Peritoneal dialysis solution and preparation method thereof
A technology for peritoneal dialysis liquid and B liquid, which is applied in the field of pharmaceutical technology drug preparation, can solve problems such as genotoxicity, teratogenicity, carcinogenicity, abdominal discomfort, etc., and achieves the effects of stable quality, reduced damage, and guaranteed stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
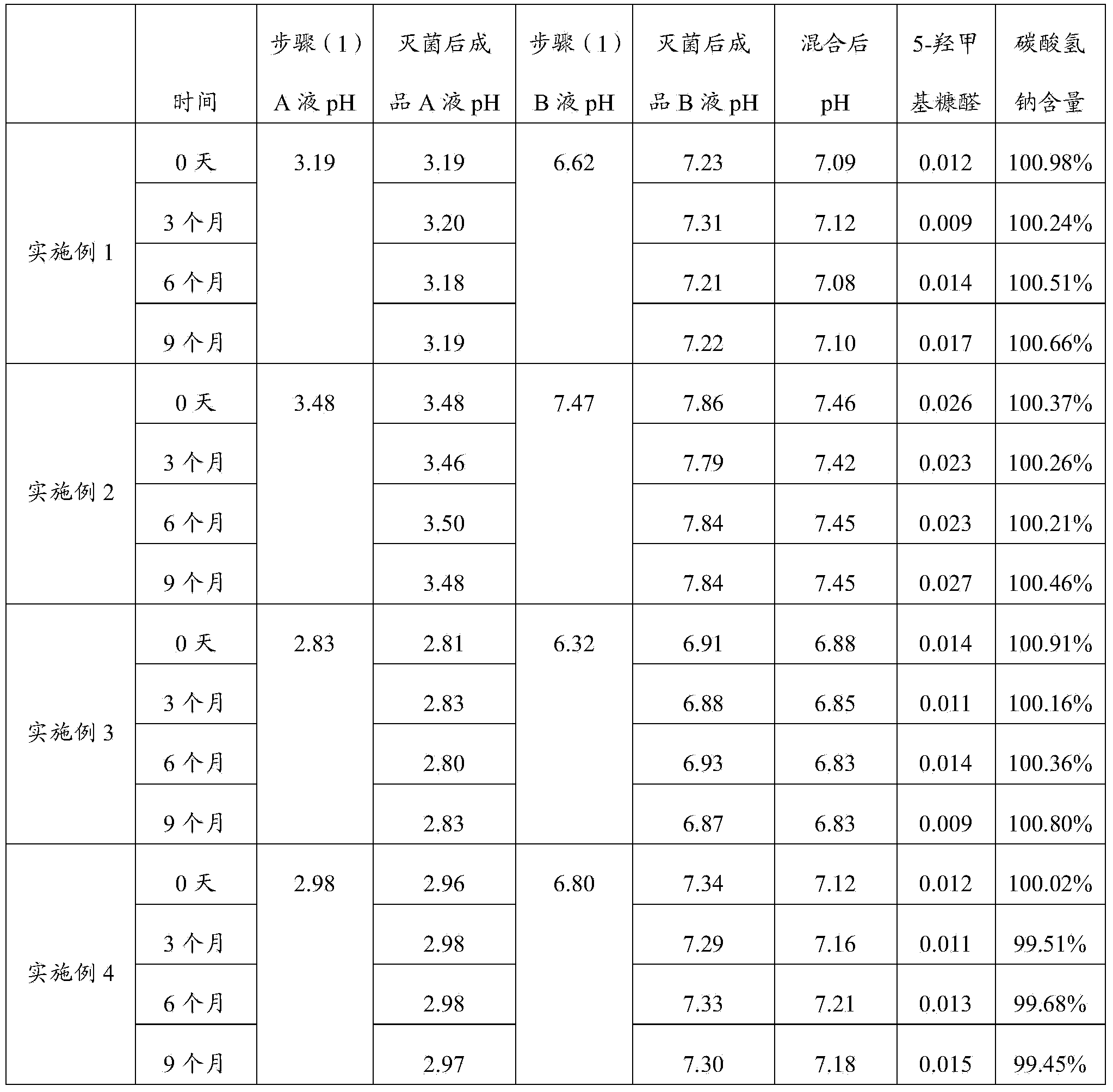
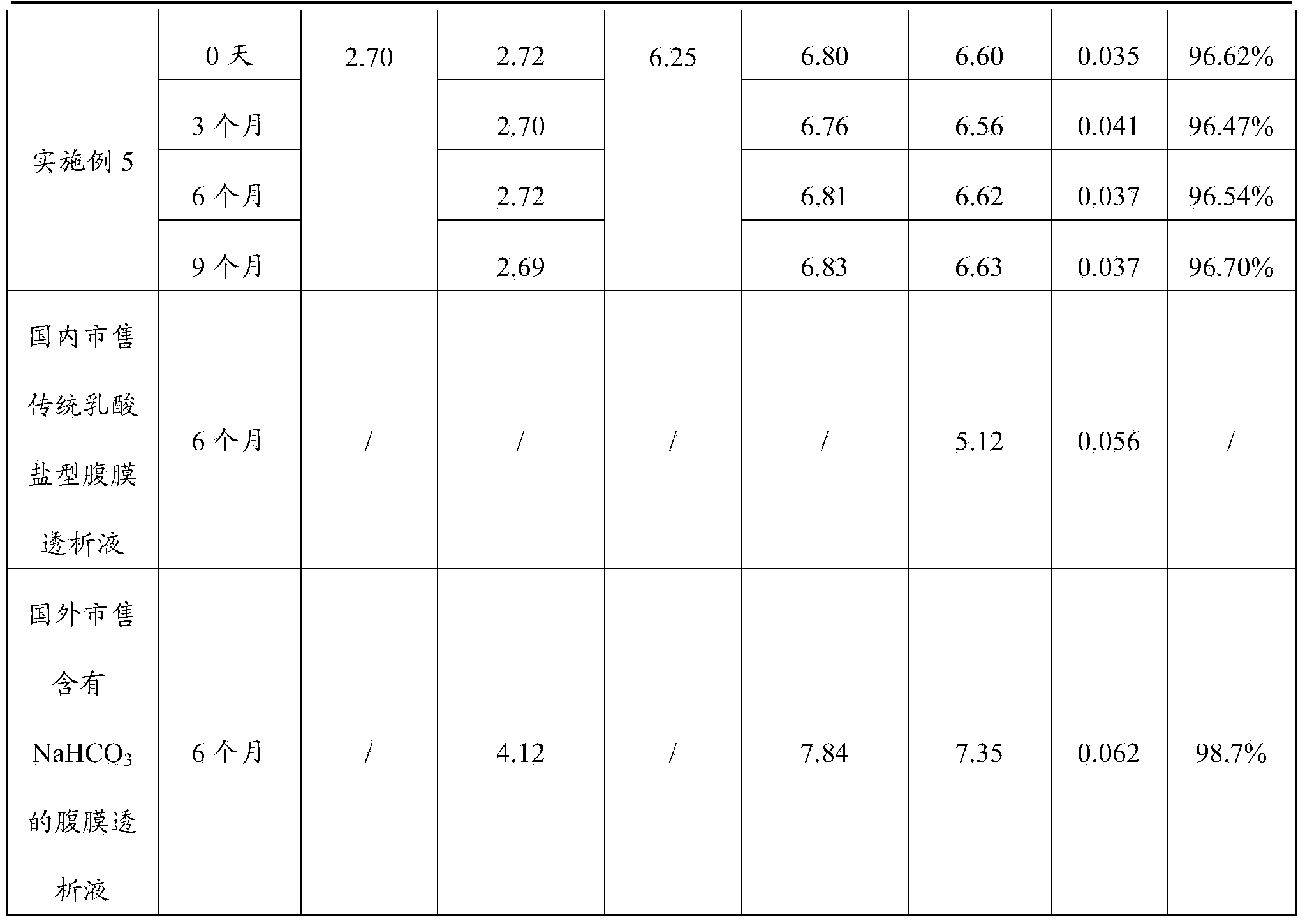
Embodiment 1
[0045] In this embodiment, the scale of producing 200 bags of peritoneal dialysis solution is taken as an example, wherein the prepared volume of liquid A is 200 L, and the prepared volume of liquid B is 300 L.
[0046] Described peritoneal dialysis fluid includes A liquid and B liquid;
[0047] Wherein, the A liquid component is as follows:
[0048] Anhydrous glucose 12.52kg,
[0049] Magnesium chloride 0.0280kg,
[0050] Calcium chloride 0.1014kg,
[0051] Add water for injection to 200L;
[0052] The B liquid components are as follows:
[0053] Sodium chloride 2.53kg,
[0054] Sodium bicarbonate 0.9870kg,
[0055] Sodium lactate 0.7890kg,
[0056] Add water for injection to 300L;
[0057] The specific preparation method is as follows:
[0058] (1) Preparation of liquid A
[0059] Dissolve the above-mentioned anhydrous glucose, calcium chloride and magnesium chloride in 60°C water for injection according to the formula, stir thoroughly for 15 minutes to dissolve comp...
Embodiment 2
[0065] A peritoneal dialysis solution, including liquid A and liquid B;
[0066] Wherein, the A liquid component is as follows:
[0067] Anhydrous glucose 77.21g,
[0068] Magnesium chloride 0.812g,
[0069] Calcium chloride 0.588g,
[0070] Add water for injection to 725mL;
[0071] The B liquid components are as follows:
[0072] Sodium chloride 17.55g,
[0073] Sodium bicarbonate 7.56g,
[0074] Sodium Lactate 10.08g,
[0075] Add water for injection to 1275mL;
[0076] The specific preparation method is as follows:
[0077] (1) Preparation of liquid A
[0078] Dissolve the above-mentioned anhydrous glucose, calcium chloride and magnesium chloride in 70°C water for injection according to the formula, stir well for 15 minutes to dissolve completely, add 70°C water for injection to 725mL, measure the pH, and use a pharmaceutical grade hydrochloric acid solution with a concentration of 3mol / L Adjust the pH to 3.48, mix well, and turn on the liquid medicine pump, so t...
Embodiment 3
[0084] A peritoneal dialysis solution, including liquid A and liquid B;
[0085] Wherein, the A liquid component is as follows:
[0086] Anhydrous glucose 27.19g,
[0087] Magnesium chloride 0.041g,
[0088] Calcium chloride 0.294g,
[0089] Add water for injection to 725mL;
[0090] The B liquid components are as follows:
[0091] Sodium chloride 9.36g,
[0092] Sodium bicarbonate 3.36g,
[0093] Sodium Lactate 2.24g,
[0094] Add water for injection to 1275mL;
[0095] The specific preparation method is as follows:
[0096] (1) Preparation of liquid A
[0097] Dissolve the above-mentioned anhydrous glucose, calcium chloride and magnesium chloride in 50°C water for injection according to the formula, stir well for 15 minutes to dissolve completely, add 70°C water for injection to 725mL, measure the pH, and use a pharmaceutical grade hydrochloric acid solution with a concentration of 3mol / L Adjust the pH to 2.83, mix well, and turn on the liquid medicine pump, so tha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com