Treatment and prevention of abnormal scar formation in keloids and other cutaneous or internal wounds or lesions
A scar, abnormal technology, applied in the field of treatment and inhibition of abnormal scar formation, can solve the problems of side effects, seriousness, recurrence, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0087] Materials and methods
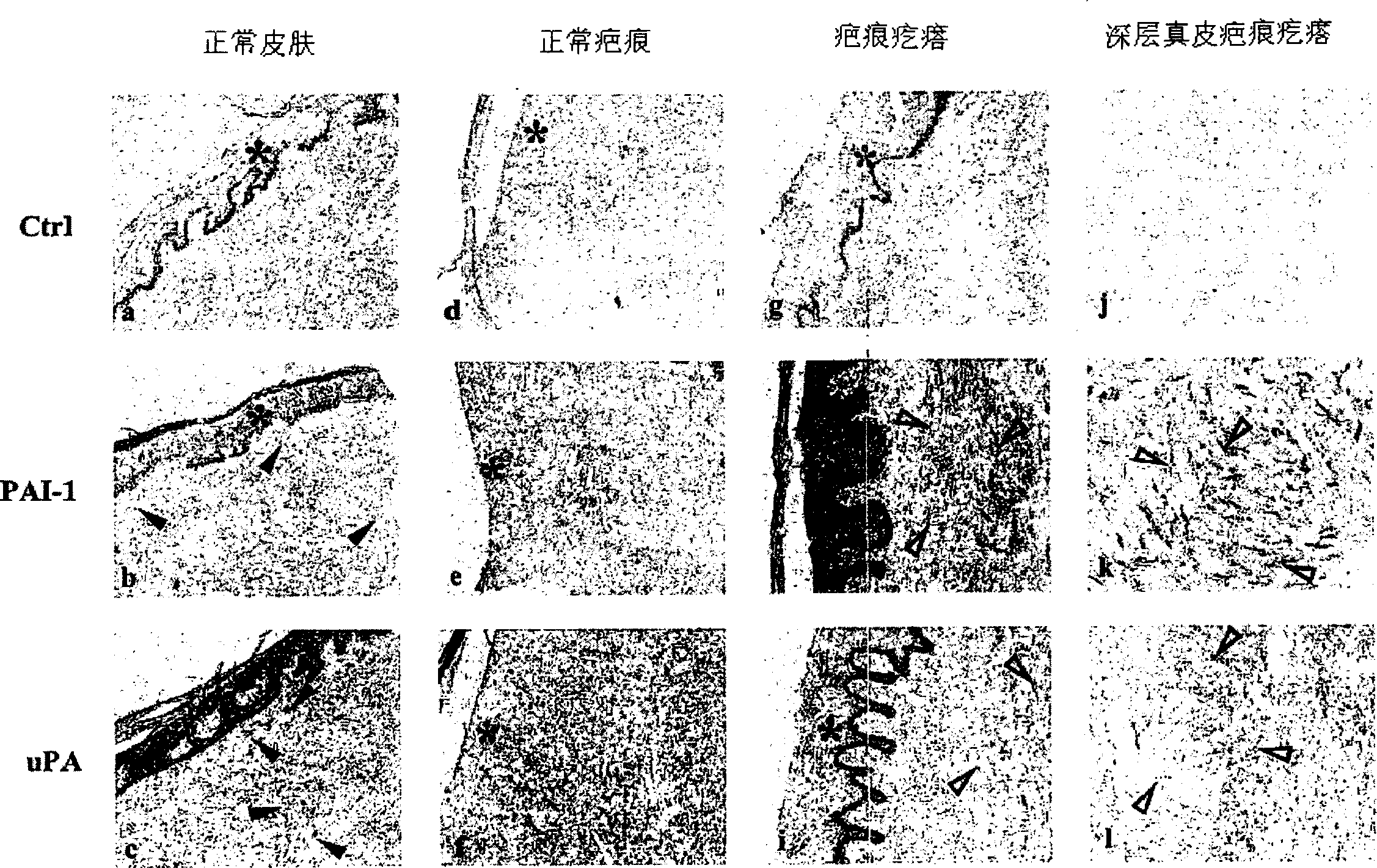
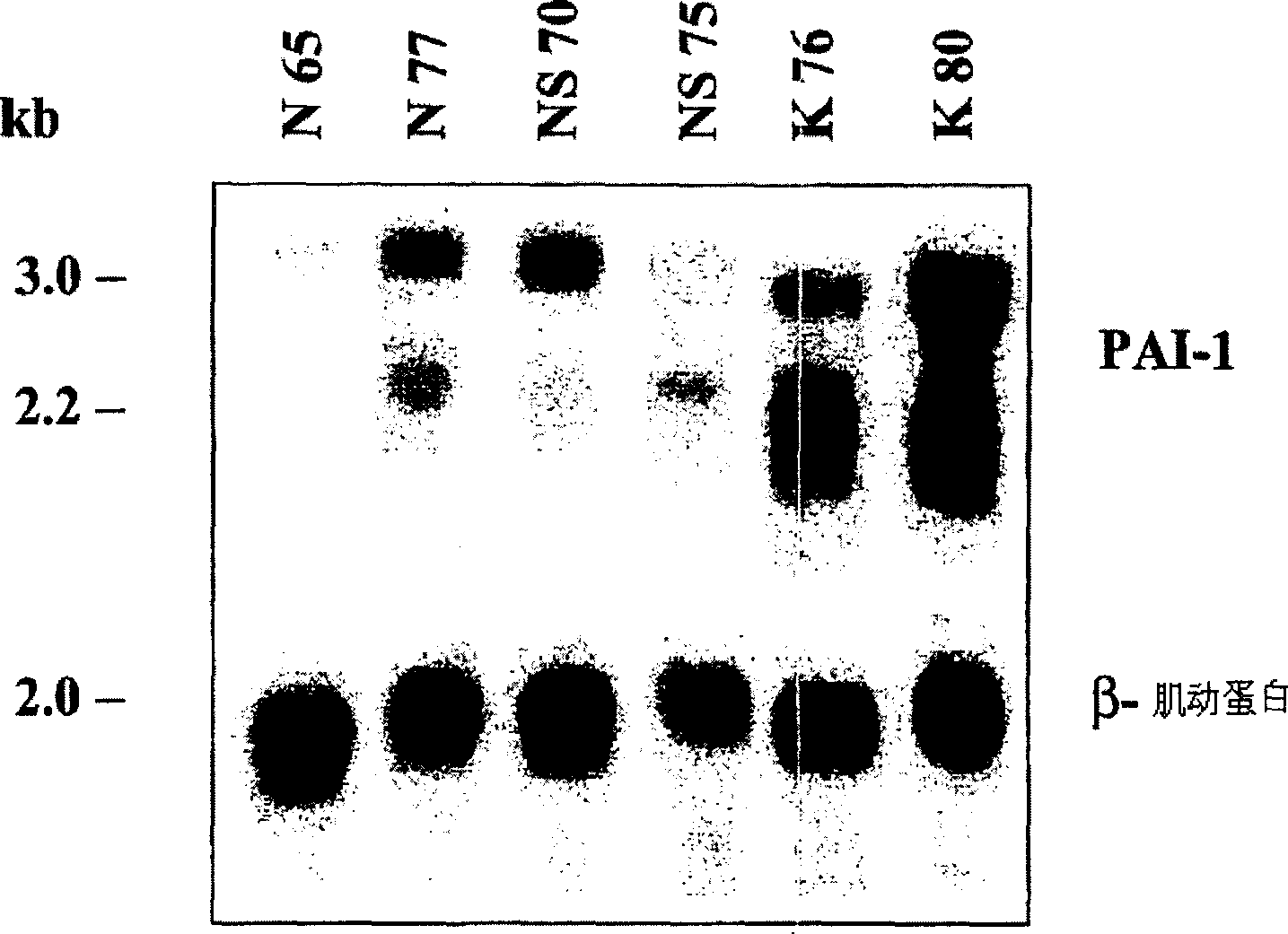
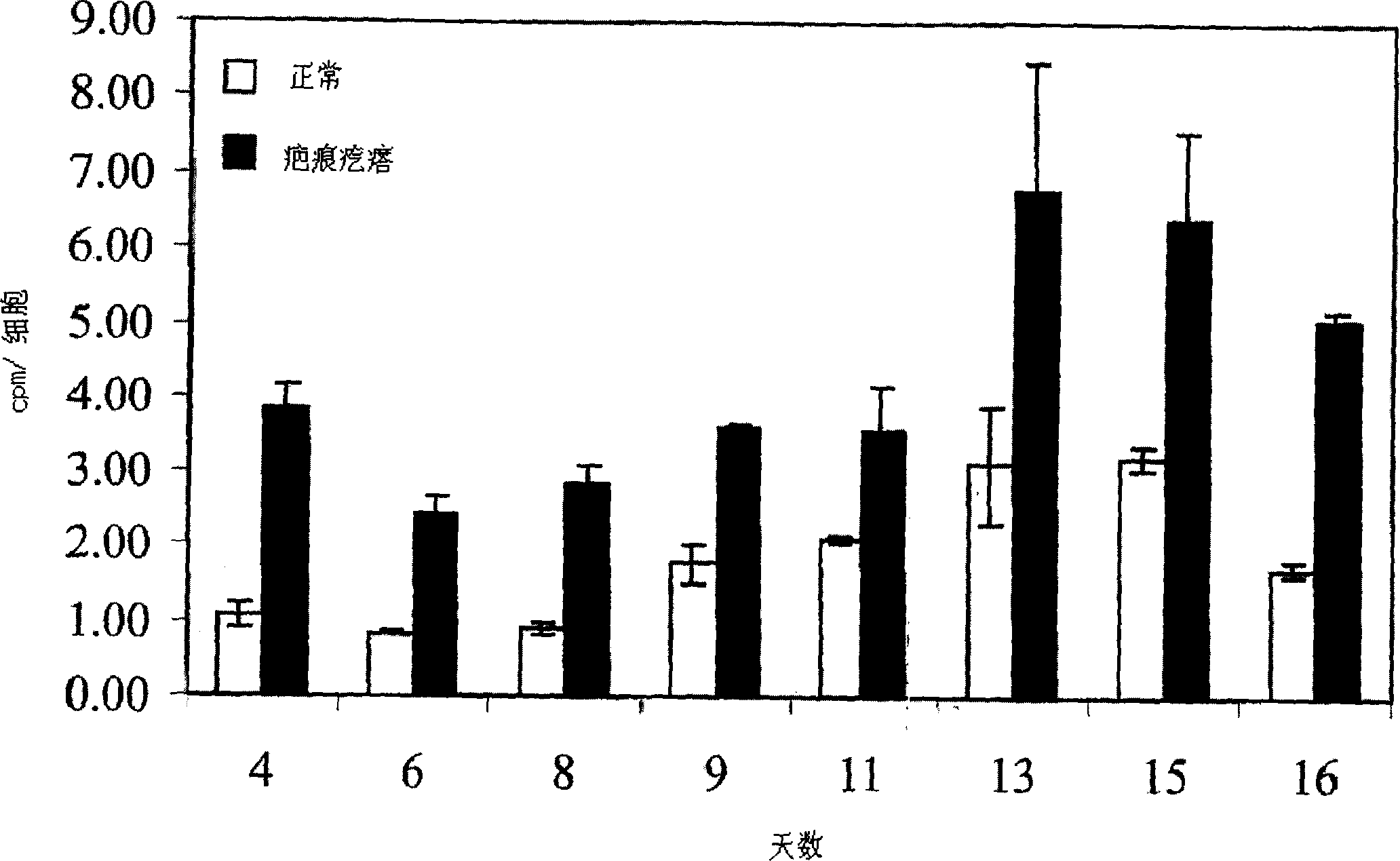
[0088] cell separation : Fibroblasts come from human normal skin, scars and keloids, and adopt the method of transplantation. The skin and scar collection protocol is accredited by Children's Hospital of Los Angeles and Charles-Medical University. The raised core area of the keloid is used to isolate fibroblasts. Fibroblasts were cultured in DMEM (Life Technologies, Inc., Grand Island, NY) containing 100 U / ml penicillin, 100 ug / ml streptomycin, and 10% fetal bovine serum (Life Technologies, Inc.). Cells in 5% CO 2 and 95% air in a humidified incubator. Fibroblasts were digested and passaged in Hank's solution (Life Technologies) containing 0.05% ethylenediaminetetraacetic acid and 0.25% trypsin once a week. Cells of passage 2-10 were used in the experiments. Cell passaging was defined as weekly expansion from primary cultures. The source of each fibroblast strain used in the present invention is listed in Table 1. These samples repre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com