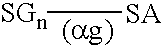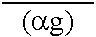Patents
Literature
181 results about "Peritoneal dialysate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
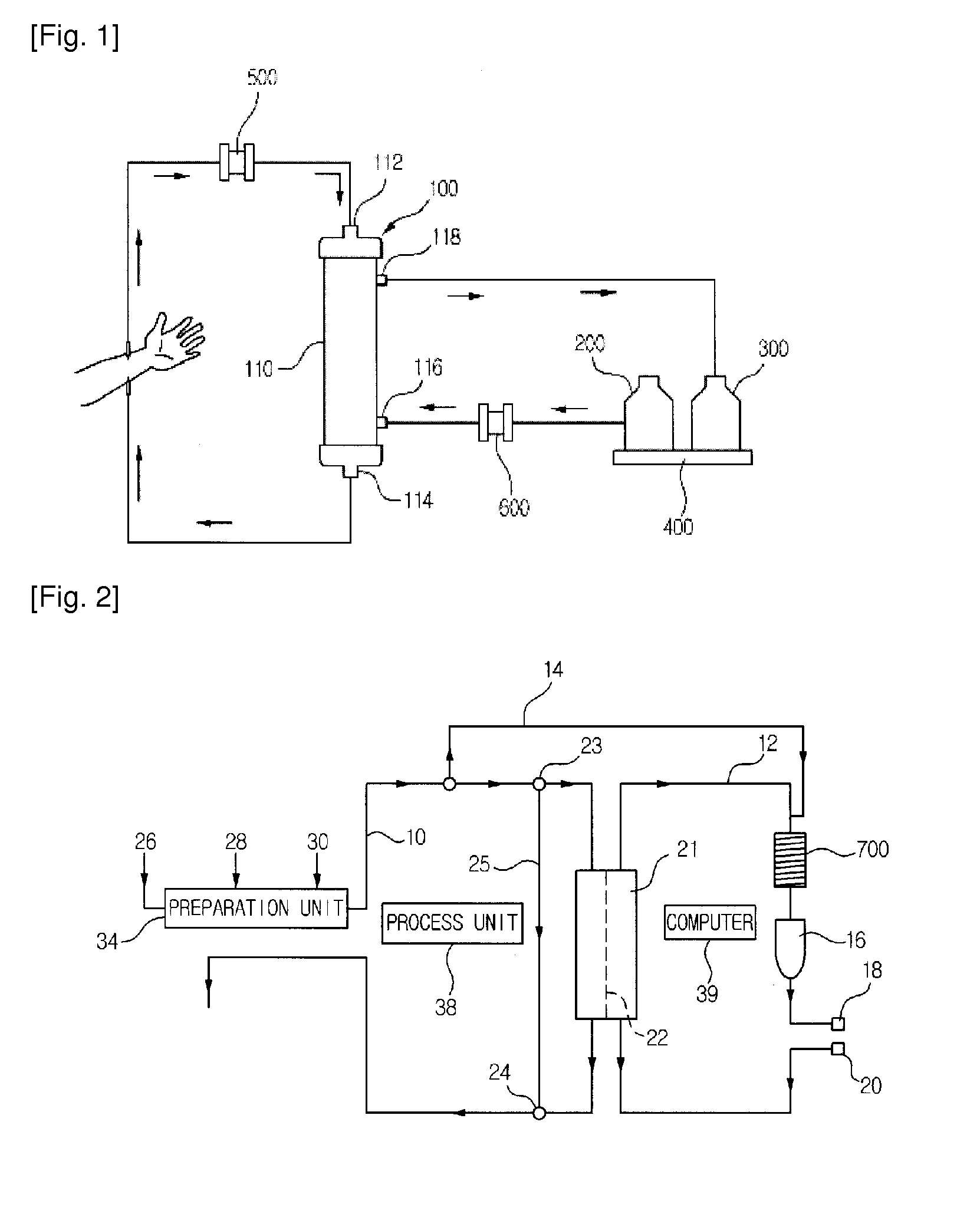
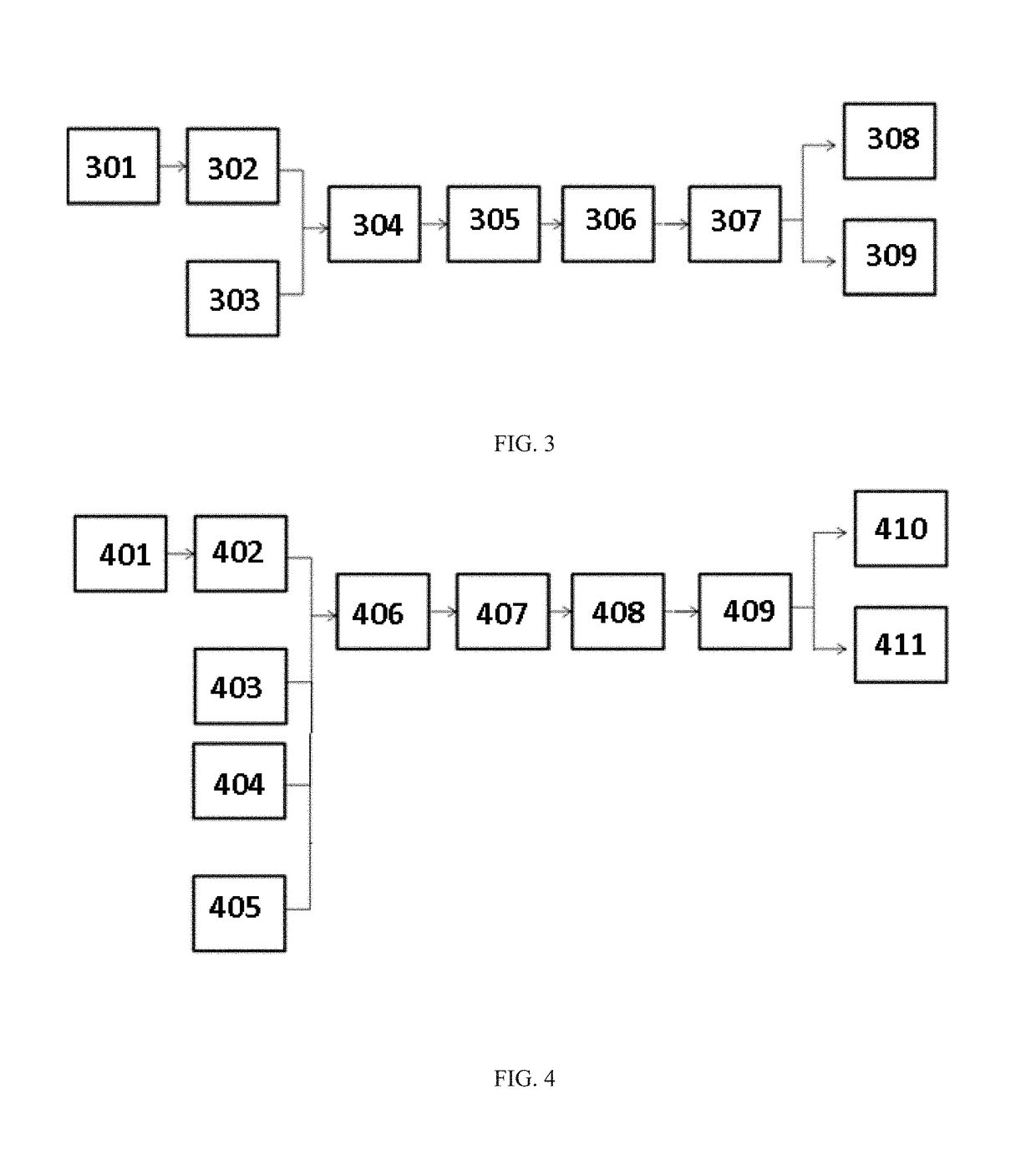
Automatic exchanger for peritoneal dialysis
InactiveUS6293921B1Avoid misuseMinimize the possibilityPeritoneal dialysisTube connectorsPeritoneal dialysis fluidEngineering
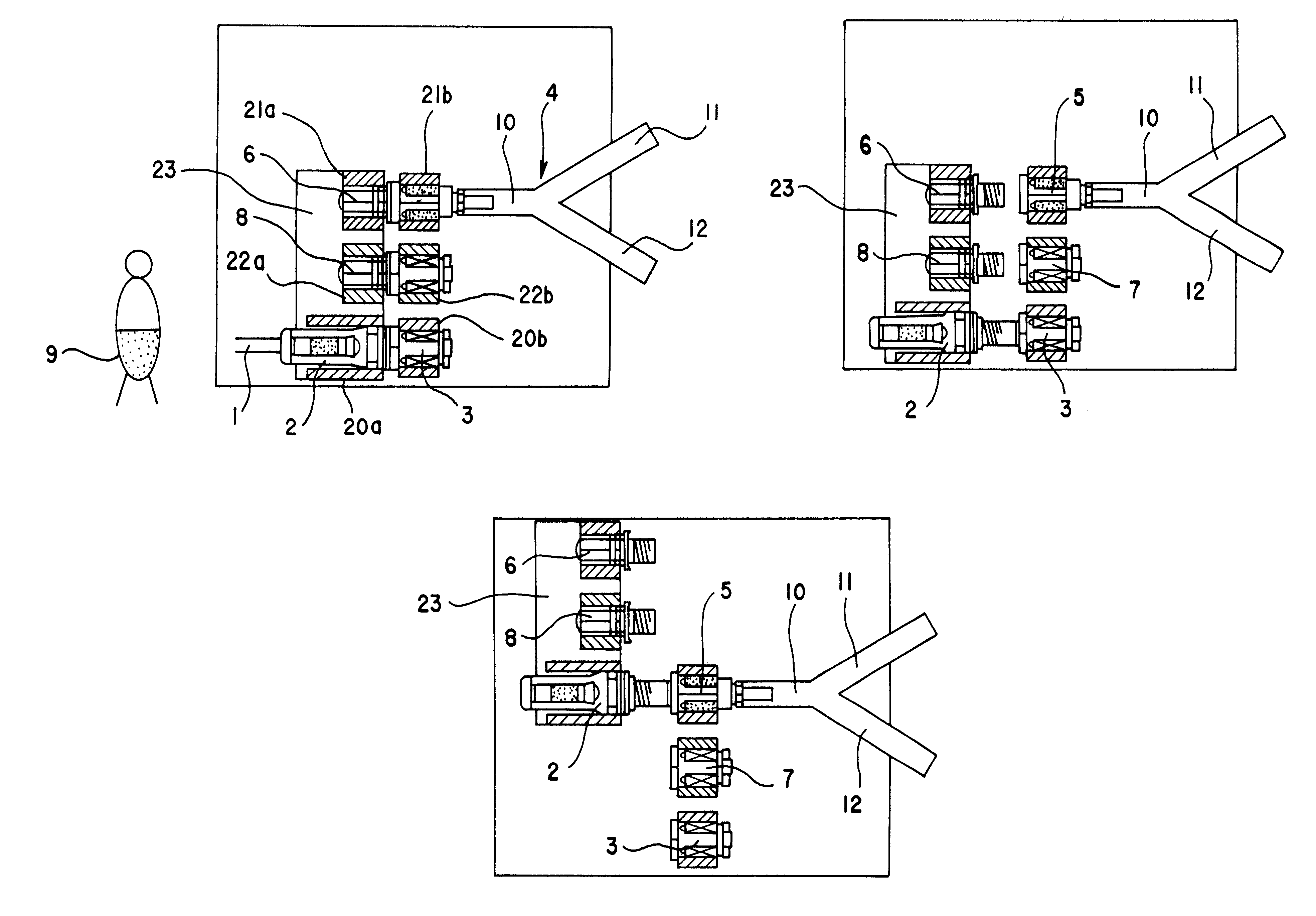
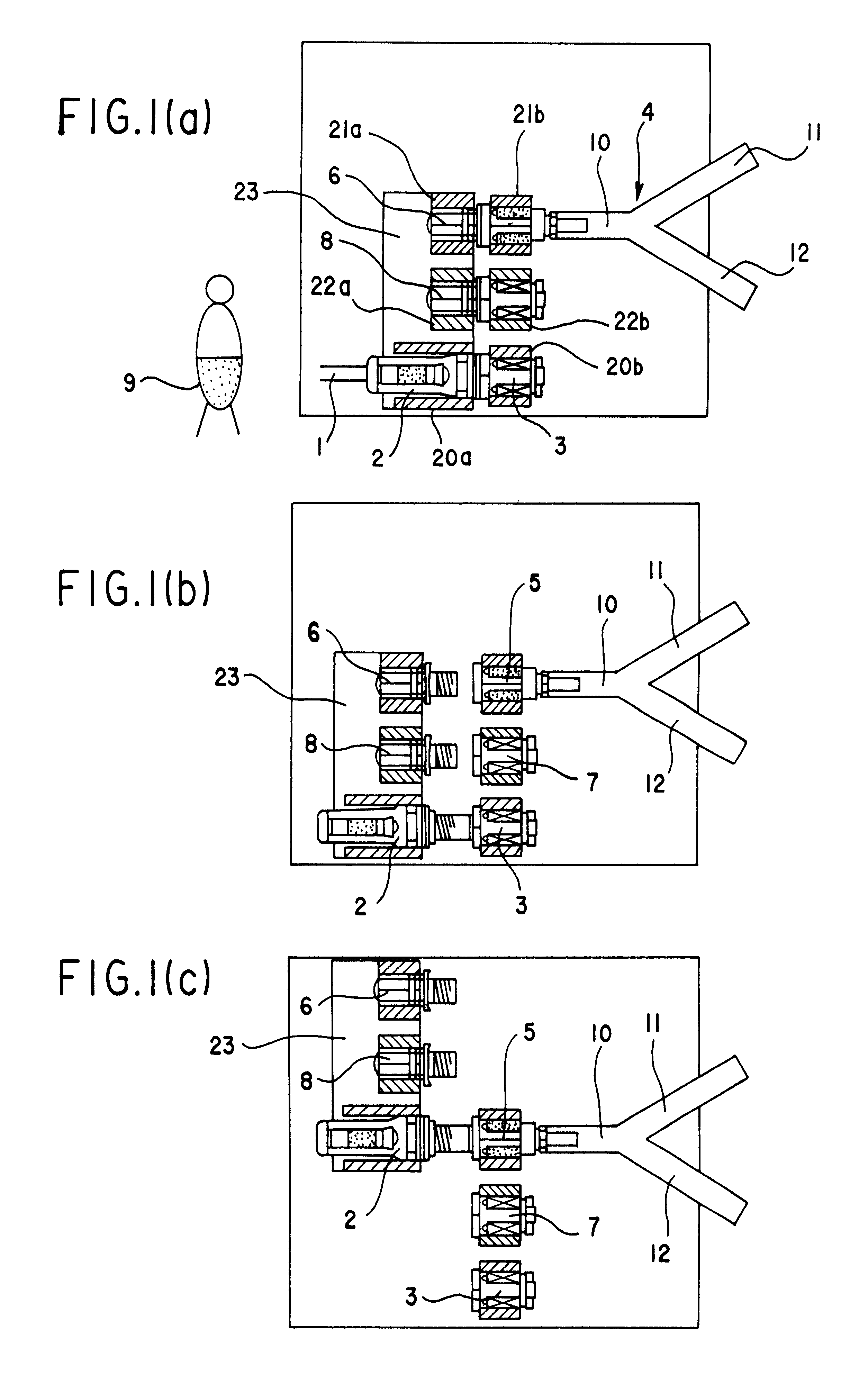
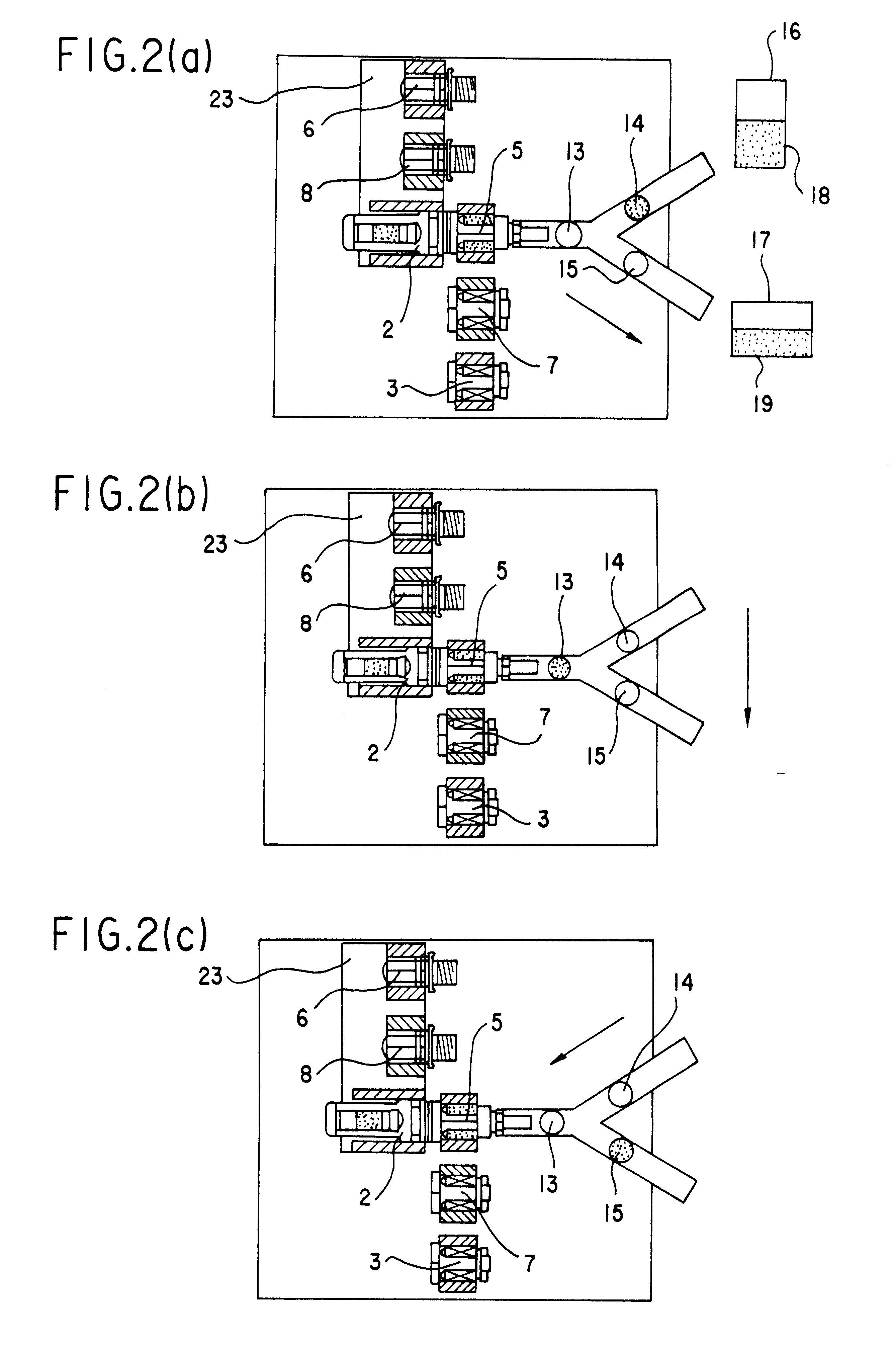
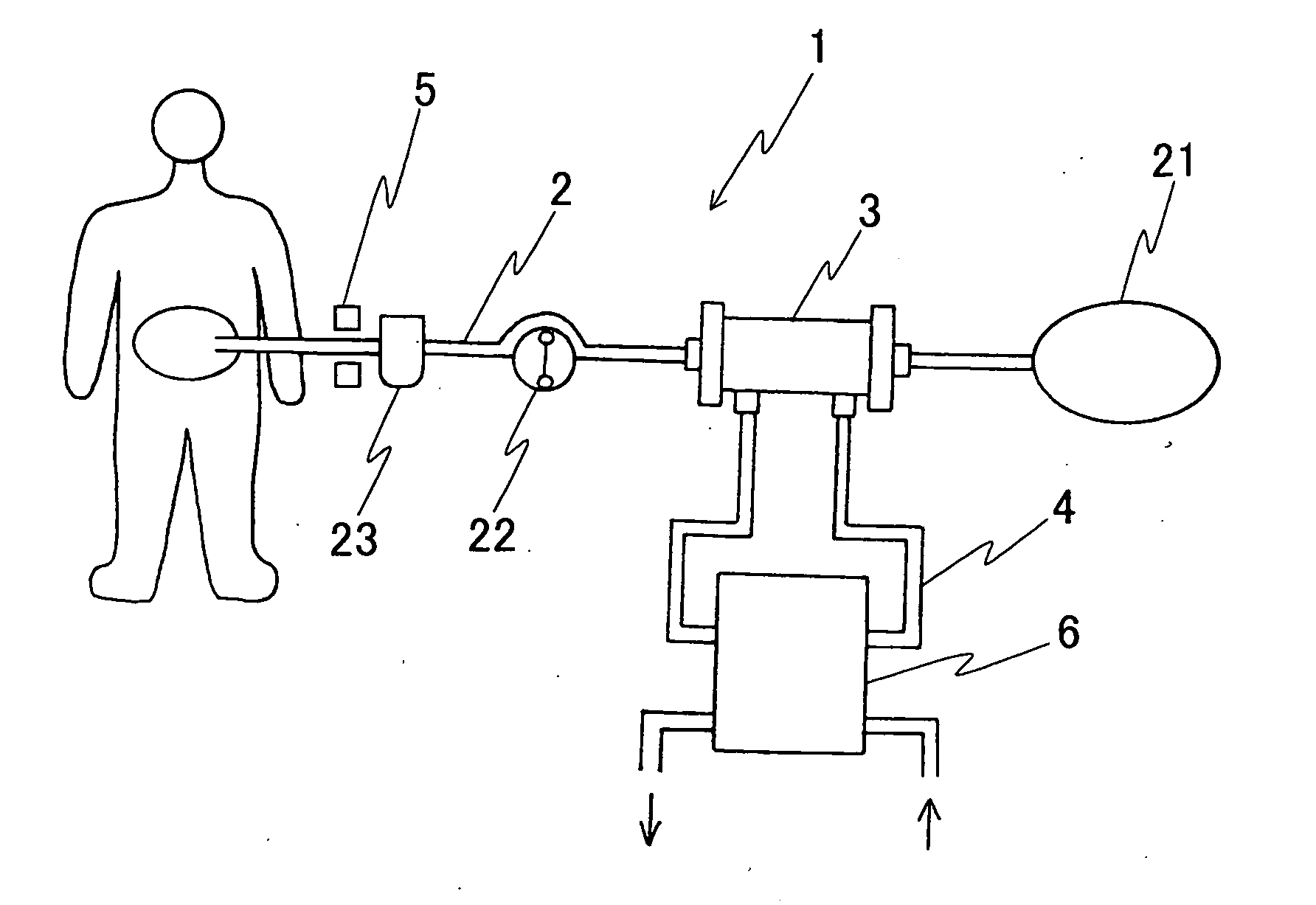
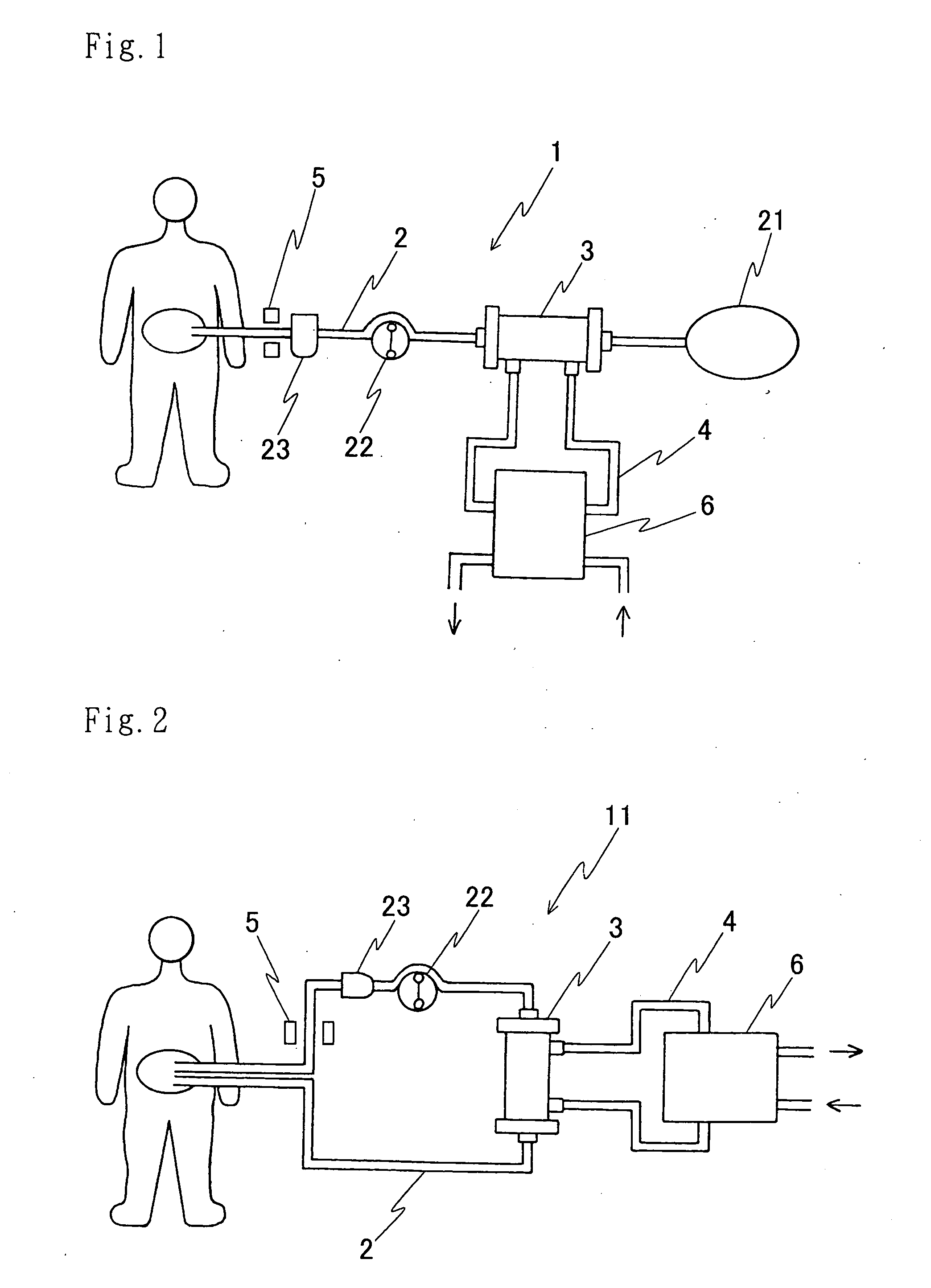
An automatic exchanger apparatus for peritoneal dialysis fluids is provided having a dialysis fluid bag and a drained fluid bag and arranged for connecting and disconnecting between the end of a peritoneal dialysis circuit equipped with a branching point and the end of a tube extending from a patient to drain the waste dialysis fluid from the cavity of the patient and fill the peritoneal cavity of the patient with a fresh peritoneal dialysis fluid for exchange, and in particular comprises: means A, B, and C for carrying out respectively a step (A) of connecting the end of the patient side tube to the end of the peritoneal dialysis circuit, a step (B) of delivering and draining the waste fluid, and a step (C) of, disconnecting the two ends and connecting the end of the patient side tube to its shut-off member, arranged for carrying out their respective steps (A) and (C) automatically; and a controlling means for controlling the respectively means to execute their respectively steps in a sequence. The apparatus is simple in the construction while carrying out, with much ease, the connection and disconnection of the tubes and the exchange of the fluids including a waste and a fresh supply one, hence avoiding operational errors of the operator and minimizing the possibility of infection and contamination.
Owner:JMS CO LTD
Dialysis solution for peritoneal dialysis
InactiveUS6284140B1Easy to degradeDwell timeBiocideSolvent extractionHydroxyethyl starchUltrafiltration
The present invention relates to dialysis solutions for peritoneal dialysis, containing hydroxyethyl starch as the osmotically-active substance, electrolytes and, optionally, conventional additives, where the hydroxyethyl starch has a molecular weight Mw in the range from 10,000 to 150,000, a substitution MS in the range from 0.10 to 0.40, a substitution DS in the range from 0.09 to 0.35 and a substitution ratio C2 / C6>=8. With this peritoneal dialysis solution it is possible, with an outstanding ultrafiltration, to maintain a longer dwell time, for example the dialysis solution can be utilized for a period of 12 hours in the CAPD without replacement. In addition, the inventive dialysis solution is also particularly advantageous for patients with residual kidney function. The resorption of the osmotically active substance is clearly diminished and even after a dwell time of 12 hours it amounts to a maximum of 60-70%.
Owner:FRESENIUS AG
Artificial kidney dialysis system
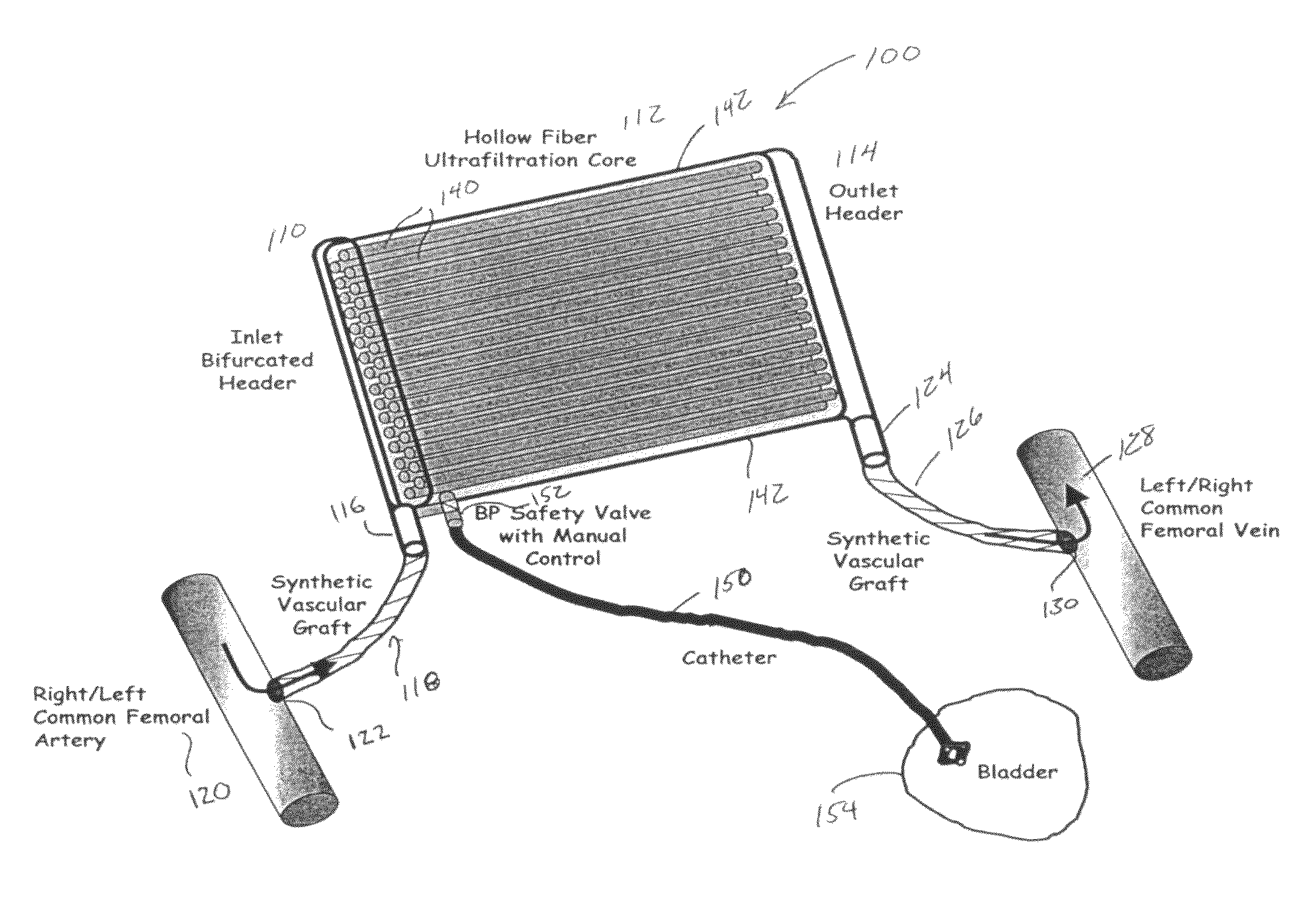
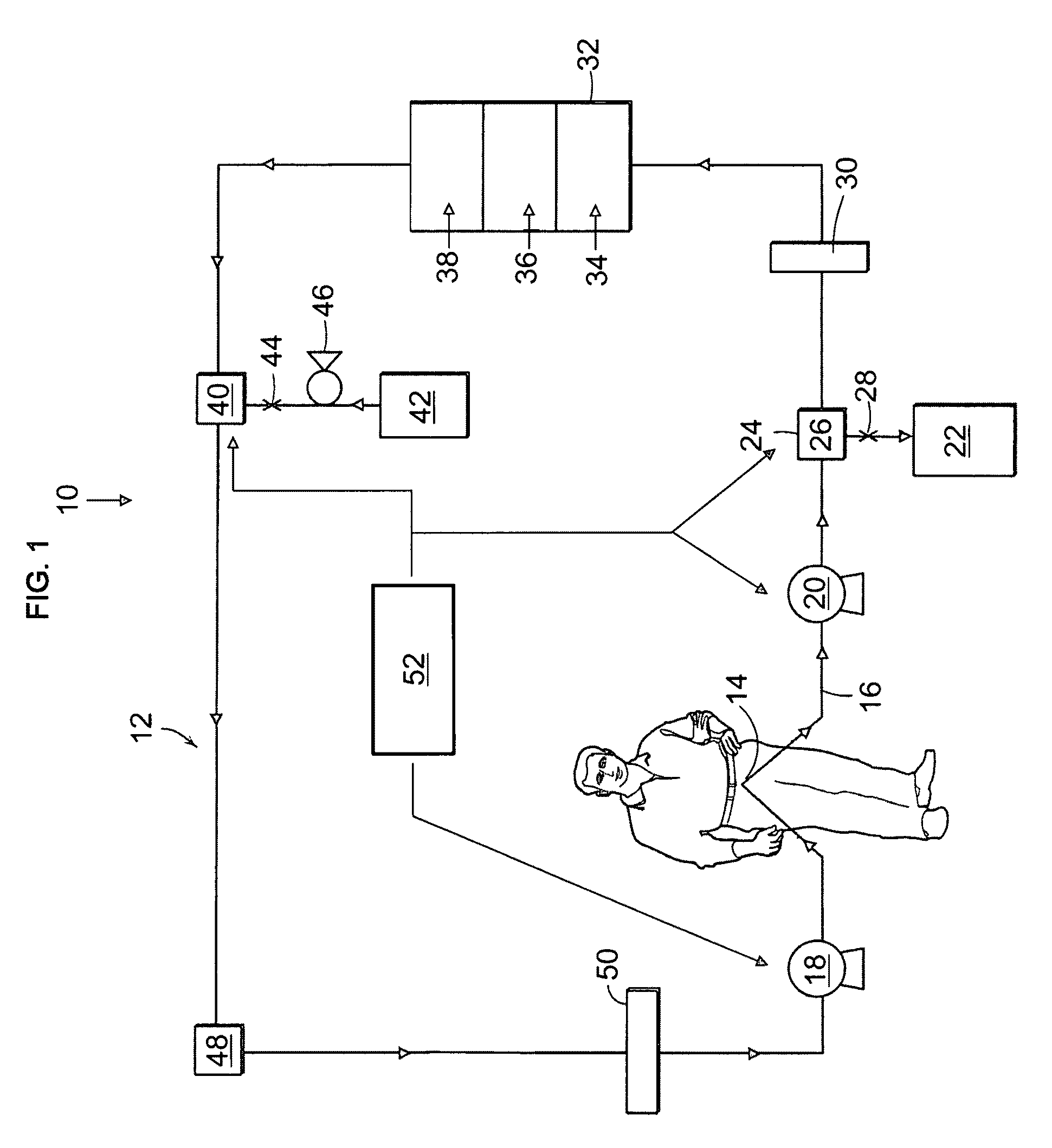
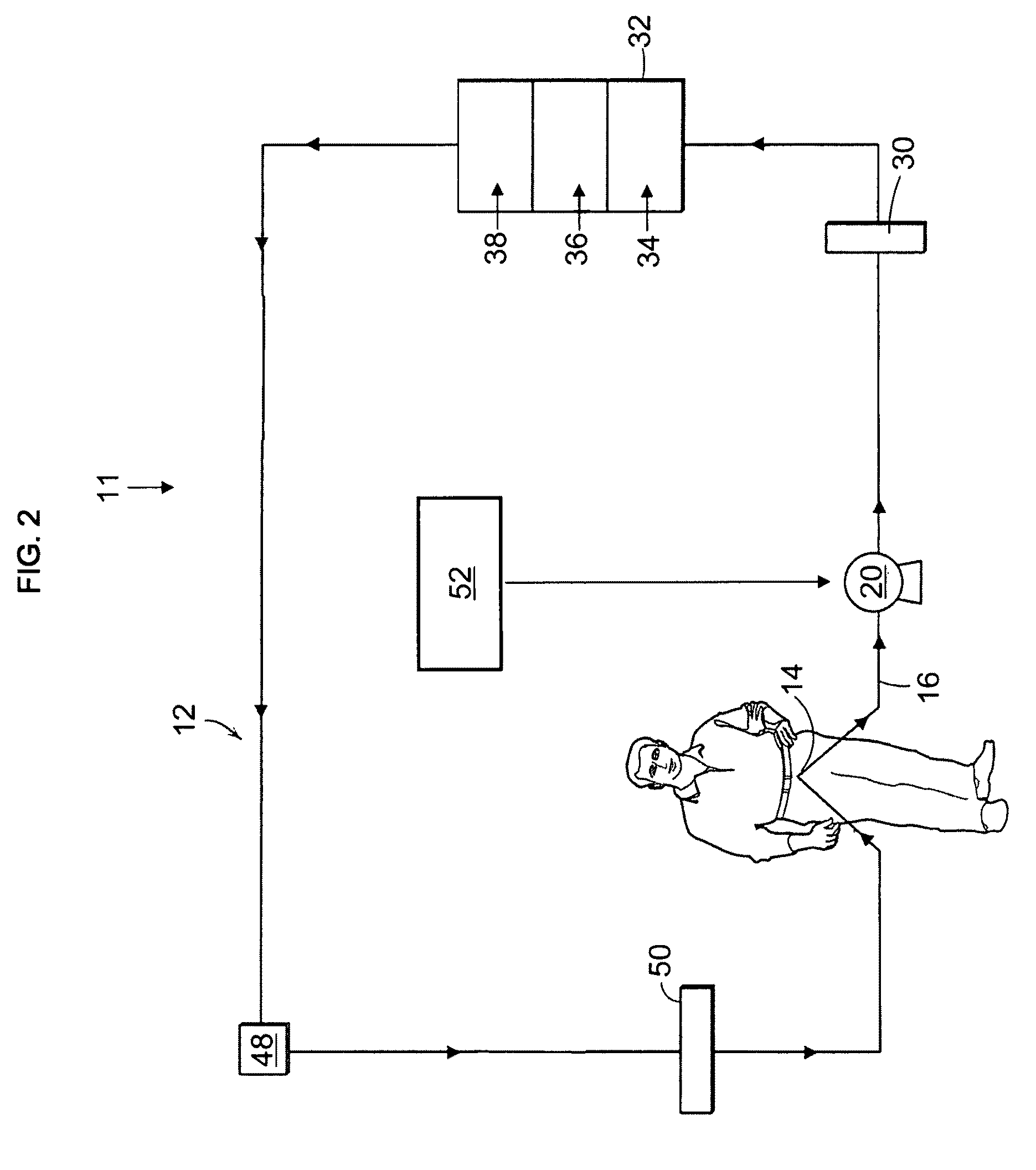
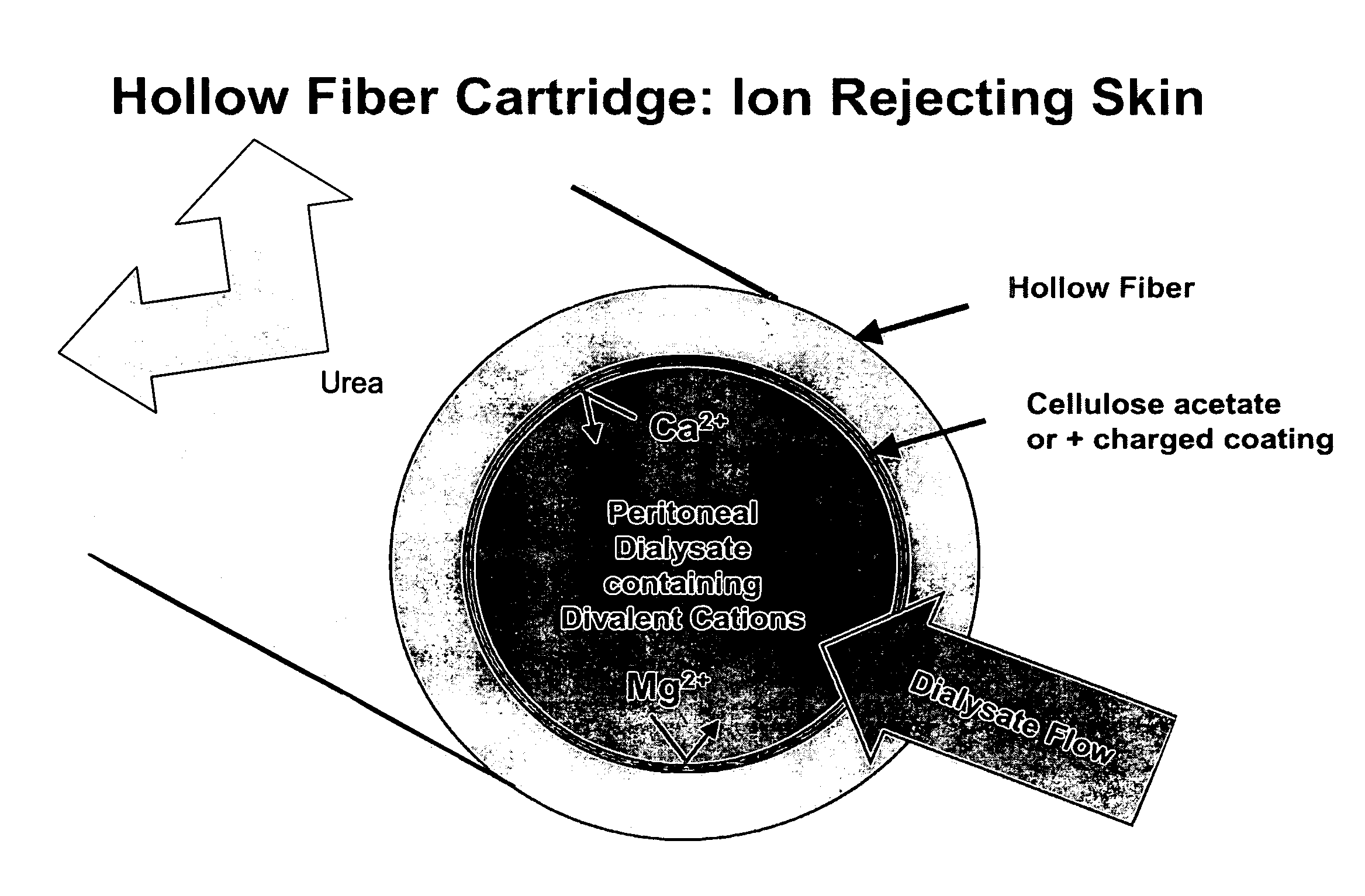
The present disclosure relates to a wearable dialysis system and method for removing uremic waste metabolites and fluid from a patient suffering from renal disease. Uremic waste metabolites can be removed by a wearable peritoneal dialysis device that regenerates the peritoneal dialysis solution without removing positively charged, essential ions from the solution and, consequently, the patient. Fluids can be removed from the blood of the patient by an implantable fluid removing device. Fluids are delivered to the bladder and preferably removed from the body of the patient through urination. The wearable dialysis system may be operated continuously or semi-continuously and be comfortably adapted to the body of the patient while allowing the patient to perform normal activities.
Owner:FRESENIUS MEDICAL CARE HLDG INC
Peritoneal dialysis method
InactiveUS20090186850A1Avoid excessive injuryAvoid injuryOrganic active ingredientsAntipyreticPeritoneal dialysateIntensive care medicine
Owner:KOWA CO LTD
Peritoneal dialysis fluid
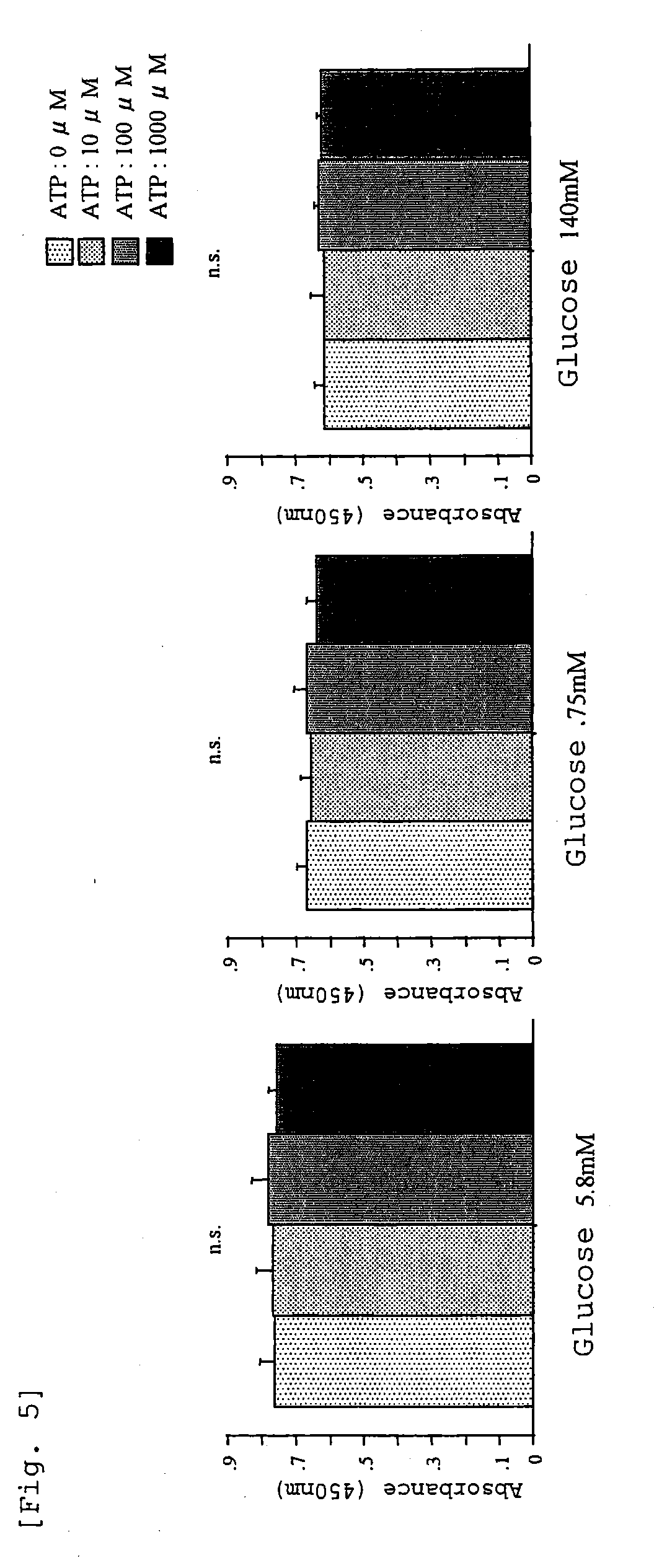
Peritoneal dialysis fluids and the use thereof for performing peritoneal dialysis are disclosed. The peritoneal dialysis fluid comprises a physiologically acceptable aqueous solution containing physiologically acceptable inorganic anions and cations and, as an osmotic agent, at least one sugar derivative, at concentrations sufficient for the removal of water and solutes from a patient by peritoneal dialysis. The sugar derivative is a compound of formulawherein each SG, which may be the same or different, represents a residue of a physiologically acceptable metabolizable sugar, SA represents a residue of a physiologically acceptable metabolizable sugar alcohol, n is from 1 to 4 andrepresents a glycoside linkage which is cleavable by an alpha-glycosidase enzyme.
Owner:ALLIED THERAPEUTICS LTD
Biochemically balanced peritoneal dialysis solutions
InactiveUS7011855B2Improved peritoneal dialysis solutionAvoid lostBiocideSolvent extractionMedicinePeritoneal dialysis solutions
A peritoneal dialysis solution that is biochemically balanced to correct metabolic acidosis associated with chronic renal failure in a more physiological manner. The peritoneal dialysis solution has a physiological pH, e.g., pH of 7.0 to 7.4, and contains bicarbonate at a concentration that is found in normal blood. Additionally, the solution contains carbon dioxide at a partial pressure that is similar to partial pressure of carbon dioxide found in normal blood. The peritoneal dialysis solution also contains a weak acid with a pKa of less than 5.0.
Owner:BAXTER INT INC
Artificial kidney dialysis system
InactiveCN101784292AGood for healthImprove the quality of lifeHaemofiltrationMedical devicesDiseaseMetabolite
The present disclosure relates to a wearable dialysis system and method for removing uremic waste metabolites and fluid from a patient suffering from renal disease. Uremic waste metabolites can be removed by a wearable peritoneal dialysis device (10) that regenerates the peritoneal dialysis solution without removing positively charged, essential ions from the solution and, consequently, the patient. Fluids can be removed. from the blood of the patient by an implantable fluid removing device (100). Fluids are delivered to the bladder and preferably removed from the body of the patient through urination. The wearable dialysis system may be operated continuously or; semi-continuously and be comfortably adapted to the body of the patient while allowing the patient to perform normal activities.
Owner:FRESENIUS MEDICAL CARE HLDG INC
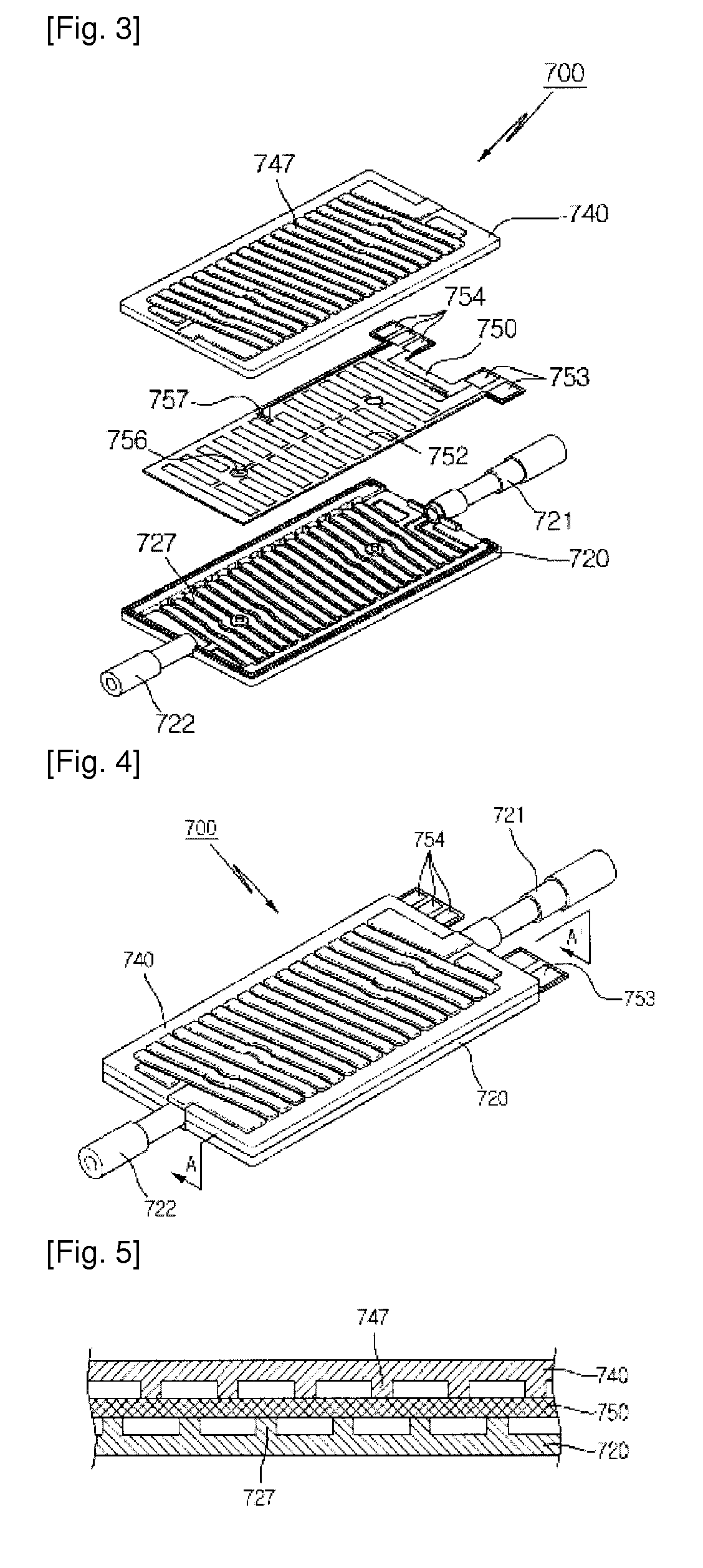
Apparatus relating to hemodialysis, hemodiafiltration, hemofiltration or peritoneal dialysis having function for rise temperature
InactiveUS20140216994A1Avoid side effectsEfficient heatingOther blood circulation devicesHaemofiltrationSide effectMedicine
Disclosed is an apparatus having a heating function for hemodialysis, hemodiafiltration, hemofiltration or peritoneal dialysis, wherein the apparatus includes at least one of pipe for transferring at least one fluid of blood and dialsate, and a heating unit for heating at least one fluid of blood and dialsate, wherein the fluids to be heated by the heating unit are substances to be injected into human body; and the heating unit is arranged for measuring flow rates of the fluids to be heated, and injecting temperatures related with the flow rate to heat the fluids to be heated. The heating unit comprises: flow passages through which the fluids to be heated are flowed; a heater formed as a part of the flow passages, for generating heat; and a cover means including a first connection portion through which the fluids enter the flow passages, and a second connection portion through which the fluids come out from the flow passages. Present invention provides one effect in that blood having the same or nearly same temperature as that of human body can be injected into human body to prevent side effects caused by the dialysis and another effect in that blood can be heated effectively because the shapes of flow passages around the heater is deformed in improved manner.
Owner:WOOYOUNG MEDITECH CO LTD
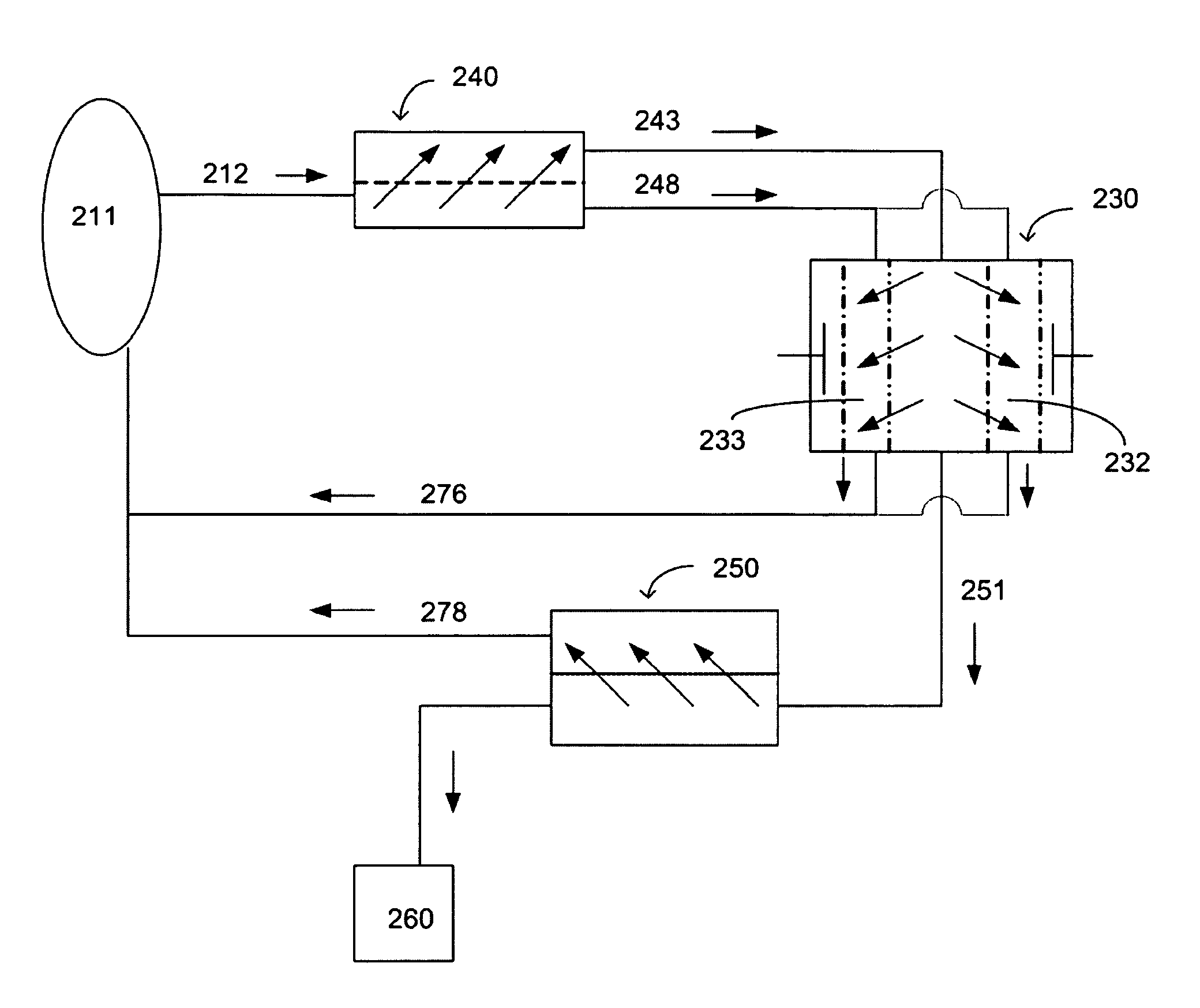
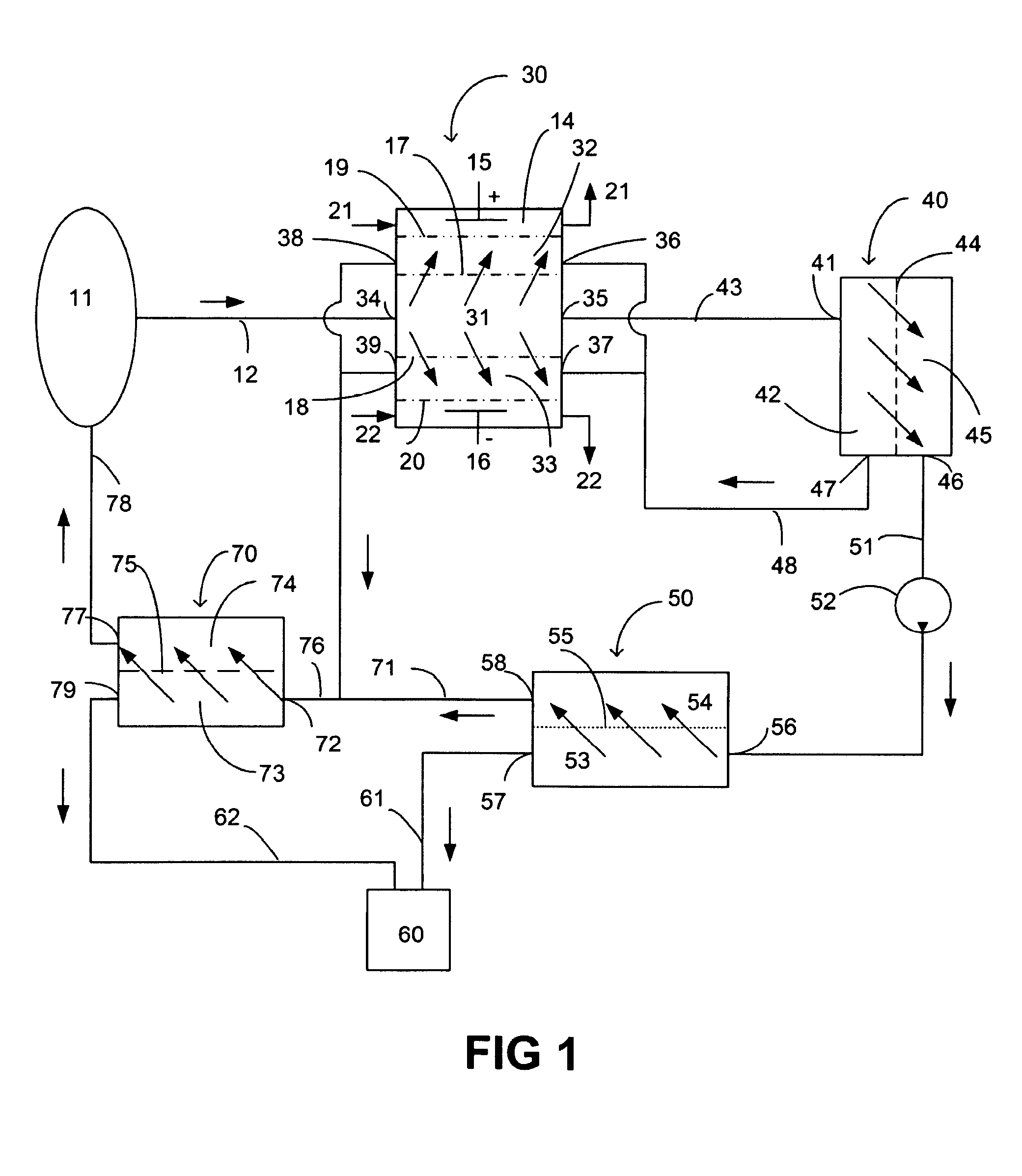
System and method for regeneration of a fluid
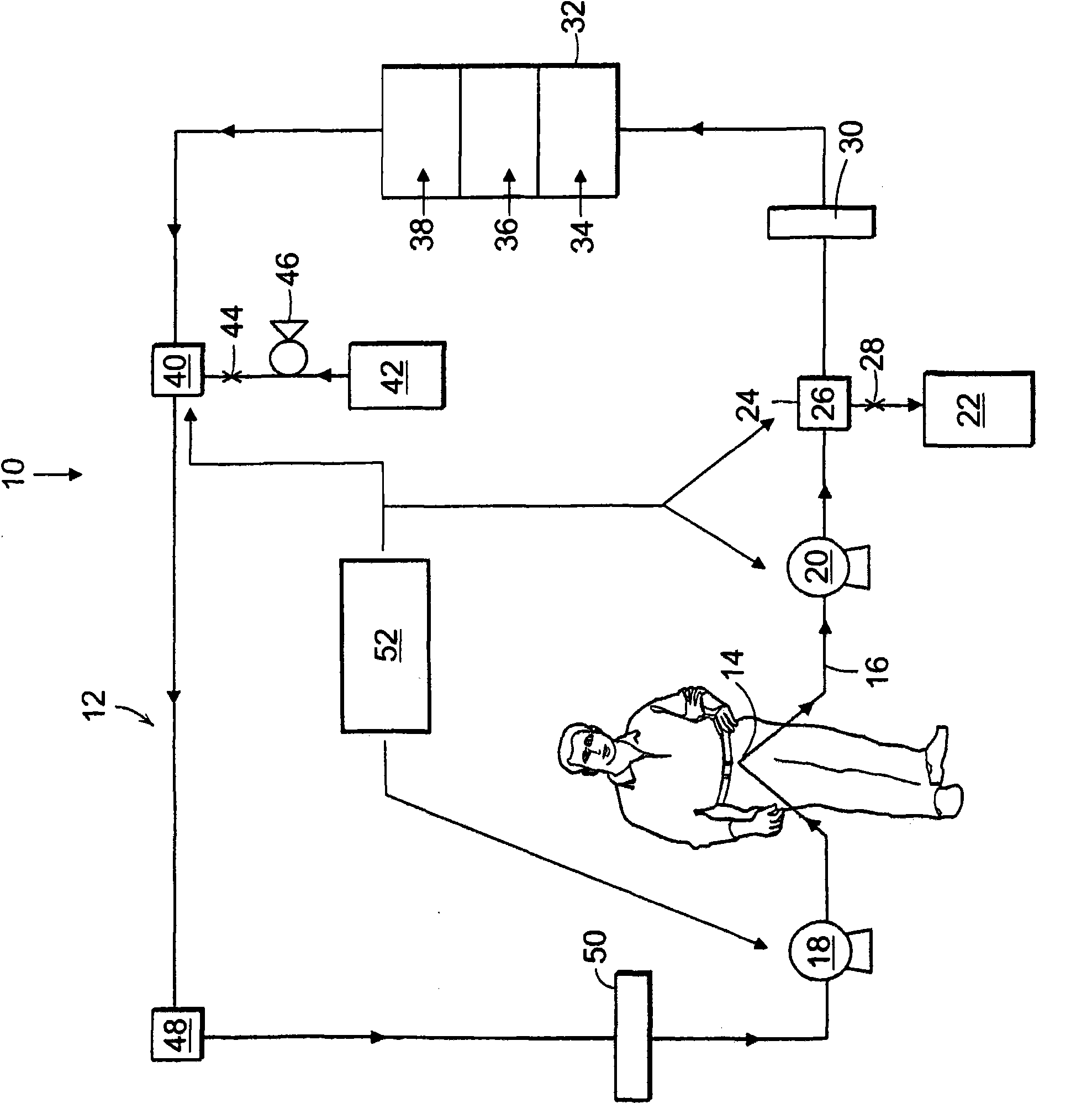
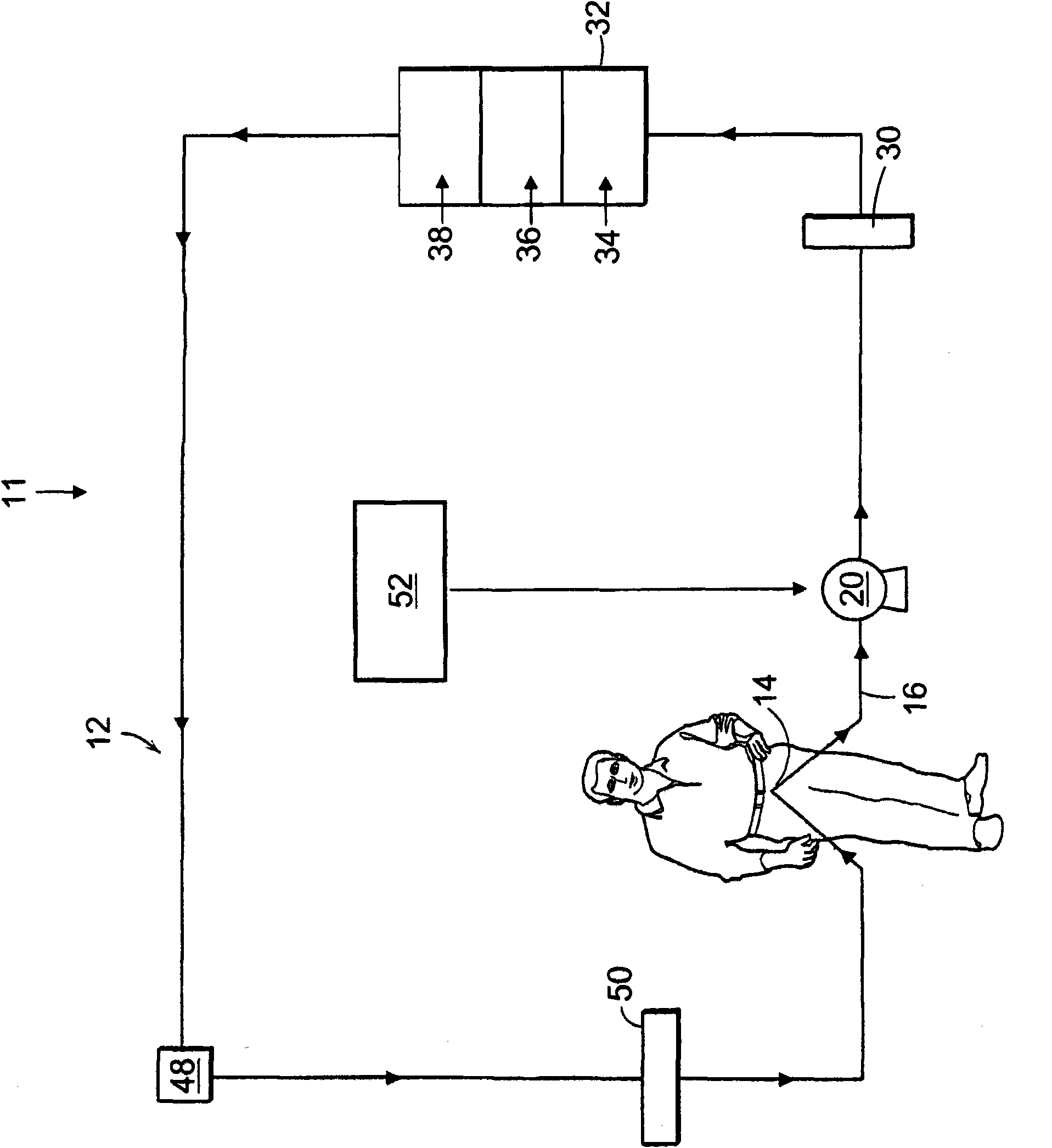
A method and a system for regenerating a body fluid, such as a peritoneal dialysis fluid. The body fluid is removed into an extracorporeal circuit comprising an electrofilter for removing charged ions from the body fluid, a nanofilter for removing large molecules, such as Dextran 40, and a reverse osmosis filter for concentrating the body fluid, for producing a synthetic urine to be discarded. The removed ions and large molecules are returned to the patient together with pure water from the reverse osmosis filter through an ultrafilter.
Owner:TRIOMED AB
Apparatus and method for preparation of a peritoneal dialysis solution
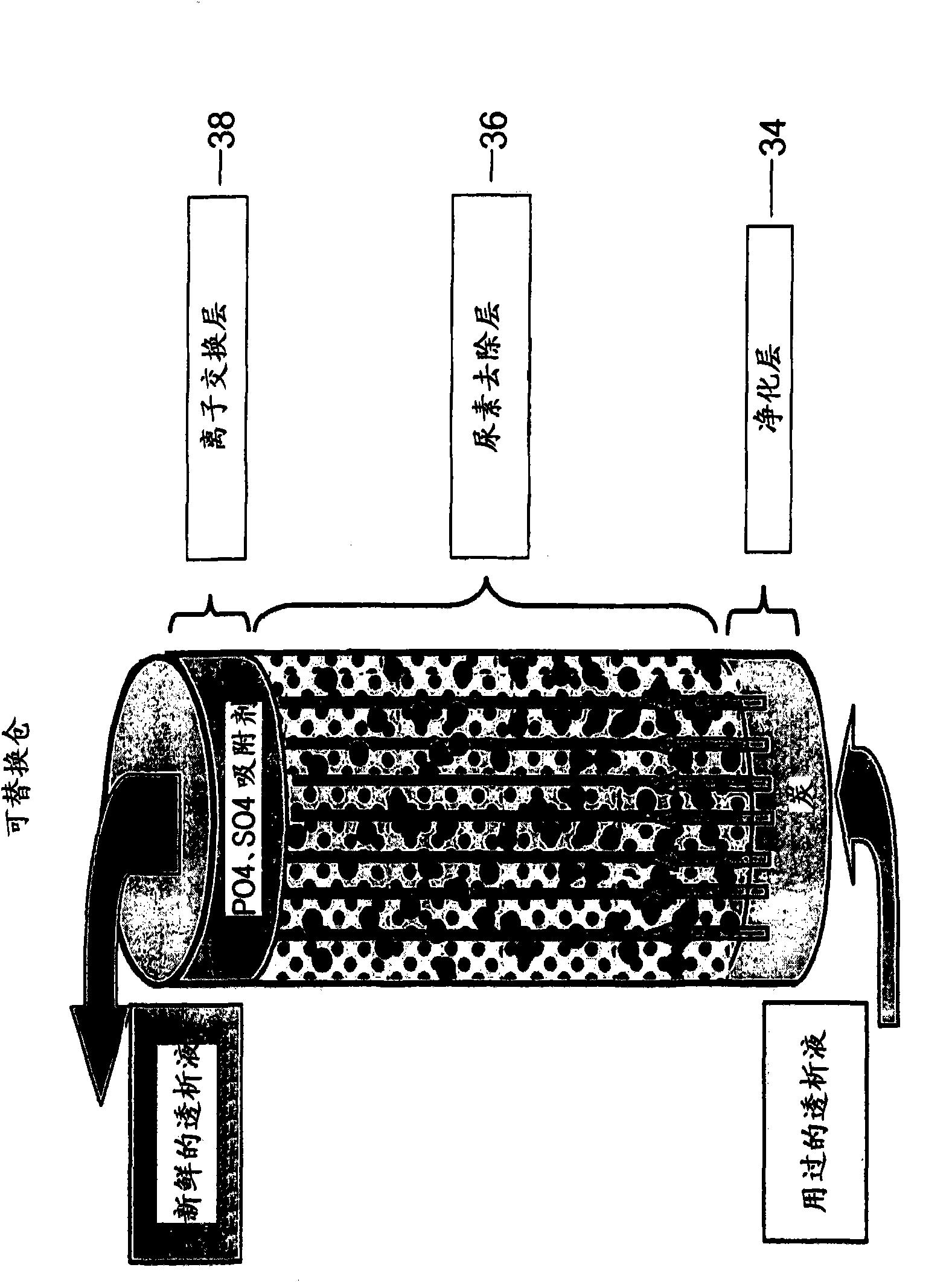
InactiveUS6986872B2Minimize the possibilityEasy to separateGeneral water supply conservationDiagnosticsPeritoneal dialysateMagnesium salt
The invention provides an apparatus and method for storing and transporting peritoneal dialysate in dry or lyophilized form, and for forming a deliverable peritoneal dialysis solution therefrom. In one embodiment, a dry reagent bed, including reagents sufficient to produce a dialysis solution, is suspended in a diluent flow path through the apparatus housing. Continuous pressure on the reagent bed causes the bed to compact as it erodes when purified water is passed through the housing. The pressure ensures complete and even dissolution of the reagents. Through dry storage and simple dissolution, even in a home, the invention enables a wider variety of solution constituents, including reduced acid content and the use of bicarbonate as a stable buffer component. The latter is illustrated in a double-bed embodiment, where bicarbonate is stored separately from calcium or magnesium salts within a single housing.
Owner:PRISMEDICAL CORP
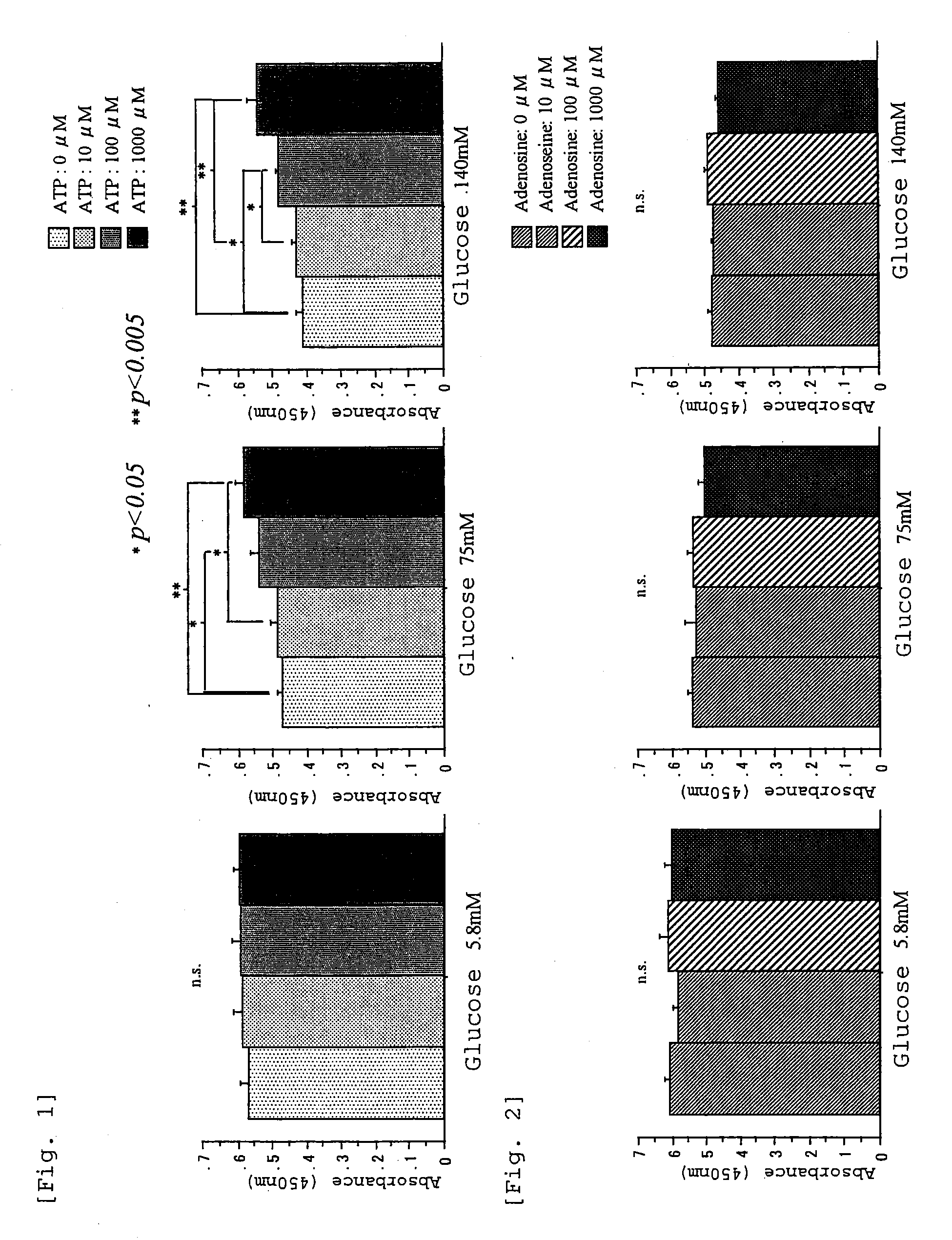
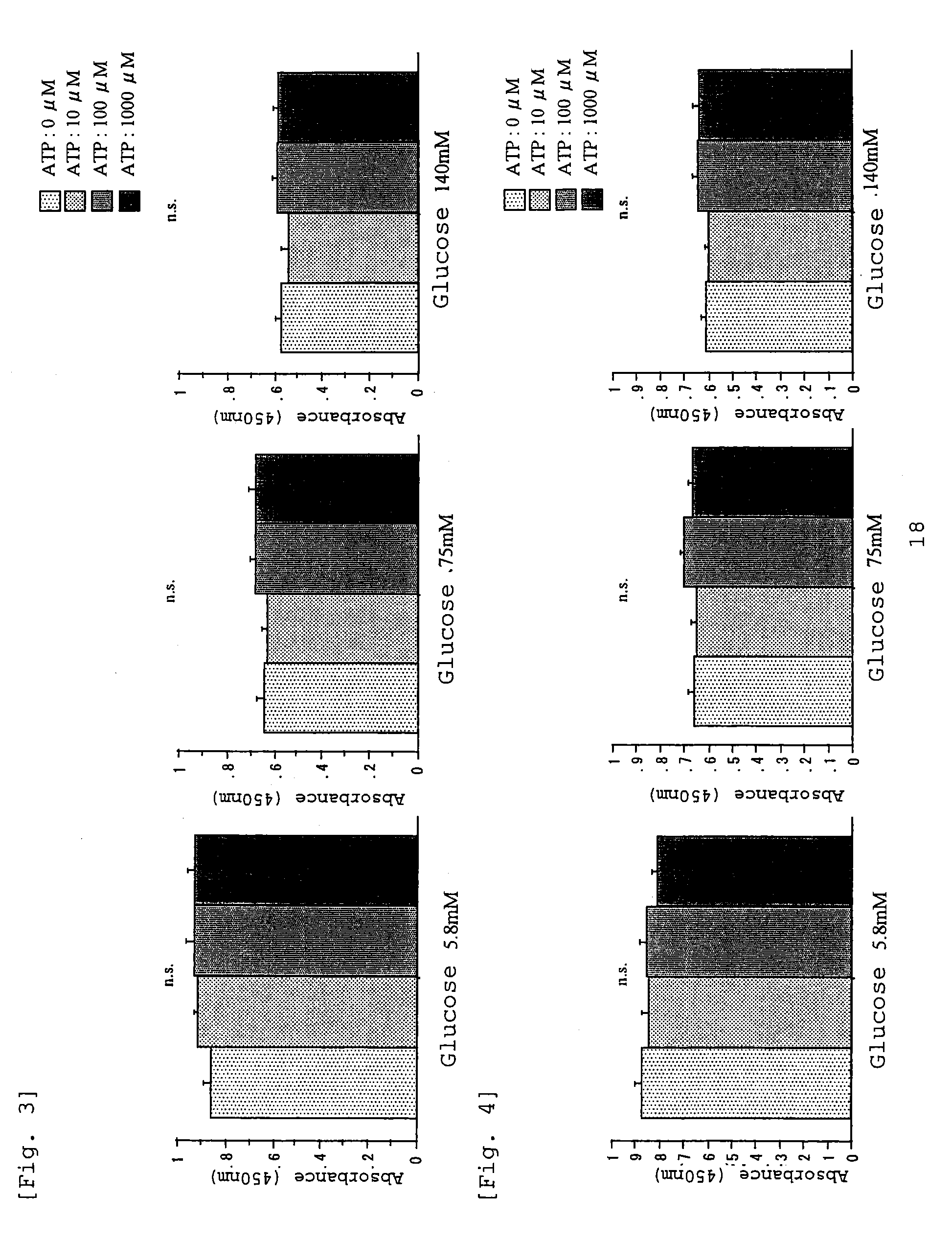
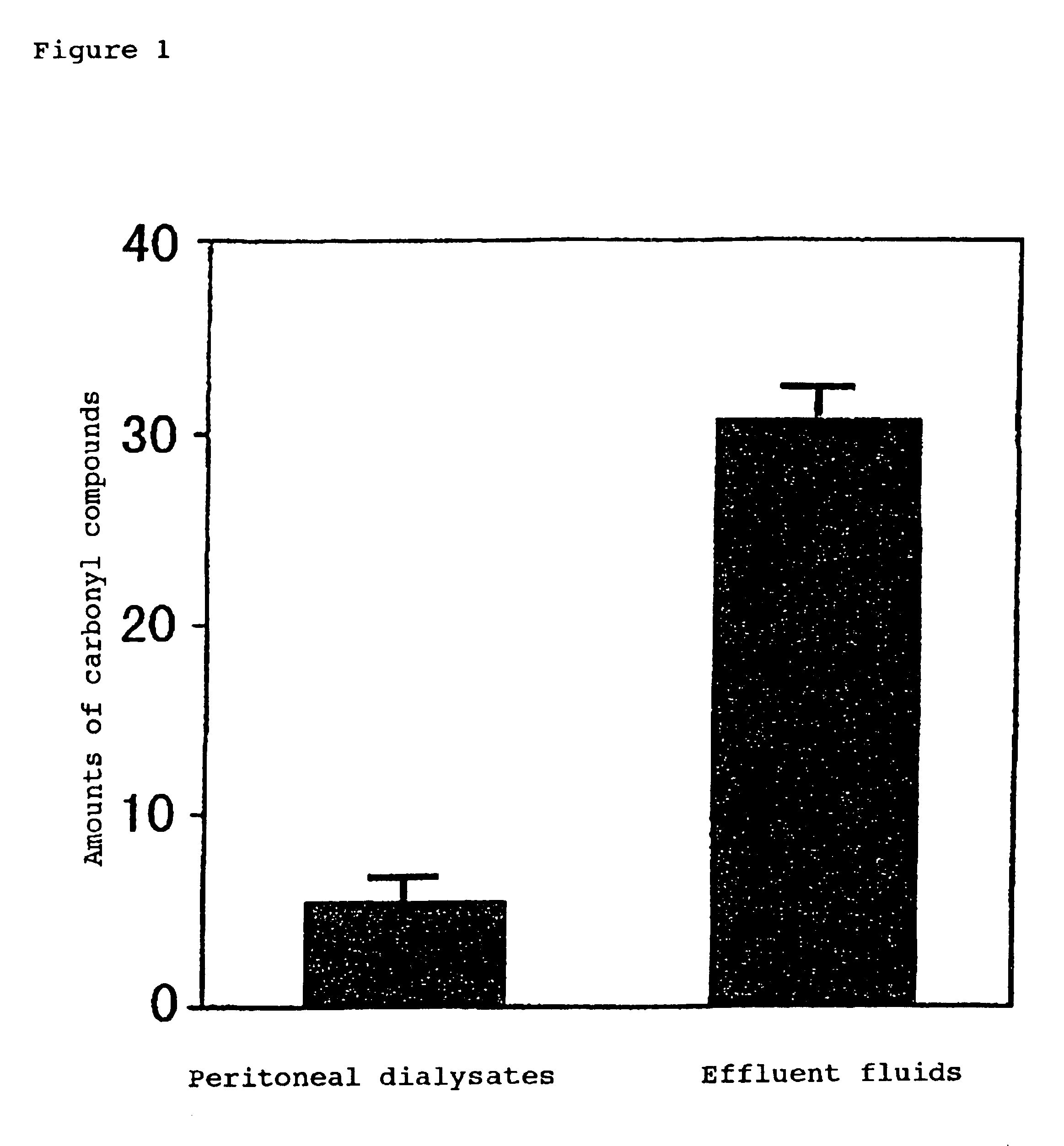
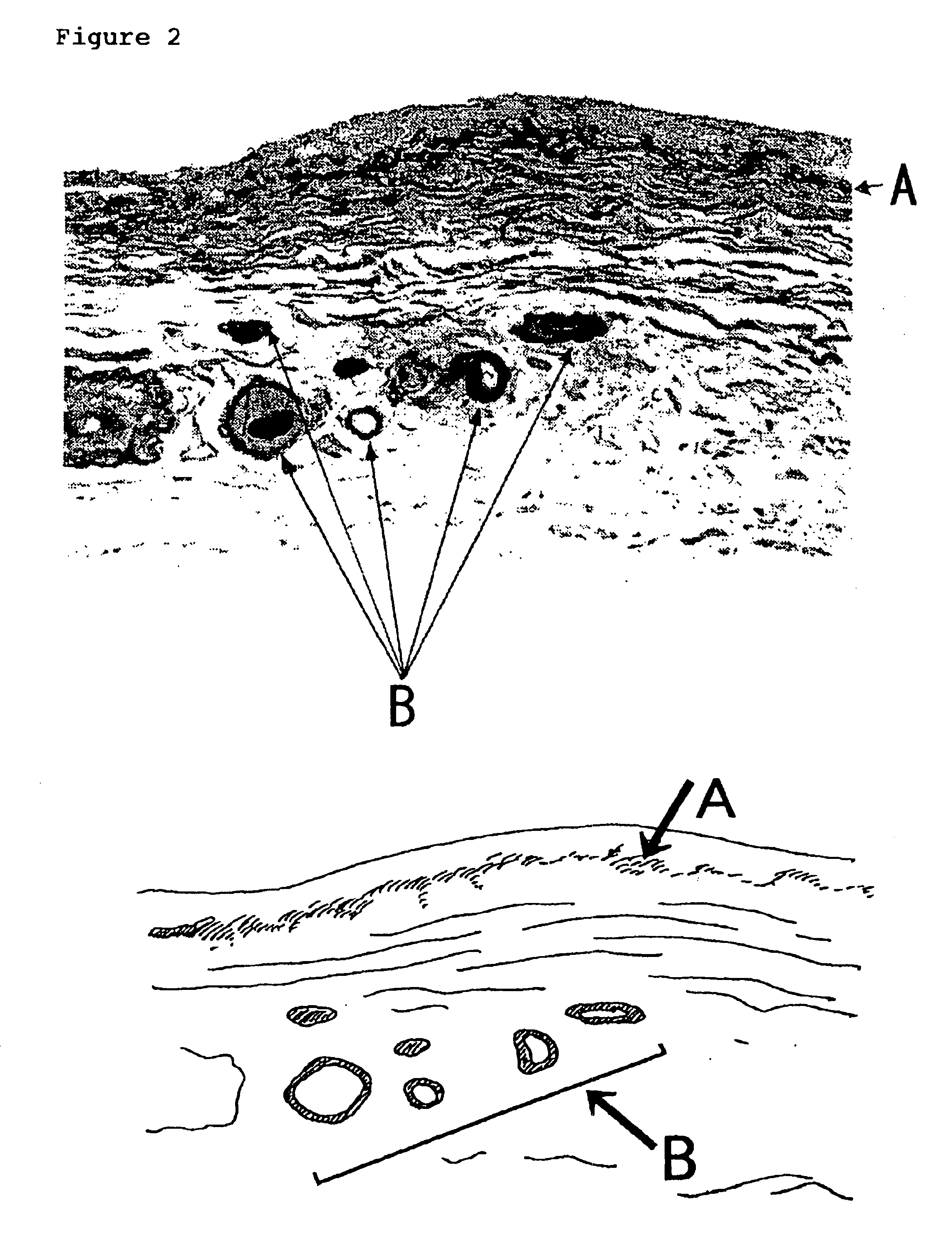
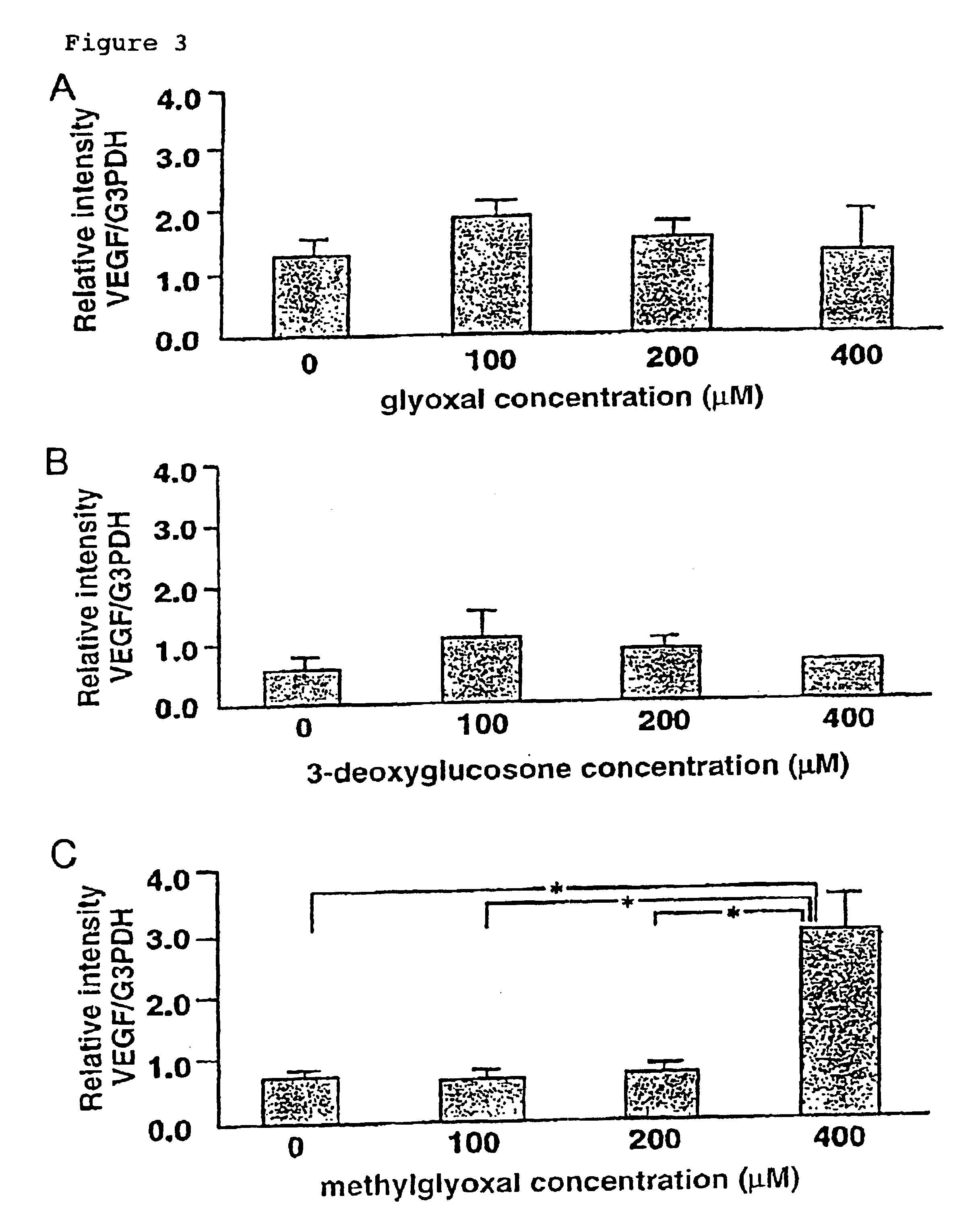
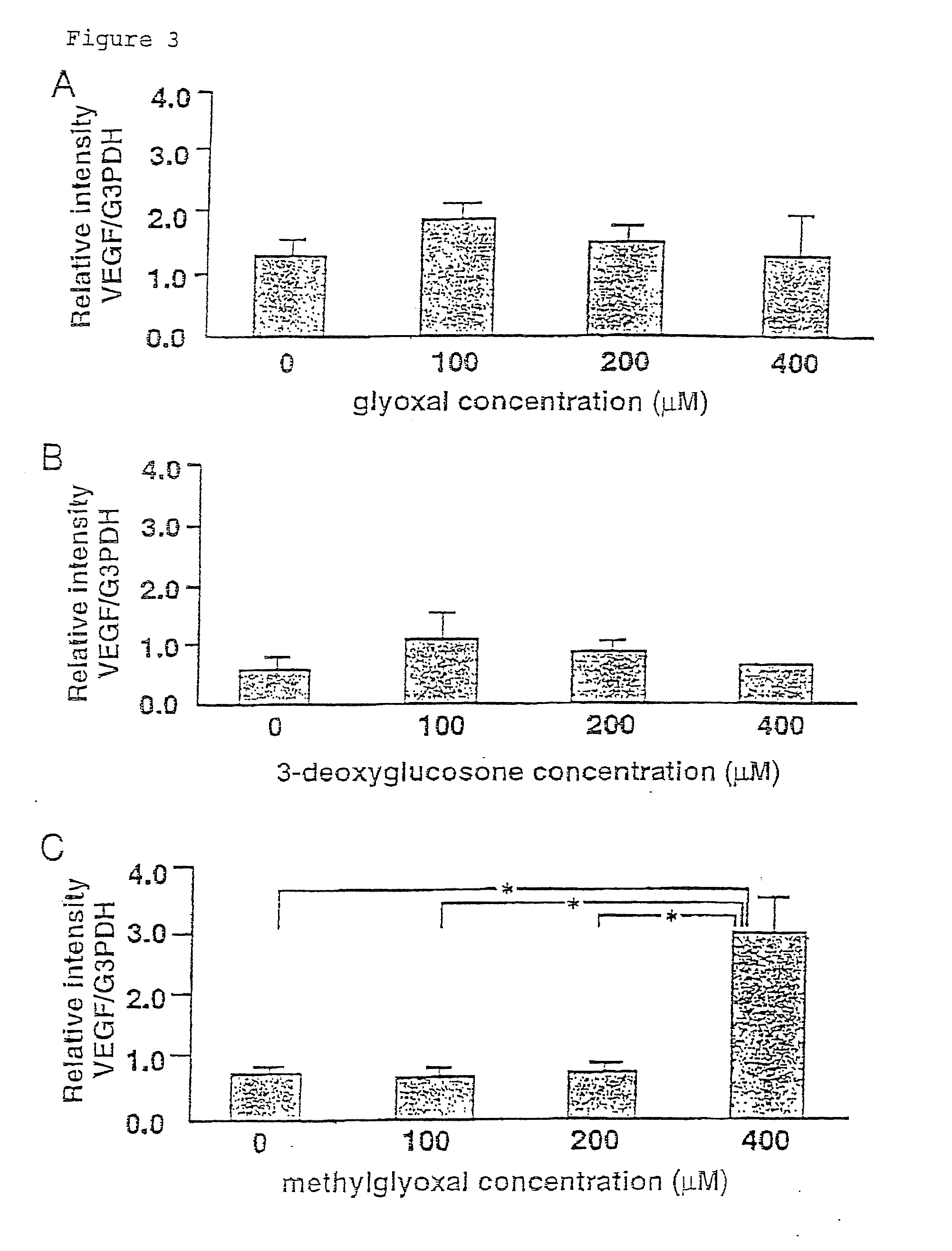
Carbonyl-stress improving agent and peritoneal dialysate
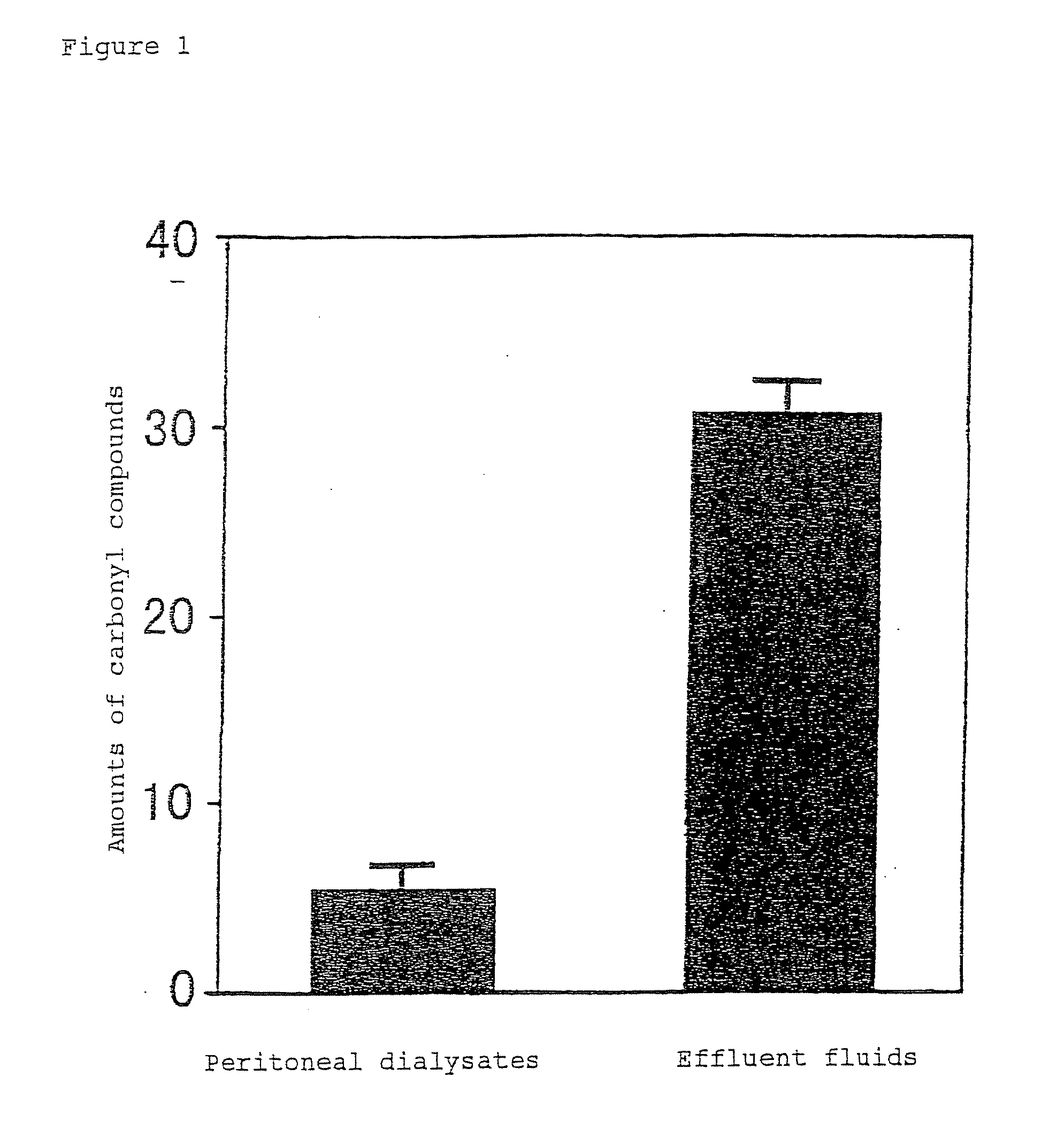
InactiveUS6919326B1Reduce harmImproving carbonyl-stress stateBiocideInorganic active ingredientsPeritoneal dialysateIntensive care medicine
Carbonyl compounds generated and accumulated in the peritoneal dialysate can be inactivated or eliminated by a carbonyl compound-trapping agent such as aminoguanidine. Carbonyl compounds generated during sterilization and storage of the peritoneal dialysate can be eliminated by pre-contacting with the trapping agent. Further, it is possible to eliminate carbonyl compounds transferred from the blood to the peritoneal cavity of the patient during peritoneal dialysis treatment, by adding the trapping agent to the peritoneal dialysate or by circulating the fluid through a carbonyl compound-trapping cartridge. Intraperitoneal protein modification by carbonyl compounds is inhibited by the present invention, thereby sufficiently reducing peritoneal disorders associated with peritoneal dialysis treatment.
Owner:MIYATA TOSHIO +3
Regenerative peritoneal dialysis system
PendingUS20170281847A1Organic active ingredientsInorganic active ingredientsEngineeringMechanical engineering
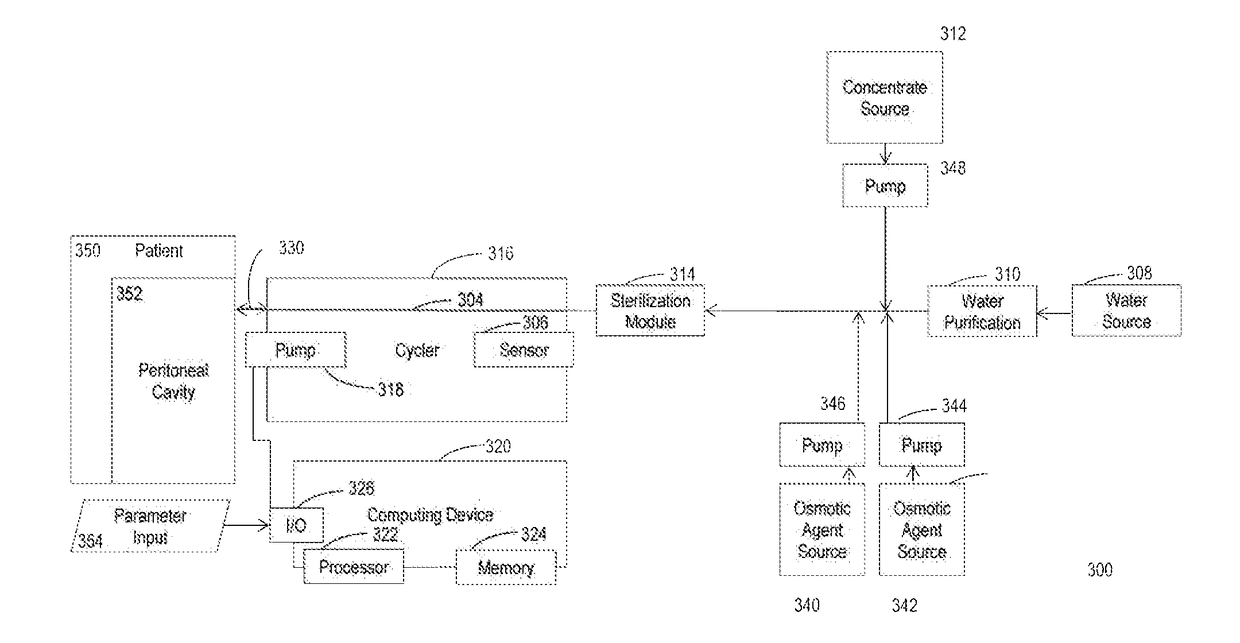
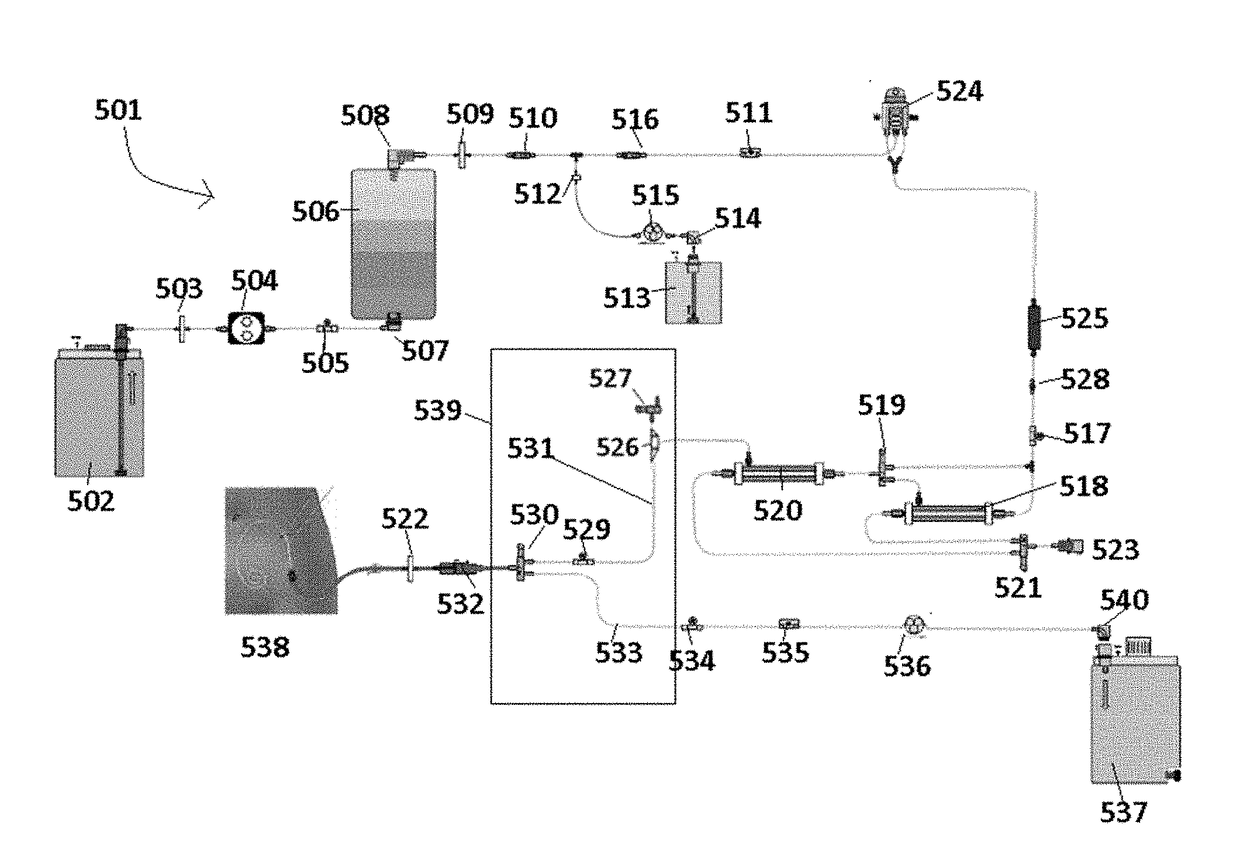
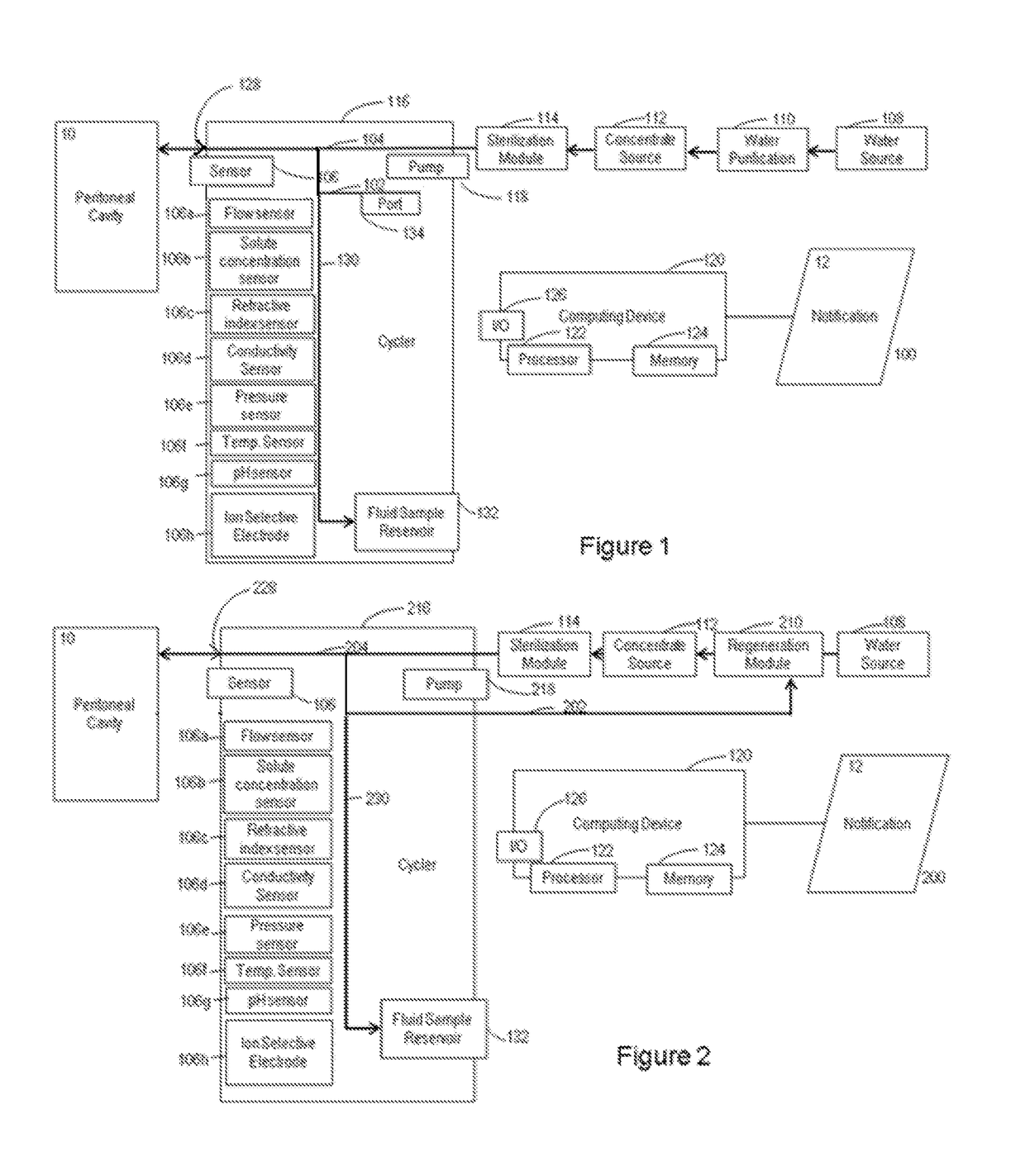
Systems and methods of generating and regenerating peritoneal dialysate are provided. The systems and methods use a dialysate regeneration module, a sterilization module and concentrates to prepare peritoneal dialysate from used peritoneal dialysate or source water. An optional integrated cycler for direct infusion of the generated peritoneal dialysate is included. Optional dialysate storage containers are provided for storage of the peritoneal dialysate prior to use.
Owner:MOZARC MEDICAL US LLC
Peritoneal dialysate fluid generation system with integrated cycler
PendingUS20170281846A1Specific water treatment objectivesMedical devicesPeritoneal dialysis solutionsDialysis fluid
Systems and methods of generating peritoneal dialysate and using the peritoneal dialysate with an integrated cycler are provided. The systems and methods use a water purification module, a sterilization module and concentrates to prepare peritoneal dialysate from source water and infuse the prepared peritoneal dialysate into a patient with an integrated cycler. Optional dialysate storage containers are provided for storage of the peritoneal dialysate prior to use.
Owner:MOZARC MEDICAL US LLC
Peritoneal dialysis fluid
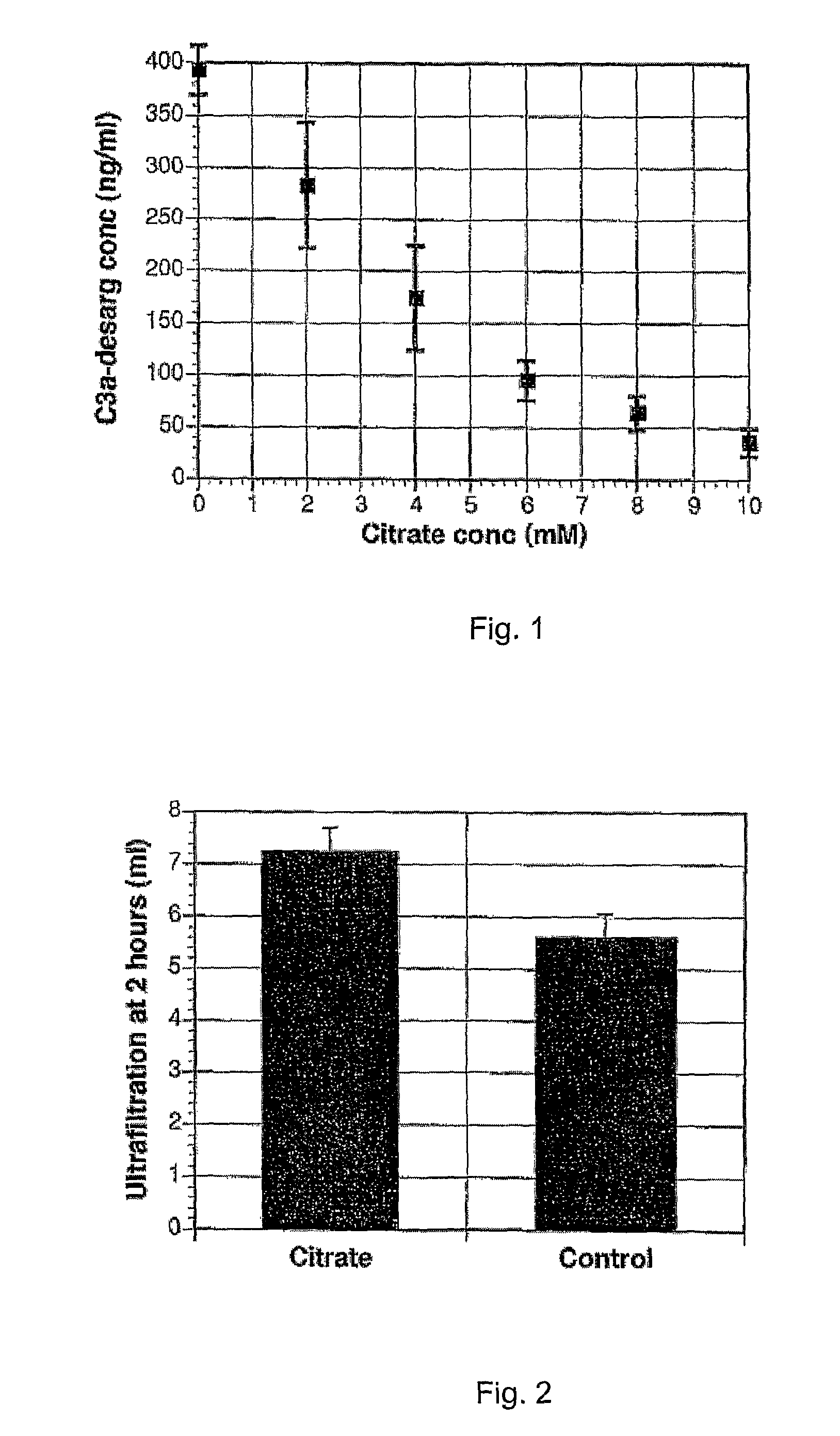
ActiveUS8367731B2Less tendency to become absorbedUltrafiltration during a dwell is enhancedBiocidePharmaceutical delivery mechanismCITRATE ESTERPeritoneal dialysis fluid
The present invention concerns a peritoneal dialysis fluid with enhanced ultrafiltration during the dialysis dwell period. According to the present invention this is achieved by a peritoneal dialysis fluid comprising sodium ions, osmotic agent and a buffer, characterised in that it comprises citrate at a level of 4 to 10 mM in a final solution ready for use.
Owner:FRESENIUS MEDICAL CARE DEUTSCHLAND GMBH
Method for preparing peritoneal dialysate
InactiveUS20070020341A1Reduce harmMinimize damageBiocideInorganic active ingredientsPeritoneal dialysateIntensive care medicine
Carbonyl compounds generated and accumulated in the peritoneal dialysate can be inactivated or eliminated by a carbonyl compound-trapping agent such as aminoguanidine. Carbonyl compounds generated during sterilization and storage of the peritoneal dialysate can be eliminated by pre-contacting with the trapping agent. Further, it is possible to eliminate carbonyl compounds transferred from the blood to the peritoneal cavity of the patient during peritoneal dialysis treatment, by adding the trapping agent to the peritoneal dialysate or by circulating the fluid through a carbonyl compound-trapping cartridge. Intraperitoneal protein modification by carbonyl compounds is inhibited by the present invention, thereby sufficiently reducing peritoneal disorders associated with peritoneal dialysis treatment.
Owner:KUROKAWA KIYOSHI +2
Fluid for peritoneal dialysis
InactiveUS20140148409A1Good biocompatibilityImprove removabilityBiocideOrganic active ingredientsBiocompatibility TestingBody fluid
The present invention has an object to provide a fluid for peritoneal dialysis with satisfactory body fluid removability, high biocompatibility, and improved storage stability, and the object is attained by a fluid for peritoneal dialysis containing one or more saccharides selected from cyclonigerosylnigerose, cyclomaltosylmaltose, and L-ascorbic acid 2-glucoside.
Owner:HAYASHIBARA BIOCHEMICAL LAB INC
Peritoneal dialysis method
InactiveUS8222229B2Avoid excessive injuryAvoid injuryBiocideAntipyreticPeritoneal dialysateIntensive care medicine
Owner:KOWA CO LTD
Methods and compositions for detection of microbial contaminants in peritoneal dialysis solutions
InactiveUS7118857B2Simple methodPrevent peritonitisAntibacterial agentsOrganic active ingredientsBiologyPeritoneal dialysis solutions
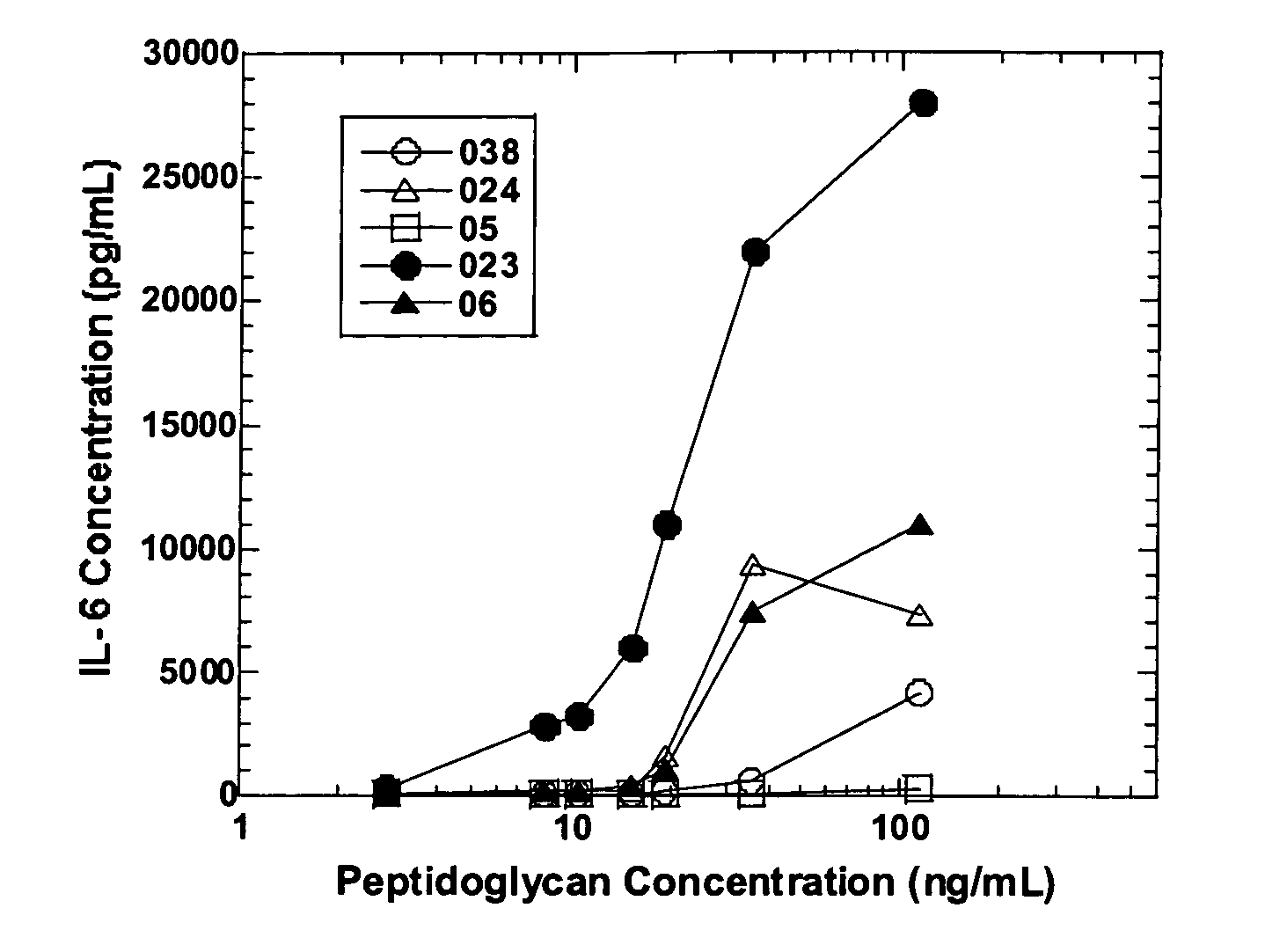
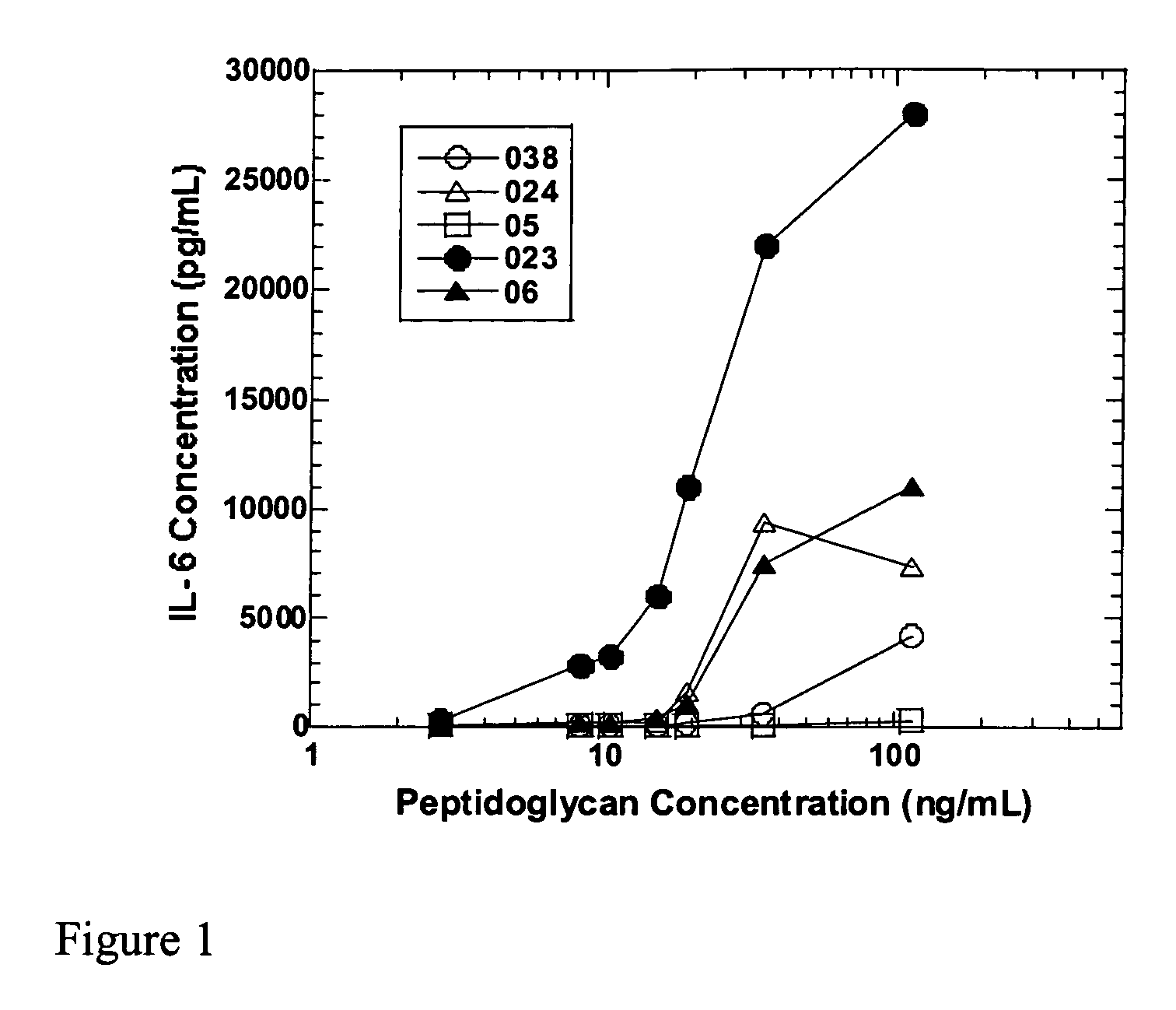
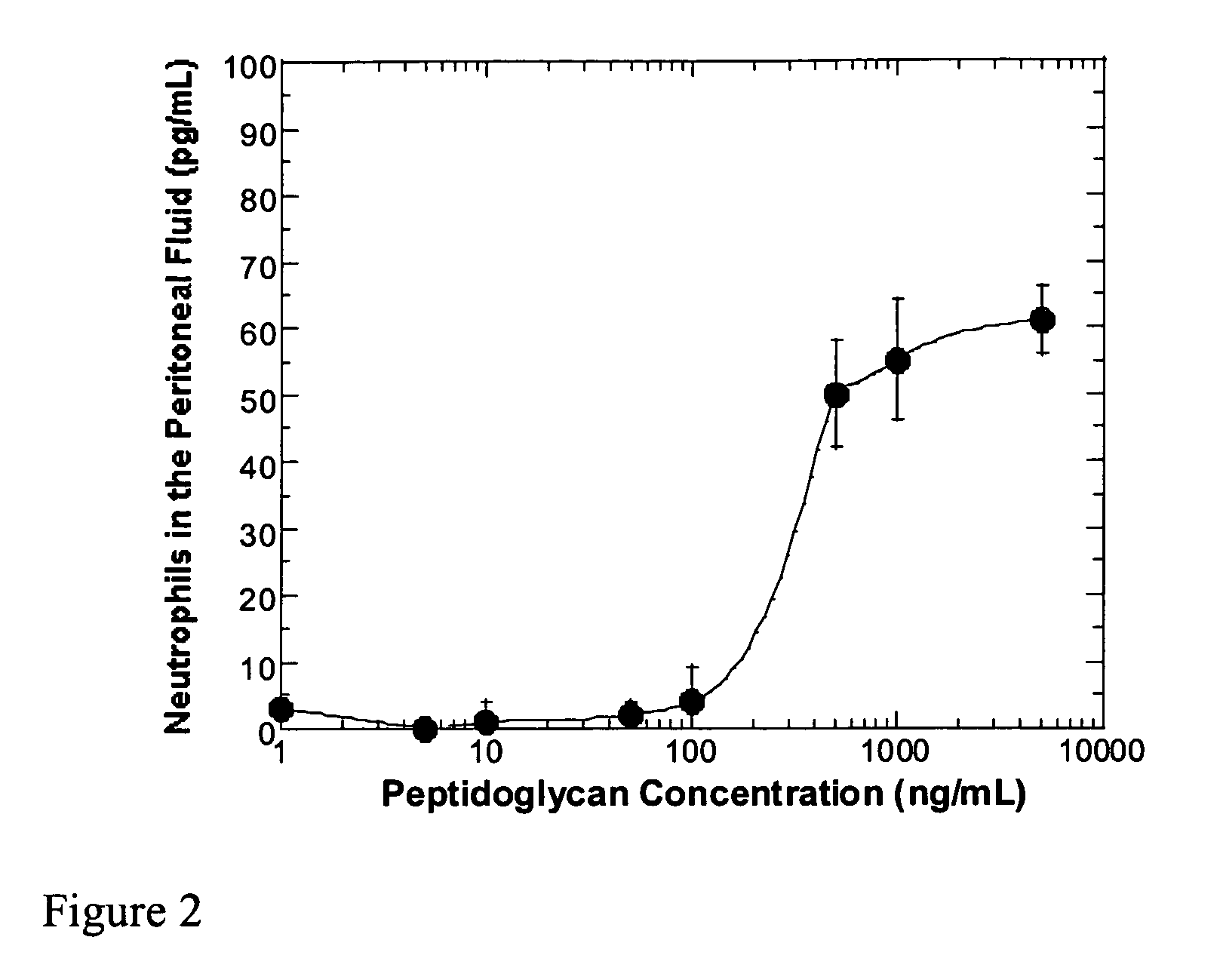
Methods and compositions for detection of microbial contaminants in peritoneal dialysis solutions are provided. The methods and compositions employ modified bioburden testing and the detection of peptidoglycan. A novel cause of aseptic peritonitis is provided—aseptic peritonitis associated with gram positive microbial contamination of a dialysis solution. Peptidoglycan is a major component of a gram positive bacterial cell wall and thus can serve as a marker for gram positive bacteria. In this regard, testing for peptidoglycans can be utilized to effectively prevent peritonitis in patients that use the peritoneal dialysis solutions, such as peritoneal dialysis solutions that contain a glucose polymer including an icodextrin and the like.
Owner:BAXTER INT INC +1
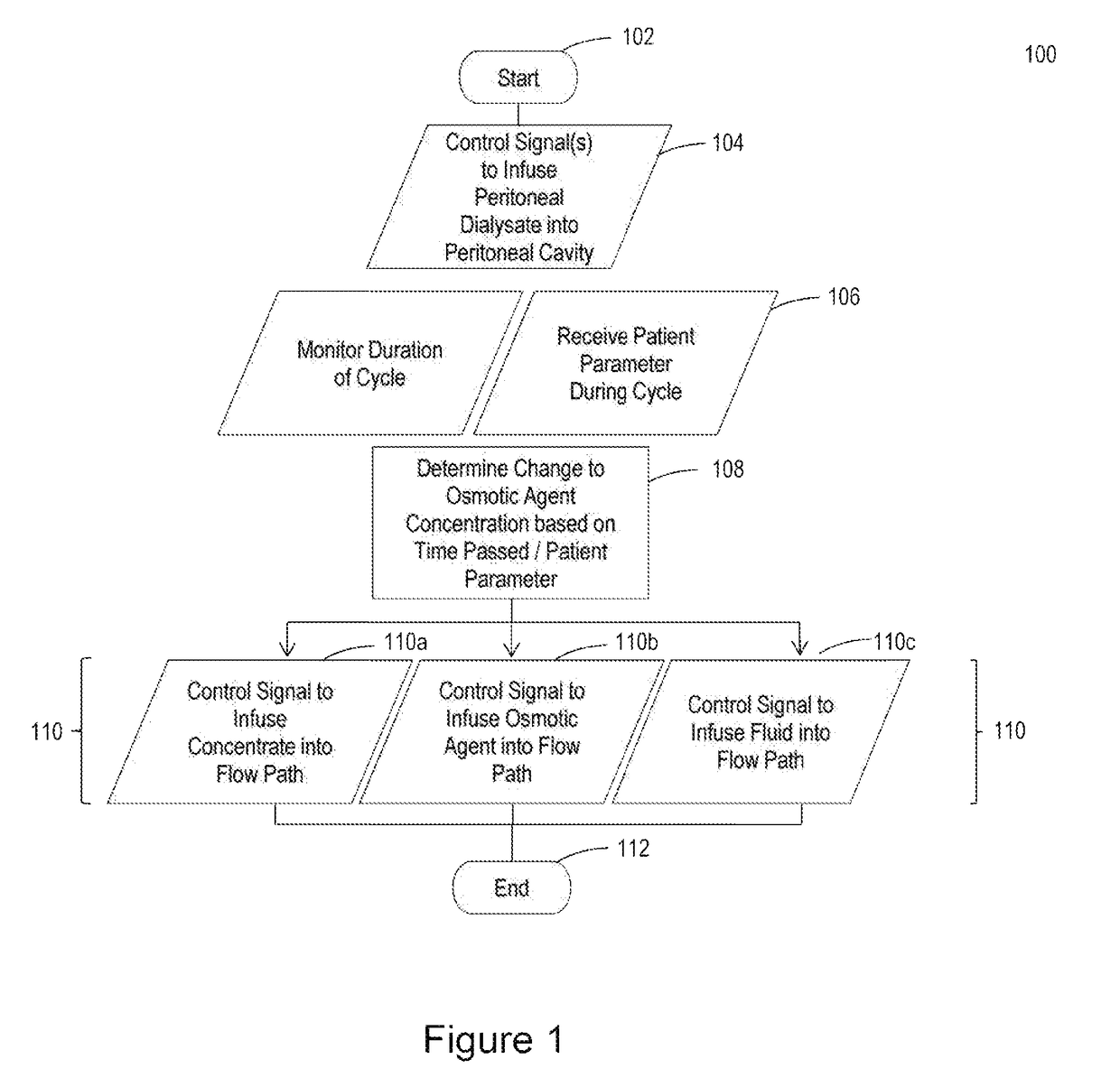
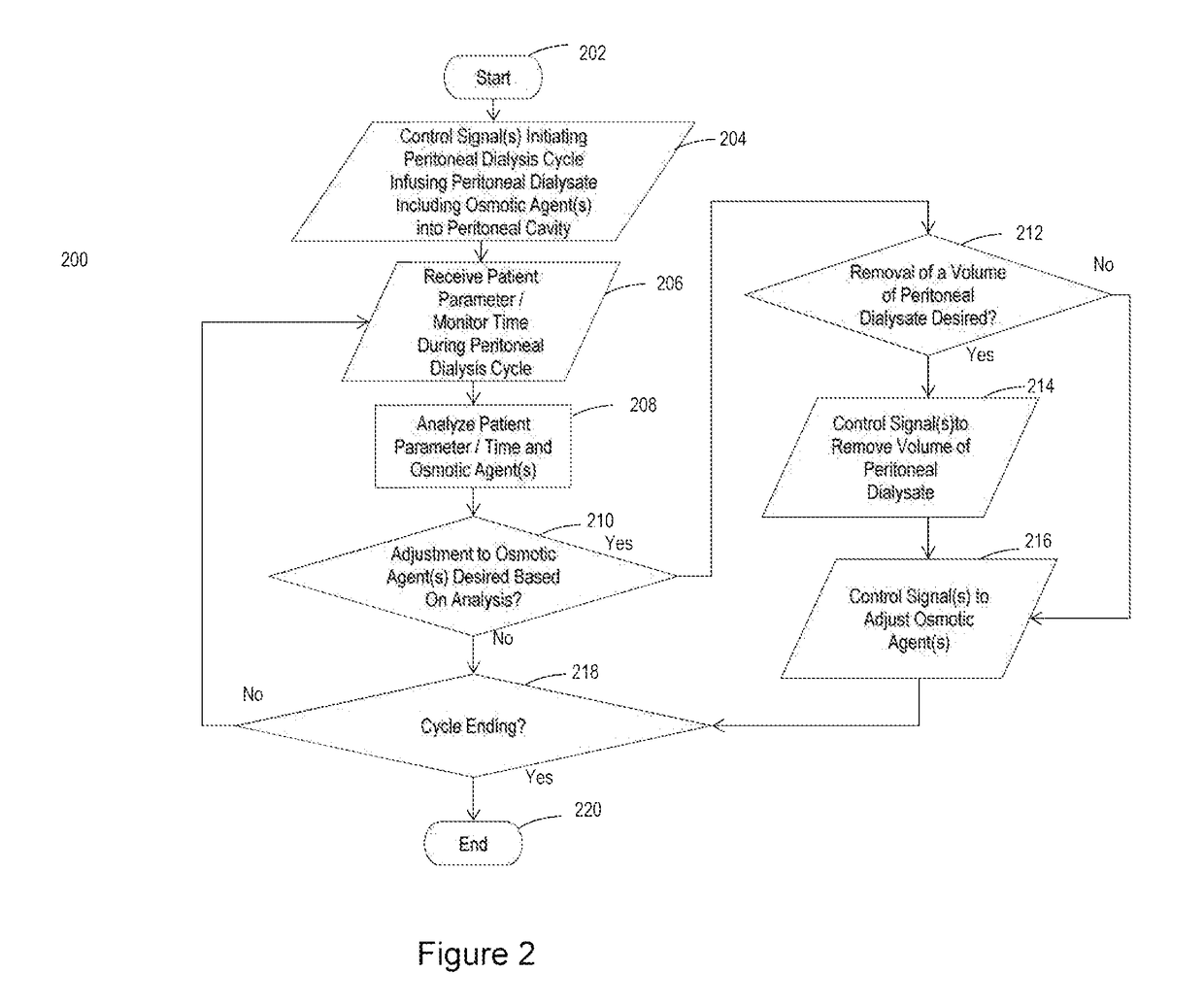
Peritoneal dialysis intracycle osmotic agent adjustment
The invention relates to systems and methods for making intracycle adjustments to an osmotic agent concentration of peritoneal dialysate inside the peritoneal cavity of a patient. The systems and methods include osmotic agent sources, flow paths, and processors to adjust the osmotic agent concentration of dialysate in the peritoneal cavity of the patient. The method can include infusing peritoneal dialysate containing an osmotic agent into the peritoneal cavity of a patient; monitoring one or more patient parameters; and adjusting the osmotic agent concentration of the peritoneal dialysate in the peritoneal cavity of the patient by infusing a concentrated osmotic agent solution or by infusing sterile fluid into the peritoneal cavity of the patient using an on-line peritoneal dialysis machine. The system can include a peritoneal dialysis cycler.
Owner:MOZARC MEDICAL US LLC
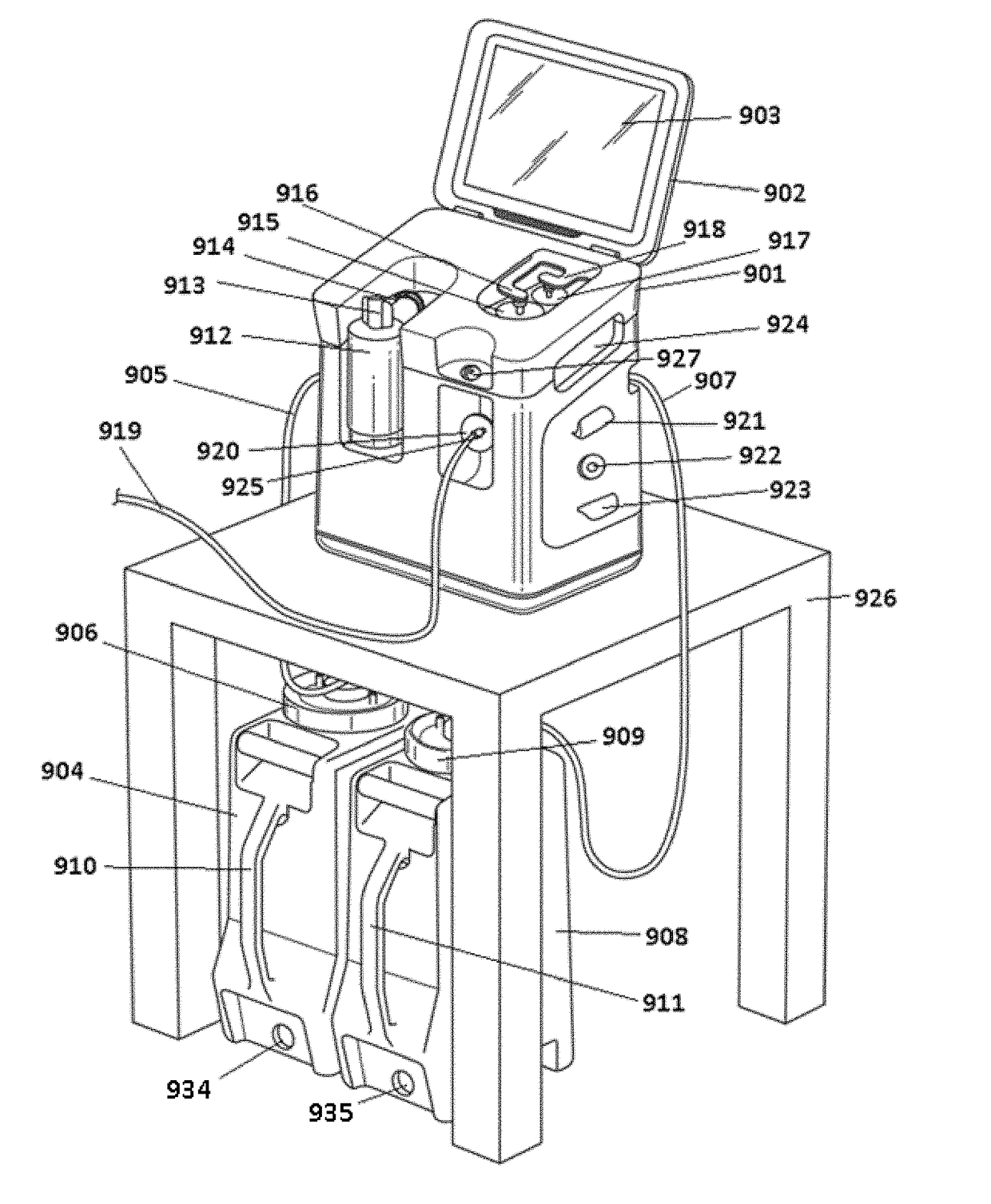
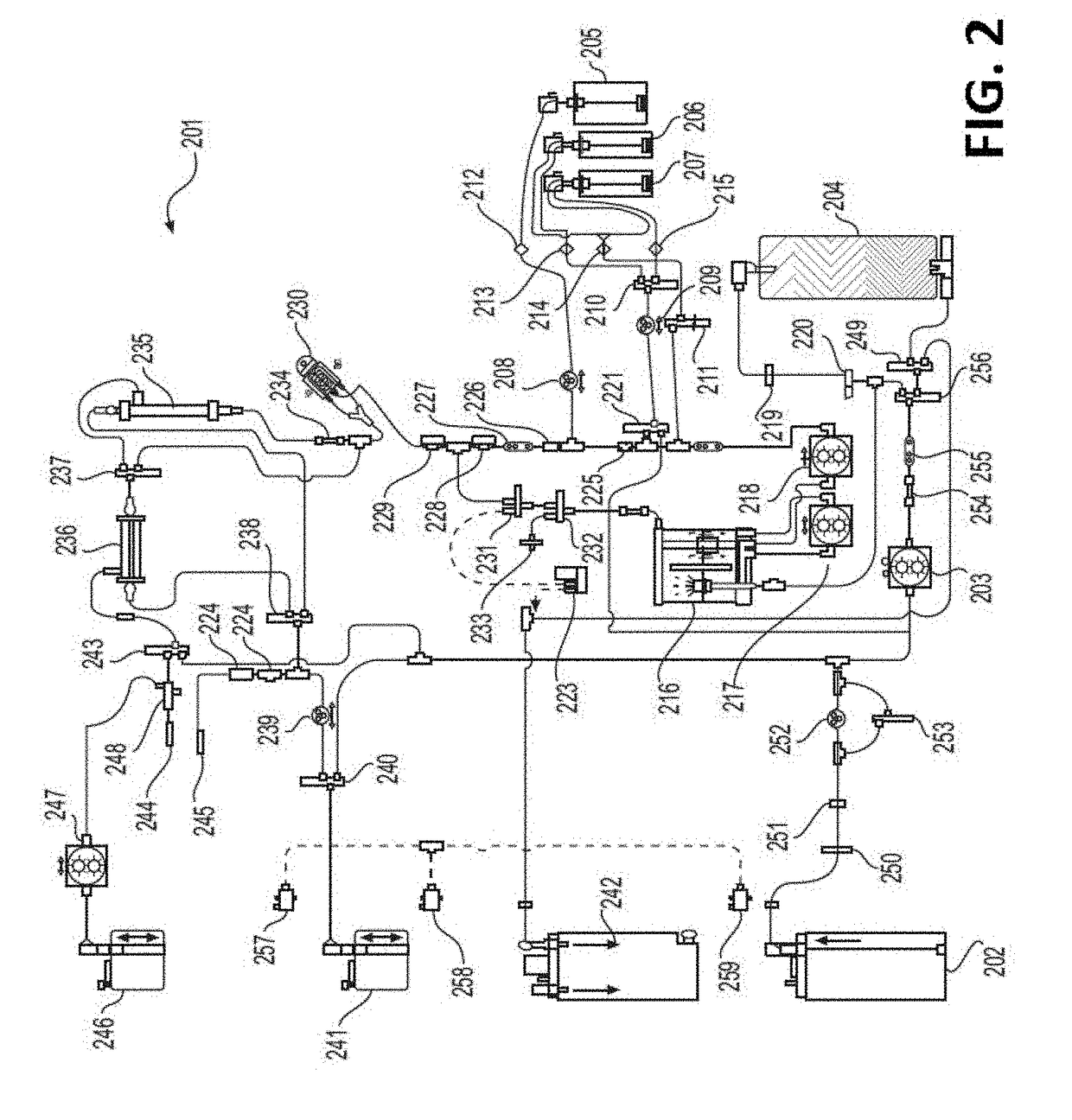
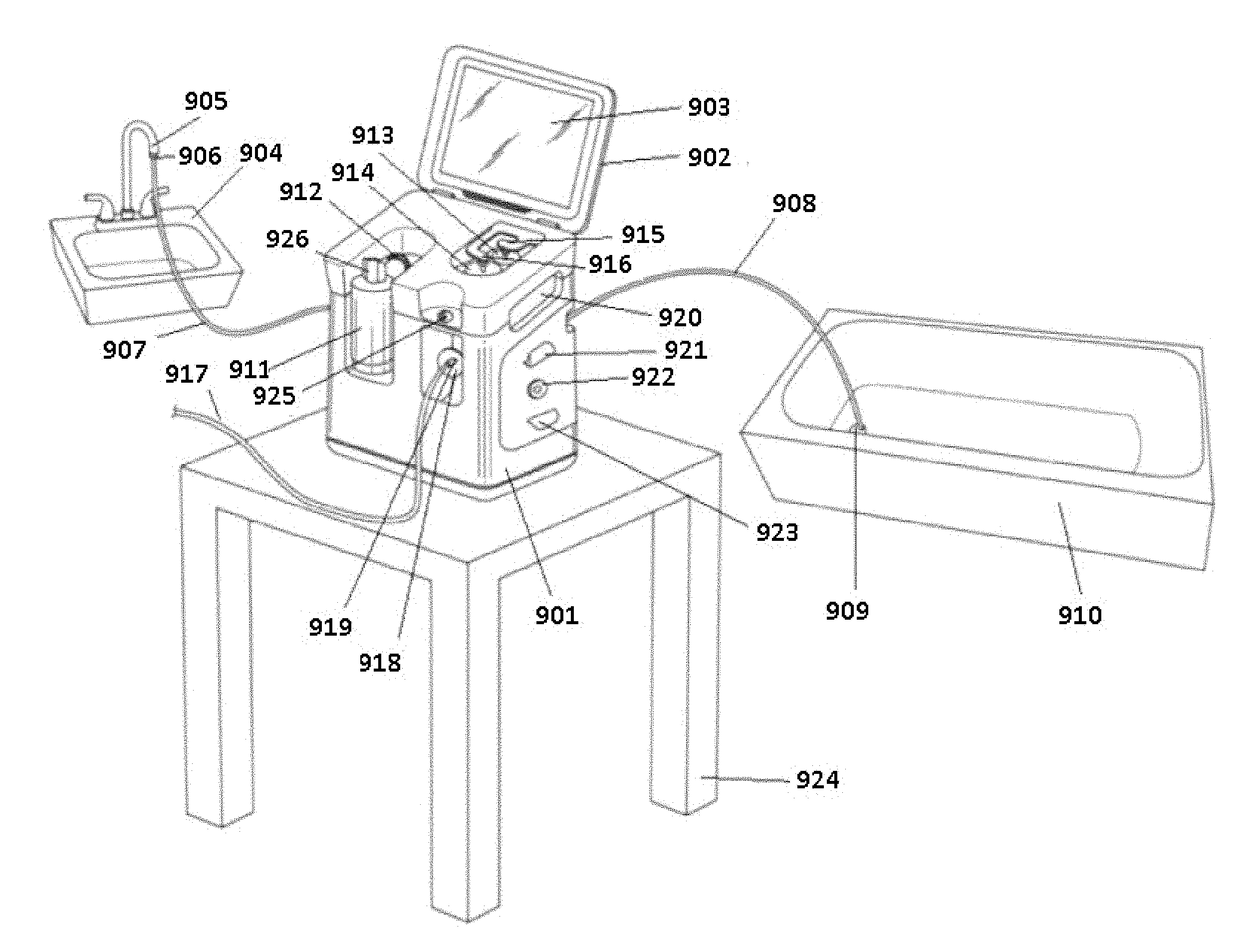
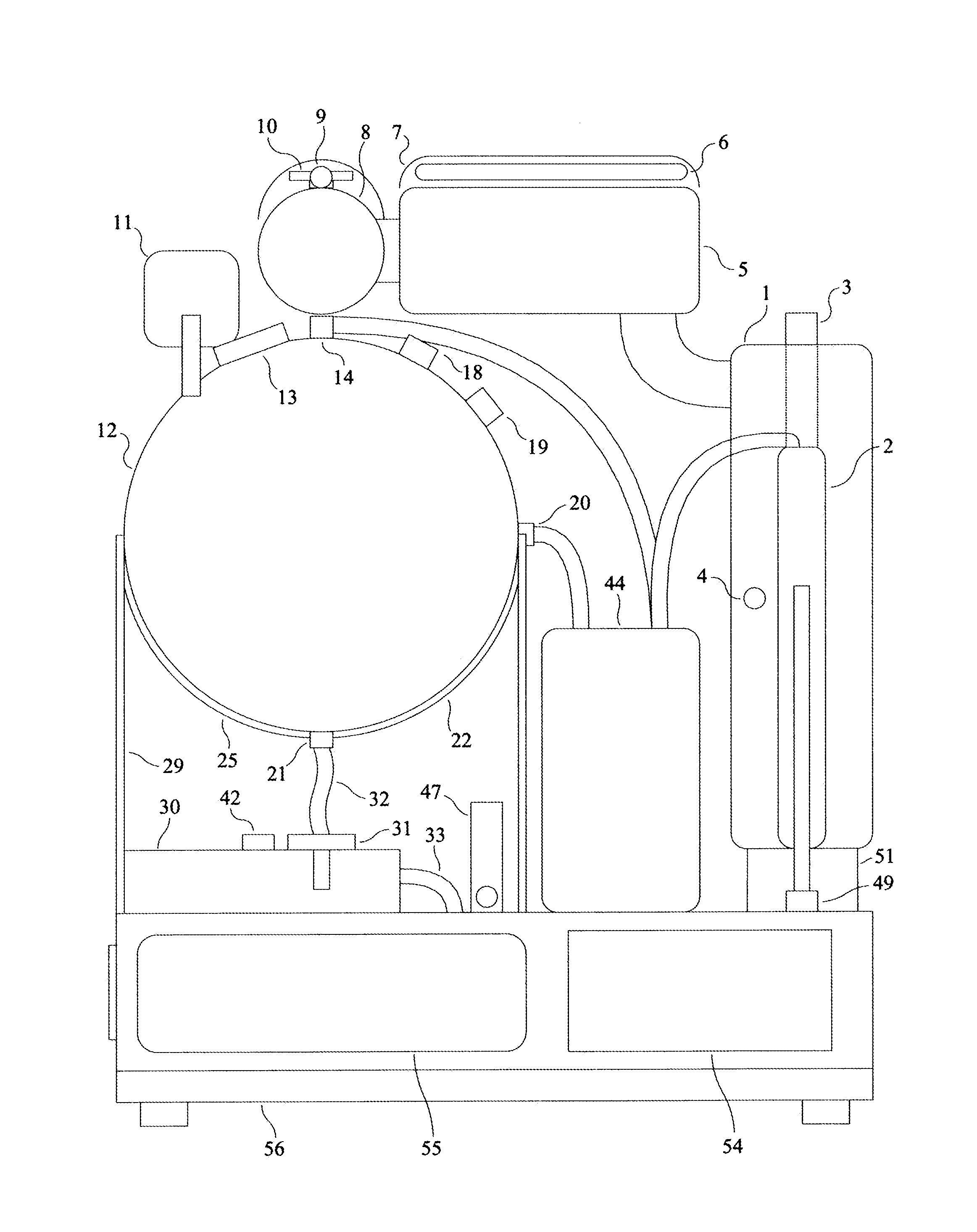
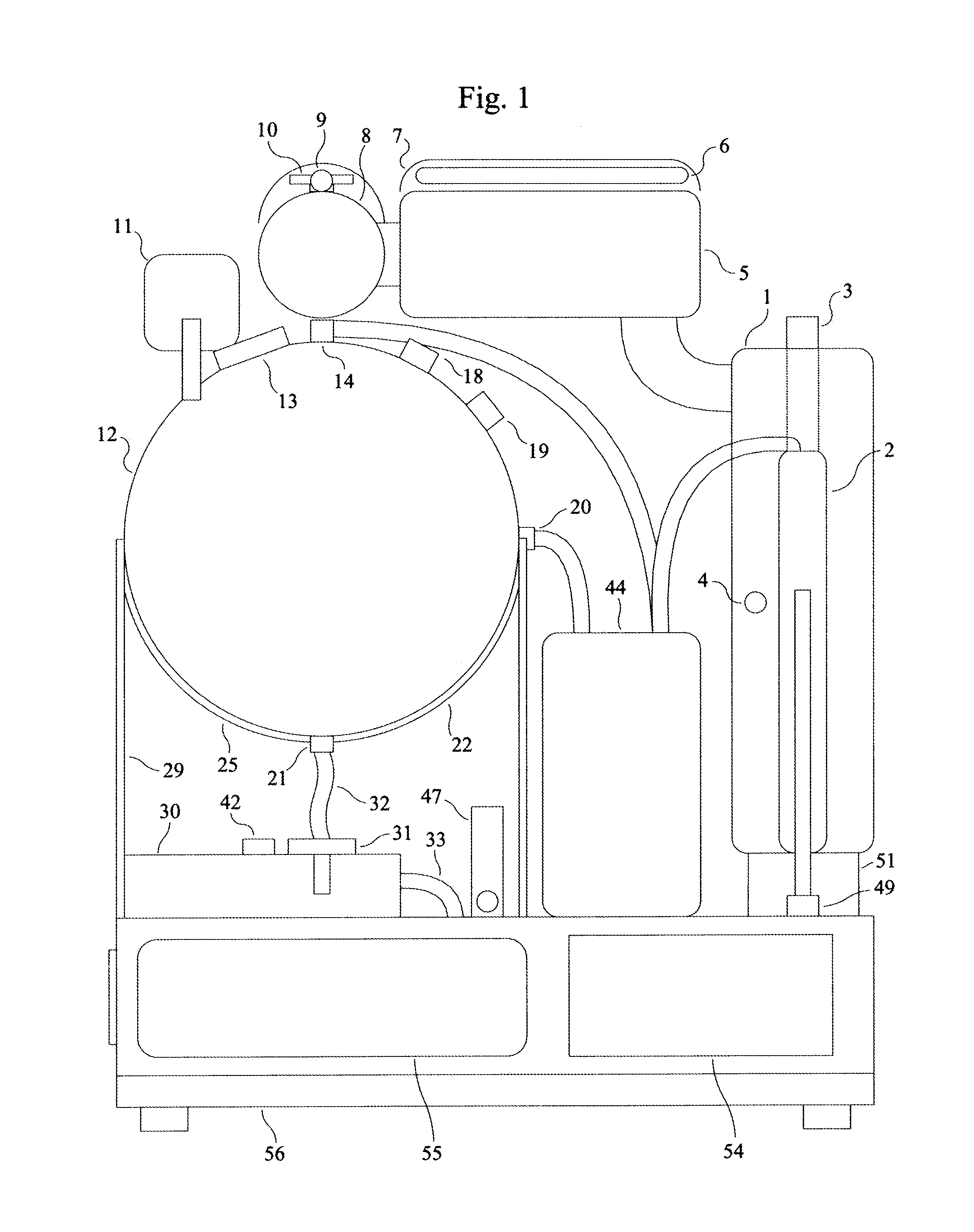
Peritoneal Dialysis System and Method
A peritoneal dialysis machine that includes a base, a boiling vessel, a condenser, a fluid storage vessel, a sterilizing UV lamp, a fluid mixer, a dialysate cassette, a boiling vessel demineralizing system, and an optional fluid storage vessel rinsing system. The peritoneal dialysis machine automatically generates a predetermined volume of distilled water each day. Pre-weighed quantities of low-endotoxin dialysate chemicals are then mixed into the warm distilled water, and the resulting peritoneal dialysate is sterilized via exposure to ultraviolet radiation. These actions are complete by the patient's indicated bed time. The peritoneal dialysis machine then exchanges spent peritoneal dialysate in the user's peritoneal cavity with fresh peritoneal dialysate throughout the night, causing each infusion of fresh dialysate to dwell in the user's peritoneal cavity for a predetermined time. All spent fluids are routed from the peritoneal dialysis machine to a toilet or a sewer drain.
Owner:HOFFMAN JOSEF C A
Peritoneal dialysis fluid testing system
ActiveUS20180193546A1Material analysis by observing effect on chemical indicatorMedical devicesPeritoneal dialysis fluidEngineering
The invention relates to a testing system and related methods for detecting peritonitis or infection in peritoneal dialysate removed from a patient. The testing system can include a fluid sensor apparatus in a fluid line of a peritoneal dialysis cycler through which spent peritoneal dialysate can be pumped. The fluid sensor apparatus can detect one or more markers associated with peritonitis or infection.
Owner:MOZARC MEDICAL US LLC
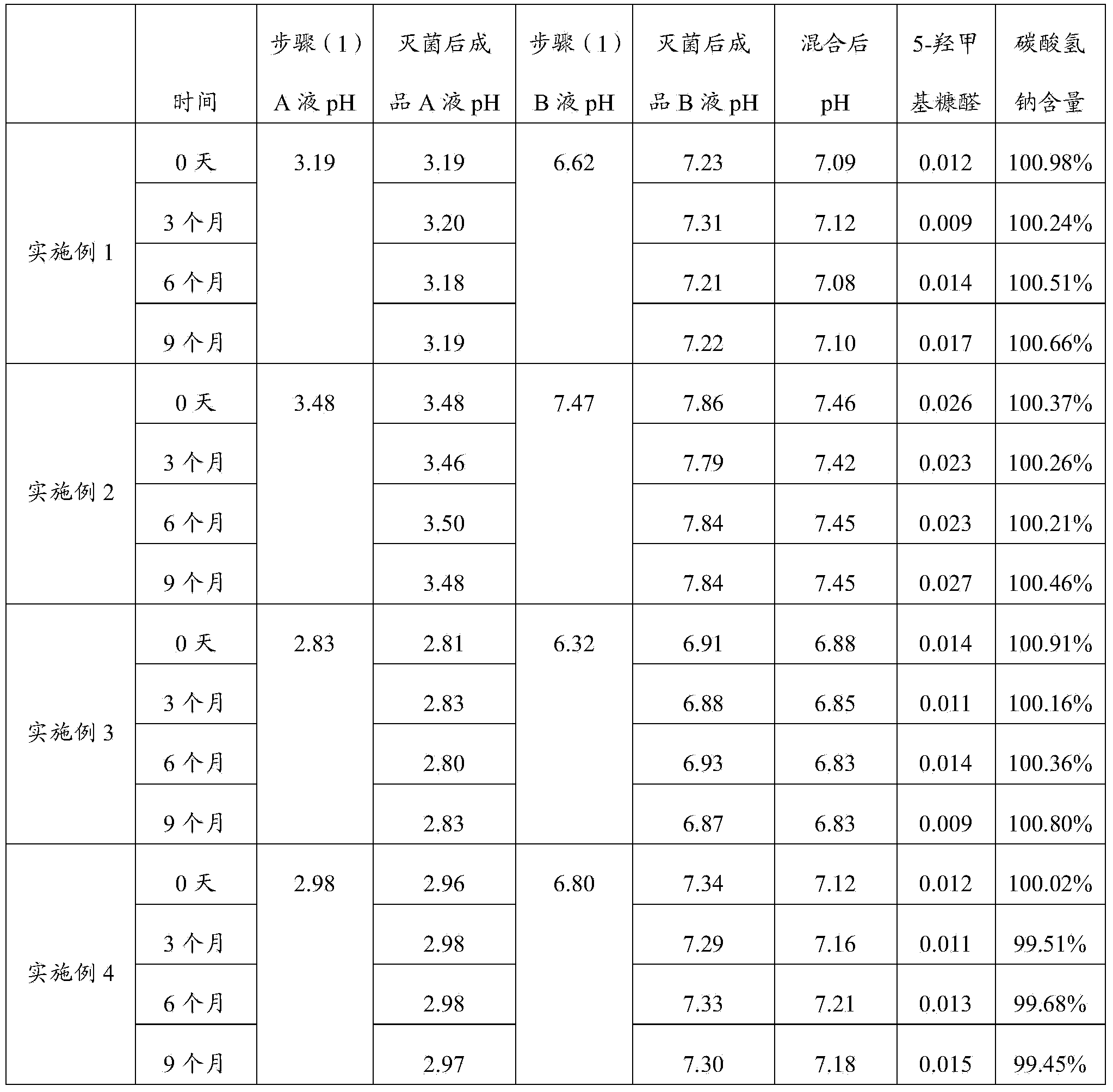
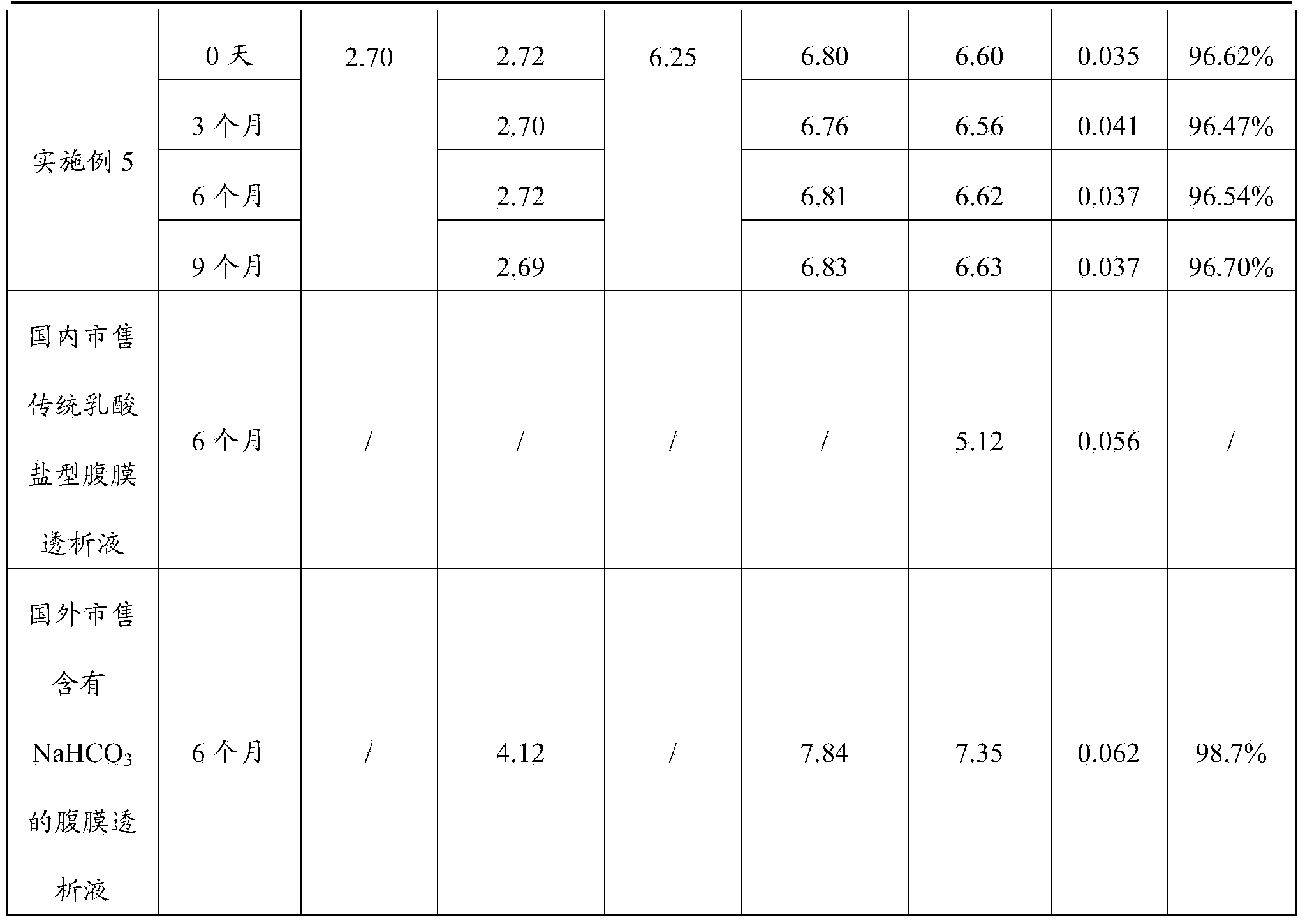
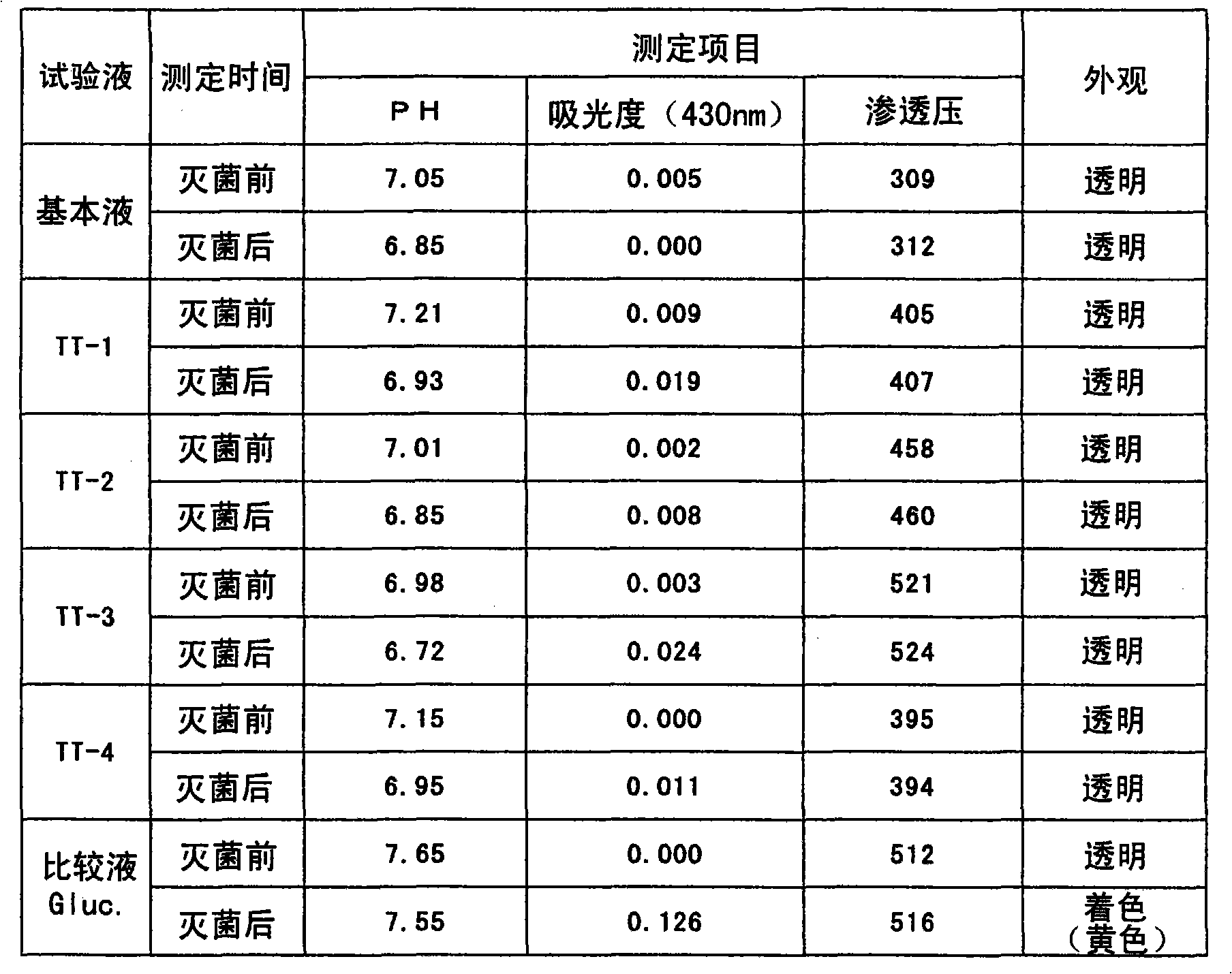
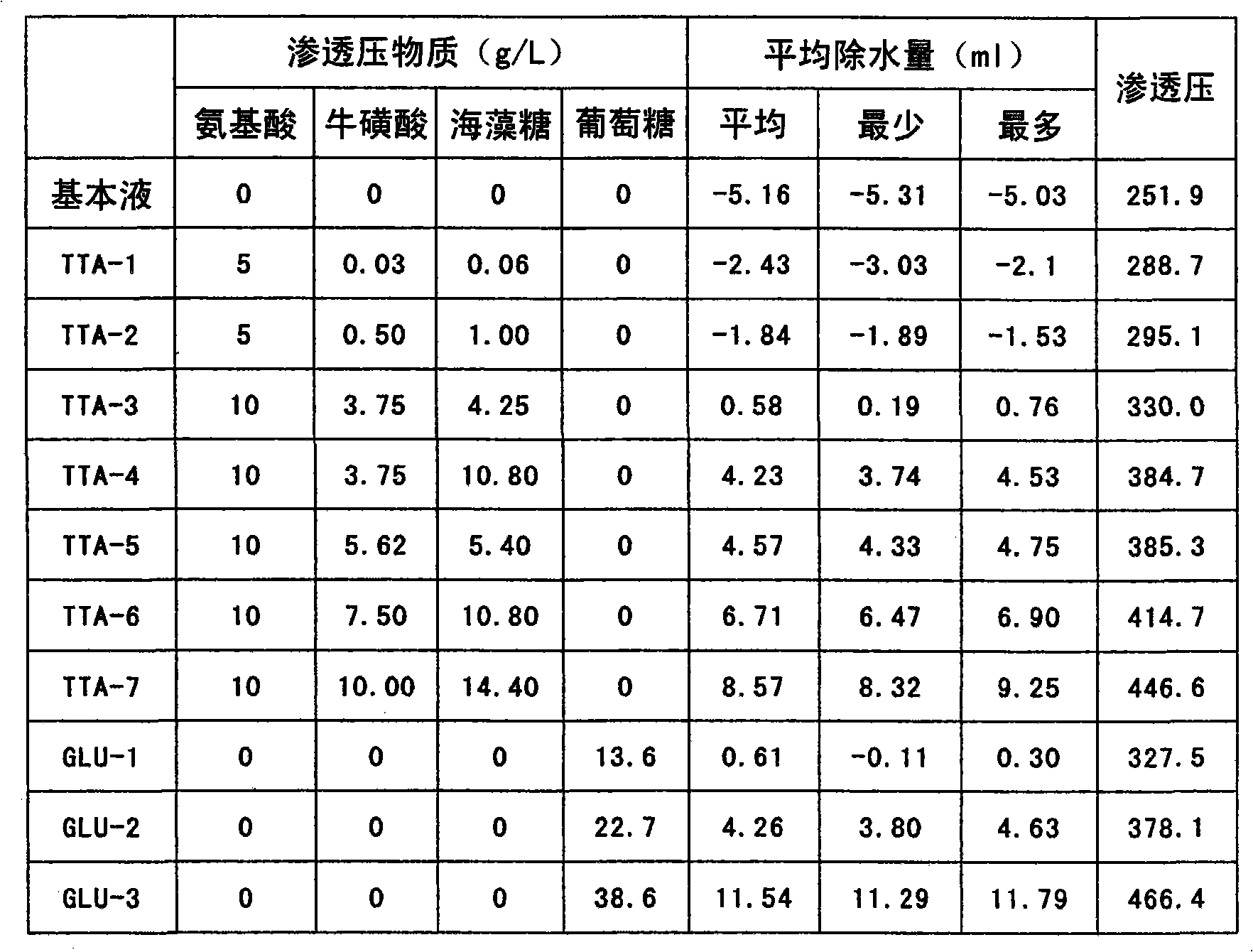
Peritoneal dialysis solution and preparation method thereof
ActiveCN103432164AAvoid damageGuaranteed stabilityPharmaceutical containersBagsSodium bicarbonateHigh resistance
The invention discloses a peritoneal dialysis solution and a preparation method thereof. The preparation method comprises the following steps: dissolving pharmaceutically acceptable amounts of anhydrous glucose, calcium chloride and magnesium chloride into water for injection, and adjusting the pH value to 2.8-3.5 to obtain a solution A; dissolving pharmaceutically acceptable amounts of sodium chloride, sodium bicarbonate and sodium lactate into water for injection, introducing CO2, and adjusting the tank pressure to 0.05-0.2MPa and the pH value to 6.3-7.5 to obtain a solution B; and packaging by using a double-chamber bag to obtain the peritoneal dialysis solution. The peritoneal dialysis solution prepared by using the preparation method disclosed by the invention is few in degradation products of glucose and stable in quality of sodium bicarbonate; and a non-PVC (Polyvinyl Chloride) double-chamber bag and an external packaging bag with high resistance are adopted, so that the stability of sodium bicarbonate in high-temperature sterilization and storage processes can be further ensured.
Owner:HUAREN PHARMACEUTICAL CO LTD
Peritoneal dialysate
InactiveCN101861153AArbitrary adjustmentAvoid damagePeritoneal dialysisAnhydride/acid/halide active ingredientsPeritoneal dialysateTrehalose
It is intended to provide a safe and highly stable peritoneal dialysate which causes neither peritoneal membrane disorders nor peritoneal sclerosis in frequently repeated peritoneal dialysis treatment, can protect the residual renal functions in chronic renal failure and inhibit the progress of renal damage over a long period of time, and enables peritoneal dialysis treatment in a diabetic patient. A peritoneal dialysate which contains 0.05 to 3.5 w / v% of taurine and 0.1 to 6.5 w / v% of trehalose as osmotic agents and having a pH value regulated to 6.5 to 7.5.
Owner:CHEIRON JAPAN CO
Wearable kidney
ActiveUS8715221B2Small sizeReduce loadMedical devicesPeritoneal dialysisMetaboliteIntensive care medicine
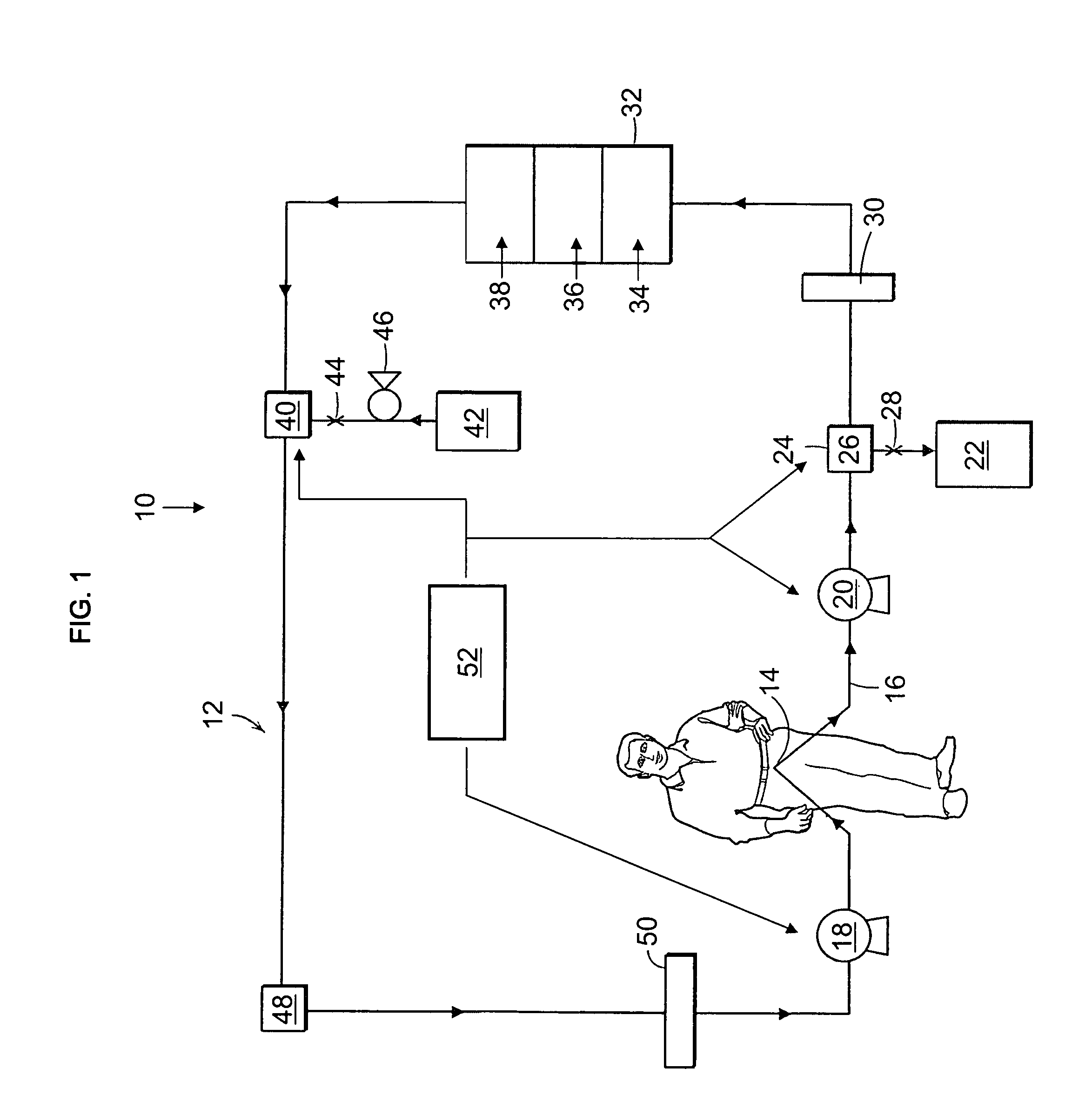
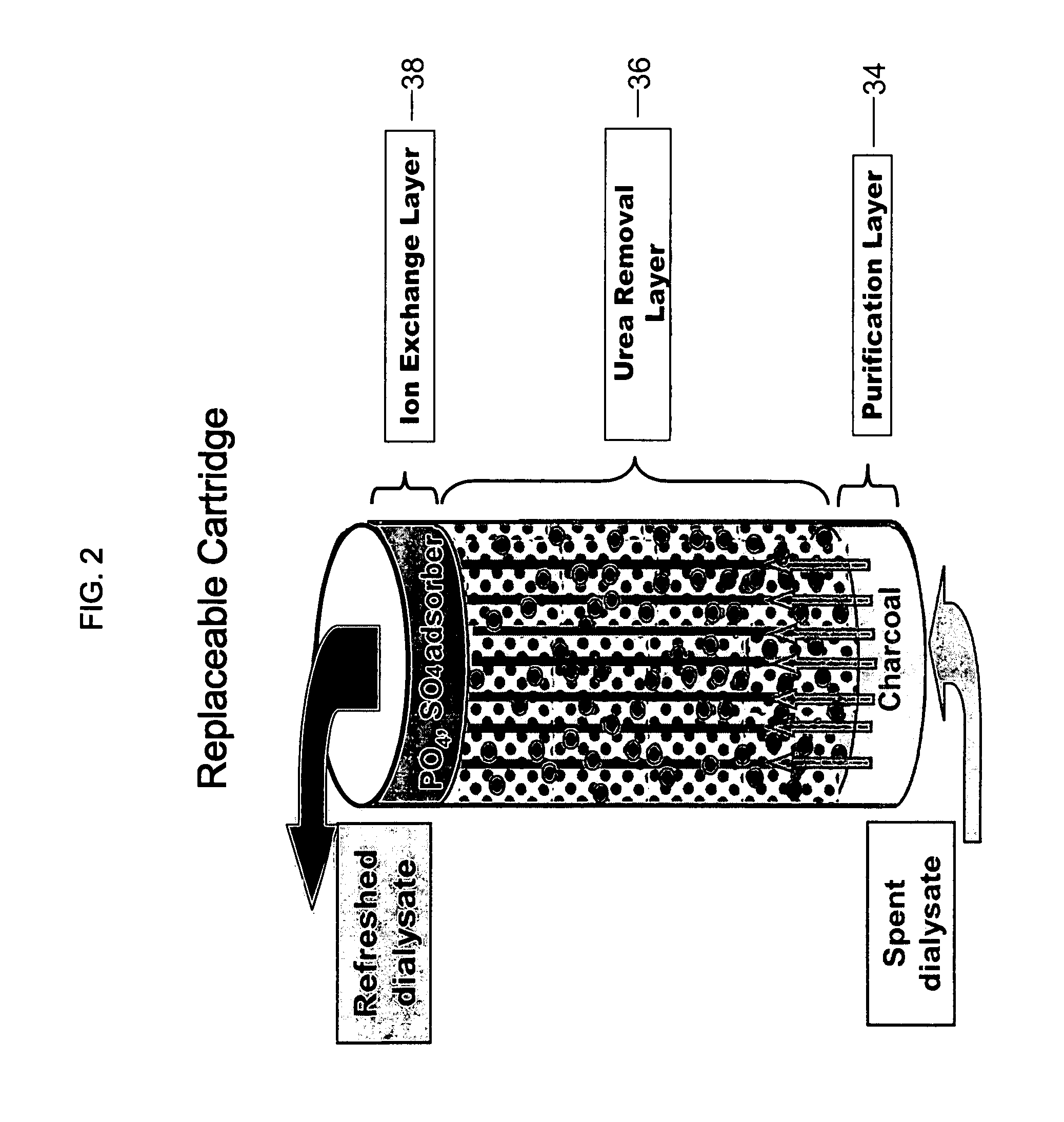
The present invention relates to a wearable peritoneal dialysis system and a replaceable cartridge in the wearable peritoneal dialysis system that regenerates the peritoneal dialysis solution without removing essential ions from the solution and, consequently, the patient. The invention also relates to methods of removing uremic waste metabolites from a patient using the wearable peritoneal dialysis system. A source of one or more enzymes that degrades uremic waste metabolites can be administered orally in conjunction with use of the wearable peritoneal dialysis system such that the load of toxins needing to be eliminated by the wearable peritoneal dialysis system is reduced. The wearable peritoneal dialysis system is meant to operate continuously or semi-continuously, its components small and light enough that it can be comfortably worn by a patient constantly, without burden.
Owner:FRESENIUS MEDICAL CARE HLDG INC
Preparation process of peritoneal dialysate with physiologic pH value
ActiveCN103446181AExtended service lifeExtended treatment timePharmaceutical containersPharmaceutical delivery mechanismSodium lactateWater baths
The invention provides a preparation process of peritoneal dialysate with a physiologic pH value. The process comprises raw material proportioning and a preparation process. The process is characterized in that the preparation process comprises the following steps: adding fresh water for injection into a proportioning container; inputting glucose, sodium chloride, calcium chloride, and magnesium chloride in prescription amount; after stirring and dissolving, adding water for injection to full dose to prepare a liquid A; adding fresh water for injection into another proportioning container; inputting sodium lactate; after stirring and dissolving, adding water for injection to full dose to prepare a liquid B; after uniformly stirring; respectively adding a pH adjustor into the liquid A and the liquid B to adjust the liquid medicine to appropriate pH values; after pre-filtering and terminal-filtering, respectively filling the liquid A and the liquid B to two chambers of a double-chamber transfusion bag in volume ratio of 1:1; plugging; sterilizing by water bath; sterilizing to obtain the peritoneal dialysate with low glucose degradation products and the physiologic pH value, wherein the pH value of the mixture of the liquid A and the liquid B reaches 6.8-7.4.
Owner:HUAREN PHARMACEUTICAL CO LTD
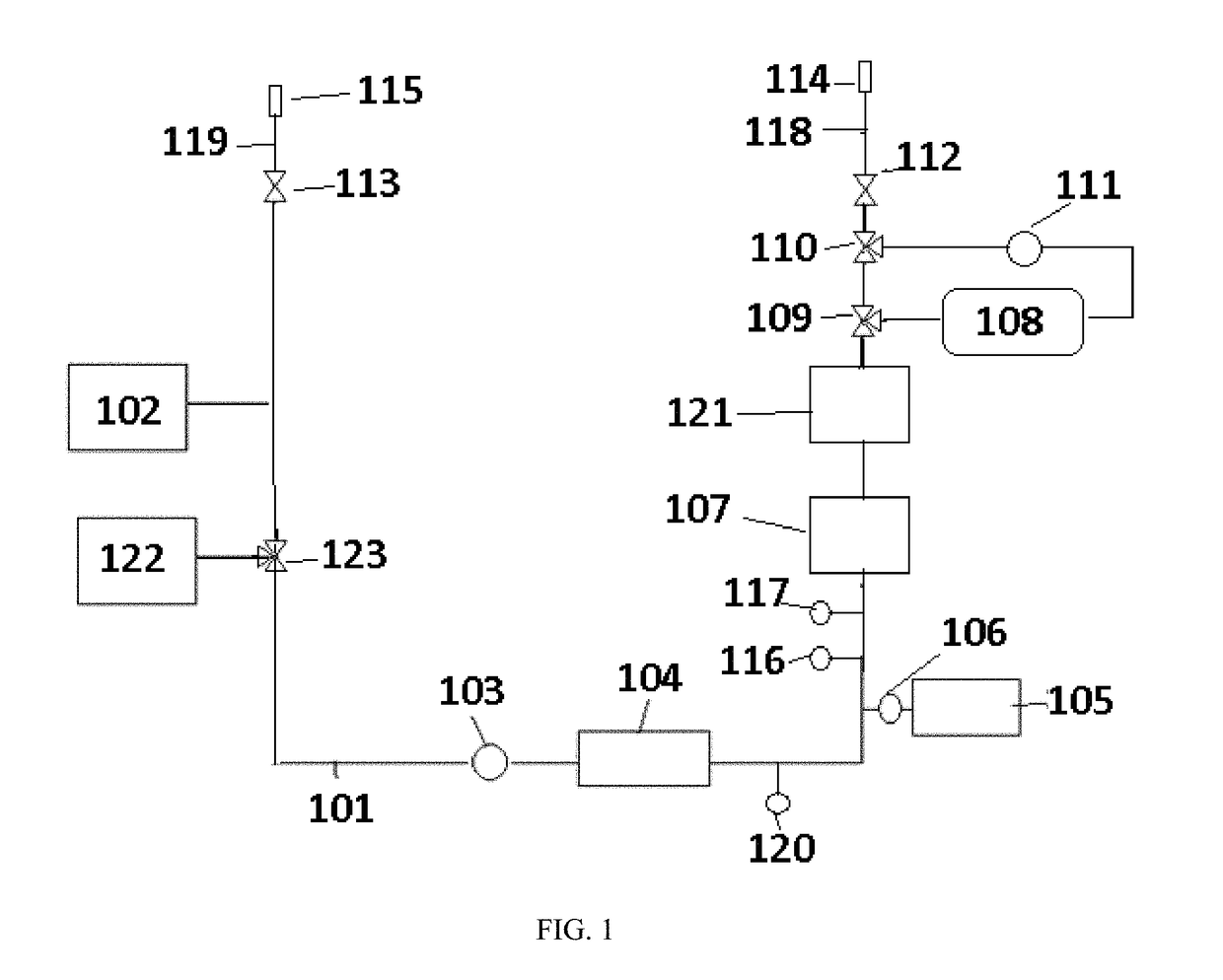
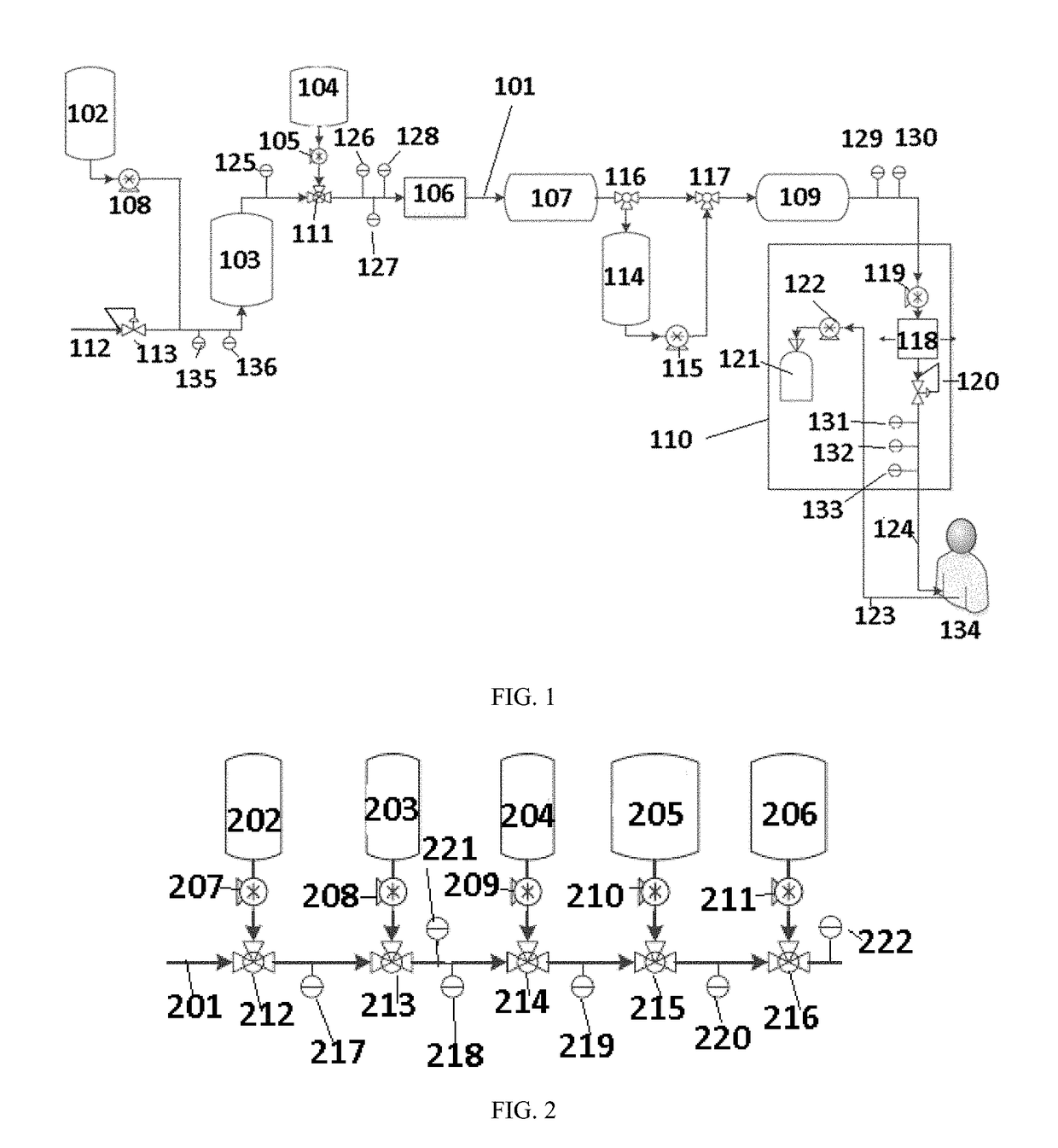
Peritoneal dialyzer and method of peritoneal dialysis
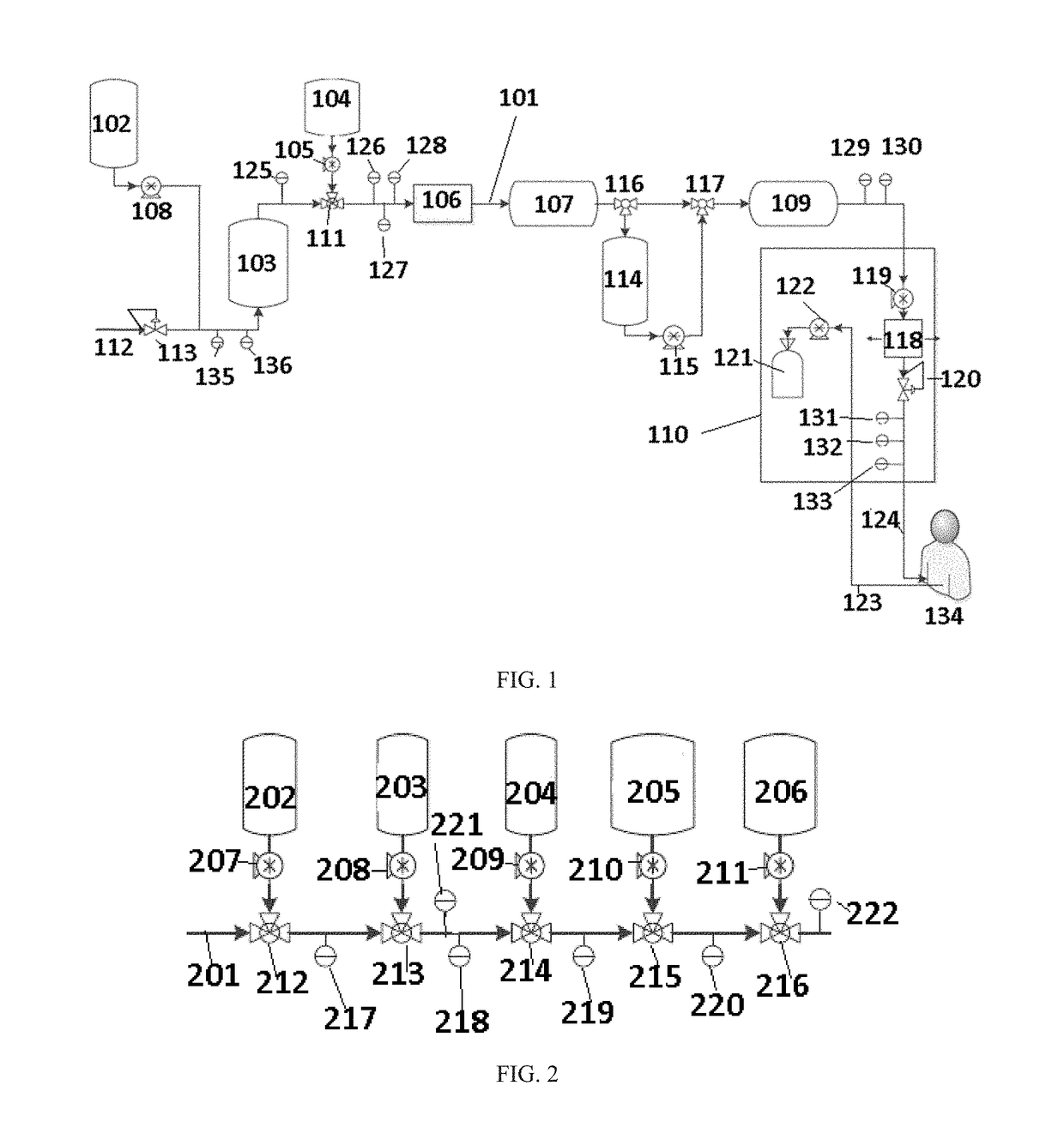
InactiveUS20050234392A1Concentration adjustableDialysisPeritoneal dialysisHemodialysisPeritoneal dialysate
A peritoneal dialyzer including: a catheter capable of injecting and discharging peritoneal dialysate in an abdominal cavity of a patient; a peritoneal dialysate circuit connected to the catheter; and a dialyzer provided in the peritoneal dialysate circuit, the dialyzer including a hemodialysate circuit connected so that peritoneal dialysate passing through the inside can come into contact with a hemodialysate via a hollow fiber membrane, characterized in that means capable of measuring an osmotic agent concentration in the peritoneal dialysate is provided on the peritoneal dialysate circuit on the side of the end at which the catheter is connected with respect to the dialyzer, and a mechanism for dehydrating the peritoneal dialysate according to the osmotic agent concentration measured by the aforementioned means is provided on the hemodialysate circuit, and a method of peritoneal dialysis using the peritoneal dialyzer.
Owner:NIPRO CORP
Peritoneal dialysate temperature regulation system
PendingUS20180021500A1Specific water treatment objectivesMedical devicesPeritoneal dialysis fluidIntensive care medicine
The invention relates to devices, systems, and methods for temperature regulation in generating peritoneal dialysate and using a peritoneal dialysis (PD) system. The system can use a heater and temperature sensors to regulate the temperature of fluid within the PD system.
Owner:MOZARC MEDICAL US LLC
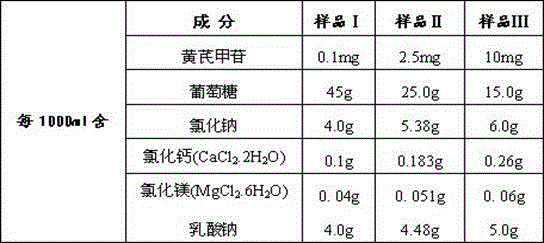
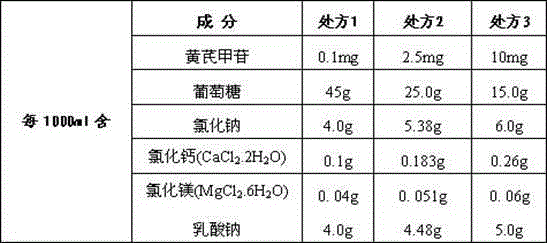
Astragaloside peritoneal dialysis solution
The invention relates to an astragaloside peritoneal dialysis solution preparation prescription and a preparation method thereof, and is characterized in that an effective monomer-astragaloside extracted from astragalus membranaceus and used as an essential component, and glucose, sodium chloride, calcium chloride, magnesium chloride and sodium lactate as auxiliary components are dissolved in an appropriate amount of water for injection, and an astragaloside peritoneal dialysis solution is prepared and is dedicated to treatment of renal failure.
Owner:SHANGHAI TREEFUL PHARMA
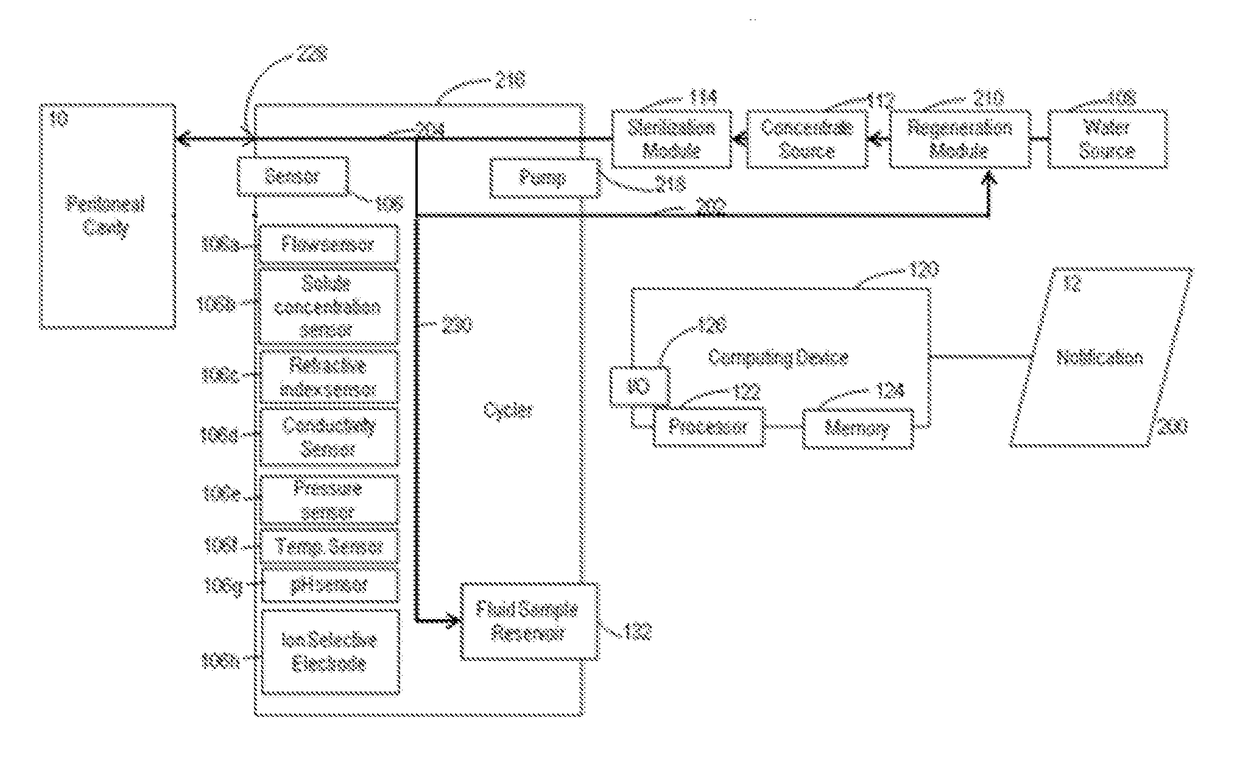
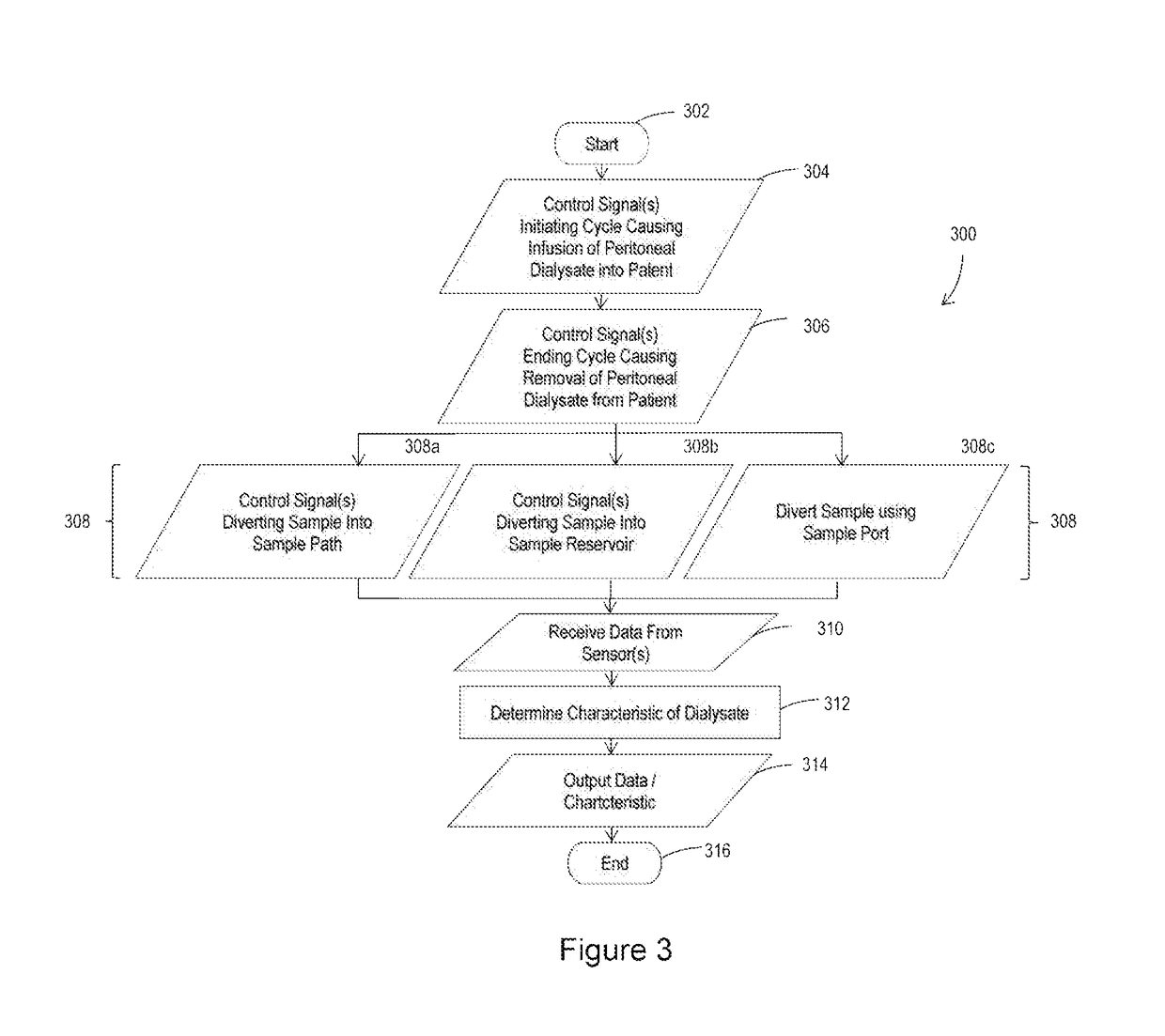
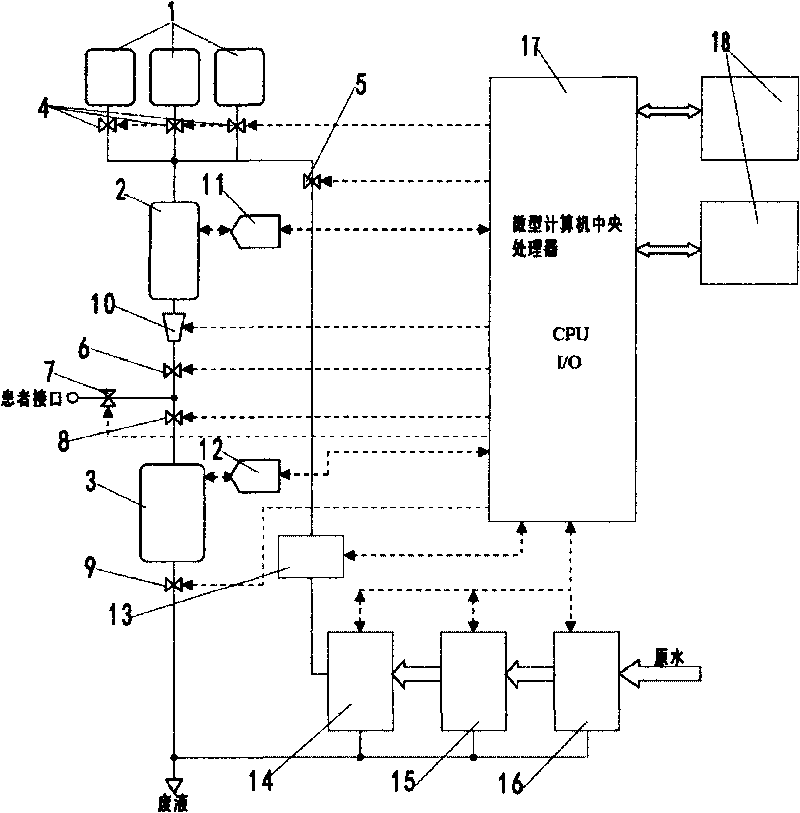
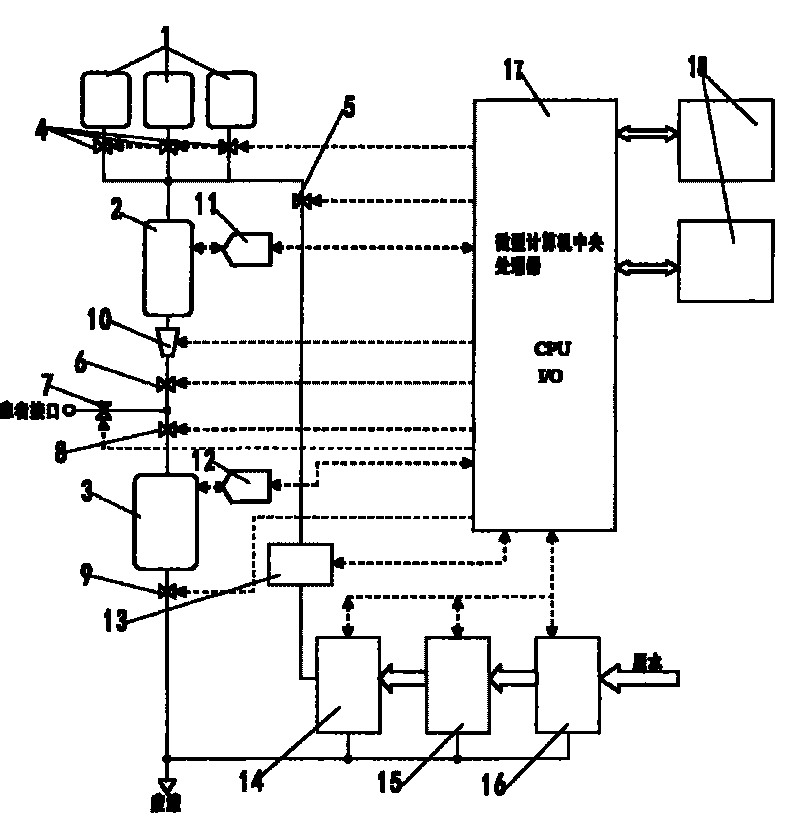
Peritoneal dialysate flow path sensing
The invention relates to systems and methods for sensing fluid characteristics of peritoneal dialysate infused into and removed from a patient during treatment. The systems and methods include sensors, processors, and flow paths for determining patient health based on the fluid characteristics of the peritoneal dialysate. The system can be a peritoneal dialysis cycler which can include an infusion line; an effluent line; at least one pump positioned in the infusion and / or effluent line; and at least one sensor fluidly connected to the effluent line. The sensor can be at least one of a flow sensor, an ion selective electrode, a pH sensor, a pressure sensor, a refractive index sensor, and a temperature sensor. The method can include infusing peritoneal dialysate through an infusion line; removing peritoneal dialysate through an effluent line; and determining at least one fluid characteristic of the peritoneal dialysate in the effluent line.
Owner:MOZARC MEDICAL US LLC
Online peritoneal dialysis unit and preparation method
InactiveCN101745157AReduce storage capacityReduce capacityPeritoneal dialysisSquare meterWater processing
The invention discloses an online peritoneal dialysis unit and preparation method which integrates computer automation control technique, antiosmosis water processing technology and peritoneal dialysis solution concentrating technology. The unit can online prepare peritoneal dialysis solution equal to or more than 30L in 10-20 hour curing process with a peritoneal dialysis solution preparing speed which is equal to or more than 200ml / min, which meets the requirement that the clinic care exchange capacity reaches 50ml / min; automatically completes exchange operating process more than 15-18 times, solves the defects that using the current CAPD technology to realize large-volume exchange is difficult to operate frequently and the APD equipment does not have the function of 30L-36L large-volume exchange function, and provides technical supports for realizing the KT / V value of most patients to be equal to or more than 2.1, realizing the Ccr of more than 70 percent of patients to be equal to or more than 60ml / 1.73 square meters, realizing the elimination rate of carbamide to be more than 12ml / min, and improves the survive rate of the patients.
Owner:贺心雅
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com