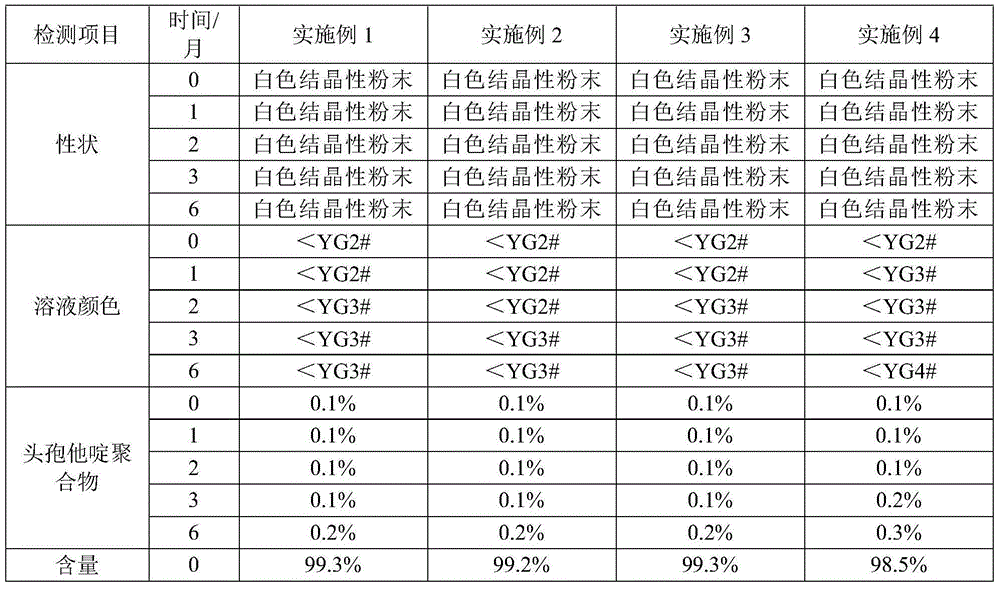
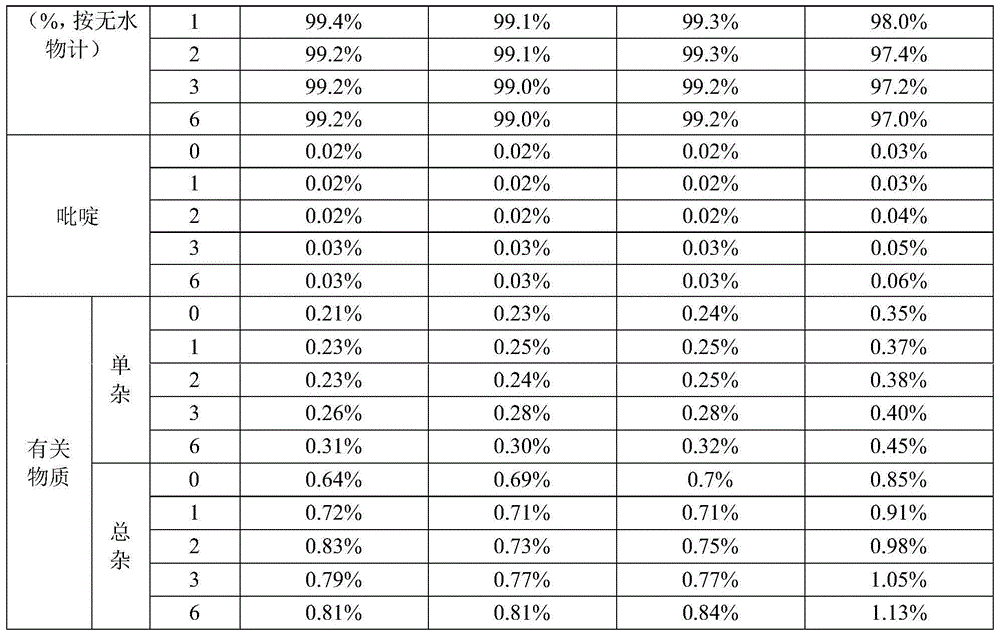
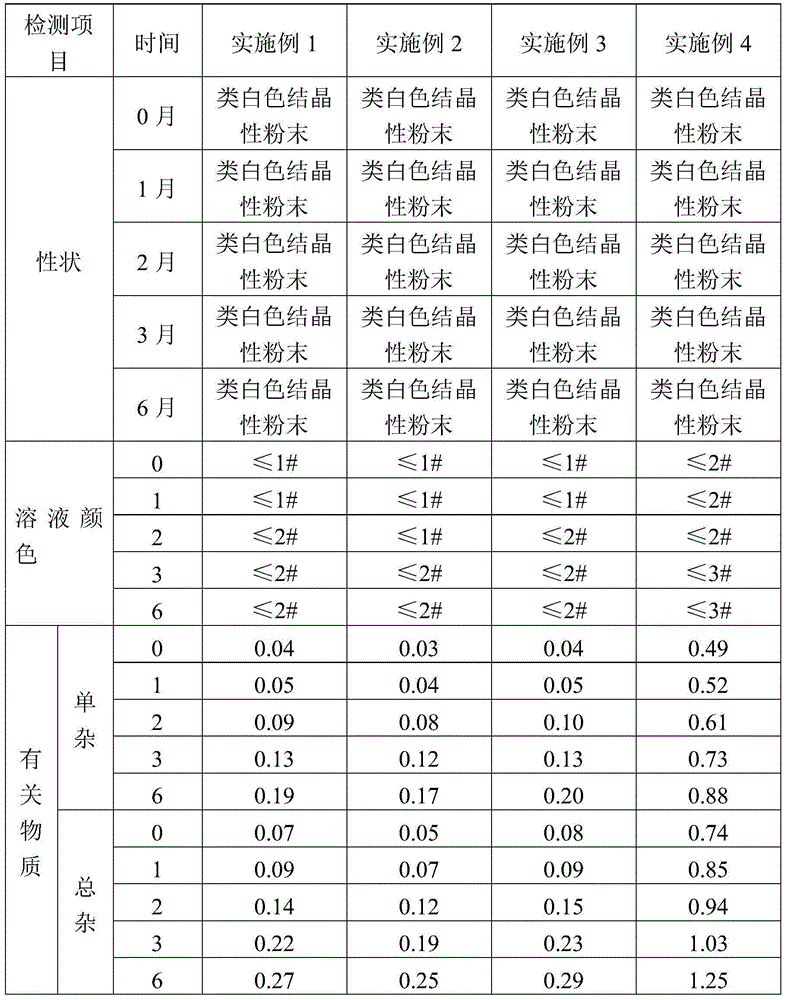
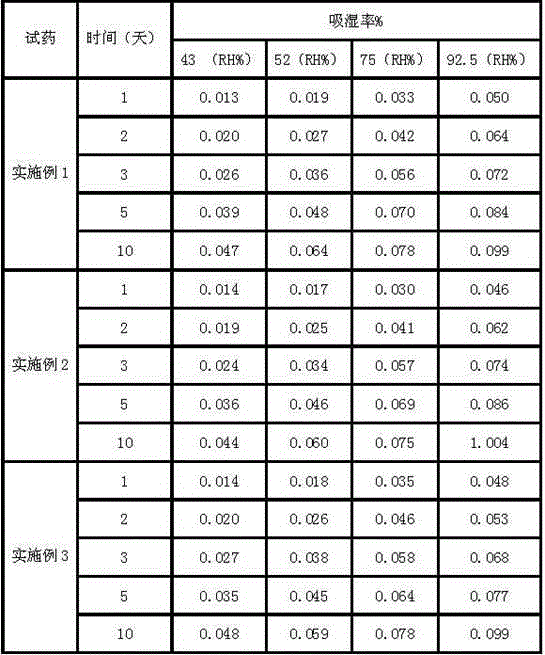
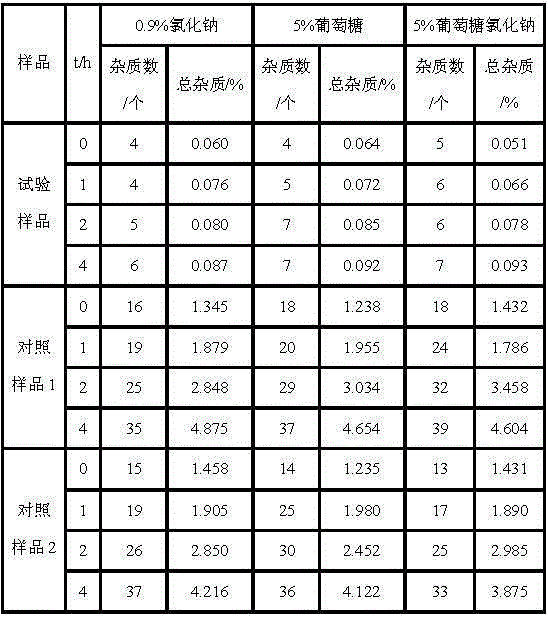
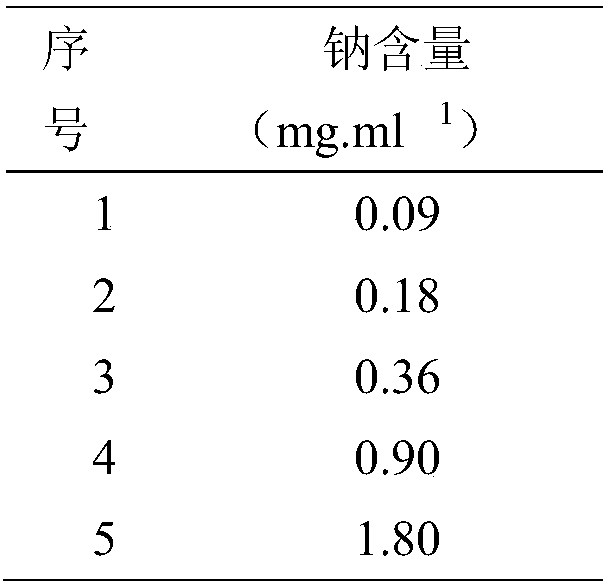
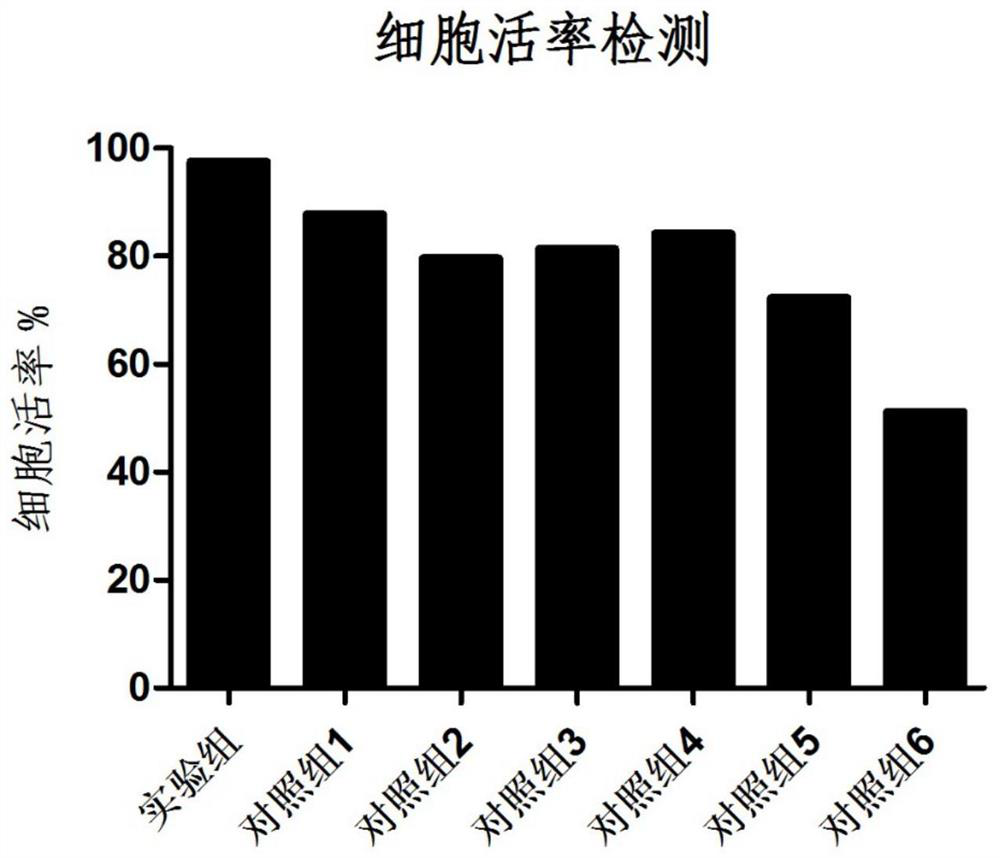
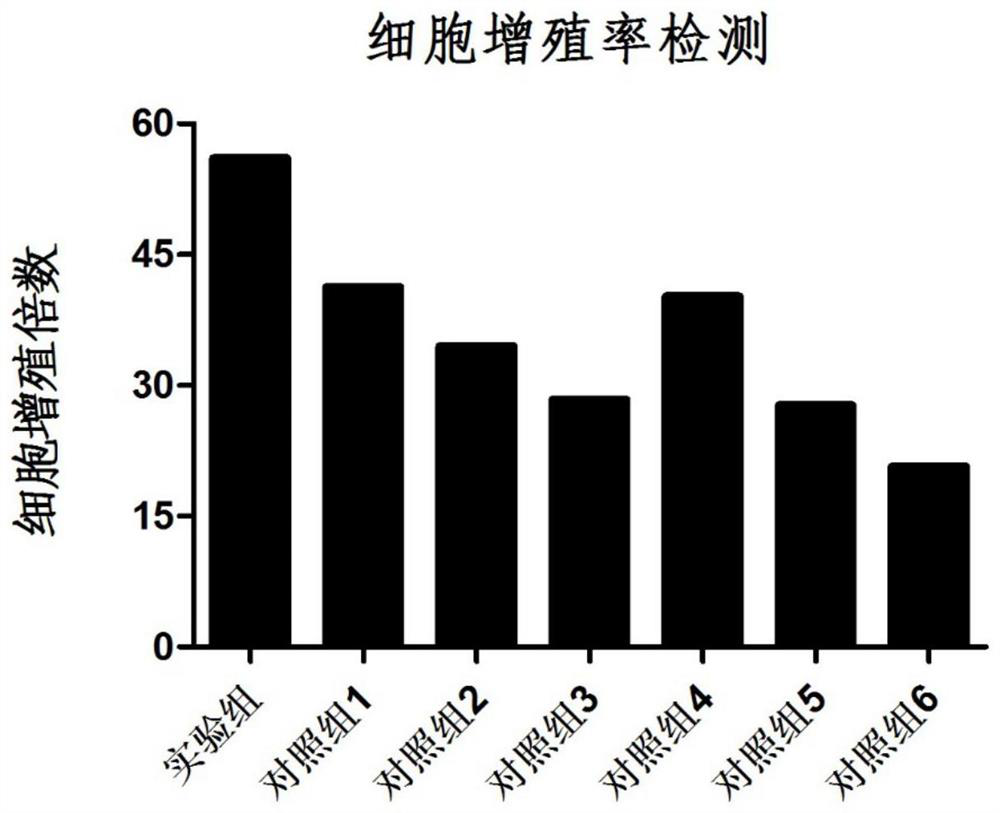
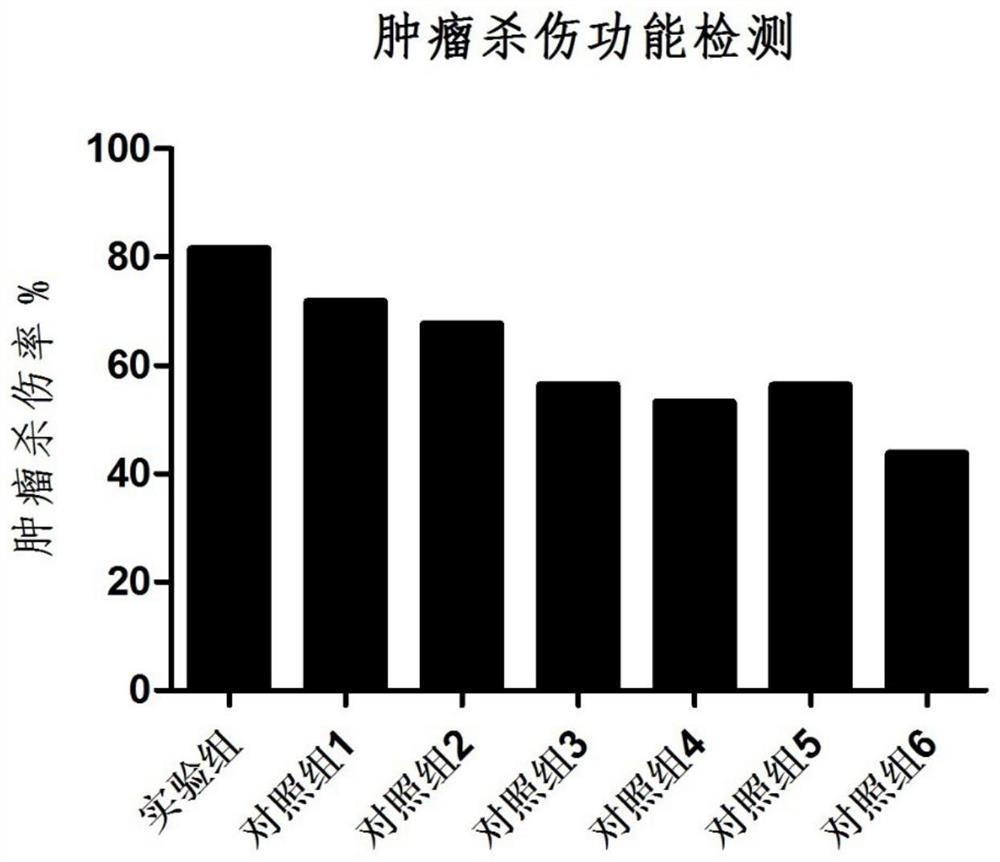
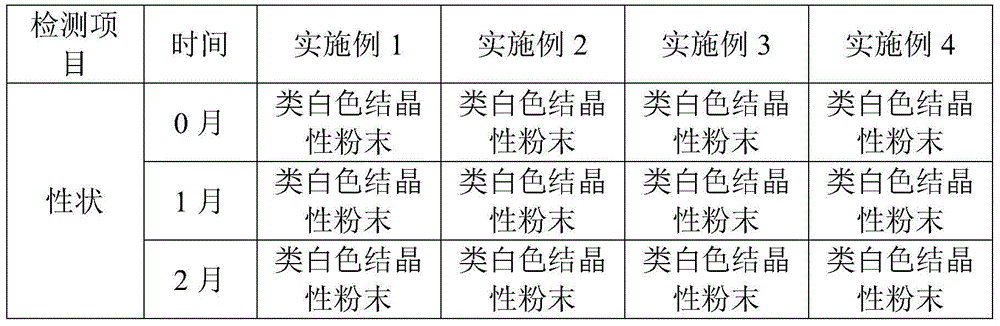
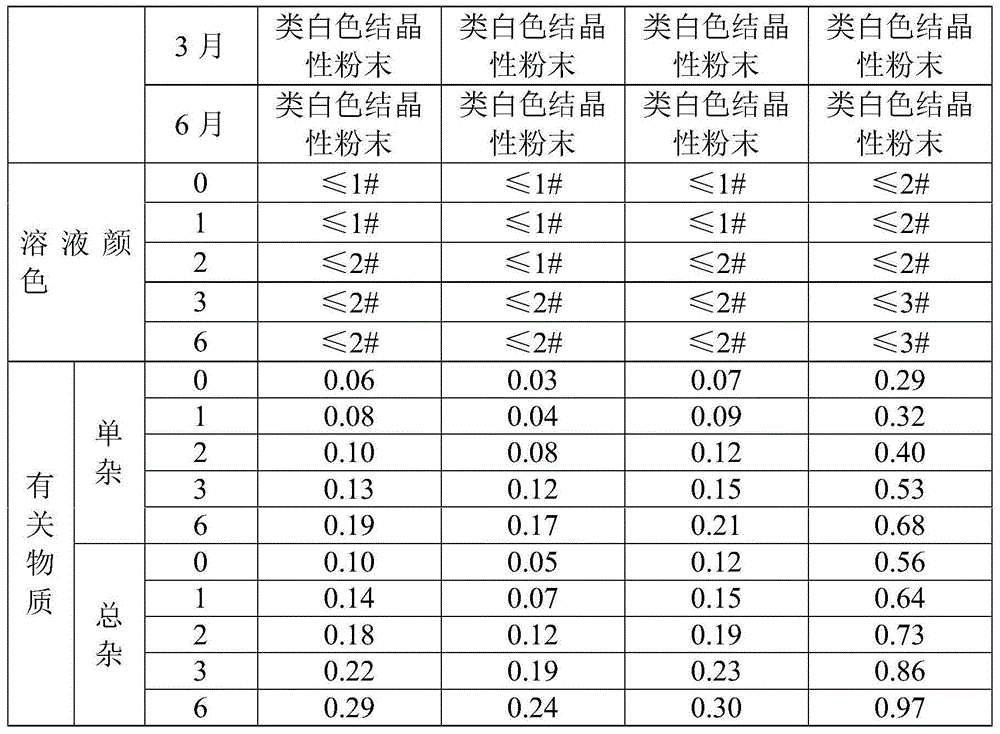
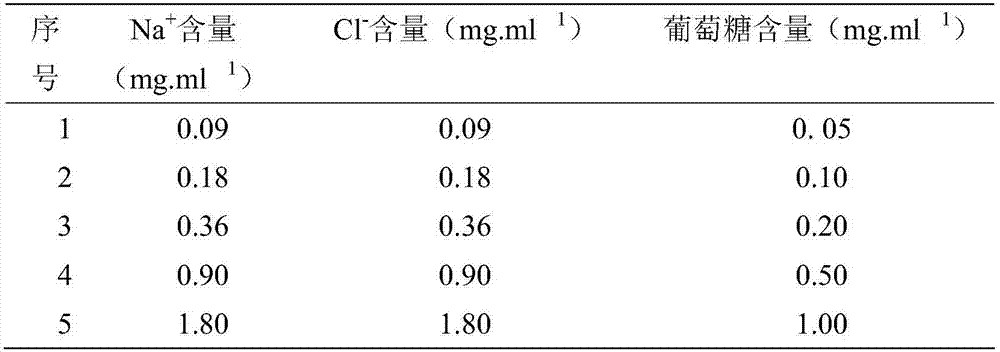
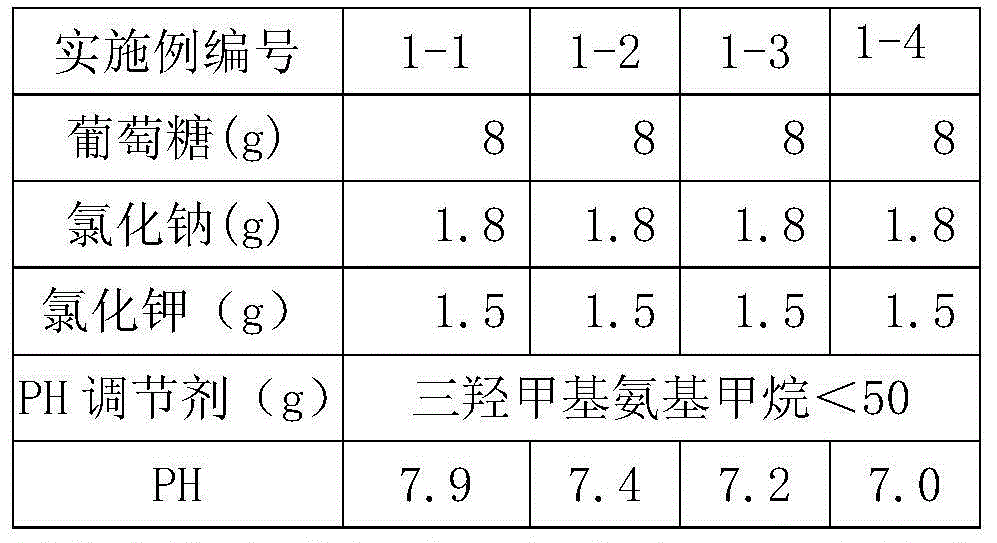
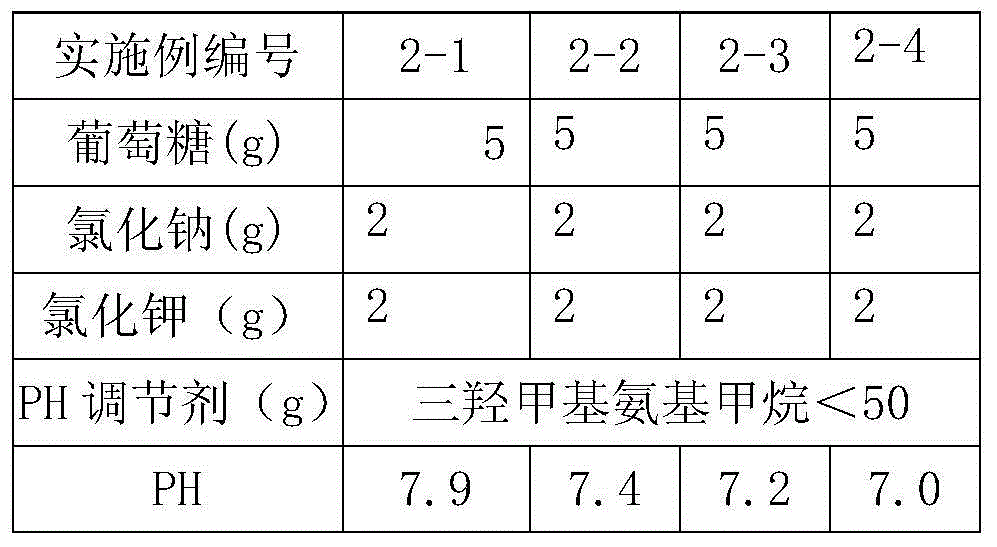
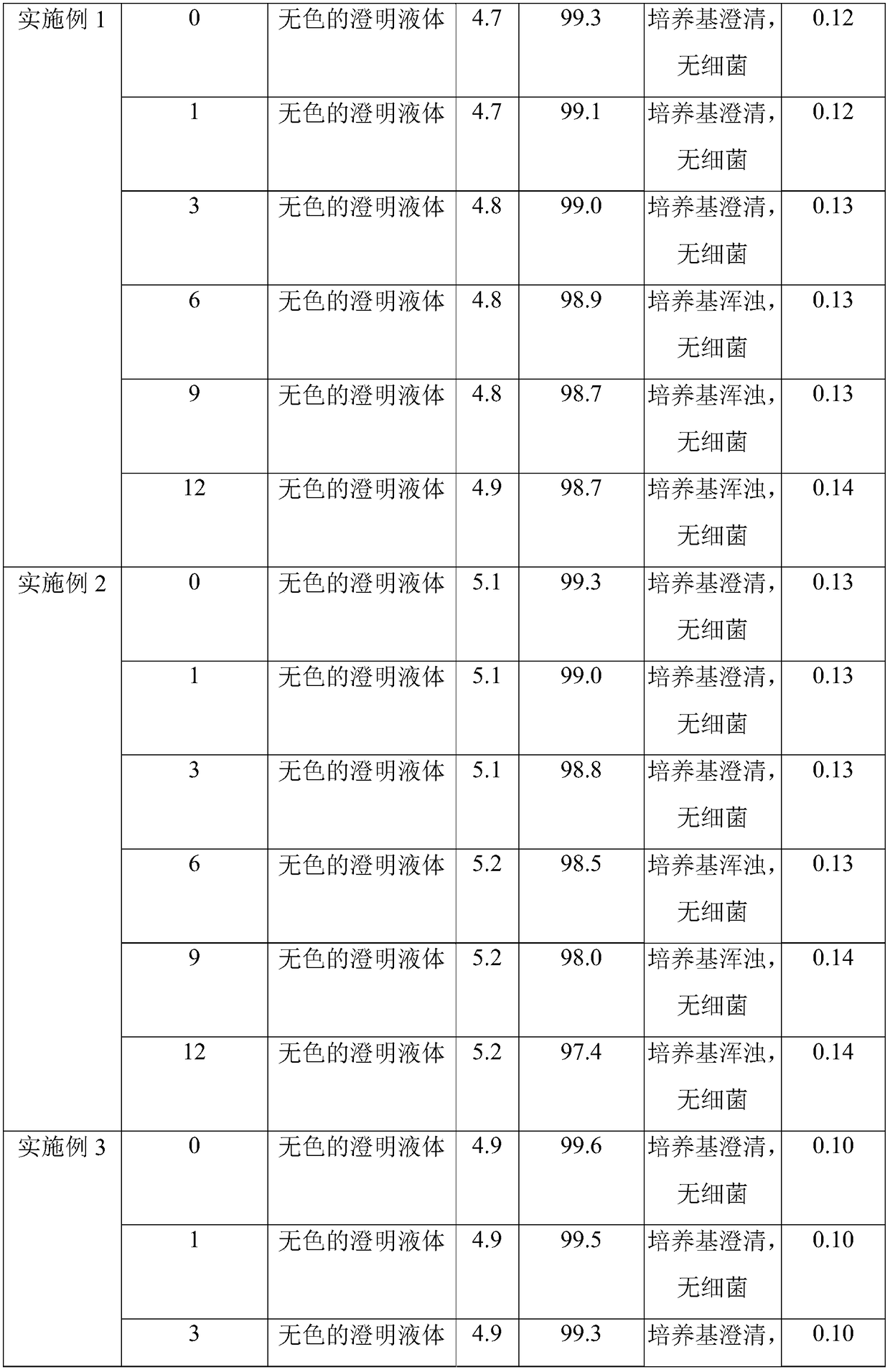
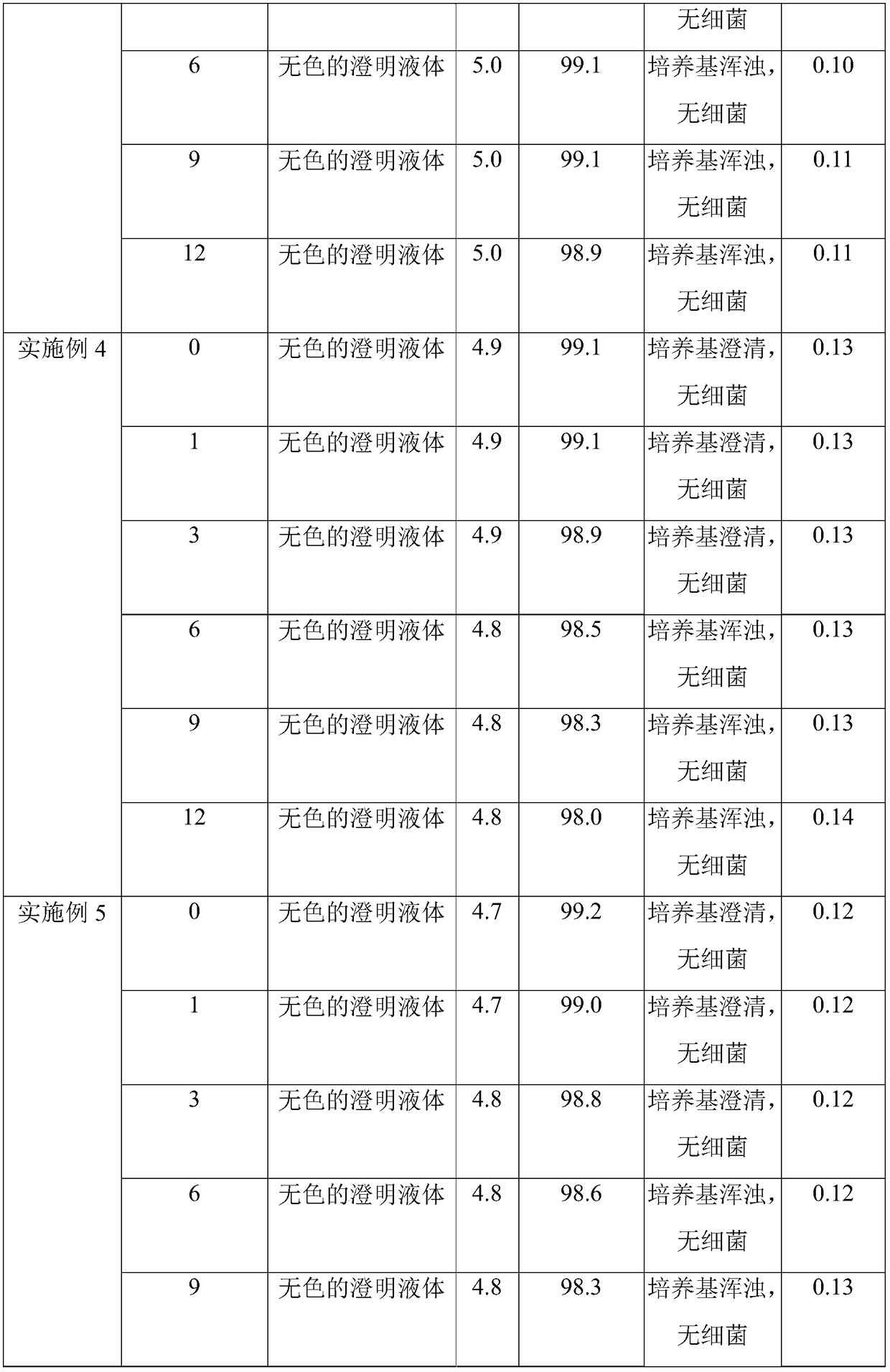
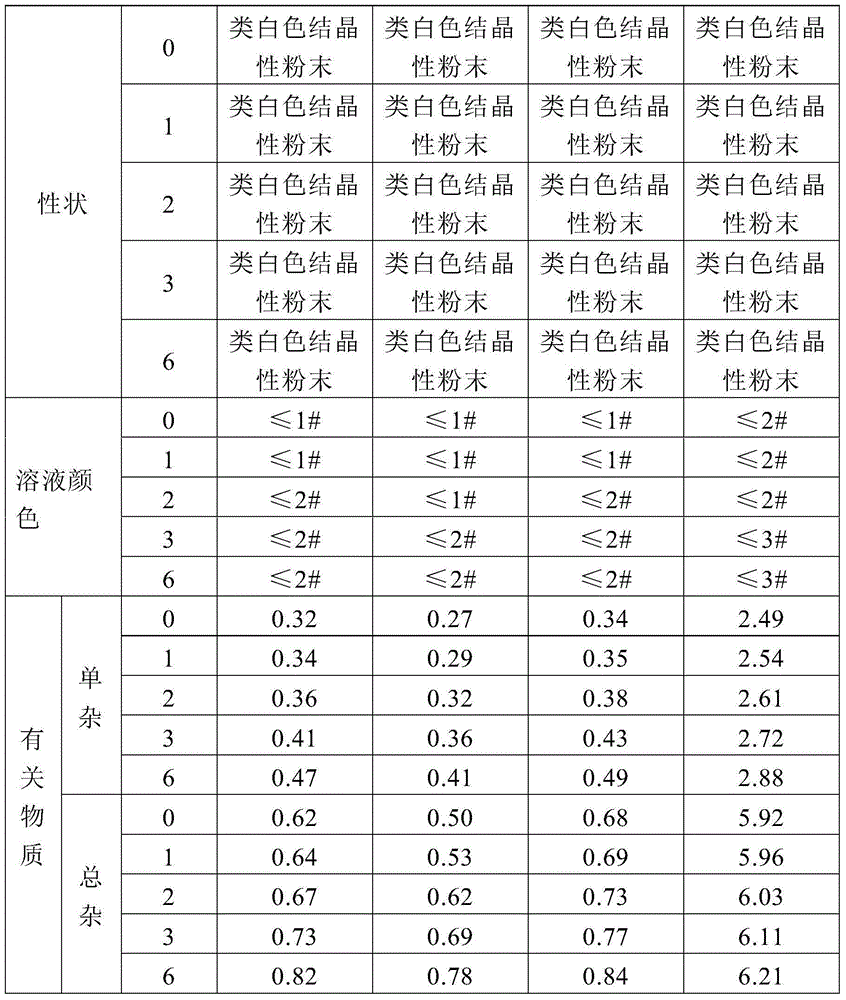
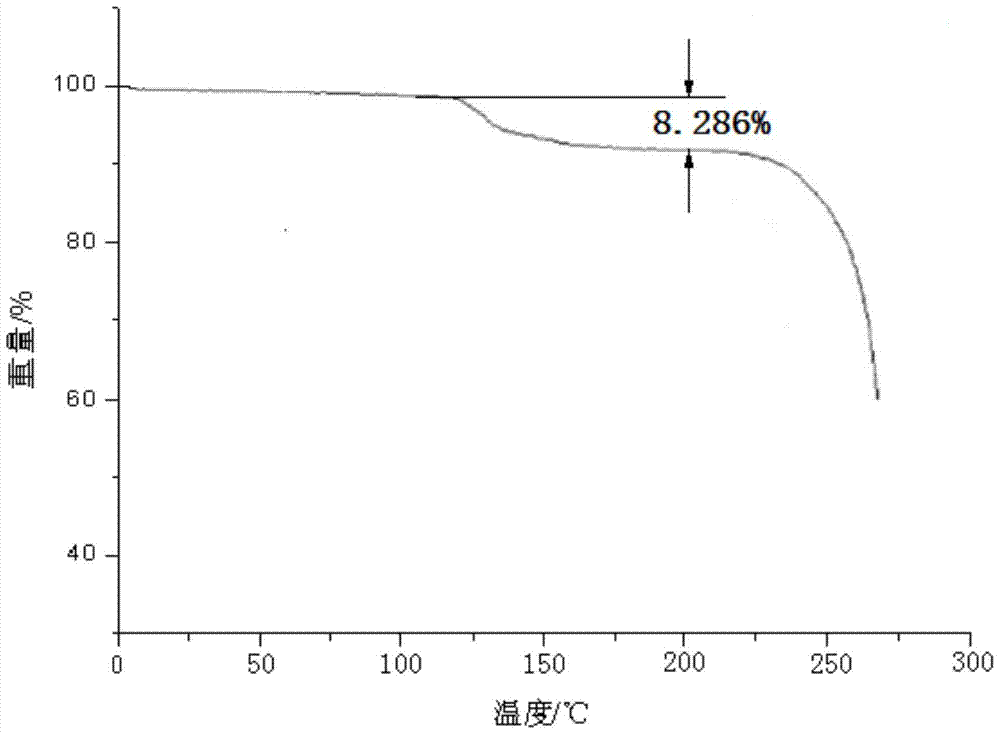
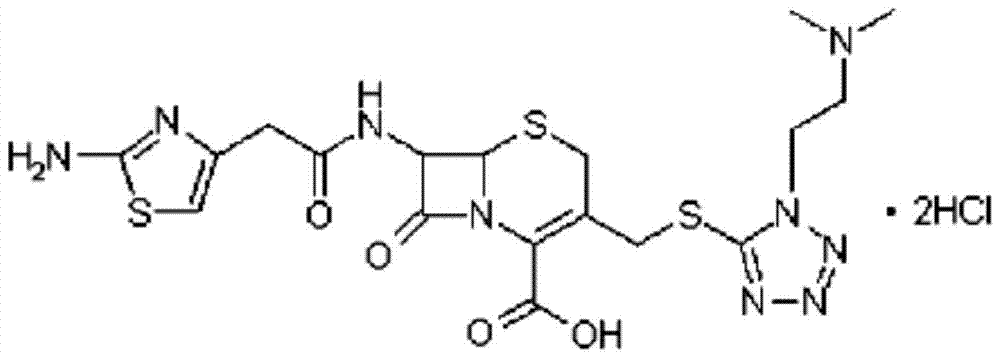
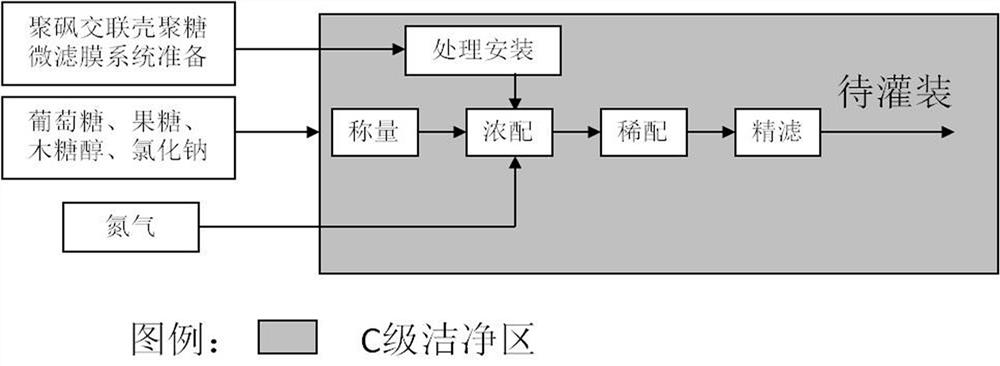
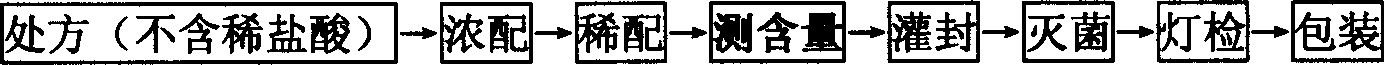Patents
Literature
37 results about "Glucose / Sodium Chloride Injection" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Process for preparing glucose injection and glucose sodium chloride injection
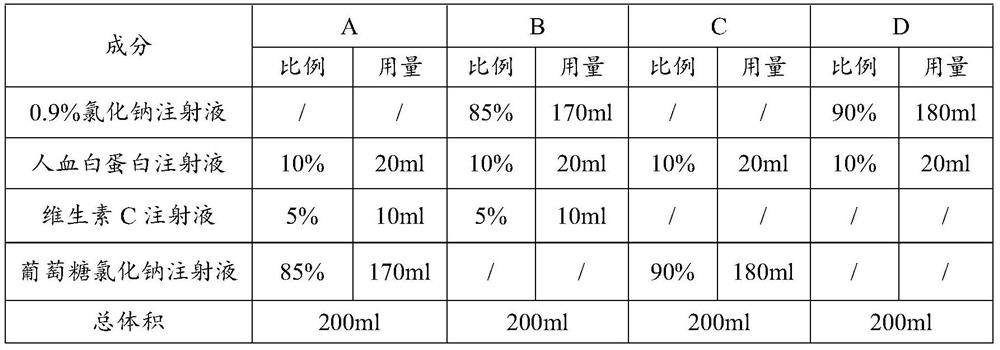
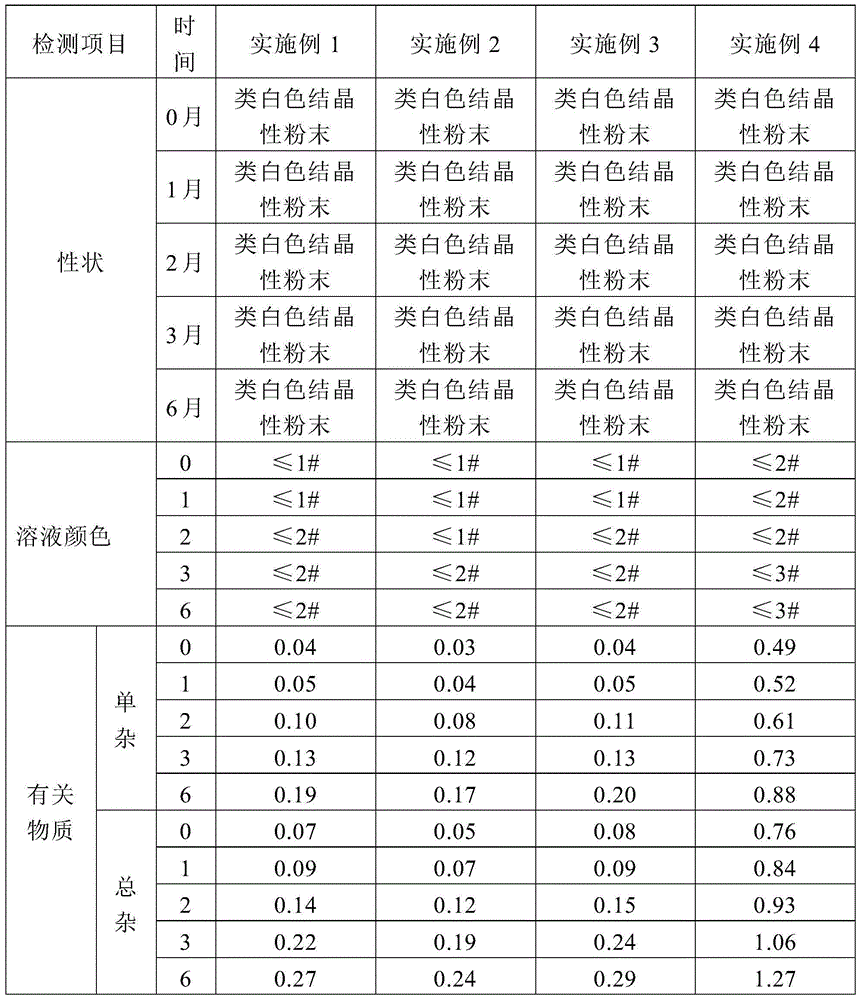
InactiveCN1799551AOvercoming technical biasImprove productivityOrganic active ingredientsMetabolism disorderSodium Chloride InjectionD-Glucose
Owner:樊武良
Children pharmaceutical composition containing ceftriaxone sodium and low-sodium carrier
InactiveCN104887621AIncrease internal pressureImprove solubilityAntibacterial agentsOrganic active ingredientsSodium Chloride InjectionKidney
The invention relates to a children pharmaceutical composition containing ceftriaxone sodium, namely a pharmaceutical combined preparation containing ceftriaxone sodium and a low-sodium carrier infusion solution, and particularly relates to a combined package. The children pharmaceutical composition comprises ceftriaxone sodium for injection and the low-sodium carrier infusion solution. The low-sodium carrier infusion solution contains a glucose and sodium chloride injection (15-200):1, a glucose and sodium chloride potassium chloride injection (15-200):1: (0-1) and the like. Compared with a mixture of ceftriaxone sodium and the low-sodium carrier infusion solution, the children pharmaceutical composition has the advantages that clinical application steps are simplified, clinical risks generated because kidneys of children are not developed to be mature and overmuch sodium in blood cannot be metabolized are reduced, and the clinical application quality and safety performance of children medication are improved.
Owner:ZHEJIANG CHANGDIAN PHARMA
Ambroxol hydrochloride containing hydroxypropyl beta-cyclodextrin and its preparation
InactiveCN1424026ASolve the problem of water solubilityGood for clinical useAmine active ingredientsRespiratory disorderFreeze-dryingWater soluble
An ambroxol hydrochloride injection containing hydroxypropyl beta-dextrin in the form of "liquid injection", "freeze dried powder injection", "aseptic powder injection", etc is composed of the ambroxol hydrochloride and 2-hydroxypropyl beta-dextrin in Wt ratio of 1:(3-50). Its advantage is high solubility in water, especially in high-pH water.
Owner:SHENYANG PHARMA UNIVERSITY
Process for preparing glucose and sodium chloride injection.
ActiveCN106309482AFast dissolutionReduce 5-HMF contentOrganic active ingredientsMetabolism disorderSodium acetateFiltration
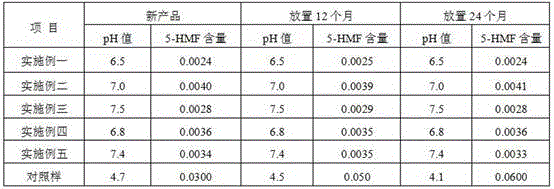
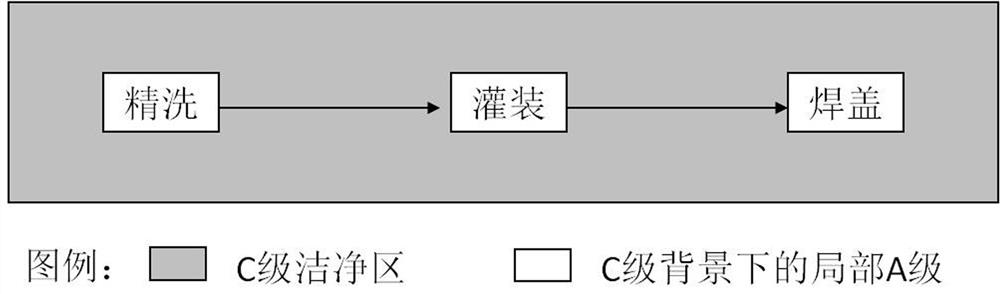
The invention discloses a process for pretreating glucose and sodium chloride injection, and belongs to the technical field of injection. The method comprises the following steps: (1) weighing raw materials; (2) dissolving the raw materials, namely a) adding sodium hydrogen sulfite, magnesium chloride, calcium chloride, sodium chloride and zinc sulfate into injecting water at 30-40 DEG C; (b) adding dipotassium phosphate, glucose, fructose, xylitol and sodium acetate into the injecting water at 50-60 DEG C; and (c) mixing the solution, adding citric acid and activated carbon, insulating and adsorbing; (3) decarburizing, delivering to a diluting tank; (4) complementing injecting water, and regulating the pH value to 6.5-7.5; (5) filling bags after rough filtration and fine filtration; and (6) sterilizing at 122 DEG C for 3-5 minutes. According to the process, raw materials are dissolved in batches with different dissolving temperatures, the dissolving speed is increased, the temperature is reasonably controlled, energy consumption and cost are reduced, the solution is uniformly mixed, and generation of glucose degraded products can be effectively reduced.
Owner:HUAREN PHARMACEUTICAL CO LTD
NK cell low-temperature preservation re-infusion liquid
InactiveCN111838137AReduce oxidative damageInhibition formationDead animal preservationVitamin CTissue repair
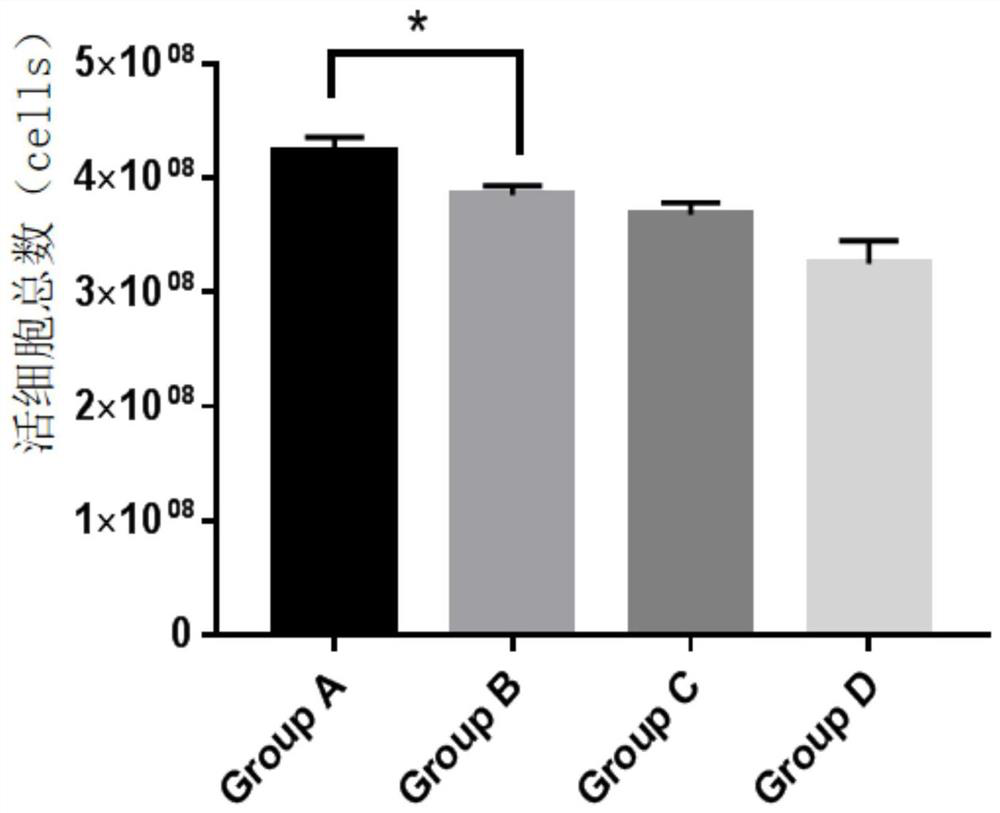
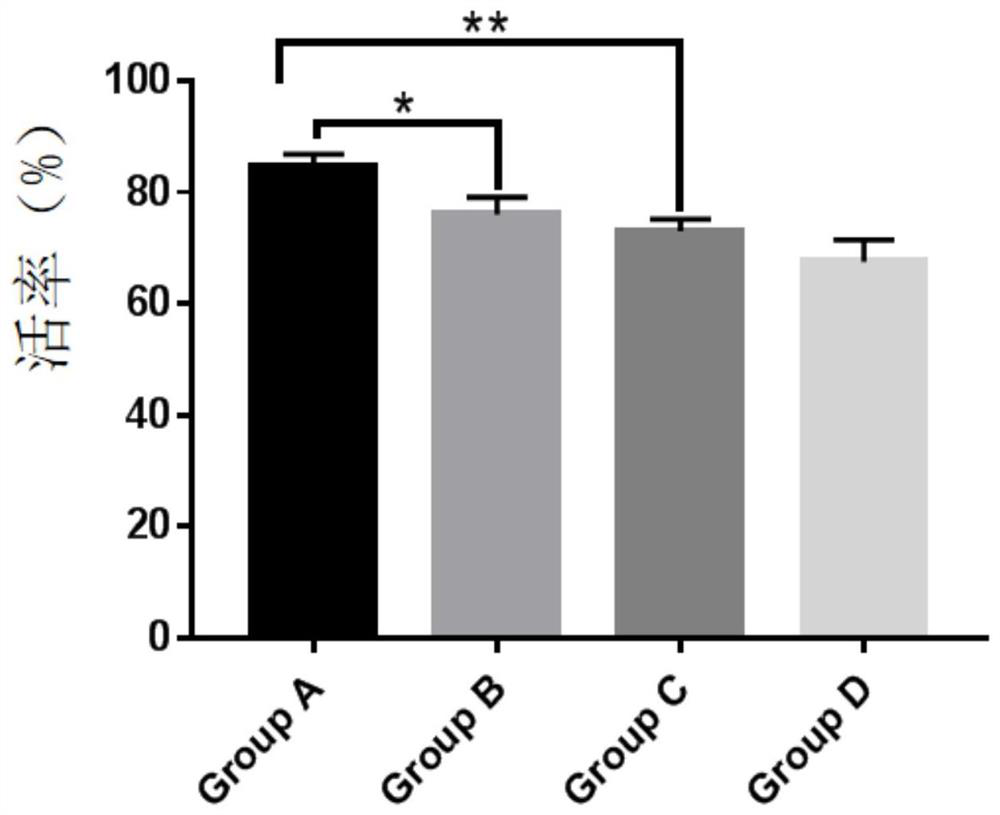
The invention provides an NK cell low-temperature preservation re-infusion liquid. According to the invention, a glucose and sodium chloride injection is added into the NK cell low-temperature preservation re-infusion liquid to serve as a diluent / buffer liquid, so that the effects of maintaining the pH value, the ion osmotic pressure and the colloid osmotic pressure and providing energy glucose for cells are achieved; the added human serum albumin can maintain the constant osmotic pressure of plasma colloid and supply nutrition; the added vitamin C can promote antibody and collagen formation and tissue repair, and meanwhile, the vitamin C also has the effects of resisting oxidation, resisting free radicals and inhibiting tyrosinase formation, so that the oxidative damage of cells can be reduced, and the accumulation of peroxides in the cells can be quickly relieved. According to the NK cell low-temperature preservation re-infusion liquid, the survival rate of NK cells can be kept at 80% or above after the NK cells are preserved for 48 h at the temperature of 4 DEG C, and a preparation can be directly re-infused.
Owner:SHENZHEN PREGENE BIOPHARMA CO LTD
Cefotiam hydrochloride compound, method for preparing same and pharmaceutical composition with cefotiam hydrochloride compound
ActiveCN104926835AImprove liquidityImprove stabilityAntibacterial agentsOrganic active ingredientsCefotiam HydrochlorideSodium Chloride Injection
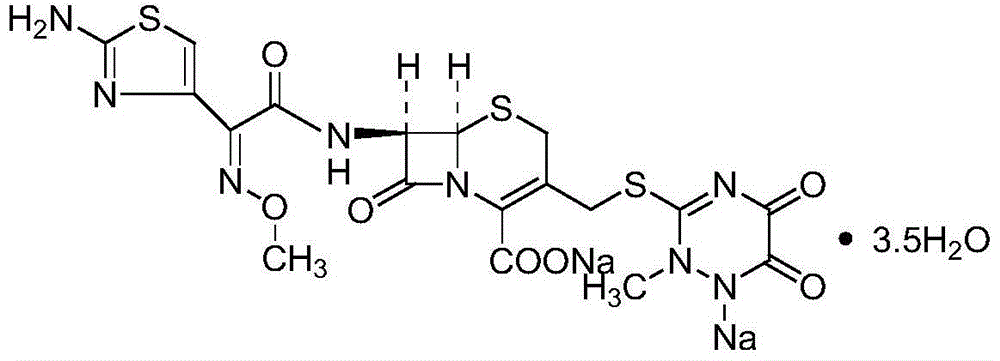
The invention belongs to the technical field of medicine, and particularly relates to a cefotiam hydrochloride compound, a method for preparing the same and a pharmaceutical composition with the cefotiam hydrochloride compound. The cefotiam hydrochloride compound is cefotiam hydrochloride trihydrate, and a structural formula of the cefotiam hydrochloride compound is shown. The cefotiam hydrochloride compound, the method and the pharmaceutical composition have the advantages that crystals of the cefotiam hydrochloride trihydrate are good in flowability, injection cefotiam hydrochloride made of the crystals of the cefotiam hydrochloride trihydrate, 0.9% sodium chloride injection, 5% glucose injection and 5% glucose and sodium chloride injection can be compatible with one another to obtain liquor, and the liquor is excellent in stability after being placed at the room temperature for 4h.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD +2
Method for determining content of glucose and sodium chloride in sodium chloride and dextrose injection
ActiveCN104931602AAvoid environmental problemsAvoid inaccuraciesComponent separationD-GlucoseGlucose polymers
The invention discloses a method for determining content of glucose and sodium chloride in sodium chloride and dextrose injection. The method comprises the following steps: step1, performing sample pretreatment, and diluting 50mg of sodium chloride and dextrose injection with 40ml of water, and adding acetonitrile to constant volume of 100ml; step2, analyzing by high performance liquid chromatography; parameters of the high performance liquid chromatography are set as follows: mobile phase: acetonitrile-water; flow rate: 1ml / min; column temperature: 40 DEG C; chromatographic column: Thermo Acclaim mixed-matrix chromatographic column, 4.6mum*250mm, 5mum. The invention aims at providing the method for determining the content of the glucose and the sodium chloride in the sodium chloride and dextrose injection, which is accurate in determination and simple in operation.
Owner:WUZHOU INST FOR FOOD & DRUG CONTROL
Child type amoxicillin sulbactam sodium and low-sodium carrier medicine composition
InactiveCN104958253AHigh puritySmall side effectsAntibacterial agentsOrganic chemistryPharmacyAmoxicillin+Sulbactam
The invention relates to a child type amoxicillin sulbactam sodium medicine composition, namely an amoxicillin sulbactam sodium and low-sodium carrier transfusion medicine combination preparation, and in particular to combined application packaging. The medicine composition comprises amoxicillin sulbactam sodium for injection and a low-sodium carrier transfusion solution. The low-sodium carrier transfusion solution contains a sodium chloride and dextrose injection (15-200:1), a glucose and sodium chloride potassium chloride injection (15-200:1:0-1) and the like. According to the composition, compared with the mode that the amoxicillin sulbactam sodium and the low-sodium carrier transfusion are mixed and used in a compatibility manner, the clinical application steps are simplified; and clinic risks caused by the fact that the kidney of a child is not mature, so that the child does not have the ability to metabolize excessive sodium in blood are reduced, and the clinic application quality and the clinic application safety of child pharmacy are improved.
Owner:ZHEJIANG CHANGDIAN PHARMA
Production technology of glucose sodium chloride injection
ActiveCN109833330AIncrease dissolution rateImprove solubilityOrganic active ingredientsMetabolism disorderAntioxidantNitrogen
The invention discloses a production technology of glucose sodium chloride injection. The method comprises the following steps of step I, performing weighing; step II, performing thick blending; stepIII, performing thin blending; step IV, performing washing, stuffing and sealing; step V, performing sterilization; step VI, performing lamp inspection; and step VII, performing packing. According tothe production technology of glucose sodium chloride injection provided by the invention, the quality of the raw materials is strictly controlled, the technological process is adjusted and optimized,and the content of insoluble granules, heavy metal, and bacterial endotoxin in products is obviously reduced. Because nitrogen filling for encapsulation is adopted, the products are good in stability,antioxidants do not need to be added, the safety of the injection is further improved, and the production efficiency of an optimized technology and the qualified rate of finished products are both obviously increased.
Owner:NANYANG LIXIN PHARMA
Children pharmaceutical composition containing ceftazidime and low-sodium carrier
InactiveCN104887683AIncrease internal pressureImpurities increaseAntibacterial agentsOrganic active ingredientsSodium Chloride InjectionCeftazidime
The invention relates to a children pharmaceutical composition containing ceftazidime, namely a pharmaceutical combined preparation containing ceftazidime and a low-sodium carrier infusion solution and particularly relates to a combined package. The children pharmaceutical composition comprises ceftazidime for injection and the low-sodium carrier infusion solution. The low-sodium carrier infusion solution contains a glucose and sodium chloride injection (15-200):1, a glucose and sodium chloride potassium chloride injection (15-200):1: (0-1) and the like. Compared with a mixture of ceftazidime and the low-sodium carrier infusion solution, the children pharmaceutical composition has the advantages that clinical application steps are simplified, clinical risks generated because kidneys of children are not developed to be mature and overmuch sodium in blood cannot be metabolized are reduced, and the clinical application quality and safety performance of children medication are improved.
Owner:ZHEJIANG CHANGDIAN PHARMA
Child-type medicinal composition with ampicillin sulbactam sodium and low-sodium carrier
InactiveCN104922678ALess impuritiesHigh purityAntibacterial agentsOrganic chemistryAmpicillin/sulbactamSodium Chloride Injection
The invention relates to a child-type ampicillin-sulbactam-sodium medicinal composition, i.e. a medicinal combined preparation with ampicillin sulbactam sodium and low-sodium carrier infusion fluid, especially combined application package comprising the ampicillin sulbactam sodium and the low-sodium infusion carrier fluid. The low-sodium carrier infusion fluid comprises glucose and sodium chloride injection fluid (15-200:1) and glucose and sodium-chloride potassium-chloride injection fluid (15-200:1:0-1) and the like. The child-type medicinal composition has the advantages that compared with the compatible and mixing use of the ampicillin sulbactam sodium and the low-sodium carrier infusion fluid, the clinical application steps are simplified, the clinical risks caused by incapability of metabolizing excessive sodium in blood since the kidney of a child is not matured are reduced and the clinical application quality and safety of child administration are improved.
Owner:ZHEJIANG CHANGDIAN PHARMA
Pharmaceutical cefotiam composition for treating infectious diseases
InactiveCN105085548AAntibacterial agentsOrganic active ingredientsSodium Chloride InjectionInfective disorder
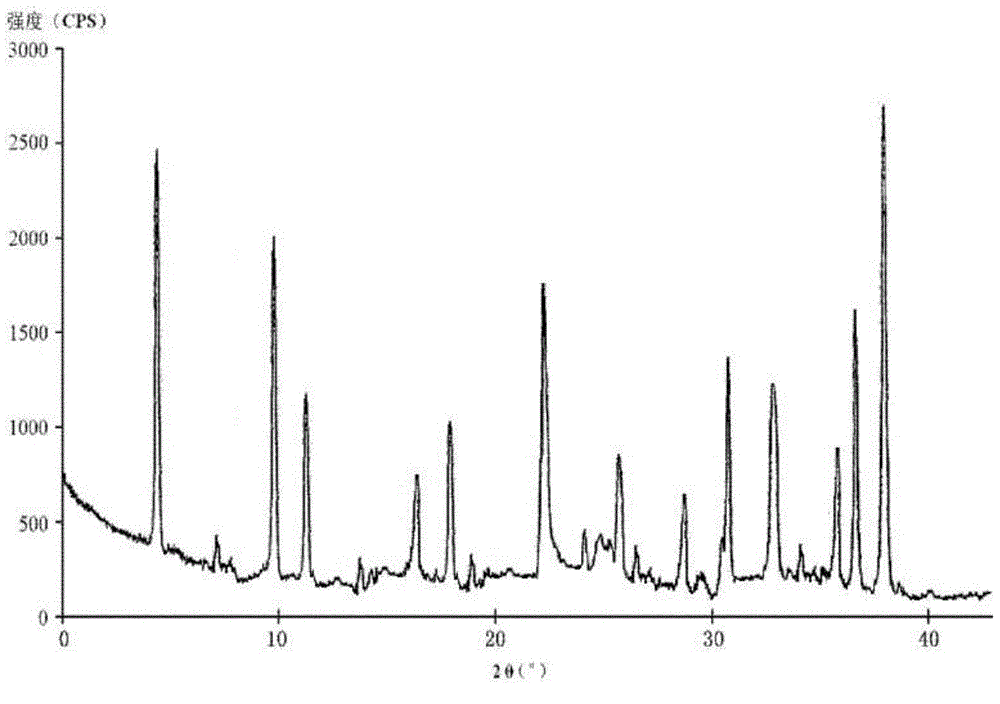
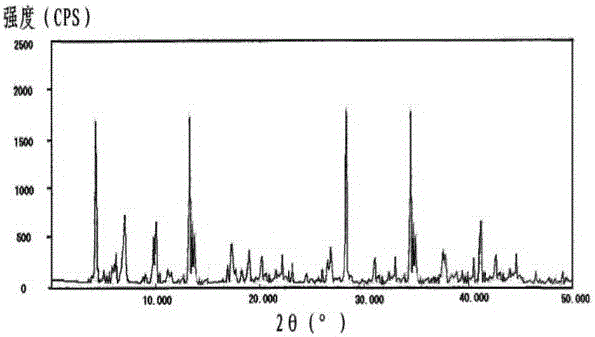
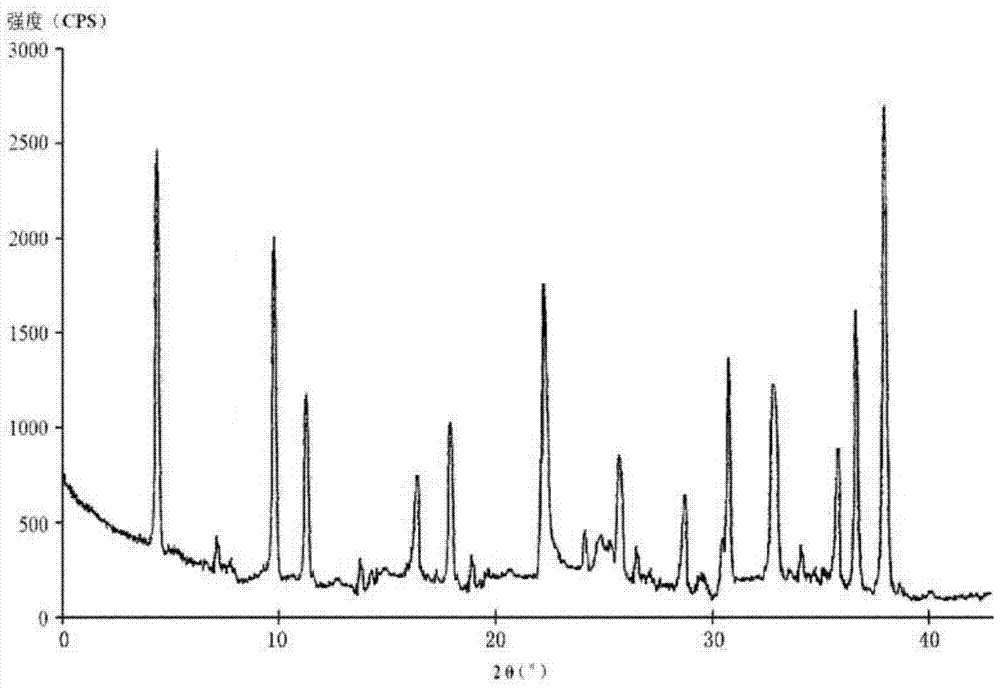
The invention relates to a pharmaceutical cefotiam hydrochloride composition for treating infectious diseases, belonging to the technical field of medicine. The composition is composed of cefotiam hydrochloride and anhydrous sodium carbonate. The cefotiam hydrochloride is a crystal, and can obtain an X-ray powder diffractogram disclosed as Figure 1 by using Cu-Kalpha ray measurement. The cefotiam hydrochloride new crystal form provided by the invention is different from the crystal form structure in the prior art. The experimental verification surprisingly detects that the crystal compound has the advantages of high purity, favorable flowability, favorable stability, low polymer content and no hygroscopicity; and the solution, which is prepared by mixing the powder injection, a 0.9% sodium chloride injection, a 5% glucose injection and a 5% glucose sodium chloride injection and standing at room temperature for 4 hours, has favorable stability.
Owner:QINGDAO LANSHENGYANG PHARMA & BIOTECH CO LTD
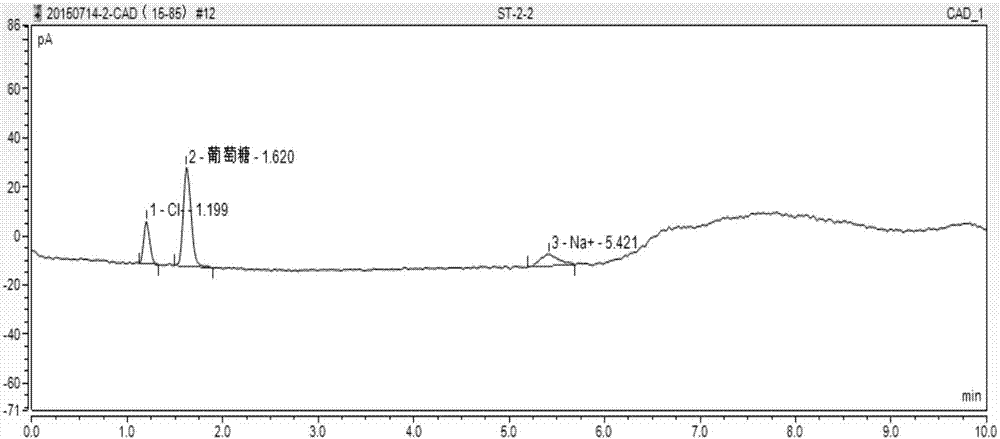
HPLC-CAD method used for measuring content of sodium in glucose and sodium chloride injection
InactiveCN108344813AAvoid environmental problemsAvoid inaccuraciesComponent separationSodium Chloride InjectionTest object
The invention discloses a HPLC-CAD method used for measuring content of sodium in glucose and sodium chloride injection, and belongs to the field of chemical detection. The HPLC-CAD method used for measuring content of sodium in glucose and sodium chloride injection is accurate in measuring, and simple in operation, and comprises following steps successively: 1, preparation of a tested object solution, wherein 1ml of glucose and sodium chloride injection is diluted with methanol to 1000ml, an obtained mixture is shaken to be uniform, and is filtered; 2, preparation of a reference solution, wherein 15g of a sodium chloride reference substrate is weighted accurately, and is introduced into a 250ml measuring flask, methanol is added for dissolving and dilution to a constant volume, and an obtained mixed solution is shaken to be uniform; and 3, high performance liquid chromatography analysis. The HPLC-CAD method can be used for replacing a conventional detection method in measuring of thecontent of sodium in glucose and sodium chloride injection.
Owner:WUZHOU INST FOR FOOD & DRUG CONTROL
A kind of antigen-specific T lymphocyte cryopreservation liquid and its preparation method and application
ActiveCN107148967BReduce operating proceduresLow pollution rateDead animal preservationSodium Chloride InjectionT lymphocyte
The invention provides an antigen-specific T lymphocyte freezing medium, comprising freezing medium A and freezing medium B; the freezing medium A includes, by volume, 30-40% of plasmalyte electrolyte injection, 30-40% of glucose and sodium chloride injection, 5-15% of dextran glucose injection and 15-25% of human albumin solution; the freezing medium B includes, by volume, 20-30% of plasmalyte electrolyte injection, 20-30% of glucose and sodium chloride injection, 5-15% of dextran glucose injection, 15-25% of human albumin solution and 10-20% of dimethyl sulfoxide; the freezing medium A and the freezing medium B are stored separately; in use, the freezing medium A and the freezing medium B are mixed in a ratio of 1:(0.5-2), forming the antigen-specific T lymphocyte freezing medium. The antigen-specific T lymphocyte freezing medium is capable of enabling less crystal to form in cells, increasing cell survival rate and maintaining tumor-killing ability of cells. The invention also provides a preparation method and application of the antigen-specific T lymphocyte freezing medium and antigen-specific T lymphocyte injection.
Owner:郴州宾泽医学检验有限公司
Ambroxol hydrochloride containing hydroxypropyl beta-cyclodextrin and its preparation
InactiveCN1259906CSolve the problem of water solubilityGood for clinical usePowder deliveryLyophilised deliverySodium Chloride InjectionGlucose polymers
The present invention is a hydroxypropyl β-cyclodextrin-containing ambroxol hydrochloride injection and a preparation method thereof, which completely solves the problem of water solubility, so that various injection preparations can be prepared. Ambroxol hydrochloride injection contains hydroxypropyl beta-cyclodextrin. The injection is realized by adding "2-hydroxypropyl β-cyclodextrin" to solve the solubility of ambroxol hydrochloride in high pH water, wherein "ambroxol hydrochloride" and "2-hydroxypropyl β-cyclodextrin" The weight ratio of "cyclodextrin" is 1:3 to 1:50. Said injection includes "injection", "lyophilized powder injection", "sterile powder injection", "ambroxol hydrochloride glucose, sodium chloride injection or glucose sodium chloride injection". The present invention can make ambroxol hydrochloride compatible with basic drugs, and is convenient for clinical medication.
Owner:SHENYANG PHARMA UNIVERSITY
Children type cefoxitin sodium and low-sodium carrier pharmaceutical composition
InactiveCN104940208AIncrease internal pressureHigh purityAntibacterial agentsOrganic active ingredientsSodium Chloride InjectionGlucose polymers
The present invention relates to a children type cefoxitin sodium pharmaceutical composition, especially to a combination application package, wherein the composition is a cefoxitin sodium and low-sodium carrier infusion pharmaceutical combination preparation, which comprises cefoxitin sodium for injection and a low-sodium carrier infusion, wherein the low-sodium carrier infusion contains a glucose and sodium chloride injection (15-200:1), a glucose, sodium chloride and potassium chloride injection (15-200:1:0-1) and the like. According to the present invention, the clinical application steps are simplified compared with the compatible mixing use of the cefoxitin sodium and the low-sodium carrier infusion; and the clinical risk caused by the excessive sodium positioned in the blood and incapable of being metabolized due to the children kidney achieving the immature development state is reduced so as to improve the clinical application quality and the safety of the children medication are improved.
Owner:ZHEJIANG CHANGDIAN PHARMA
Children type azlocillin sodium and low-sodium carrier pharmaceutical composition
InactiveCN104940130AHigh puritySmall side effectsAntibacterial agentsOrganic chemistryAzlocillin SodiumSodium Chloride Injection
The present invention relates to a children type azlocillin sodium pharmaceutical composition, especially to a combination application package, wherein the composition is an azlocillin sodium and low-sodium carrier infusion pharmaceutical combination preparation, which comprises azlocillin sodium for injection and a low-sodium carrier infusion, wherein the low-sodium carrier infusion contains a glucose and sodium chloride injection (15-200:1), a glucose, sodium chloride and potassium chloride injection (15-200:1:0-1) and the like. According to the present invention, the clinical application steps are simplified compared with the compatible mixing use of the azlocillin sodium and the low-sodium carrier infusion; and the clinical risk caused by the excessive sodium positioned in the blood and incapable of being metabolized due to the children kidney achieving the immature development state is reduced so as to improve the clinical application quality and the safety of the children medication are improved.
Owner:ZHEJIANG CHANGDIAN PHARMA
Children type sulbenicillin sodium and low-sodium carrier pharmaceutical composition
InactiveCN104940129AHigh purityIncrease internal pressureAntibacterial agentsOrganic chemistrySulbenicillinSodium Chloride Injection
The present invention relates to a children type sulbenicillin sodium pharmaceutical composition, especially to a combination application package, wherein the composition is a sulbenicillin sodium and low-sodium carrier infusion pharmaceutical combination preparation, which comprises sulbenicillin sodium for injection and a low-sodium carrier infusion, wherein the low-sodium carrier infusion contains a glucose and sodium chloride injection (15-200:1), a glucose, sodium chloride and potassium chloride injection (15-200:1:0-1) and the like. According to the present invention, the clinical application steps are simplified compared with the compatible mixing use of the sulbenicillin sodium and the low-sodium carrier infusion; and the clinical risk caused by the excessive sodium positioned in the blood and incapable of being metabolized due to the children kidney achieving the immature development state is reduced so as to improve the clinical application quality and the safety of the children medication are improved.
Owner:ZHEJIANG CHANGDIAN PHARMA
Cefmenoxime hydrochloride and low-sodium carrier drug composition for children
InactiveCN104906035AIncrease internal pressureHigh purityAntibacterial agentsOrganic active ingredientsSodium Chloride InjectionCefmenoxime Hydrochloride
The invention relates to a cefmenoxime hydrochloride drug composition for children, namely, a drug composition preparation of cefmenoxime hydrochloride and a low-sodium carrier infusion solution, in particular to a composition application package. The composition comprises the cefmenoxime hydrochloride and the low-sodium carrier infusion solution for injection. The low-sodium carrier infusion solution comprises a dextrose and sodium chloride injection (15-200:1), a glucose, sodium chloride and potassium chloride injection (15-200:1:0-1) and the like. Relative to the compatible and mixed use of the cefmenoxime hydrochloride and the low-sodium carrier infusion solution, clinical application steps are simplified. The clinical risks caused due to the fact that the kidneys of the children are not mature and are incapable of metabolizing too much sodium existing in blood are reduced. The clinical application quality and safety of child drugs are improved.
Owner:ZHEJIANG CHANGDIAN PHARMA
Method for simultaneously measuring content of dextrose, chlorine and sodium in sodium chloride and dextrose injection through HPLC-CAD (High Performance Liquid Chromatography- Corona Chaged Aerosol Detection)
InactiveCN107014916AAvoid operabilityAvoid environmental problemsComponent separationBottleGlucose / Sodium Chloride Injection
The invention discloses a method for simultaneously measuring the content of dextrose, chlorine and sodium in sodium chloride and dextrose injection through HPLC-CAD (High Performance Liquid Chromatography- Corona Chaged Aerosol Detection) with advantages of measurement accuracy and simplicity in operation, belongs to the field of chemical detection and aims to provide a detection method which is accurate in measurement and simple in operation. The method sequentially comprises the following steps: 1) preparing a test solution, namely fixing the volume of 1ml of sodium chloride and dextrose injection to 1000ml with water, uniformly shaking, and filtering to obtain the solution; 2) preparing a reference solution, namely precisely weighing 12.5g of dextrose reference substance and 2.25g of sodium chloride reference substance, adding into a 250mL of measuring bottle, adding water for dissolving and diluting to scales, and uniformly shaking so as to obtain the reference solution; and 3) analyzing by using high performance liquid chromatography. The method can replace a traditional detection method for simultaneously measuring the content of dextrose, chlorine and sodium in the sodium chloride and dextrose injection.
Owner:WUZHOU INST FOR FOOD & DRUG CONTROL
Childhood cefodizime sodium and low-sodium carrier pharmaceutical composition
InactiveCN104940935AIncrease internal pressureImprove solubilityAntibacterial agentsOrganic active ingredientsCefodizime SodiumLow sodium
The invention relates to a childhood cefodizime sodium pharmaceutical composition, namely a pharmaceutical composition preparation of cefodizime sodium and low-sodium carrier transfusion, and in particular relates to a combined application package. The pharmaceutical composition comprises cefodizime sodium for injection and low-sodium carrier transfusion; the low-sodium carrier transfusion comprises a sodium chloride and dextrose injection ((15-200) to 1), a glucose and sodium chloride potassium chloride injection ((15-200) to 1 to (0-1)) and the like. Compared with combined and mixed application of the cefodizime sodium for injection and the low-sodium carrier transfusion, the clinical application steps are simplified; the clinical risk caused by immature development of kidneys of children and no metabolic capability on excessive sodium in blood is reduced; and the clinical application quality and safety of medicines for children are improved.
Owner:ZHEJIANG CHANGDIAN PHARMA
Juvenile Cefamandole Nafate and low-sodium carrier pharmaceutical composition
InactiveCN105030786AIncrease internal pressureHigh purityAntibacterial agentsOrganic active ingredientsComposite applicationPotassium
The invention relates to a juvenile Cefamandole Nafate pharmaceutical composition, namely a pharmaceutical composite preparation of Cefamandole Nafate and low-sodium carrier infusion, in particular to a composite application package, comprising Cefamandole Nafate for injection and low-sodium carrier infusion. The low-sodium carrier infusion comprises glucose and sodium chloride injection ((15-200):1), glucose, sodium chloride and potassium chloride ((15-200):1:(0-1)) and the like. Compared with compatible mixed use of the Cefamandole Nafate and the low-sodium carrier infusion, the pharmaceutical composition has the advantages that clinical application step is simplified, clinical risks caused by a child with underdeveloped kidneys being unable to metabolize extra sodium in blood are decreased, and clinical application of medication for children is better in quality and safety.
Owner:ZHEJIANG CHANGDIAN PHARMA
Method for preparing sodium chloride and dextrose injection
PendingCN109498647AAvoid turbidityReduce turbidityOrganic active ingredientsMetabolism disorderSodium Chloride InjectionDrug product
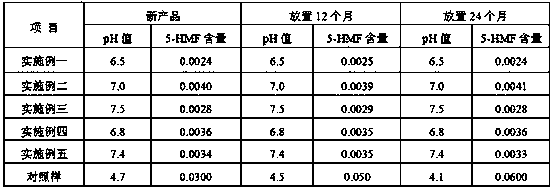
The invention relates to the technical field of medicinal preparation, and discloses a method for preparing a sodium chloride and dextrose injection. The method comprises the following steps: (a) adding sodium chloride and glucose into 60% to 70% configuration amount of water for injection to prepare a concentrated compound fluid; (b) adding the concentrated compound fluid into the water for injection until configuration amount, and carrying out potting to obtain potted liquid medicine; and (c) carrying out sterilization treatment on the potted liquid medicine, wherein the sterilization treatment comprises irradiation and / or microwave sterilization. According to the method, sterilization is carried out by using the sodium chloride and dextrose injection after irradiation and / or microwave potting, and killing of bacteria inside a sodium chloride injection can be realized without using a conventional moist heat sterilization method of increasing the temperature of the liquid medicine, the reduction of the clarity of the sodium chloride injection caused by boiling of the liquid medicine cannot be caused, and simultaneously, the content of 5-hydroxymethyl furfural inside the injectionis reduced, and the quality of the medicine is guaranteed.
Owner:江西润泽药业有限公司
Child type piperacillin tazobactam sodium and low-sodium carrier pharmaceutical composition
InactiveCN104906101AHigh puritySmall side effectsAntibacterial agentsPharmaceutical delivery mechanismPediatric drugGlucose polymers
The invention relates to a child type piperacillin tazobactam sodium pharmaceutical composition, namely a piperacillin tazobactam sodium and low-sodium carrier pharmaceutical composited preparation for transfusion, in particular to combined application package. The pharmaceutical composition comprises piperacillin tazobactam sodium for injection and a low-sodium carrier for transfusion. The low-sodium carrier for transfusion comprises sodium chloride and dextrose injection (15-200:1), glucose and sodium chloride potassium chloride injection (15-200:1:0-1) and the like. Compared with compatible and mixed use of piperacillin tazobactam sodium and the low-sodium carrier, clinical application steps are simplified; the clinical risk which is caused by the problem that the kidneys of children are not mature and have no capacity of metabolizing excessive sodium in blood is reduced, and clinical application quality and safety of pediatric drugs are improved.
Owner:ZHEJIANG CHANGDIAN PHARMA
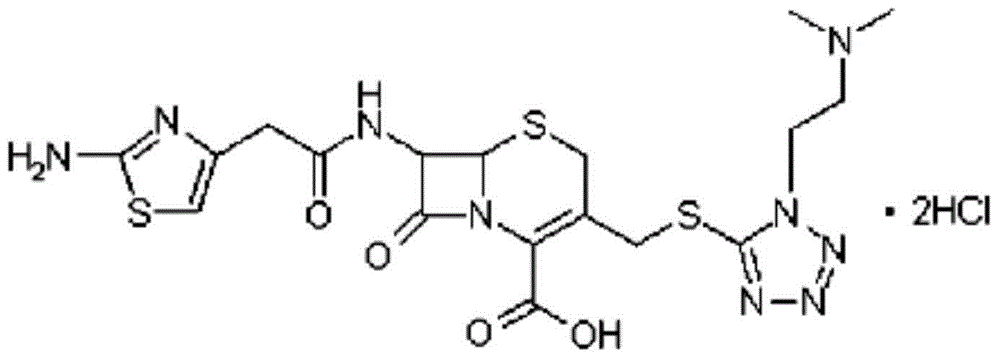
A kind of cefotiam hydrochloride compound, its preparation method and its pharmaceutical composition
ActiveCN104926835BImprove liquidityImprove stabilityAntibacterial agentsOrganic active ingredientsCefotiam HydrochlorideRoom temperature
The invention belongs to the technical field of medicine, and particularly relates to a cefotiam hydrochloride compound, a method for preparing the same and a pharmaceutical composition with the cefotiam hydrochloride compound. The cefotiam hydrochloride compound is cefotiam hydrochloride trihydrate, and a structural formula of the cefotiam hydrochloride compound is shown. The cefotiam hydrochloride compound, the method and the pharmaceutical composition have the advantages that crystals of the cefotiam hydrochloride trihydrate are good in flowability, injection cefotiam hydrochloride made of the crystals of the cefotiam hydrochloride trihydrate, 0.9% sodium chloride injection, 5% glucose injection and 5% glucose and sodium chloride injection can be compatible with one another to obtain liquor, and the liquor is excellent in stability after being placed at the room temperature for 4h.
Owner:SHANDONG LUOXIN PHARMA GRP CO LTD +2
A kind of production technology of glucose sodium chloride injection
ActiveCN109833330BIncrease dissolution rateImprove solubilityOrganic active ingredientsMetabolism disorderBiotechnologySodium Chloride Injection
The invention discloses a production process of glucose sodium chloride injection. The method comprises the following steps: step 1, weighing; step 2, concentrated preparation; step 3, thin preparation; Bacteria; Step 6: Light inspection; Step 7: Packaging. The production process of glucose sodium chloride injection provided by the present invention, the production process of glucose sodium chloride injection provided by the present invention, through strict control of the quality of raw materials, adjustment and optimization of the process flow, the content of insoluble particles, heavy metals, and bacterial endotoxins in the product Significantly reduced, due to the use of nitrogen filled potting, the product has good stability, and no additional antioxidant is needed, which further improves the safety of the injection, and the production efficiency of the optimized process and the qualified rate of the finished product are both significantly improved.
Owner:NANYANG LIXIN PHARMA
A kind of production technology of glucose sodium chloride injection
ActiveCN106309482BFast dissolutionReduce 5-HMF contentOrganic active ingredientsMetabolism disorderSodium acetateFiltration
The invention discloses a process for pretreating glucose and sodium chloride injection, and belongs to the technical field of injection. The method comprises the following steps: (1) weighing raw materials; (2) dissolving the raw materials, namely a) adding sodium hydrogen sulfite, magnesium chloride, calcium chloride, sodium chloride and zinc sulfate into injecting water at 30-40 DEG C; (b) adding dipotassium phosphate, glucose, fructose, xylitol and sodium acetate into the injecting water at 50-60 DEG C; and (c) mixing the solution, adding citric acid and activated carbon, insulating and adsorbing; (3) decarburizing, delivering to a diluting tank; (4) complementing injecting water, and regulating the pH value to 6.5-7.5; (5) filling bags after rough filtration and fine filtration; and (6) sterilizing at 122 DEG C for 3-5 minutes. According to the process, raw materials are dissolved in batches with different dissolving temperatures, the dissolving speed is increased, the temperature is reasonably controlled, energy consumption and cost are reduced, the solution is uniformly mixed, and generation of glucose degraded products can be effectively reduced.
Owner:HUAREN PHARMACEUTICAL CO LTD
Child-type medicinal composition with ceftizoxime sodium and low-sodium carrier
InactiveCN104922679AIncrease internal pressureHigh purityAntibacterial agentsOrganic active ingredientsMedicineSodium Chloride Injection
The invention relates to a child-type medicinal composition with ceftizoxime sodium, i.e. a medicinal combined preparation with ceftizoxime sodium and low-sodium carrier infusion fluid, especially combined application package comprising the ceftizoxime sodium and the low-sodium infusion carrier fluid. The low-sodium carrier infusion fluid comprises glucose and sodium chloride injection fluid (15-200:1) and glucose and sodium-chloride potassium-chloride injection fluid (15-200:10-1) and the like. The child-type medicinal composition has the advantages that compared with the compatible and mixing use of the ceftizoxime sodium and the low-sodium carrier infusion fluid, the clinical application steps are simplified, the clinical risks caused by incapability of metabolizing excessive sodium in blood since the kidney of a child is not matured are reduced and the clinical application quality and safety of child administration are improved.
Owner:ZHEJIANG CHANGDIAN PHARMA
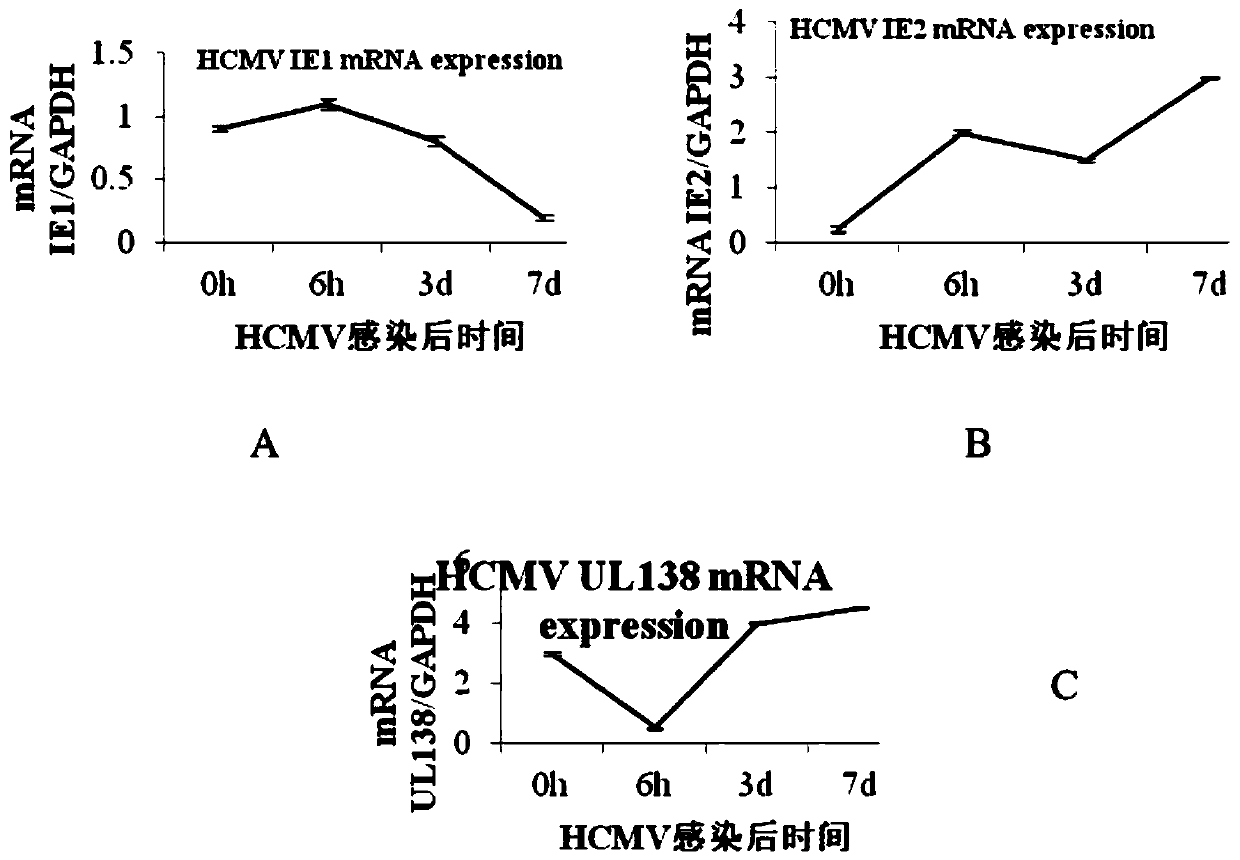
United medicine for resisting human cytomegalovirus (HCMV) and measuring method of component concentration of united medicine
InactiveCN110585144AImprove blood flowWide variety of sourcesPowder deliveryMicrobiological testing/measurementFreeze-dryingSodium Chloride Injection
The invention provides a united medicine for resisting HCMV. Each part of the medicine consists of polysaccharide sulfate, ganciclovir and a solvent, wherein the polysaccharide sulfate and the ganciclovir are freeze-dried sterilization powder, and the solvent is 0.9% sodium chloride injection or 5% glucose injection or 5% glucose and sodium chloride injection or normal saline or water for injection. When the medicine is used, the polysaccharide sulfate and the ganciclovir dissolve in the solvent, and 1mL of injection is prepared; and the concentration of the polysaccharide sulfate in the injection is 312.50 [mu]g / mL-1000.00[mu]g / mL, and the concentration of the ganciclovir is 12.50 [mu]g / mL-63.00[mu]g / mL. The invention aims to solve the problem of determining a marine medicine for resisting HCMV infected livers and of which the main component is the polysaccharide sulfate, and a measuring method of largest non-toxic concentration and the lowest valid concentration of the marine medicine, wherein the lowest dosage of the marine medicine is used in the medication range, and the toxicity of the medicine is reduced as far as possible.
Owner:WEIFANG MEDICAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com