Preparation and application method of human peripheral blood stem cell freezing medium
A technology of human peripheral blood and stem cells, applied in the field of stem cell bioengineering, can solve problems such as toxic side effects, high cost, and inconvenient clinical application, and achieve the effect of easy operation and safe use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0008] The present invention will be further described below in conjunction with specific embodiments, but not limited to the present invention.
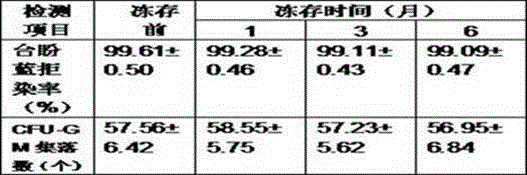
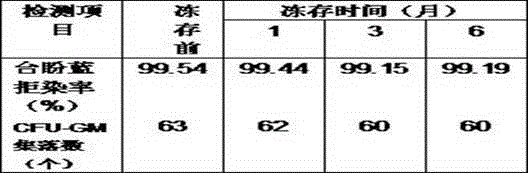
[0009] Zhang XX, non-Hodgkin's lymphoma. The blood cell separator collects 100ml of peripheral blood stem cell suspension, and the leukocyte concentration is 245.43×10 9 / L. The collection bag is placed on the ultra-clean bench. According to the fact that the stem cell suspension accounts for 25% of the final volume of the cryopreservation system, the final cryopreservation volume is calculated as 100ml÷25%=400ml; according to the mixture of compound amino acid injection (18AA-IV), DMSO and hydroxyethyl starch substitute plasma Accounting for 55% of the final volume of the cryopreservation system, the calculated volume of the mixture of the three is 400ml×55%=220ml; based on the final volume percentage of human serum albumin of 20%, the required volume of human serum albumin is calculated to be 400ml× 20%=80ml. Aseptically extra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com