Peritoneal dialysate
A peritoneal dialysis solution and osmotic pressure technology, applied in the field of peritoneal dialysis solution, can solve problems such as pH value reduction, peritoneal damage, and insufficient confirmation of body safety, etc., to achieve damage suppression, safe osmotic pressure, and protection and maintenance of peritoneal tissue Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Embodiment 1: (stability to heat sterilization)
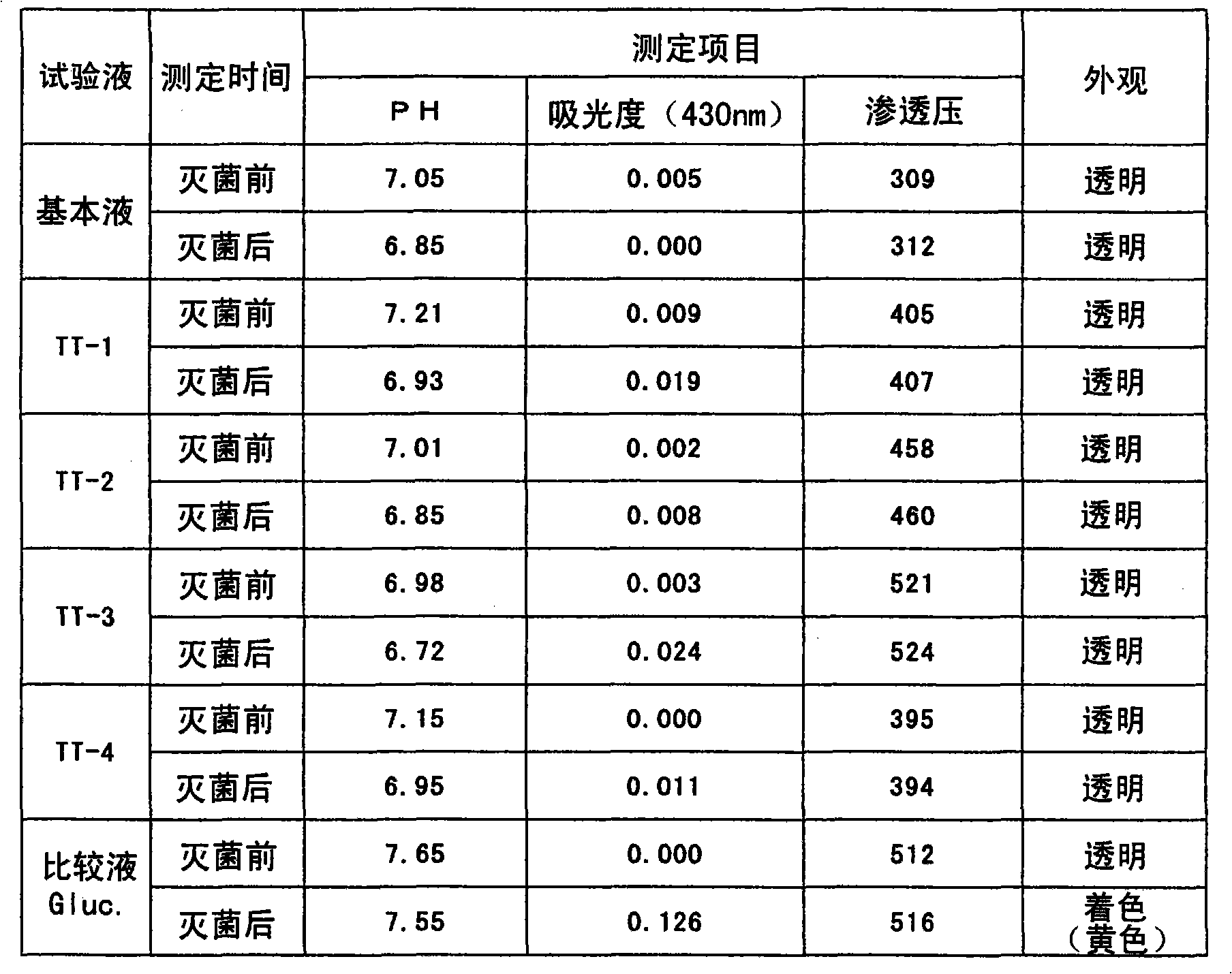
[0058] Sodium chloride 107.8g, calcium chloride dihydrate 5.14g, magnesium chloride hexahydrate 1.016g, acid sodium carbonate 5.4g and L-histidine 5.6g, L-tryptophan 3.8g, L-isoleucine Amino acid 15.0g, L-leucine 12.4g, L-valine 9.4g, L-tyrosine 4.3g, L-arginine 9.4g were dissolved in an appropriate amount of distilled water for injection, and the Adjust the pH value to 7, add water to 20L. This is the base liquid. It should be noted that, in this embodiment, a solution without adding vitamins is used as the basic solution. The test solutions TT-1, TT-2, TT-3, TT-4, and Gluc. are shown in the lower column of Table 1.
[0059] Table 1
[0060]
[0061] Basic solution: only containing salts and amino acids, pH 7.4, adjusted to 20L with distilled water for injection.
[0062] TT-1: Basic solution 1L + taurine 10.0g + trehalose 10.0g
[0063] TT-2: Basic solution 1L + taurine 15.0g + trehalose 15.0g
[0064] TT-3: ...
Embodiment 2
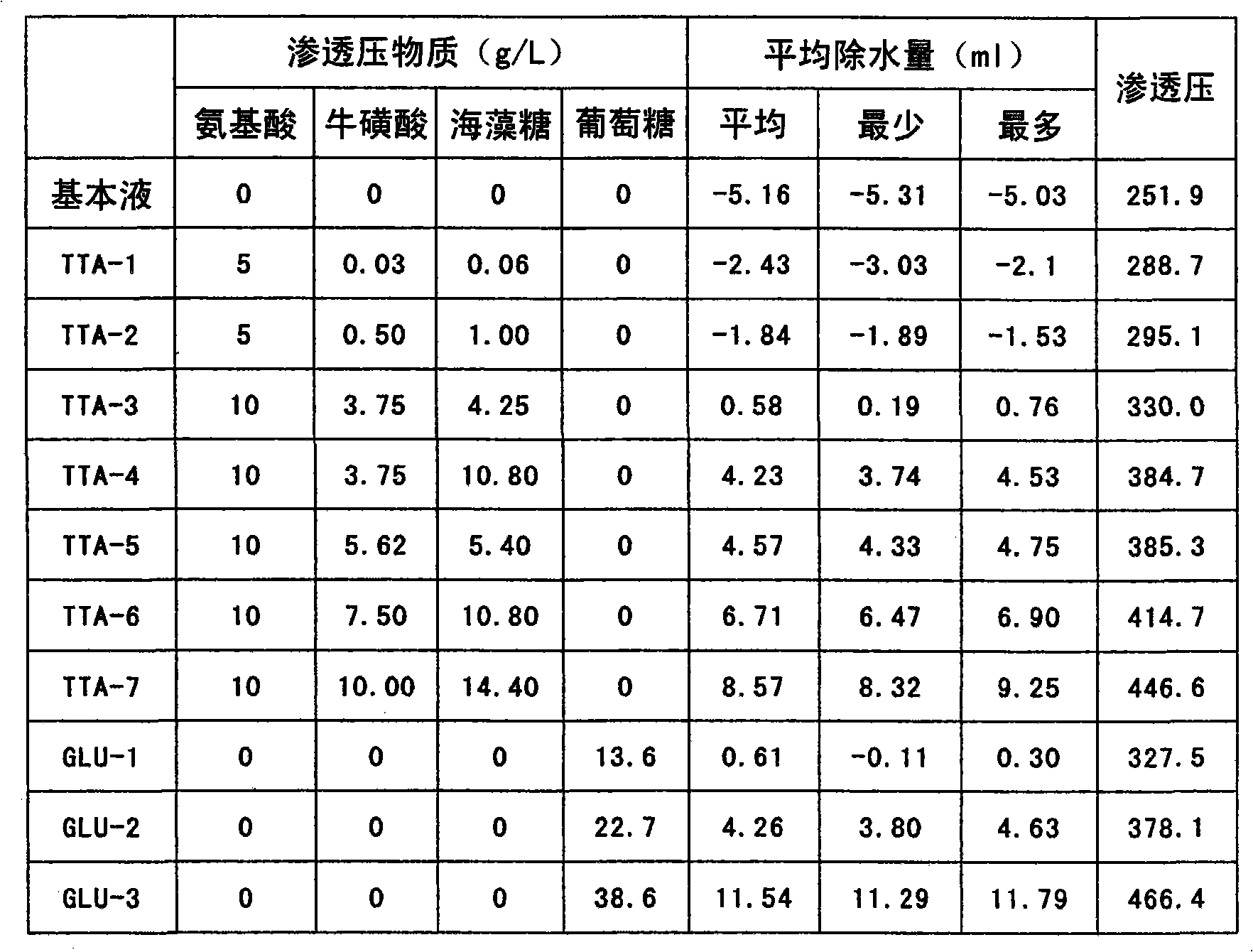
[0070] Embodiment 2: (relationship of concentration / water removal amount)
[0071] Dissolve sodium chloride (5.38 g), sodium bicarbonate (2.52 g), magnesium chloride hexahydrate (34 mg), calcium chloride dihydrate (370 mg), zinc sulfate heptahydrate (86 mg) in distilled water and adjust the pH value to 7.2, add distilled water to adjust the volume to 1L. This liquid was used as a basic liquid.
[0072] Add 2.0g of L-arginine, 0.5g of L-histidine, 3.0g of L-leucine, 2.0g of L-isoleucine, 2.0g of L-valine, and 2.0g of L-valine to 1L of basic solution. The solutions obtained by adding 0.5 g of tyrosine and adding taurine and trehalose were used as TTA-3~TTA-6, and the control solution was glucose dissolved in the basic solution and used as GLU-1, GLU-2, GLU -3. The amino acid concentration of TTA-1 and TTA-2 liquids is 1 / 2 of TTA-3-6.
[0073] 30 ml of these solutions were injected into the peritoneal cavity of SD male rats, and the difference between the measured intraperito...
Embodiment 3
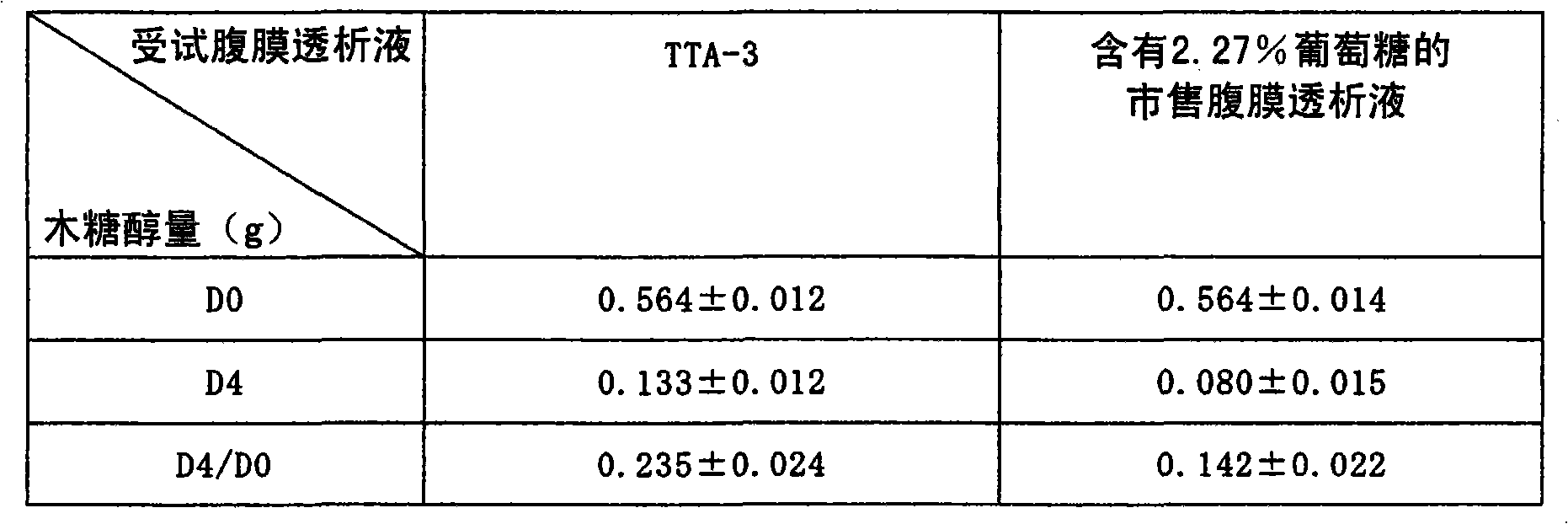
[0078] Embodiment 3: (peritoneal injury effect)
[0079] Even after repeated operations of injecting, retaining and draining the peritoneal dialysis solution into the peritoneal cavity for many times, the peritoneum will not be damaged, and the drainage function can be maintained normally for a long time, which is the basic condition that the peritoneal dialysis solution should have.
[0080] In order to conduct a comparative study on the peritoneal injury effect, the following experimental conditions were set. That is, a predetermined amount (30 ml) of the test peritoneal dialysate was injected into the abdominal cavity of the rat, and the entire amount was discharged after being retained for a predetermined time (4 hours). Then inject the new peritoneal dialysate of the same test into the peritoneal cavity with the same amount, keep it for 4 hours and then discharge it completely. This operation was performed 3 times a day for 7 days.
[0081] When the peritoneum is damage...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com