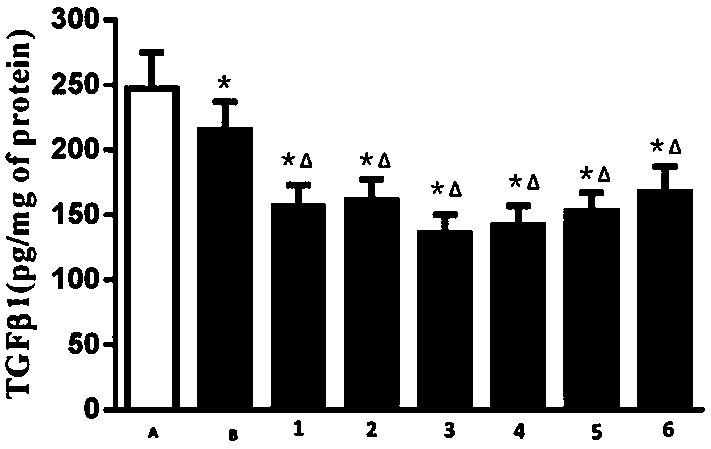
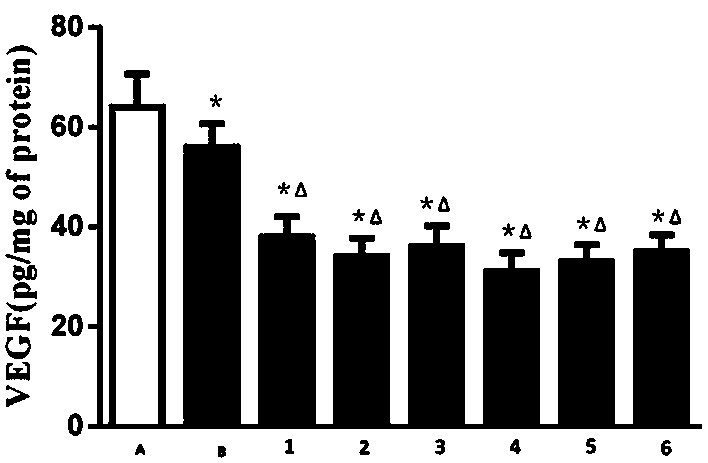
Peritoneal dialysis solution containing glucose polymer and preparation method of peritoneal dialysis solution
A technology of glucose polymer and peritoneal dialysis fluid, which is applied in the field of peritoneal dialysis fluid, can solve the problems of peritoneal injury, polydextrose peritoneal dialysis fluid bioincompatibility, etc., to reduce damage, improve biocompatibility, and ensure stability sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] A method for preparing peritoneal dialysis solution containing glucose polymers, comprising the following steps: 1) Weighing glucose polymers, weighing pharmaceutical raw materials containing calcium ions, magnesium ions and chloride ions, mixing them, adding injection water , adjust the pH value to make the first liquid medicine; 2) weigh glutamine dipeptide and buffer base respectively, mix them, add water for injection, adjust the pH value, and make the second liquid medicine; 3) respectively 1) The obtained first medicinal liquid and the second medicinal liquid obtained in step 2) are filtered and filled, and then subjected to moist heat sterilization at a sterilization temperature of 115-121° C. and a sterilization time of 8-32 minutes to obtain the product.
[0033] Specifically, in the step 3), during filling, the first medicinal liquid and the second medicinal liquid are sequentially filled into two chambers of a plastic container containing at least two chambers...
Embodiment 1
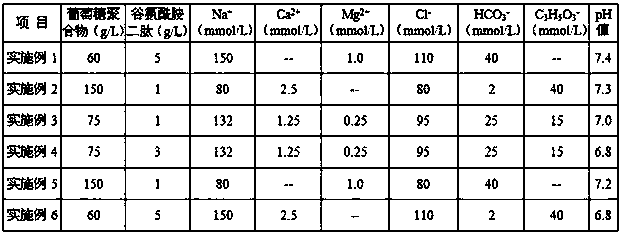
[0035] A three-layer co-extruded film bag for infusion is used to make a double-chamber bag containing two chambers A and B, which is sterilized for use; weigh the glucose polymer and MgCl 2 ·6H 2 O raw material, add water for injection, adjust the pH value to 4.5, make the first acidic liquid, filter, and put it into chamber A; weigh L-alanyl-L-glutamine Dipeptide, sodium chloride and sodium bicarbonate raw materials, add water for injection, adjust the pH value to make the pH value 7.0, make the second liquid medicine, filter, and put it into chamber B; then, put the second drug solution containing The double-chamber bags of the first liquid and the second liquid are sterilized by moist heat, the sterilization temperature is 115°C, and the sterilization time is 32 minutes. After sterilization, the finished product is obtained. The medicine solution in each chamber was mixed, and the formula of the peritoneal dialysis solution obtained after mixing was shown in Table 1.
[...
Embodiment 2
[0040] A three-layer co-extruded film bag for infusion is used to make a double-chamber bag containing two chambers A and B, which is sterilized for use; weigh the glucose polymer and CaCl 2 2H 2 O raw material, add water for injection, adjust the pH value to make the pH value 3.5, make the first acidic liquid, filter it, and put it into chamber A; weigh glycylglutamine dipeptide, chloride Sodium, sodium bicarbonate and sodium lactate raw materials, add water for injection, adjust the pH value to make it 7.5, make the second medicinal solution, filter, and put it into chamber B; then, put the first medicinal solution containing The double-chamber bag with the second liquid medicine is sterilized by moist heat, the sterilization temperature is 121°C, the sterilization time is 8min, and the finished product is obtained after sterilization. The medicine solution was mixed, and the formula of the peritoneal dialysis solution obtained after mixing was shown in Table 1.
[0041] W...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com