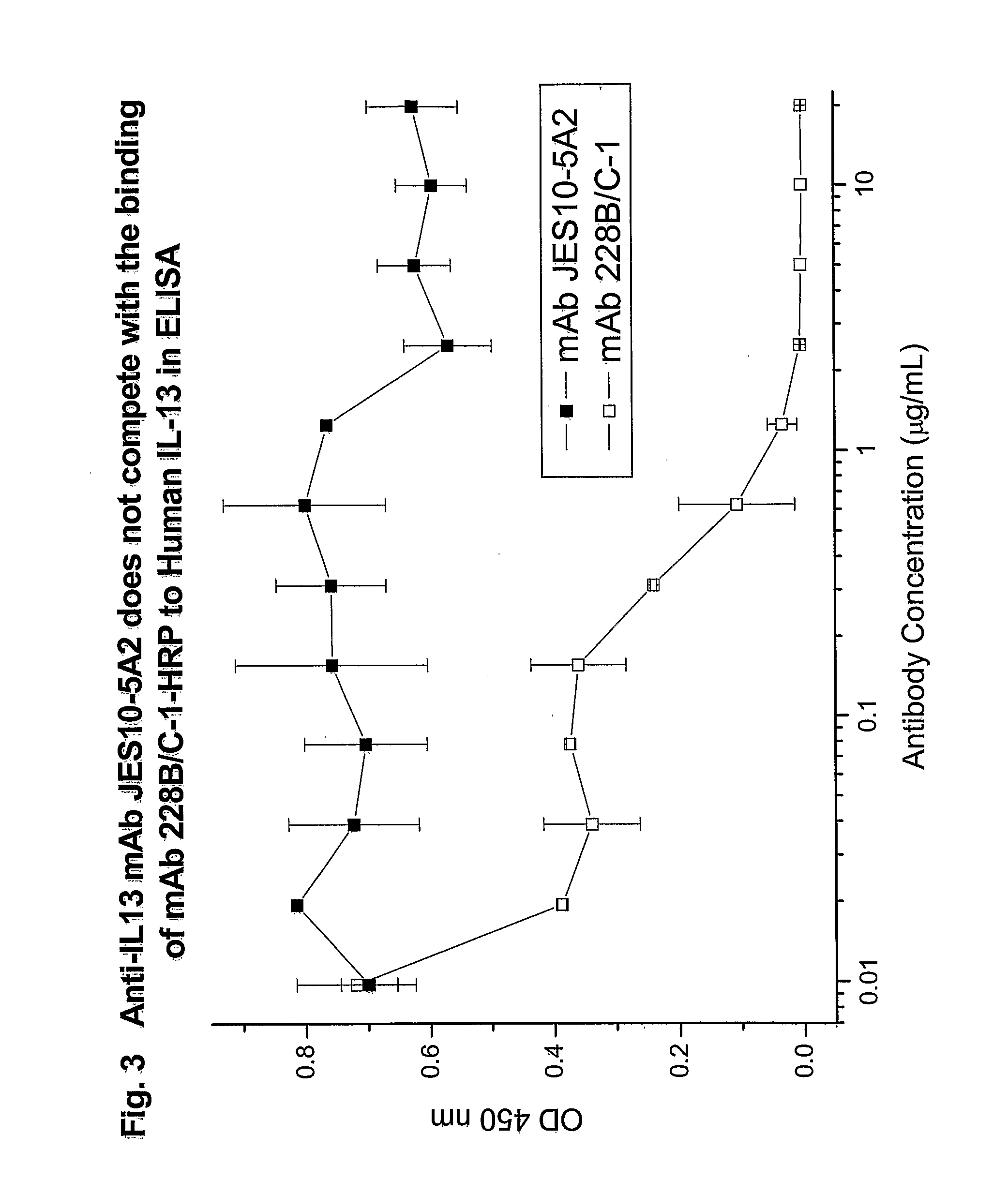
Novel anti-IL13 antibodies and uses thereof
ActiveUS20090214523A1Inhibiting antibody productionRelieve symptomsSenses disorderAntipyreticUveitisNonallergic rhinitis
The present invention relates to anti-IL13 antibodies that bind specifically and with high affinity to both glycosylated and non-glycosylated human IL13, does not bind mouse IL13, and neutralize human IL13 activity at an approximate molar ratio of 1:2 (MAb:IL13). The invention also relates to the use of these antibodies in the treatment of IL13-mediated diseases, such as allergic disease, including asthma, allergic asthma, non-allergic (intrinsic) asthma, allergic rhinitis, atopic dermatitis, allergic conjunctivitis, eczema, urticaria, food allergies, chronic obstructive pulmonary disease, ulcerative colitis, RSV infection, uveitis, scleroderma, and osteoporosis.
Owner:GENENTECH INC
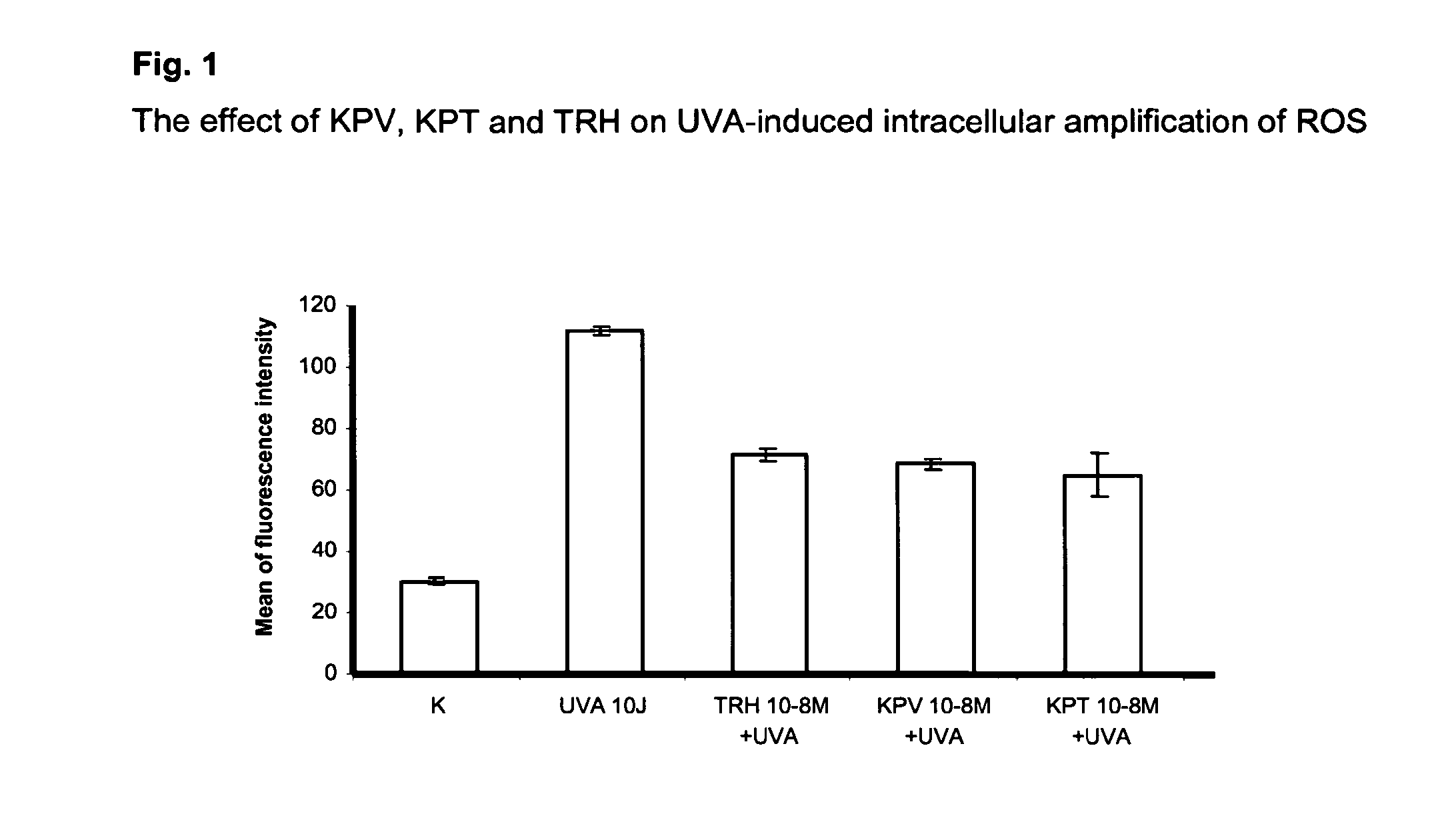
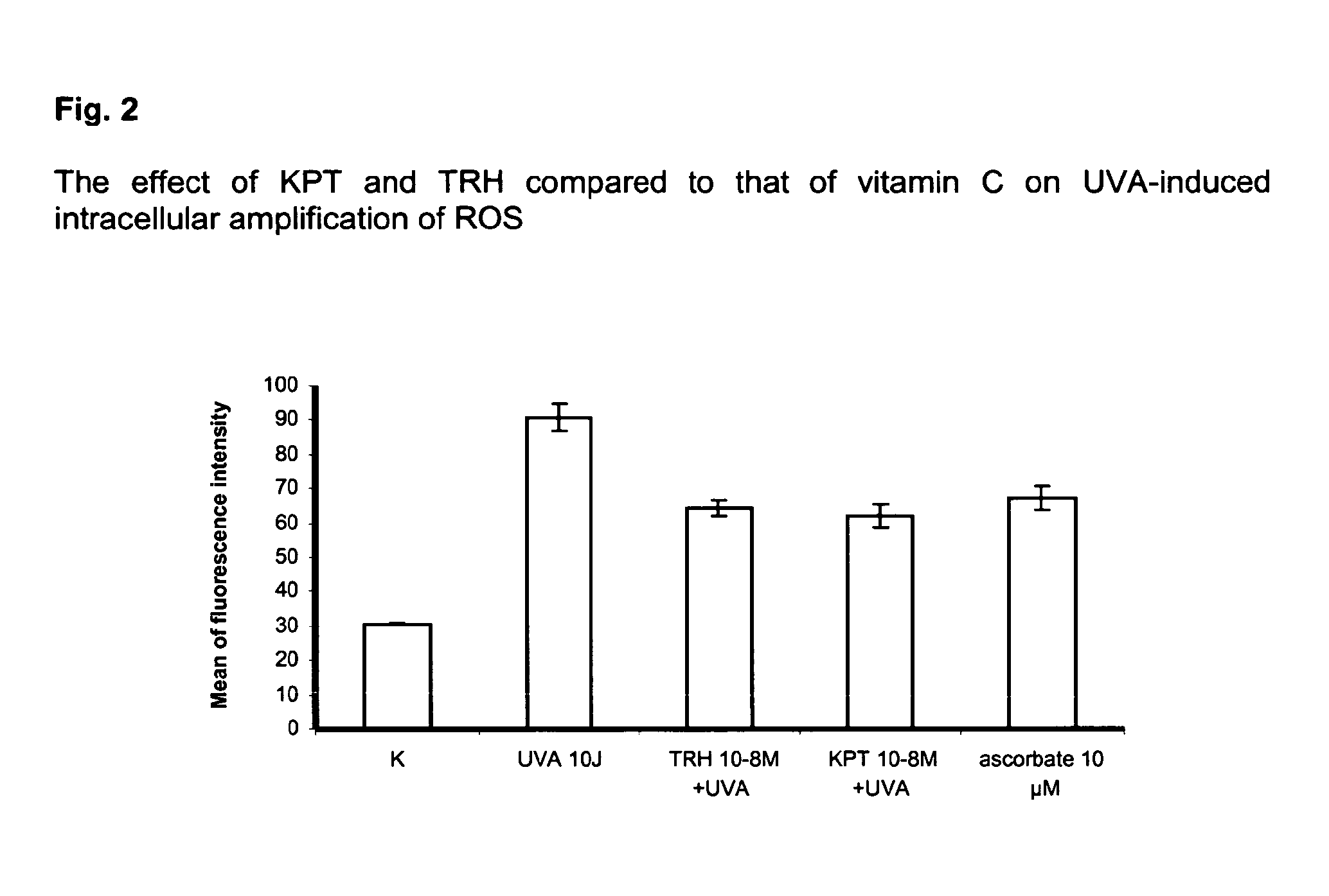
Compositions for reducing oxidative stress and uses thereof
InactiveUS20100286056A1Reduce oxidative stressGood dispersionCosmetic preparationsHair cosmeticsDiseaseMedicine
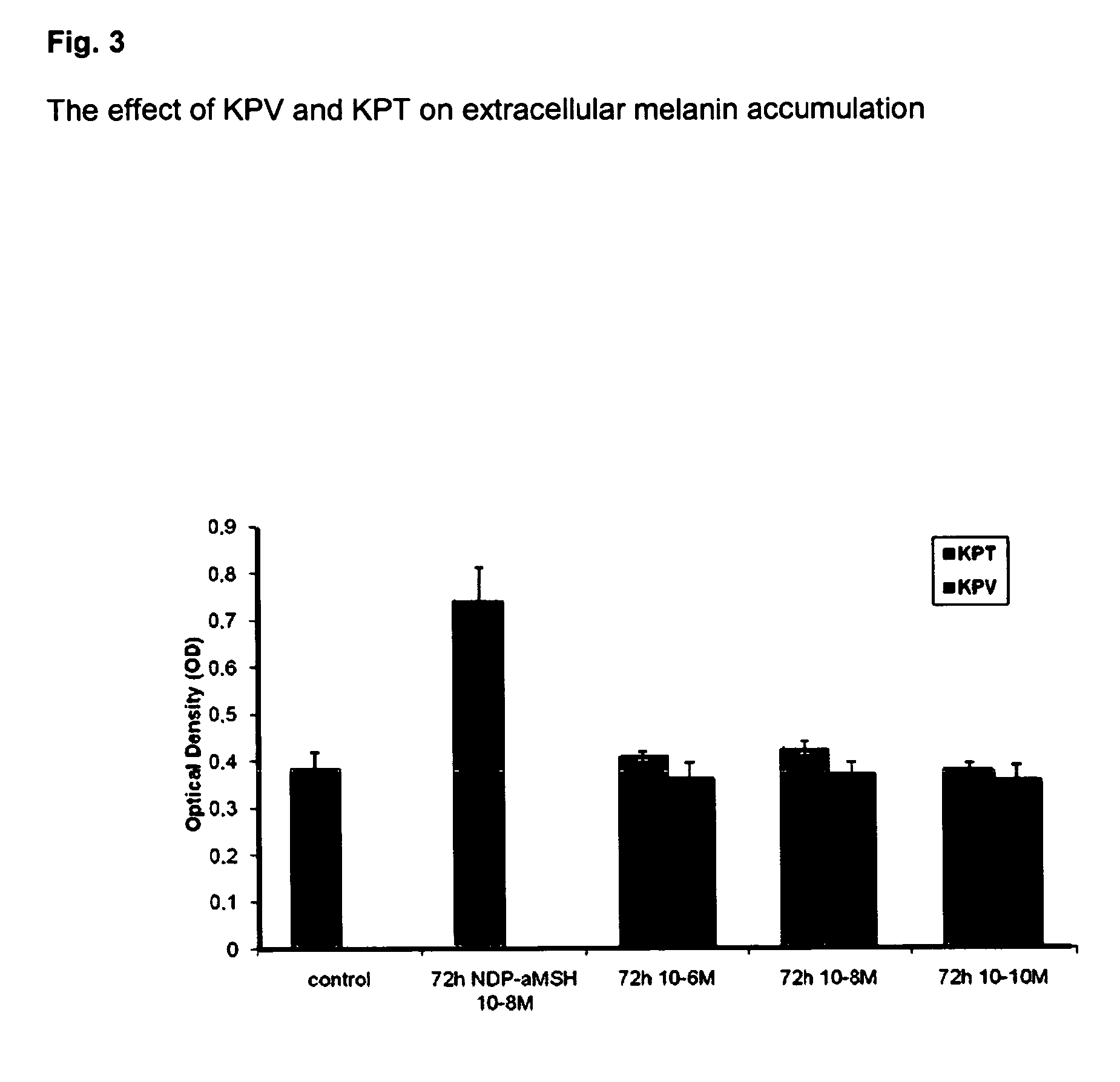
The present invention relates to the use of one or more tripeptides selected from the group consisting of NLys-Pro-ValC, NLys-Pro-ThrC and NpGLu-His-ProC for the reduction of oxidative stress. The above tripeptides are particularly useful for the treatment of a disease or damage caused by oxidative stress; such as vitiligo, scleroderma, necrosis, or erythema; furthermore, a disease or damage of the hair, like premature hair loss or premature formation of grey hair. Furthermore the invention relates the cosmetic use of the above tripeptides, in particular against skin aging. Further the invention relates cosmetic compositions containing at least one of said tripeptides.
Owner:UNIVSKLINIKUM MUNSTER
Toxin peptide therapeutic agents
ActiveUS7833979B2Preventing and mitigating relapseAvoid it happening againNervous disorderAntipyreticHalf-lifeFibrosis
Disclosed is a composition of matter of the formula(X1)a—(F1)d—(X2)b—(F2)e—(X3)c (I)and multimers thereof, in which F1 and F2 are half-life extending moieties, and d and e are each independently 0 or 1, provided that at least one of d and e is 1; X1, X2, and X3 are each independently -(L)f-P-(L)g-, and f and g are each independently 0 or 1; P is a toxin peptide of no more than about 80 amino acid residues in length, comprising at least two intrapeptide disulfide bonds; L is an optional linker; and a, b, and c are each independently 0 or 1, provided that at least one of a, b and c is 1. Linkage to the half-life extending moiety or moieties increases the in vivo half-life of the toxin peptide, which otherwise would be quickly degraded. A pharmaceutical composition comprises the composition and a pharmaceutically acceptable carrier. Also disclosed are a DNA encoding the inventive composition of matter, an expression vector comprising the DNA, and a host cell comprising the expression vector. Methods of treating an autoimmune disorder, such as, but not limited to, multiple sclerosis, type 1 diabetes, psoriasis, inflammatory bowel disease, contact-mediated dermatitis, rheumatoid arthritis, psoriatic arthritis, asthma, allergy, restinosis, systemic sclerosis, fibrosis, scleroderma, glomerulonephritis, Sjogren syndrome, inflammatory bone resorption, transplant rejection, graft-versus-host disease, and lupus and of preventing or mitigating a relapse of a symptom of multiple sclerosis are also disclosed.
Owner:AMGEN INC
Copper lowering treatment of inflammatory and fibrotic diseases
InactiveUS6855340B2Treatment safetyLower Level RequirementsBiocideHeavy metal active ingredientsDithiomolybdateLiver disease
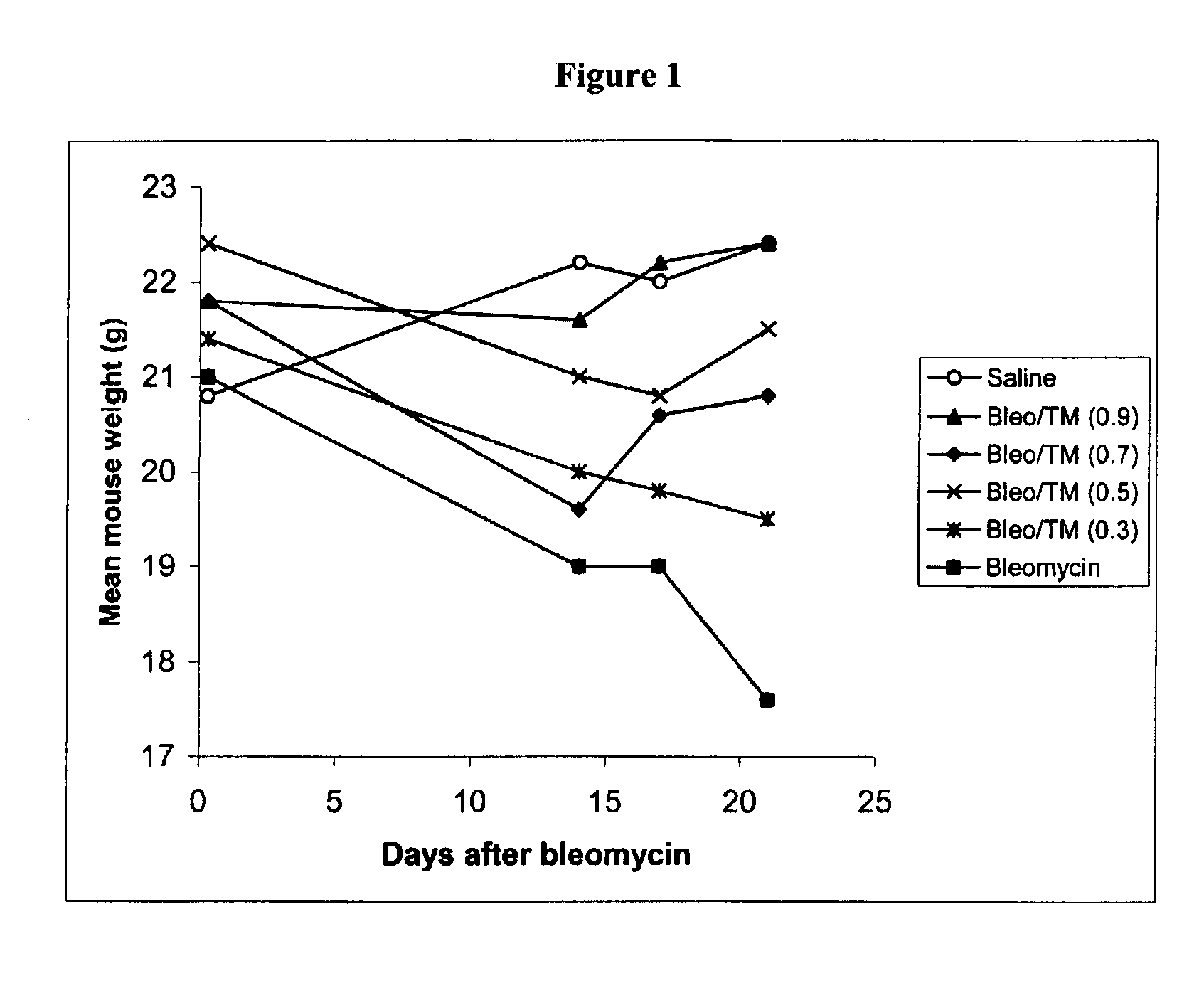
The present invention relates generally to the field of prophylaxis and therapy for inflammatory and / or fibrotic diseases which include responses to injuries. In particular, the present invention is related to agents that can bind or complex copper such as thiomolybdate, and to the use of these agents in the prevention and treatment of inflammatory and / or fibrotic diseases. Exemplary thiomolybdates include mono-, di-, tri- and tetrathiomolybdate; these agents are administered to patients to prevent and / or treat inflammatory and / or fibrotic diseases, such as pulmonary disease including pulmonary fibrosis and acute respiratory distress syndrome, liver disease including liver cirrhosis and hepatitis C, kidney disease including renal interstitial fibrosis, scleroderma, cystic fibrosis, pancreatic fibrosis, keloid, secondary fibrosis in the gastrointestinal tract, hypertrophic burn scars, myocardial fibrosis, Alzheimer's disease, retinal detachment inflammation and / or fibrosis resulting after surgery, and graft versus host and host versus graft rejections.
Owner:RGT UNIV OF MICHIGAN
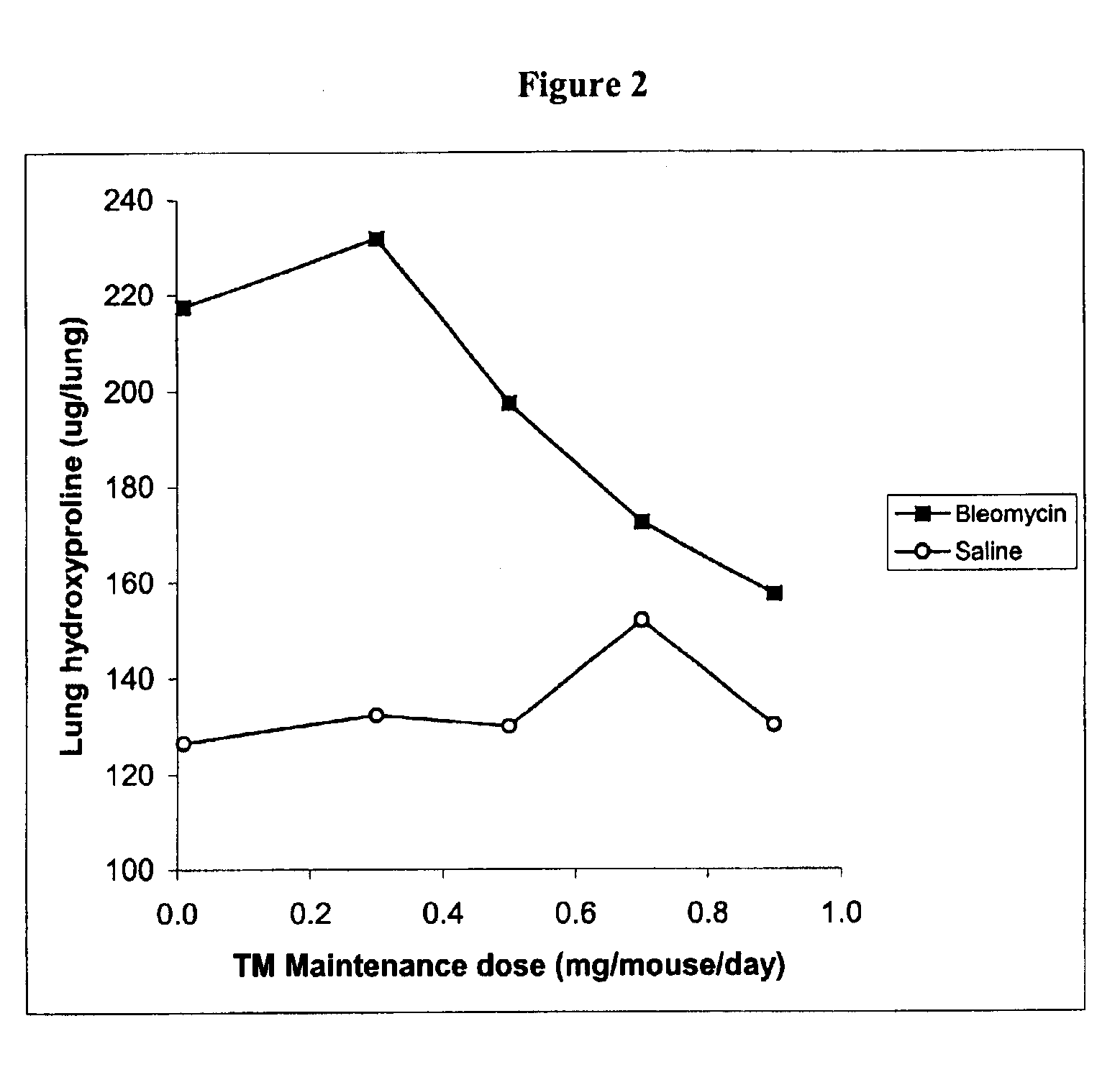
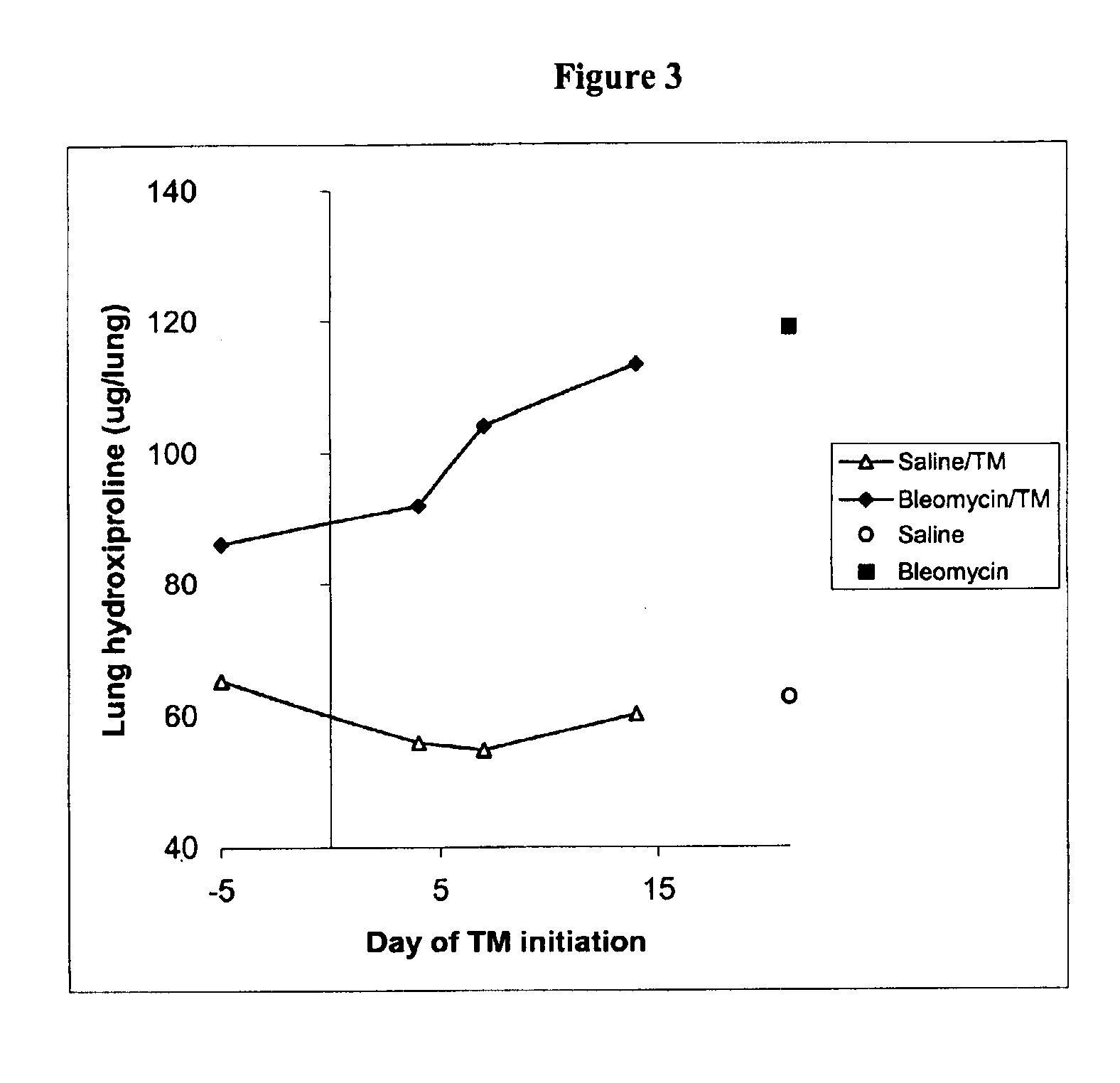
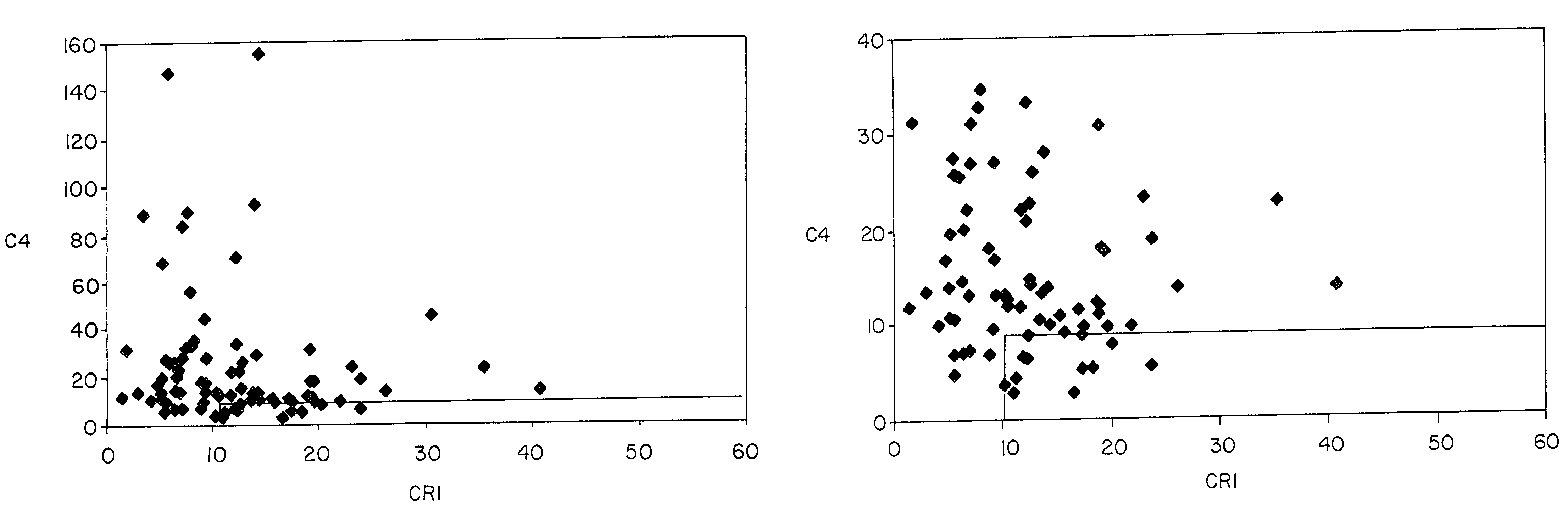
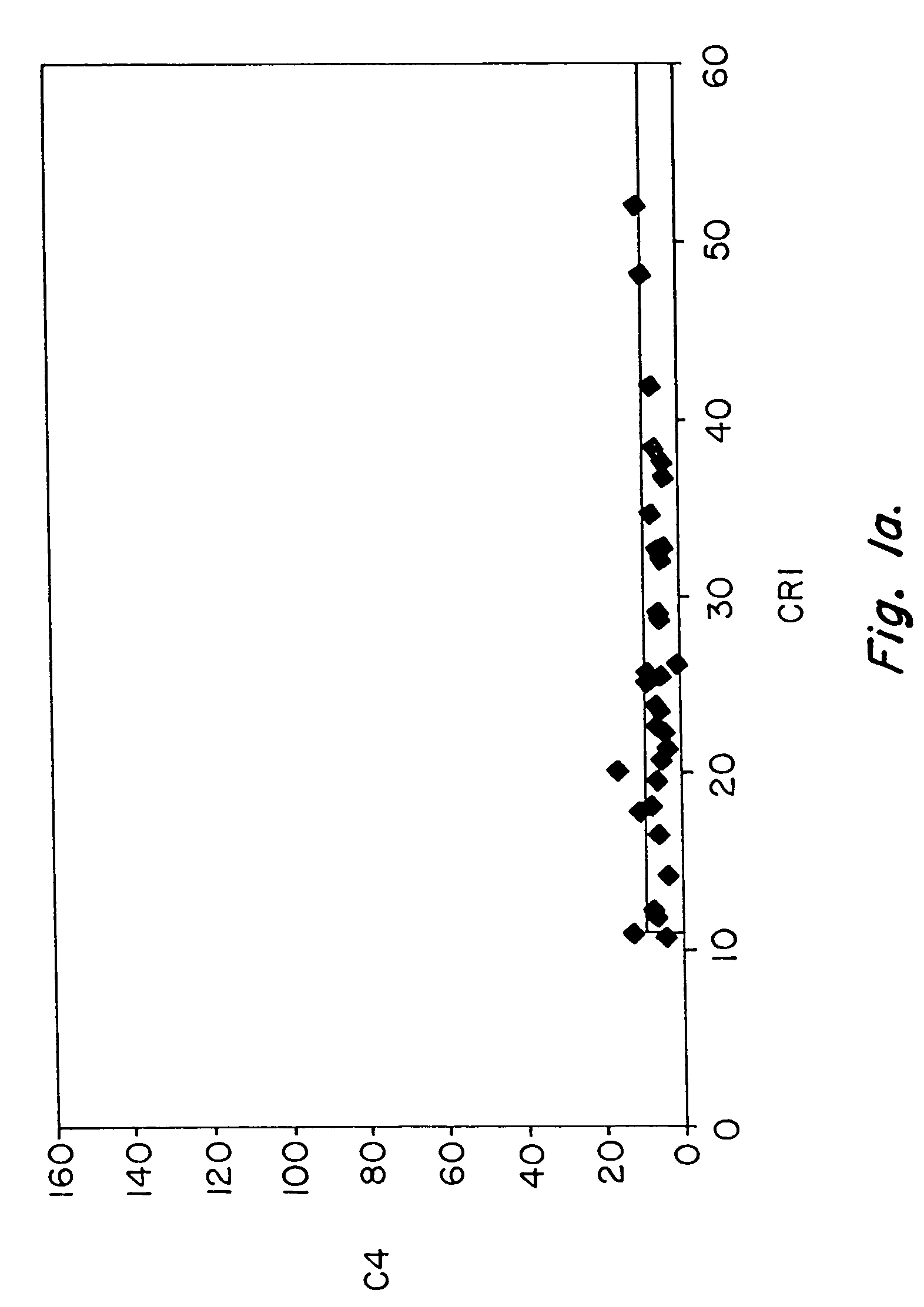
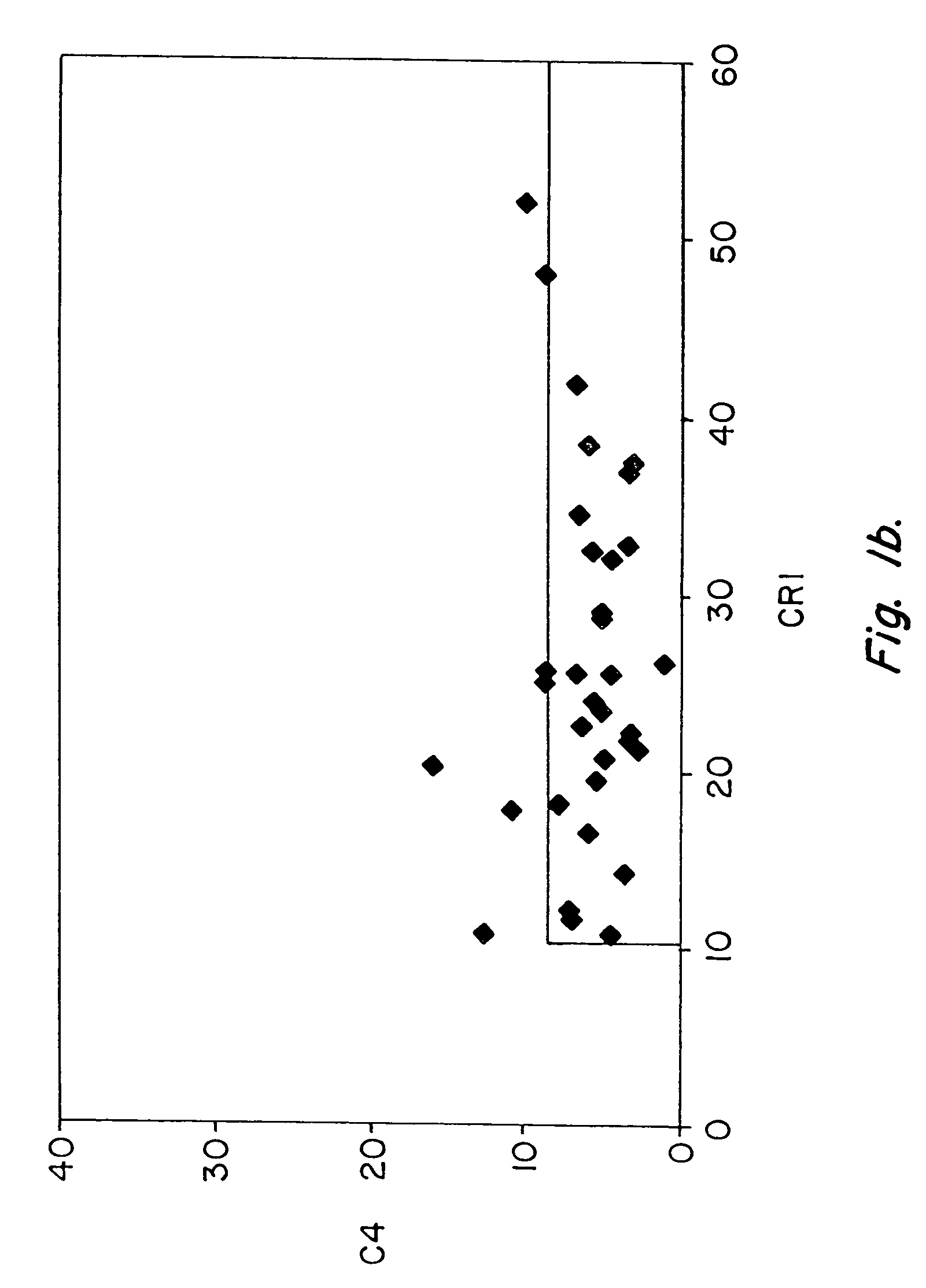
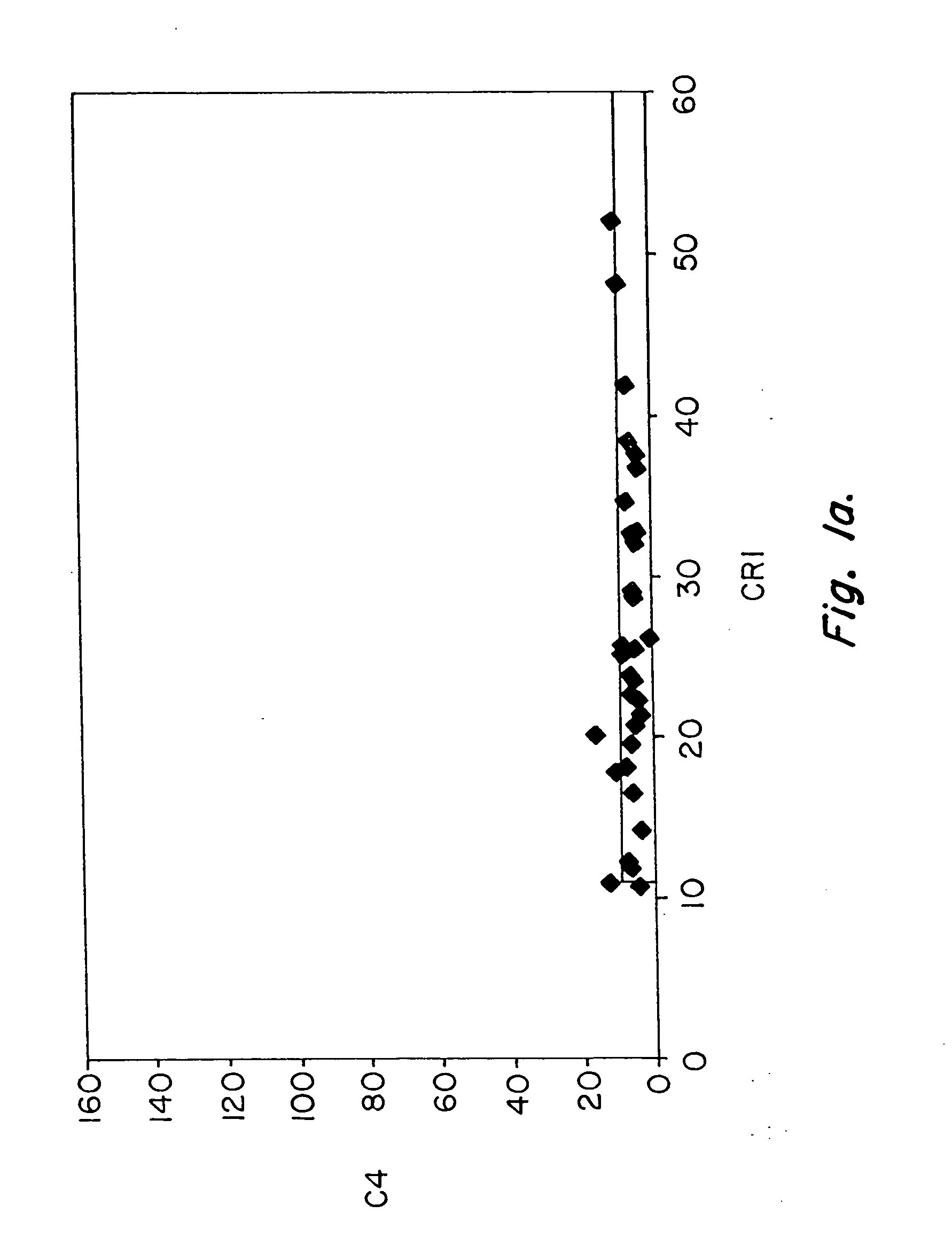
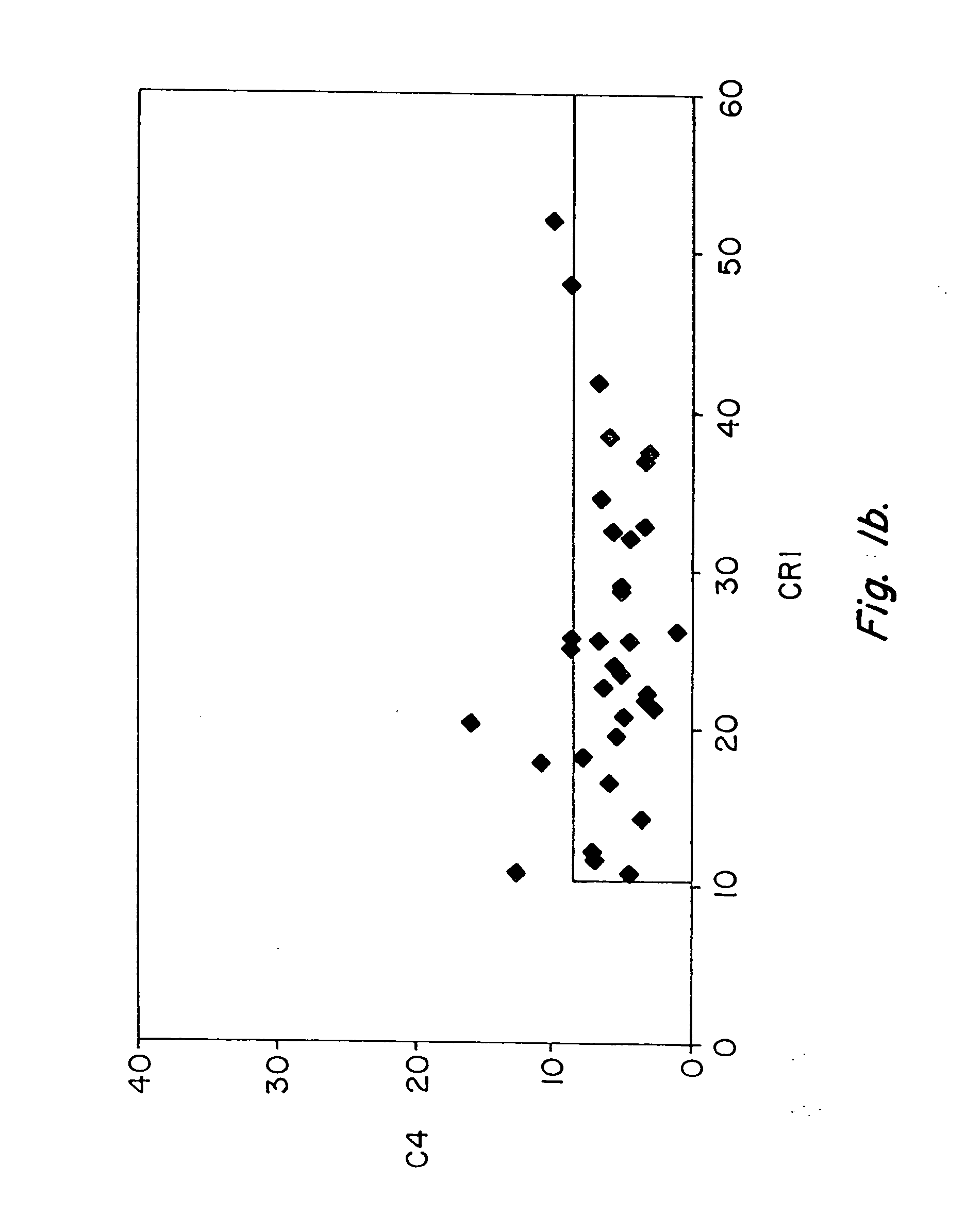
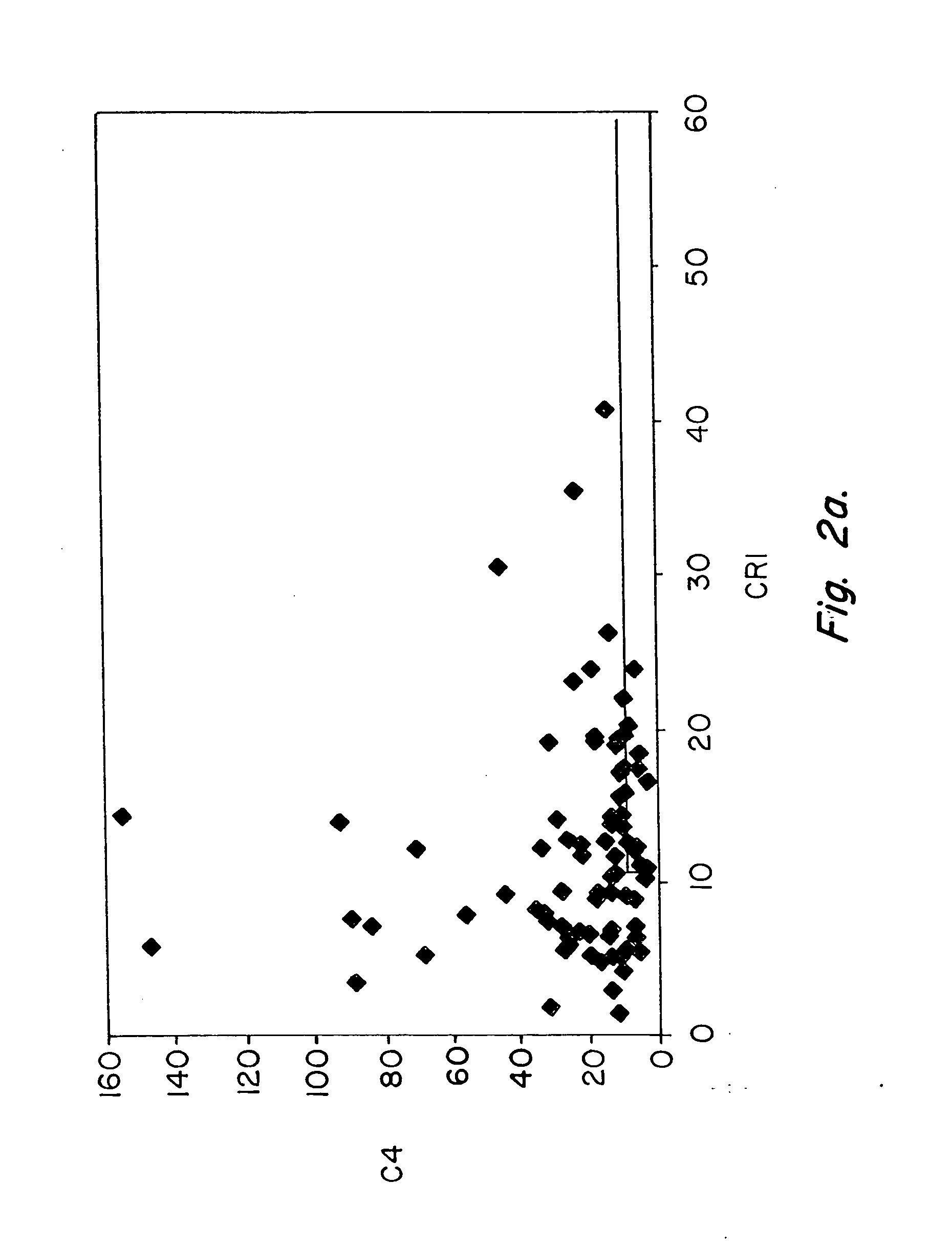
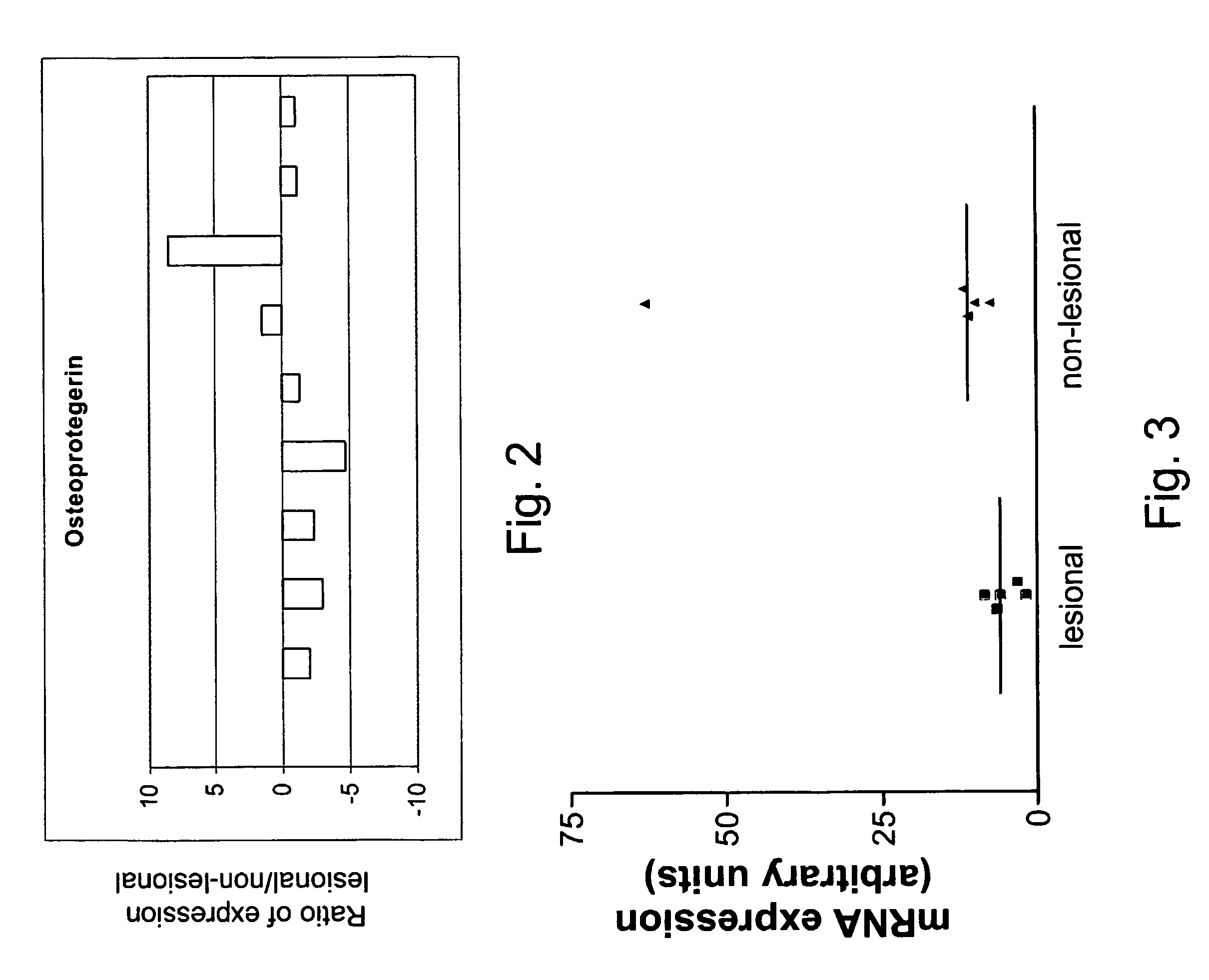
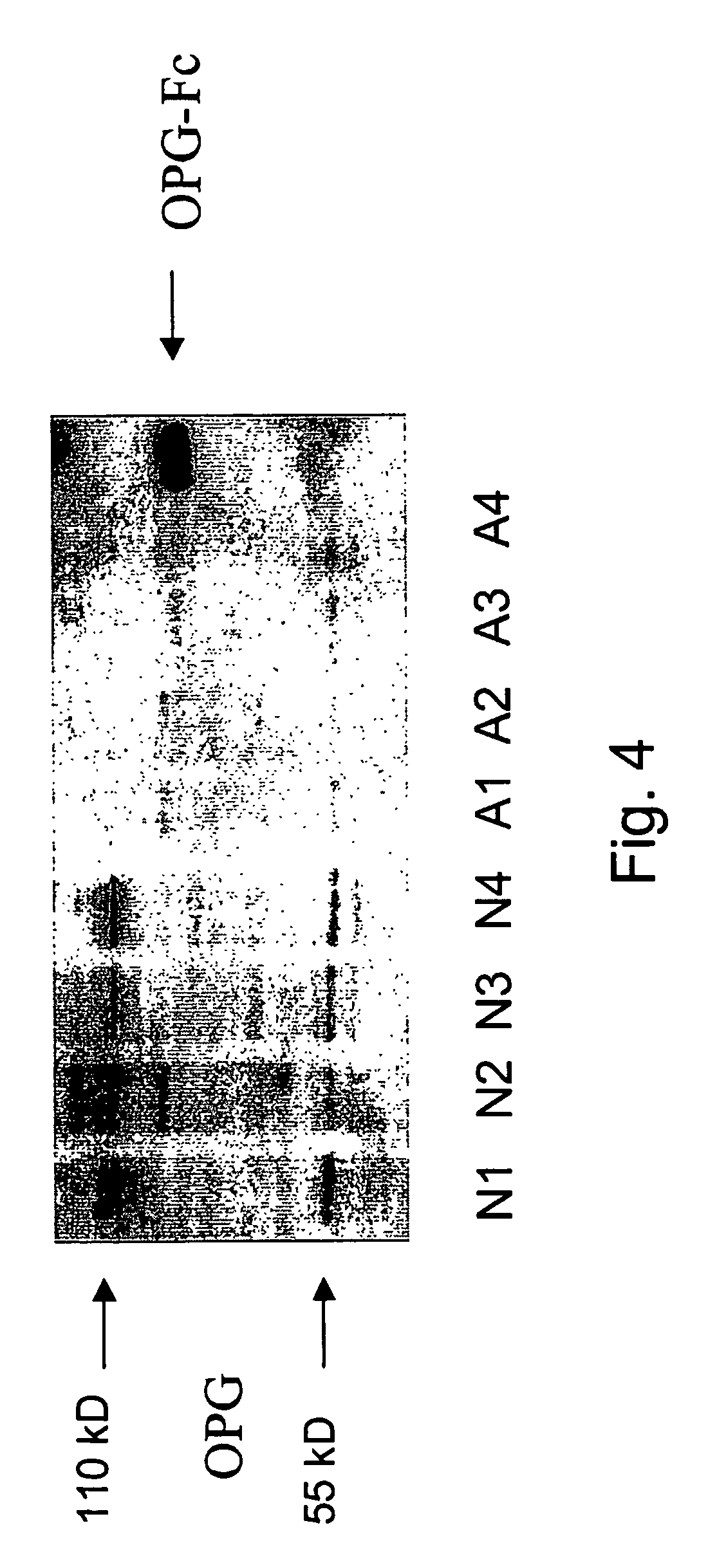
Diagnosis and monitoring of systemic lupus erythematosus and of scleroderma
ActiveUS7390631B2Bioreactor/fermenter combinationsBiological substance pretreatmentsRed CellSclerosis skin
Methods for diagnosing and monitoring systemic lupus erythematosus (SLE) or scleroderma by determining, in a blood sample from the individual being diagnosed or monitored, complement component C4d deposited on surfaces of red blood cells in the sample, and optionally also determining complement receptor CR1 deposited on the red blood cell surfaces. For diagnosis this is compared with the quantity of C4d (and optionally CR1) present on red blood cells of normal individuals. For monitoring it is compared with a value in a sample or samples previously obtained from the individual patient. The comparison may be made with individual values for C4d and CR1 and / or with a ratio of the two found in normal individuals.
Owner:UNIVERSITY OF PITTSBURGH
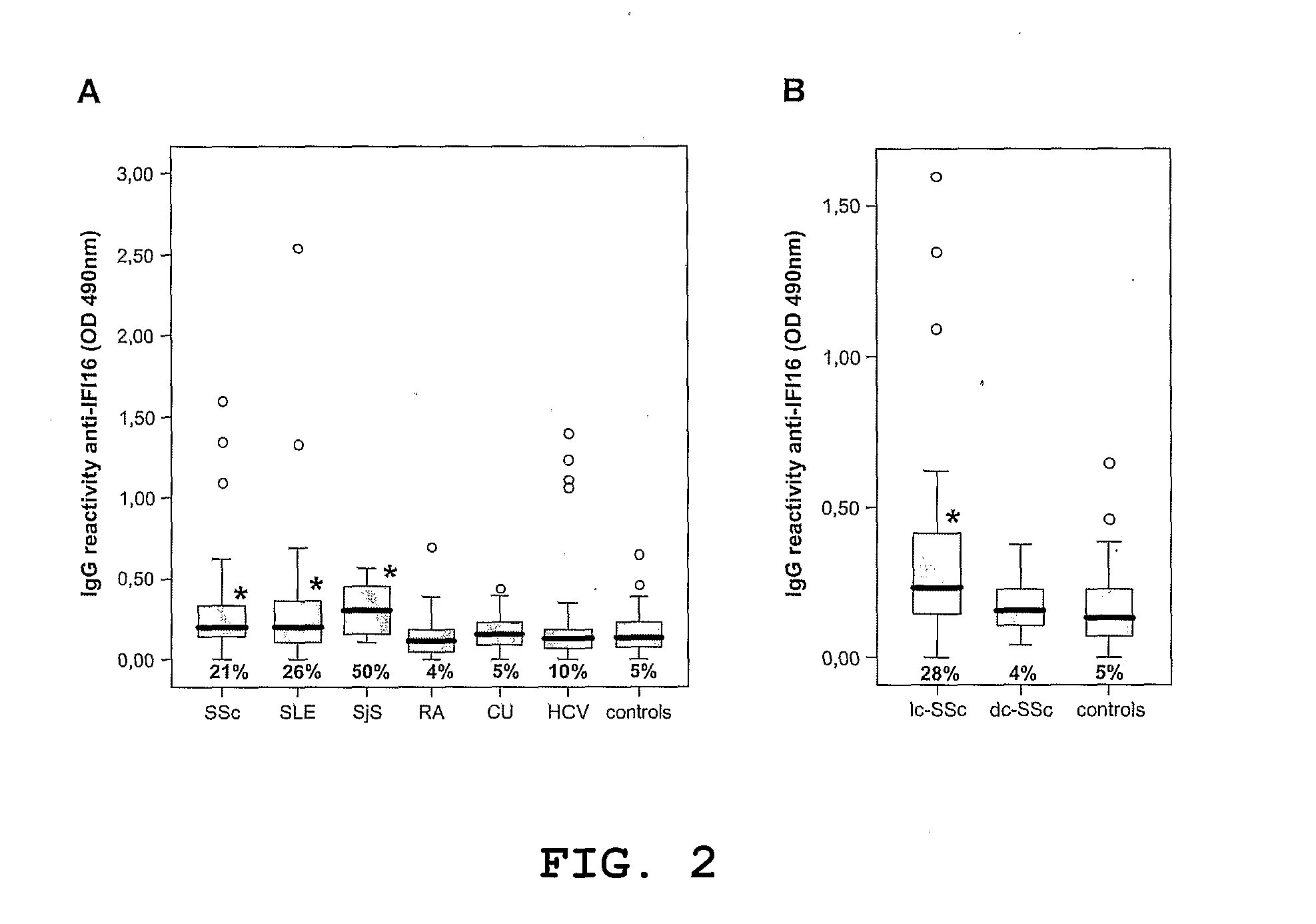
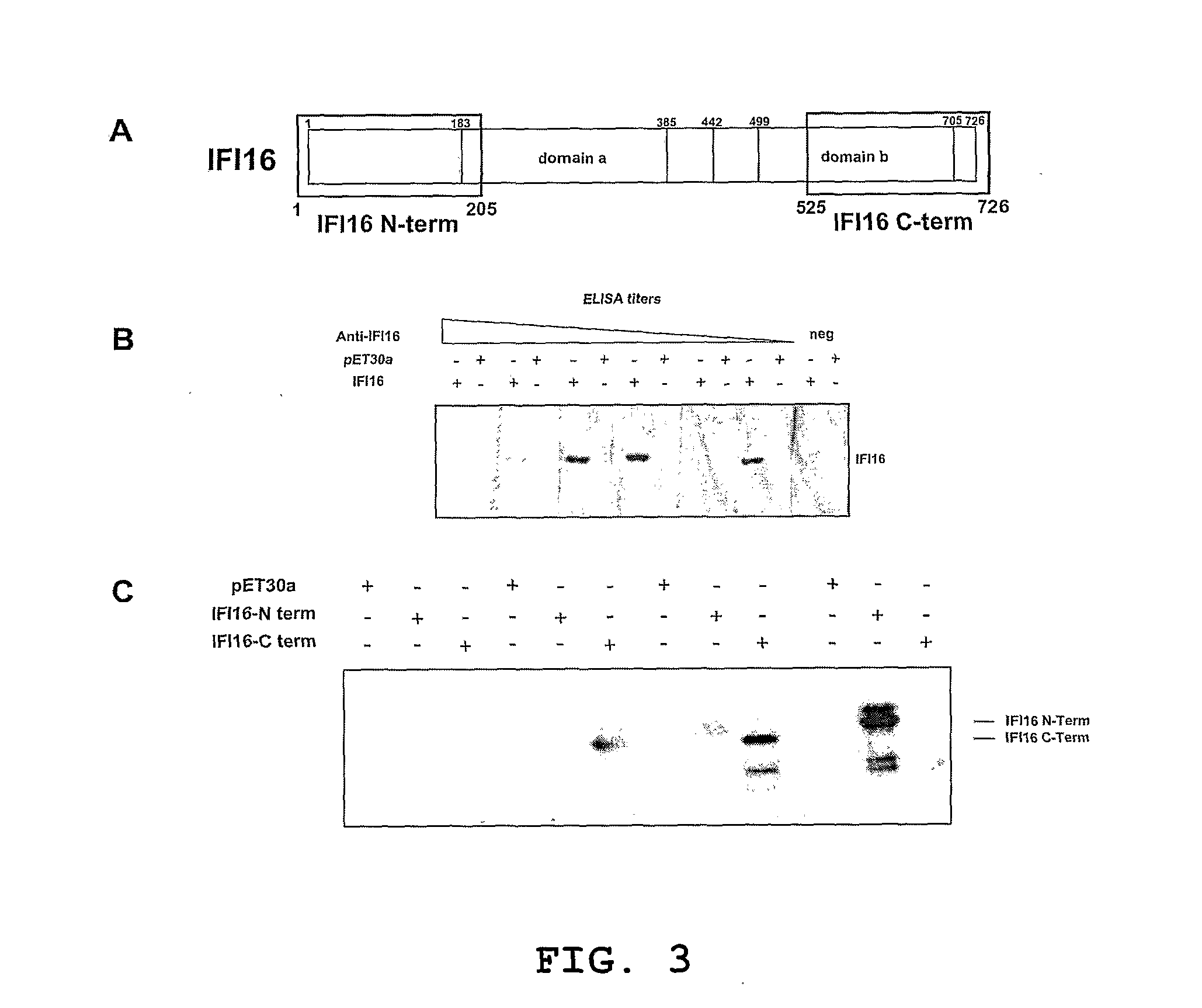
Differential diagnosis for scleroderma
Use of IFI16 protein, fragments or peptides thereof for differential diagnosis of the limited cutaneous form of scleroderma (Ic-SSc) in a subject suspected of or at risk of having an autoimmune disease and the corresponding method of diagnosis and kit.
Owner:UNIV DEGLI STUDI DEL PIEMONTE ORIENTALEAMEDEO AVOGADRO
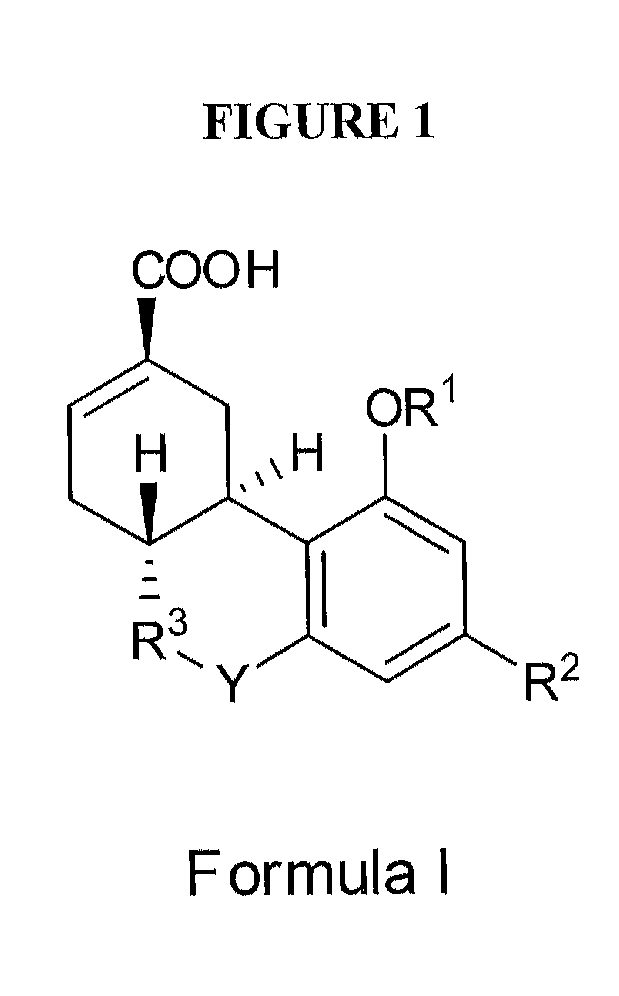
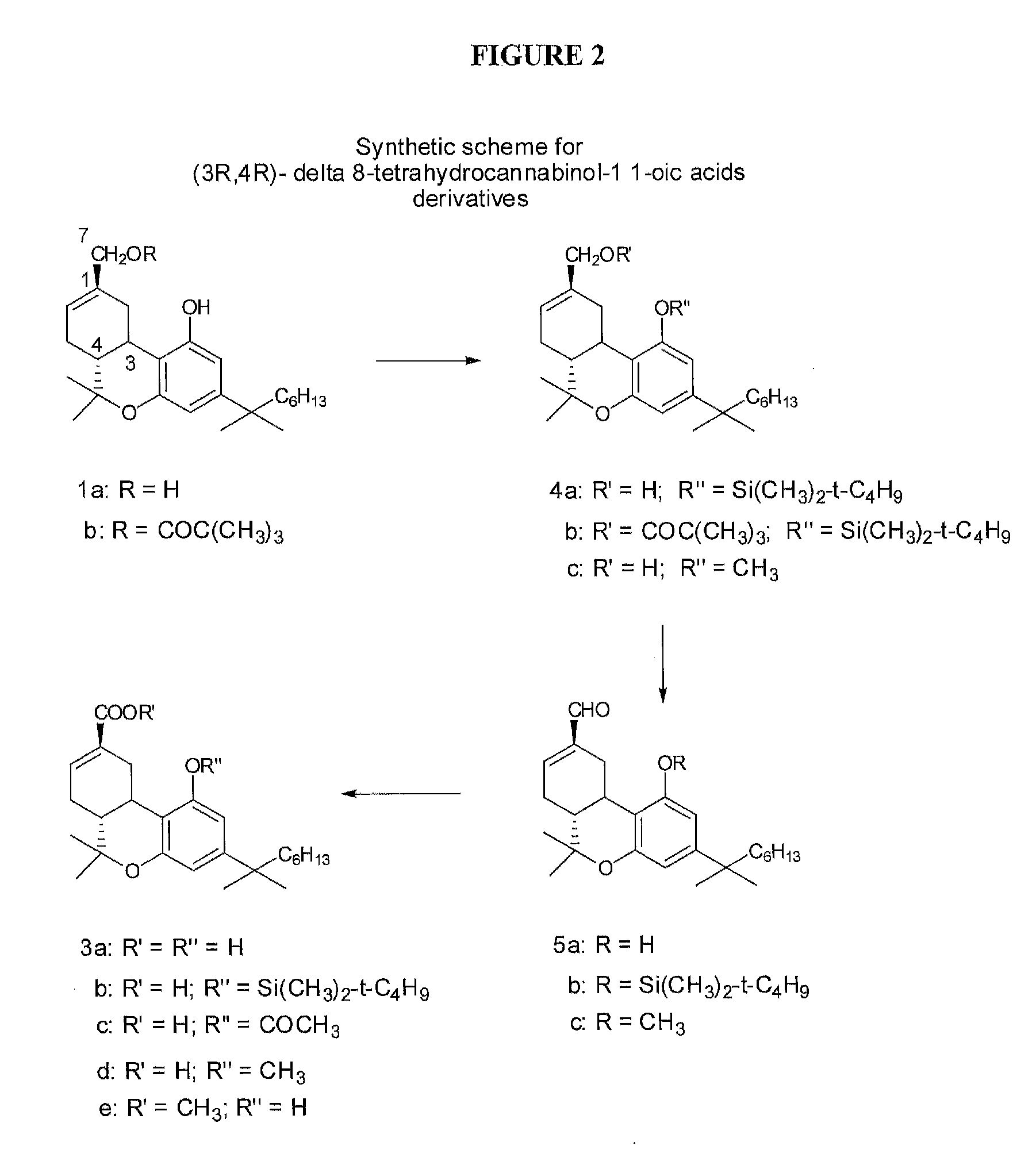
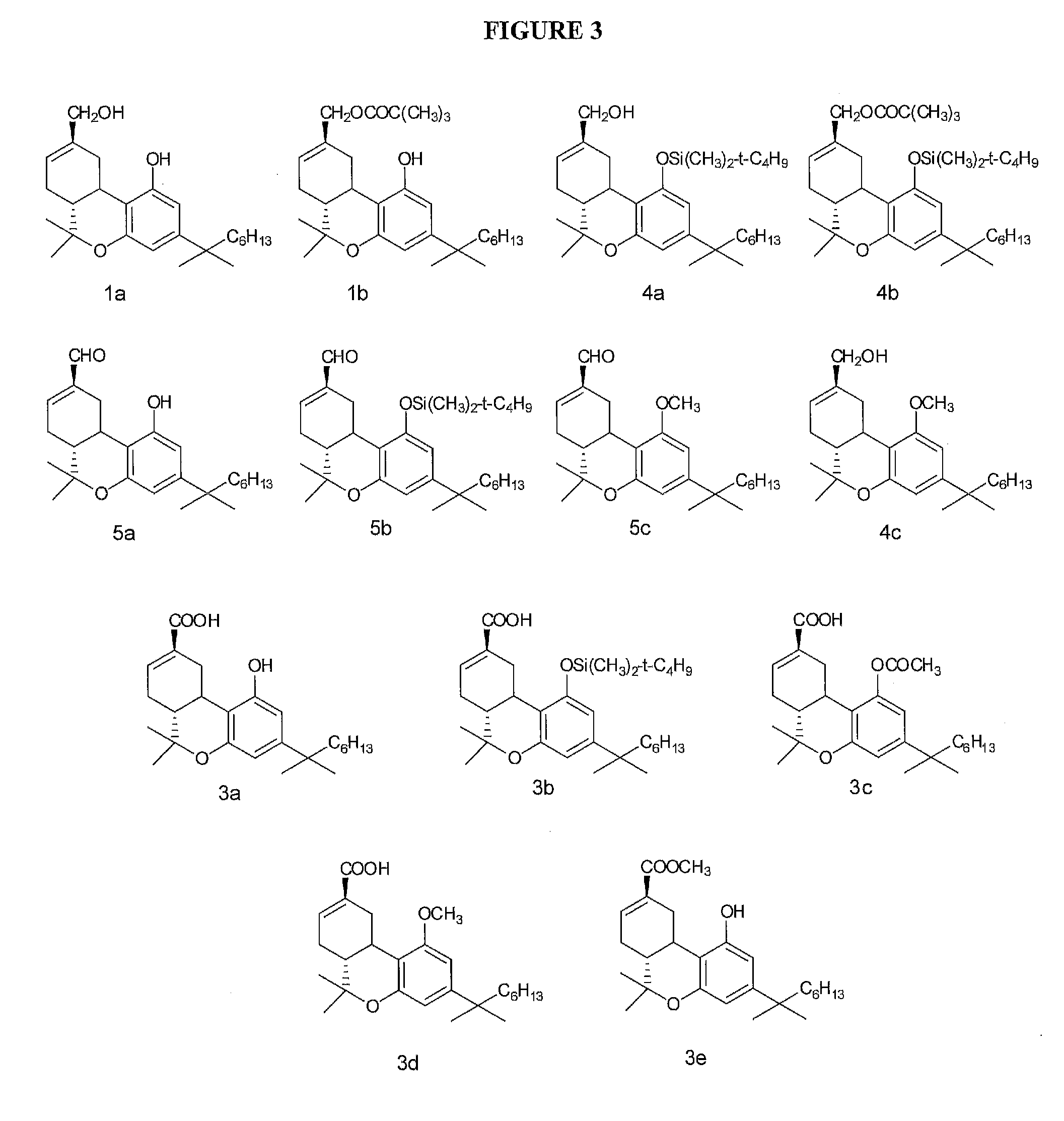
Methods of treating fibrotic diseases using tetrahydrocannabinol-11-oic acids
This invention is in the field of medicinal chemistry and relates to novel compounds, and pharmaceutical compositions and methods of use thereof for the treatment and / or prevention of fibrotic diseases including scleroderma, systemic sclerosis, scleroderma-like disorders, sine scleroderma, liver cirrhosis, interstitial pulmonary fibrosis, Dupuytren's contracture, keloids, chronic kidney disease, chronic graft rejection, and other scarring / wound healing abnormalities, post operative adhesions, and reactive fibrosis. The invention also relates to methods of using the compounds and pharmaceutical compositions of this invention to treat fibrotic conditions.
Owner:CORBUS PHARM INC
Diagnosis and monitoring of systemic lupus erythematosus and of scleroderma
ActiveUS20050037441A1Bioreactor/fermenter combinationsBiological substance pretreatmentsRed CellSclerosis skin
Methods for diagnosing and monitoring systemic lupus erythematosus (SLE) or scleroderma by determining, in a blood sample from the individual being diagnosed or monitored, complement component C4d deposited on surfaces of red blood cells in the sample, and optionally also determining complement receptor CR1 deposited on the red blood cell surfaces. For diagnosis this is compared with the quantity of C4d (and optionally CR1) present on red blood cells of normal individuals. For monitoring it is compared with a value in a sample or samples previously obtained from the individual patient.The comparison may be made with individual values for C4d and CR1 and / or with a ratio of the two found in normal individuals.
Owner:UNIVERSITY OF PITTSBURGH
A3 adenosine receptor antagonists and partial agonists
InactiveUS20110171130A1High potencyHighly selectiveBiocideSenses disorderHypersensitive responseArthritis
Disclosed are A3 adenosine receptor antagonists and / or partial agonists of formula (I): wherein R1 to R5 are as described herein, as well as pharmaceutical compositions thereof and methods of use thereof. The antagonists or partial agonists find use in treating a number of diseases including cancer, glaucoma, inflammatory diseases, asthma, stroke, myocardial infarction, allergic reactions, rhinitis, poison ivy induced responses, urticaria, scleroderma, arthritis, brain arteriole diameter constriction, bronchoconstriction, and myocardial ischemia, as well as in preventing cardiac ischemia. Also disclosed are radiolabeled compounds of formula (I) and the use thereof in diagnostic imaging of tissues and organs.
Owner:UNITED STATES OF AMERICA
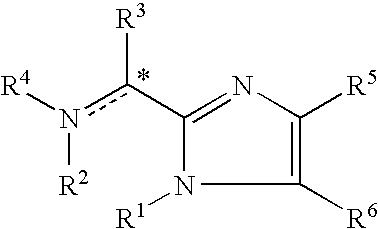
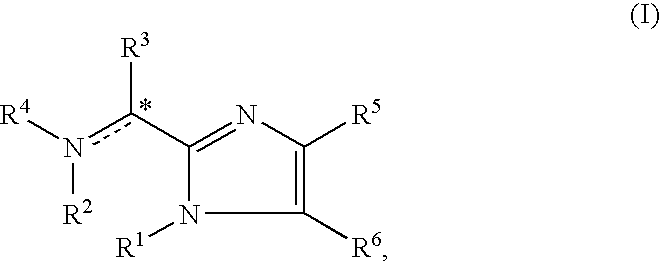
Imidazolyl derivatives
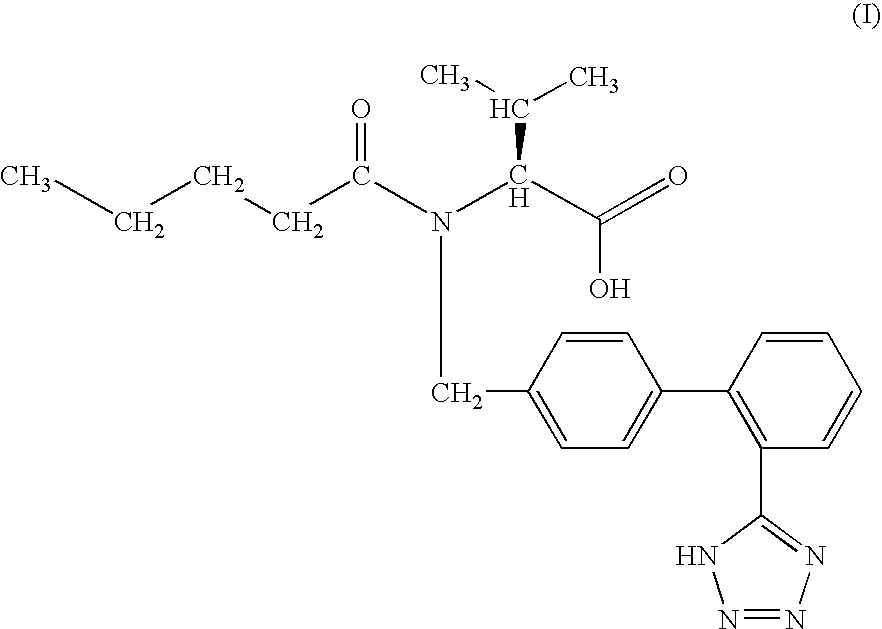
The present invention is directed to imidazolyl derivatives of the formula: where the substituents are defined in the specification, or a pharmaceutically acceptable salt thereof. The derivatives bind selectively to the somatostatin subtype receptors and elicit either an agonist or antagonist effect from the somatostatin subtype receptors. The derivatives are useful for treating a variety of diseases including acromegaly, restenosis, Crohn's disease, systemic sclerosis, external and internal pancreatic pseudocysts and ascites, VIPoma, nesidoblastosis, hyperinsulinism, gastrinoma, Zollinger-Ellison Syndrome, diarrhea, AIDS related diarrhea, chemotherapy related diarrhea, scleroderma, Irritable Bowel Syndrome, pancreatitis, small bowel obstruction, gastroesophageal reflux, duodenogastric reflux, Cushing's Syndrome, gonadotropinoma, hyperparathyroidism, Graves' Disease, diabetic neuropathy, Paget's disease, polycystic ovary disease, cancer, cancer cachexia, hypotension, postprandial hypotension, panic attacks, GH secreting adenomas or TSH secreting adenomas.
Owner:IPSEN PHARMA SAS
Topical medicament
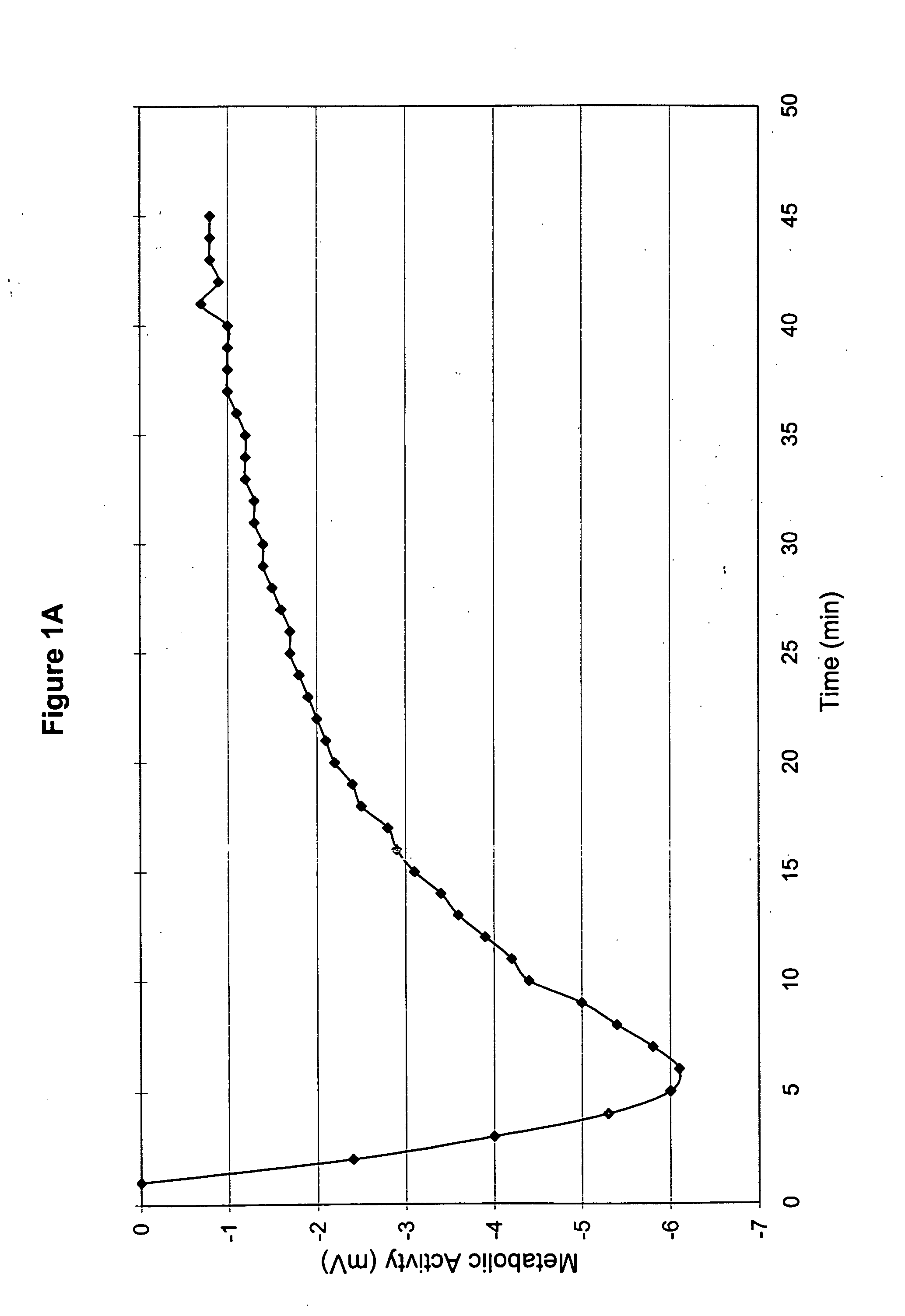
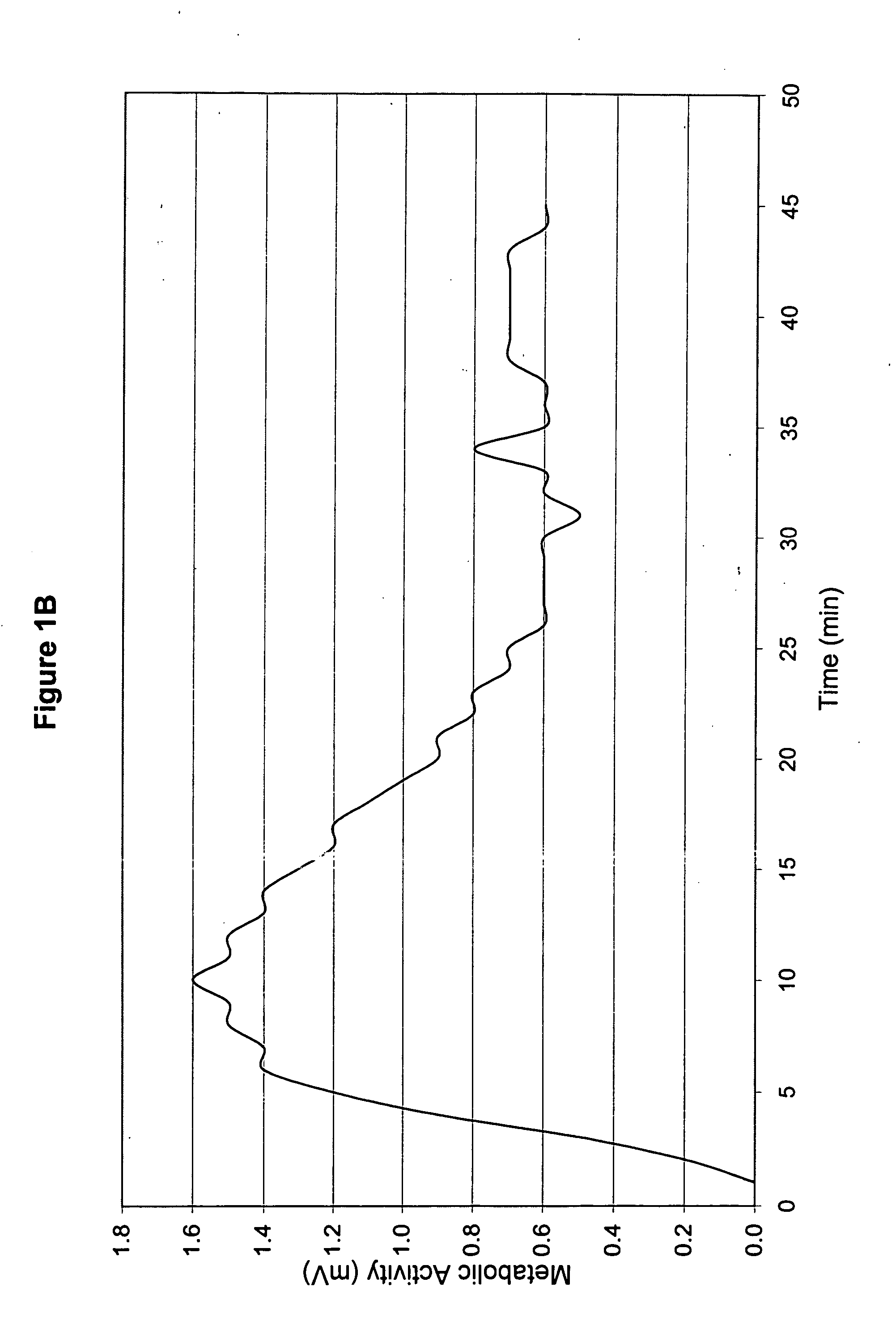
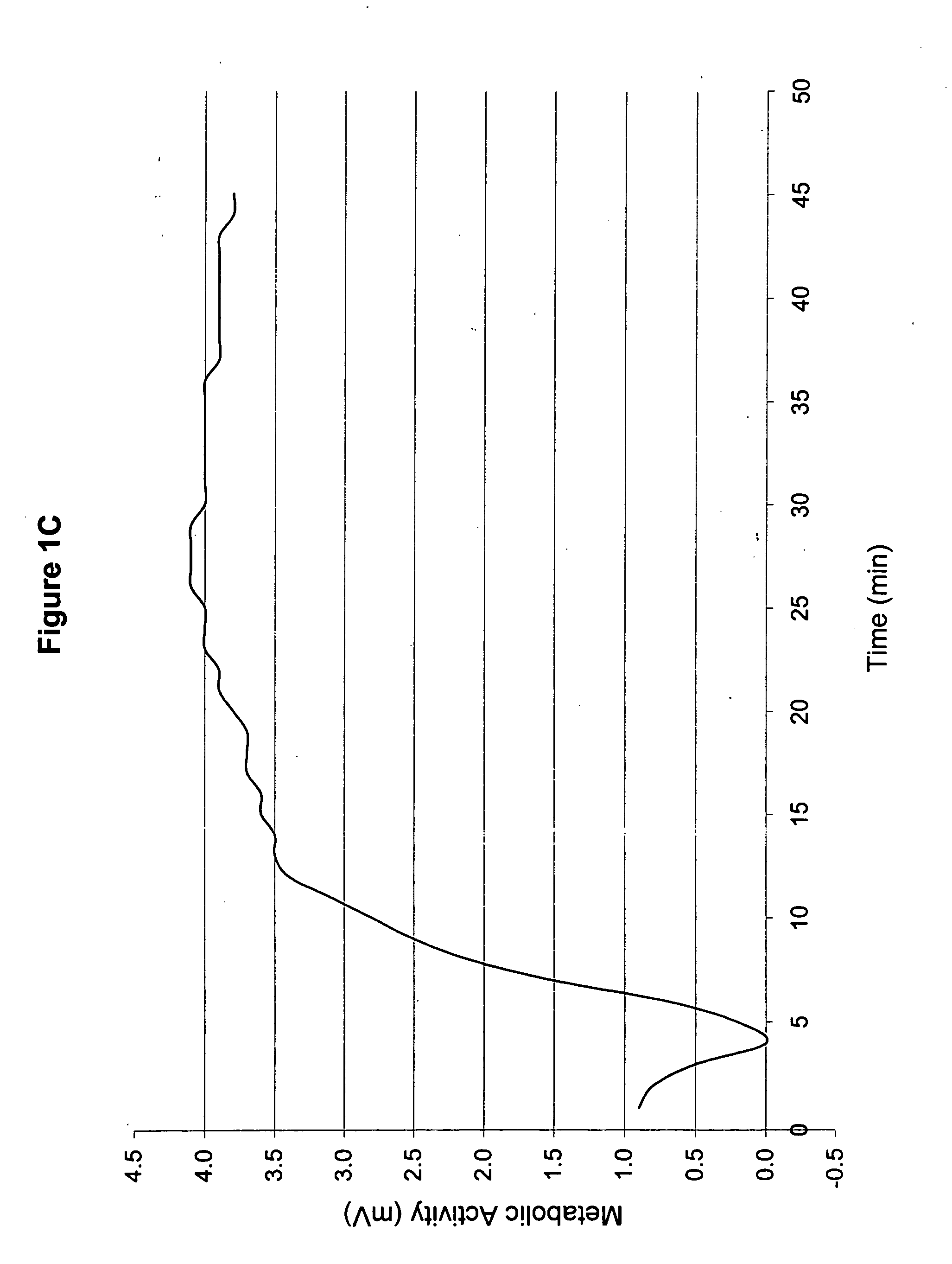
InactiveUS20060051432A1Improve metabolic activityStimulates the nervous systemBiocideNervous disorderDiseaseSinusitis
New topical medicaments are provided. The medicaments comprise menthol and camphor, preferably provided as part of a base gel, supplemented with potassium and a source of oxygen. The most preferred base gel is sold under the name SOMBRA, while the most preferred source of oxygen is a chlorite (e.g., sodium chlorite) and / or spirulina. The medicaments provide high metabolic activities and sustain those activities over prolonged periods of time, thus being useful for treating a large variety of ailments, including diabetic neuropathy, post hepatic neuralgia, scleroderma, psoriasis, strain, spasticity, headaches, neuropathy secondary to drugs, peripheral neuropathy, leg pain, muscle cramps, muscle aches and pains, bruise, sinusitis, sprain, arthritis, joint pain (arthralgia), and edema.
Owner:MORGAN CLYDE
Methods of treating autoimmune diseases using IL-21
ActiveUS20050095223A1Mitigating autoimmune responseReduced responseNervous disorderPeptide/protein ingredientsArthritis osteoarthritisAutoimmune condition
Owner:ZYMOGENETICS INC
Herbal composition for treating various disorders including psoriasis, a process for preparation thereof and method for treatment of such disorders
InactiveUS20030194456A1Safe and well-toleratedMinimal effectBiocideAntipyreticPhosphodiesteraseEnzyme inhibition
The invention provides a novel herbal composition containing the extracts of the leaves and / or stem of <italic>Argemone mexicana < / highlight>plant, optionally containing the extracts of the fruits of <italic>Cuminum cyminum< / highlight>, which exhibits useful in vitro, in vivo and interesting immunological and pharmacological activities; a process for preparation thereof; and a method of treatment of psoriasis and related immunological and biological disorders by administration of the said novel herbal composition. The useful in vitro, in vivo and interesting immunological and pharmacological activities exhibited by the extracts and fractions of the leaves and / or stem of <italic>Argemone mexicana < / highlight>plant include immunosuppression, lymphoproliferation inhibition, cytokine modulation such as IL-2 inhibition, IFNgamma inhibition, IL-10 induction, keratinocyte proliferation inhibition, keratolytic activity, endothelial cell proliferation inhibition, inhibition of cell adhesion molecule expression such as ICAM-1, MEST inhibition, and enzymes inhibition such as p60src Tyrosine kinase, which are known to be involved in anti-psoriatic activity. The novel herbal composition(s) is useful in the treatment of various disorders, such as psoriasis including plaque psoriasis, gutatte psoriasis, pustular psoriasis and psoriasis of the nails; dermatitis and scleroderma; eczema; inflammatory disorders and other autoimmune diseases like psoriatic arthritis, rheumatoid arthritis, Crohn's disease, multiple sclerosis, irritable bowel disease, ankylosing spondilitis, systemic lupus erythremetosus and Sjogren's syndrome; allergies like asthma and chronic obstructive pulmonary disease and is safe, well-tolerated, non-toxic, with minimal and reversible adverse reactions or side effects, and most importantly, with minimal relapse or recurrence of the disease following completion of a treatment regimen. The invention also describes the presence of phosphodiesterase (III, IV and V) inhibition and 5-Lipoxygenase inhibition in the aqueous, ethanolic or aqueous-ethanolic extracts of fruits of <italic>Cuminum cyminum < / highlight>plant.
Owner:LUPIN LTD
Methods of treatment and pharmaceutical composition
The invention relates a pharmaceutical composition comprising a combination of:(i) the AT 1-antagonist valsartan or a pharmaceutically acceptable salt thereof; and(ii) a NEP inhibitor or a pharmaceutically acceptable salt thereof and optionally a pharmaceutically acceptable carrier and to a method for the treatment or prevention of a condition or diseaseselected from the group consisting of hypertension, heart failure, such as (acute and chronic) congestive heart failure, left ventricular dysfunction and hypertrophic cardiomyopathy, diabetic cardiac myopathy, supraventricular and ventricular arrhythmias, atrial fibrillation, atrial flutter, detrimental vascular remodeling, myocardial infarction and its sequelae, atherosclerosis, angina (whether unstable or stable), renal insufficiency (diabetic and non-diabetic), heart failure, angina pectoris, diabetes, secondary aldosteronism, primary and secondary pulmonary hypertension, renal failure conditions, such as diabetic nephropathy, glomerulonephritis, scleroderma, glomerular sclerosis, proteinuria of primary renal disease, and also renal vascular hypertension, diabetic retinopathy, the management of other vascular disorders, such as migraine, peripheral vascular disease, Raynaud's disease, luminal hyperplasia, cognitive dysfunction, such as Alzheimer's, glaucoma and stroke, comprising administering a therapeutically effective amount of the pharmaceutical composition to a mammal in need thereof.
Owner:NOVARTIS PHARM CORP
Jixuedai gel with action of promoting wound reunion, and prepn. thereof
A gel of asiaticoside for promoting healing of wound and treating burn, wound, skin ulcer, scleroderma, etc is prepared from asiaticoside and proper auxiliary. Its preparing process is also disclosed.
Owner:BEIJING YINKERUISI BIOLOGICAL PODUCTS RES INST
Treatment and prevention of abnormal scar formation in keloids and other cutaneous or internal wounds or lesions
InactiveCN1668312AReduced uPA activityImmunoglobulins against animals/humansMuscular disorderVitamin CFibrosis
Owner:CHILDRENS HOSPITAL OF LOS ANGELES +1
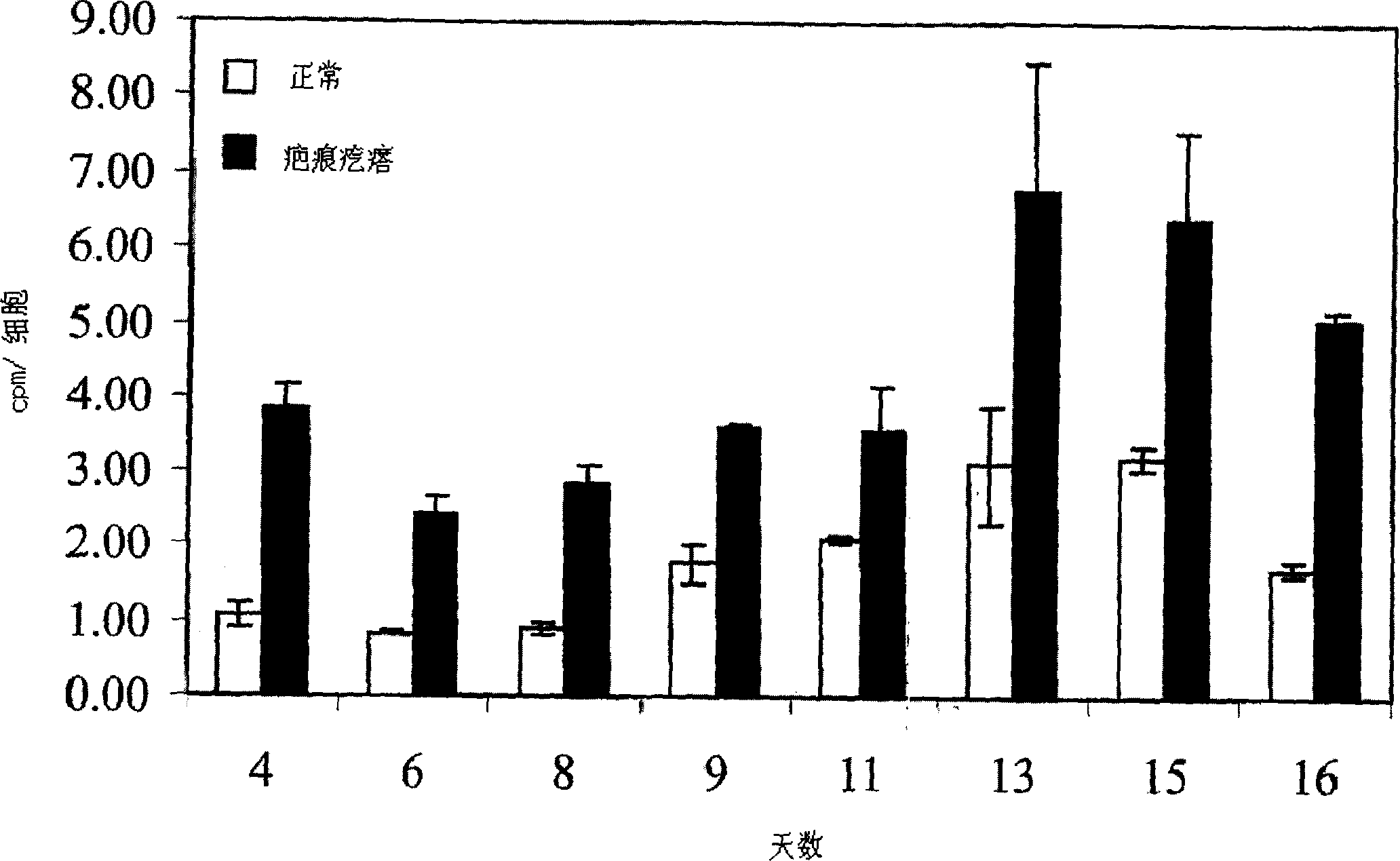
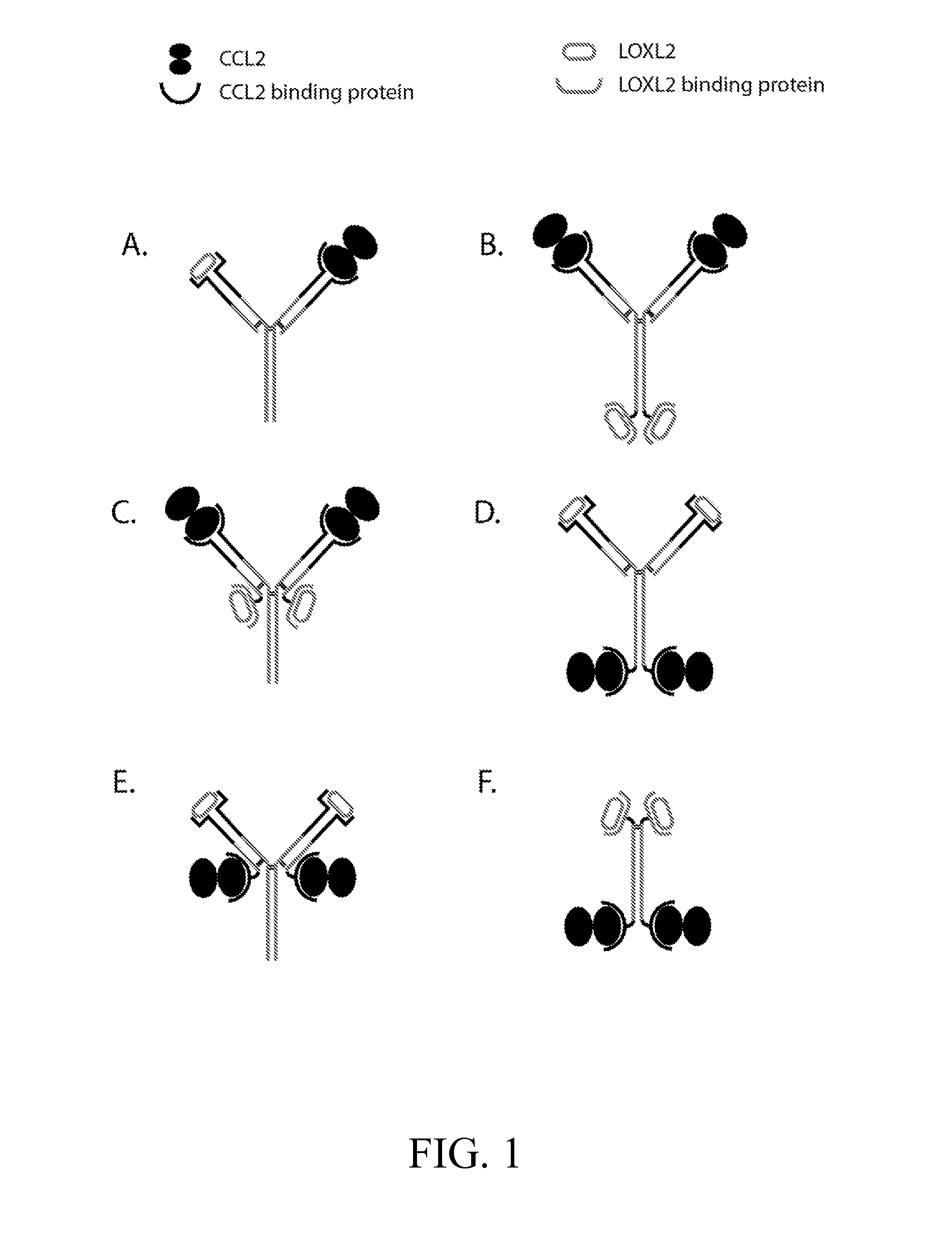
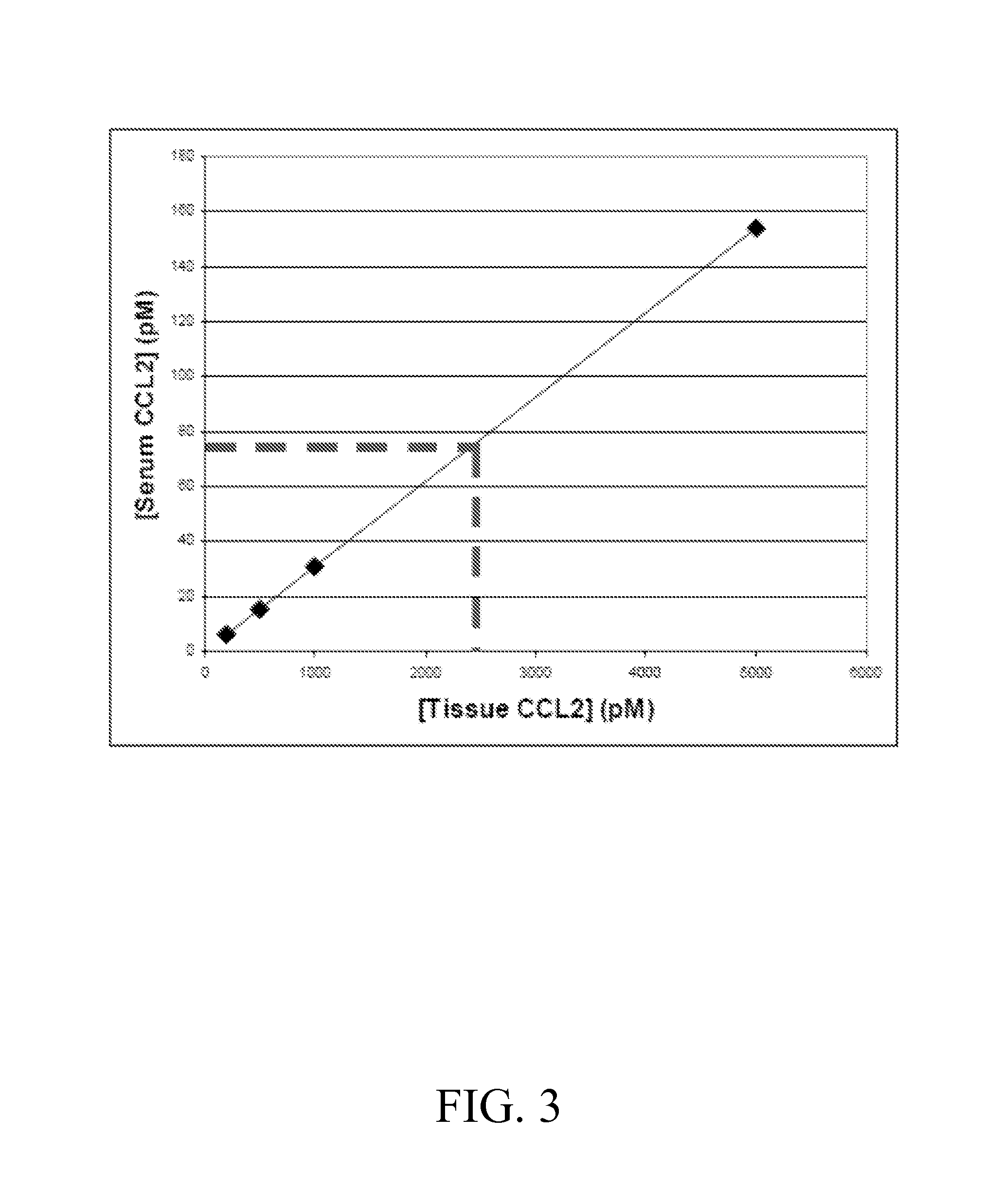
Anti-ccl2 and Anti-loxl2 combination therapy for treatment of scleroderma
ActiveUS20160083482A1Enhance physical fitnessIneffective targetingNervous disorderAntipyreticCCL2Fibrosis
The present invention provides, among other things, bi-specific molecules including, but not limited to, antibodies, fynomers, aptamers, fuproteins, and protein binding domains that bind both CCL2 and LOXL2 and uses thereof, in particular, for treatment of scleroderma and related fibrotic and / or inflammatory diseases, disorders and conditions. In some embodiments, the present invention further provides methods and compositions for treatment of scleroderma and related fibrotic and / or inflammatory diseases, disorders and conditions based on the combination of mono-specific anti-CCL2 and anti-LOXL2 molecules.
Owner:TAKEDA PHARMA CO LTD
Lupus preparation and new preparing method
The present invention relates to a Chinese medicine composition, and especially a kind of Chinese medicine composition for treating lupus erythematosus, systemic scieroderm, dermatomyositis, panniculitis, behcets disease and connective tissue disease and its preparation process. The Chinese medicine composition is preferably prepared into dripping pill and soft capsule.
Owner:FUKANGREN BIO PHARMA
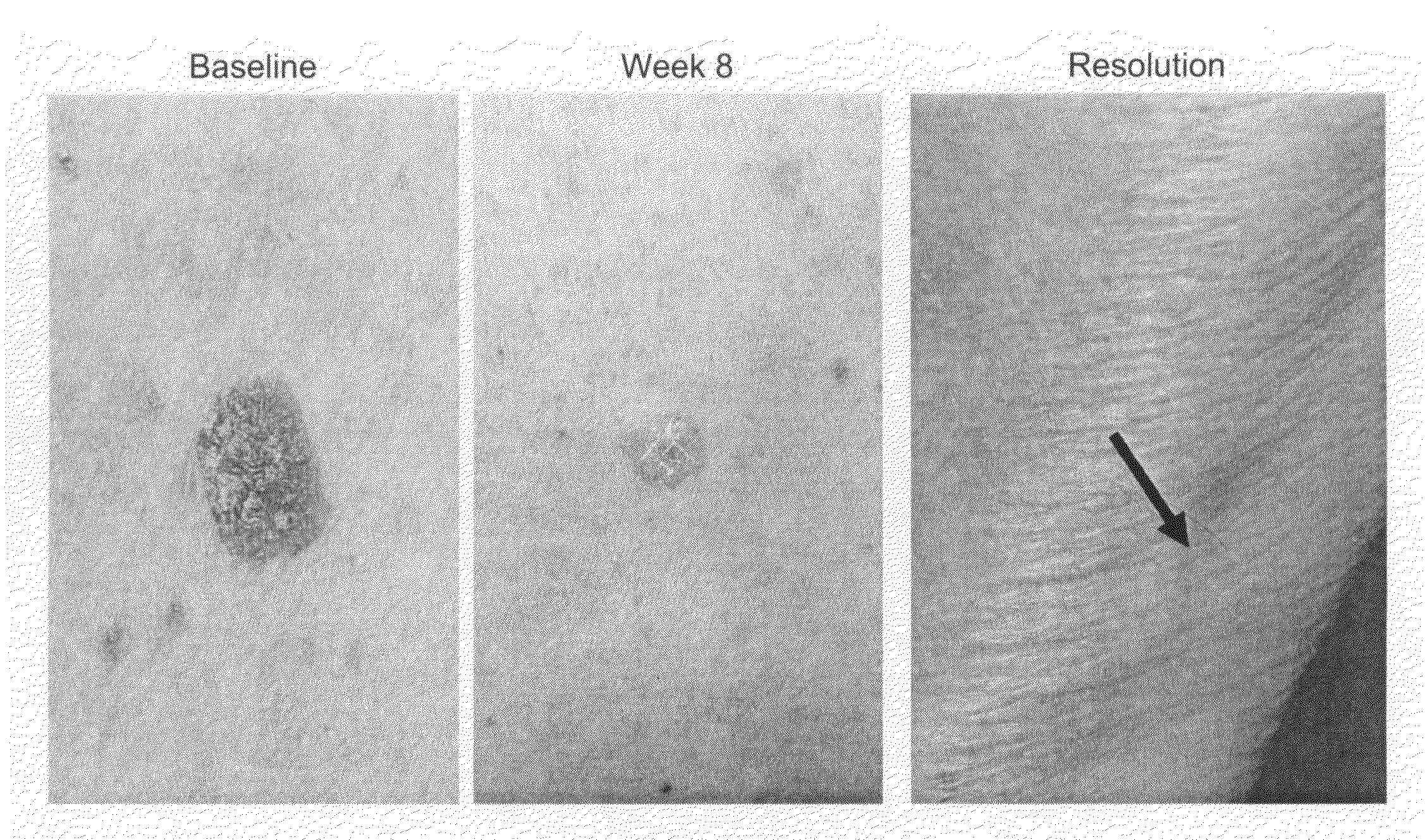
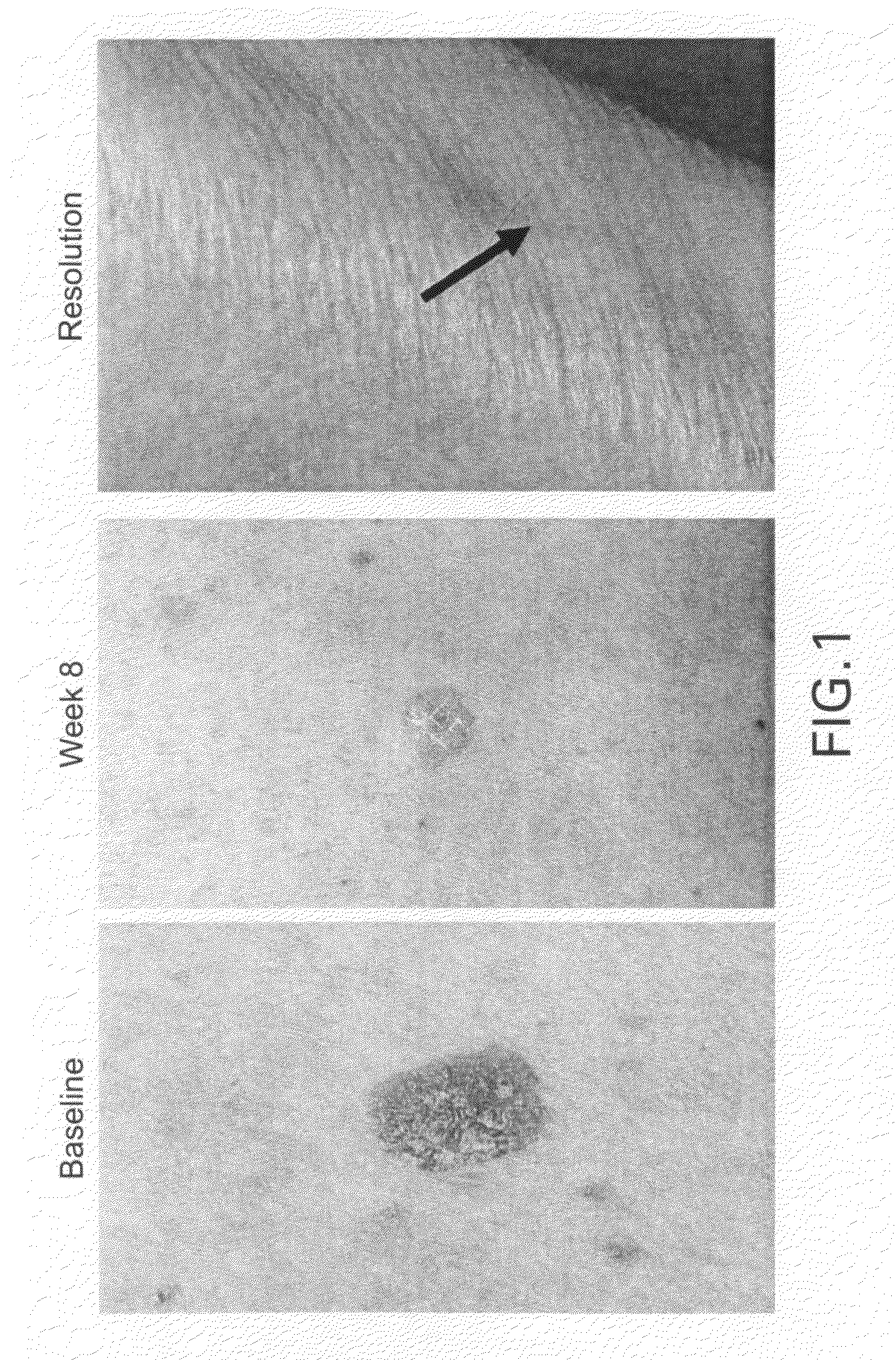
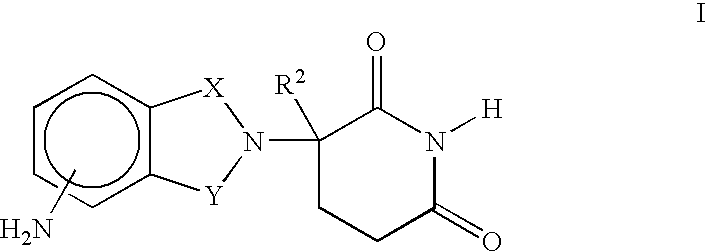
Methods for the treatment of scleroderma using 1-oxo-2-(2,6-dioxopiperidin-3-yl)-4-methylisoindoline
Methods of treating, preventing, correcting and / or managing skin diseases or disorders characterized by overgrowths of the epidermis, keratoses, scleroderma, cutaneous vasculitis, acne or wrinkles are disclosed. Specific embodiments encompass the administration of an immunomodulatory compound, or a pharmaceutically acceptable salt, solvate, hydrate, stereoisomer, clathrate, or prodrug thereof, alone or in combination with a second active agent. Specific second active ingredients are capable of affecting or inhibiting cell growth or proliferation, removing or improving acne scars, or reducing or correcting wrinkle lines. Pharmaceutical compositions, single unit dosage forms, and kits suitable for use in methods of the invention are also disclosed.
Owner:CELGENE CORP
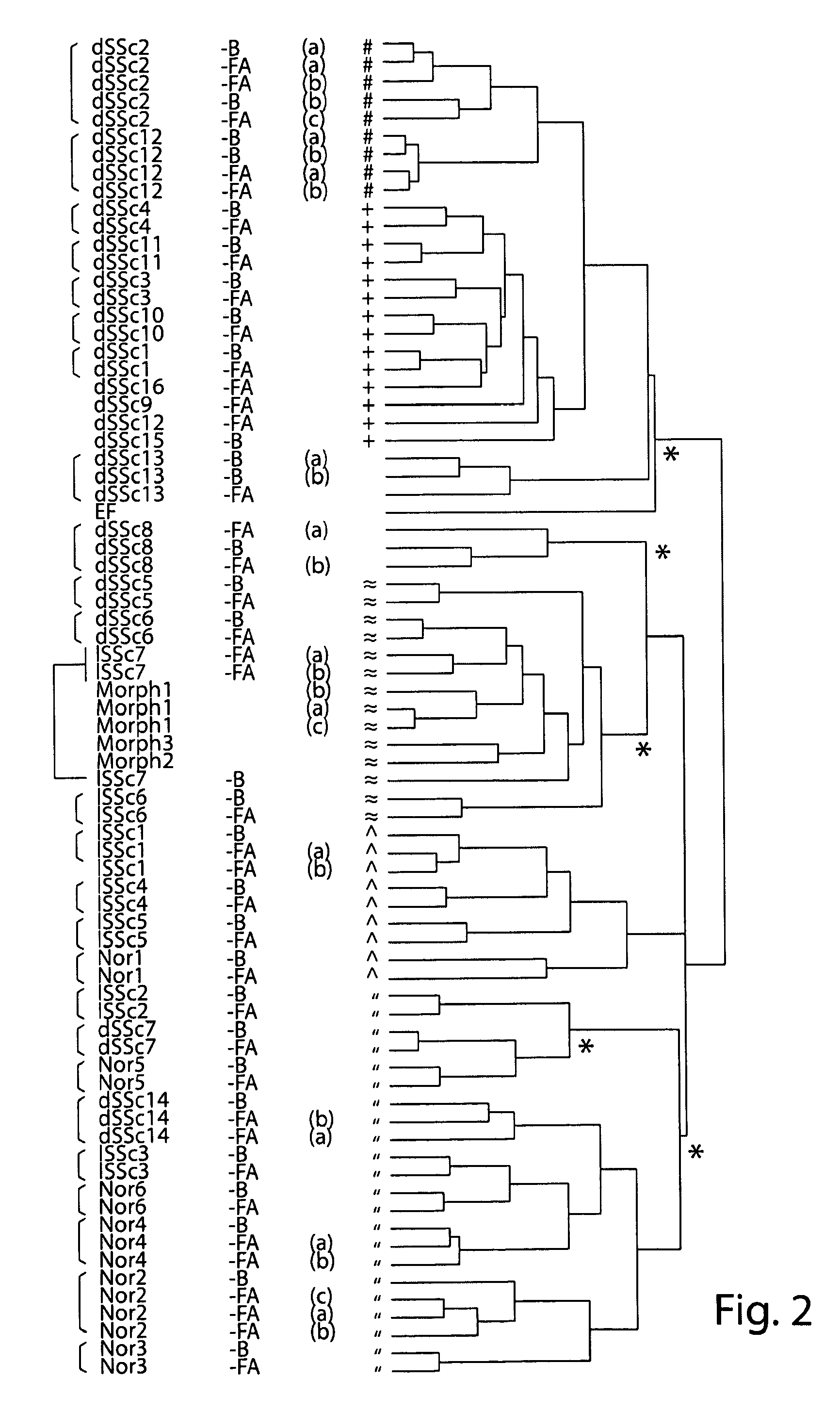
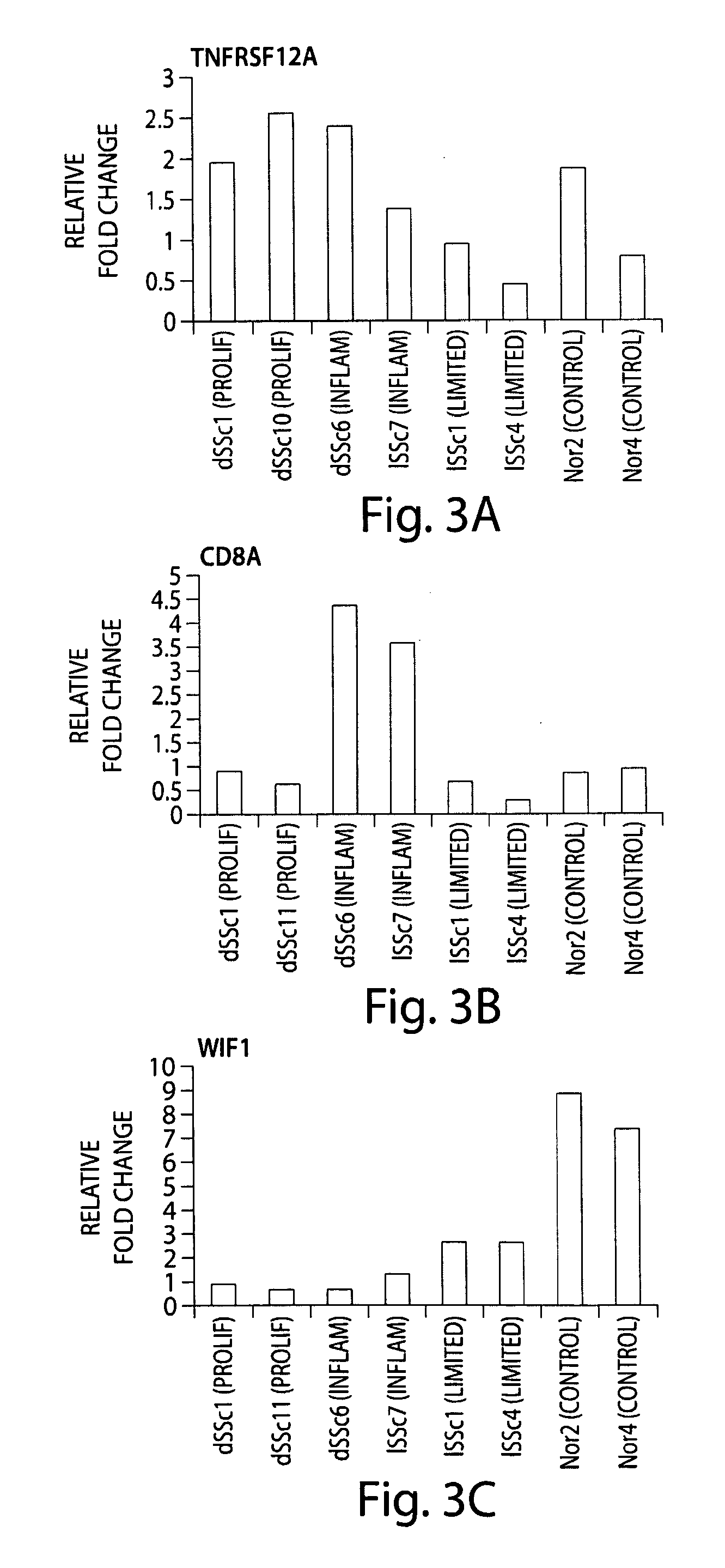
Molecular signatures for diagnosing scleroderma
Owner:TRUSTEES OF DARTMOUTH COLLEGE THE
Use of sGC stimulators, sGC activators, alone and combinations with PDE5 inhibitors for the treatment of systemic sclerosis (SSc).
The use of sGC stimulators, sGC activators alone, or in combination with PDE5 inhibitors for the prevention and treatment of fibrotic diseases, such as systemic sclerosis, scleroderma, and the concomitant fibrosis of internal organs.
Owner:ADVERIO PHARMA
Application of norisoboldine in preparing medicament for treating autoimmune disease
InactiveCN101375850AOrganic active ingredientsImmunological disordersImmunologic disordersUlcerative colitis
The invention relates to the medicine filed, in particular to a medicinal purpose of norisoboldine. The medicinal purpose is characterized in that the norisoboldine can be used in the application of preparing drugs for the treatment of autoimmune diseases. The autoimmune diseases are: rheumatoid arthritis, multiple sclerosis, systemic lupus erythematosus, multiple sclerosis, type-I diabetes, psoriasis, ulcerative colitis, Sjogren syndrome, scleroderma, polymyositis, chronic active hepatitis, mixed connective tissue disease, primary biliary cirrhosis, autoimmune hemolytic anemia and other diseases.
Owner:CHINA PHARM UNIV
Novel Sphingosine 1-Phosphate Receptor Antagonists
InactiveUS20150045332A1Improve featuresRaise the concentration levelBiocideSenses disorderChronic heart diseaseDiabetic retinopathy
The present invention relates to sphingosine-1-phosphate (S1P) receptors and compounds of the general formula:that are useful in the treatment and prevention of conditions associated with such receptors. More specifically, the present invention relates to the synthesis and use of sphingosine 1-phosphate receptor 2 (S1P2) antagonists that are useful in the treatment of cancer, atherosclerosis, diabetic retinopathy, and other inflammatory diseases. Among these inflammatory diseases that could be treated with these S1P2 antagonist are those characterized by fibrosis including chronic lung disease, chronic kidney and liver disease, chronic heart disease, and skin diseases such as sclerosis / scleroderma. The S1P2 antagonists can also be used in the treatment of glioblastoma multiforme (brain cancer), pediatric neuroblastoma, and other cancers.
Owner:ARROYO BIOSCI L L C
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com