Method for preparing sodium chloride and dextrose injection
A glucose sodium chloride and injection technology, which is applied in the field of preparation of glucose sodium chloride injection, can solve the problems of lower clarity, lower quality of injection, and lower content of 5-hydroxymethylfurfural, etc., so as to avoid glucose degradation The effect of reaction, excellent stability, and excellent sterilization effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0028] The present invention provides a kind of preparation method of glucose sodium chloride injection, wherein, described method comprises the following steps:
[0029] (a) Sodium chloride and glucose are dropped into the concentrated preparation tank that contains the water for injection of 60-70% configuration amount, stir until dissolving completely, add activated carbon I for injection, leave standstill after stirring 10-30min, filter, obtain concentrated dosing;
[0030] (b) Transfer the concentrated solution to the diluted formulation tank, add activated carbon II for injection, add water for injection to the configured volume into the diluted formulation tank, stir for 10-60 minutes after the adsorption reaction, and perform decarbonization treatment to obtain Medicinal solution: take a sample to detect the sodium chloride, glucose content and pH value of the medicinal solution, and the qualified medicinal solution is filtered through a 0.45 μm precision filter and a ...
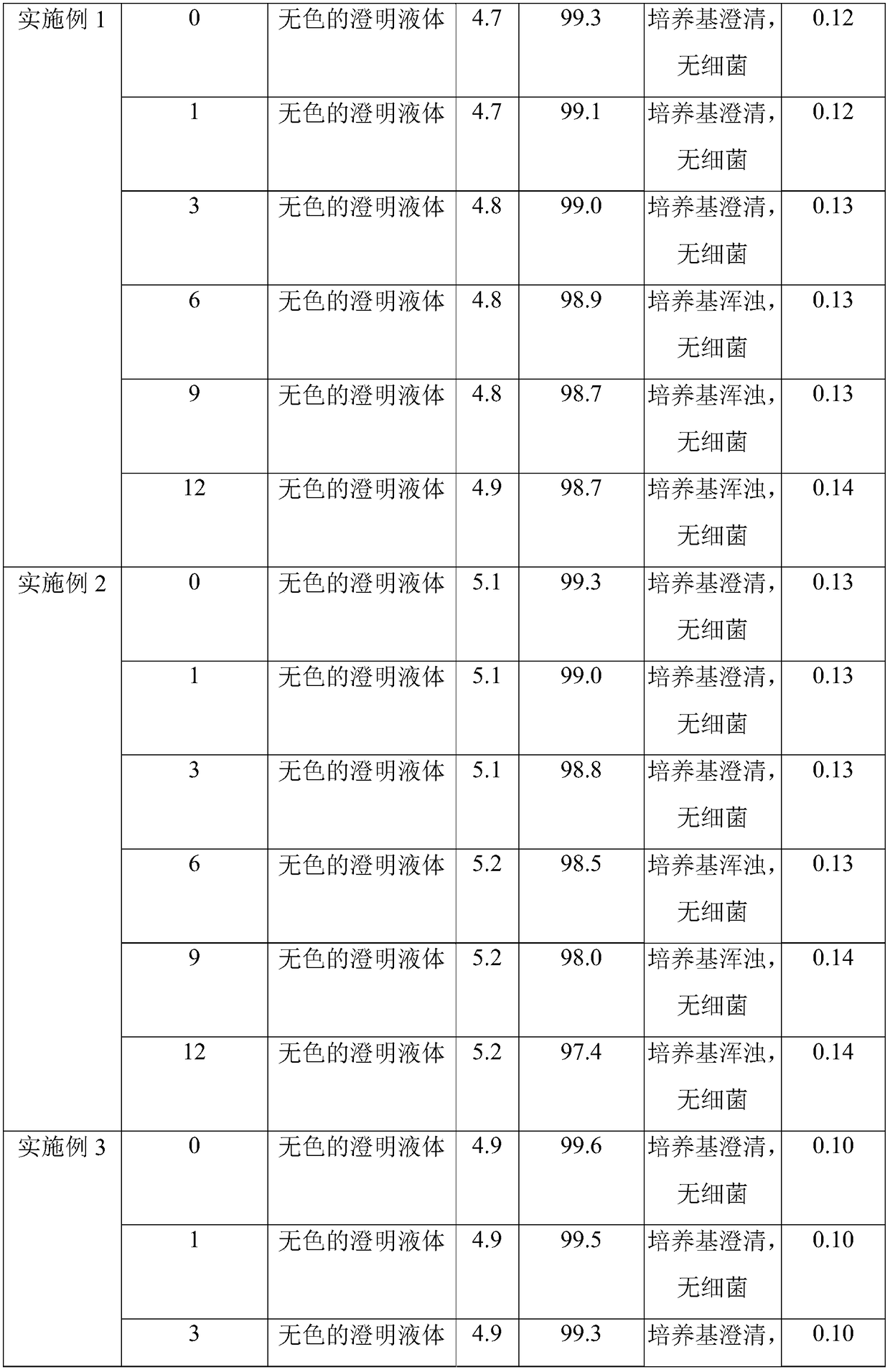
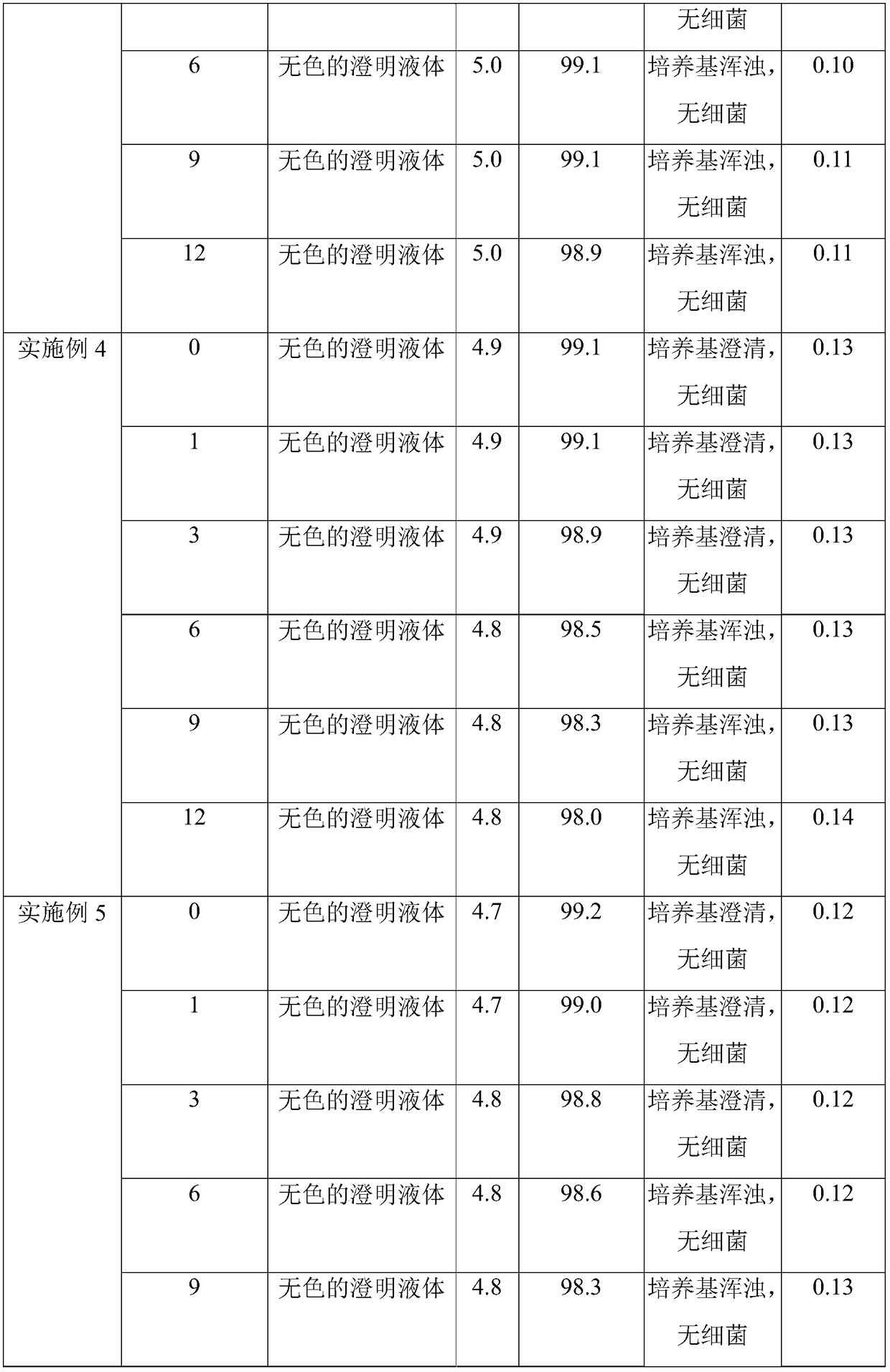
Embodiment 1
[0061] (1) Accurately weigh raw materials;
[0062] (2) Add 70% water for injection, sodium chloride and glucose in the concentrated preparation tank, stir until fully dissolved, add activated carbon for injection 1, leave standstill after stirring for 30min, filter, and obtain concentrated dosing;
[0063] (3) Transfer the thick dosing solution to the dilute dosing tank, add activated carbon II for injection, add water for injection to the prepared volume in the dilute dosing tank, stir for adsorption reaction for 30 minutes, perform decarbonization treatment, and take samples to detect chlorination The content of sodium and glucose and the pH of the medicinal solution, the qualified medicinal solution is filtered through a 0.45 μm precision filter and a 0.2 μm sterilizing filter in sequence, and then canned. Wherein, the total consumption of activated carbon for injection is 0.06w / v%, the consumption ratio of activated carbon I for injection and activated carbon II for injec...
Embodiment 2
[0068] (1) Accurately weigh raw materials;
[0069] (2) Add 60% water for injection, sodium chloride and glucose in the concentrated preparation tank, stir until completely dissolved, add activated carbon I for injection, leave standstill after stirring for 10min, filter, and obtain concentrated dosing;
[0070] (3) Transfer the thick dosing solution to the dilute dosing tank, add activated carbon II for injection, add water for injection to the configured volume in the dilute dosing tank, stir for adsorption reaction for 40 minutes, then perform decarbonization treatment, and take samples to detect chlorination The sodium content and the pH of the medicinal solution, the qualified medicinal solution is filtered through a 0.45 μm precision filter and a 0.2 μm sterilizing filter in sequence, and then canned. Wherein, the total dosage of activated carbon for injection is 0.02w / v%, the dosage ratio of activated carbon for injection I and activated carbon for injection II is 1:2, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com