Mesenchymal stem cell cryopreserved liquid and injection liquid
A technology of mesenchymal stem cells and cryopreservation solution, which is applied in the field of mesenchymal stem cell cryopreservation solution and injection, can solve the problems of high cost, difficulty in large-scale application, difficulty in taking into account cell viability and clinical direct infusion, etc., and achieve the goal of preparing The effect of simple method, low cost and good clinical application prospect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] Example 1 Preparation of Injection with Mesenchymal Stem Cell Freezing Solution of the present invention
[0082] 1. Preparation of mesenchymal stem cell injection of the present invention
[0083] The formulation of the cryopreservation solution is shown in Table 1. Take Bomaili A solution, 18AA5% compound amino acid injection and 20% human serum albumin, mix well, and add umbilical cord mesenchymal stem cells until the cell density is 1×10 7 pcs / ml, then add DMSO.
[0084] Table 1 Mesenchymal stem cell cryopreservation liquid formula of the present invention
[0085]
[0086] In group 6, the albumin content was high, and the cost was higher than other groups.
[0087] Control group: take 90ml of complete medium (90% (v / v) DMEM / F12+10% (v / v) fetal calf serum), mix well, add umbilical cord mesenchymal stem cells to a cell density of 1×10 7 pcs / ml, then add 10ml DMSO. The formula of the control group is a commonly used cell cryopreservation solution in China.
[...
Embodiment 2
[0099] Example 2 Detection of various properties of the mesenchymal stem cell injection of the present invention after one year of frozen storage
[0100] 1. Experimental method
[0101] The mesenchymal stem cell injection of the present invention: Take another batch of cells that are prepared in the same way as Group 3 of Example 1, freeze them for 1 year and revive them in a water bath at 37°C, and store them in a refrigerator at 2-8°C.
[0102] Control group: with embodiment 1.
[0103] (1) Recovery after 1 year of cryopreservation, detection of cell viability at different time points after recovery
[0104] Take the cells before freezing to test the cell viability; test the cell viability of the mesenchymal stem cell injection of the present invention and the cells of the control group at 0 hours, 24 hours, 48 hours and 72 hours after recovery. The detection method was classical trypan blue staining.
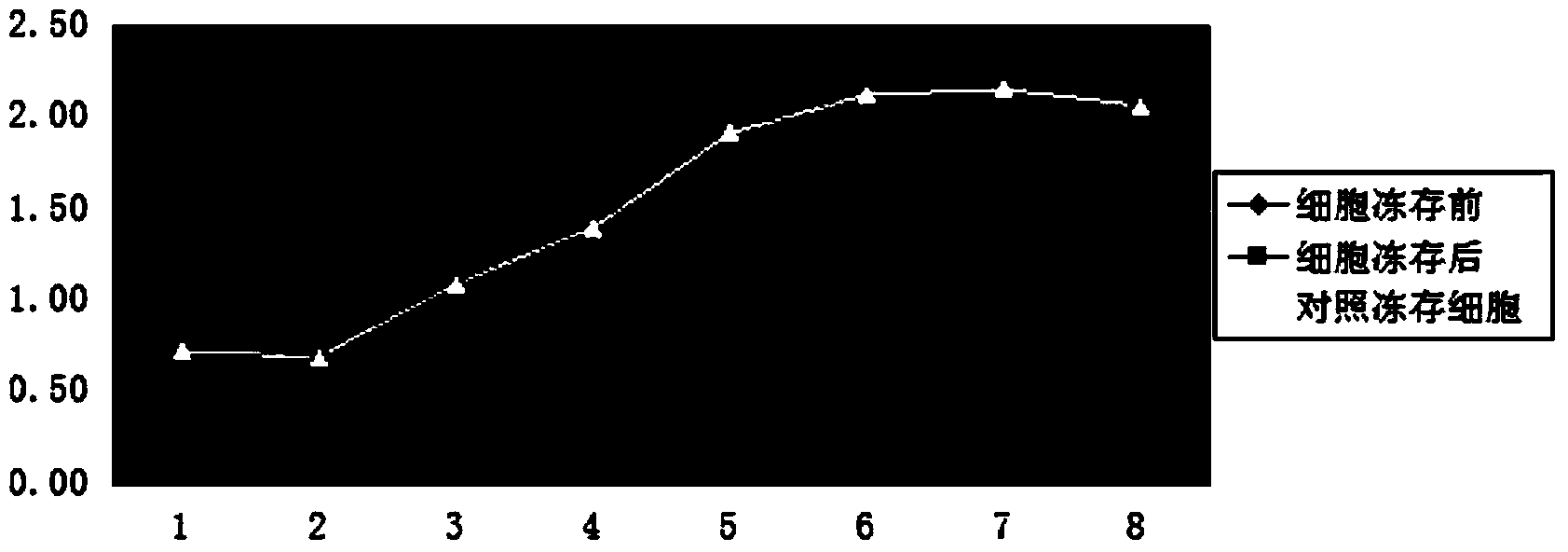
[0105] (2) Comparison of growth curves
[0106] Take the cells be...
Embodiment 3
[0133] Example 3 The relationship between cell viability and freezing time of mesenchymal stem cell injection of the present invention
[0134] 1. Experimental method
[0135] The mesenchymal stem cell injection of the present invention: prepare the mesenchymal stem cell injection according to the proportion of the first group and 3 in the embodiment, and store it in a liquid nitrogen tank after program-controlled cooling.
[0136] Contrast: with embodiment 1.
[0137] After 1, 6, and 12 months of frozen storage, they were revived in a 37°C water bath, and the cell viability was tested respectively. The detection method was the classic trypan blue staining method.
[0138] 2. Experimental results
[0139] The experimental results are shown in Table 5:
[0140] Table 5 The relationship between cell viability and freezing time
[0141]
[0142] As can be seen from Table 5, after 1, 6, and 12 months of cryopreservation, the cell viability of the mesenchymal stem cell injec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com