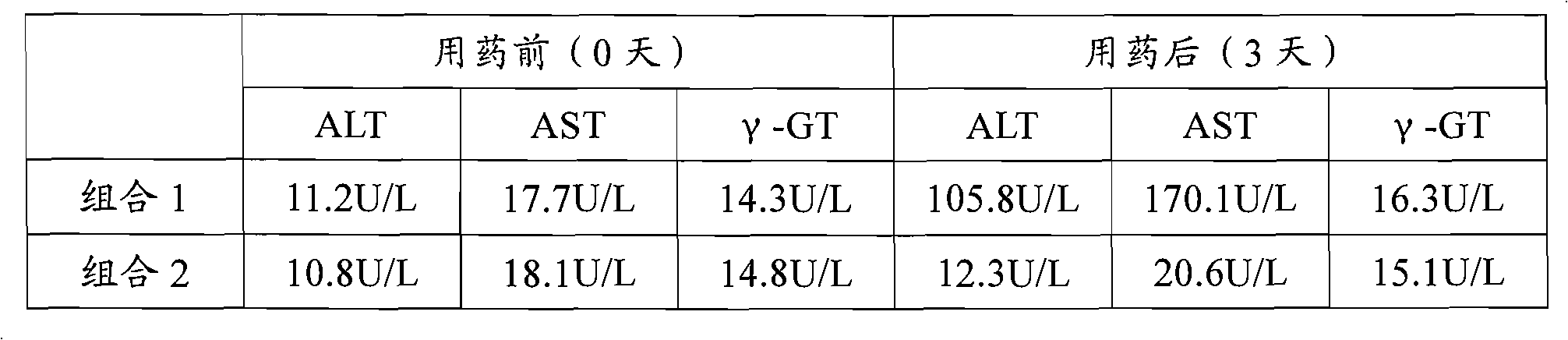
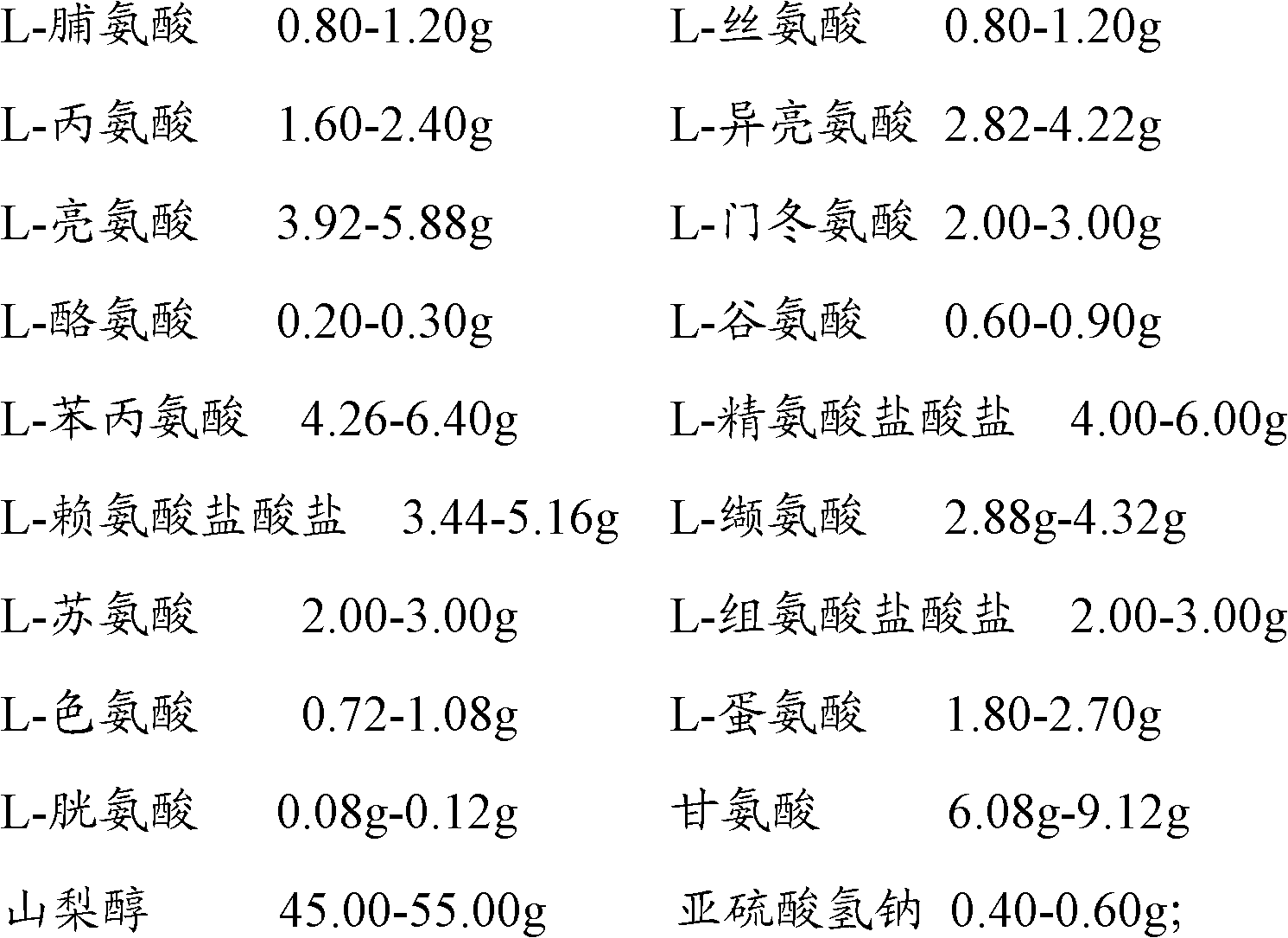
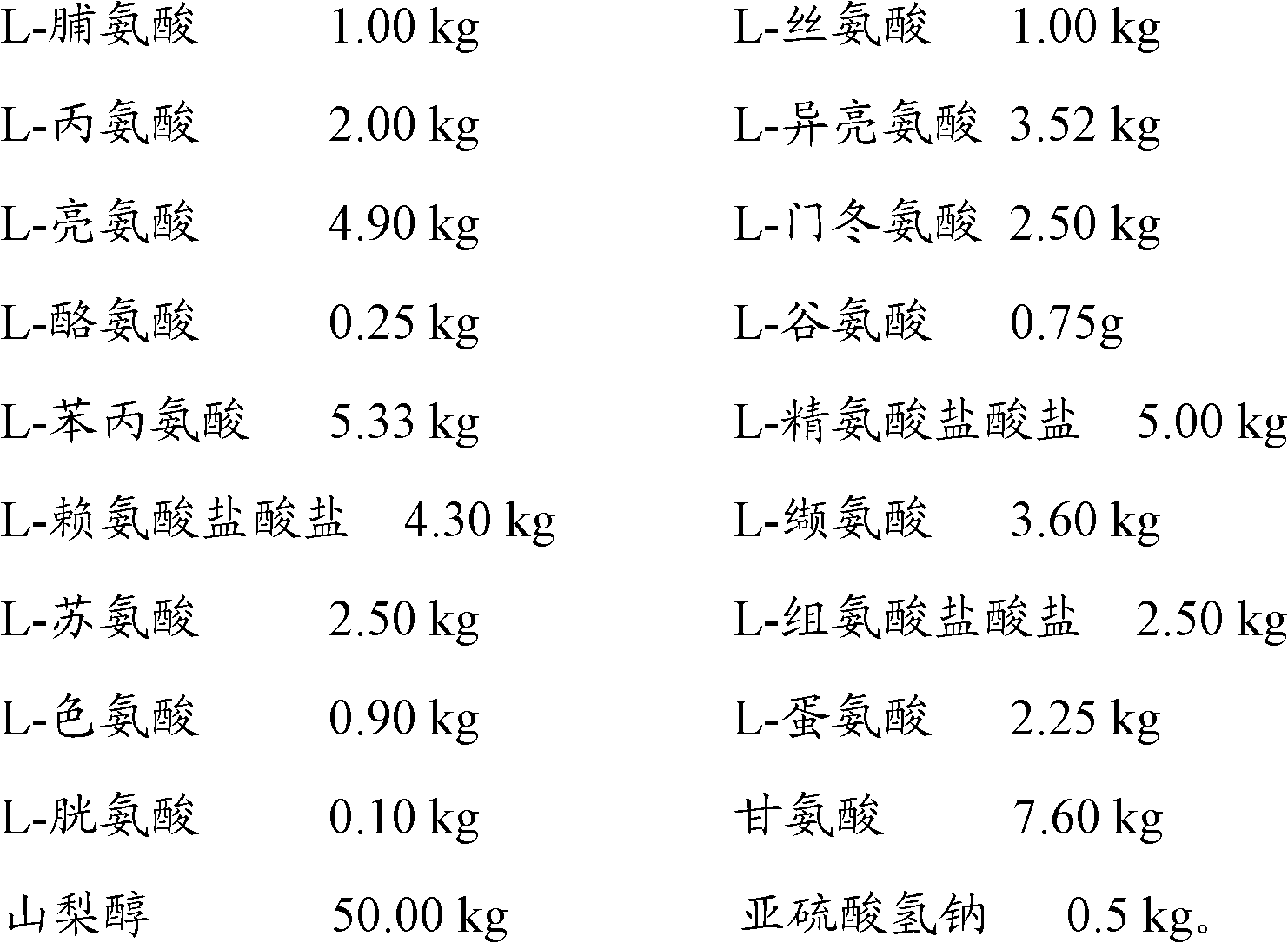
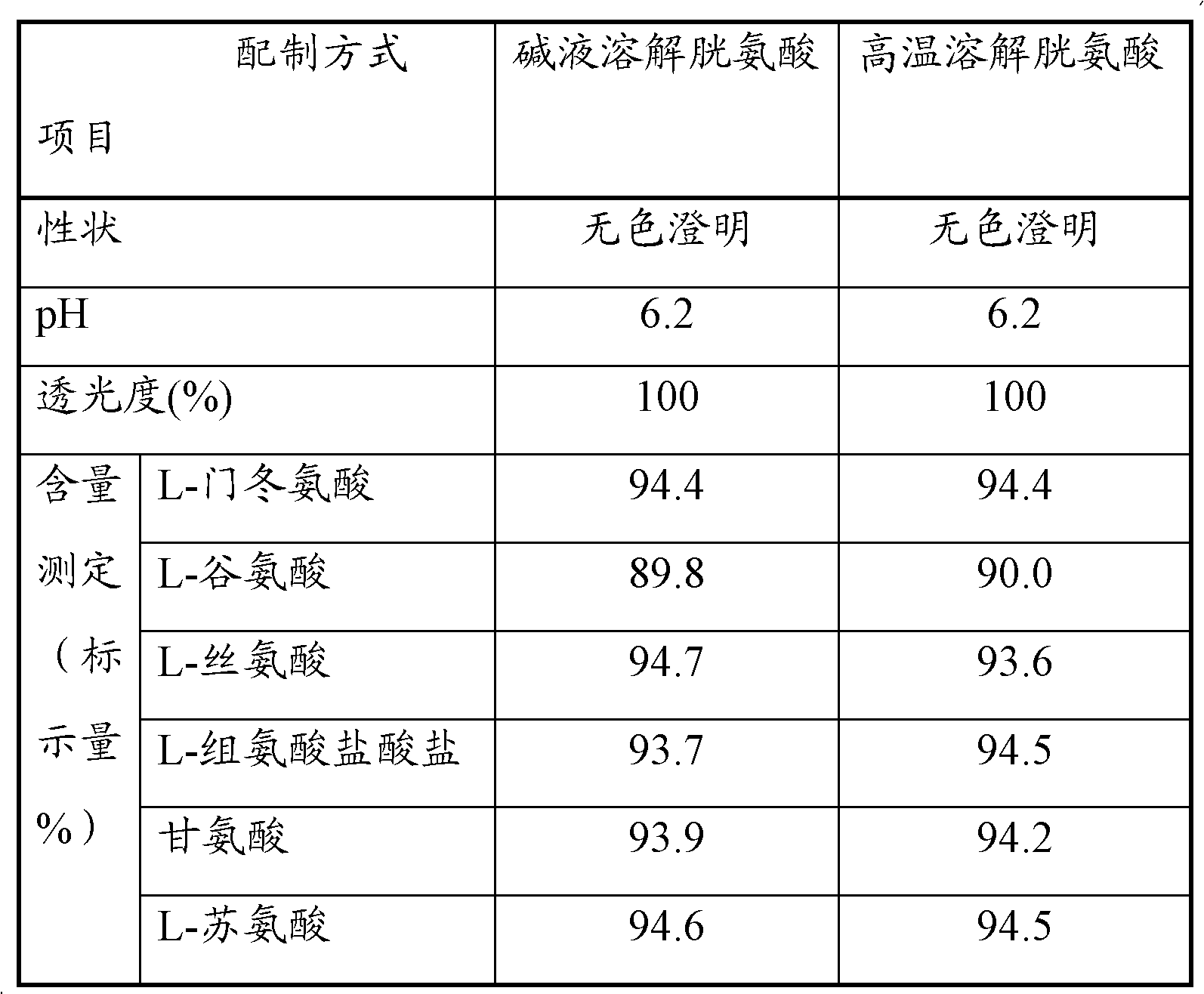
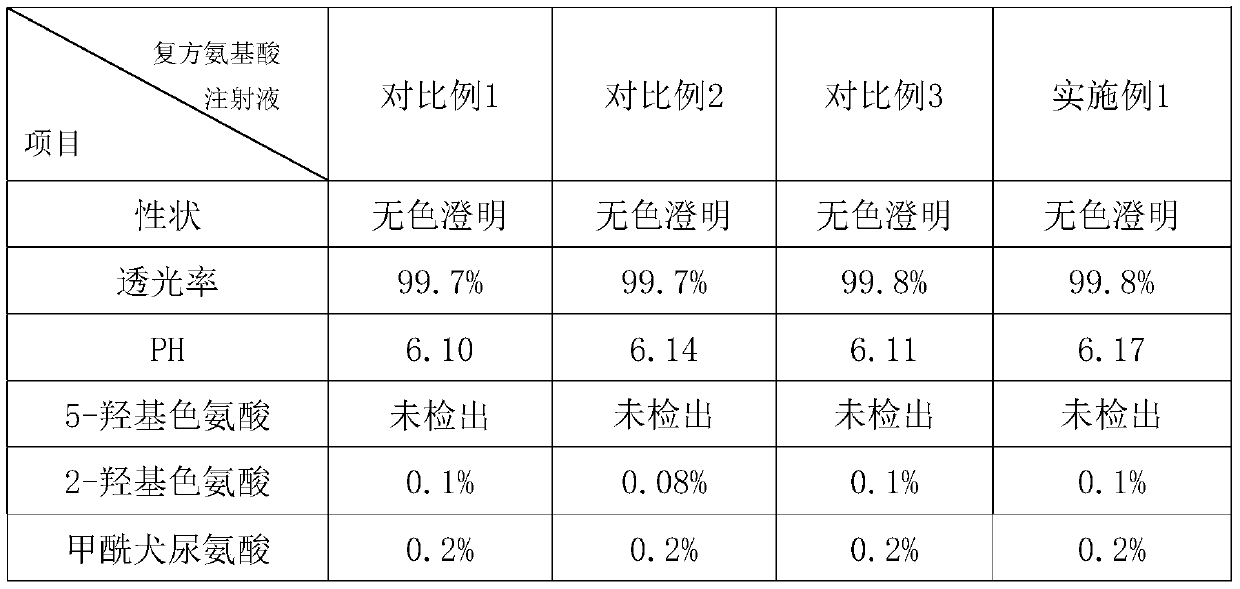
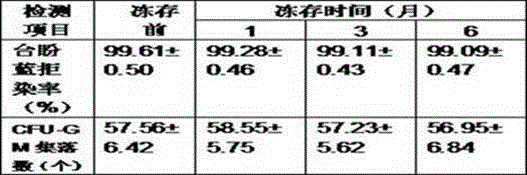
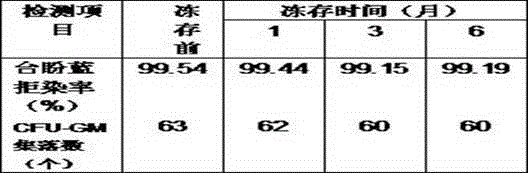
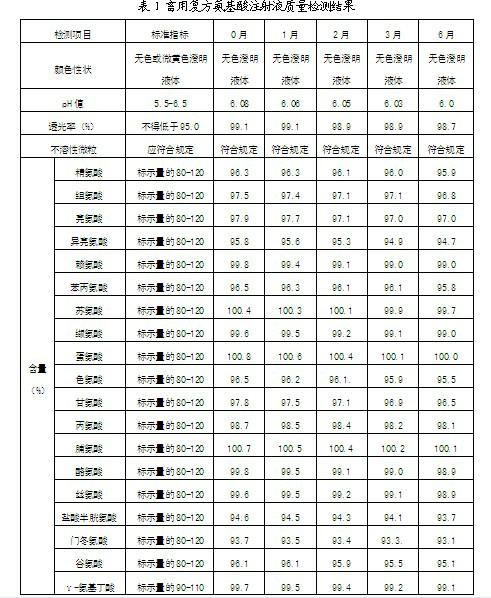
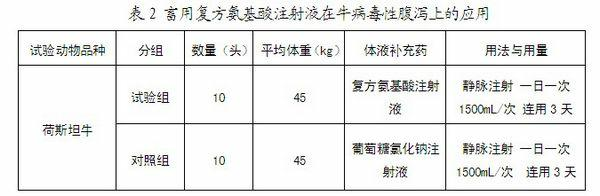
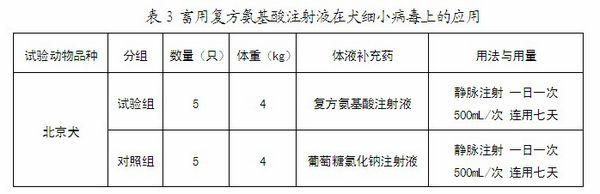
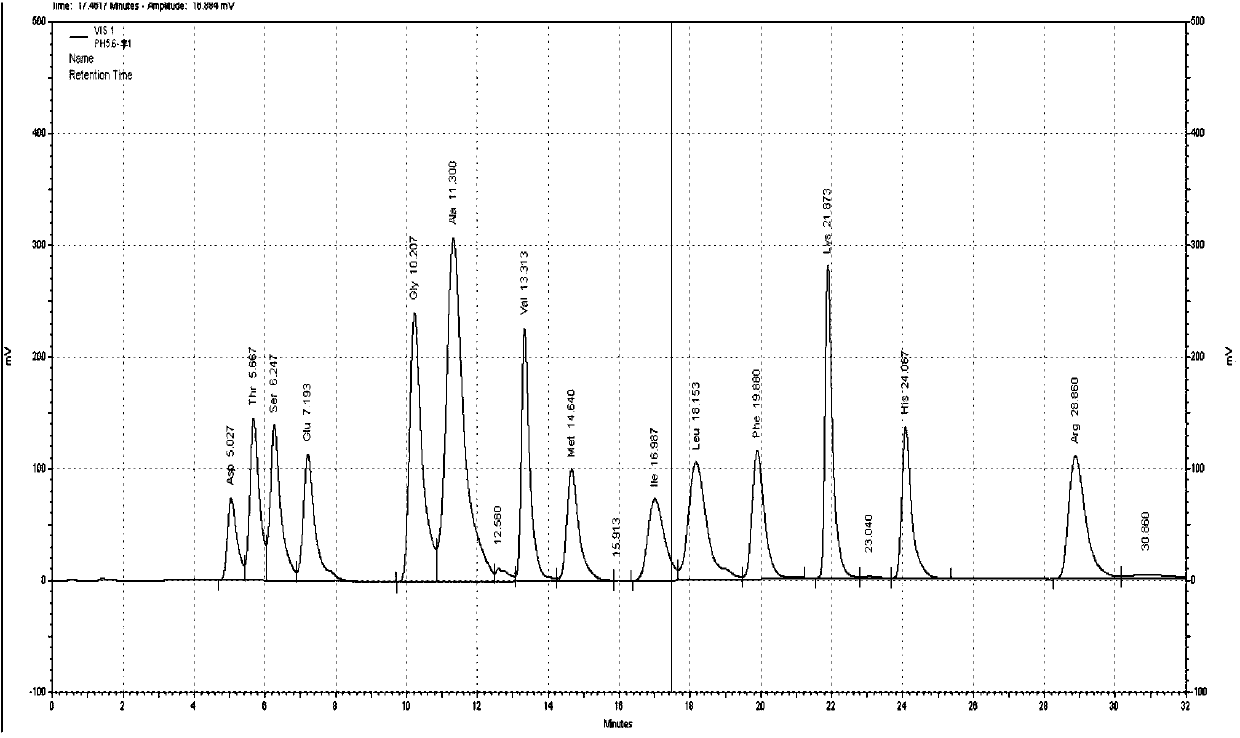
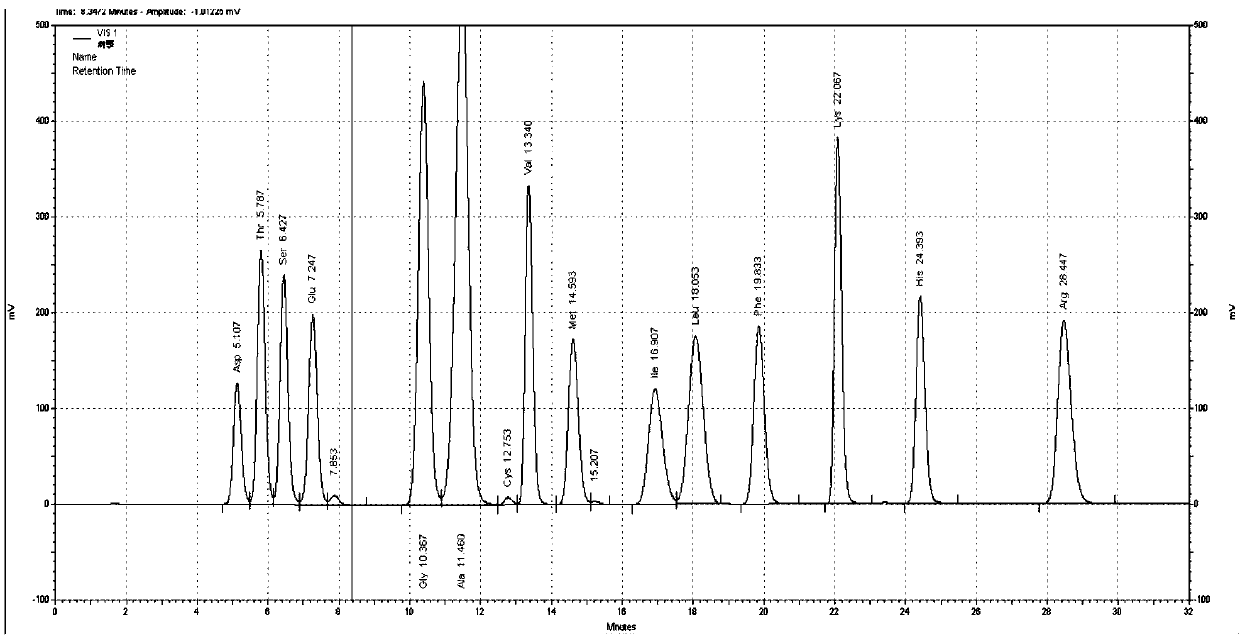
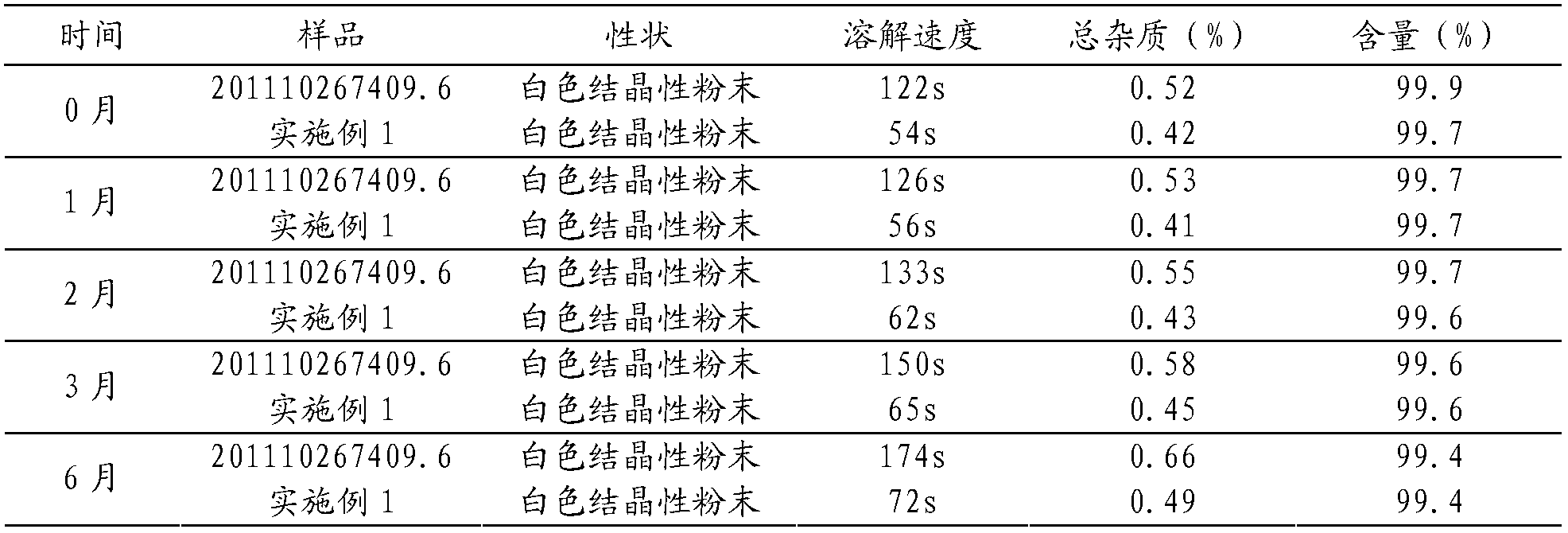
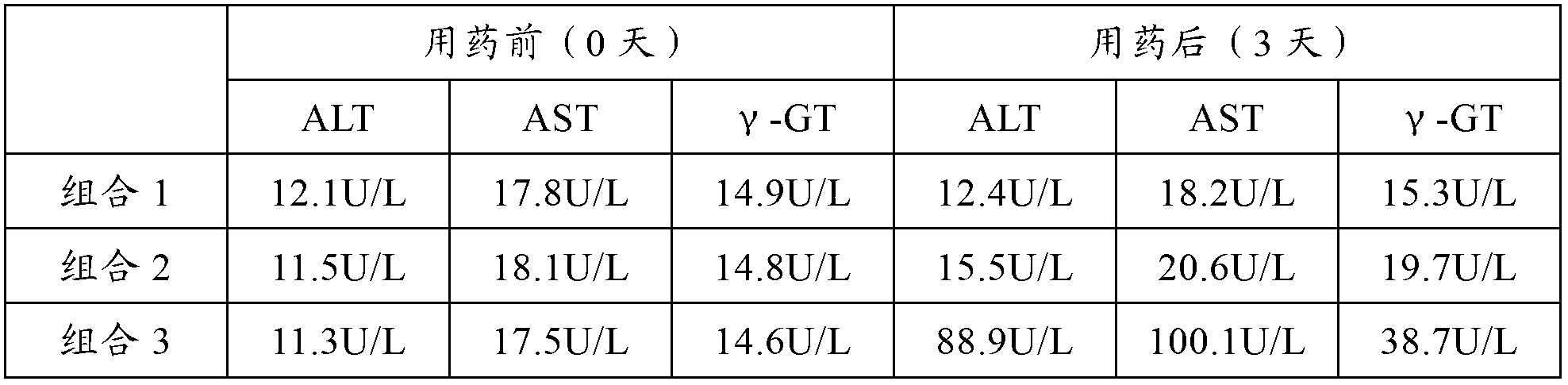
Patents
Literature
202 results about "Amino Acid Injection" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
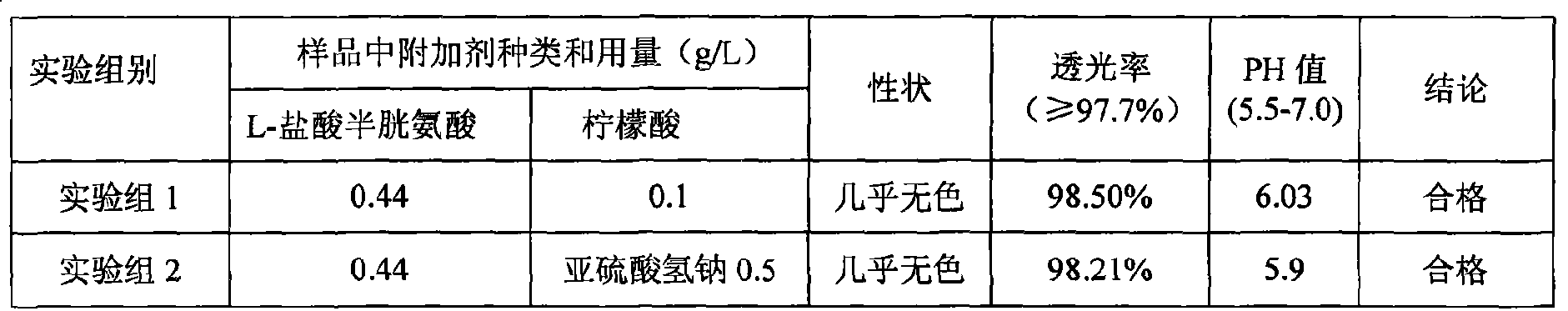
A concentrated dietary supplement for injection containing the essential amino acids leucine, isoleucine, lysine, valine, phenylalanine, histidine, threonine, methionine and tryptophan as well as the non-essential amino acids alanine, arginine, glycine, proline, serine and tyrosine, with potential anabolic and anti-catabolic activities. Upon dilution and intravenous infusion of the amino acid nutritional supplement, the amino acids serve as protein building blocks, promote protein synthesis in muscle cells and prevent protein breakdown.
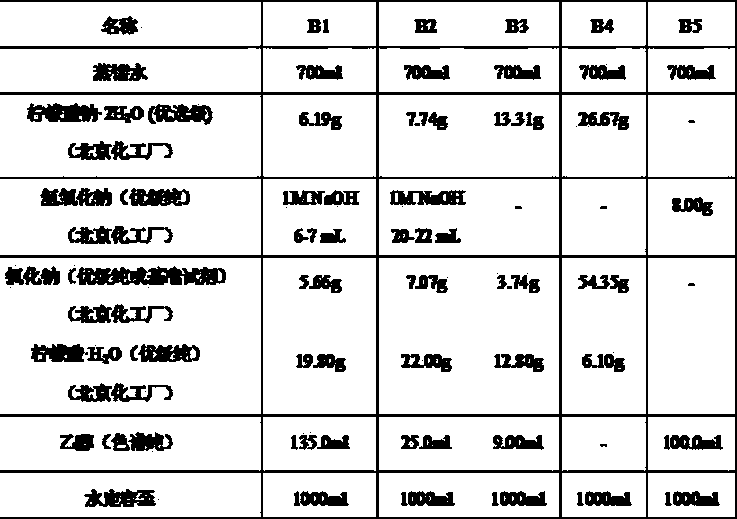
Cell preservation solution and preparation method and applications thereof
ActiveCN104542578AExcellent maintenance of biological characteristicsMaintain biological characteristicsDead animal preservationVitamin CAmino Acid Injection
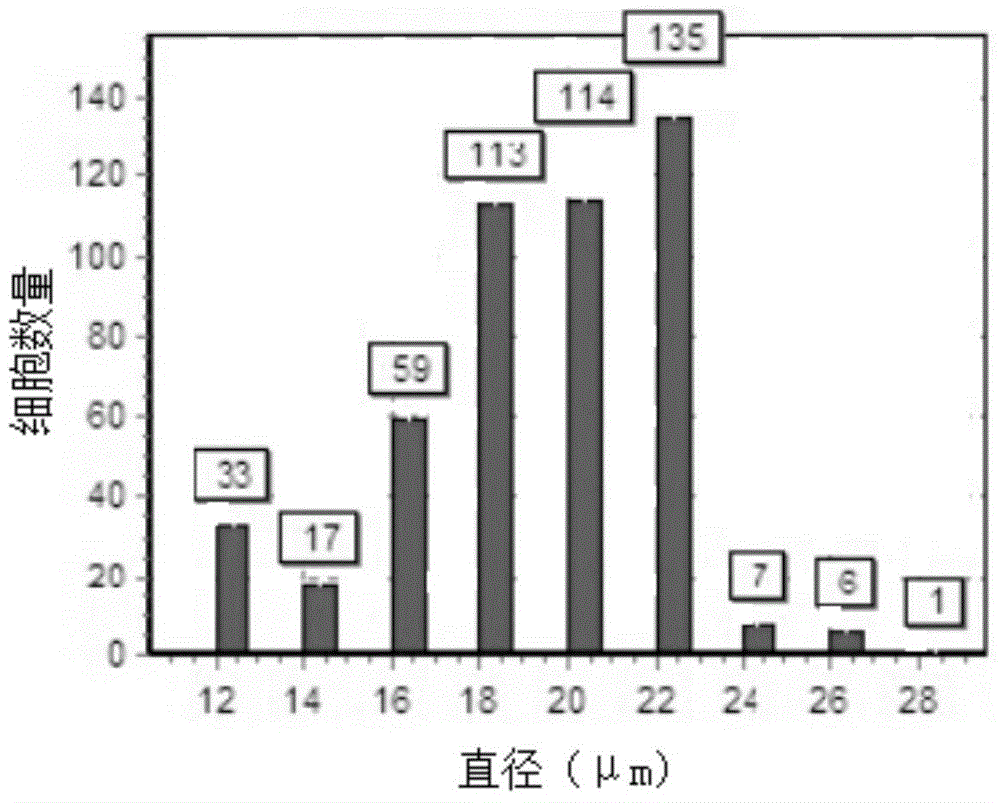
The invention relates to the technical field of clinical medicines, and particularly relates to a cell preservation solution and a preparation method and applications thereof. The cell preservation solution comprises albumin, glucose, vitamin C and basic preservation liquid, wherein the basic preservation liquid is a mixture of compound electrolyte injection and compound amino acid injection. The cell preservation solution disclosed by the invention achieves a good maintaining effect on the viability and morphology of more than two kinds of seed cells, and can preserve the seed cells for a long time, and maintain the biological characteristics of the seed cells, thereby significantly improving the therapeutic effect of the seed cells.
Owner:GUANGZHOU SALIAI STEMCELL SCI & TECH CO LTD
Compound amino acid injecta, and preparation method and detection method thereof
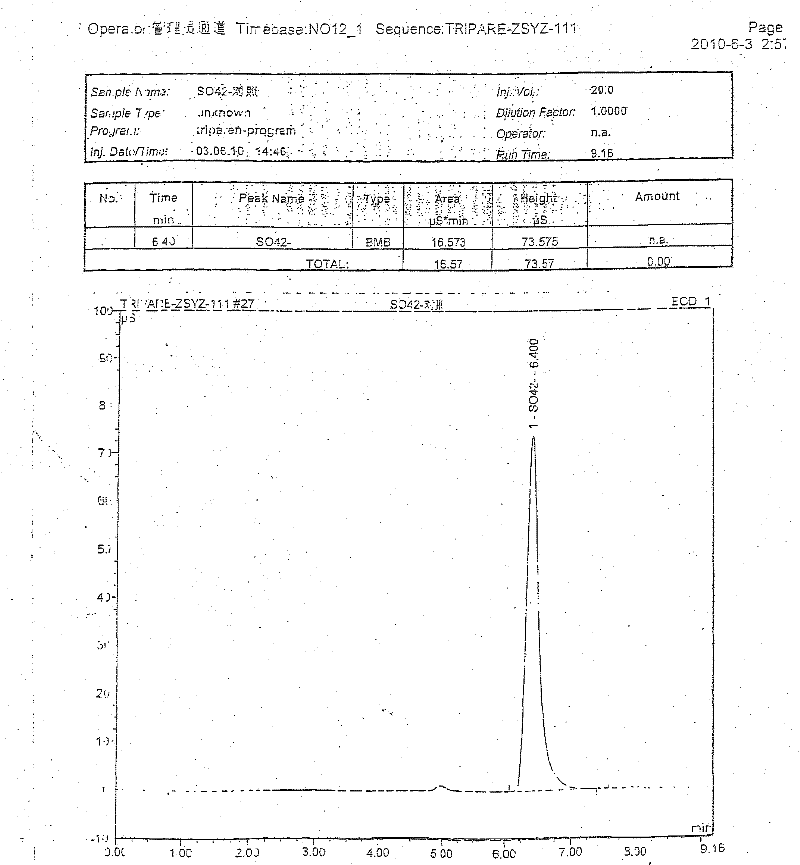
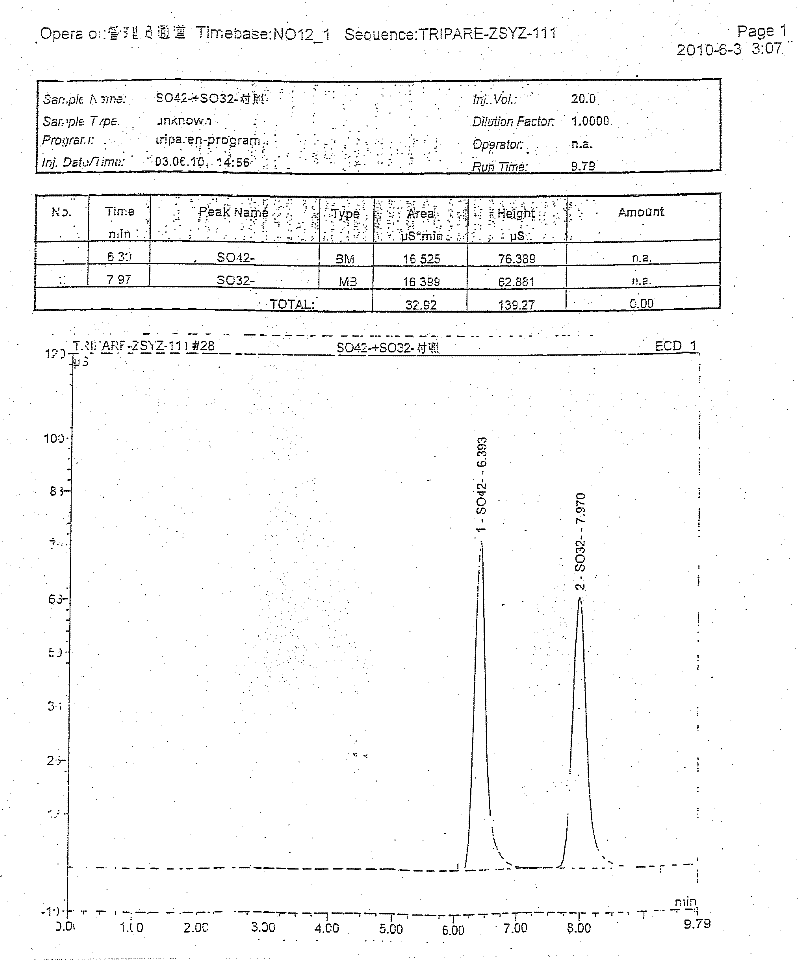
ActiveCN102440989AAccurate measurementExact reproductionOrganic active ingredientsMetabolism disorderIon chromatographySulfate radicals
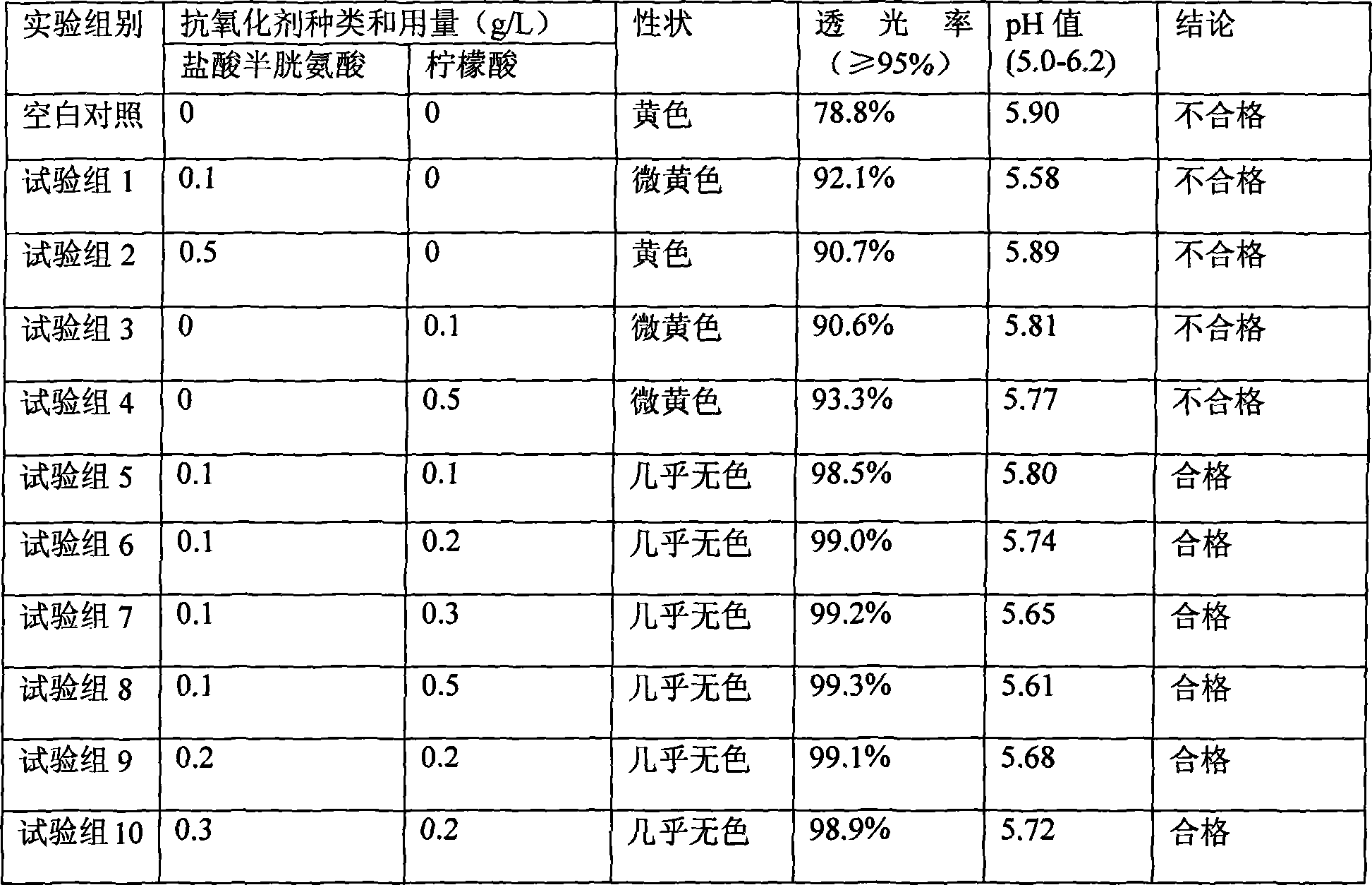
The invention discloses compound amino acid injecta, which contains arginine hydrochloride, histidine monohydrochloride, leucine, isoleucine, lysine hydrochloride and the like; meanwhile, the invention further discloses a preparation method of the compound amino acid injecta (18AA); in the event of ensuring the product quality, the compound amino acid injecta has the characteristics of saving energy and enhancing efficacy; by means of repetitive verification on precision, detection limit, quantitation limit, linear range and the like, a simple, convenient, rapid and reliable method with cleaning verification is ensured; furthermore, the residual quantity of antioxygen, namely sodium hydrogensulfite, in the compound amino acid injecta and sulphate ion generated by degrading sodium hydrogensulfite are detected according to an ion chromatography principle, thus, the amount of the antioxygen charged in the raw material proportioning can be accurately measured, and the stability of the raw material proportioning process is judged according to the measurement result; and a detection method of the compound amino acid injecta provided by the invention is an online derivative amino acid content measuring method, the speed is rapid, and the method is applied to large-scale detection.
Owner:SICHUAN KELUN PHARMA RES INST CO LTD
Pharmaceutical composition containing 18 kinds of amino acid
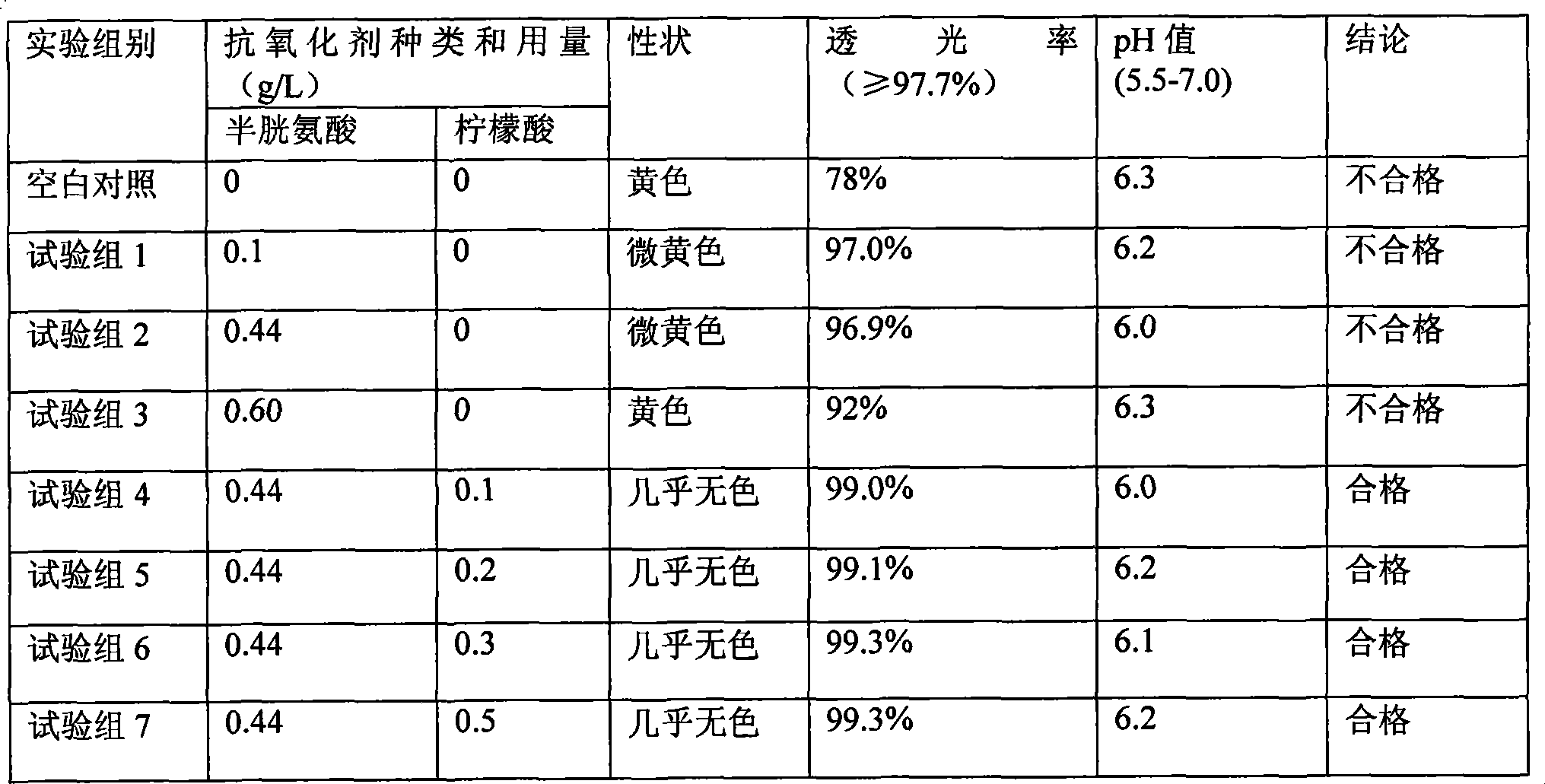
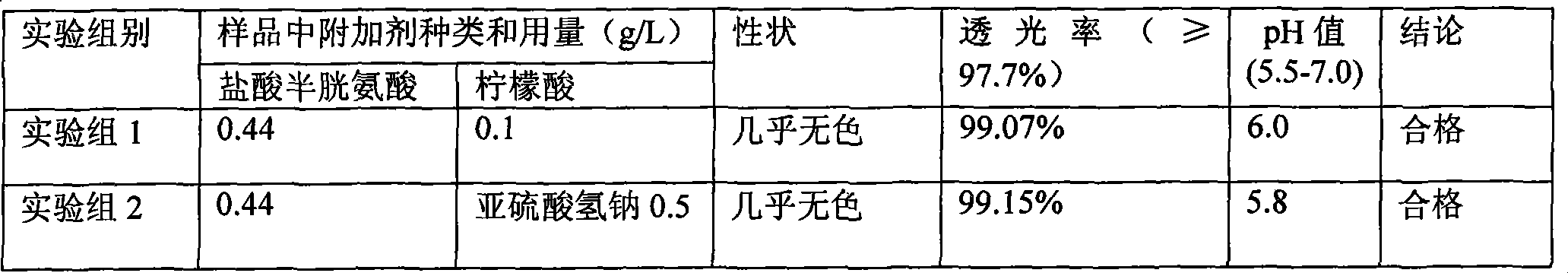
ActiveCN101439036AInhibition of oxidative decomposition reactionsQuality assuranceOrganic active ingredientsMetabolism disorderAntioxidantTryptophan
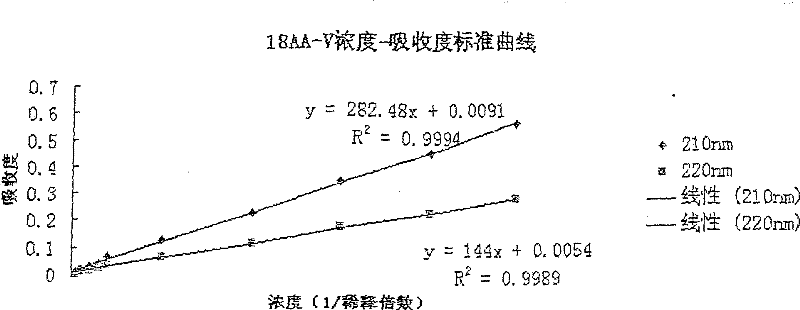
The invention discloses a pharmaceutical composition containing 18 amino acids. The pharmaceutical composition is characterized in that a compound amino acid injection (18AA-V) with varied concentration is prepared by using the following components according to the following ratios of parts by weight: 2.89 of arginine hydrochloride, 2.46 of histidine hydrochloride, 3.79 of leucine, 1.70 of isoleucine, 3.33 of lysine hydrochloride, 2.83 of phenylalanine, 1.97 of threonine, 1.36 of valine, 1.06 of methionine, 0.39 of tryptophan, 3.24 of glycine, 1.88 of alanine, 1.00 of proline, 0.11 of tyrosine, 0.67 of serine, 0.44 of cysteine hydrochloride, 1.15 of aspartic acid, 1.97 of glutamic acid, 50 of xylitol, 0.10 to 0.30 of citric acid and injection water with proper amount. The pharmaceutical composition does not contain a sulfite antioxidant so that the pharmaceutical composition is clinically used in a safer manner. After an accelerated test and a quality test, results show that the pharmaceutical composition is as stable as or more stable than like products (18AA-V) which are sold in the markets and contain sulfites.
Owner:福州凯瑞医药咨询有限公司
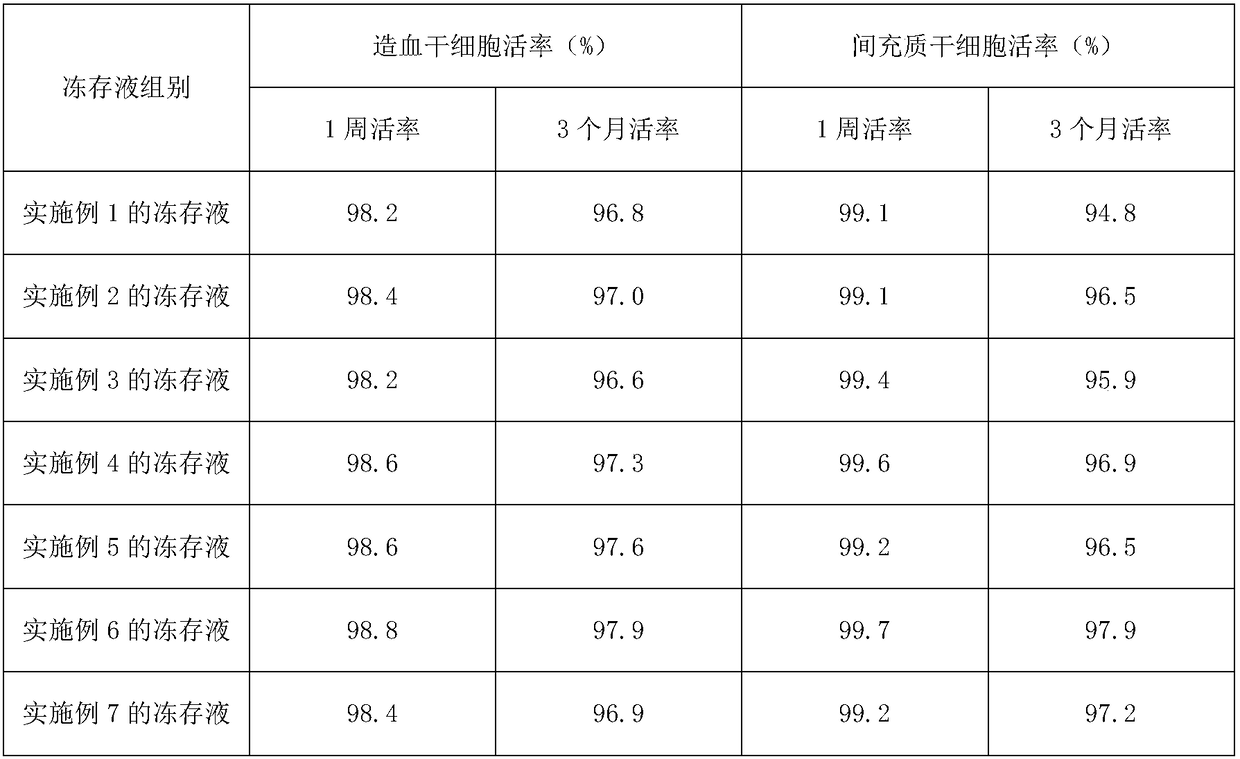
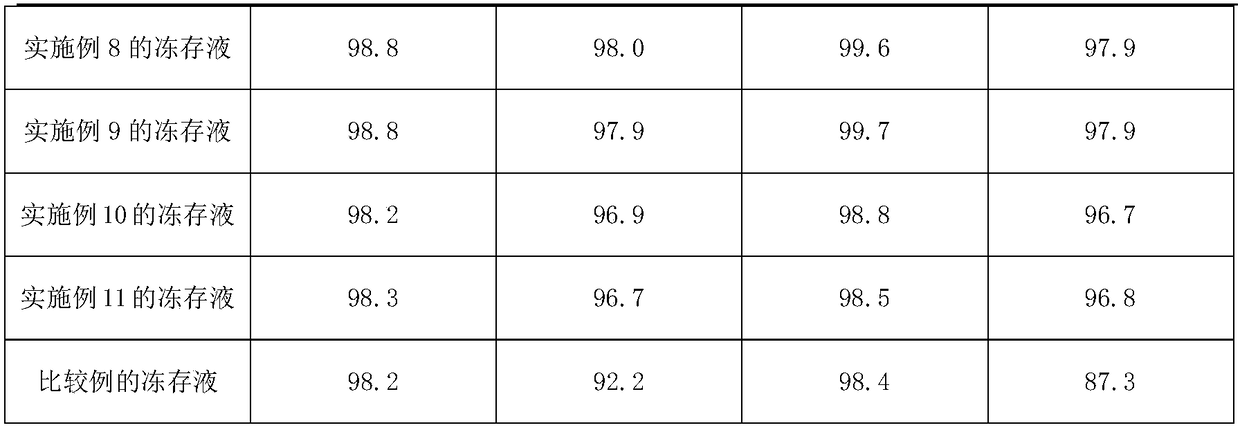
Mesenchymal stem cell cryopreserved liquid and injection liquid
The invention discloses a mesenchymal stem cell cryopreserved liquid which comprises the following ingredients in percentage by volume: 25%-75% of plasmalyte-A, 10%-35% of an 18AA5% compound amino acid injection liquid, 1%-50% of 20% human serum albumin and 5%-20% of DMSO (dimethylsulfoxide). The invention further provides a mesenchymal stem cell injection liquid and a preparation method thereof. According to the mesenchymal stem cell cryopreserved liquid, the defects of a conventional mesenchymal stem cell cryopreserved liquid can be overcome, not only can the motility rate of the mesenchymal stem cell be maintained for a long time, but also the direct clinical infusion can be performed, and accordingly, the mesenchymal stem cell cryopreserved liquid has a good application prospect and economic benefits.
Owner:SICHUAN NEO LIFE STEM CELL BIOTECH
Injection composition of fat milk, amino acid and glucose packed with three-cavity bag and preparing method thereof
ActiveCN101015501AAvoid contamination riskEasy to useOrganic active ingredientsMetabolism disorderMicroorganismParticulate pollution
The invention discloses an injection composition packaged in a three-cavity bag. The three cavities separated by diaphragm are respectively filled with fattiness milk injection, compound amino acid injection, and glucose injection. The using method is crimping each cavity in turns, or divulging the isolating bar in each cavity to mix the injections for venoclysis so as to avoid the venture of microorganism and particulates pollution in traditional mix processing. The mix processing can be carried out under any environmental condition which qualifies the small hospital without aseptic preparation condition to use the nutrition, highly reduce the untoward reaction such as transfusion heater response, sanguis infection, and blood poisoning due to traditional mix processing.
Owner:费森尤斯卡比华瑞制药有限公司
Pharmaceutical composition containing 18 kinds of amino acid
ActiveCN101439031ASolve the problem of trace oxygenSolve the oxygen redissolution problemOrganic active ingredientsMetabolism disorderAntioxidantArginine
The invention discloses a pharmaceutical composition containing 18 amino acids. The pharmaceutical composition is characterized in that a compound amino acid injection (18AA-II) with varied concentration is prepared by using the following components according to the following ratios of parts by weight: 1.50 of aspartic acid, 2.50 of glutamic acid, 1.90 of serine, 3.00 of histidine, 3.50 of glycine, 2.50 of threonine, 7.20 of alanine, 4.90 of arginine, 0.20 of tyrosine, 0.20 of cystine, 3.20 of valine, 2.50 of methionine, 0.85 of tryptophan, 3.50 of phenylalanine, 2.50 of isoleucine, 3.40 of leucine, 5.50 of lysine acetate, 2.90 of proline, 0.10 of cysteine hydrochloride and 0.20 of lemon acid. The composition does not contain a sulfite antioxidant so that the pharmaceutical composition is clinically used in a safer manner. After an accelerated test, a test result shows that the pharmaceutical composition containing 18 amino acids is as stable as or more stable than like products which are sold in the markets and contain sulfites.
Owner:郑飞雄
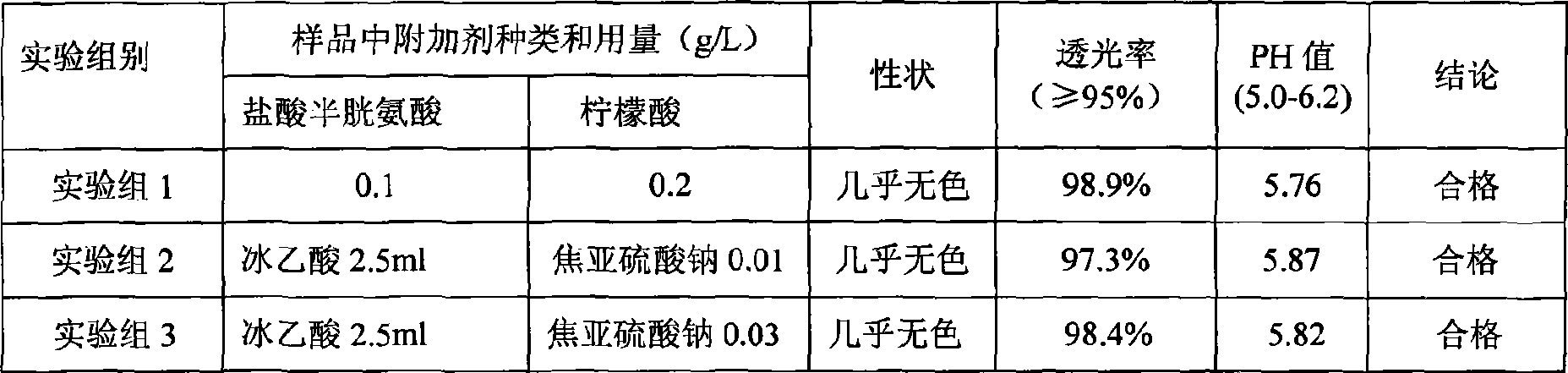
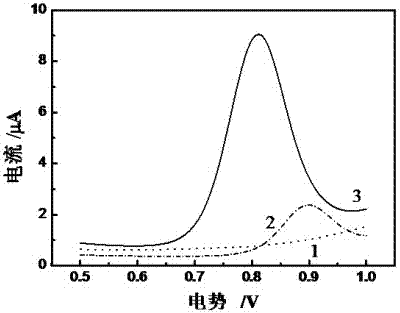
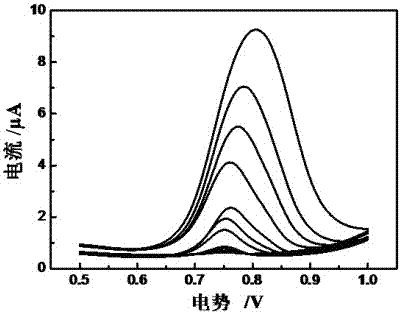
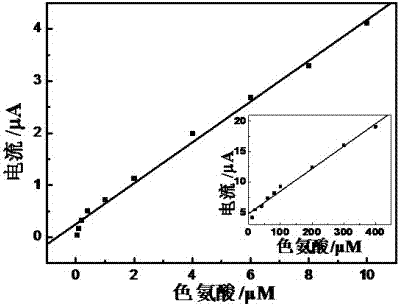
Method for preparing electropolymerized sulfosalicylic acid modified glassy carbon electrode and application of glassy carbon electrode in measurement of tryptophan
InactiveCN102507686AIncreased current responseHigh sensitivityMaterial electrochemical variablesPhosphateSalicylic acid
The invention relates to a method for preparing an electropolymerized sulfosalicylic acid modified glassy carbon electrode, and use of the glassy carbon electrode as a tryptophan electrochemical sensor in high-sensitivity measurement of tryptophan in compound amino acid injection, and belongs to the technical field of electrochemical analysis and detection. The invention mainly aims to prepare the electropolymerized sulfosalicylic acid modified glassy carbon electrode to perform sensitive quantitative analysis and measurement on the tryptophan by a differential pulse voltammetry. Experimental results show that: the modified electrode has an obvious sensitization effect on the tryptophan in a phosphate buffer solution at the concentration of 0.1mol / L and the pH of 3.5, and under the optimal conditions, the differential pulse voltammetry is adopted for measurement, and the tryptophan at the concentration in a range of 5.0*10<-8>-4.0*10<-4> and peak current of the tryptophan have a good linear relation, and the detection limit is 4.0*10<-8>. When the method is used for measuring the tryptophan content in a medicine, results are satisfactory.
Owner:SHANGHAI UNIV
Culture method, application and combined preparation of hypoxia mesenchymal stem cell
InactiveCN101792737AHigh activityImprove survival rateOrganic active ingredientsMammal material medical ingredientsCell massTherapeutic effect
The invention discloses a culture method, application and a combined preparation of a hypoxia mesenchymal stem cell. By adopting an umbilical or placenta mesenchymal stem cell and combining the means of transfusing compound amino acid injection and the like, the vitality, the implantation survival rate and the treating effect of the mesenchymal stem cell in vivo can be improved; and in order to avoid the possibility of conglutination, conglomerating and cell mass embolism of the mesenchymal stem cell in blood vessel, the matching of saline containing heparin during treatment can be carried out by a mode of intravenous injection or intravenous drip. With more than three times of treatment, physical conditions of curee can be remarkably improved and objective indicators such as immunity, blood fat, blood sugar and the like can be remarkably improved.
Owner:晏泽
Compound amino acid injection composition suitable for liver disease patient
InactiveCN101120918AReduce contentIncrease contentDigestive systemPharmaceutical delivery mechanismAntioxidantArginine
The present invention comprises a compound amino acid injection and the preparation method. The characteristics of the injection are as follows. The prescription comprises most human essential amino acid and non essential amino acid. Content of branched chain amino acid (BCAA) in each amino acid is high, and content of the aromatic amino acid (AAA) is low. Content of arginine is high. The prescription comprises the acetylcysteine. The compound amino acid injection is prepared under strict control of aerobic environment, which makes the product in accordance with quality requirement without any antioxidant. The production is applicable in the patients with hepatic disease, which is in accordance with specific requirement for AAA restriction. No antioxidant is added in the prescription, which can effectively reduce incidence rate of adverse reaction in clinical application.
Owner:费森尤斯卡比华瑞制药有限公司
Amino acid injection and preparation method thereof
ActiveCN101401785APain reliefReduce clinical adverse reactionsOrganic active ingredientsMetabolism disorderAntioxidantThreonine
The invention discloses an amino acid injection, commonly known as propranolol, which relates to a pharmaceutical preparation containing 18 kinds of amino acids. The amino acid injection contains the following components per 1,000 milliliters: isoleucine, leucine, methionine, phenylalanine, threonine, tryptophan, valine, glycine, alanine, glutamic acid, aspartic acid, proline, serine, cystine, lysine hydrochloride or lysine acetate, arginine hydrochloride or arginine, histidine hydrochloride or histidine, tyrosine or acetyl tyrosine, and sodium bisulfite or sodium meta-bisulphite. The osmotic pressure ratio of the amino acid injection is lower than 1.8, which effectively reduces the pain for a patient in infusion; the added quantity of antioxidant is small, which can reduce the clinical adverse reactions; and an infusion bottle body is large, which is convenient to mix liquid in clinical application.
Owner:GUANGDONG LITAI PHARM CO LTD
Method for organic acid hydrolytic producing amino-acid
InactiveCN1757633ASolve the separation problemReduce solubilityOrganic compound preparationAmino-carboxyl compound preparationFiltrationAmino Acid Injection
The invention relates to a method of amino-acid with hydrolysis of organic acid or mixed acid (mixing an organic acid and hydrogen chloride with different proportion based on various salt-containing of different products), including the following steps: (1) pre-treatment of raw material; (2) hydrolysis, neutralization; (3) produced amino-acid solution after blending and filtration; (4) going to market after sterilizing, filling in tins, labelling, inspecting and packing. More steps needed for producing amino-acid powder: after adding additives and fillings needed, dissolving, stirring, appropriate concentrating and drying in drying machine, the amino-acid powder is produced, then going to market after packing, labelling, inspecting and packing in boxes. Based on different kind of raw material hydrolysis animal albumen (HAP) and hydrolysis plant albumen (HVP) and various kind of amino-acid preparation may be made with amino-acid. The amino-acid met pharmaceutical grade may be provided as raw material of amino-acid injection solution.
Owner:陈贵卿
Composite glycyrrhizic acid amino acid injection, and preparation method as well as applications thereof
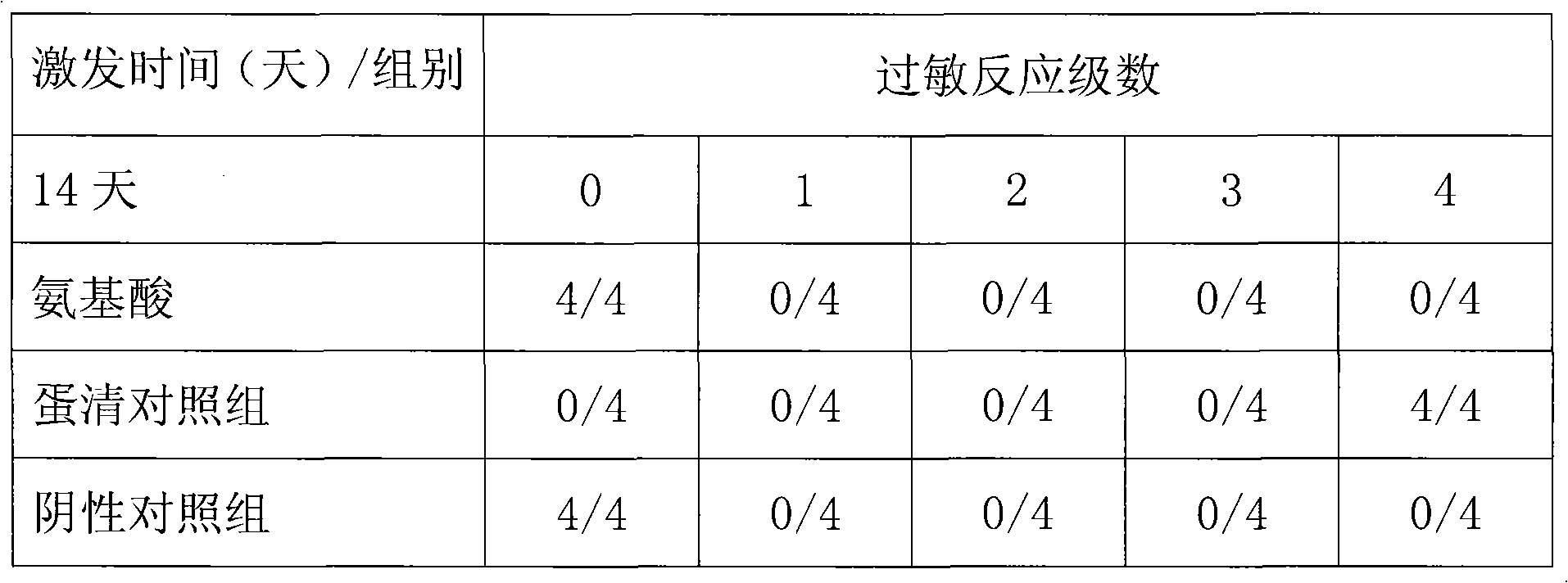
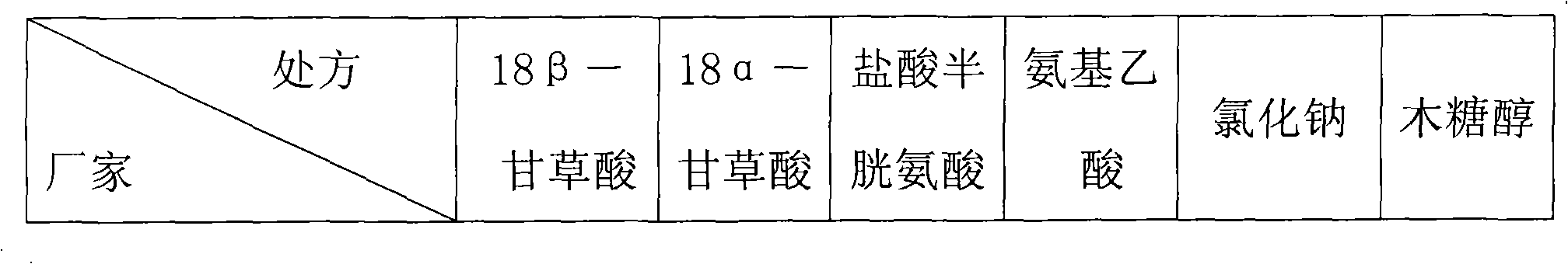
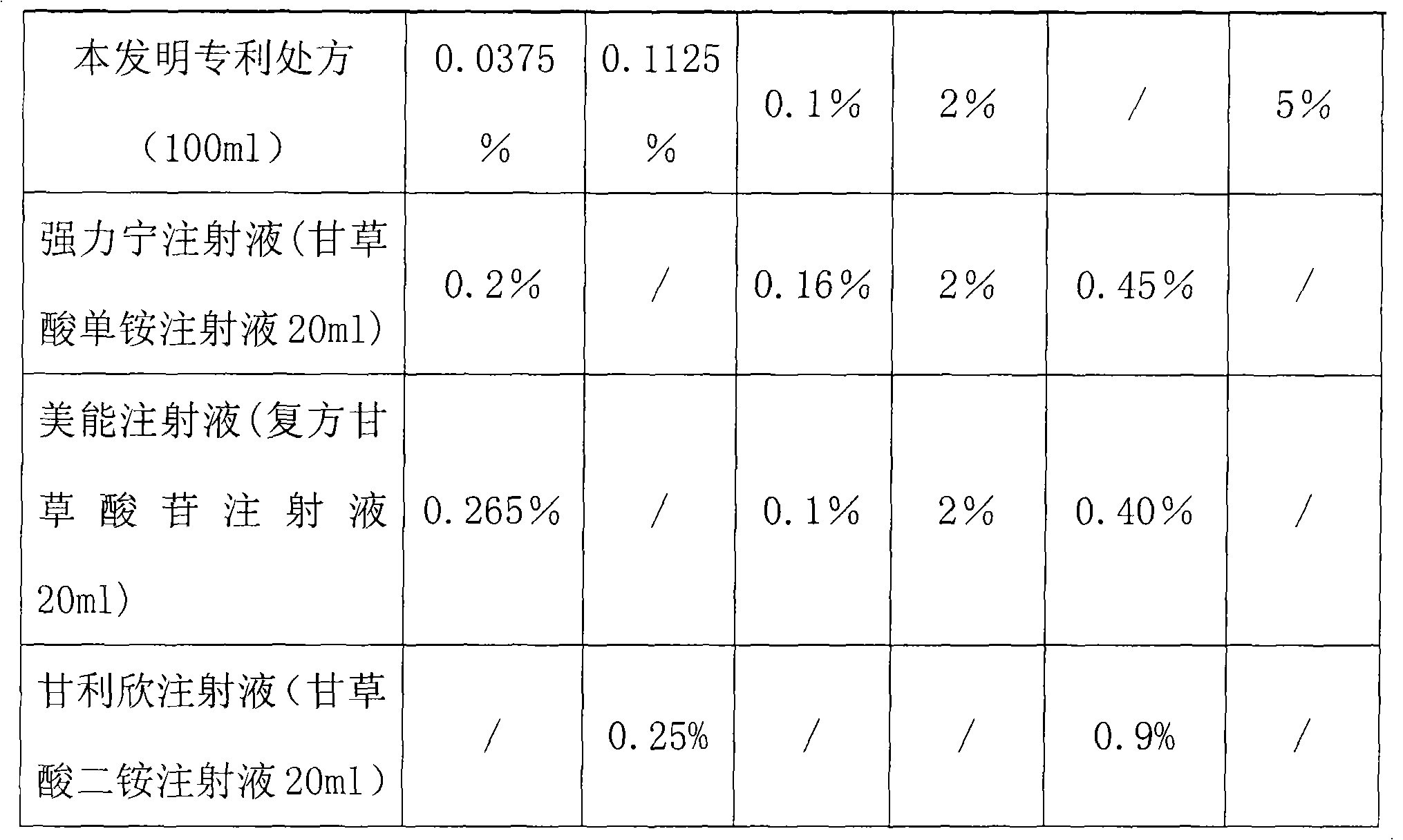
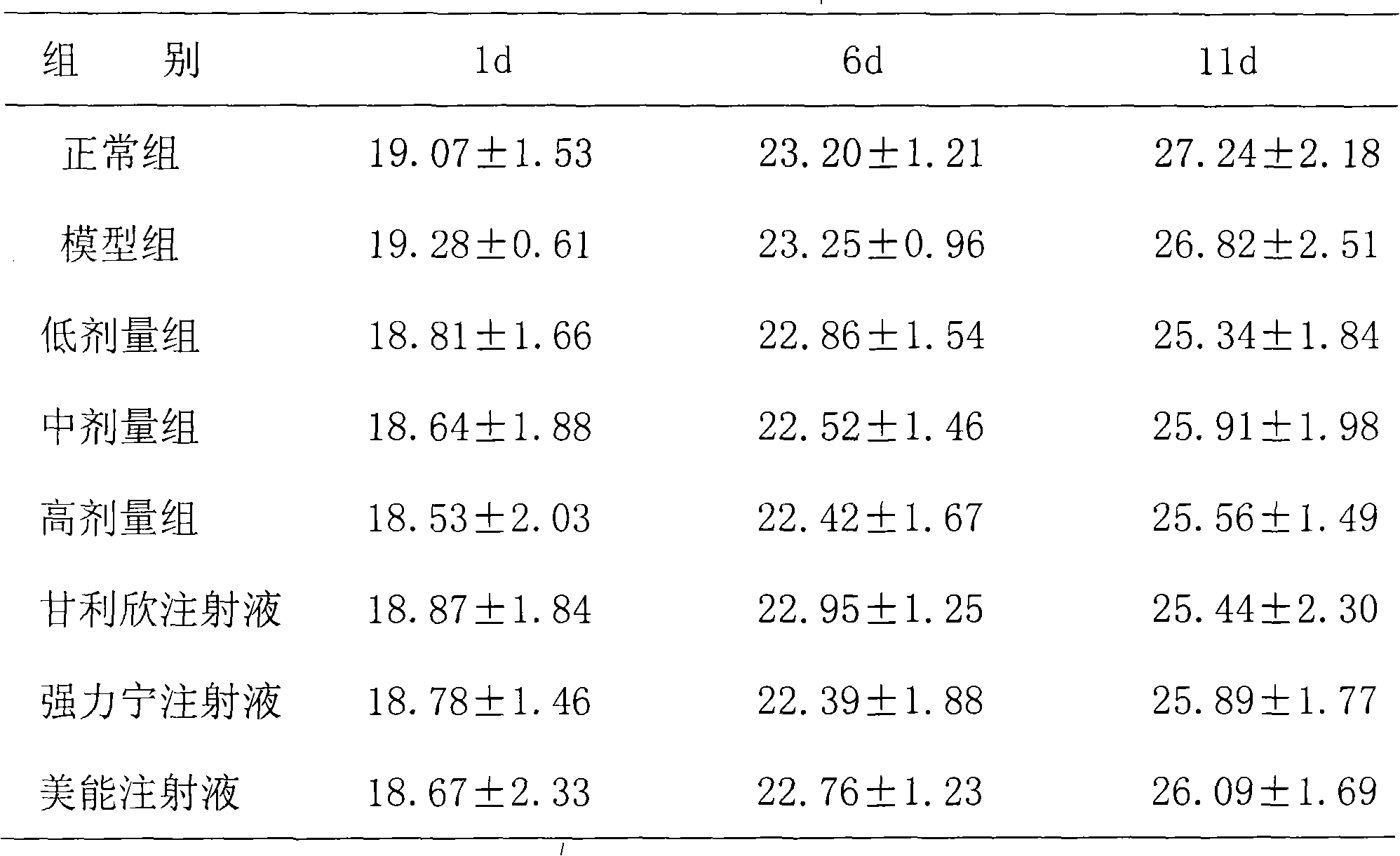
InactiveCN101669962AReduced functionalityImprove functionalityAntipyreticMetabolism disorderAmino Acid InjectionDrug product
The invention belongs to the technical field of medicines, in particular to a composite glycyrrhizic acid amino acid injection, and simultaneously, the invention also relates to a method for preparingthe injection and applications thereof in medicines. The invention aims at providing the composite glycyrrhizic acid amino acid injection which has obvious functions of anti-inflammation, antianaphylaxis, oxidation resistance, antiatheroscloresis, immune regulation and detoxication, and also has the characteristics of good product quality, safe and reliable taking and the like; and the method hasthe characteristics of simple technological process and high product yield. The technical scheme provides the composite glycyrrhizic acid amino acid injection which is characterized by being preparedby following crude drug by weight percent: 0.05-0.4% of composite glycyrrhizic acid, 1.05-3.2% of acid amino, 3-8% of xylitol, 0.01-0.05% of pH modifier, and the balance of water for injection.
Owner:杭州市第六人民医院
Pharmaceutical composition of alanyl glutamine and compound amino acid
InactiveCN102429902AImprove securityAvoid quality impactOrganic active ingredientsPowder deliveryFreeze-dryingAmino Acid Injection
The invention relates to a pharmaceutical composition of alanyl glutamine aseptic frozen-dried powder for injection and compound amino acid injection, particularly a combined application package, comprising alanyl glutamine aseptic frozen-dried powder for injection, water for injection, and compound amino acid injection. When the pharmaceutical composition is used, the alanyl glutamine aseptic frozen-dried powder for injection is dissolved with water for injection, and then is mixed with compatible amino acid injection or transfusion containing amino acid, and intravenous infusion is used. The combined application package disclosed herein avoids the influence to quality caused by quality discrepancy of different factories when the combination is used, and greatly increases the safety of application of patients.
Owner:HAINAN LINGKANG PHARMA CO LTD
Preparation method of compound amino acid injection
ActiveCN102302489ASolve the yellowing of the colorSolve the problem of decreased amino acid contentOrganic active ingredientsMetabolism disorderNitrogenWater soluble
The invention provides a preparation method of a compound amino acid injection. Due to the adoption of vacuumization and nitrogen injection in an injection preparation process, oxygen in preparation equipment and water-soluble dissolved oxygen are removed, and the problems that amino acid is instable and is vulnerable to oxidizing in a solution so as to enable the color of the liquid medicine to turn yellow and the content of the amino acid to be decreased are solved. The preparation method provided by the invention is characterized in that the most insoluble amino acid composition, i.e. cystine, is dissolved by low-concentration alkali liquor independently and then is subjected to batch feeding, other amino acid components are subjected to batch feeding at a time, and multi-group batch feeding or high-temperature batch feeding of each component is not needed; and the preparation method has the advantages of simple and convenient batch feeding way, low preparation temperature, short preparation time and the like, has lower requirement on production facilities, and can be easily operated by production staff.
Owner:HUAREN PHARMACEUTICAL CO LTD
Separately packed fatty milk, amino acid and glucose injection composition and the prepn process
InactiveCN101019822AEasy to synthesizeScientific and reasonable clinical useMetabolism disorderPharmaceutical delivery mechanismAmino Acid InjectionMedical prescription
The present invention is one kind of three-cavity packed injection composition, and has medium / long chain fatty milk injection, compound amino acid injection and glucose injection packed separately in three diaphragm separated cavities. Before intravenous infusion, the medium / long chain fatty milk injection, the compound amino acid injection and the glucose injection are squeezed out and mixed in common operation condition. The injection composition can be used to patient for meeting the requirement in protein and saccharide.
Owner:费森尤斯卡比华瑞制药有限公司
Separately packed structural fatty milk, aminoacid and glucose injection composition and the prepn process
ActiveCN101019823AMeet metabolic needsAvoid Metabolic AcidosisMetabolism disorderPharmaceutical delivery mechanismAmino Acid InjectionMedical prescription
The present invention is one kind of three-cavity packed injection composition, and has structural fatty milk injection, compound amino acid injection and glucose injection packed separately in three diaphragm separated cavities. Before intravenous infusion, the structural fatty milk injection, the compound amino acid injection and the glucose injection are squeezed out and mixed in common operation condition. The injection composition can be used to patient for meeting the requirement in protein and saccharide.
Owner:费森尤斯卡比华瑞制药有限公司
Clinical mesenchymal stem cell cryopreservation solution and preparation method thereof
InactiveCN109329271AImprove the living environmentImprove protectionDead animal preservationAmino Acid InjectionTrehalose
The invention discloses clinical mesenchymal stem cell cryopreservation solution and a preparation method thereof. The preservation solution comprises the following components in percentage by weight:1-5% of autologous plasma lysate mixed liquor, 1-2% of human serum albumin, 0-1% of VC injection, 5-10% of glucose injection, 20-30% of electrolyte-like solution, 20-30% of glucosamine injection, 20-30% of compound amino acid injection, 2-5% of dimethyl sulfoxide and 2.5-5% of trehalose. The prepared cryopreservation solution can be used for deep low-temperature and long-term storage of cells, and can realize long-distance transportation, so that the distribution range of cells is expanded, the long-distance treatment of stem cells is realized, and the cells can be directly returned after recovery so as to reduce the potential safety hazards caused by complicated operations.
Owner:CHENGDU QINGKE BIOTECH
Method for preparing compound amino acid injection liquid (20AA)
InactiveCN102961377AOrganic active ingredientsPeptide/protein ingredientsPhysical chemistryAmino Acid Injection
The invention mainly relates to a method for preparing compound amino acid injection liquid (20AA), and belongs to the technical field of medicines. The problems that the amino acid is oxidized and degraded and solution color is deepened because the solution is in contact with oxygen for a long time during preparation and filling are solved by vacuumizing and filling nitrogen during preparation of the injection liquid. In the method, the injection liquid is dissolved in batch or in several times according to the dissolution difficulty of amino acid, so that the problem that the indissolvable amino acid crystal separates out during preparation is solved, and the stability and the quality of the amino acid injection liquid are guaranteed to the maximum limit. The method has the advantages of simple operation, scientific and reasonable process, low cost and low requirement on production equipment.
Owner:湖北一半天制药有限公司
Pharmaceutical composition of alanyl glutamine injection and compound amino acid injection
InactiveCN102631665AProne to decompositionLittle side effectsOrganic active ingredientsDipeptide ingredientsAmino Acid InjectionPharmacology
The invention relates to a pharmaceutical composition of an alanyl glutamine injection and a compound amino acid injection, in particular to combination application package. The pharmaceutical composition comprises the alanyl glutamine injection and the compound amino acid injection. When the pharmaceutical composition is in use, the alanyl glutamine injection and the compound amino acid injection are jointly utilized and are intravenously dripped. The combination application package greatly facilitates the requirement of clinical application.
Owner:灵康药业集团股份有限公司
Stem cell culture solution and injection
ActiveCN106754668AKeep aliveHealth recoveryCulture processPharmaceutical delivery mechanismDipeptideHuman albumin
A stem cell culture solution is prepared from raw materials as follows: 10% of a compound amino acid injection, 1% of water-soluble vitamins for injection, 0.5% of glutamine dipeptide, 10% of human albumin, 10% of a hydrolyzed protein injection, 0.5% of a microelement injection and 68% of a plasmalyte-A solution, wherein the pH value is 6.8-7.0, and the osmotic pressure is 310 mOsm / kg. The stem cell culture solution is used for culturing mesenchymal stem cells, and a mesenchymal stem cell culture supernatant is collected; the collected culture supernatant is filtered by a filter membrane, and filtrate is collected and is the stem cell injection. A decellularized stem cell secretion factor is used for intravenous injection, so that the dispute of stem cells in the aspect of ethic is effectively avoided; the stem cell culture solution is prepared from clinical raw materials, is safe and reliable, provides nutrition for the living environment of the stem cells, can effectively promote the stem cells to secrete a large quantity of cell factors and keeps the activity of the stem cells; through cooperation of a nutritional supplement formula and the stem cell secretion factor, the sub-health state of a body can be effectively relieved, and the body health is regained.
Owner:沈阳细胞治疗工程技术研发中心有限公司
Lovamin and its prepn process
InactiveCN1370524AGuaranteed quality of lifeImprove securityPeptide/protein ingredientsMetabolism disorderAcetic acidSide effect
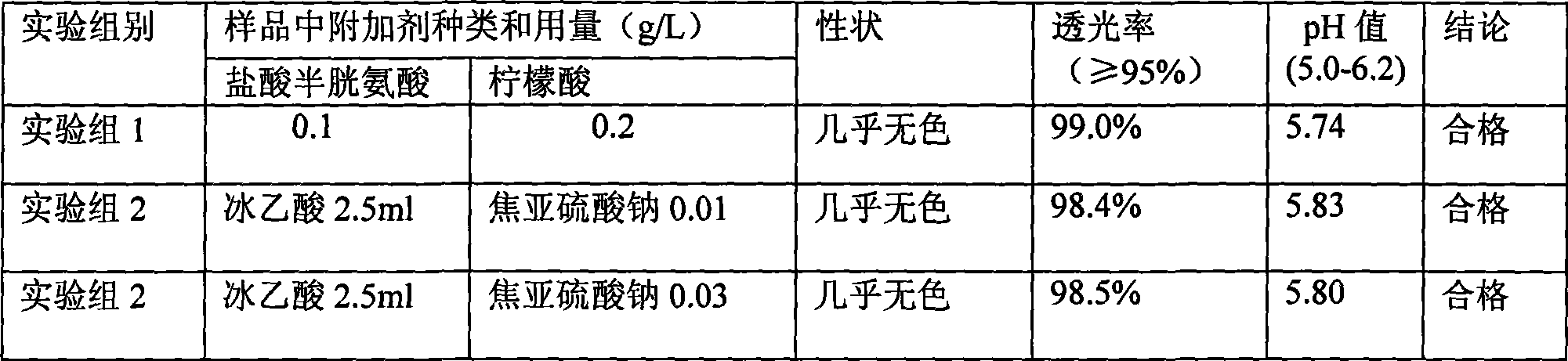
The present invention belongs to one mixed amino acid injection containing 18 kinds of amino acids and is named Lovamin. Its main components includes 18 kinds of amino acids in 8.5 wt% or 11.4 wt%, sulfite in 0.001-0.003 wt% and glacial acetic acid in small amount other than injection water. Its preparation process includes the steps of compounding of injection, filling and disinfecting in a steam sterilizer. Compared with similar product, the present invention has less side effect.
Owner:费森尤斯卡比华瑞制药有限公司
Compound amino acid injection and preparation method thereof
InactiveCN110507604AReduce degradationStable in natureOrganic active ingredientsMetabolism disorderTryptophanBottle
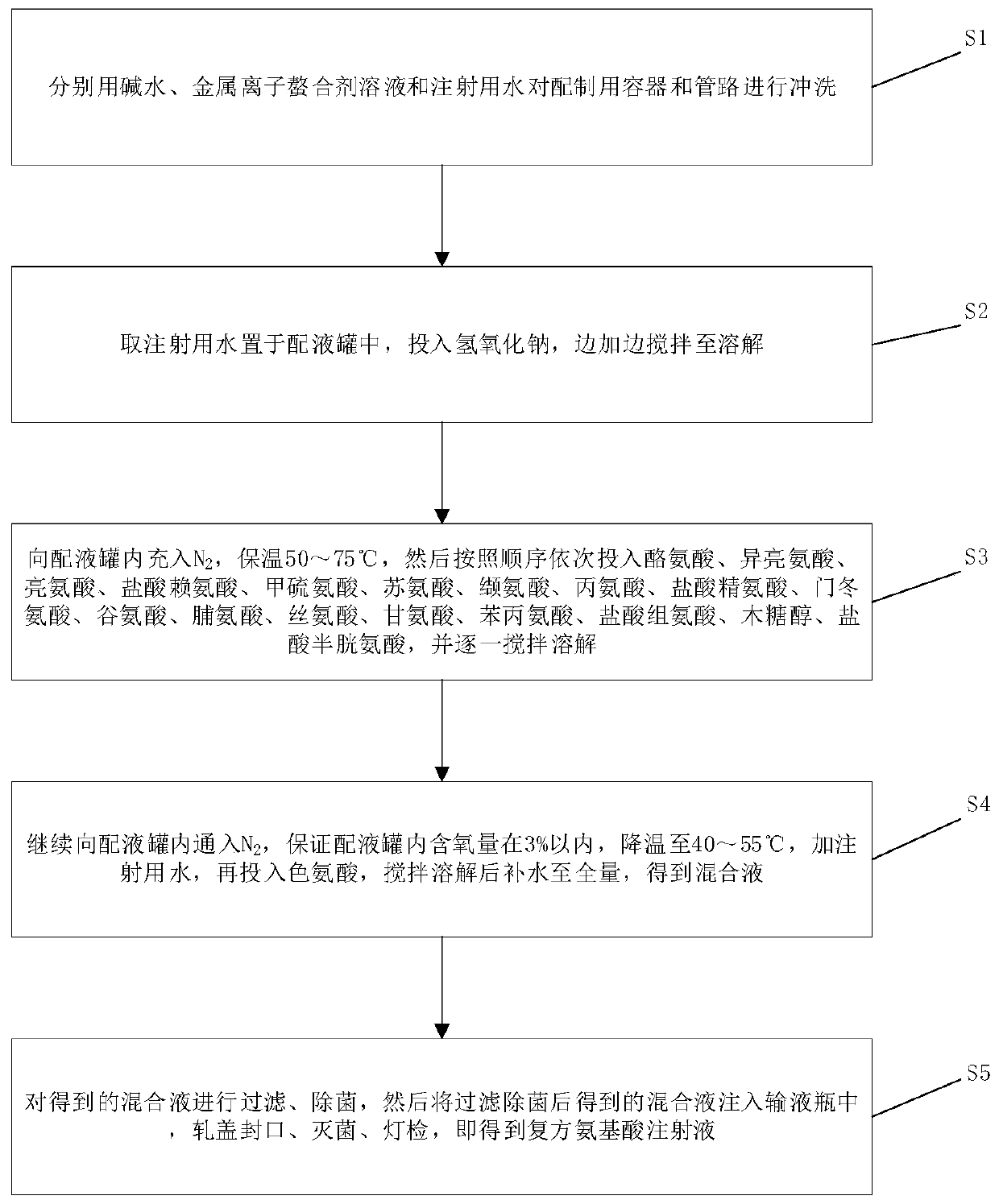
The invention provides a preparation method of a compound amino acid injection. The preparation method comprises the following steps: respectively washing a preparation container and a pipeline with alkaline water, a metal ion chelating agent solution and water for injection; putting the water for injection into a liquid preparation tank, adding sodium hydroxide, and stirring while adding until the sodium hydroxide is dissolved; introducing N2 into the liquid preparation tank, then sequentially adding tyrosine, isoleucine, leucine, lysine hydrochloride, methionine, threonine, valine, alanine,arginine hydrochloride, aspartic acid, glutamic acid, proline, serine, glycine, phenylalanine, histidine hydrochloride, xylitol and cysteine hydrochloride, and stirring and dissolving one by one; continuously introducing N2 into the liquid preparation tank, ensuring that the oxygen content is within 3%, cooling the materials, adding water for injection, adding tryptophan, stirring, dissolving, andsupplementing water to a full amount to obtain a mixed solution; and filtering and sterilizing the mixed solution, injecting the mixed solution into an infusion bottle, capping and sealing the bottle, and performing sterilizing and performing light inspection to obtain the compound amino acid injection.
Owner:武汉久安药业有限公司
Preparation and application method of human peripheral blood stem cell freezing medium
InactiveCN104430303AEffective preservationSimple and fast operationDead animal preservationHydroxyethyl starchSterile environment
The invention relates to preparation and an application method of a human peripheral blood stem cell freezing medium. The stem cell freezing medium is prepared from the following components in percentage by volume: 48.2% of hydroxyethyl starch dextran, 2% of compound amino acid injection (18AA-IV), 4.8% of dimethyl sulfoxide and 20% of human serum albumin. The stem cell freezing medium has the characteristics of high viable rate after recovery (greater than 99%) and availability for vein back transfusion. The invention further provides the preparation and the application method of the human peripheral blood stem cell freezing medium. The preparation comprises the following steps: under sterile environment, premixing all the components of the human peripheral blood stem cell freezing medium in sequence, adding a stem cell suspension, mixing uniformly, and packing into a cryopreservation bag for cryopreservation. Stem cells with the freezing medium can be directly placed into a cryogenic refrigerator at -80 DEG C for storage, a programmable cooler is not needed, the operation is simple and convenient, and the use is safe.
Owner:QINGDAO CENT HOSPITAL
Compound amino acid injection for livestock as well as preparation method and applications thereof
ActiveCN102552250AEnhance physical fitnessReduce involuntary movementOrganic active ingredientsMetabolism disorderBiotechnologyDisease
Owner:HEBEI KEXING PHARMA
Compounded amino acid injection 18AA-II content measurement method
ActiveCN105588900AEasy to separateReduce distractionsComponent separationAmino Acid InjectionAmino acid content
The invention relates to the field of medicine preparation and especially relates to a compounded amino acid injection 18AA-II content measurement method. According to the method, when the time of conversion of a buffer solution from B2 to B3 is pushed back by 0.5 min, the time of conversion of a buffer solution from B3 to B4 is pushed back by 0.8 min, and the temperature of a separation column reaches 57 DEG C, a better separation effect between the components is achieved and further separation degree between cystine and valine satisfies the demand on content measurement, and meanwhile, interference on the cystine due to conversion of a mobile phase is reduced, so that accurate quantitative measurement is achieved even under a low content, and finally, accurate measurement of contents of sixteen amino acids in the compounded amino acid injection 18AA-II with an amino acid analyzer can be achieved.
Owner:HUAREN PHARMACEUTICAL CO LTD
Tissue preserving fluid for treating HIV (human immunodeficiency virus) infection-accompanied colorectal cancer and decompensated cirrhosis complicated small liver carcinoma
The invention discloses a tissue preserving fluid. The tissue preserving fluid is composed of, by concentration, 1.0-2.5%w / v of poloamer, 1.0-2.5%w / v of polyvinylpyrrolidone, 0.2-1.0%w / v of glucose, 15-40%w / v of compound amino acid injection, 5-30%w / v of propylene glycol, 10-40%w / v of PBS (phosphate buffer saline) vitamin E, 0.1-1.5%w / v of vitamin C and 0.1-1.0%w / v of aqueous solvent for injection. Due to the fact of containing no DMSO (dimethylsulfoxide) or protein, the tissue preserving fluid can avoid toxicity produced due to DMSO reverse infusion as well as safety worries of serum and proteins on clinical application, thereby being high in safety.
Owner:南京三生生物技术股份有限公司
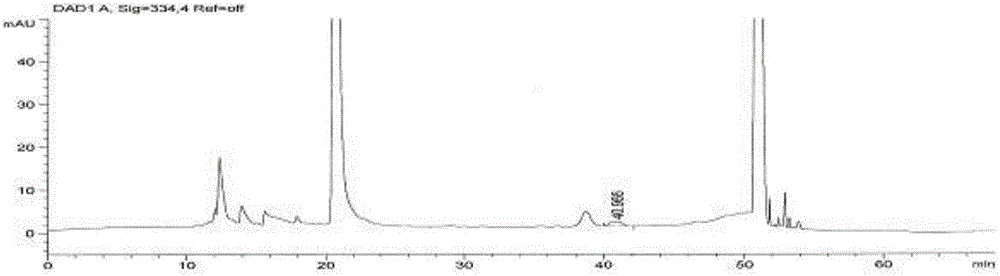
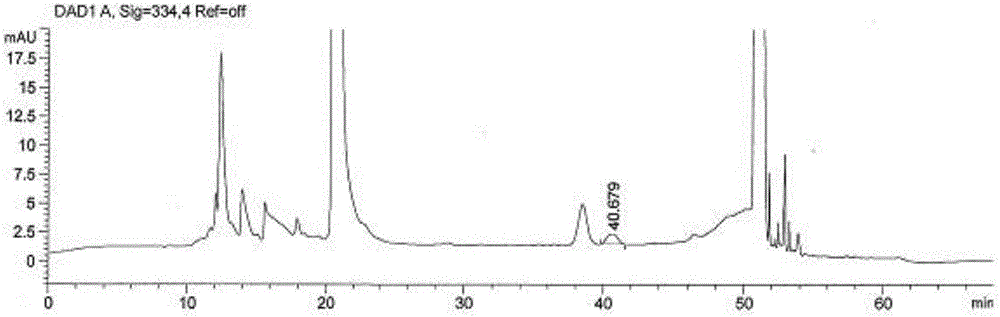
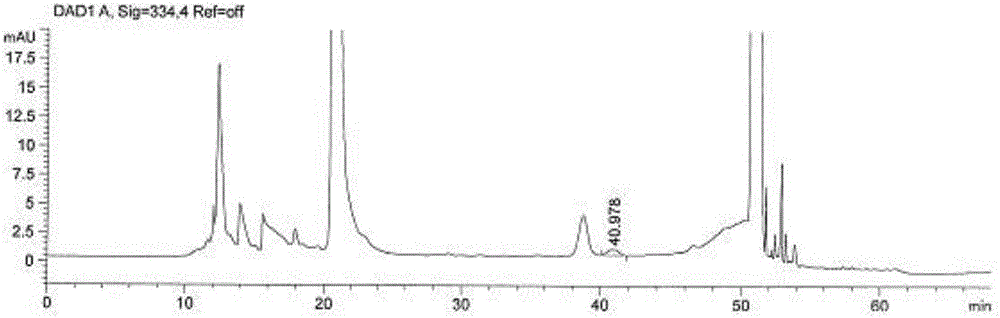
Method for measuring content of degraded impurity methionine sulfoxide in compound amino acid injection
ActiveCN106248853AEffective controlEasy to separateComponent separationSilanesMonopotassium phosphate
The invention discloses a method for measuring content of degraded impurity methionine sulfoxide in compound amino acid injection; an octadecyl silane bonded silica gel column is used, program setting is made by using Agilent sampler and derivatization is carried out, wavelength of 334 nm is detected after sampling, and the content is calculated according to an external standard method; for mobile phases, 0.038 mol / L of monopotassium phosphate (pH 6.9) and acetonitrile (88:12) acts as mobile phase A, acetonitrile acts as mobile phase B, and gradient diluting is carried out; test article diluent is water, and sampling concentration is 5 Mug / ml; according to the method of the invention, other amino acids and auxiliaries in the formulation are friendly to detection, the method has a good degree of separation and high specificity, the difficulty in detecting methionine sulfoxide in the amino acid compound is overcome, the methionine sulfoxide in a product is effectively controlled, and product quality is guaranteed.
Owner:SICHUAN KELUN PHARMA CO LTD
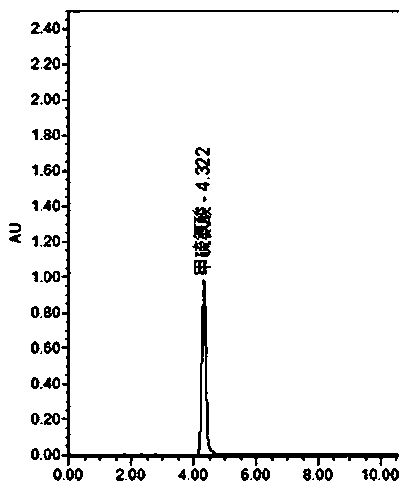
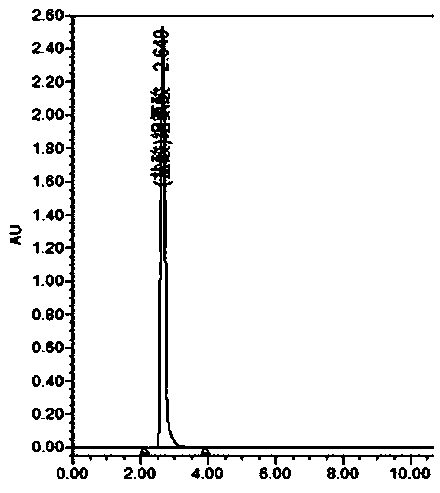
Determination method for determining tryptophan-related substances in amino acid injection
ActiveCN107907603AThe result is obviousEfficient determinationComponent separationStationary phaseRetention time
The invention provides a determination method for determining tryptophan-related substances in an amino acid injection, and relates to the technical field of medicines. The determination method is used for determining the content of the tryptophan-related substances in amino acid injection. The method adopts high-performance liquid chromatography, adopts chromatographic column using octadecylsilane bonded silica as the stationary phase, and adopts the following three mobile phases: the mobile phase A being 0.01 mol / L sodium dihydrogen phosphate and having a pH value being adjusted with sodiumhydroxide solution and being 7.2; the mobile phase B being obtained through mixing acetonitrile, methanol and water according to a volume ratio of 45:45:10; and the mobile phase C being obtained through mixing methanol and water according to a volume ratio of 10:90 to carry out gradient elution. The relative retention time of tryptophan is analyzed and calculated to separate and determine the tryptophan-related substances in the amino acid injection. The determination method has the advantages of rapid analysis speed of the tryptophan-related substances, high accuracy and low inspection cost.
Owner:湖北华仁同济药业有限责任公司
Rapid detection method of methionine sulfoxide in amino acid injection
The invention belongs to the technical filed of a drug detection method and particularly relates to a rapid detection method of methionine sulfoxide in amino acid injection. An automatic amino acid analyzer is adopted for detection; only two mobile phases are adopted for separation, R1-R3 are derivative reaction reagents, Hitachi special ion exchange resin is taken as a filling agent, and the calculation is performed by peak area according to an external standard method. Through contrastive study, as a primary standard procedure adopts six mobile phases, the consumption of the reagents is high, and the mobile phases are various. As the two mobile phases are adopted, the consumption of the reagents is low, and a spectrogram is briefer. The test of the primary standard procedure needs 53.8 minutes, and the detection method provided by the invention only needs 29.5 minutes, so that the detection time is greatly reduced, and the efficiency is higher.
Owner:湖北华仁同济药业有限责任公司
Glutamine dipeptide and compound amino acid medicinal composition prepared with solvent crystallization method
ActiveCN102698247AThe need for convenient clinical medicationConvenient sourcePowder deliveryOrganic active ingredientsDipeptideAmino Acid Injection
The invention relates to a medicinal composition of glutamine dipeptide sterile powder for injection and a compound amino acid injection, prepared with a solvent crystallization method, in particular to a combined application package. The medicinal composition comprises glutamine dipeptide sterile powder for injection, water for injection and a compound amino acid injection. Due to the adoption of the combined application package disclosed by the invention, great convenience is brought to the requirement of clinical administration; great convenience is brought to purchase and dispensation of an administration unit, and cost is saved; the combined application package is made for emergent use, and plays a great role in ensuring the stability of every single medicament, and the clinical application quality and bioavailability of a medicament can be enhanced; and the composition disclosed by the invention has the effect of remarkably reducing liver damage.
Owner:灵康药业集团股份有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com