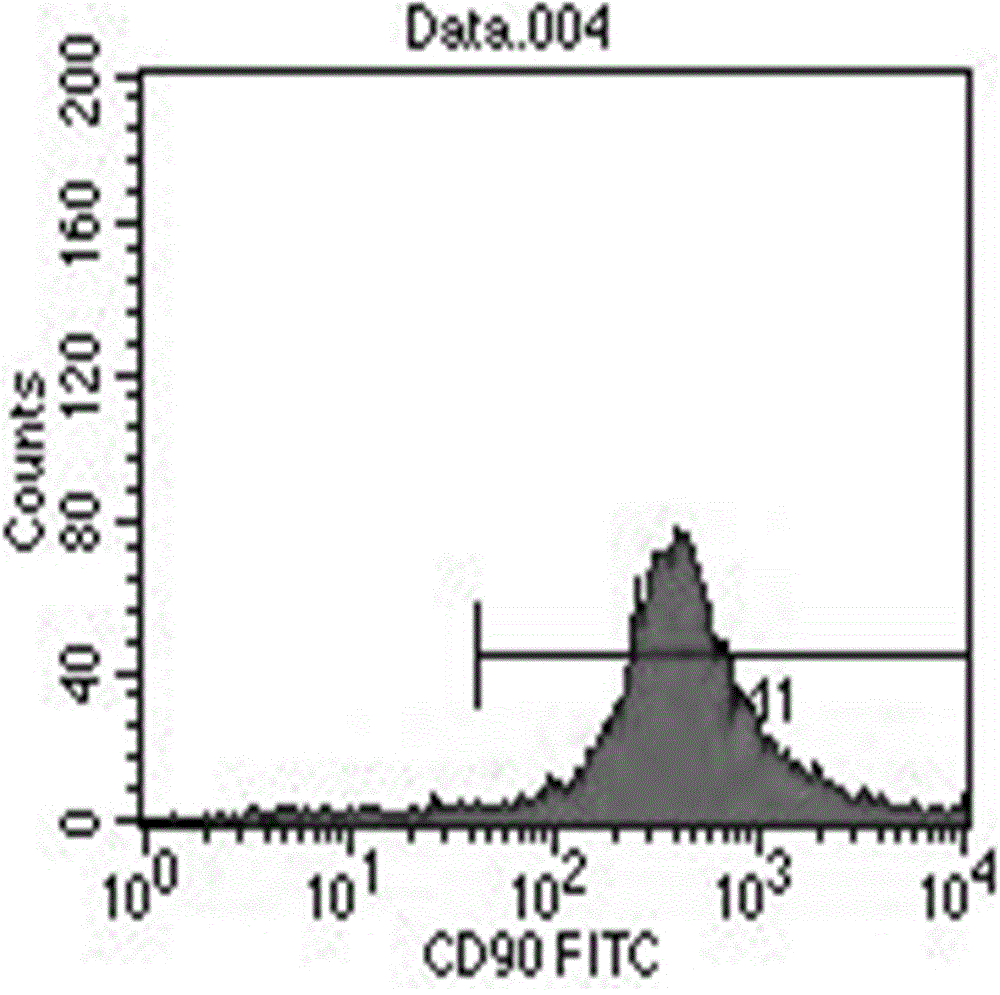
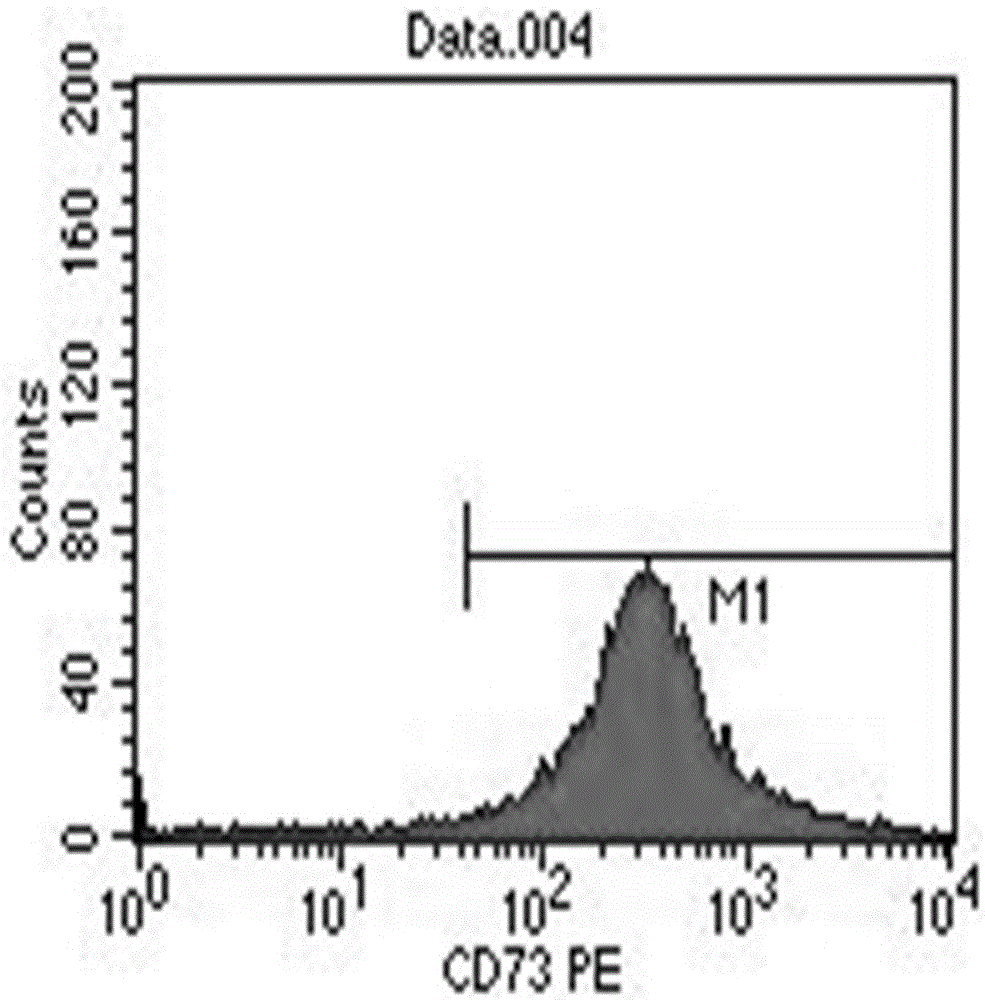
Stem cell culture solution and injection
A stem cell culture and injection technology, applied in cell culture active agents, animal cells, culture process, etc., can solve the problems of inability to meet cell nutrition, single nutritional structure, cell activity and the content of secreted factors, etc., to maintain stem cell activity. , huge application prospects, the effect of alleviating the sub-health state of the body
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] A method for preparing umbilical cord mesenchymal stem cell culture fluid and injection, comprising the following steps.
[0037] Step 1. Sample collection.
[0038] With the informed consent of the puerpera, fresh aseptic umbilical cord tissue was collected from full-term healthy fetuses, stored at 4°C, and processed within 24 hours. Maternal blood should be collected within seven days before delivery for HIV testing, hepatitis B testing, hepatitis C testing, syphilis testing, alanine aminotransferase testing, cytomegalovirus testing and mycoplasma testing. Fresh sterile umbilical cord tissue from the fetus can only be used after all tests pass.
[0039] Cleaning and disinfection: Take out the umbilical cord in the biological safety cabinet, first use 10ml of 0.9% sodium chloride injection to clean the surface, then immerse the umbilical cord in 75% alcohol sterilized by 0.22μm filter for 2 minutes; finally use 0.9% chlorine Sodium chloride injection 10ml was washed a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com