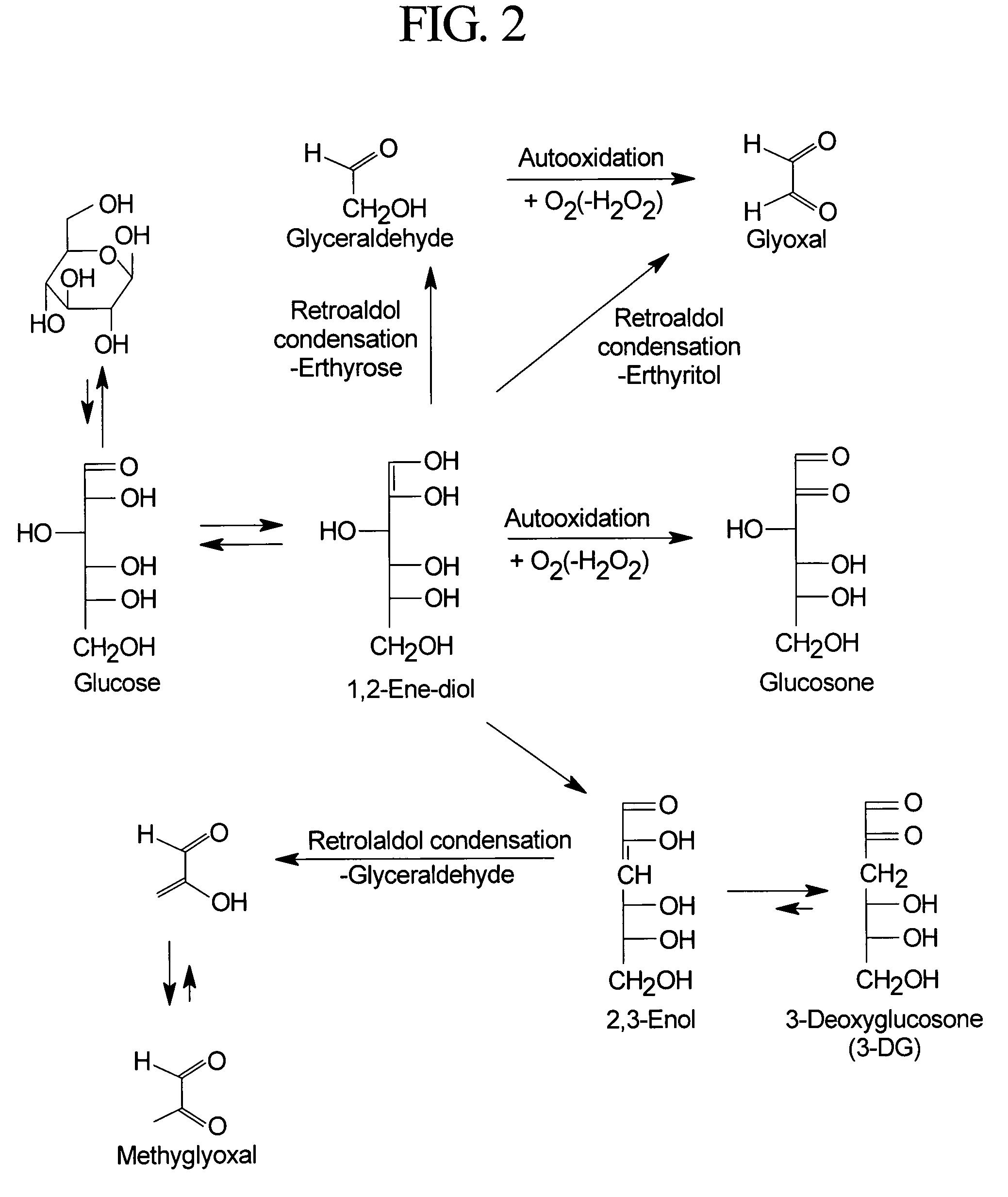
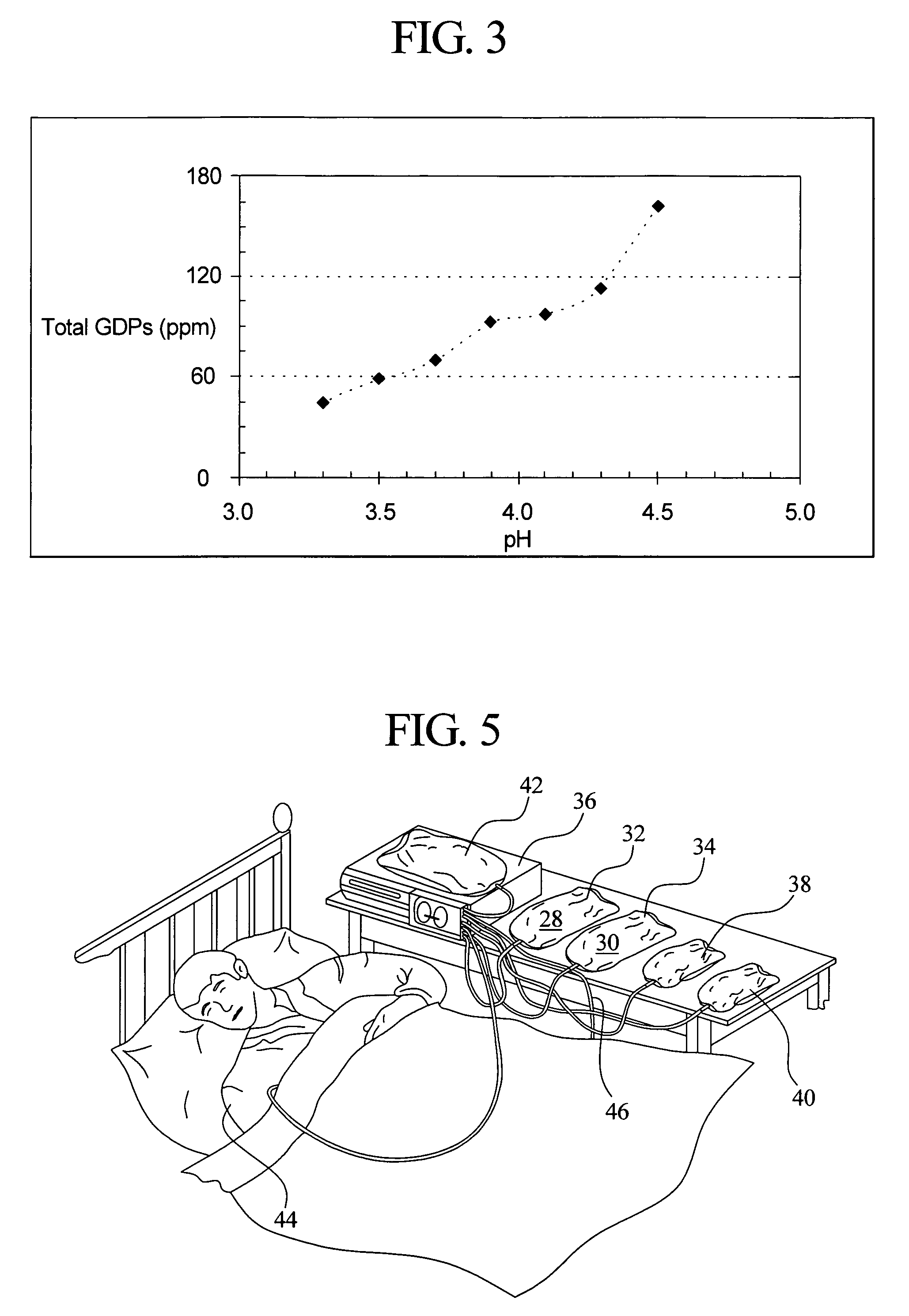
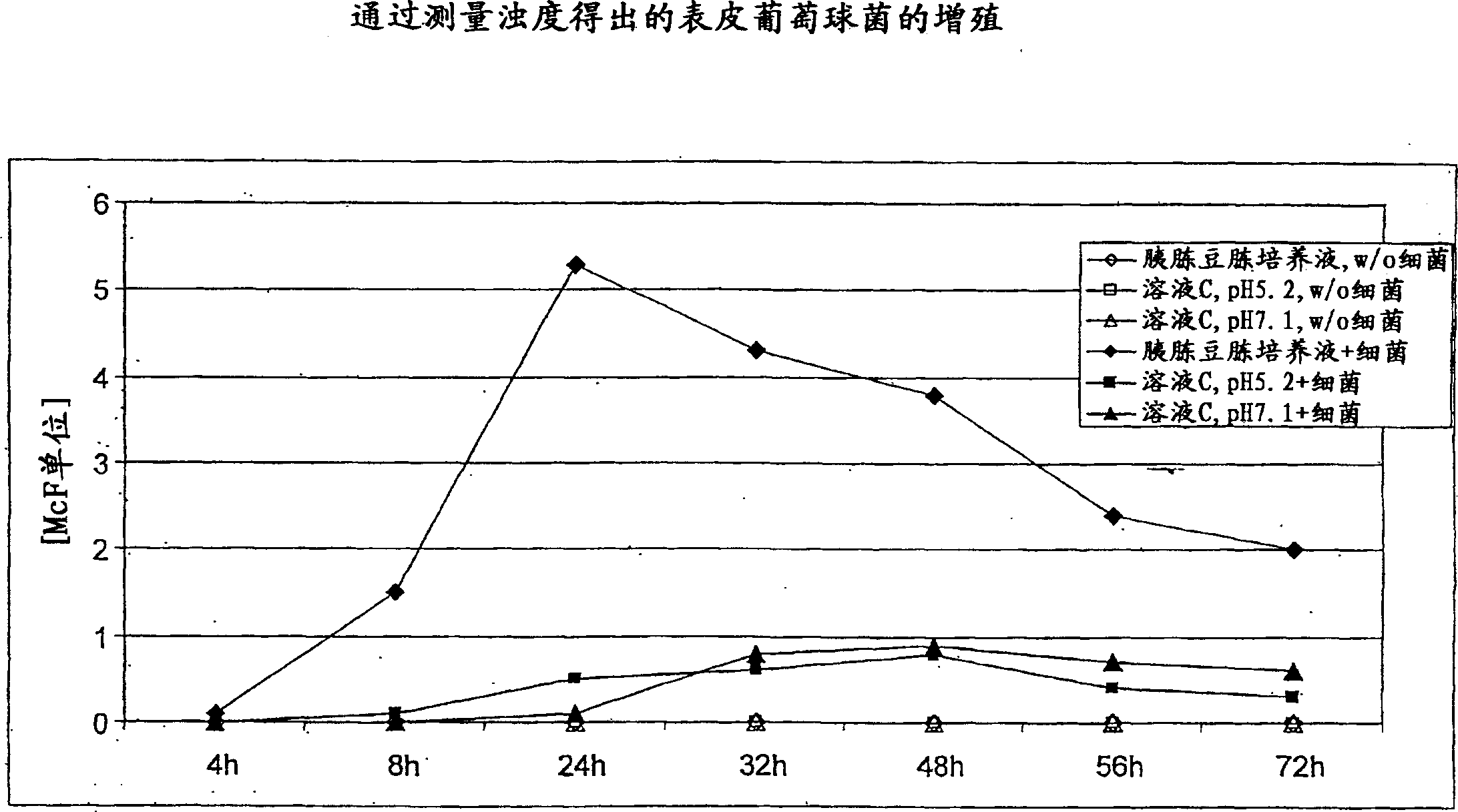
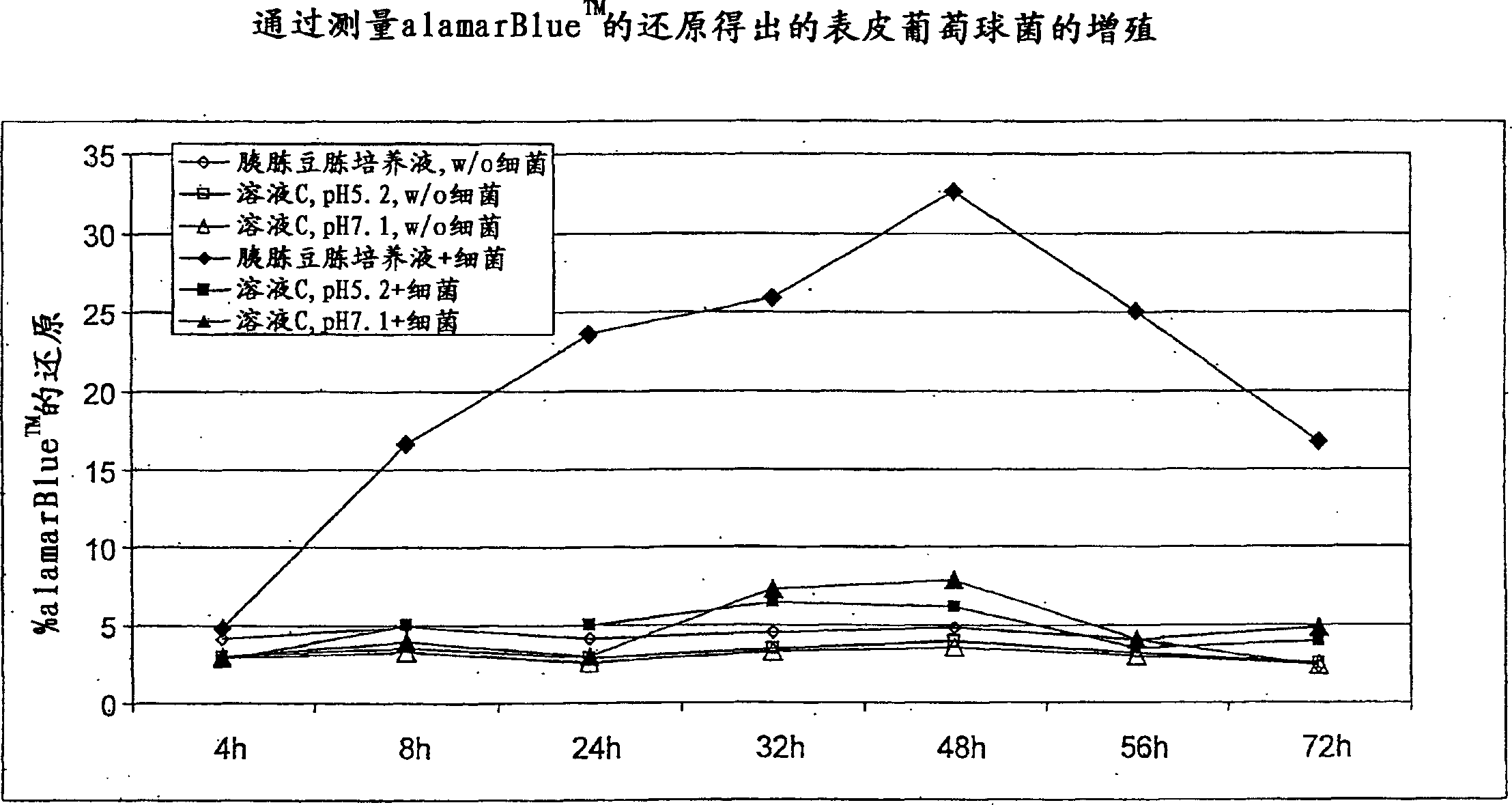
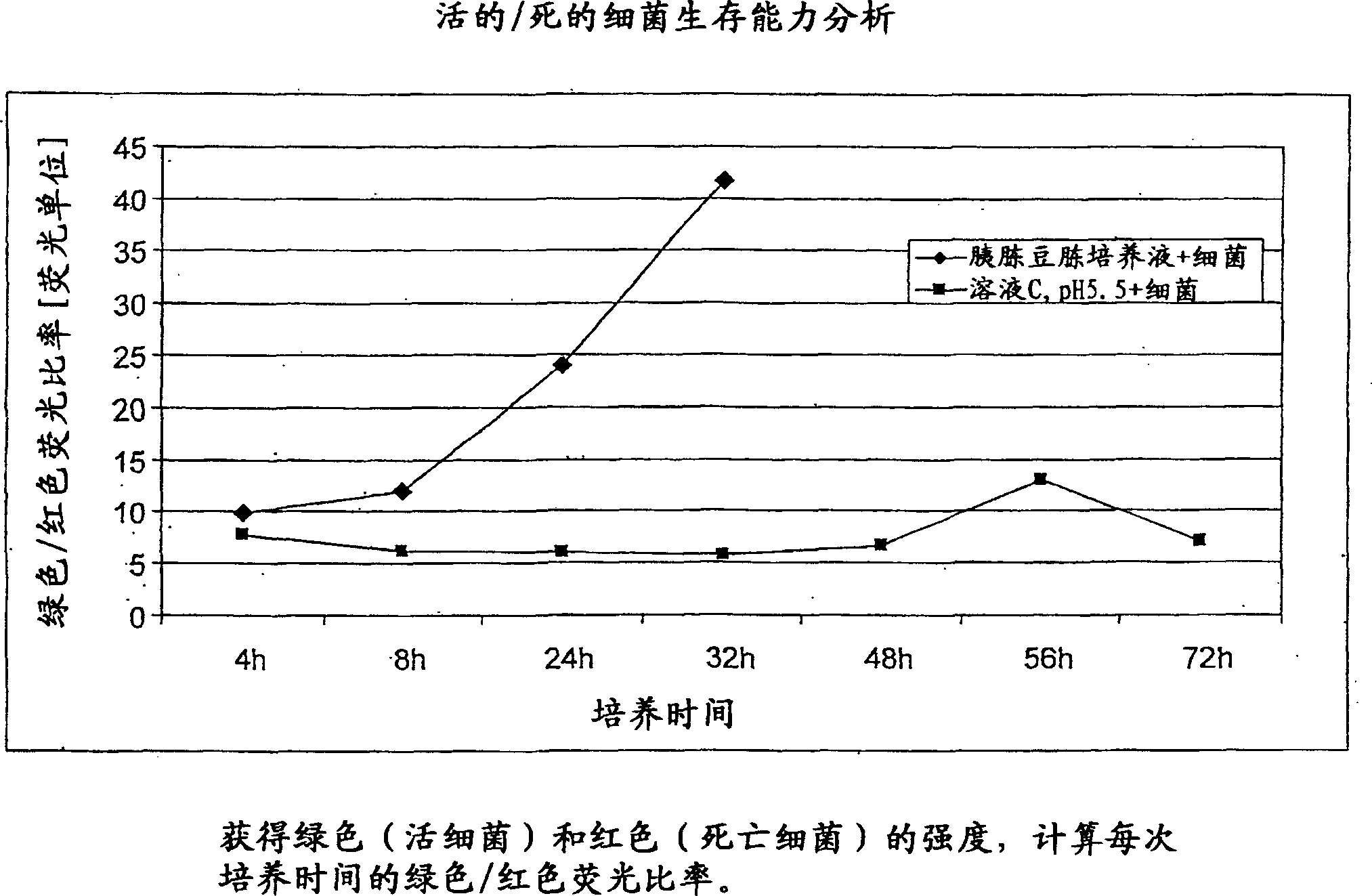
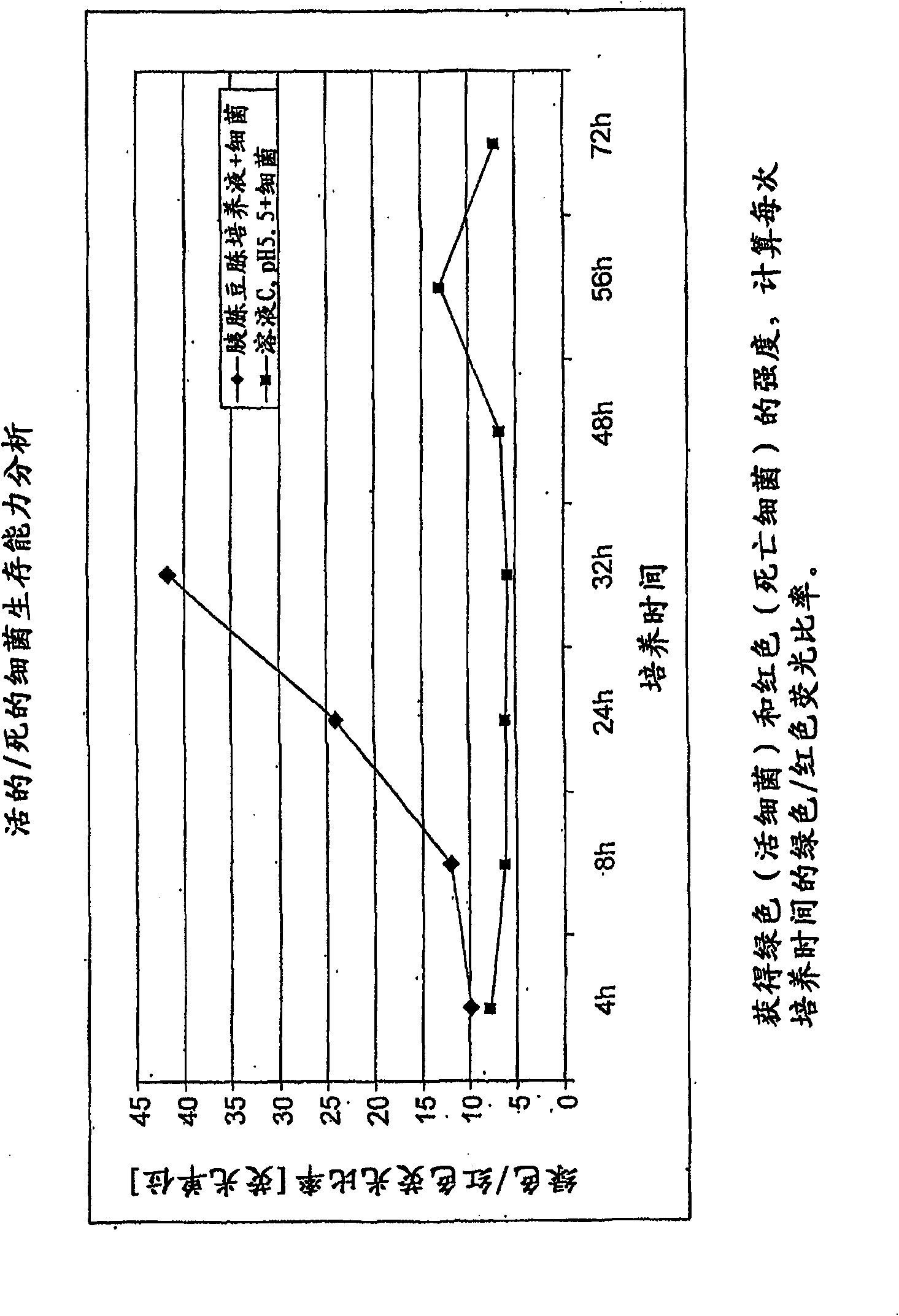
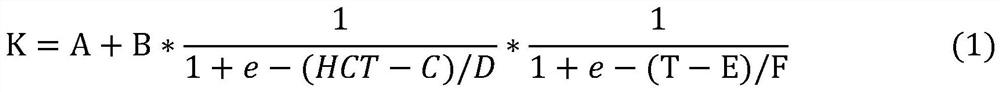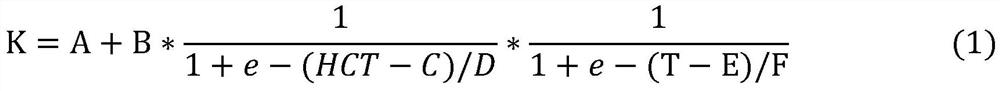Patents
Literature
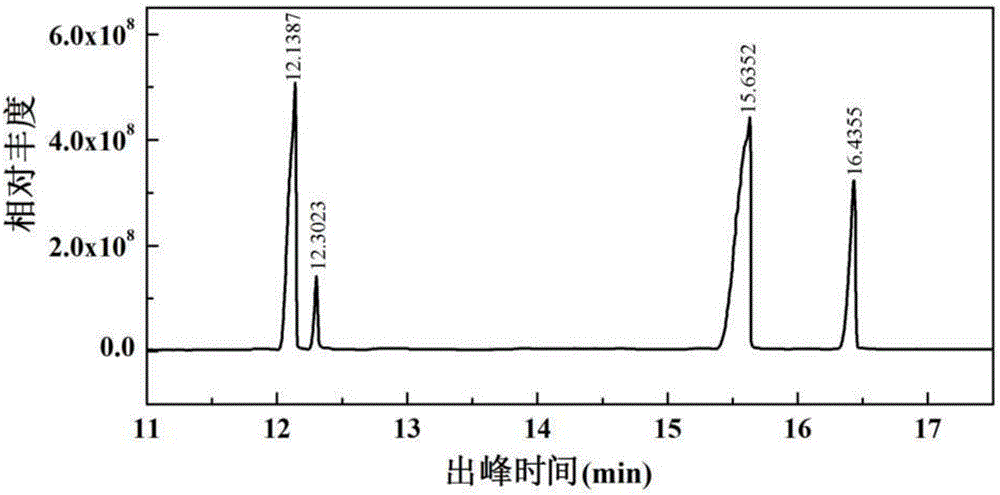
39 results about "Glucose degradation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
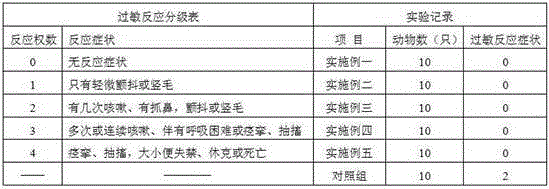
Systems and methods for dextrose containing peritoneal dialysis (PD) solutions with neutral pH and reduced glucose degradation product
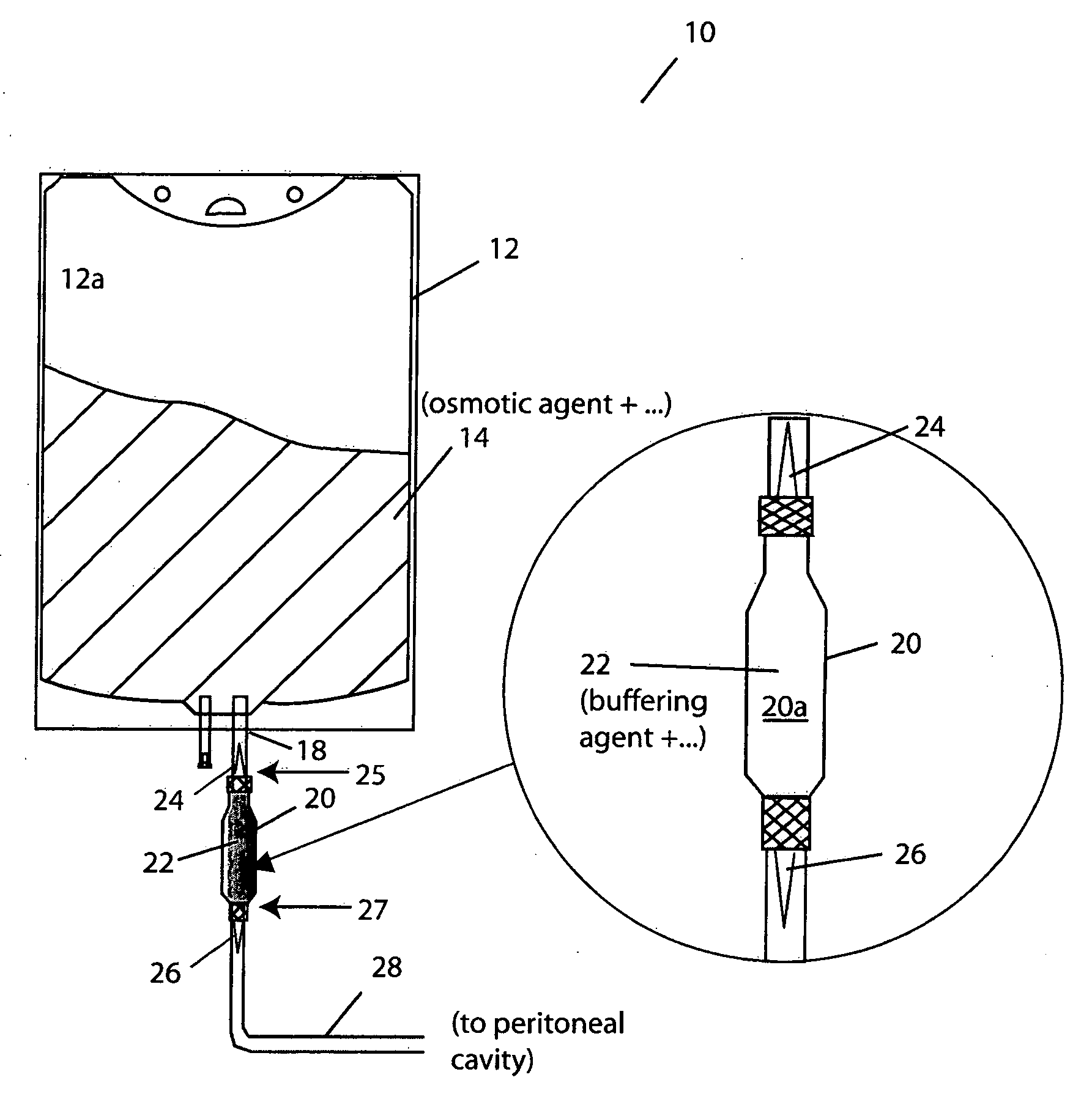
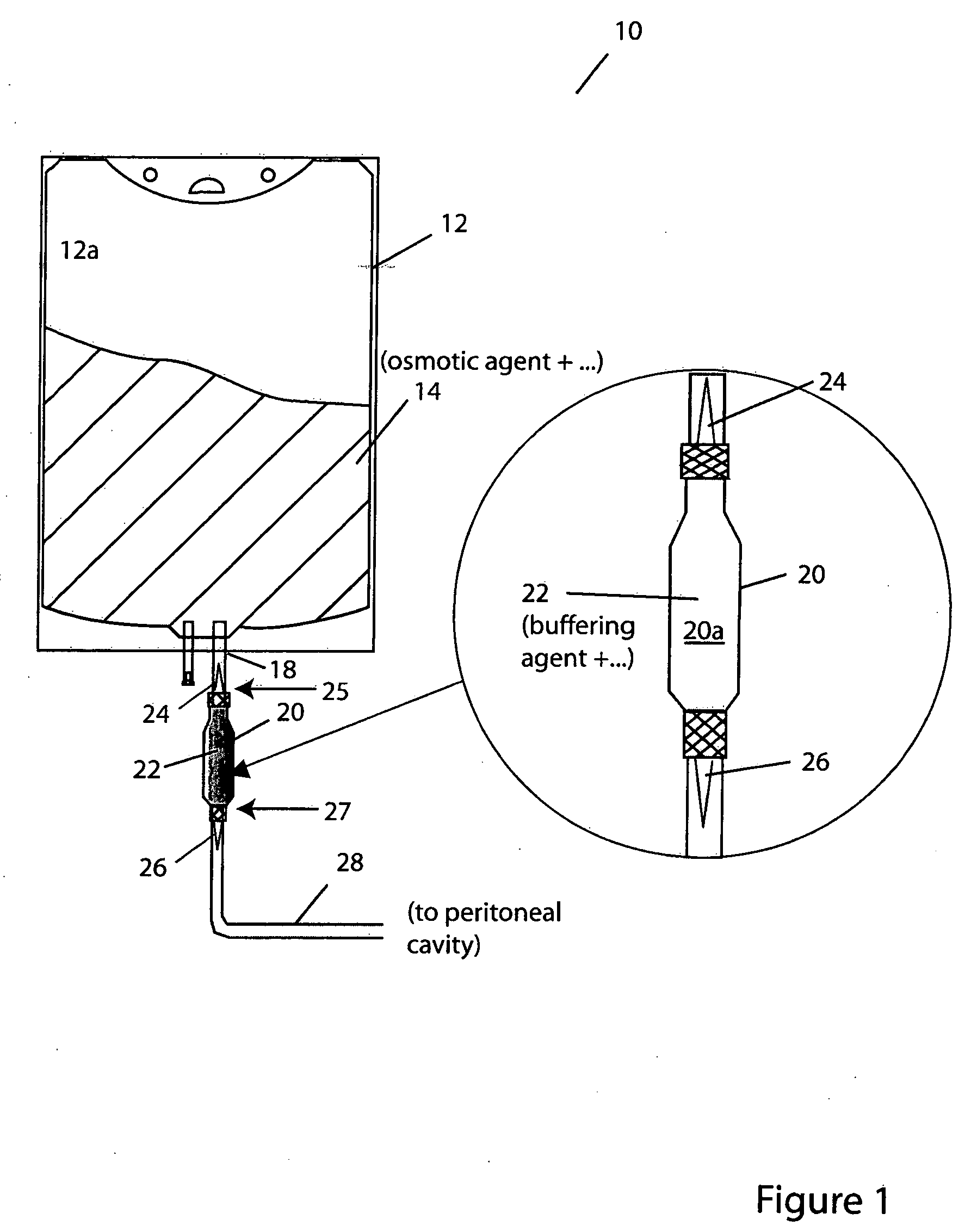
The invention provides container systems, kits and methods for peritoneal dialysis (PD) solutions. Such a system, for example, includes a first compartment that contains a Pt) osmotic agent and a second compartment that contains a PD buffer agent. The compartments maintain their respective contents separately from one another for purposes of transport, storage and / or sterilization. However, the compartments are fluidly couplable, so that their respective contents can be combined with one another, e.g., following sterilization of the agents and prior to their introduction into the patient's abdomen.
Owner:FRESENIUS MEDICAL CARE HLDG INC +1
Dialysis solutions with reduced levels of glucose degradation products
InactiveUS7053059B2Improve featuresDecrease in levelBiocidePharmaceutical delivery mechanismMedicineBiocompatibility Testing
Dialysis solutions with enhanced biocompatibility are provided. The dialysis solutions include a first acidic solution and a second acidic solution that are admixed to form a ready-to-use dialysis solution with reduced levels of glucose degradation products prior to use. The first acidic solution includes a dextrose concentrate, and the second acidic solution includes a buffer concentrate, such as a lactate-based buffer. The first and second acid solutions are separately sterilized prior to mixing to form the ready-to-use dialysis solutions. The dialysis solutions can be used in a variety of different applications, such as infusion into a patient during peritoneal dialysis.
Owner:BAXTER INT INC +1
Extraction process and application of peach gum polysaccharide
ActiveCN105061617AHigh purityThe preparation method is fineMetabolism disorderGlucose degradationPrecipitation
The invention discloses an extraction process and application of peach gum polysaccharide (PGPSD). The process includes: water extraction and alcohol precipitation, protein removal by a Sevage reagent, dialysis, passing of a column with DEAE cellulose as the filler and other steps, thus obtaining finer polysaccharide (PGPSD). The PGPSD can be dissolved in water at room temperature, and is formed by connection of arabinose, mannose and galactose through a glycosidic bond. The peach gum polysaccharide (PGPSD) prepared by the process provided by the invention can reinforce the expression of insulin related transcription factors and glucose degradation key enzyme, has no toxicity to mice, and can significantly lower the blood glucose of diabetic mice.
Owner:HUAZHONG AGRI UNIV
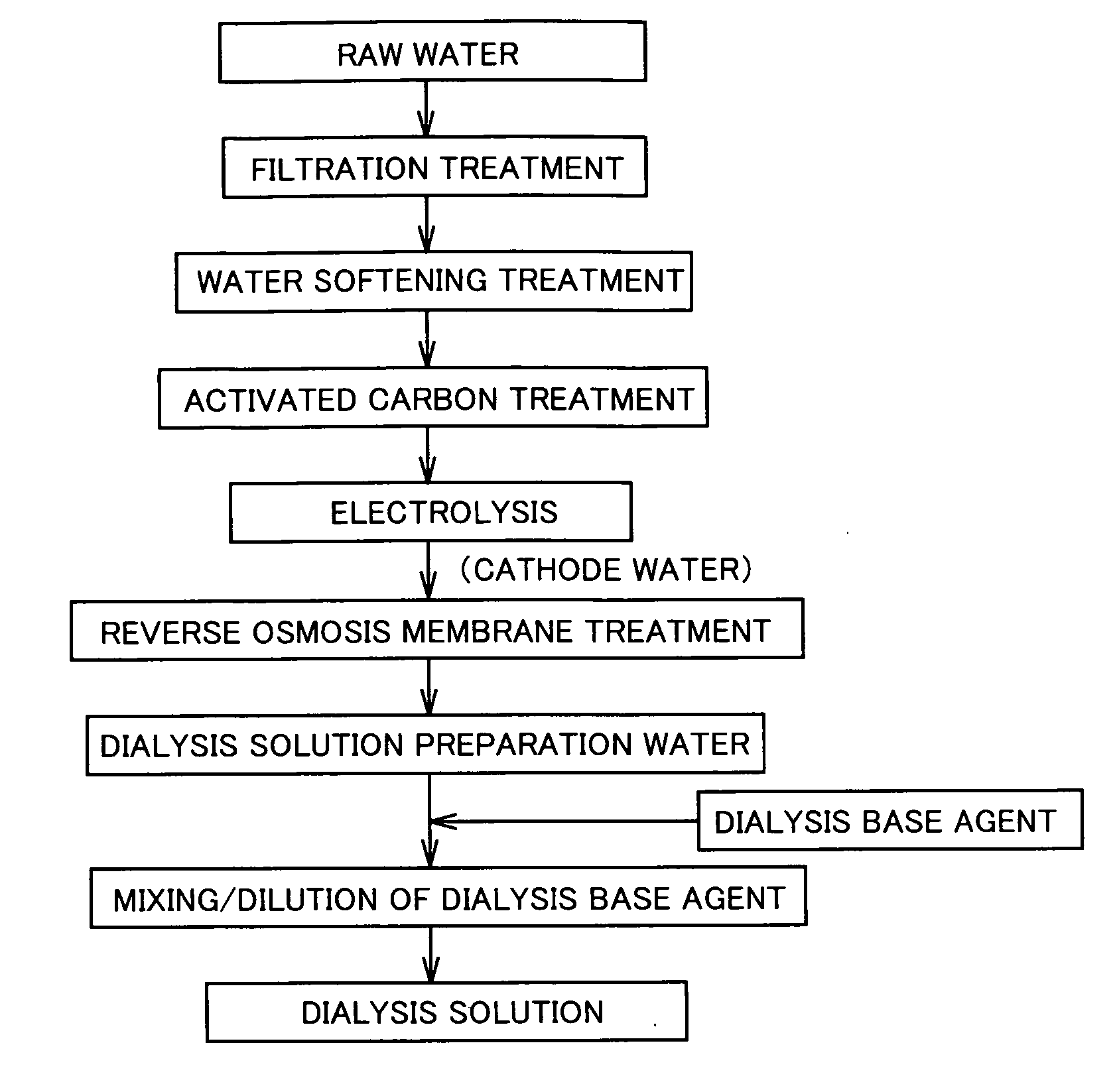
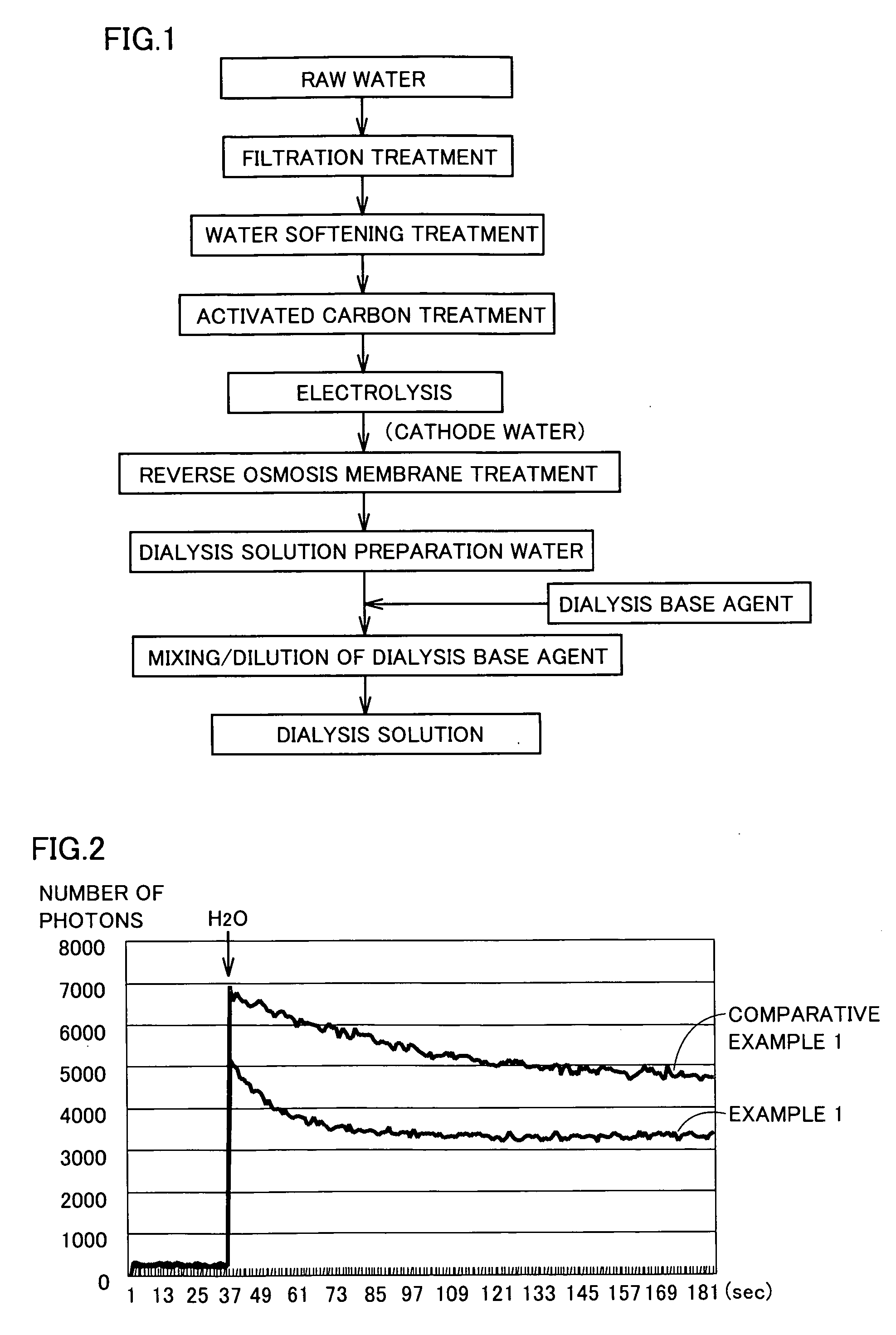
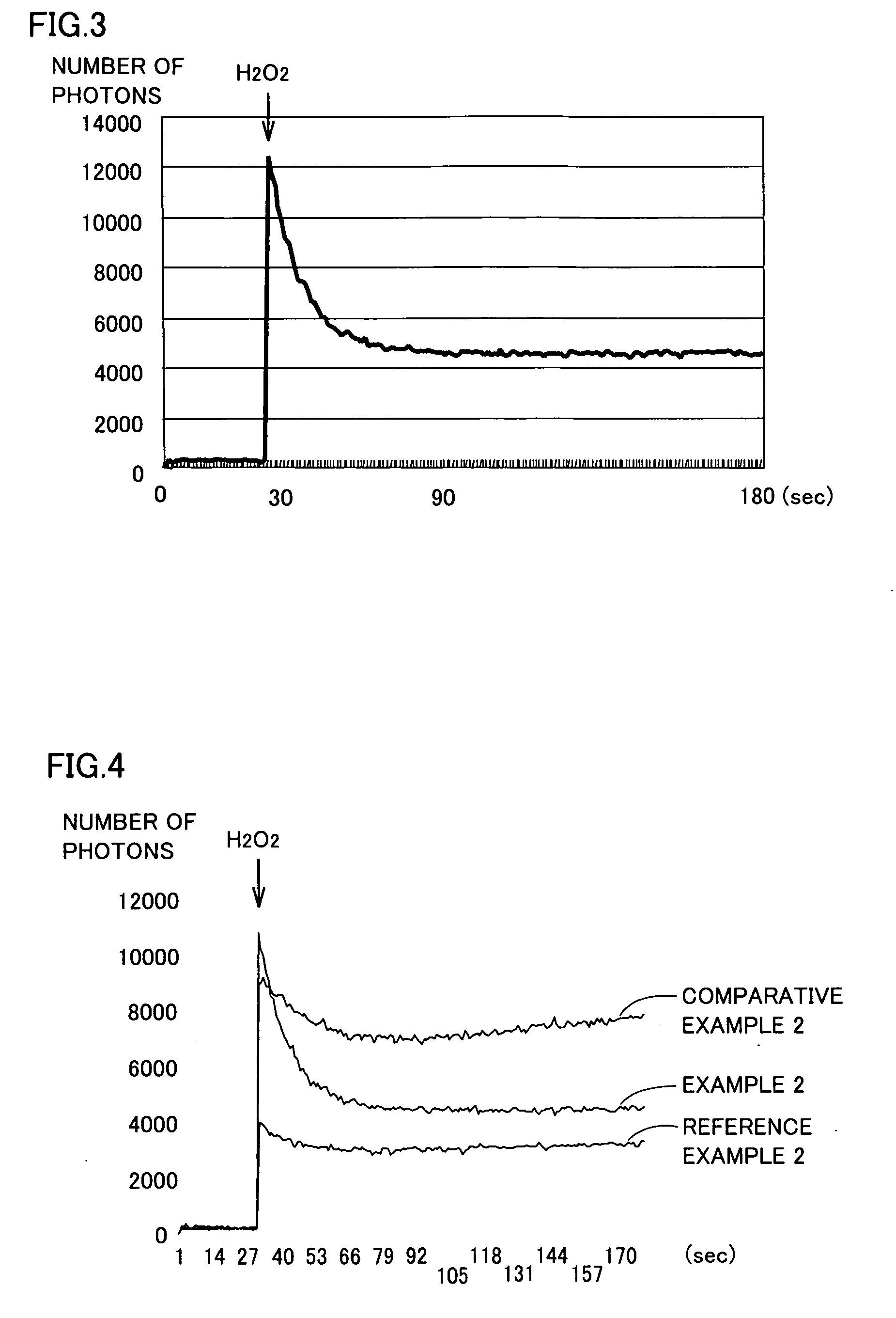
Dialysis Solution Preparation Water, Dialysis Solution Using Such Water, Method of Producing Dialysis Solution, and Dialysis Equipment
InactiveUS20090045121A1Reduce adverse effectsBiocideOrganic active ingredientsWater useBiological body
Dialysis solution preparation water having a dissolved hydrogen concentration of 50 to 600 ppb, a pH of 7 to 10, and satisfying the water quality criterion defined at ISO 13959, used to prepare a dialysis solution by diluting a dialysis base agent including at least 50 ng / mL of a glucose degradation product, a method of preparing a dialysis solution by diluting a dialysis base agent using the dialysis solution preparation water, and a dialysis solution obtained thereby. By dialysis equipment comprising means for supplying dialysis solution preparation water having a dissolved hydrogen oxygen of 50 to 600 ppb, a pH of 7 to 10, and satisfying a water quality criterion defined at ISO 13959, means for storing a dialysis base agent including at least 50 ng / mL of a glucose degradation product, and means for preparing a dialysis solution by diluting the dialysis base agent with the dialysis solution preparation water, there can be provided a dialysis solution that can prevent the adverse effect of glucose degradation products on the biological body, a dialysis solution preparation water used therefor, a method of producing a dialysis solution, and dialysis equipment.
Owner:NIKHON TRIM KO LTD
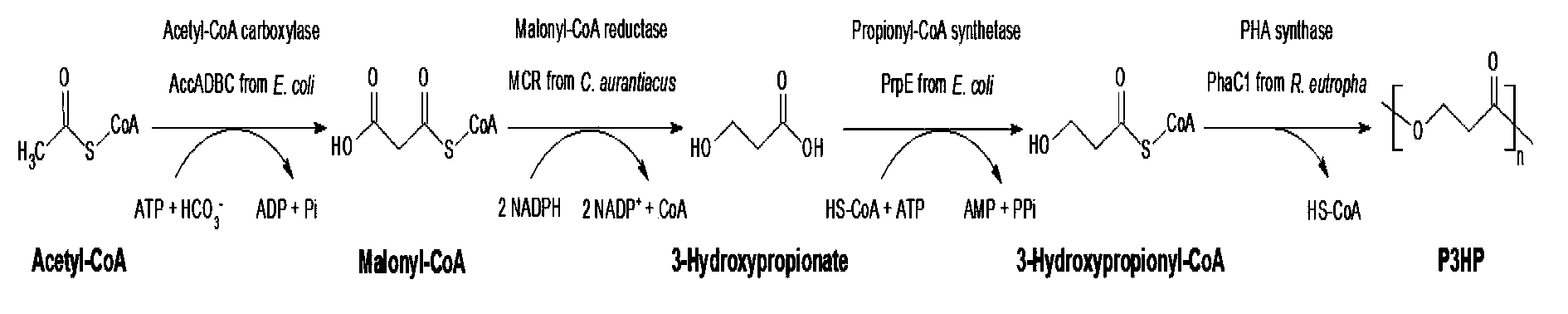
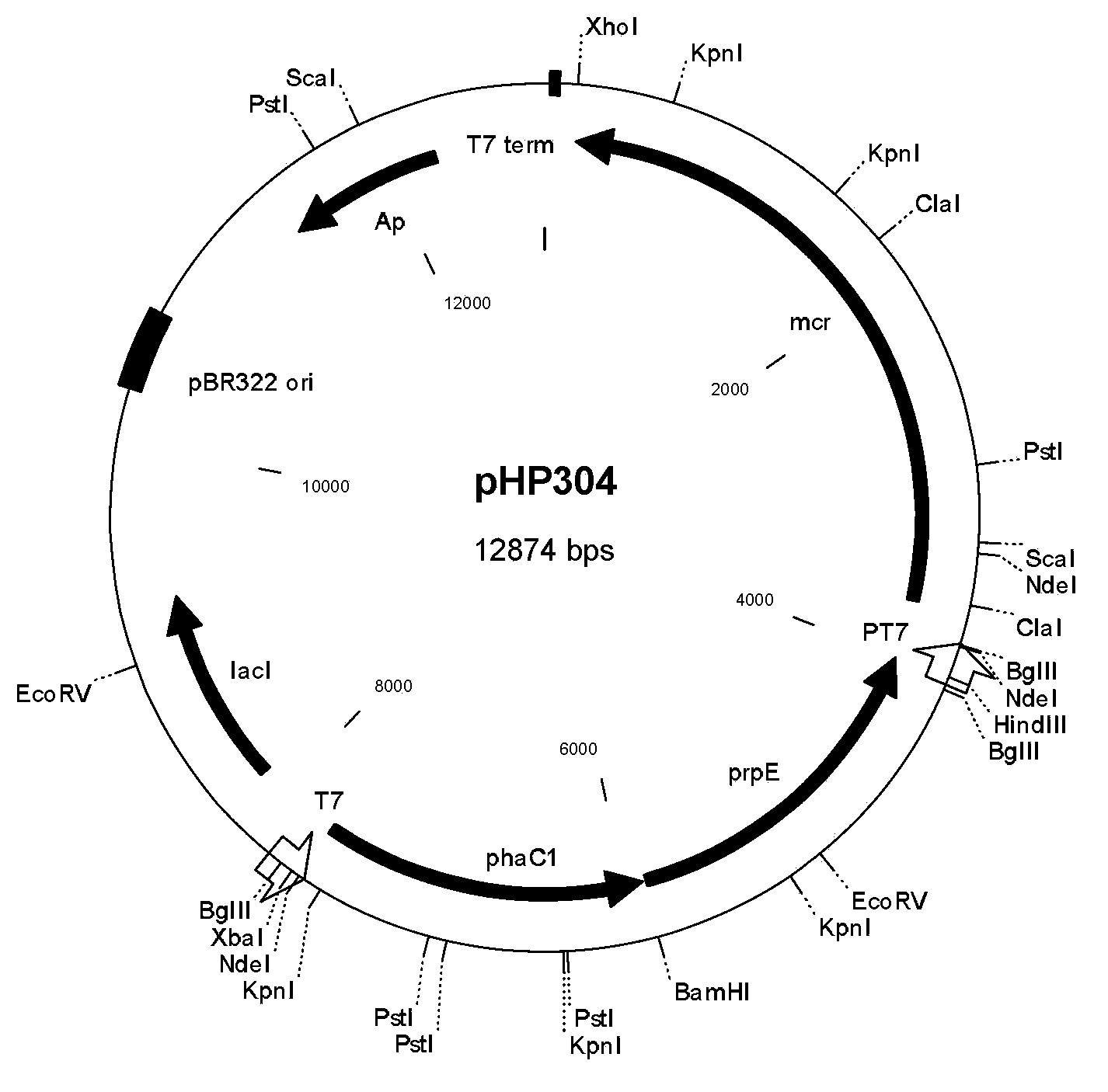
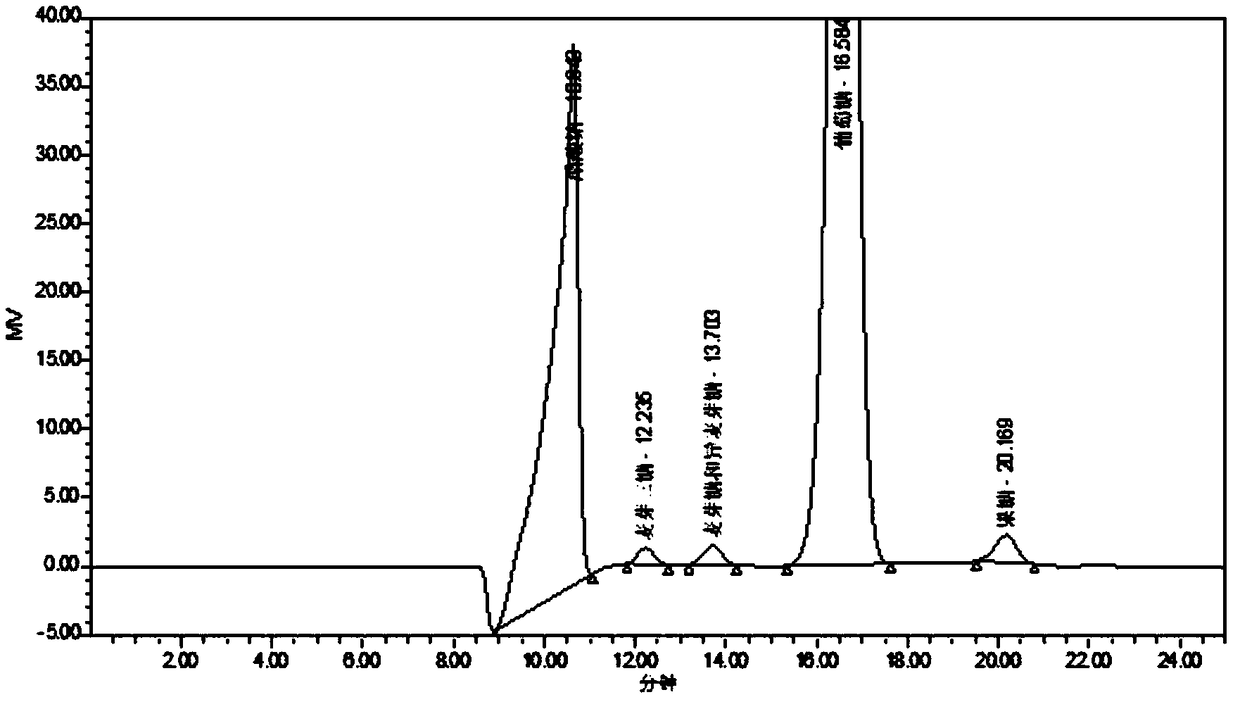
Method for biologically synthesizing poly-3-hydroxypropionic acid
InactiveCN103898034AWide variety of sourcesSimple production processBacteriaMicroorganism based processesEscherichia coli3-Hydroxypropionic acid
The invention provides a recombinant escherichia coli strain, a preparation method of the recombinant escherichia coli strain and a method for biologically synthesizing poly-3-hydroxypropionic acid from acetyl coenzyme A. The method comprises the following steps: with glucose or glycerin and the like as carbon sources, commonly over-expressing endogenous or exogenous acetyl coenzyme A carboxylase genes (acc) and propionyl coenzyme A synthetase genes (prpE) as well as exogenous malony coenzyme A reductase genes (mcr) and polyhydroxyalkanoate synthetase genes (phaC) in proper host cells (such as escherichia coli), and degrading intermediate products by virtue of glucose so as to biologically synthesize the poly-3-hydroxypropionic acid, wherein acetyl coenzyme A is finally obtained from simple starting materials such as the glucose and the like.
Owner:QINGDAO INST OF BIOENERGY & BIOPROCESS TECH CHINESE ACADEMY OF SCI
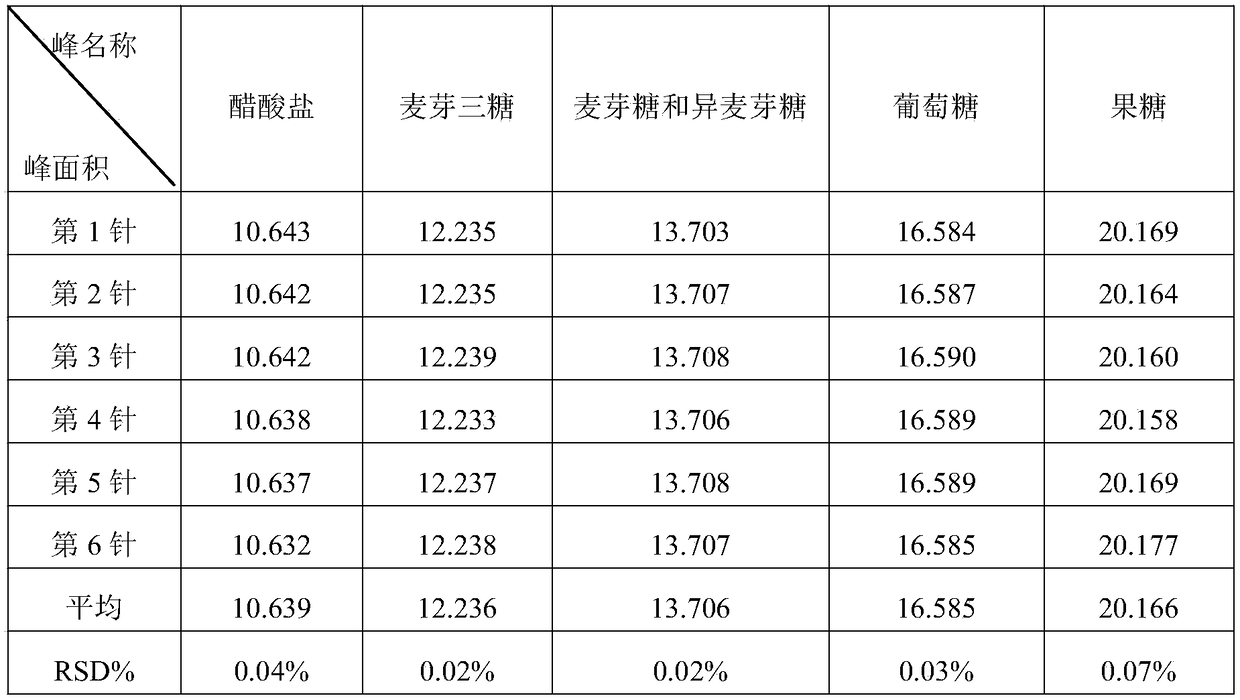
Detection method of glucose related substances in acetate compound electrolyte injection
InactiveCN109030656ASimple processQuality improvementComponent separationSodium acetateSodium acetrizoate
The invention provides a detection method of glucose related substances in an acetate compound electrolyte injection, related to the technical field of medicine. The detection method of glucose related substances in the acetate compound electrolyte injection comprises the following steps: preparing a system suitability solution: weighing a sodium acetate reference substance, a glucose reference substance and a glucose degradation product reference substance, placing in a 10ml volumetric flask, diluting to a mark with water, shaking well, and taking as the system suitability solution; preparinga test substance and a self reference solution: taking the sample directly for the test substance solution; measuring and taking the test substance solution, placing in a 50ml volumetric flask, diluting to the mark with water, shaking well as a contrast solution A; taking a portion of the contrast solution A, placing in a 100ml volumetric flask, diluting to the mark with water, and shaking well as a contrast solution B; and chromatographic conditions are as follows: Waters Sugar Park I column, 300mm x 6.5mm, 10[Mu]m; mobile phase, 50 mg / L aqueous solution of calcium ethylenediaminetetraacetate; flow rate, 0.3 ml / min; column temperature, 85+ / -5 degrees centigrade; differential detector set temperature, 40+ / -5 degrees centigrade; and injection volume, 20[Mu]l. The detection method of glucose related substances in the acetate compound electrolyte injection has the beneficial effects of effectively separating the acetate and the glucose degradation products, optimizing the process and improving the product quality and safety.
Owner:HUAREN PHARMACEUTICAL CO LTD
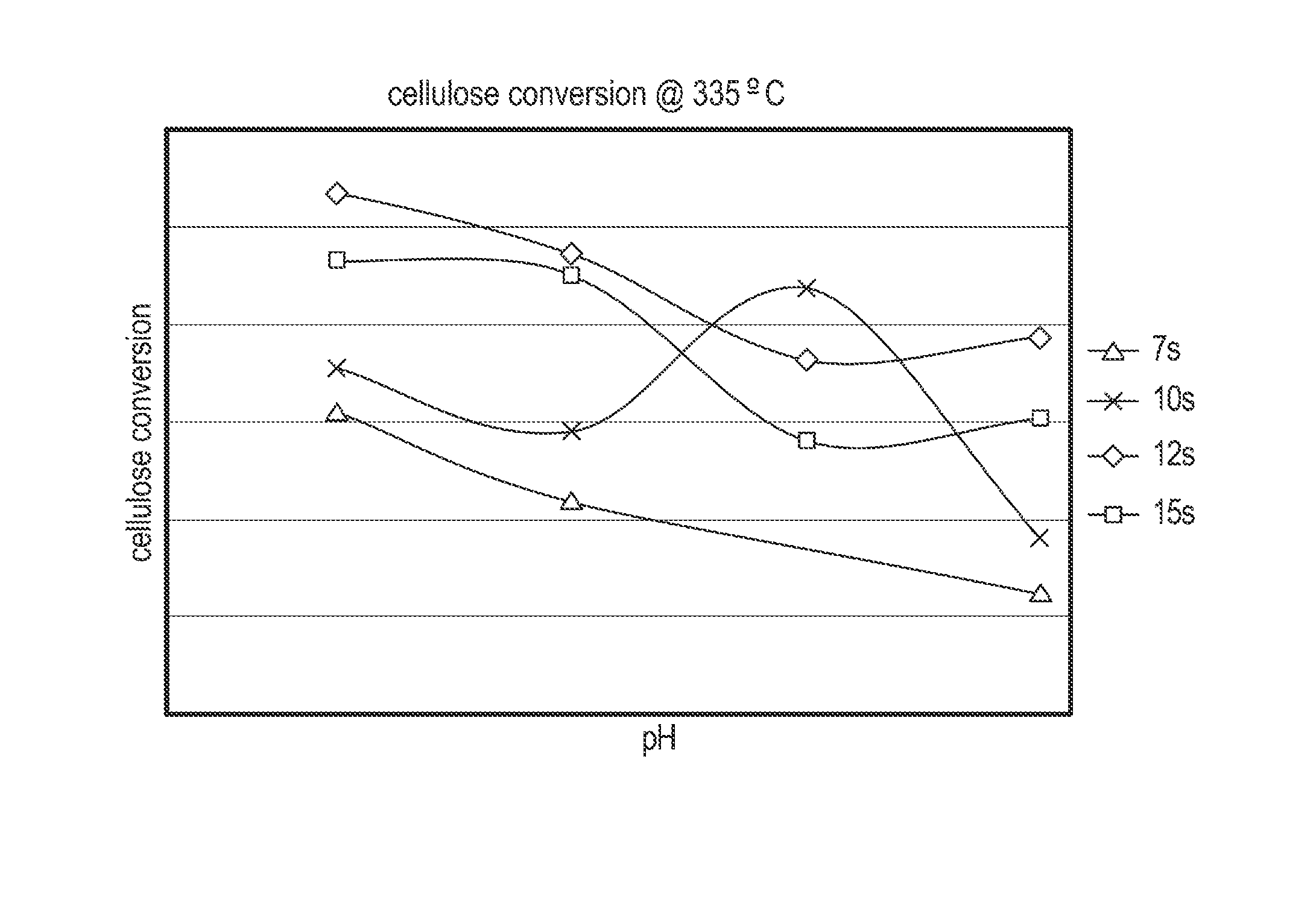
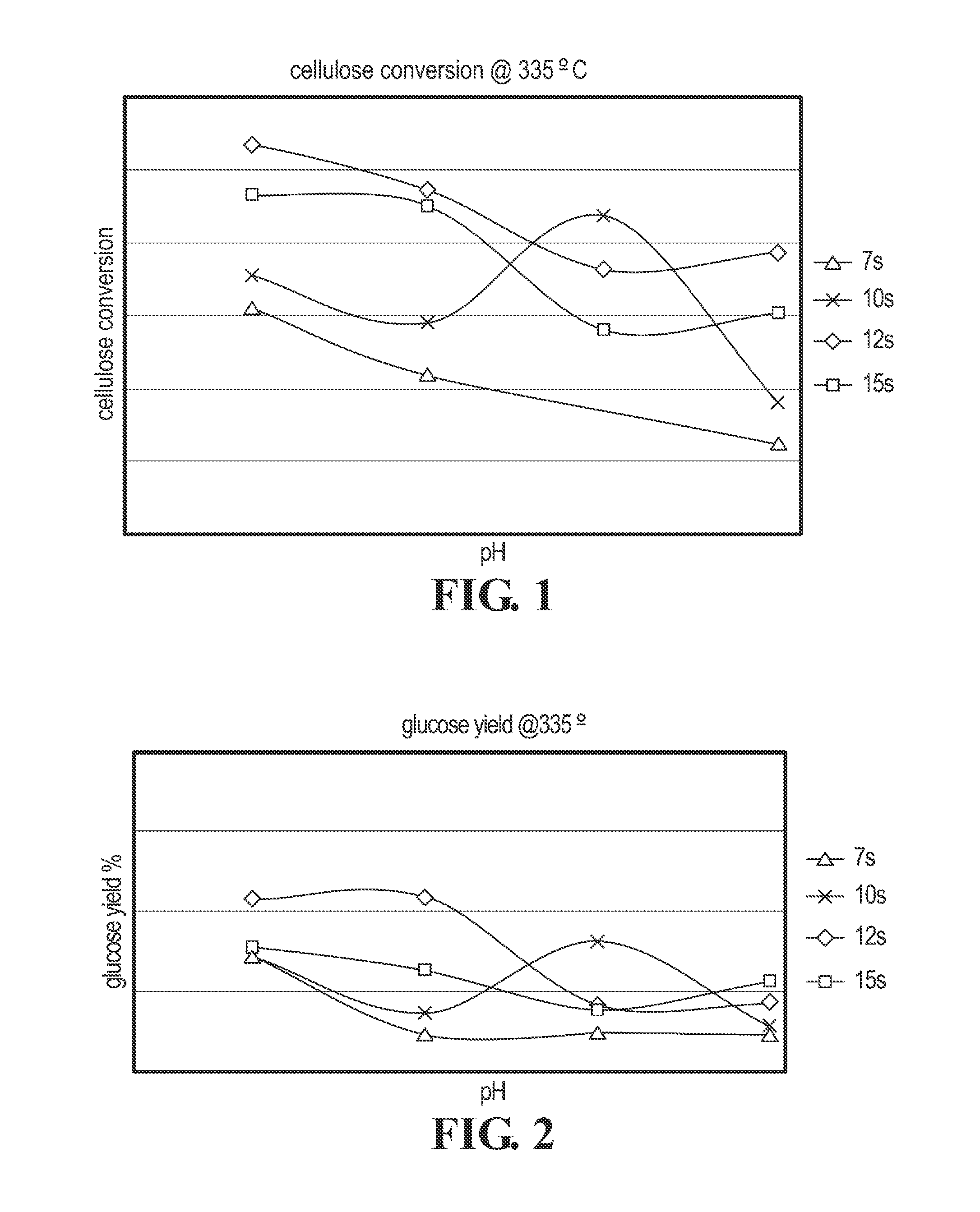
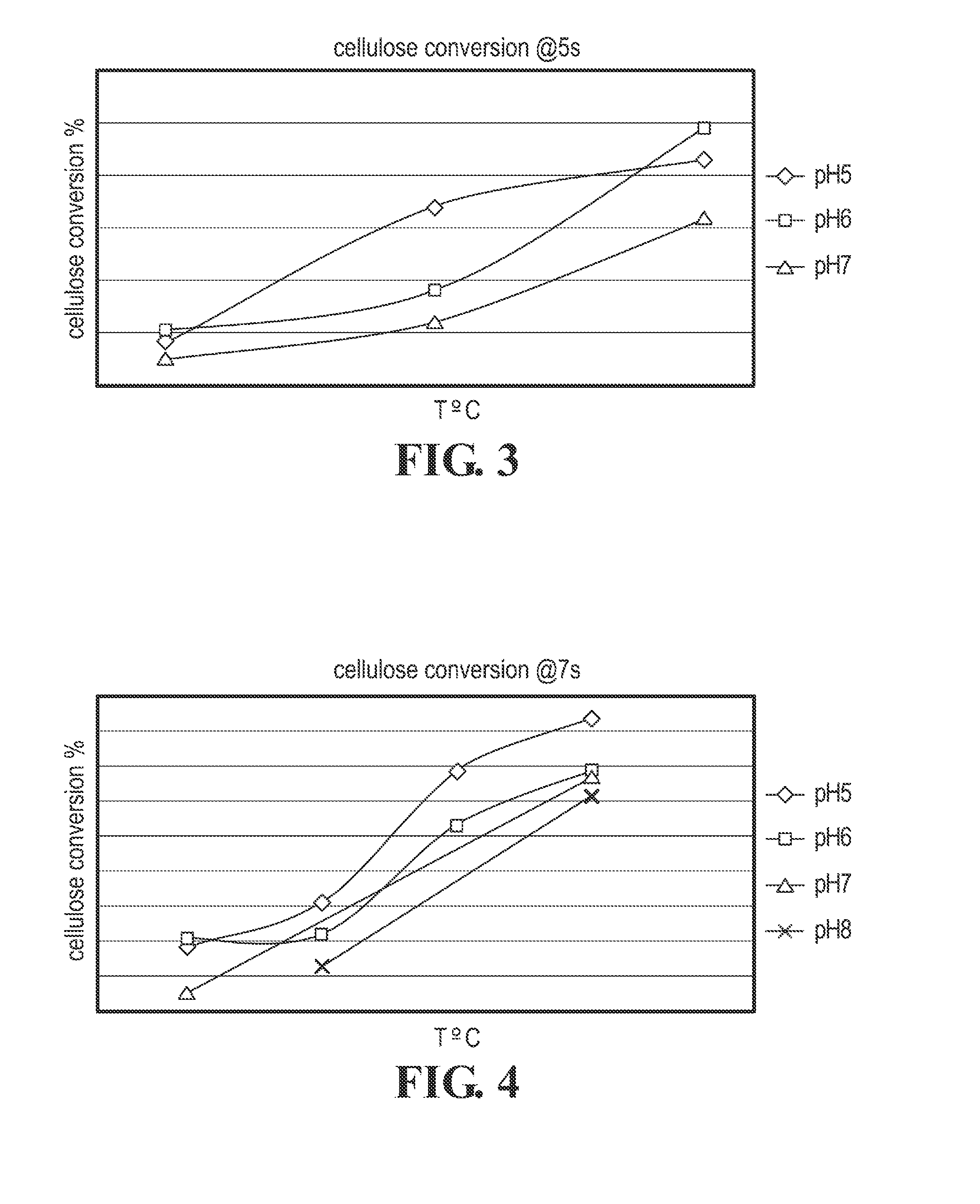
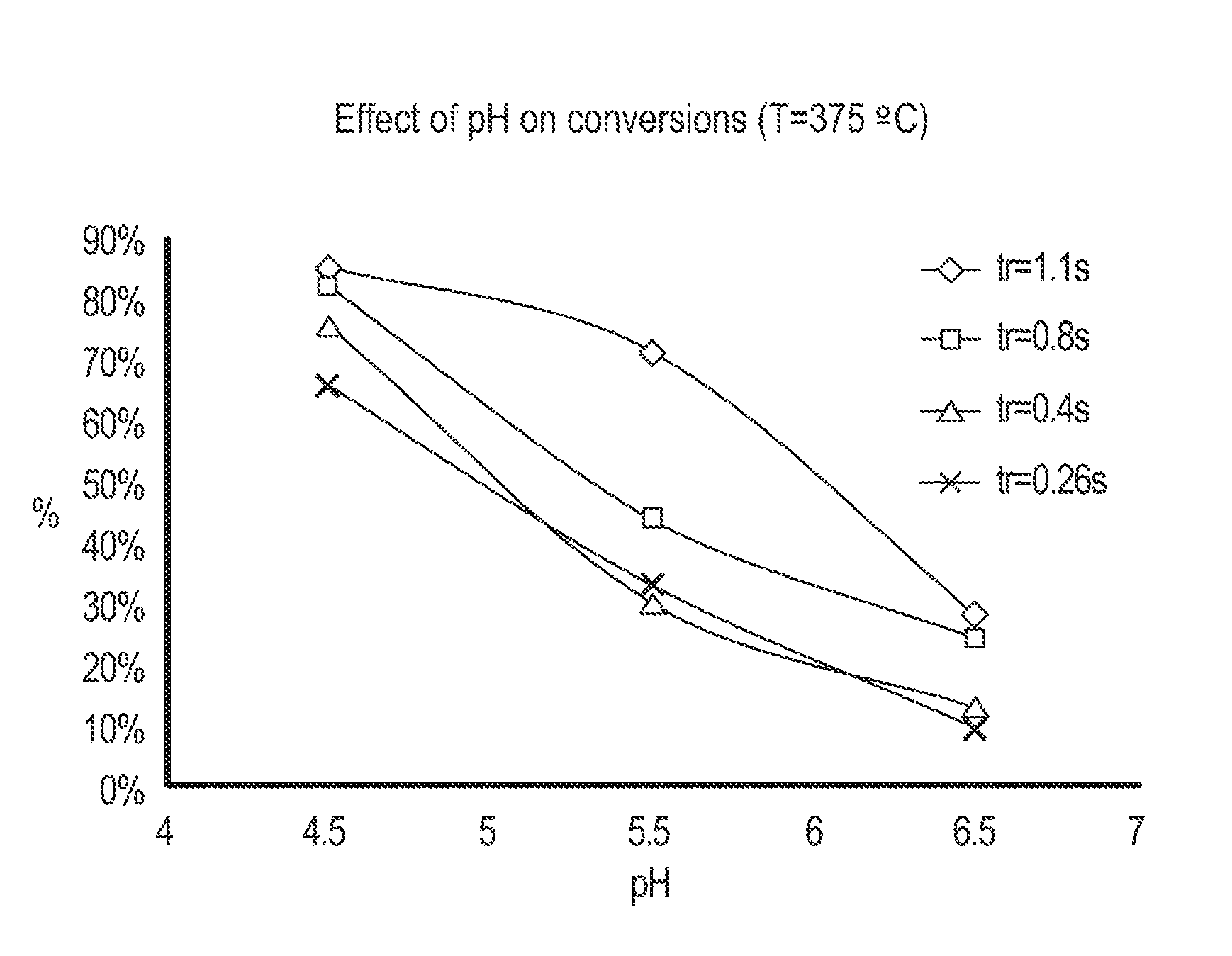
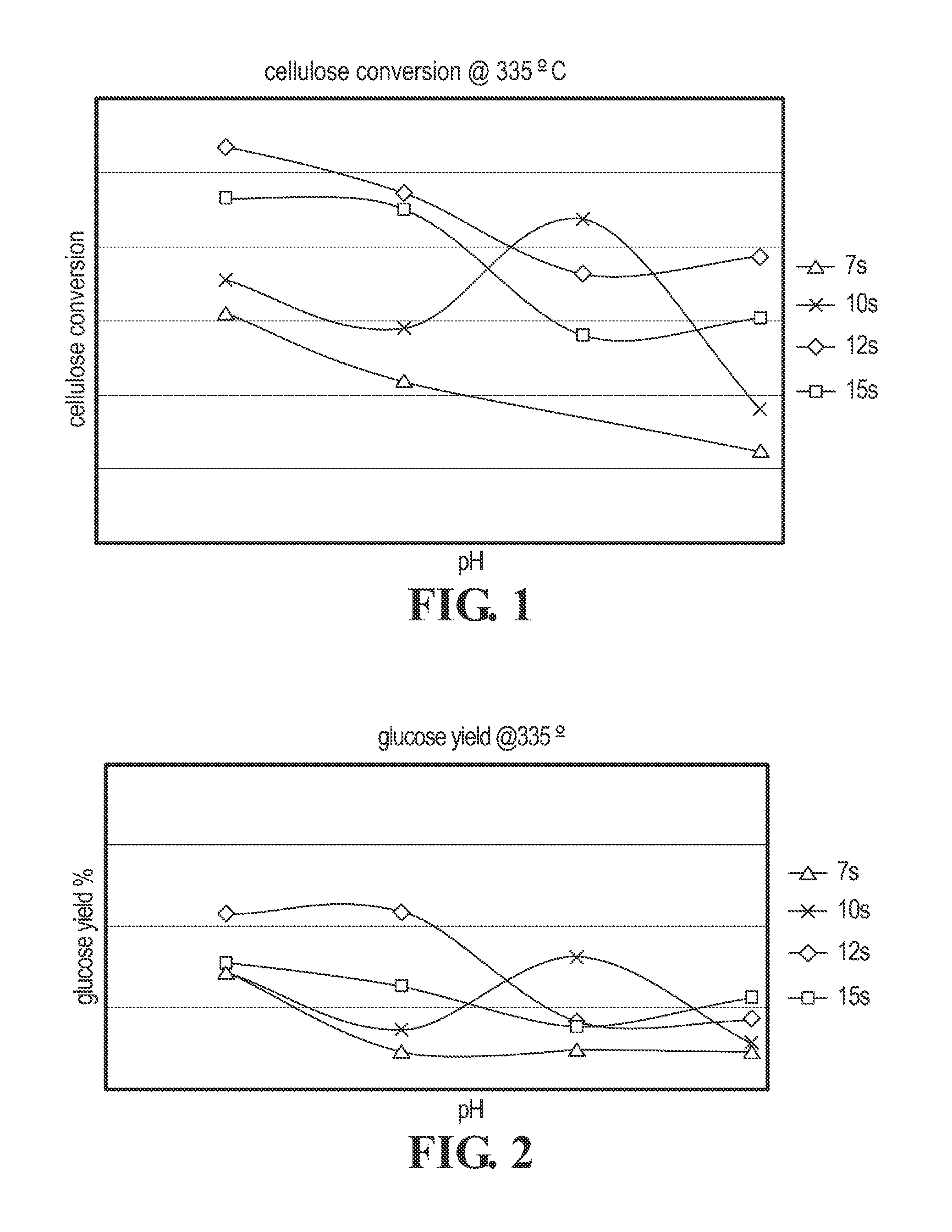
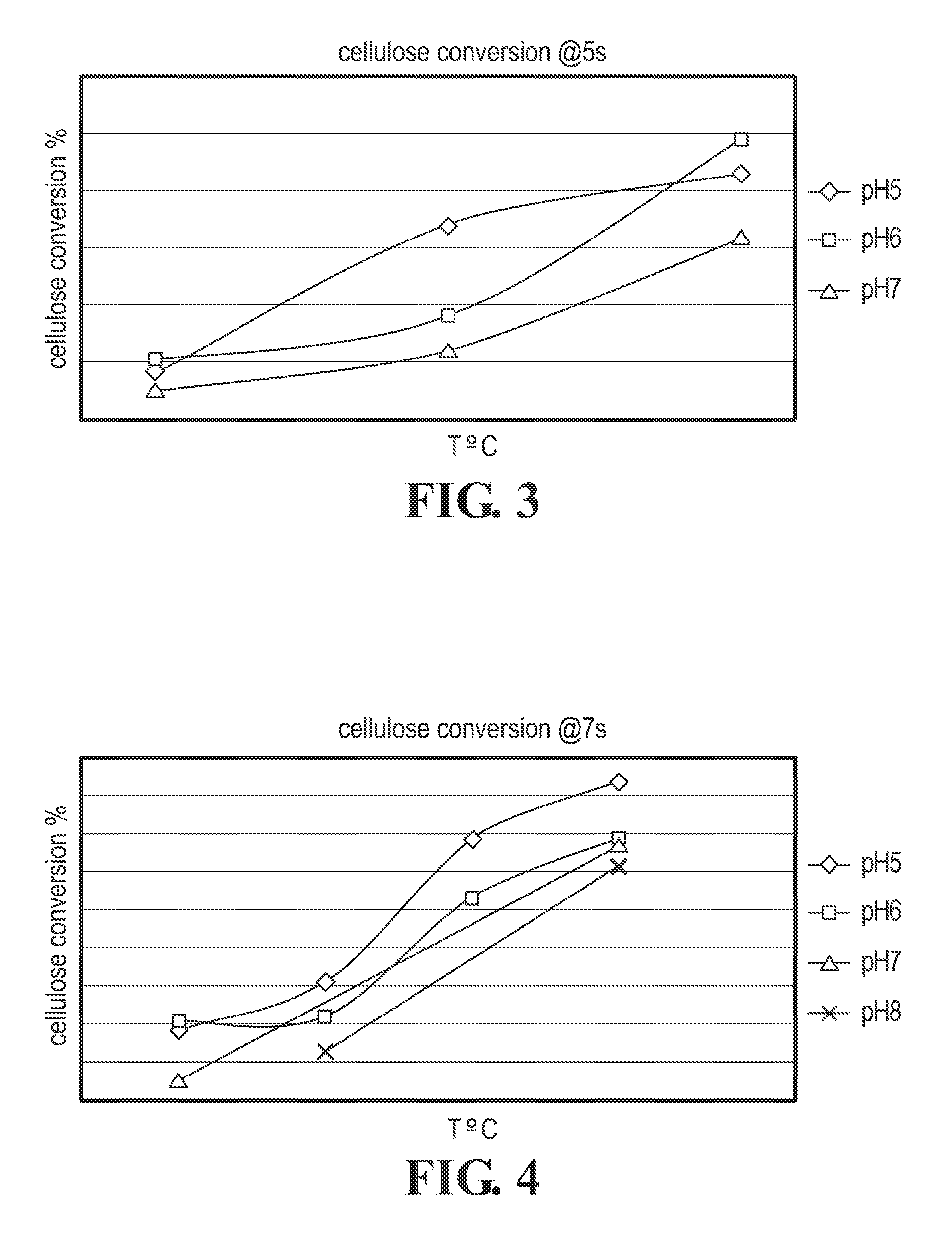
Cellulose Hydrolysis with pH Adjustment
Methods are disclosed for controlling the rate of cellulose hydrolysis and reducing the rate of glucose degradation by adjusting the pH during cellulose hydrolysis.
Owner:RENMATIX INC
Peritoneal dialysis solution and preparation method thereof
ActiveCN103432164AAvoid damageGuaranteed stabilityPharmaceutical containersBagsSodium bicarbonateHigh resistance
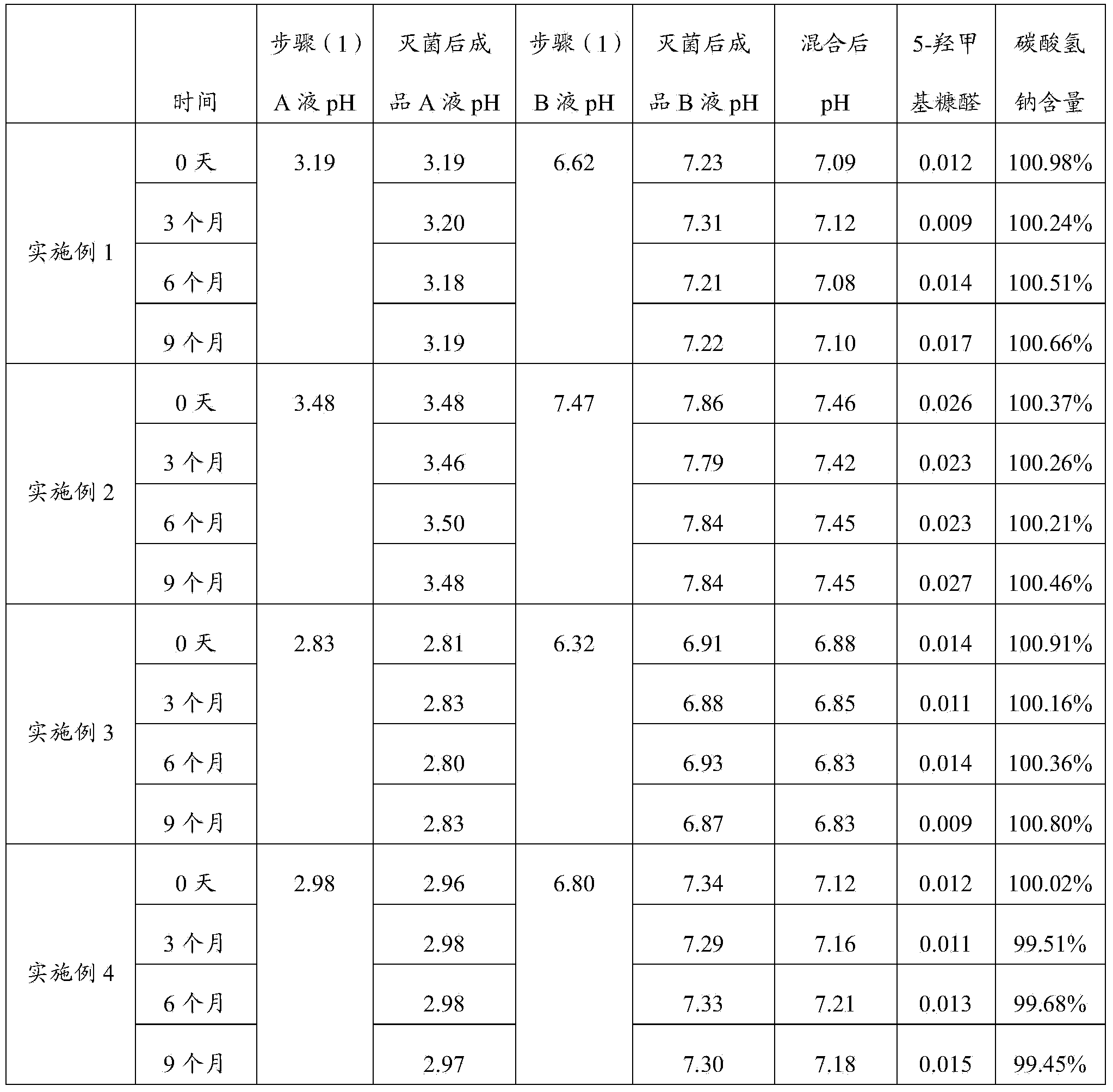
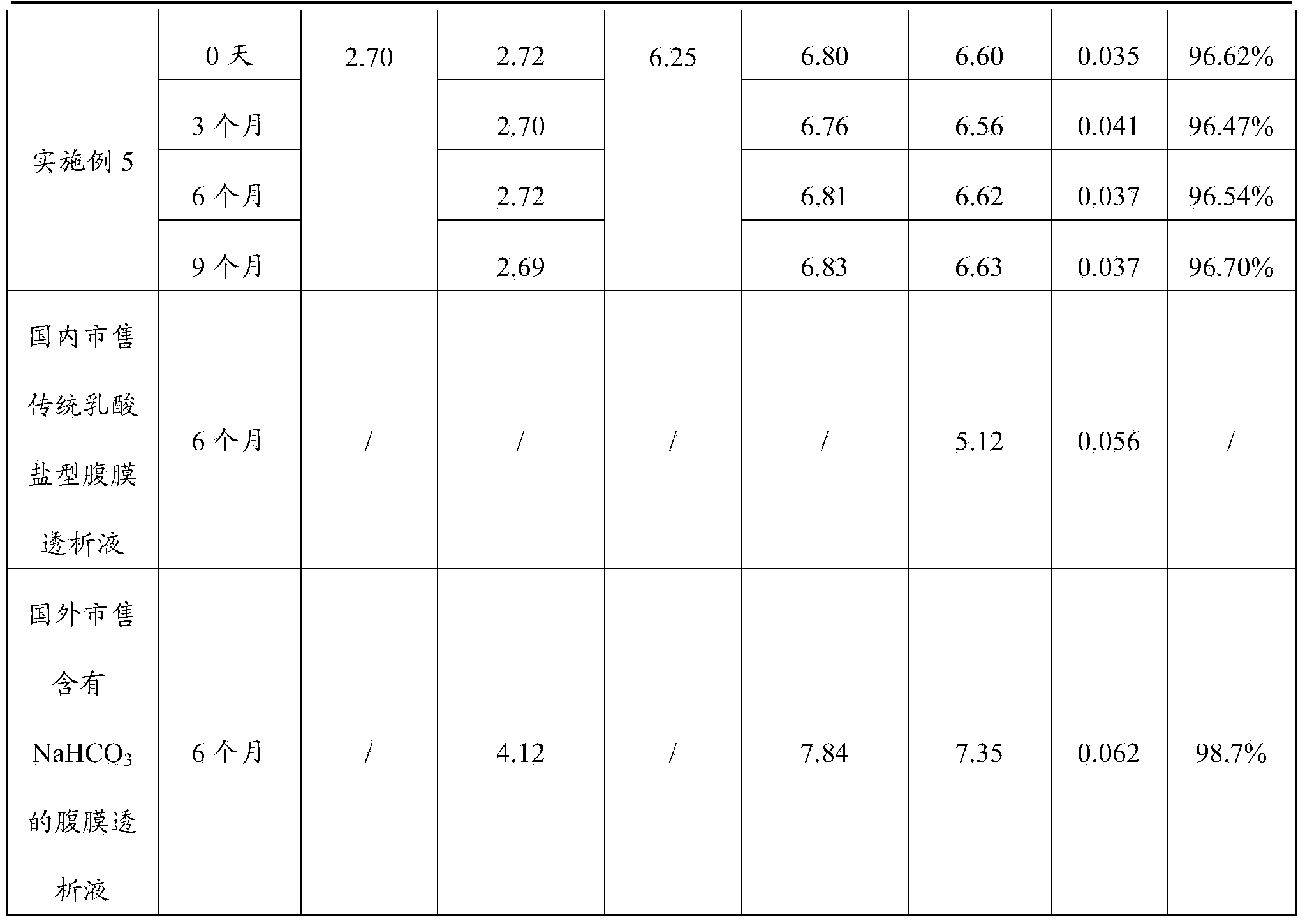
The invention discloses a peritoneal dialysis solution and a preparation method thereof. The preparation method comprises the following steps: dissolving pharmaceutically acceptable amounts of anhydrous glucose, calcium chloride and magnesium chloride into water for injection, and adjusting the pH value to 2.8-3.5 to obtain a solution A; dissolving pharmaceutically acceptable amounts of sodium chloride, sodium bicarbonate and sodium lactate into water for injection, introducing CO2, and adjusting the tank pressure to 0.05-0.2MPa and the pH value to 6.3-7.5 to obtain a solution B; and packaging by using a double-chamber bag to obtain the peritoneal dialysis solution. The peritoneal dialysis solution prepared by using the preparation method disclosed by the invention is few in degradation products of glucose and stable in quality of sodium bicarbonate; and a non-PVC (Polyvinyl Chloride) double-chamber bag and an external packaging bag with high resistance are adopted, so that the stability of sodium bicarbonate in high-temperature sterilization and storage processes can be further ensured.
Owner:HUAREN PHARMACEUTICAL CO LTD
Biomass environment-friendly non-chlorine deicing and snow-melting agent and preparation method thereof
InactiveCN109266310ALittle effect on germination rateStrong ability to melt snow and iceOther chemical processesSnow meltingMonoglyceride citrate
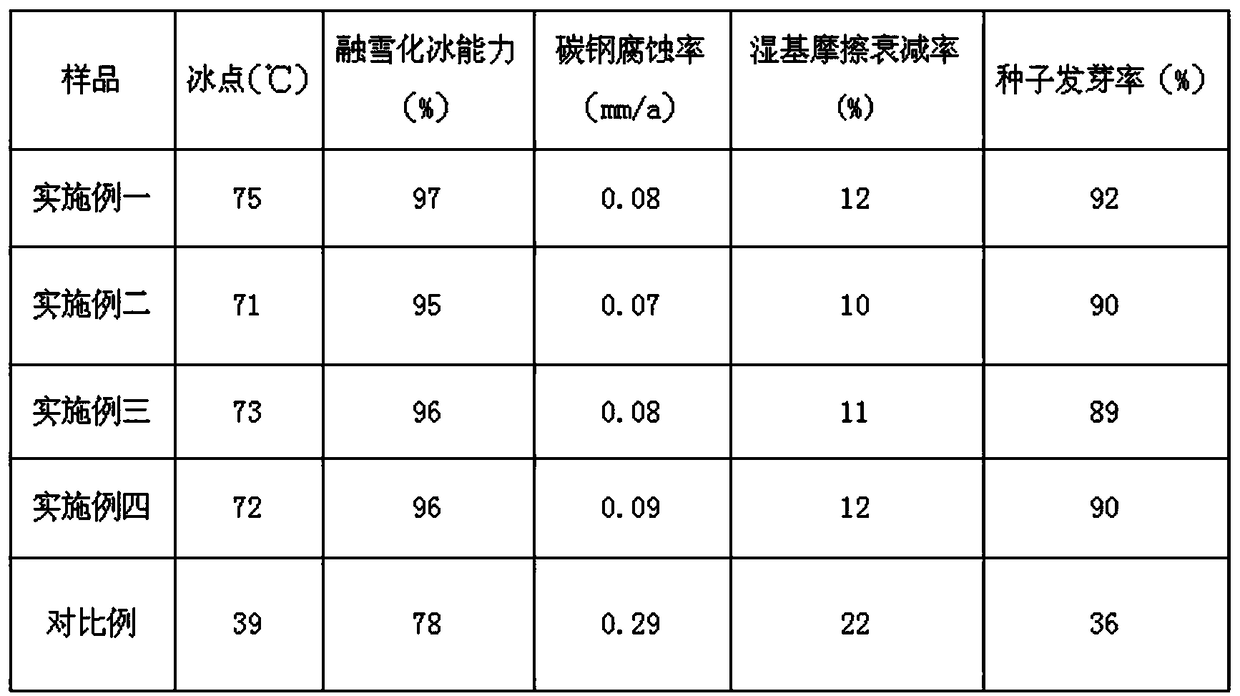
The invention belongs to the field of deicing and snow-melting agents, and discloses a biomass environment-friendly non-chlorine deicing and snow-melting agent and a preparation method thereof. The deicing and snow-melting agent is prepared from 10-30 parts of tap water, 15-35 parts of glucose degradation product, 6-20 parts of starch degradation product, 8-18 parts of saccharose, 7-18 parts of monoglyceride citrate and 2-5 parts of corrosion inhibitor; the preparation method comprises the steps that firstly, the glucose degradation product and the starch degradation product are prepared; thenthe tap water is added into a reaction kettle, the temperature rises to 60-80 DEG C, the glucose degradation product and the starch degradation product are added, and mixing reaction is carried out for 1-3 h; then the saccharose, the monoglyceride citrate and triisopropanolamine are added in sequence, and full stirring and mixing reaction is carried out for 1-2 h; and lastly, after the reactant is mixed uniformly and dissolved into one phase, cooling and discharging are carried out. The biomass environment-friendly non-chlorine deicing and snow-melting agent and the preparation method thereofhave the advantages that the obtained product has strong snow-melting and deicing abilities and a weak corrosive effect, the raw material is basically biomass, the biodegradability is high, and the biomass environment-friendly non-chlorine deicing and snow-melting agent belongs to an environment-friendly product.
Owner:黄山九星环保科技股份有限公司
Cellulose hydrolysis with pH adjustment
Methods are disclosed for controlling the rate of cellulose hydrolysis and reducing the rate of glucose degradation by adjusting the pH during cellulose hydrolysis.
Owner:RENMATIX INC
Preparation process of peritoneal dialysate with physiologic pH value
ActiveCN103446181AExtended service lifeExtended treatment timePharmaceutical containersPharmaceutical delivery mechanismSodium lactateWater baths
The invention provides a preparation process of peritoneal dialysate with a physiologic pH value. The process comprises raw material proportioning and a preparation process. The process is characterized in that the preparation process comprises the following steps: adding fresh water for injection into a proportioning container; inputting glucose, sodium chloride, calcium chloride, and magnesium chloride in prescription amount; after stirring and dissolving, adding water for injection to full dose to prepare a liquid A; adding fresh water for injection into another proportioning container; inputting sodium lactate; after stirring and dissolving, adding water for injection to full dose to prepare a liquid B; after uniformly stirring; respectively adding a pH adjustor into the liquid A and the liquid B to adjust the liquid medicine to appropriate pH values; after pre-filtering and terminal-filtering, respectively filling the liquid A and the liquid B to two chambers of a double-chamber transfusion bag in volume ratio of 1:1; plugging; sterilizing by water bath; sterilizing to obtain the peritoneal dialysate with low glucose degradation products and the physiologic pH value, wherein the pH value of the mixture of the liquid A and the liquid B reaches 6.8-7.4.
Owner:HUAREN PHARMACEUTICAL CO LTD
Lock solution for medical devices
The invention relates to a lock solution for medical devices comprising carbohydrates and / or glucose degradation products as antimicrobial agent(s).
Owner:GAMBRO LUNDIA AB
Method for preparing sodium-potassium-magnesium-calcium glucose injection
InactiveCN104622896AQuality assuranceImproving Sterility Assurance LevelsOrganic active ingredientsMetabolism disorderSodium acetatePotassium
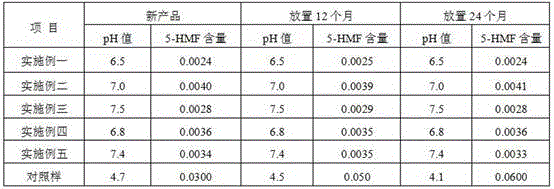
The invention discloses a method for preparing a sodium-potassium-magnesium-calcium glucose injection. The method comprises the following steps: adding water for injection into a preparation tank; sequentially adding calcium gluconate, sodium chloride, potassium chloride, magnesium chloride, glucosum anhydricum, sodium acetate and sodium citrate; dissolving, regulating the pH value to 4.5-5.5 with hydrochloric acid, filtering and filling; sterilizing for 12 minutes at 121 DEG C, inspecting with a lamp, and packaging. According to the method, after the pH value is regulated to 4.5-5.5, a durable overkilling method is carried out at 121 DEG C for 12 minutes (F0 is more than or equal to 12) for sterilizing, the content of glucose is not remarkably reduced, 5-hydroxymethylfurfural and other glucose degrading impurities are in a relatively low level, and the sterility assurance level of the sodium-potassium-magnesium-calcium glucose injection is improved.
Owner:SHANDONG QIDU PHARMA
Detection and control method of glucose degradation products in compound electrolyte injection
InactiveCN108226333AAccurate detectionEasy to detectComponent separationPhysical chemistryHydroxymethylfurfural
The invention provides a detection and control method of glucose degradation products in a compound electrolyte injection for children. The glucose degradation products is prepared from various othersubstances including 5-hydroxymethylfurfurale 5-HMF; the quality controllability of the child medicine is ensured, so that the safe medication is ensured.
Owner:青岛力腾医药科技有限公司
Process for preparing glucose and sodium chloride injection.
ActiveCN106309482AFast dissolutionReduce 5-HMF contentOrganic active ingredientsMetabolism disorderSodium acetateFiltration
The invention discloses a process for pretreating glucose and sodium chloride injection, and belongs to the technical field of injection. The method comprises the following steps: (1) weighing raw materials; (2) dissolving the raw materials, namely a) adding sodium hydrogen sulfite, magnesium chloride, calcium chloride, sodium chloride and zinc sulfate into injecting water at 30-40 DEG C; (b) adding dipotassium phosphate, glucose, fructose, xylitol and sodium acetate into the injecting water at 50-60 DEG C; and (c) mixing the solution, adding citric acid and activated carbon, insulating and adsorbing; (3) decarburizing, delivering to a diluting tank; (4) complementing injecting water, and regulating the pH value to 6.5-7.5; (5) filling bags after rough filtration and fine filtration; and (6) sterilizing at 122 DEG C for 3-5 minutes. According to the process, raw materials are dissolved in batches with different dissolving temperatures, the dissolving speed is increased, the temperature is reasonably controlled, energy consumption and cost are reduced, the solution is uniformly mixed, and generation of glucose degraded products can be effectively reduced.
Owner:HUAREN PHARMACEUTICAL CO LTD
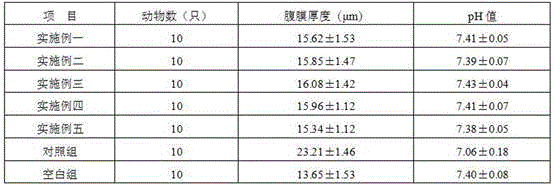
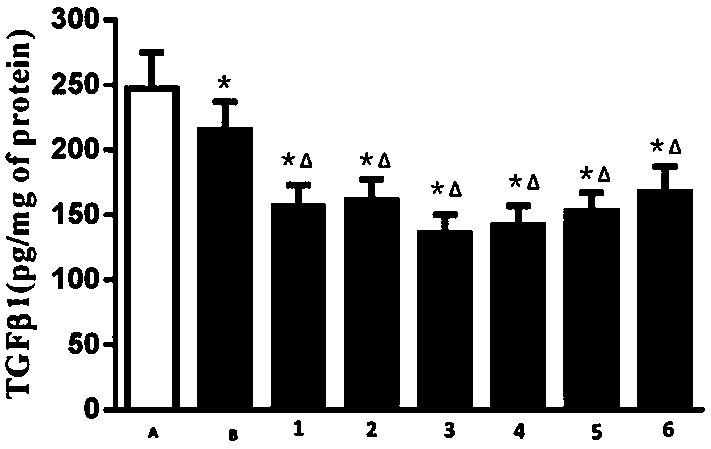
Peritoneal dialysis solution containing glucose polymer and preparation method of peritoneal dialysis solution
ActiveCN108144042AImprove stabilityGood biocompatibilityDipeptide ingredientsUrinary disorderDipeptideIrritation
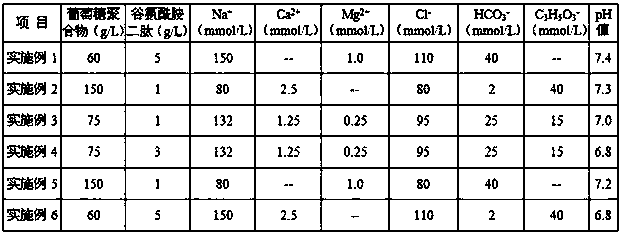
The invention discloses a peritoneal dialysis solution containing a glucose polymer and a preparation method of the peritoneal dialysis solution, and belongs to the technical field of peritoneal dialysis solutions. The peritoneal dialysis solution consists of first acid medicinal liquid and second acid medicinal liquid which are packaged in a separated mode; the first medicinal liquid consists ofthe glucose polymer; the second medicinal liquid consists of glutamine dipeptide and buffer base; and the first medicinal liquid and the second medicinal liquid, after getting mixed, consist of the following components: 60-150g / L of the glucose polymer, 1-5g / L of the glutamine dipeptide, 2-42mmol / L of the buffer base, 0-2.5mmol / L of calcium ions, 0-1.0mmol / L of magnesium ions and 80-110mmol / L of chloride ions. According to the peritoneal dialysis solution provided by the invention, the glucose polymer is kept in an environment that pH value is relatively low, so that the generation of glucosedegradation products is reduced and the bio-incompatibility of the glucose polymer to a peritoneum is obviously improved; and the pH value is kept within a physiological range as the first medicinal liquid and the second medicinal liquid get mixed, so that local irritation of the acid peritoneal dialysis solution to the peritoneum is avoided.
Owner:HUAREN PHARMACEUTICAL CO LTD
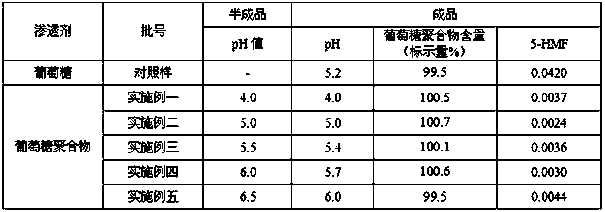
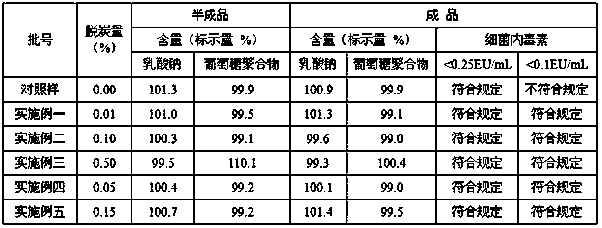
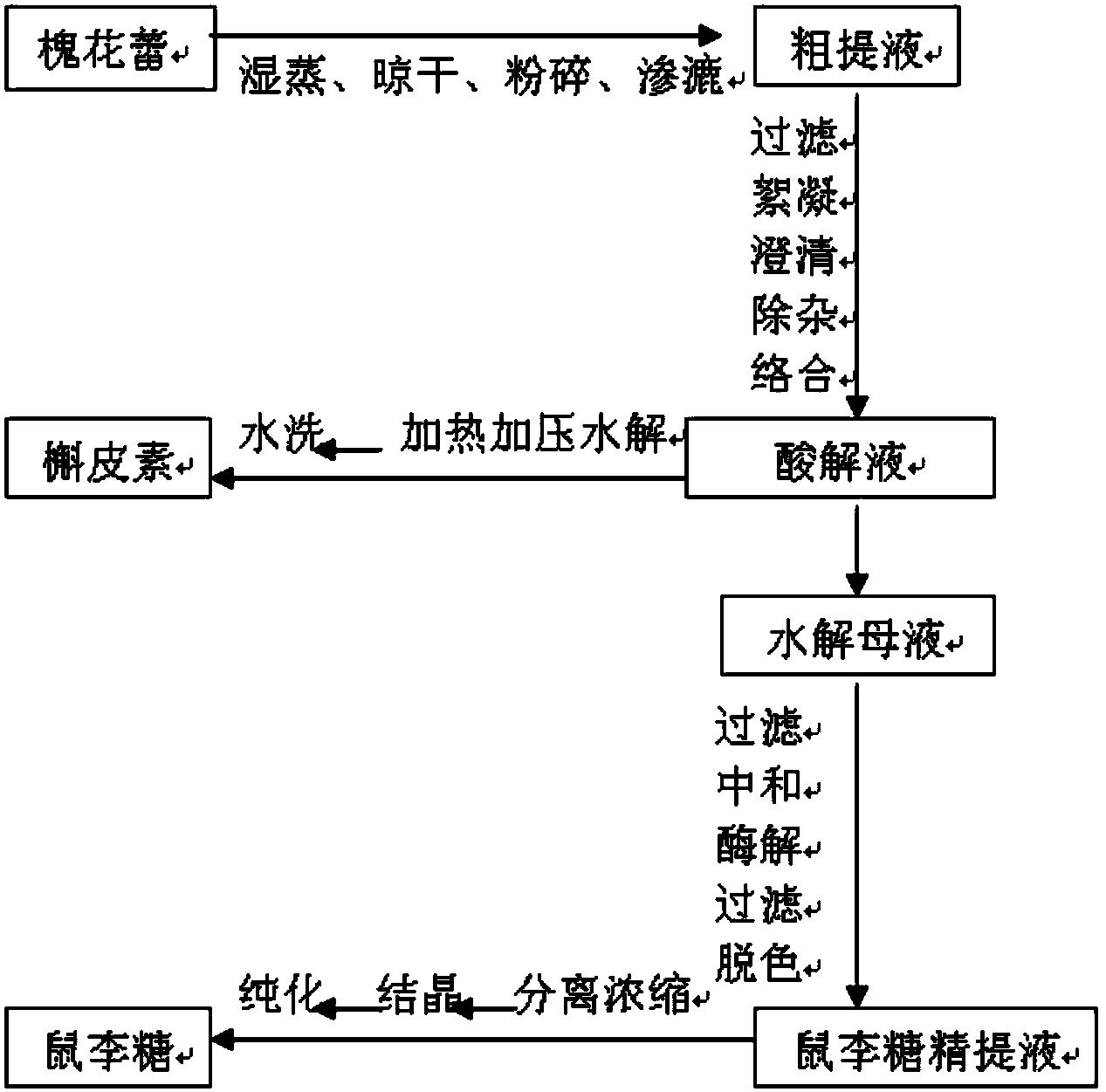
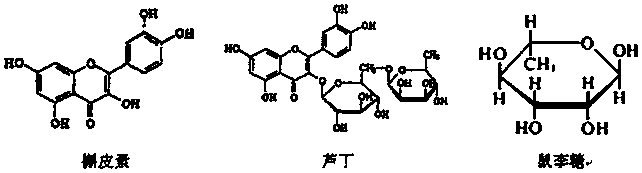
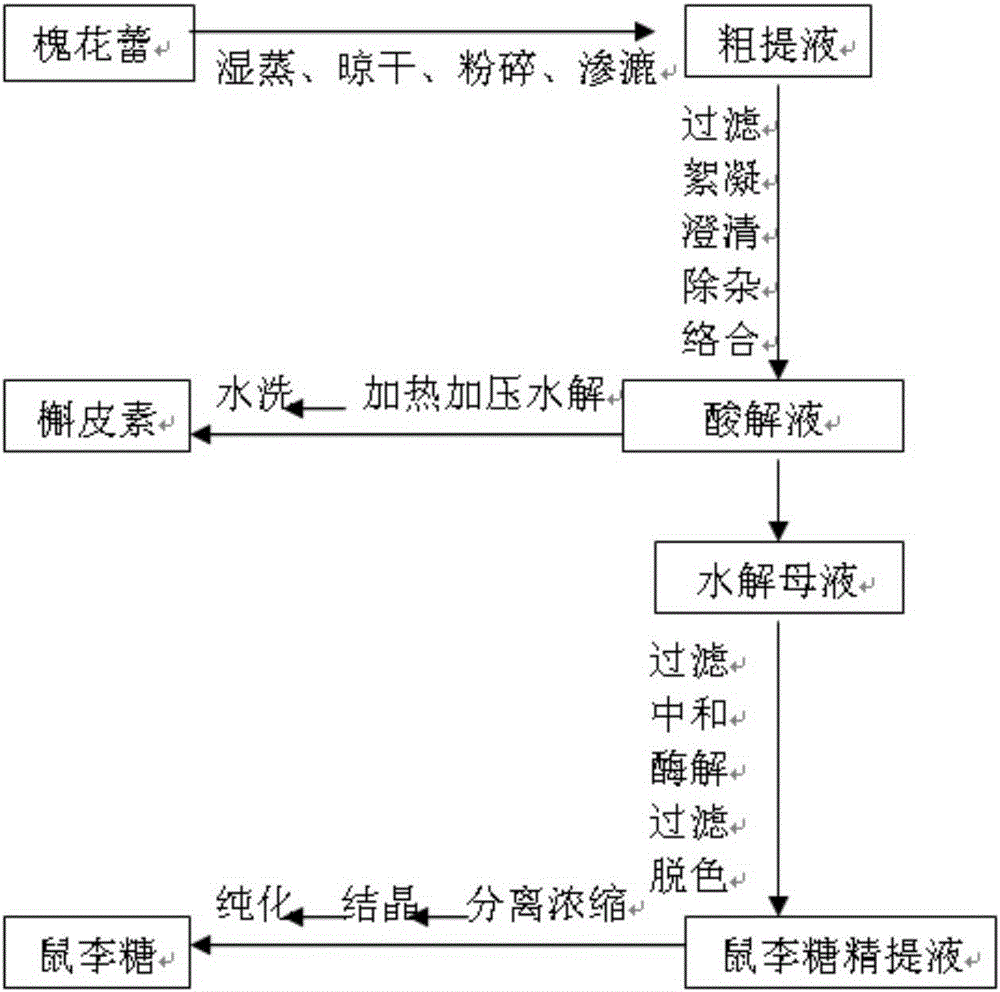
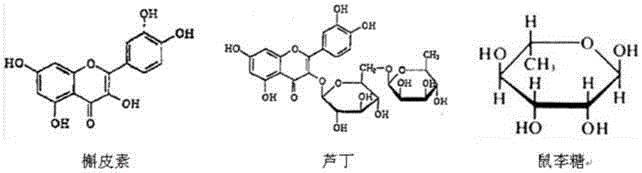
Method for preparing rhamnose by using rutin
InactiveCN103937854AShort processReduce manufacturing costOrganic chemistryFermentationFiltrationRutin
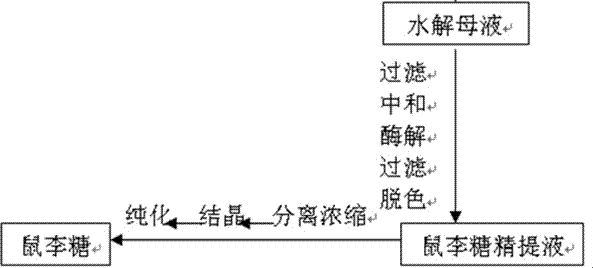
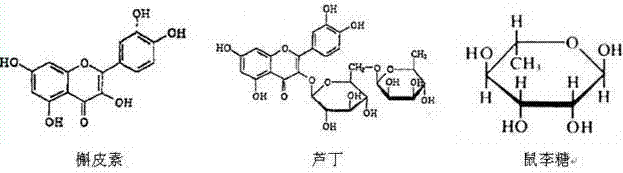
The invention belongs to the field of extraction of flavonoid compounds, and in particular relates to a method for preparing rhamnose by using rutin. The method comprises the steps of extracting quercetin from collected sophora flower bud, producing hydrolyzed mother liquor, performing filtration, impurity removal and neutralizing treatment on the hydrolyzed mother liquor, degrading glucose by using glucose degrading enzyme, filtering and separating a solution, and then performing decolorization, vacuum centrifugal concentration and crystallization to obtain a product namely rhamnose. In the technological process, the rutin hydrolyzed mother liquor is subjected to comprehensive treatment at the same time, and is used for producing rhamnose; the hydrolyzed mother liquor from which quercetin is separated out is recovered, so that the production cost of the product is reduced, the possibilities of causing environment pollution are avoided, and the environment-friendly and energy-saving effects of the production are realized.
Owner:徐大鹏
Glucose polymer peritoneal dialysis solution and preparation process thereof
InactiveCN107550928AAvoid damageImprove ultrafiltration effectAluminium/calcium/magnesium active ingredientsAnhydride/acid/halide active ingredientsThermal insulationUltrafiltration
The invention discloses a glucose polymer peritoneal dialysis solution and a preparation process of the glucose polymer peritoneal dialysis solution, belonging to the technical field of peritoneal dialysis. The glucose polymer peritoneal dialysis solution is prepared from the following components in parts by weight: 50-150 g / L of glucose polymer, 80-150 mEq / L of sodium ions, 80-110 mEq / L of chloride ions, 0-4.0 mEq / L of calcium ions, 0-4.0 mEq / L of magnesium ions, 10-45 mEq / L of lactate radical ions, and an appropriate amount of water for injection. The components are mixed and dissolved, active carbon is added, thermal insulation and adsorption are carried out, decarburization and filtering are carried out, the pH value is adjusted, filling is carried out, opening sealing is carried out,sterilization is carried out, and thus the product is obtained. Compared with the traditional peritoneal dialysis solution, the product provided by the invention has the advantage that little glucosedegradation products, especially 5- hydroxymethylfurfural, are generated, and injuries caused by the product to the peritoneum during clinical use of the product are reduced; meanwhile, the stabilityof the molecular weight of the glucose polymer is guaranteed, and the effective ultrafiltration during the long abdomen indwelling period is realized.
Owner:HUAREN PHARMACEUTICAL CO LTD
Method for preparing quercetin and rhamnose through rutin
InactiveCN103965153AAvoid pollutionRealize the effect of environmental protection and energy savingSugar derivativesSugar derivatives preparationFiltrationBud
The invention belongs to the field of flavonoids compound extraction, and particularly relates to a method for preparing quercetin and rhamnose through rutin. The preparation method comprises the following steps: steaming collected sophora flower buds through steam, air-drying, smashing and seeping the steamed sophora flower buds, removing colloid and chlorophyll impurities through flocculation, performing protection complexing treatment through an antioxidant and a complexing agent, adding a mixed acid to adjust the mixture to be acidic, and heating, pressurizing and hydrolyzing to obtain the quercetin; performing impurity filtration and neutralization on hydrolysis mother liquor subjected to quercetin extraction, degrading glucose through glucose degrading enzyme, and performing decoloring vacuum centrifugal concentration and crystallization after a solution is filtered and separated to obtain a product, namely the rhamnose. Under detection of HPLC (high performance liquid chromatography), the content of the quercetin is over 95 percent; all impurity requirements meet an international standard requirement; in a technical process, rutin hydrolysis mother liquor is also subjected to comprehensive treatment, and rhamnose is obtained through production; the production cost of the production is lowered, and the environmental pollution is alleviated.
Owner:罗俊
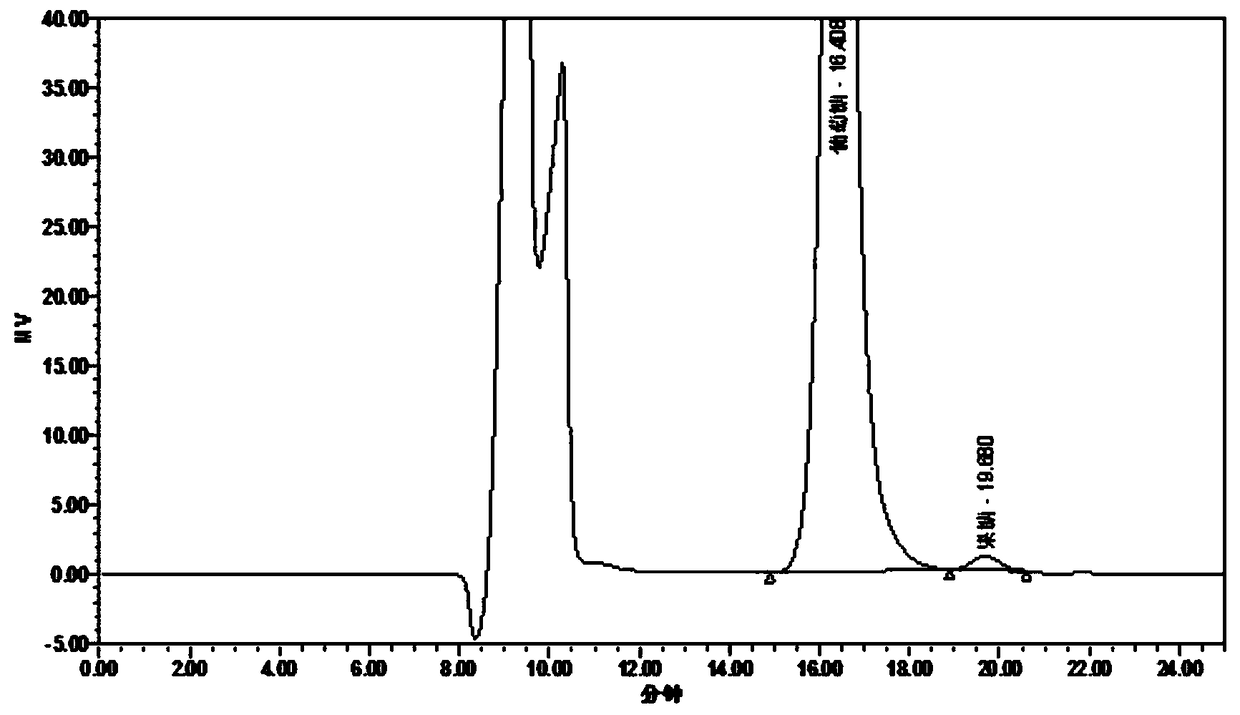
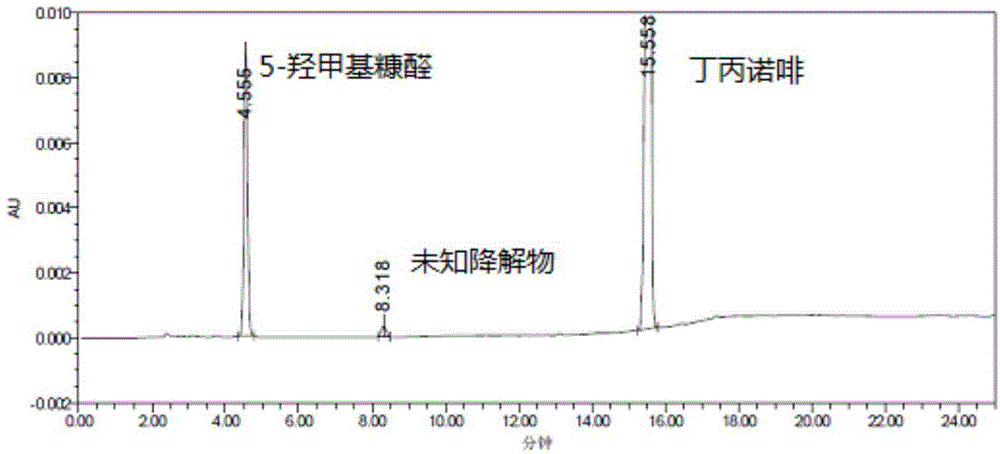
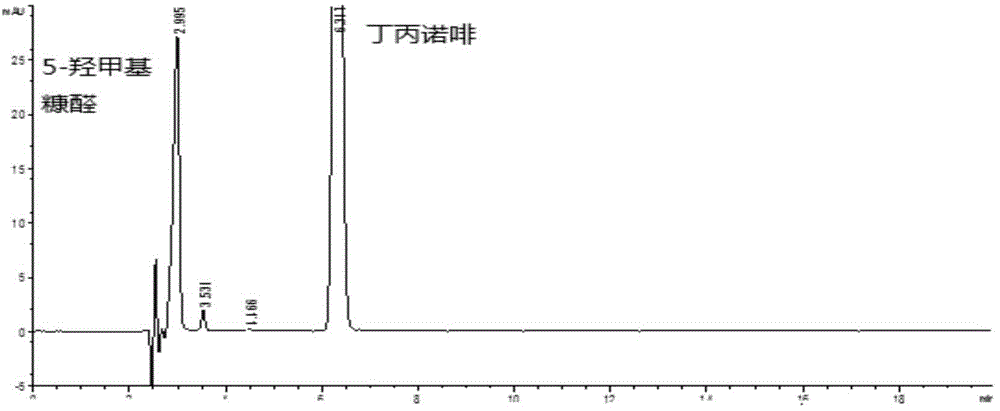
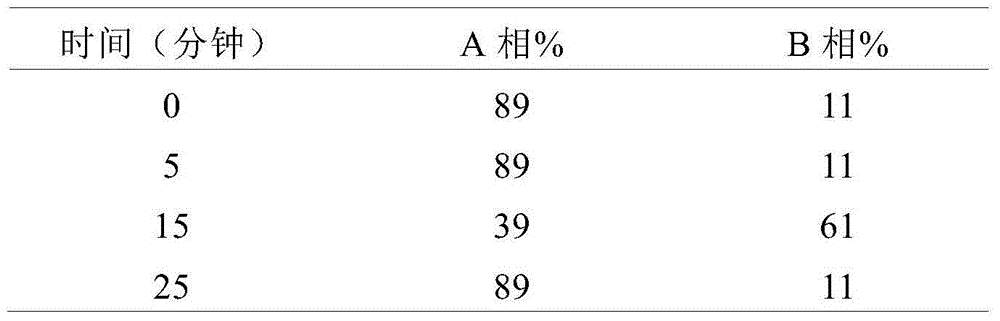
Method of detecting glucose degradation products in buprenorphine hydrochloride injection
The invention relates to a method of detecting glucose degradation products in a buprenorphine hydrochloride injection. The analysis method is mainly used for detecting the glucose degradation products except 5-hydroxymethyl furfural and can effectively separate the chromatographic peaks of the 5-hydroxymethyl furfural, unknown glucose degradation products and buprenorphine in the buprenorphine hydrochloride injection, and especially, completely separate the 5-hydroxymethyl furfural from the unknown glucose degradation products. The method has strong specificity and can control the contents of the 5-hydroxymethyl furfural and the unknown glucose degradation products accurately.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
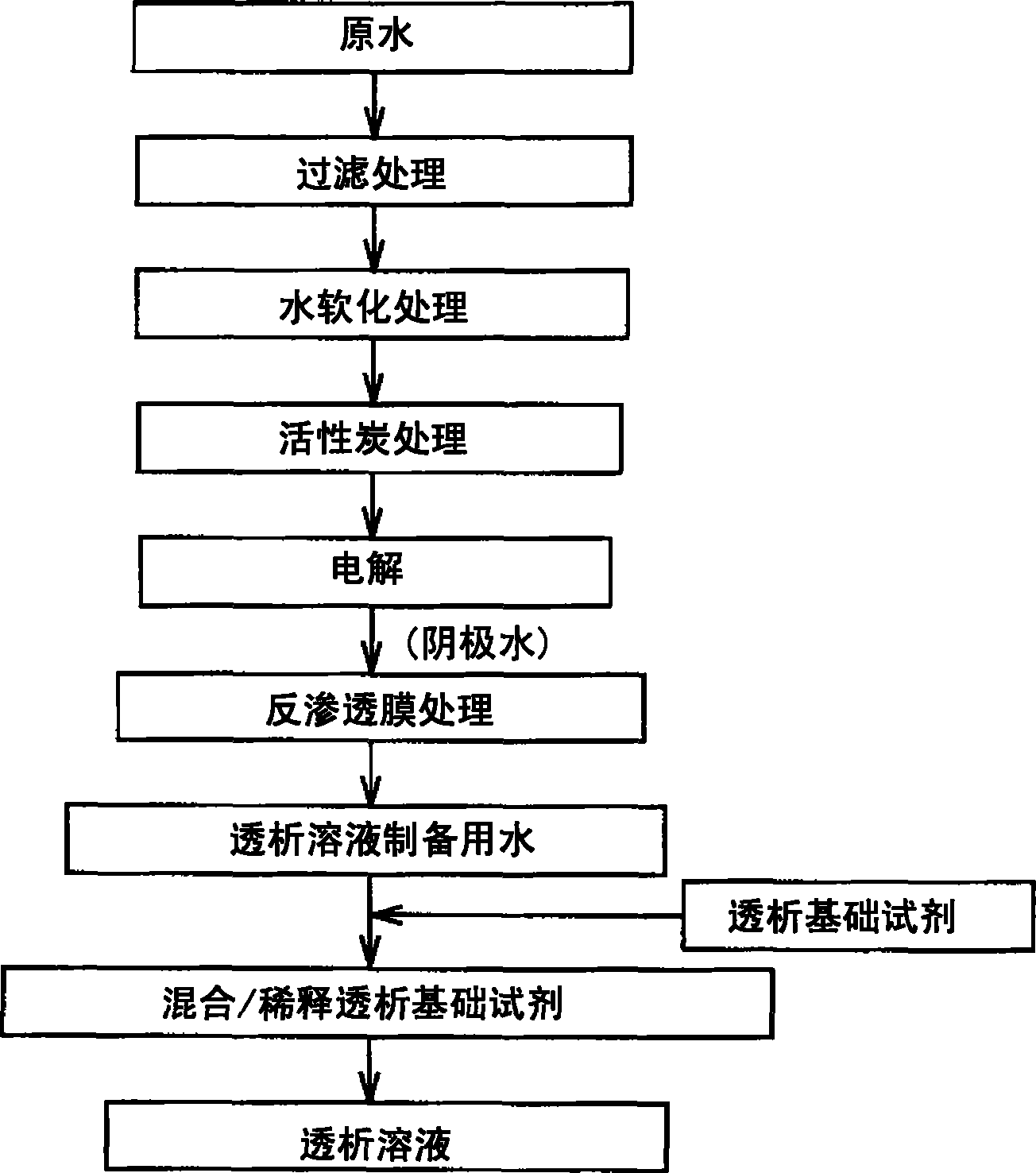
Water for preparing dialysate and dialysate and method of producing dialysate using the same, and dialyzer
ActiveCN101522231AReduce adverse effectsOrganic active ingredientsInorganic active ingredientsBiological bodyDecomposition
The invention provides a water for preparing a dialysate which is a water having a dissolved hydrogen concentration of from 50 to 600 ppb and a pH value of from 7 to 10 and satisfying the water quality standards as specified in ISO 13959 and to be used in preparing a dialysate by diluting a stock agent for a dialysate containing at least 50 ng / mL of glucose decomposition products; a method of preparing a dialysate by diluting a stock agent for a dialysate by using the above water; and the dialysate thus obtained. It is possible to provide a dialysate by which undesirable effects of glucose decomposition products on the living body can be inhibited, a water for preparing a dialysate to be used therefore, a method of producing a dialysate and a dialyzer can be provided by using a dialyzer having a means of supplying a water for preparing a dialysate, which is a water having a dissolved hydrogen concentration of from 50 to 600 ppb and a pH value of from 7 to 10 and satisfying the water quality standards as specified in ISO 13959, a means of storing a stock agent for a dialysate containing at least 50 ng / mL of glucose decomposition products, and a means of preparing a dialysate by diluting the stock agent for a dialysate with the water for preparing a dialysate.
Owner:NIKHON TRIM KO LTD
Dual-chamber bag for peritoneal dialysis
InactiveCN106821748AAchieving Physiological pHReduce generationPharmaceutical containersMedical packagingSolderingBuffering agent
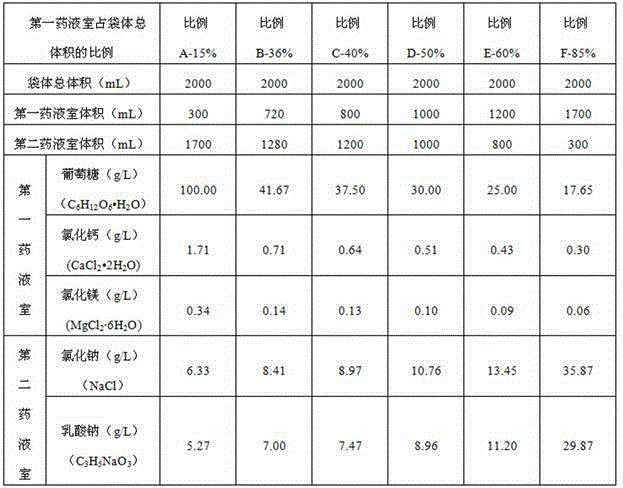
The invention discloses a dual-chamber bag for peritoneal dialysis, and belongs to the technical field of peritoneal dialysis. The dual-chamber bag for the peritoneal dialysis comprises a bag body, a first medicine liquid chamber and a second medicine liquid chamber which are provisionally partitioned are formed in the bag body, a penetrating agent is arranged in the first medicine liquid chamber, and the volume of the first medicine liquid chamber accounts for 15% to 40% of the total volume of the bag body; a buffering agent is arranged in the second medicine liquid chamber, and the volume of the second medicine liquid chamber accounts for 60% to 85% of the total of the bag body. According to the dual-chamber bag for the peritoneal dialysis, the volume and the ratio of the first medicine liquid chamber and the volume and the ratio of the second medicine liquid chamber are scientifically designed, medicine liquid with the penetrating agent is located in the medicine liquid chamber with the small volume, the pH value is 2.5 to 4.0, small generation of glucose degradation products in the low-pH-value environment is achieved, and the physiological pH after the two medicine liquid chambers are mixed is also achieved; the large medicine liquid chamber and the small medicine liquid chamber are reasonable in design, energizing is easy, pseudo soldering is easy to break, and using is convenient; folding of the bag body is convenient, pseudo soldering is protected, and the product scraping phenomenon generated by pseudo soldering breaking in the transportation process and the placing process of products is avoided.
Owner:HUAREN PHARMACEUTICAL CO LTD
Nicotine selectively degrading bacterium construction method and application in waste tobacco water extract of nicotine selectively degrading bacteria
ActiveCN108531501AWater contaminantsVector-based foreign material introductionEscherichia coliInformation analysis
The invention discloses a nicotine selectively degrading bacterium construction method and application of nicotine selectively degrading bacteria in a waste tobacco water extract. Pseudomonas sp.JY-Qis used as an original bacterial strain, through whole genome information analysis of the Pseudomonas sp.JY-Q, the following five target genes are determined, knockout vectors of the target genes areconstructed, by means of a thermal transferring method, the knockout vectors are transferred into an escherichia coli deleted bacterial strain WM3064, and by means of Kanamycin resistance genes on thevectors, recons of first exchange are screened out; after the bacterial strain is subjected to overnight propagation, by means of anti-screen marker SacB genes carried by the vectors, recons, namelythe target genes, of secondary exchange fall off along with plasmids which are integrated into a genome to achieve seamless gene knockout until all the target genes are knocked out. The phenotypic difference between the bacterial strain obtained through the method and a wild type JY-Q mainly comprises growth difference and nicotine and glucose degrading difference.
Owner:ZHEJIANG UNIV OF TECH
Method for preparing quercetin and rhamnose by using rutin
InactiveCN103965153BShort processReduce manufacturing costSugar derivativesFermentationFiltrationBud
The invention belongs to the field of flavonoids compound extraction, and particularly relates to a method for preparing quercetin and rhamnose through rutin. The preparation method comprises the following steps: steaming collected sophora flower buds through steam, air-drying, smashing and seeping the steamed sophora flower buds, removing colloid and chlorophyll impurities through flocculation, performing protection complexing treatment through an antioxidant and a complexing agent, adding a mixed acid to adjust the mixture to be acidic, and heating, pressurizing and hydrolyzing to obtain the quercetin; performing impurity filtration and neutralization on hydrolysis mother liquor subjected to quercetin extraction, degrading glucose through glucose degrading enzyme, and performing decoloring vacuum centrifugal concentration and crystallization after a solution is filtered and separated to obtain a product, namely the rhamnose. Under detection of HPLC (high performance liquid chromatography), the content of the quercetin is over 95 percent; all impurity requirements meet an international standard requirement; in a technical process, rutin hydrolysis mother liquor is also subjected to comprehensive treatment, and rhamnose is obtained through production; the production cost of the production is lowered, and the environmental pollution is alleviated.
Owner:罗俊
Sugar-free fruit product with fortified calcium and zinc nutrition, and production technology thereof
InactiveCN107334036AHigh sweetnessUnparalleled nutritional valueAnimal feeding stuffAccessory food factorsFructoseSucrose
The invention relates to a sugar-free fruit product, and is characterized in that inherent sucrose in the fruit product is decomposed into fructose and glucose; moreover, the glucose produced by decomposition of the sucrose and inherent glucose in the fruit product are further converted into calcium gluconate and / or zinc gluconate. The sugar-free fruit product disclosed by the invention is free of any addition of glucose and sucrose; moreover, inherent sucrose and glucose in the fruits are converted into calcium gluconate and / or zinc gluconate which are high in nutritional values, and thus, the sugar-free fruit product has relatively high health-care effects and nutritional values.
Owner:山东优杰姆食品科技有限公司
A kind of extraction technology of peach gum polysaccharide and its application
ActiveCN105061617BHigh purityThe preparation method is fineMetabolism disorderGlucose degradationGlycoside
The invention discloses an extraction process of peach gum polysaccharide and its application. The present invention adopts steps such as water extraction and alcohol precipitation, Sevage reagent to remove protein, dialysis, and DEAE cellulose as filler to pass through the column to prepare finer polysaccharide PGPSD. The polysaccharide PGPSD is soluble in water at normal temperature, and is connected by arabinose, mannose and galactose through glycosidic bonds. The peach gum polysaccharide PGPSD prepared by the invention can enhance the expression of insulin-related transcription factors and glucose degradation key enzymes, has no toxic effect on mice, and can significantly reduce the blood sugar of diabetic mice.
Owner:HUAZHONG AGRI UNIV
A lock solution for medical devices
InactiveCN1905906AReduce adhesionReduce proliferationPharmaceutical containersMedical packagingMedical deviceGlucose degradation
The present invention relates to a lock solution for medical devices comprising carbohydrate and / or glucose degradation products as antimicrobial agents.
Owner:GAMBRO LUNDIA AB
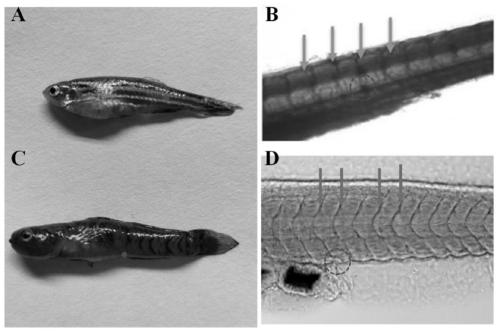
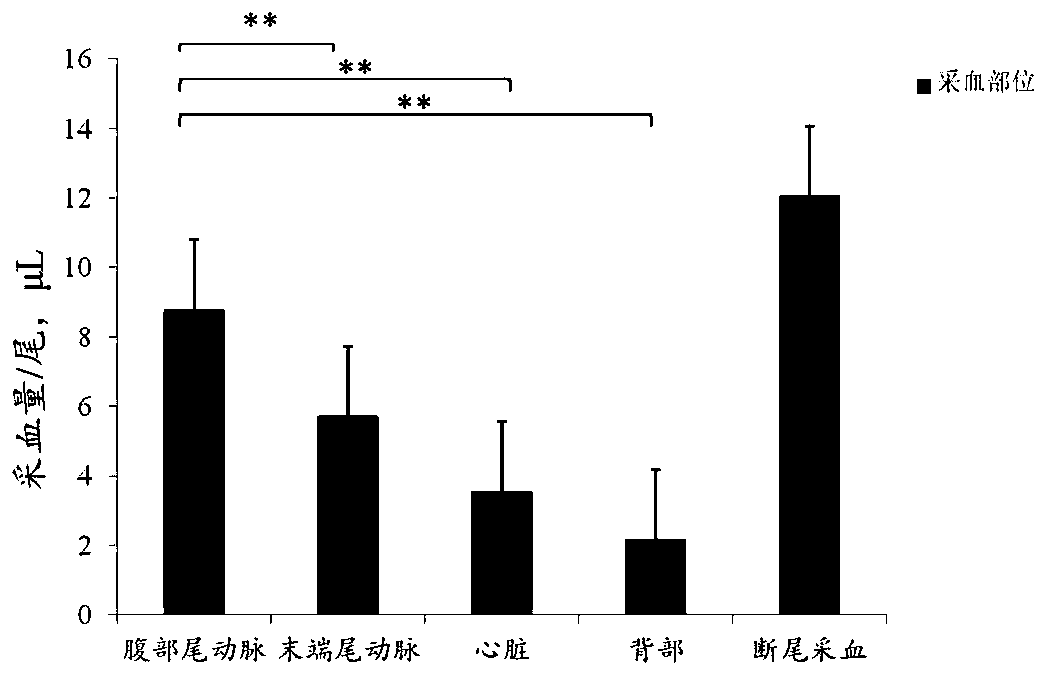
Method for collecting blood of small-size experimental fish
ActiveCN111096755ABlood volume expansionAvoid pollutionClimate change adaptationPharmaceutical containersBlood collectionHemolysis
The invention discloses a method for collecting blood of a small-size experimental fish. According to the method, an optimal blood collection position of mugilogobius chulae is confirmed through experiments such as early-stage blood vessel coloring, a capillary pipet which is applicable to rapid blood collection of the rimental fish within an appropriate range is prepared, and thus hemolysis and bacterial pollution can be effectively prevented. Meanwhile, the invention provides a solution formula which is particularly applicable to blood collection of a small-size seawater fish. By adopting the solution formula, platelet aggregation and thrombosis can be effectively prevented, an anticoagulation function can be achieved, blood glucose degradation can be well inhibited, and accuracy of sugar tolerance experiments can be ensured. On premise that survival of a model fish is ensured, the positive rate of a pancreas fat infiltration model can be evaluated through a method with the combination of sugar tolerance experiments and growth and blood indexes, and practical significances can be achieved.
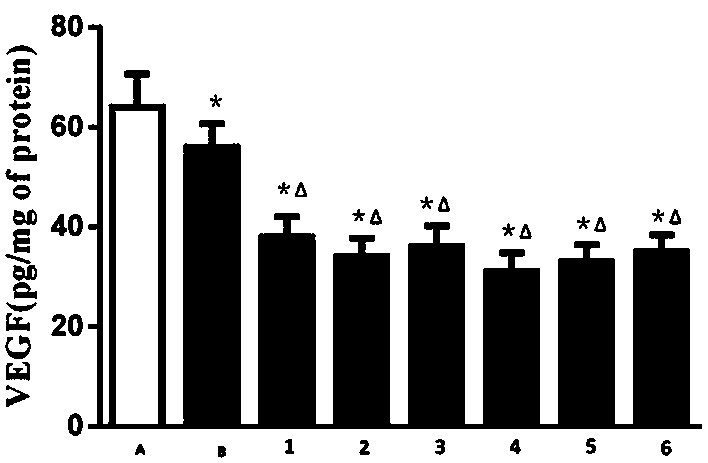
Owner:GUANGDONG LAB ANIMALS MONITORING INST
A lock solution for medical devices and its uses
InactiveCN100574813CReduce adhesionReduce proliferationPharmaceutical containersMedical packagingMedical deviceGlucose degradation
Owner:GAMBRO LUNDIA AB
Method for detecting blood glucose concentration in real time in in-vitro blood glucose degradation process
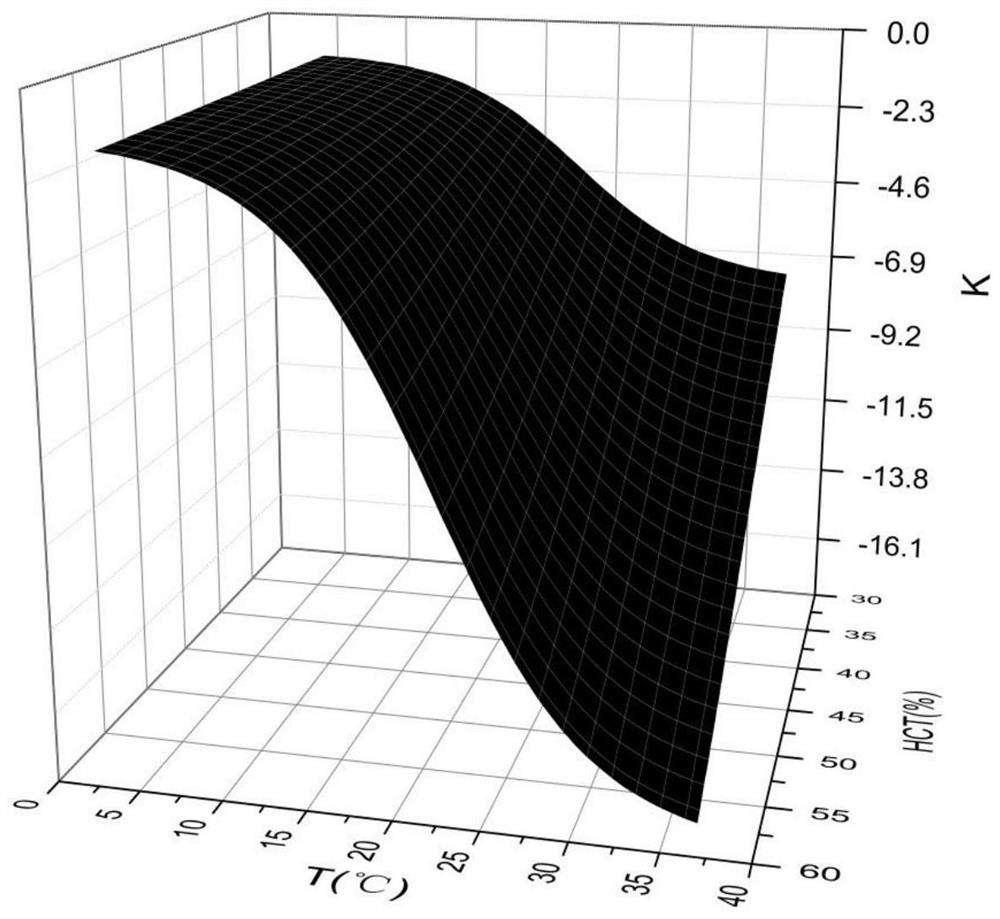
PendingCN114235906AEasy to operateReliable Calculation MethodMaterial electrochemical variablesRed blood cellHematological test
A method for detecting blood glucose concentration in real time in an in-vitro blood glucose degradation process comprises the following steps: step 1, acquiring hematocrit HCT parameters in an in-vitro blood sample, and then adjusting the hematocrit HCT parameters to be within a preset range; 2, acquiring the initial blood glucose concentration C < initial > of the in-vitro blood sample treated in the step 1; and step 3, setting the temperature T of the sample chamber, with the unit being Celsius degree, and calculating the blood glucose degradation rate K through an empirical formula according to the adjusted hematocrit HCT parameter, the initial blood glucose concentration C initial and the temperature T. 4, degrading the in-vitro blood sample for a period of time t; 4 < = Tlt; when 17, 0 < = tlt; 1; 4 < = Tlt; 17, and t > = 1; 17 < = T < = 37; if T and t are in different ranges, corresponding formulas are selected to calculate the real-time blood glucose concentration C < real > of the in-vitro blood sample.
Owner:江苏鱼跃凯立特生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com