Detection and control method of glucose degradation products in compound electrolyte injection
A technology for compound electrolytes and degradation products is applied in the field of detection and control of glucose degradation products in electrolyte injections, which can solve problems such as influence of drug quality control, toxic and side effects, and achieve the effects of ensuring safe medication and controllability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
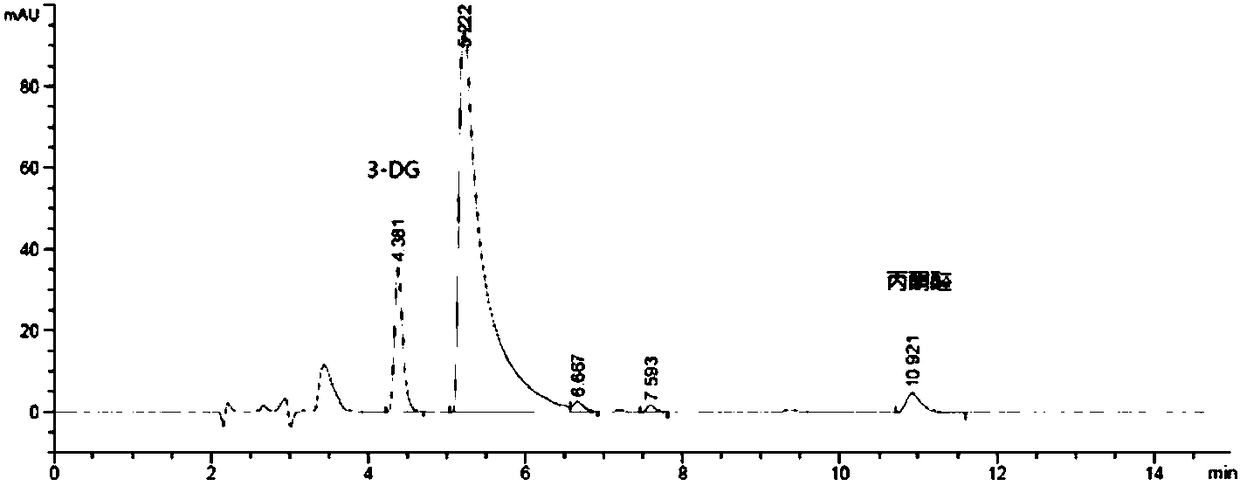
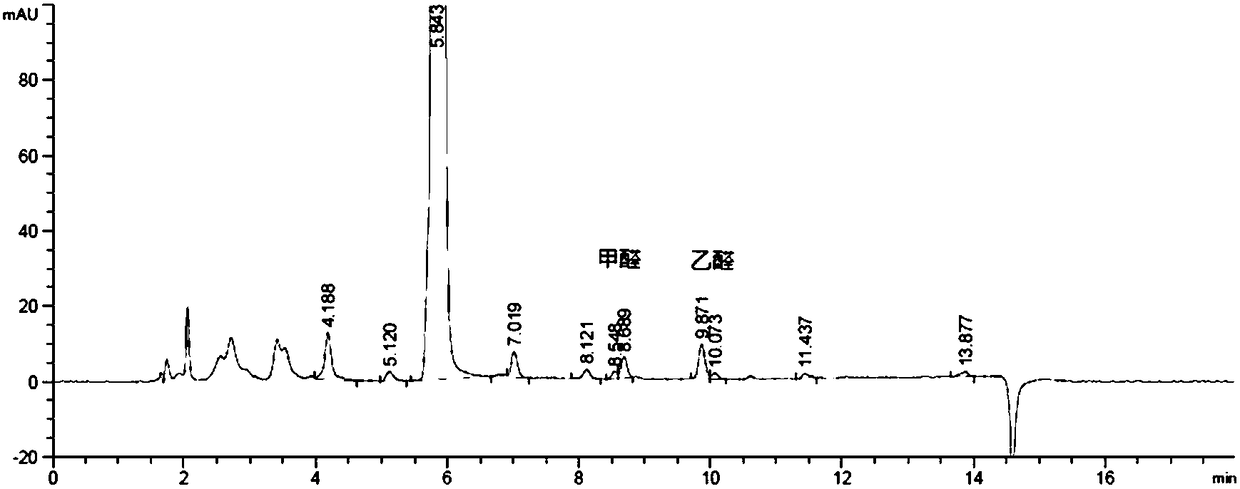
[0055] The HPLC determination of embodiment 1 glucose degradation product—3-DG, glyoxal and methylglyoxal
[0056] 1) Preparation of reference substance solution: Prepare a solution containing 3-deoxyglucosone, glyoxal and methylglyoxal at a concentration of 20 μg / ml, accurately measure 1.0ml, place them in 10ml measuring bottles, add water to the mark, and shake well Standby; take 1ml of reference substance solution (3-deoxyglucosone, glyoxal and methylglyoxal concentration is 2μg / ml), add water 1ml, then add o-phenylenediamine solution (4mg / ml) 1.2ml, mix well, adjust When the pH value reached 7.0, it was obtained by filtering at room temperature for 1 hour in the dark.
[0057] 2) Preparation of the test solution: Take 1ml of the glucose-containing electrolyte injection for children to be tested, add 0.6ml of o-phenylenediamine solution (4mg / ml), mix well, adjust the pH value to 7.0, and place it at room temperature for 1h Filter and serve.
[0058] 3) HPLC detection: C...
Embodiment 2
[0061] The methodological investigation of the HPLC detection of embodiment 2 3-DG, glyoxal and methylglyoxal
[0062] 1) Limit of detection and limit of quantitation
[0063] According to the method of Example 1, quantitatively dilute each impurity solution until the signal-to-noise ratio of each impurity peak is about 3, the calculated detection concentration of 3-deoxyglucosone is 0.2 μg / ml, the injection volume is 20 μl, and the detection limit is 4ng; The detection concentration of glyoxal was 0.2μg / mL, the injection volume was 15μl, and the detection limit was 3ng; the detection concentration of methylglyoxal was 0.2μg / mL, the injection volume was 20μl, and the detection limit was 4ng.
[0064] 2) Linearity and range
[0065] According to the method of Example 1, prepare a solution containing 3-deoxyglucosone, glyoxal and methylglyoxal at a concentration of 20 μg / ml, accurately measure 0.25, 0.5, 1.0, 2.0, 4.0ml, and place them in 10ml measuring bottles Add water to ...
Embodiment 3
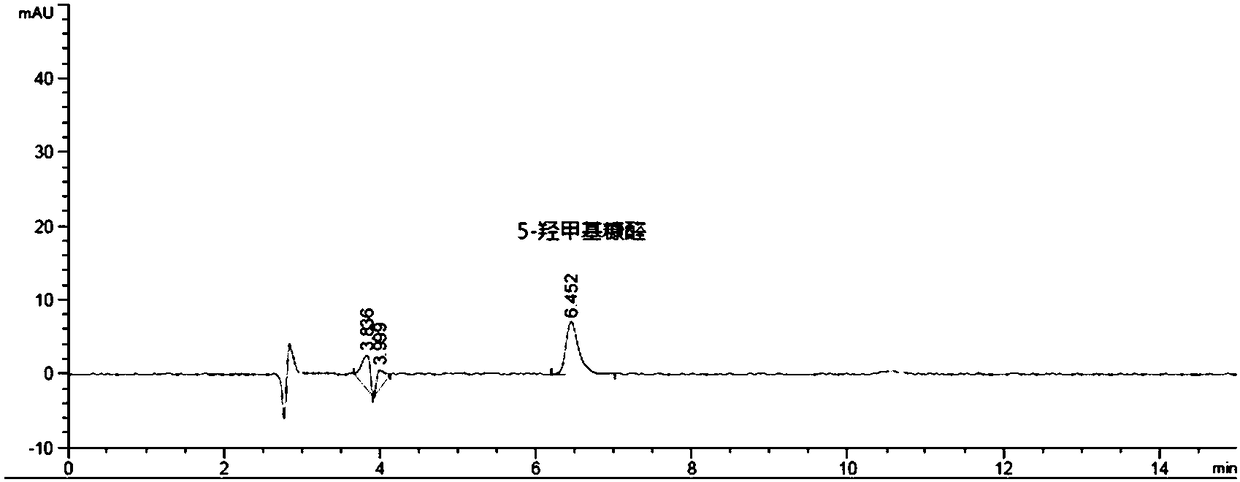
[0100] Embodiment 3 Degradation products of glucose—5-hydroxymethylfurfural, furfural, levulinic acid determination
[0101] 1) Preparation of reference solution: Precisely prepare a solution containing 2 μg / ml of 5-hydroxymethylfurfural and furfural and 200 μg / ml of levulinic acid.
[0102] 2) Test solution: 1ml of glucose-containing electrolyte injection sample for children to be tested.
[0103] 3) HPLC detection:
[0104] Mobile phase: 0.015mol / L sodium dihydrogen phosphate (adjust pH2.60 with phosphoric acid): acetonitrile (90:10 volume ratio)
[0105] Detection wavelength: 283nm
[0106] Flow rate: 0.8ml / min
[0107] Column temperature: 30°C
[0108] Injection volume: 20μl
[0109] 4) Determination method: draw respectively 20 μ l of reference substance solution and need testing solution, inject high performance liquid chromatograph, measure, obtain high performance liquid chromatogram ( image 3 with Figure 4 ), measure the peak areas of the components to be t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| mobile phase | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com