Clinical mesenchymal stem cell cryopreservation solution and preparation method thereof
A cryopreservation solution and mesenchymal stem cell technology, which is applied in the field of clinical mesenchymal stem cell cryopreservation solution and its preparation, can solve the problems of low cell activity and inability to be directly applied, achieve stable cell structure, realize long-distance treatment, reduce Effect of exogenous infection risk
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
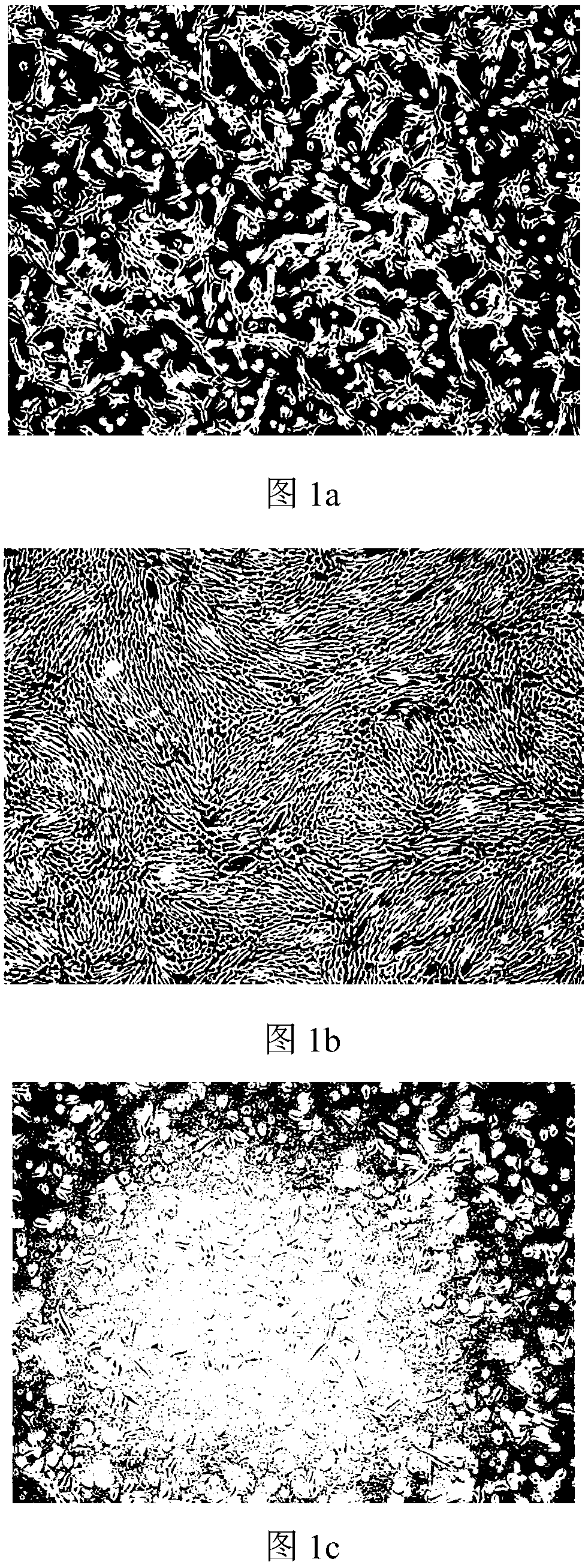
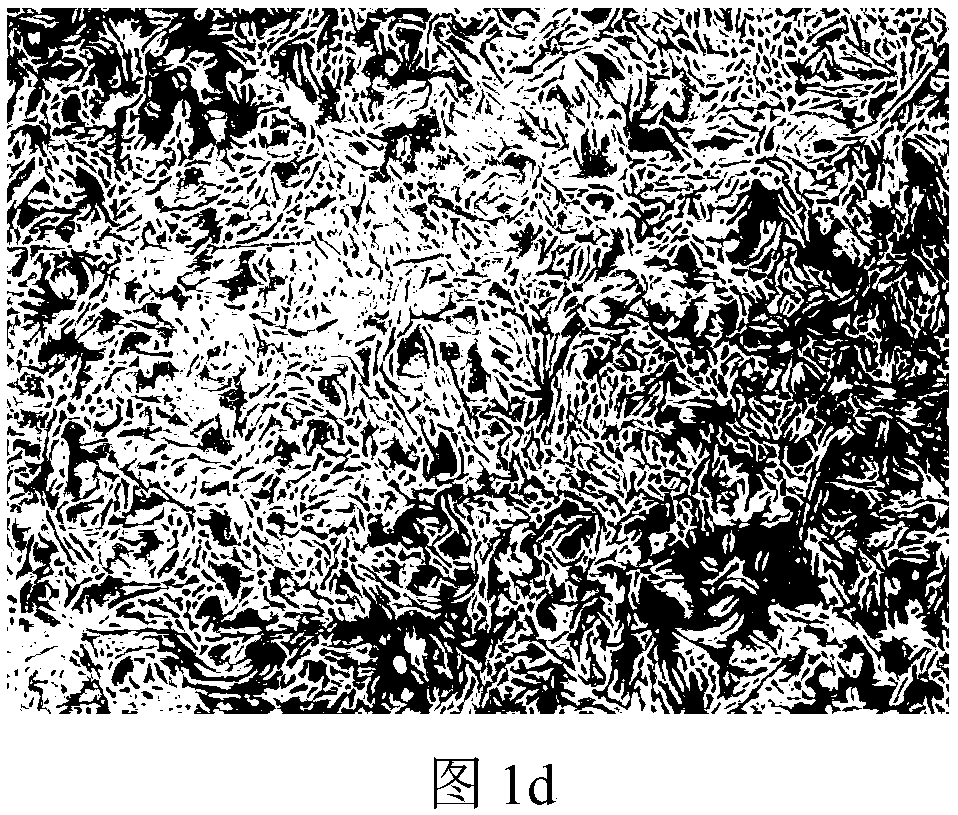
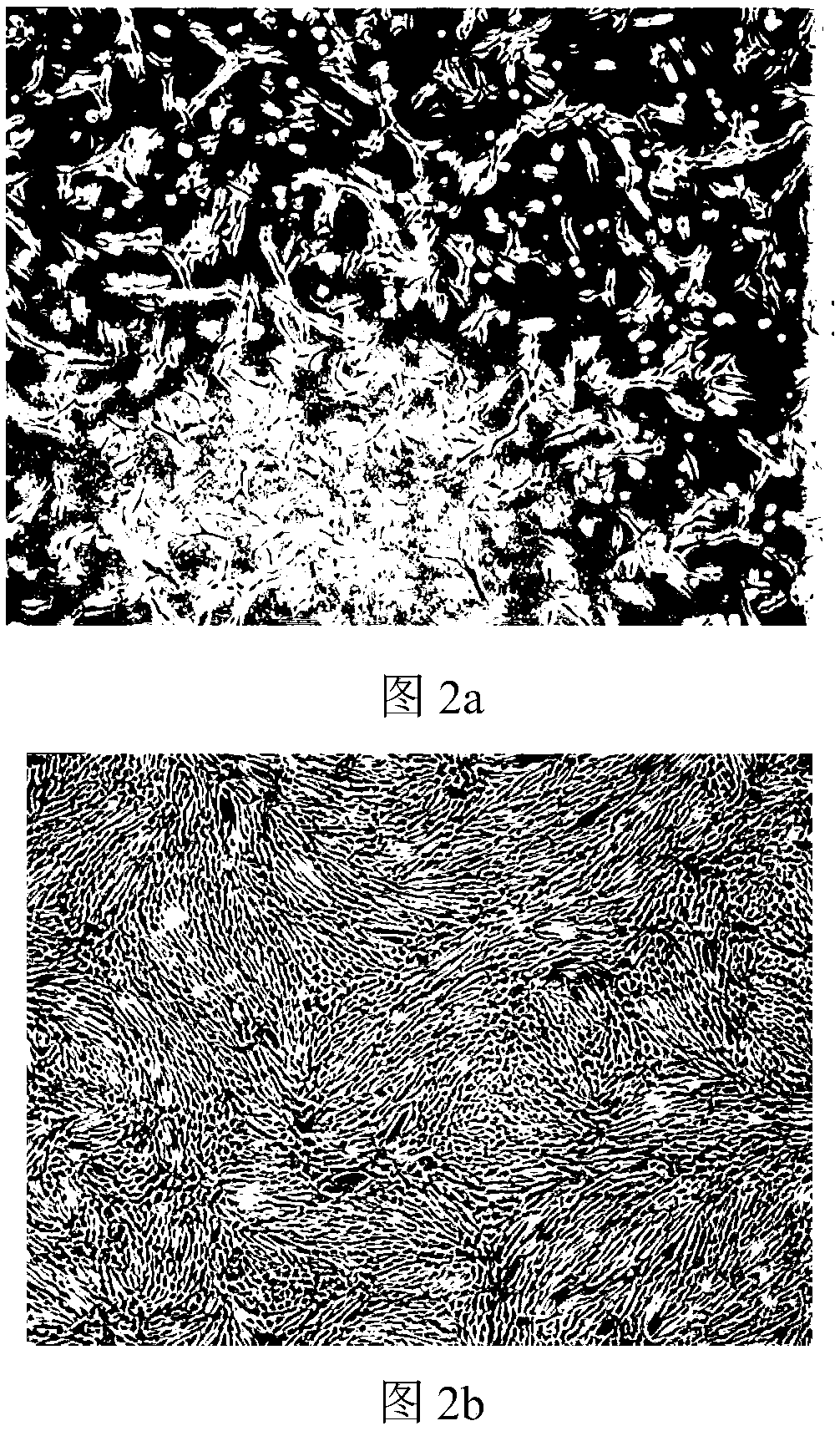
Image
Examples
Embodiment 1
[0038] A cryopreservation solution of mesenchymal stem cells for clinical use, comprising the following components by weight percentage:
[0039] Autologous plasma lysate mixture 5%, human serum albumin 1%, VC injection 0.5%, 10% low molecular weight dextran glucose injection 5%, compound electrolyte injection 24%, glucose sodium chloride injection 28%, compound Amino acid injection 27%, dimethyl sulfoxide 5% and trehalose 4.5%.
[0040] Wherein, the preparation method of autologous plasma lysate mixture is:
[0041] (1) Collect 16 mL of peripheral blood from the donor in EDTA anticoagulant blood collection tubes, and complete the plasma preparation within 2 hours of collection;
[0042] (2) Centrifuge at 4000r / min for 8min, absorb the upper layer of plasma and gently blow the buffy coat to mix the buffy coat and plasma evenly, transfer the plasma and buffy coat to cryopreservation tubes, and temporarily store them in a -80° ultra-low temperature refrigerator stay overnight;...
Embodiment 2
[0049] A cryopreservation solution of mesenchymal stem cells for clinical use, comprising the following components by weight percentage:
[0050] Autologous plasma lysate mixture 1%, human serum albumin 2%, 10% low molecular weight dextran glucose injection 10%, compound electrolyte injection 30%, glucose sodium chloride injection 20%, compound amino acid injection 30%, Dimethyl sulfoxide 2% and trehalose 5%.
[0051] Wherein, the preparation method of autologous plasma lysate mixture is:
[0052] (1) Collect 16 mL of peripheral blood from the donor in EDTA anticoagulant blood collection tubes, and complete the plasma preparation within 2 hours of collection;
[0053] (2) Centrifuge at 4000r / min for 8min, absorb the upper layer of plasma and gently blow the buffy coat to mix the buffy coat and plasma evenly, transfer the plasma and buffy coat to cryopreservation tubes, and temporarily store them in a -80° ultra-low temperature refrigerator stay overnight;
[0054] (3) Resus...
Embodiment 3
[0060] A cryopreservation solution of mesenchymal stem cells for clinical use, comprising the following components by weight percentage:
[0061] Autologous plasma lysate mixture 5%, human serum albumin 2%, VC injection 1%, glucose injection 5%, compound electrolyte injection 23.5%, glucose salt injection 25%, compound amino acid injection 30%, two Methyl sulfoxide 4% and trehalose 4.5%.
[0062] Wherein, the preparation method of autologous plasma lysate mixture is:
[0063] (1) Collect 16 mL of peripheral blood from the donor in EDTA anticoagulant blood collection tubes, and complete the plasma preparation within 2 hours of collection;
[0064] (2) Centrifuge at 4000r / min for 8min, absorb the upper layer of plasma and gently blow the buffy coat to mix the buffy coat and plasma evenly, transfer the plasma and buffy coat to cryopreservation tubes, and temporarily store them in a -80° ultra-low temperature refrigerator stay overnight;
[0065] (3) Resuscitate the plasma prep...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com