Patents
Literature
782 results about "Peritoneal membrane" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Peritoneal Membrane: A sac, resembling cellophane with tiny holes that serves as a lining of the abdominal cavity and holds organs in place within the peritoneal cavity. Peritoneum: The lining of the peritoneal cavity.
Electrosurgical instrument
InactiveUS7278994B2Lower impedanceReduced effectivenessCannulasDiagnosticsGynecologyPeritoneal cavity
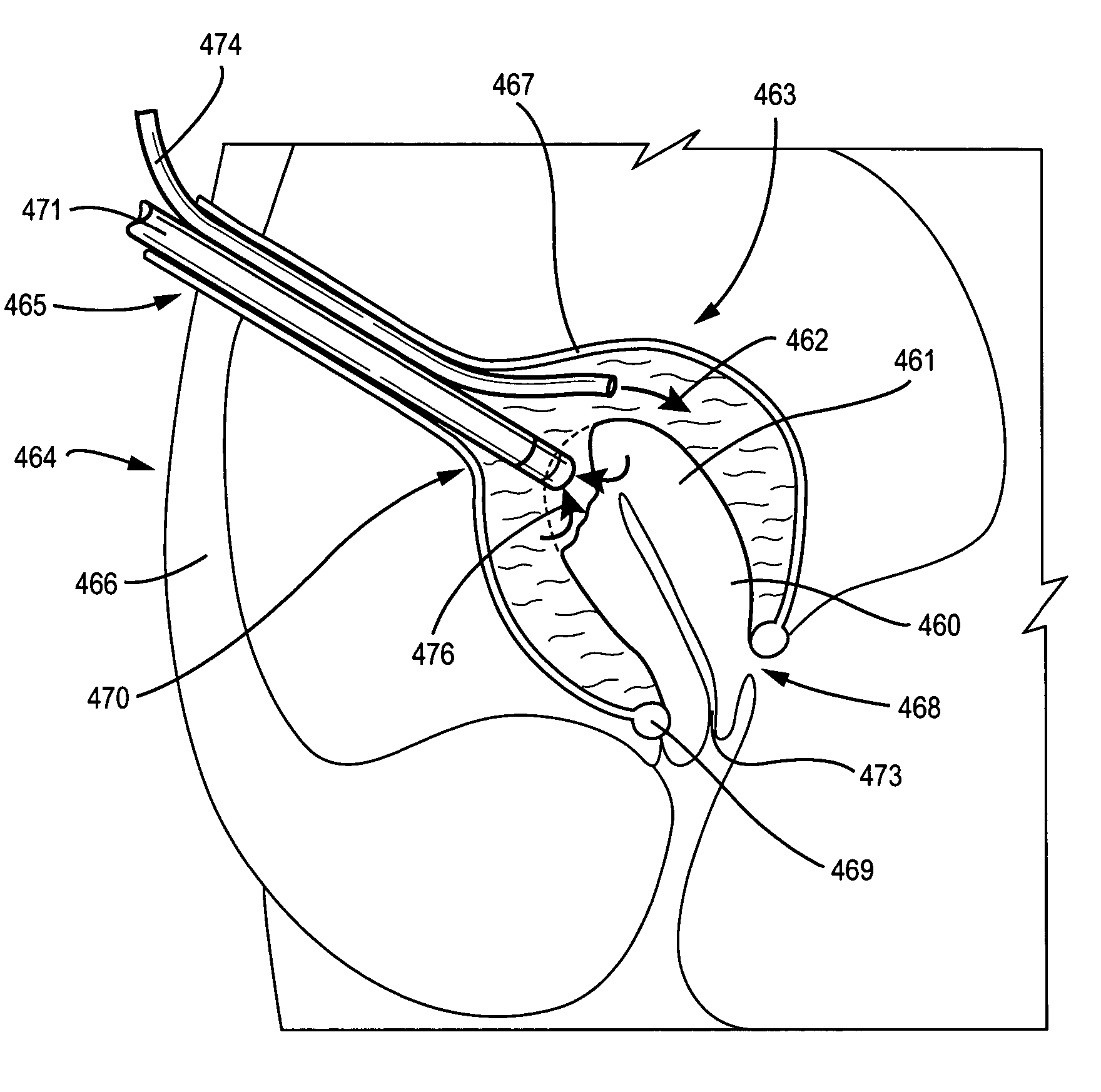
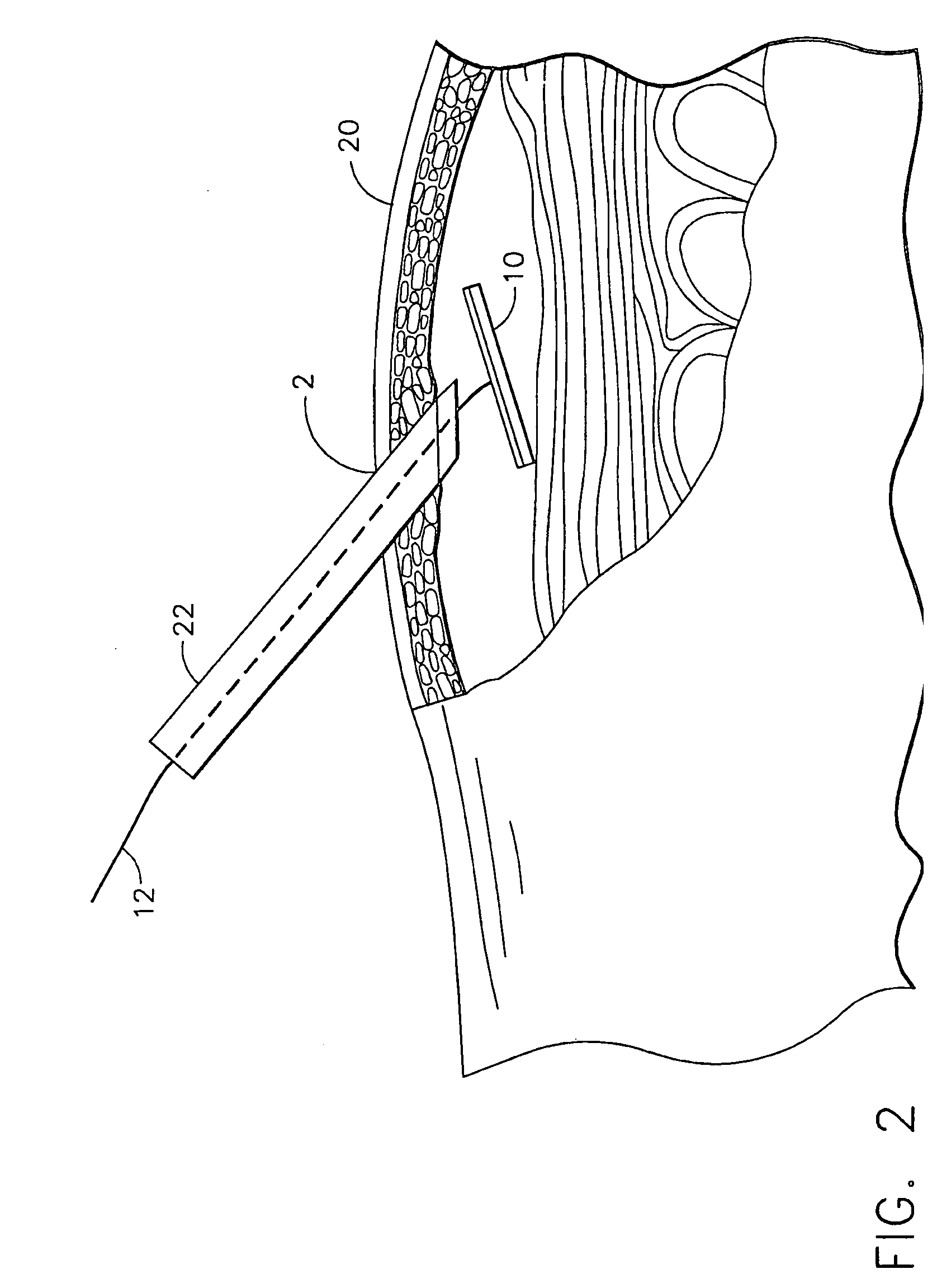
A system and method are disclosed for removing a uterus using a fluid enclosure inserted in the peritoneal cavity of a patient so as to enclose the uterus. The fluid enclosure includes a distal open end surrounded by an adjustable loop, that can be tightened, a first proximal opening for inserting an electrosurgical instrument into the fluid enclosure, and a second proximal opening for inserting an endoscope. The loop is either a resilient band extending around the edge of the distal open end or a drawstring type of arrangement that can be tightened and released. The fluid enclosure is partially inserted into the peritoneal cavity of a patient in a deflated condition and then manipulated within the peritoneal cavity over the body and fundus of the uterus to the level of the uterocervical junction. The loop is tightened around the uterocervical junction, after which the enclosure is inflated using a conductive fluid. The loop forms a pressure seal against the uterocervical junction to contain the conductive fluid used to fill the fluid enclosure. Endoscopically inserted into the fluid enclosure is an electrosurgical instrument that is manipulated to vaporize and morcellate the fundus and body of the uterus. The fundus and body tissue that is vaporized and morcellated is then removed from the fluid enclosure through the shaft of the instrument, which includes a hollow interior that is connected to a suction pump The fundus and body are removed after the uterus has been disconnected from the tissue surrounding uterus.
Owner:GYRUS MEDICAL LTD
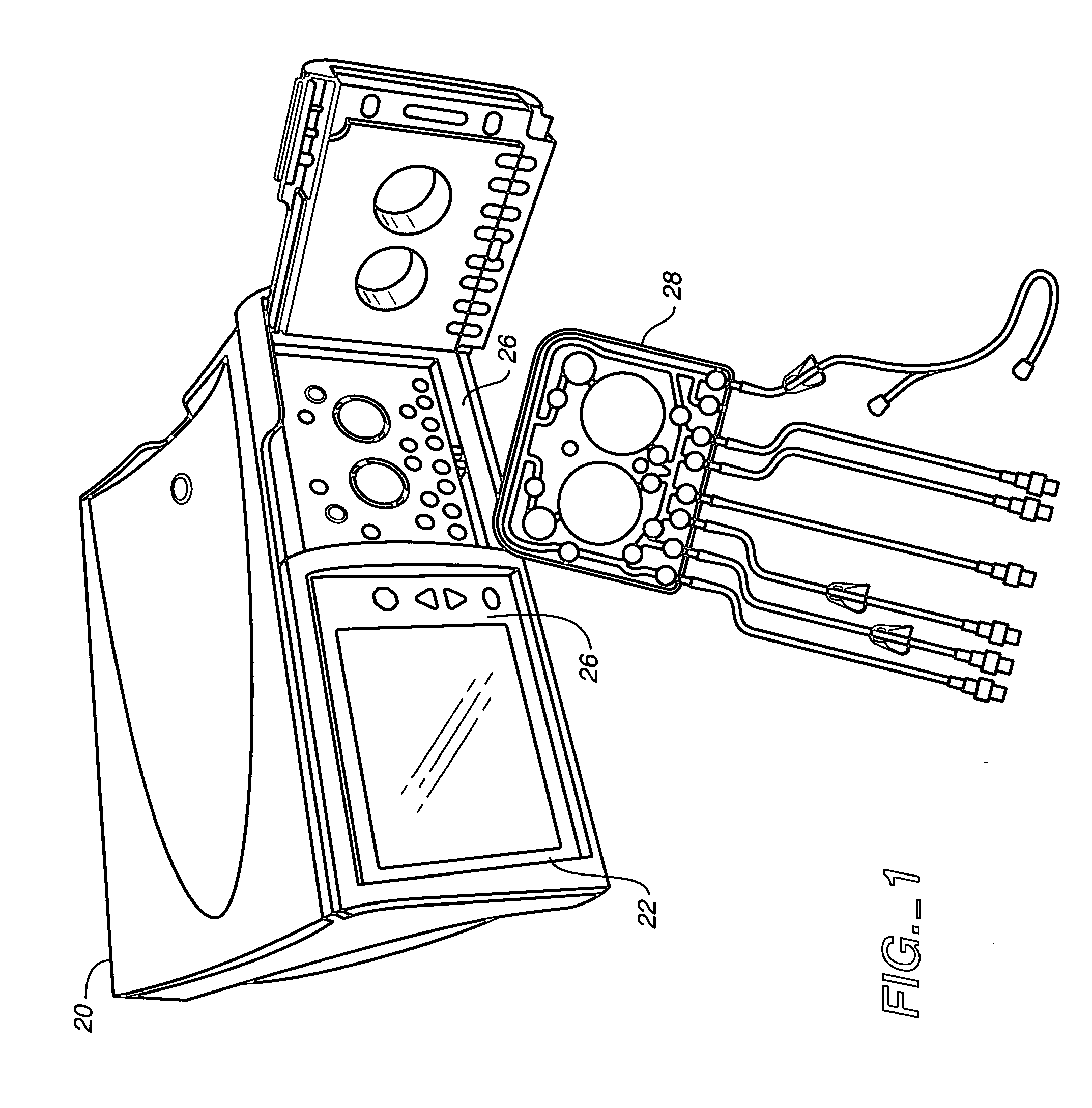
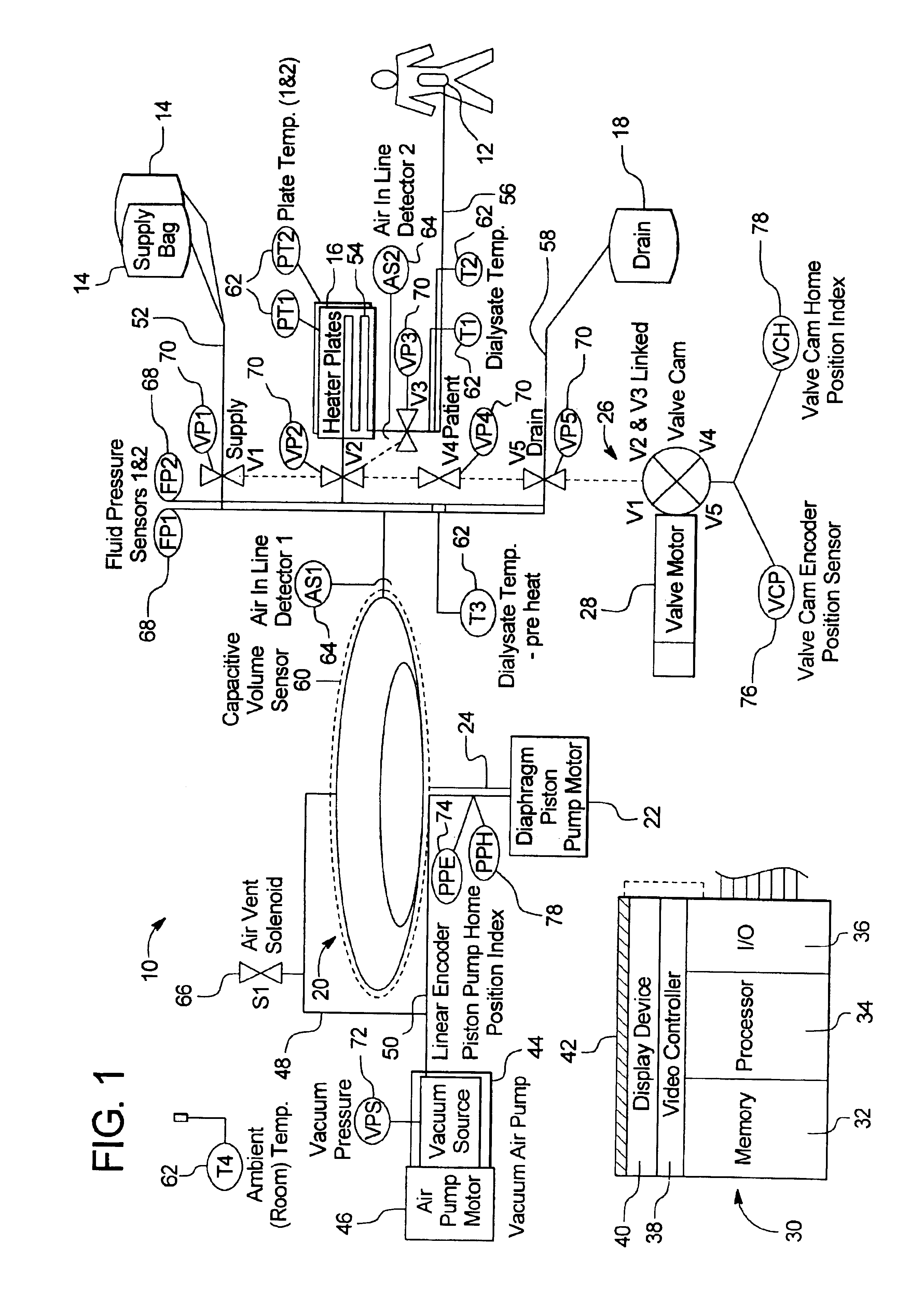
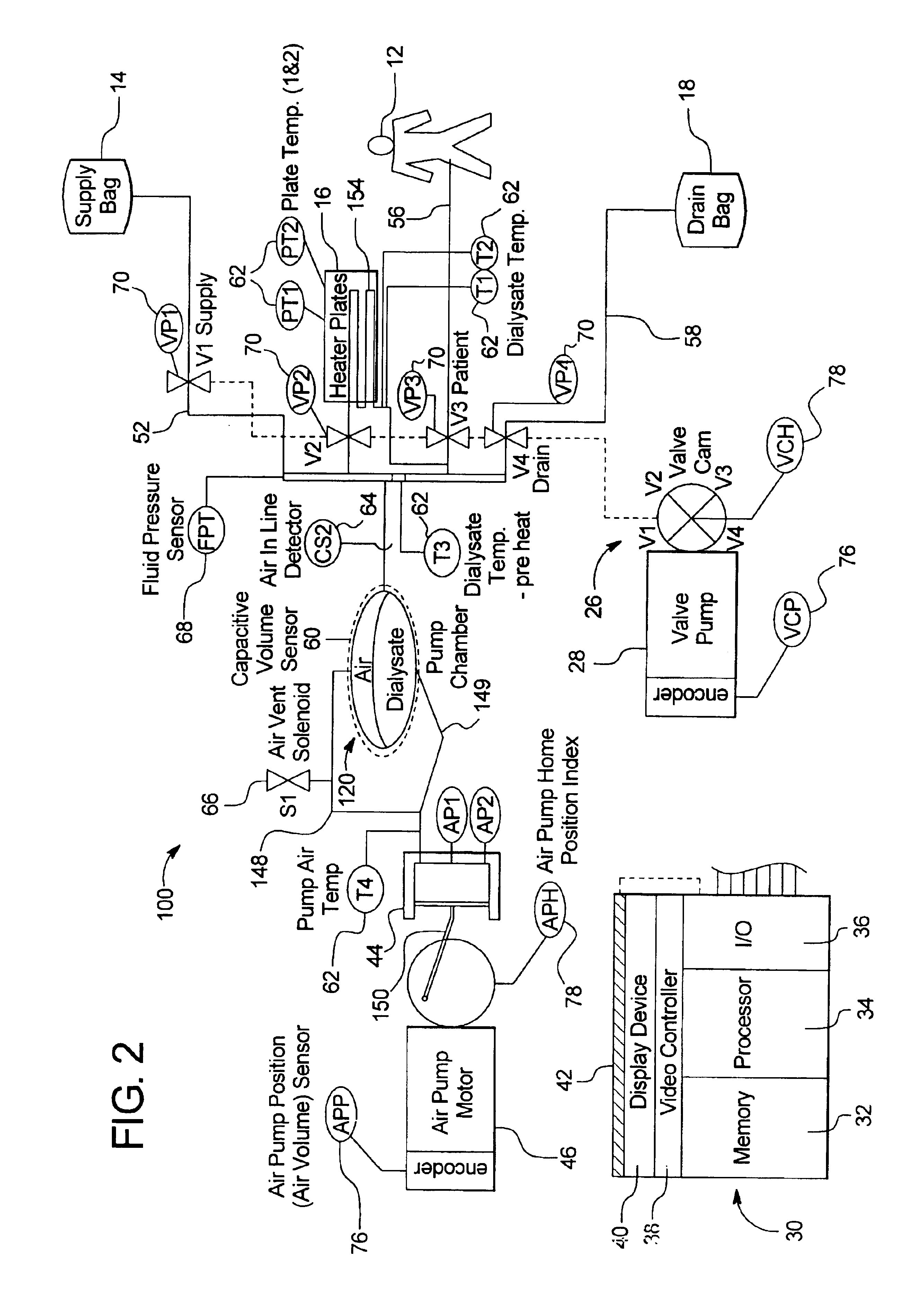
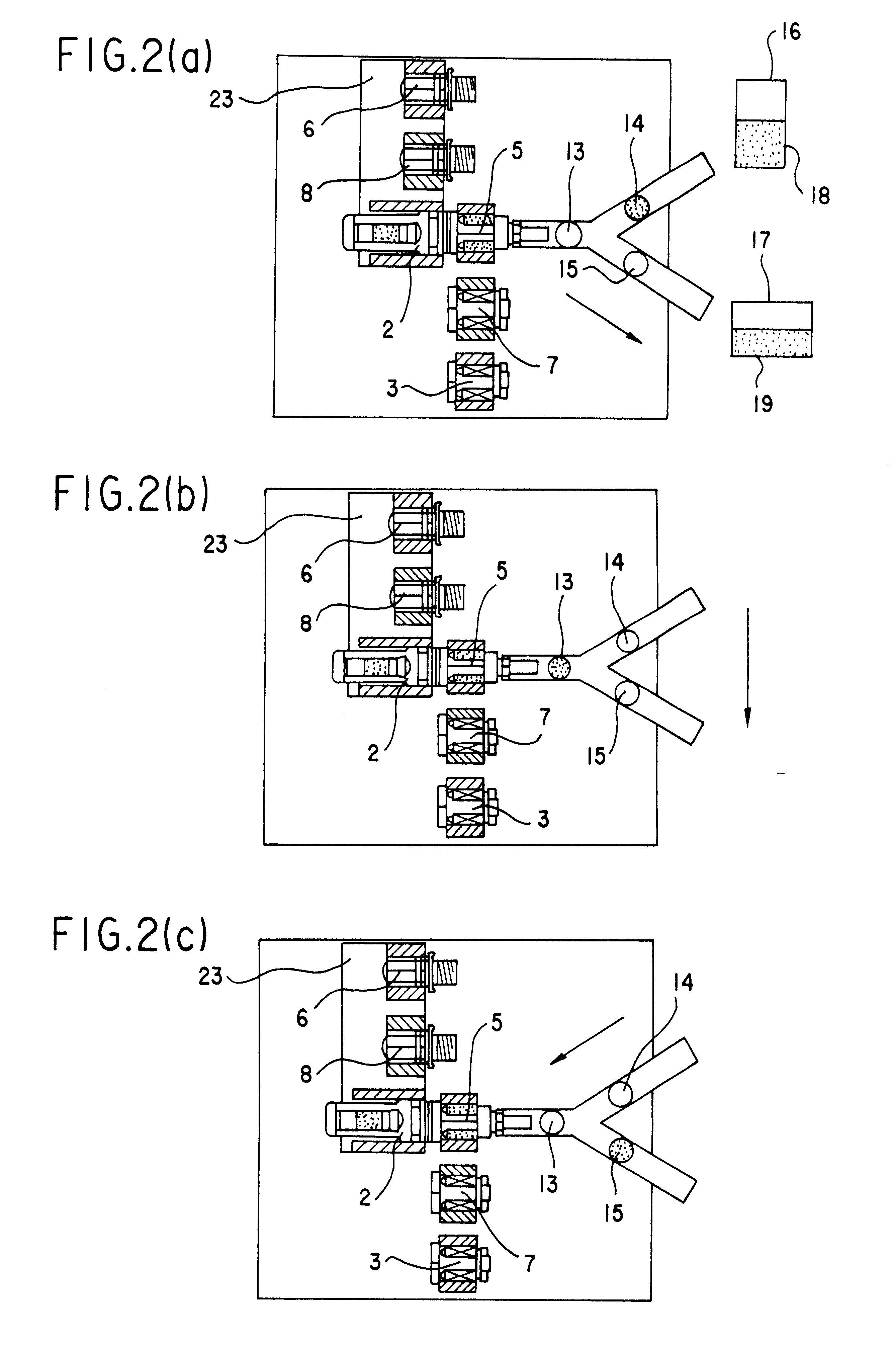
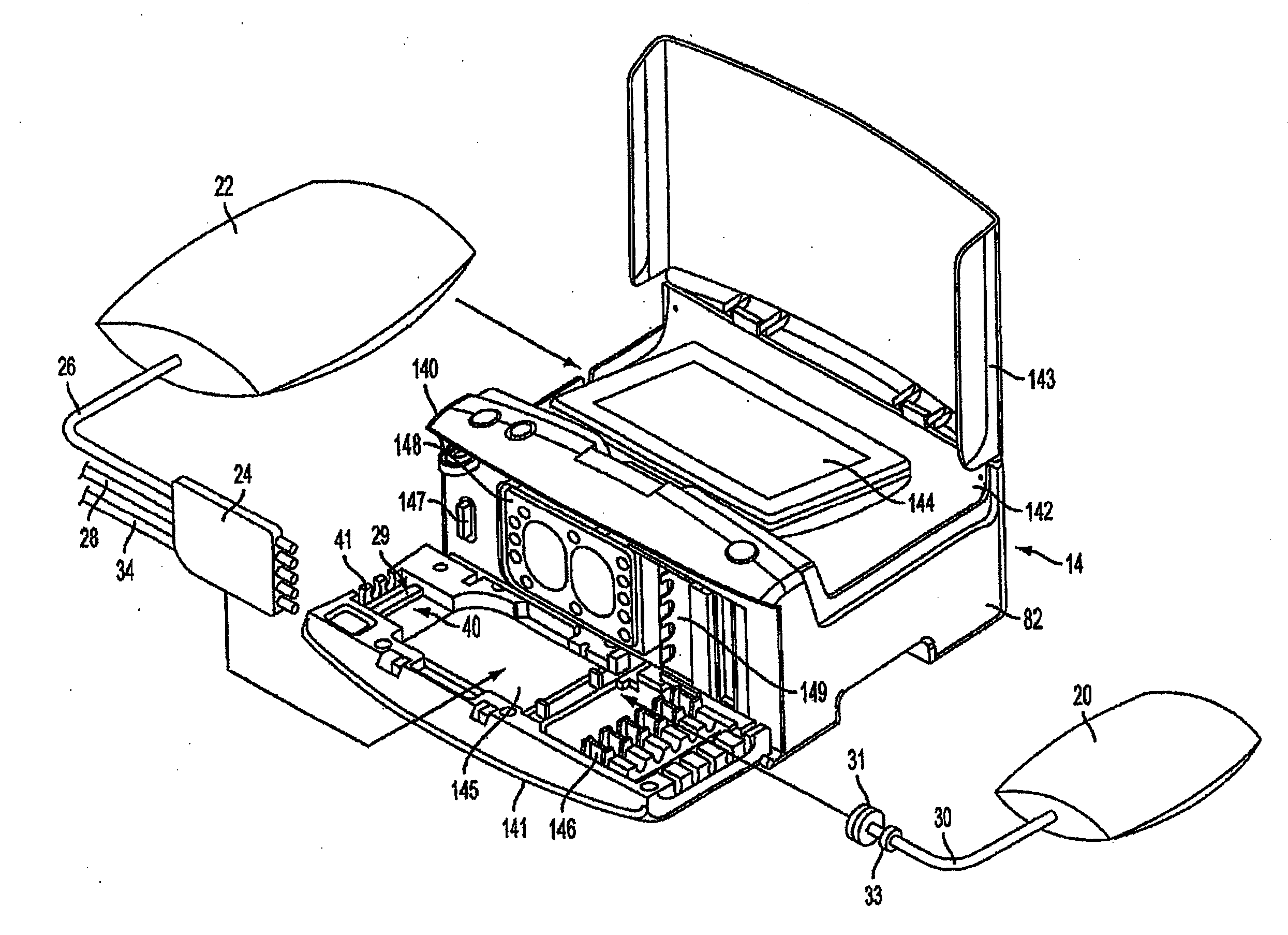
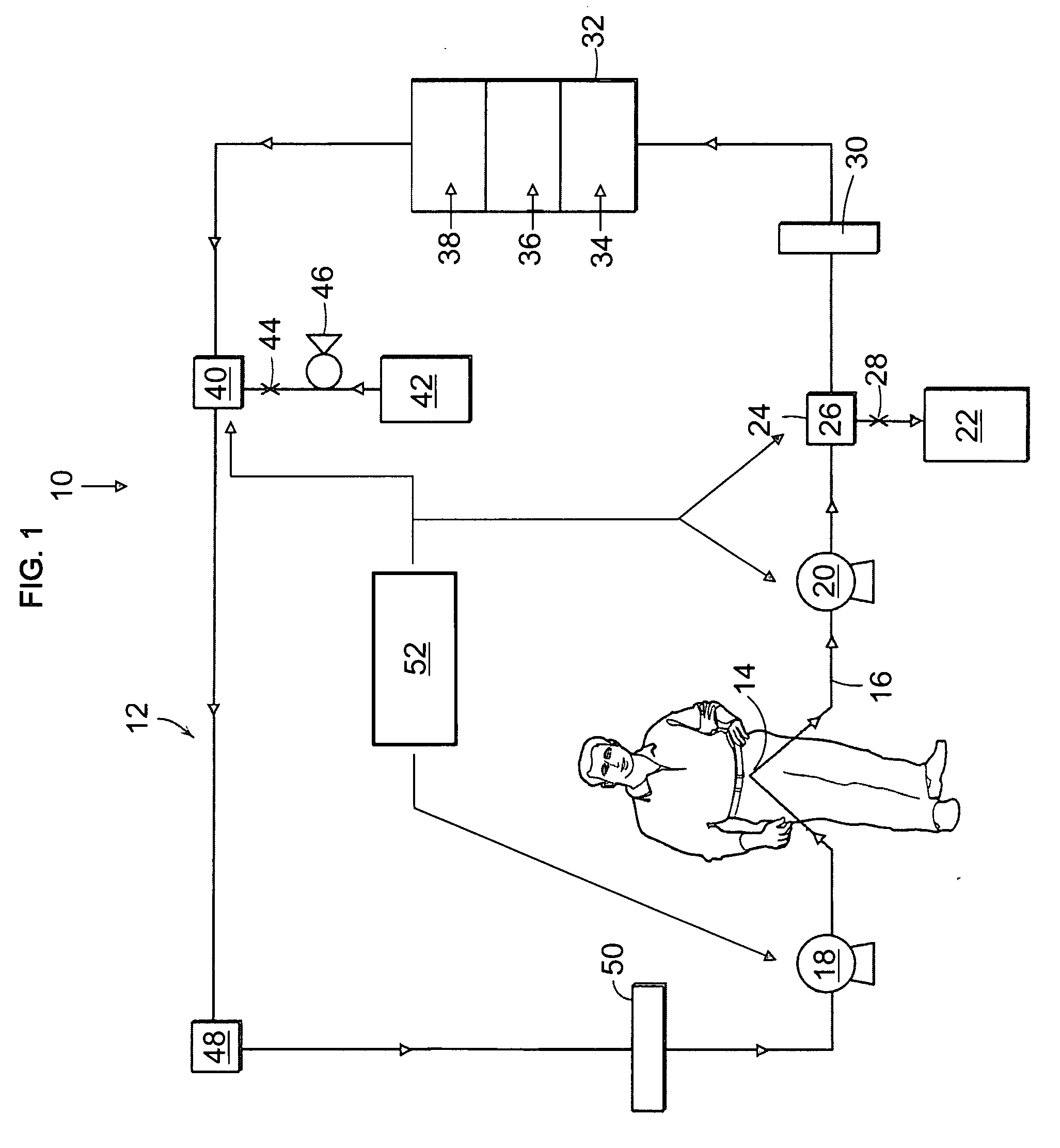
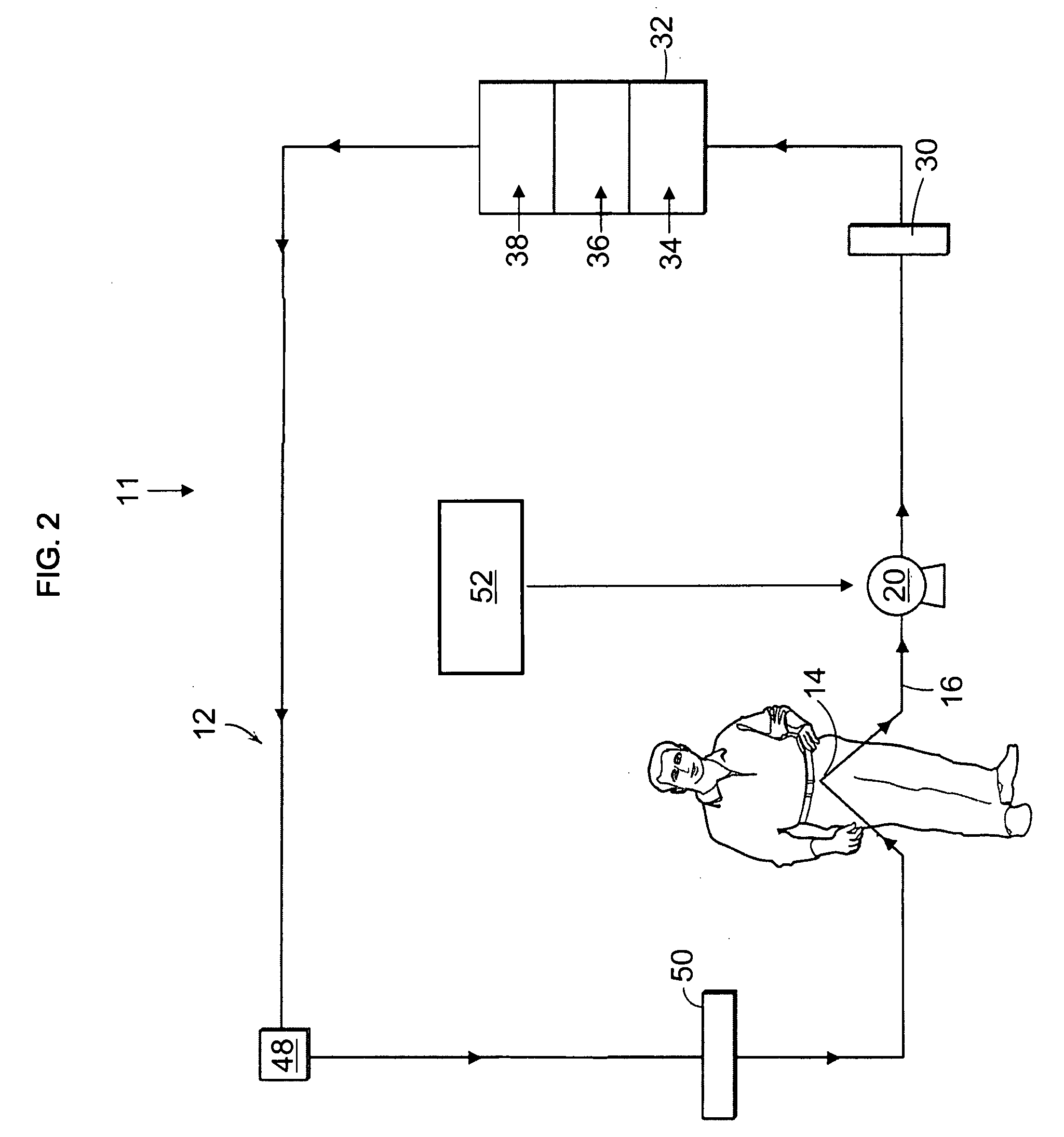
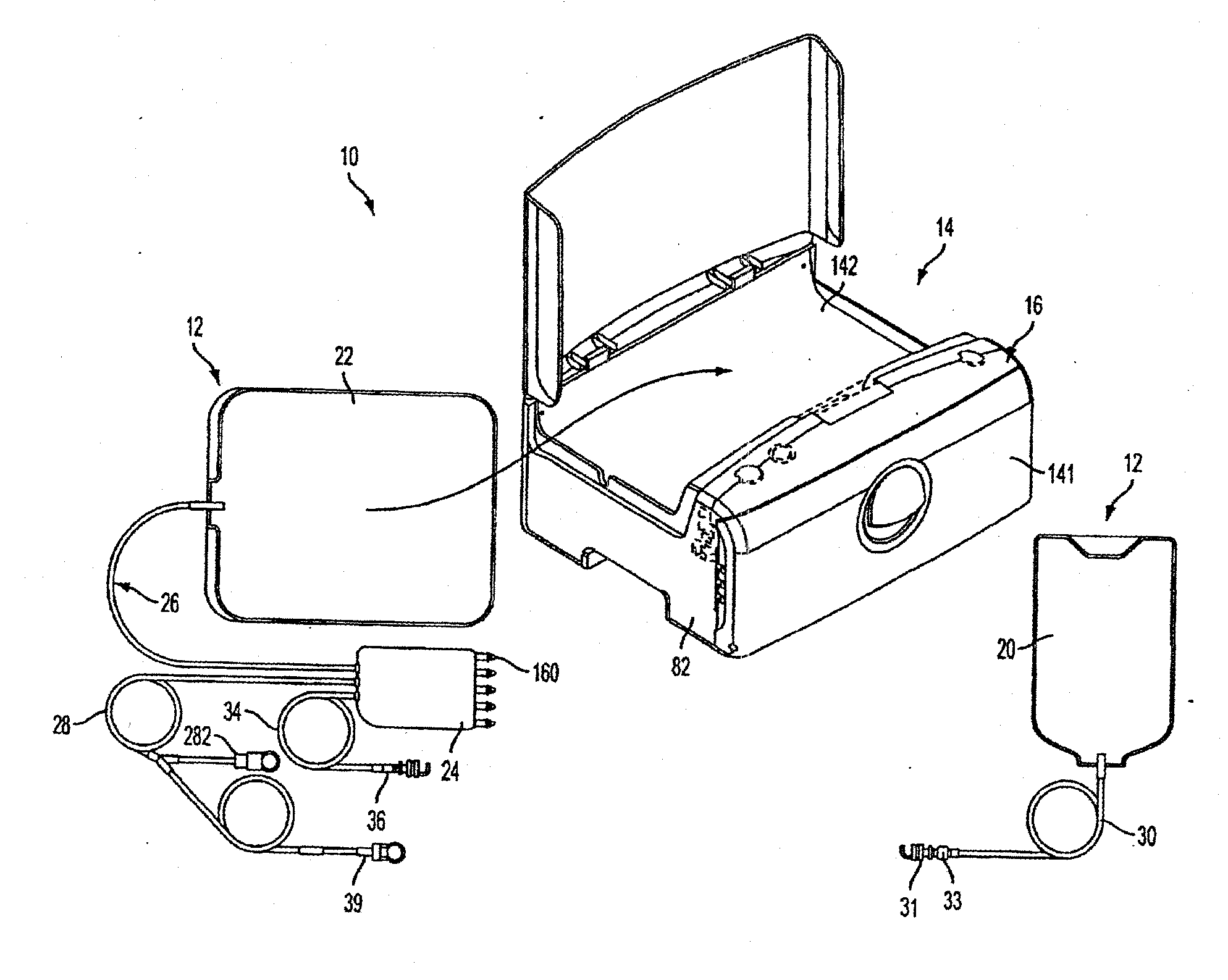
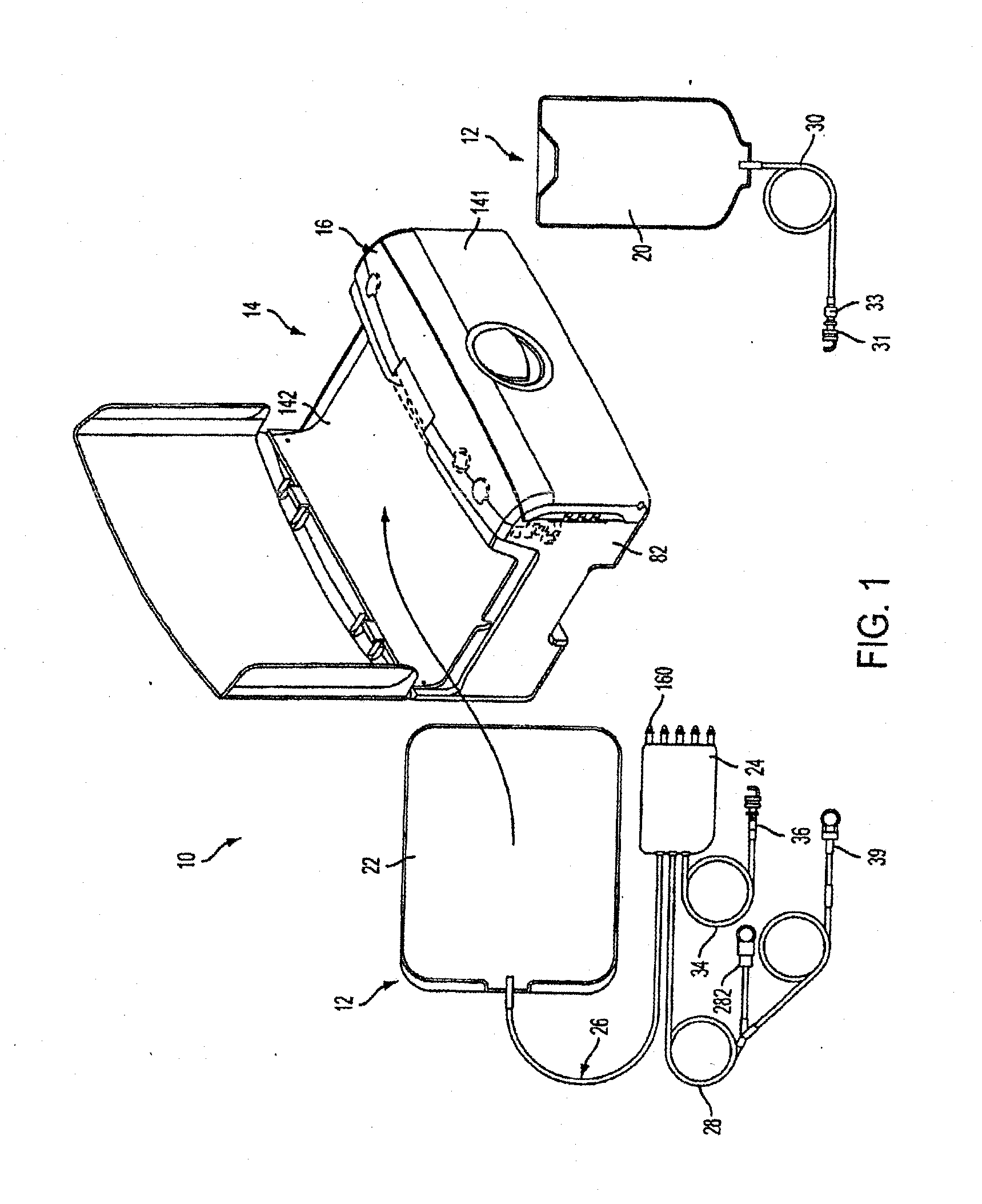
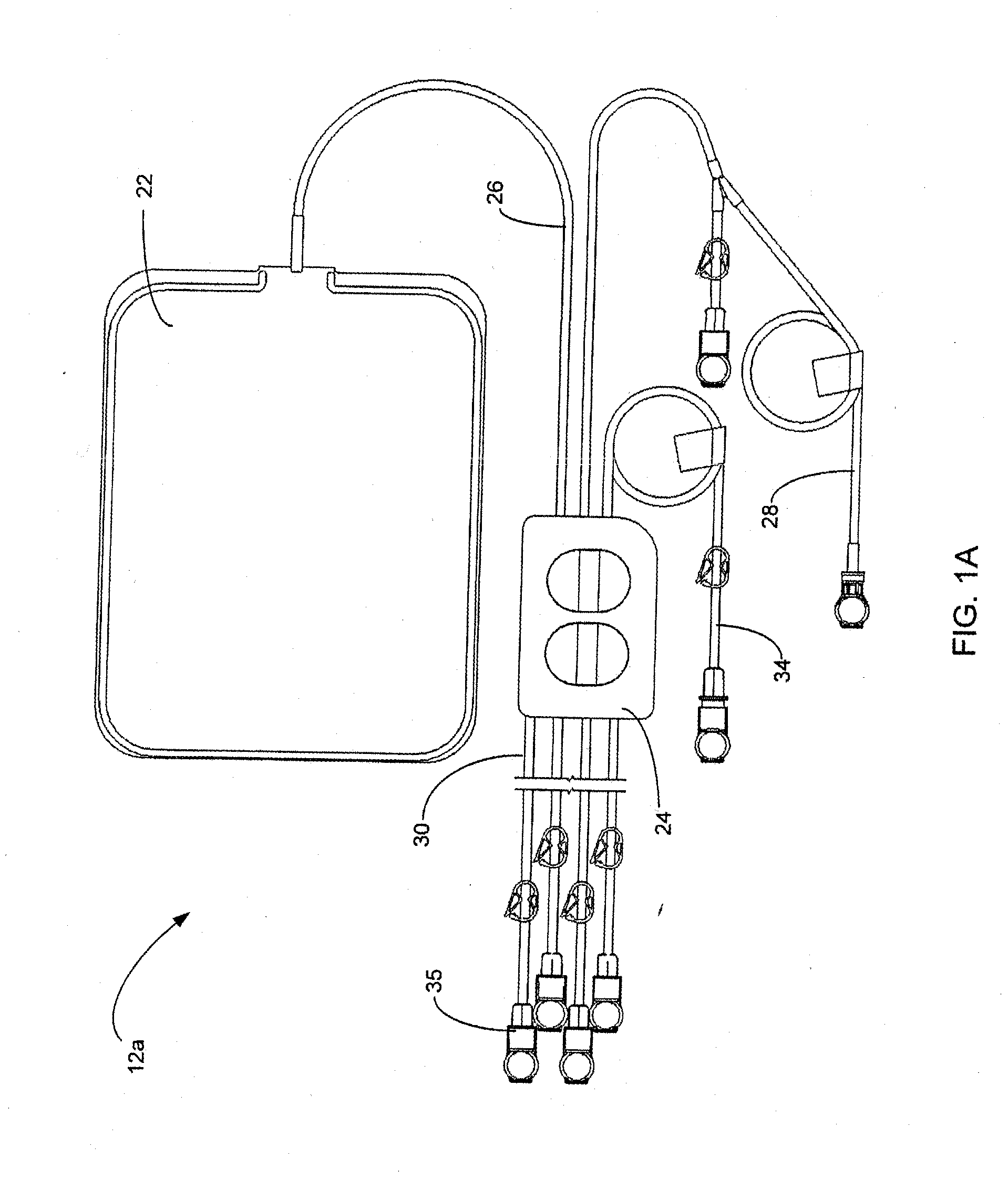
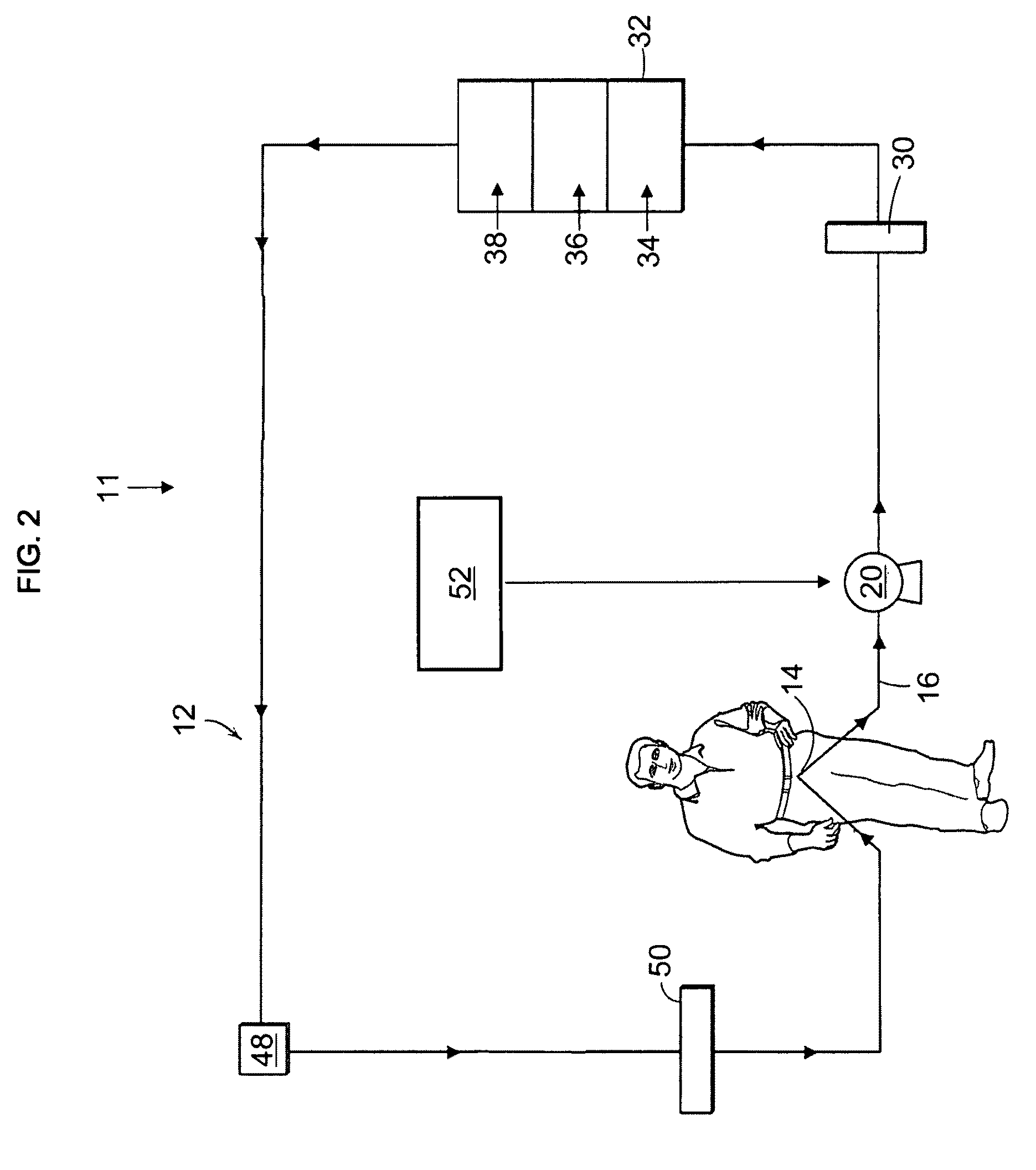
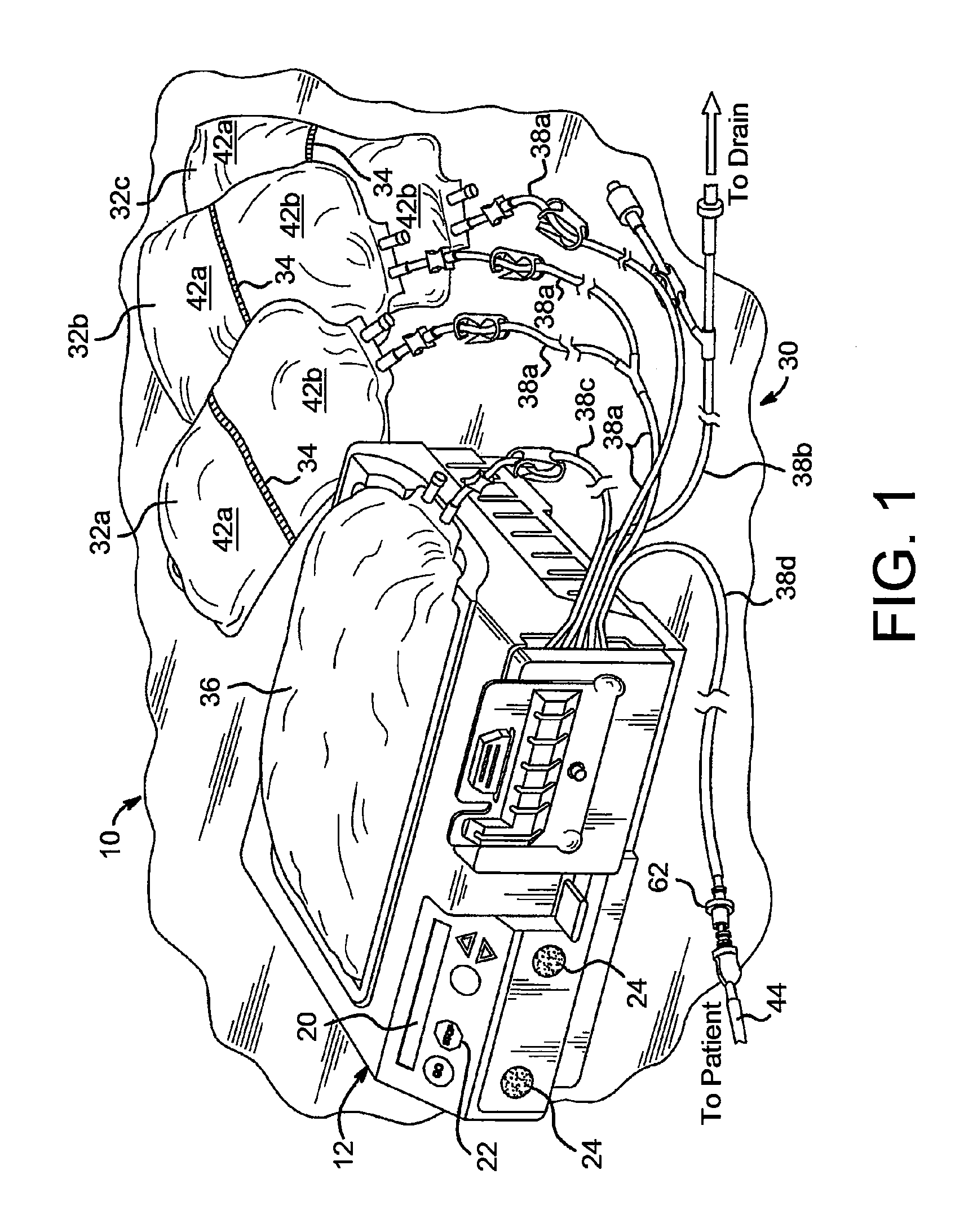
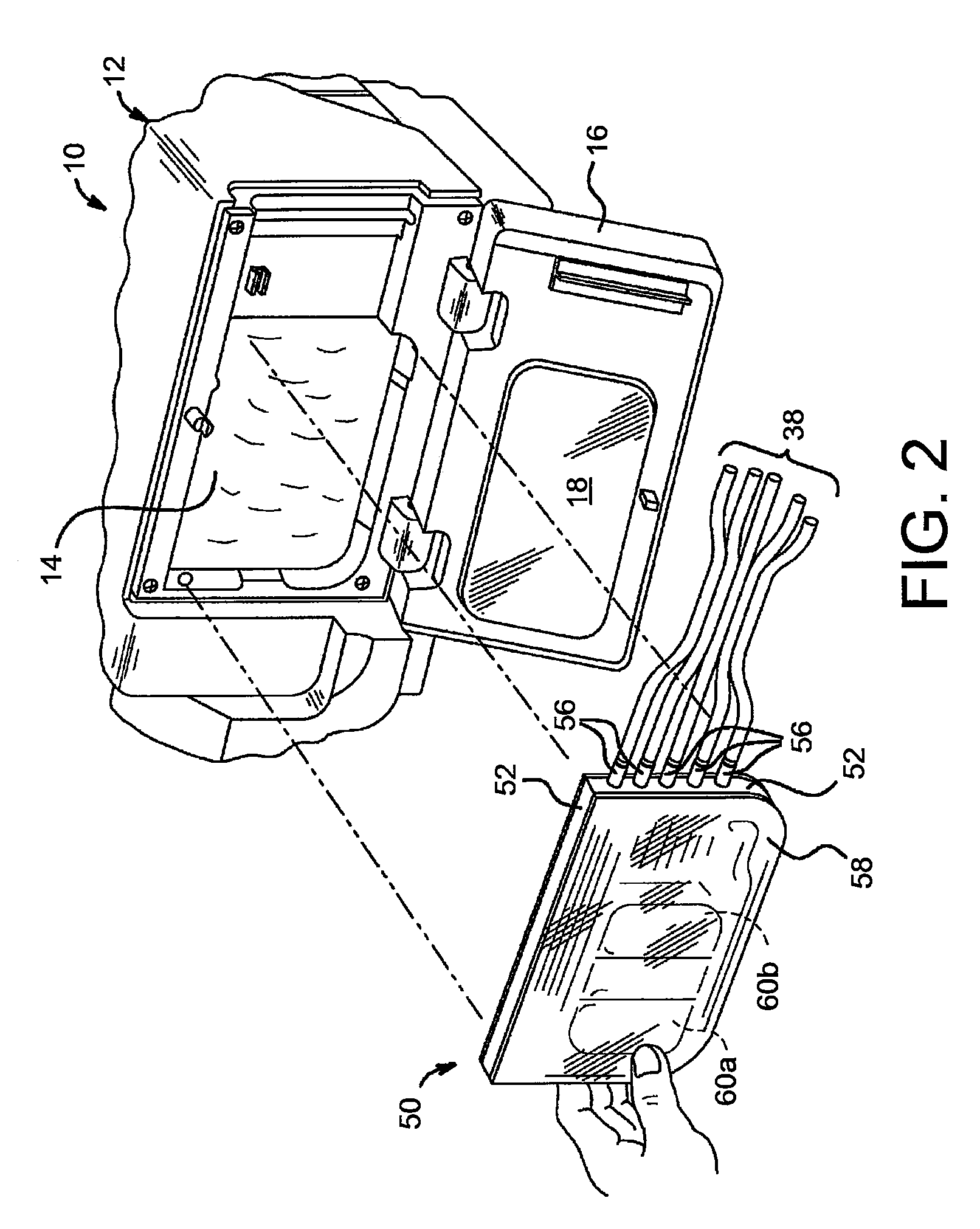
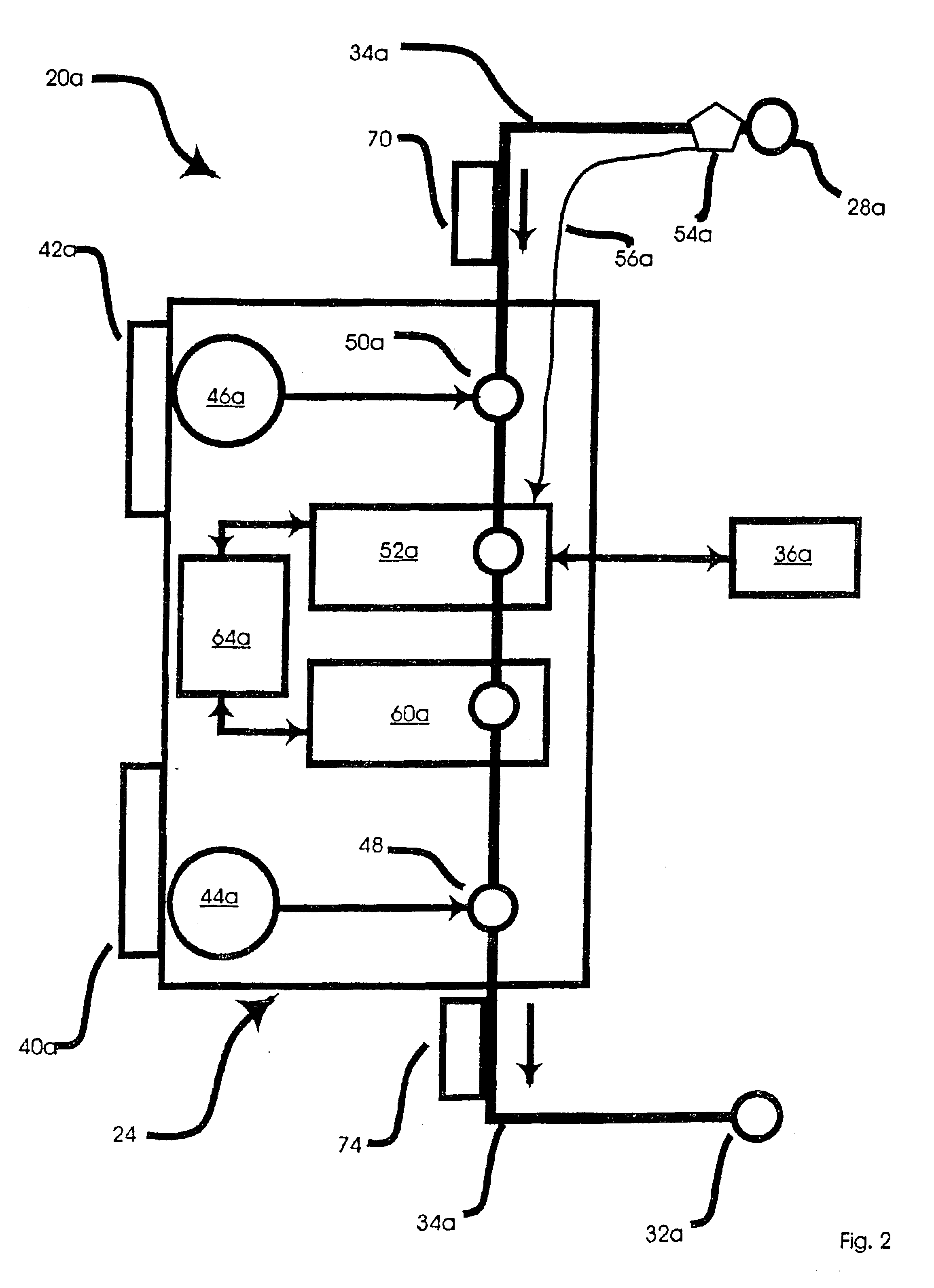
Portable apparatus for peritoneal dialysis therapy
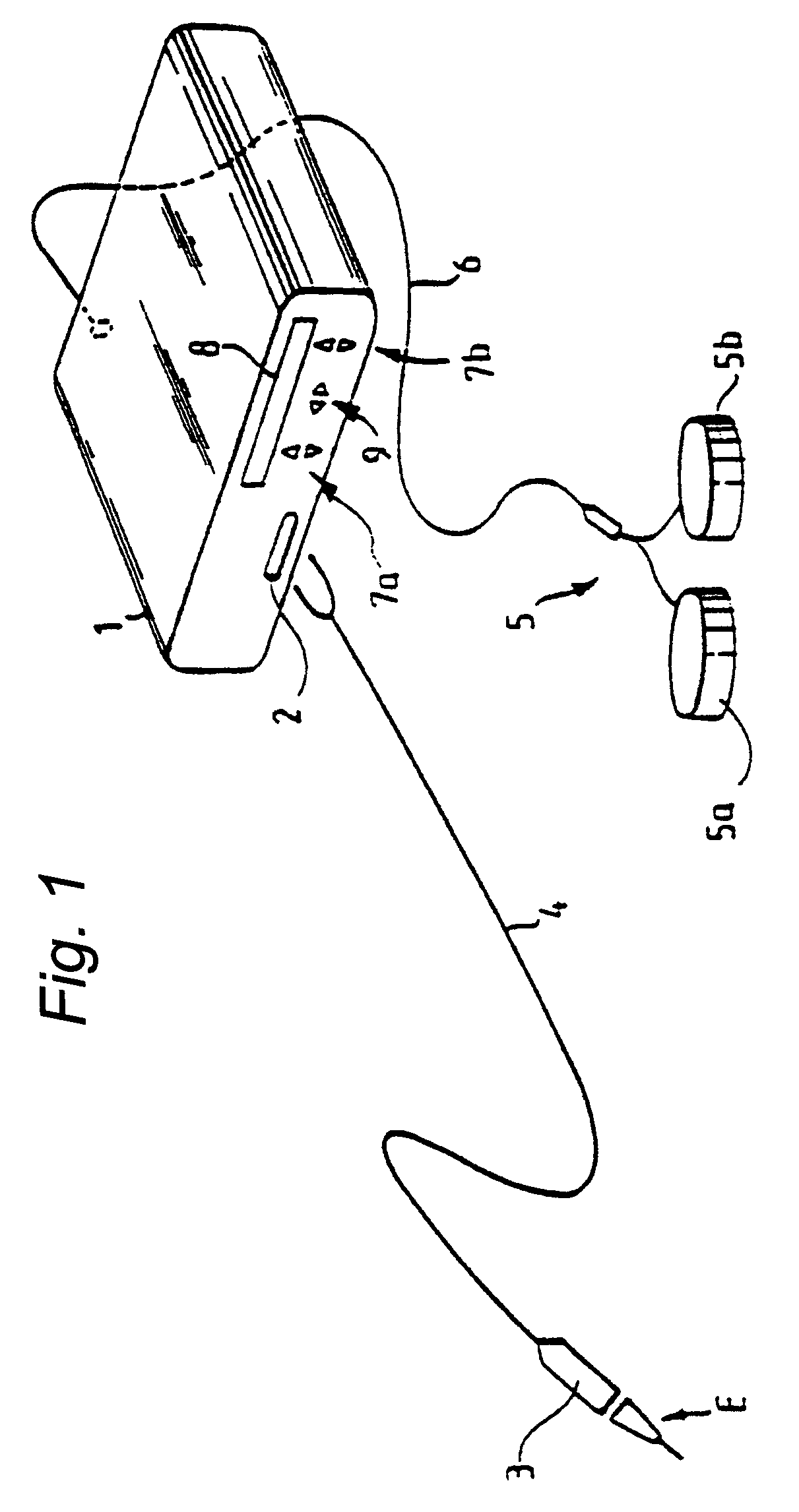
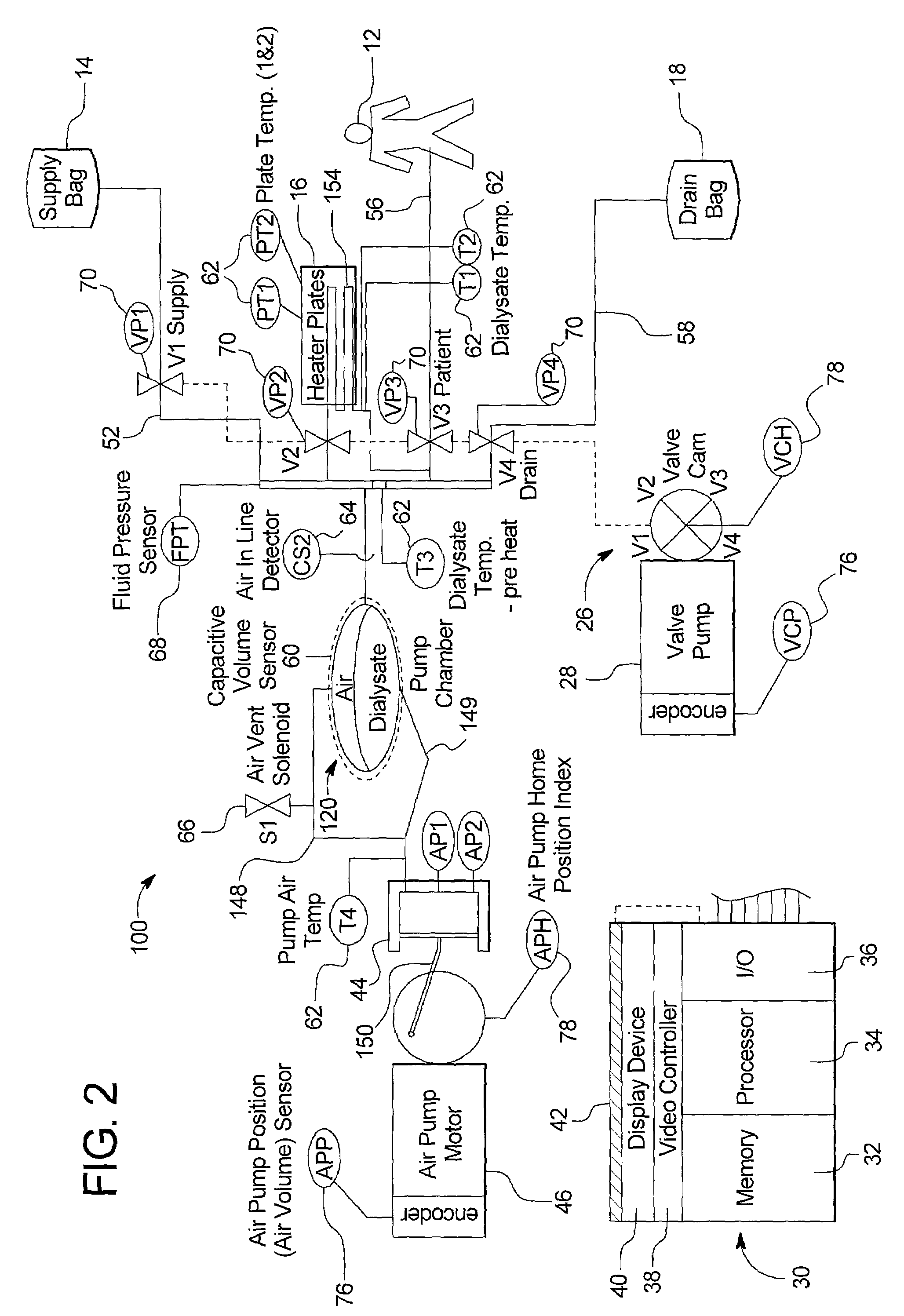
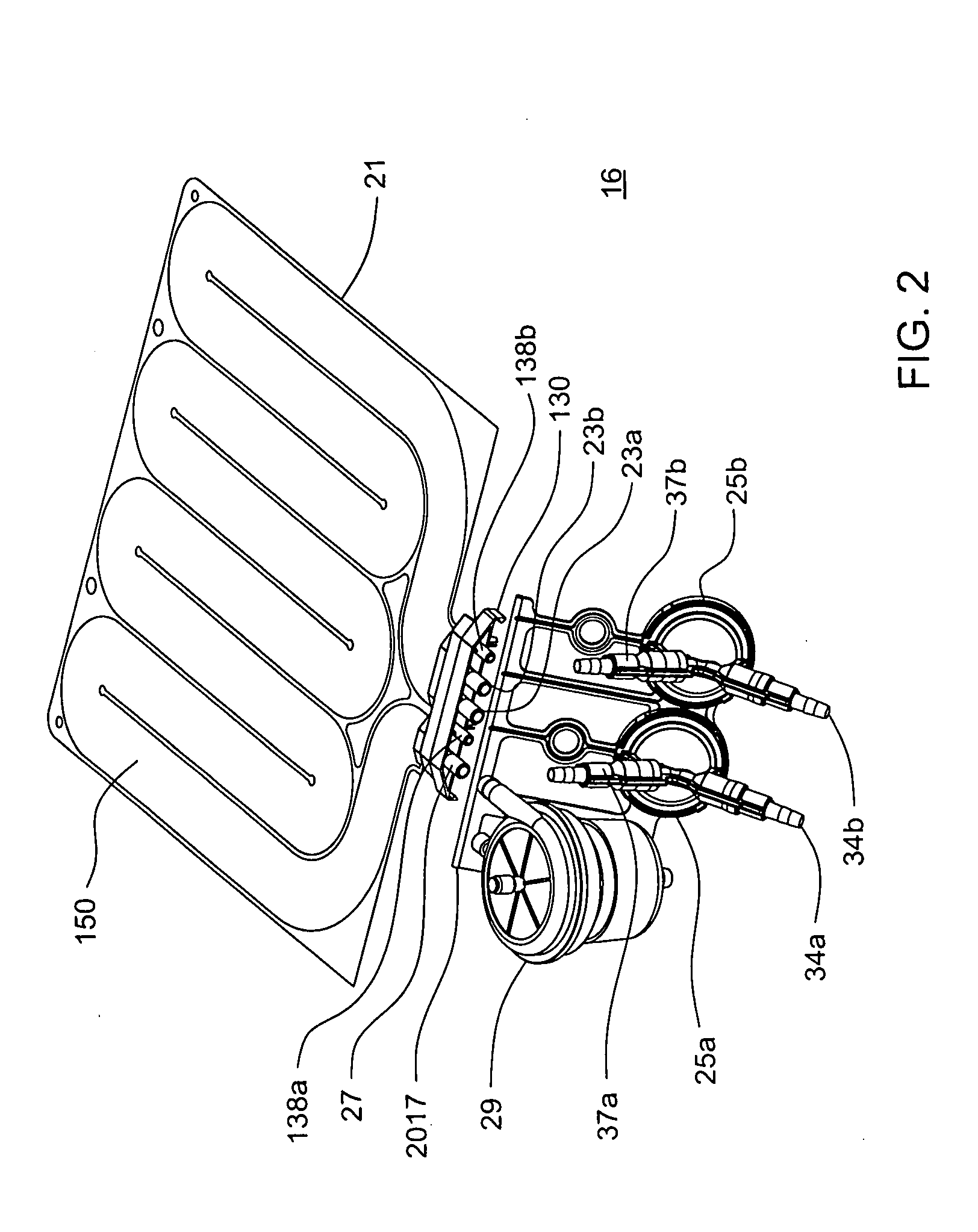
A portable peritoneal dialysis apparatus having (1) a hinged door for enclosing a disposable cassette that seals tightly shut using air pressure; (2) accurate pressure sensing of pressures applied to the patient through an enclosure in the disposable cassette; (3) two pumps that can operate separately or in tandem actuated by two separate stepper motors; and (4) a touch screen user interface where indicia of the operating mode is always visible along with indicia for the other possible operating modes and the mode can be changed by touching one of these indicia.
Owner:FRESENIUS MEDICAL CARE HLDG INC
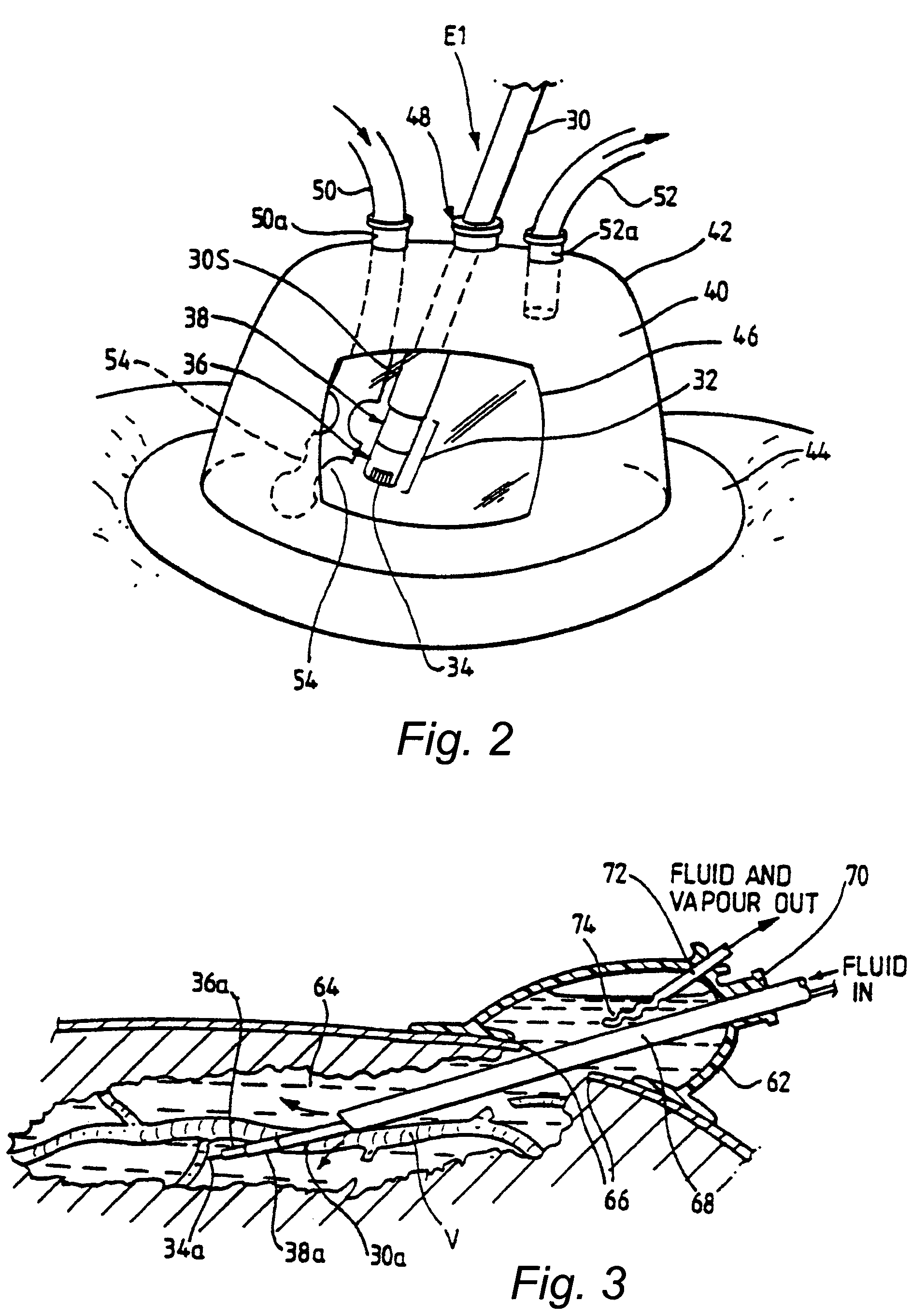
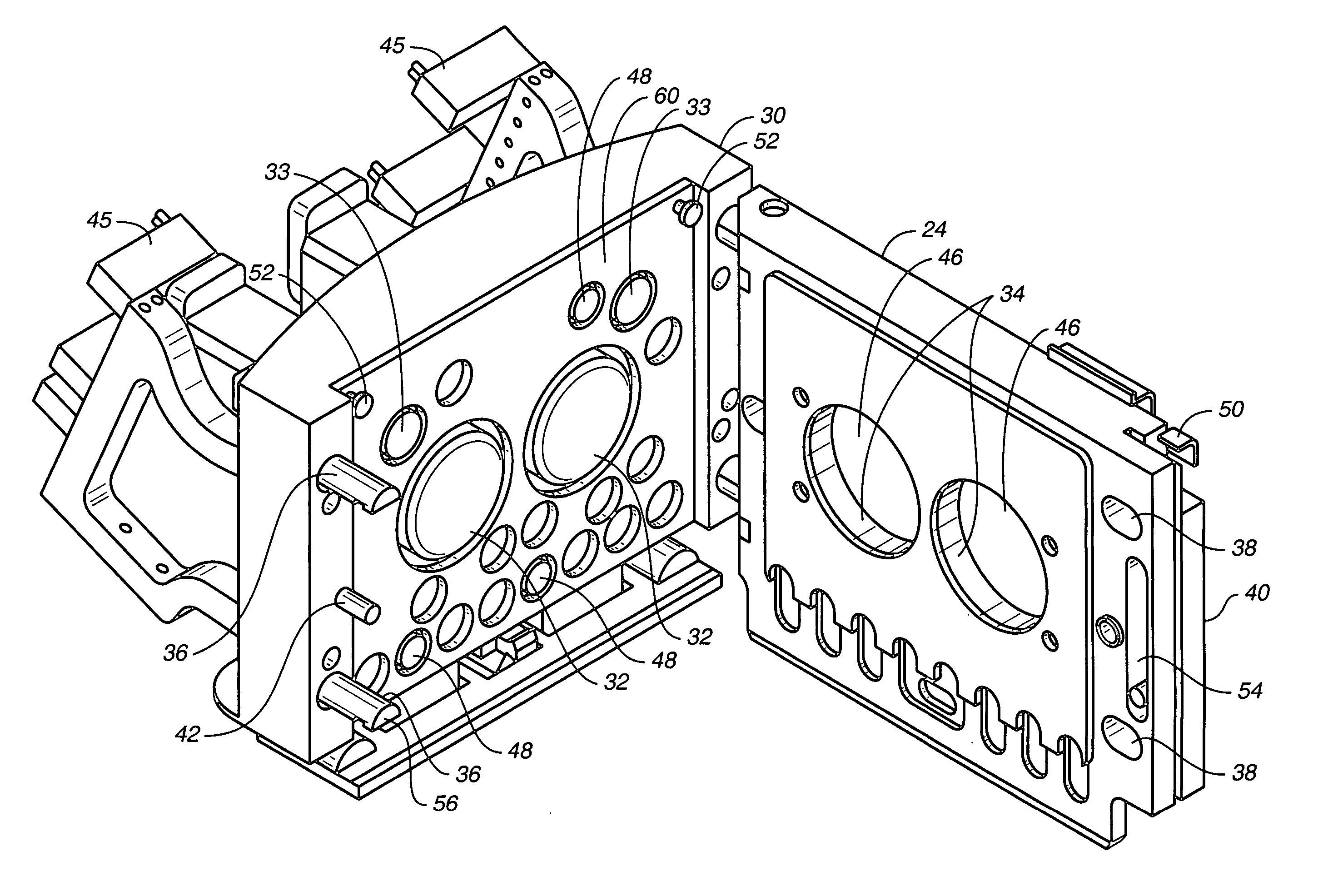
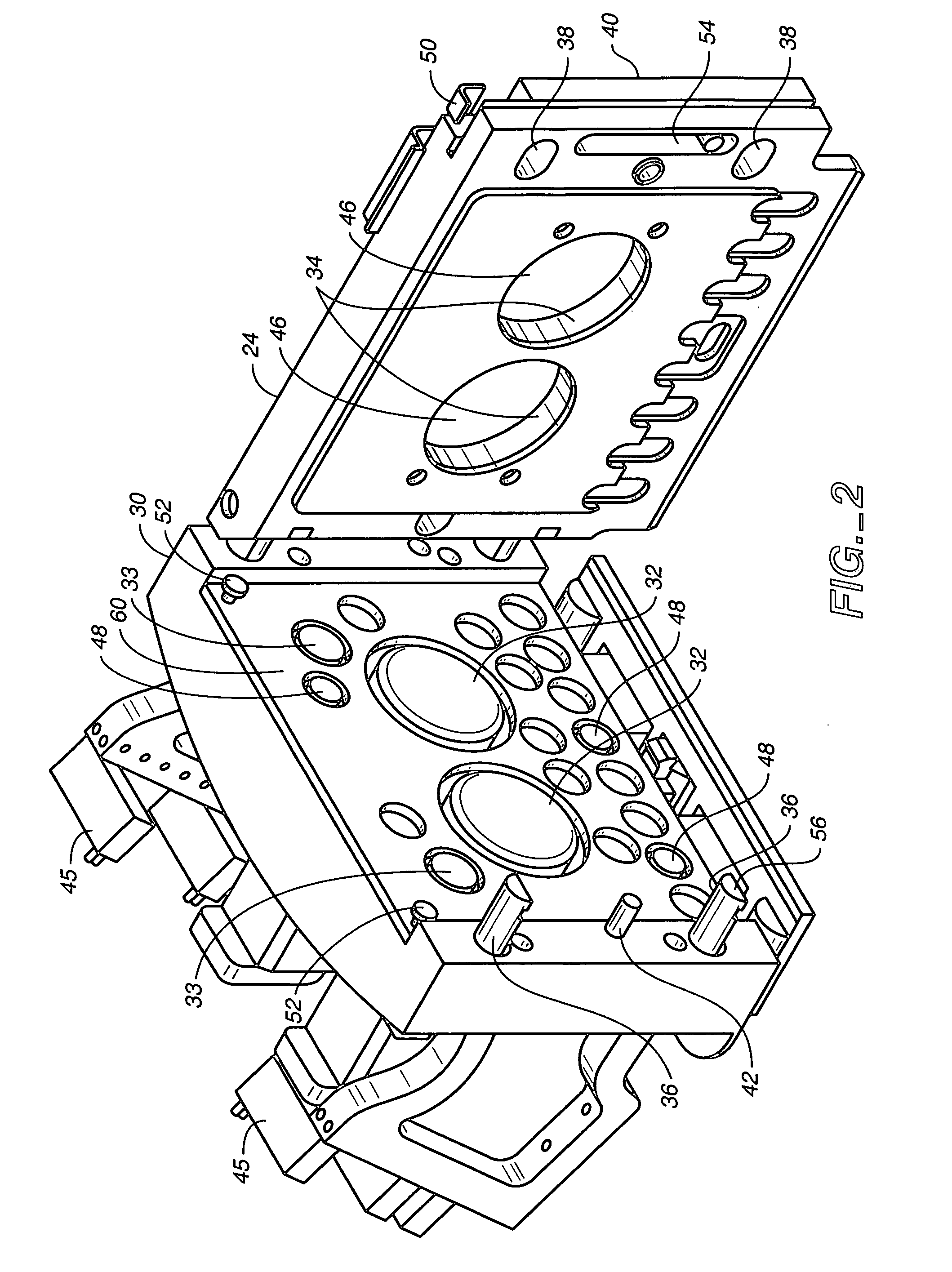
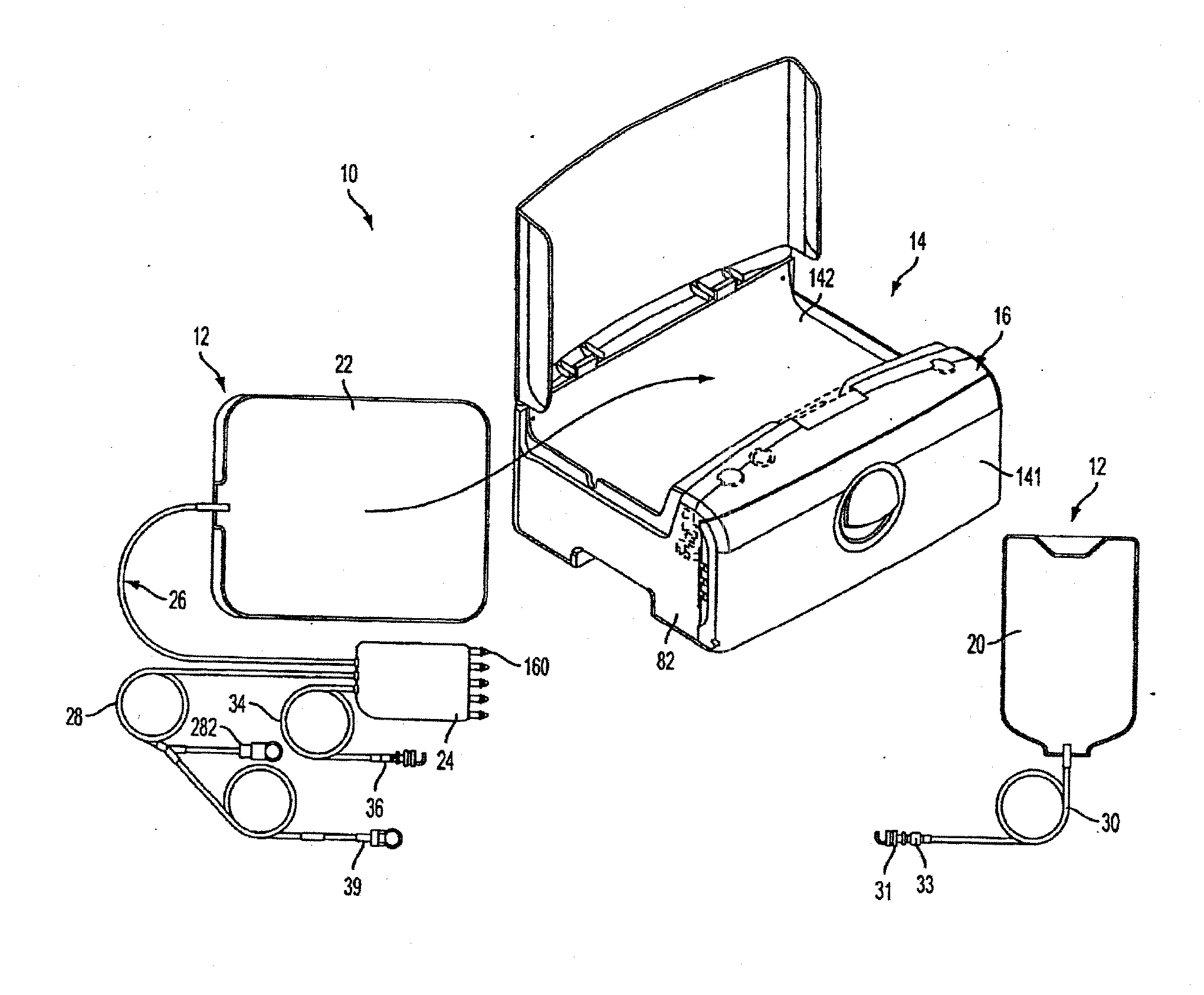
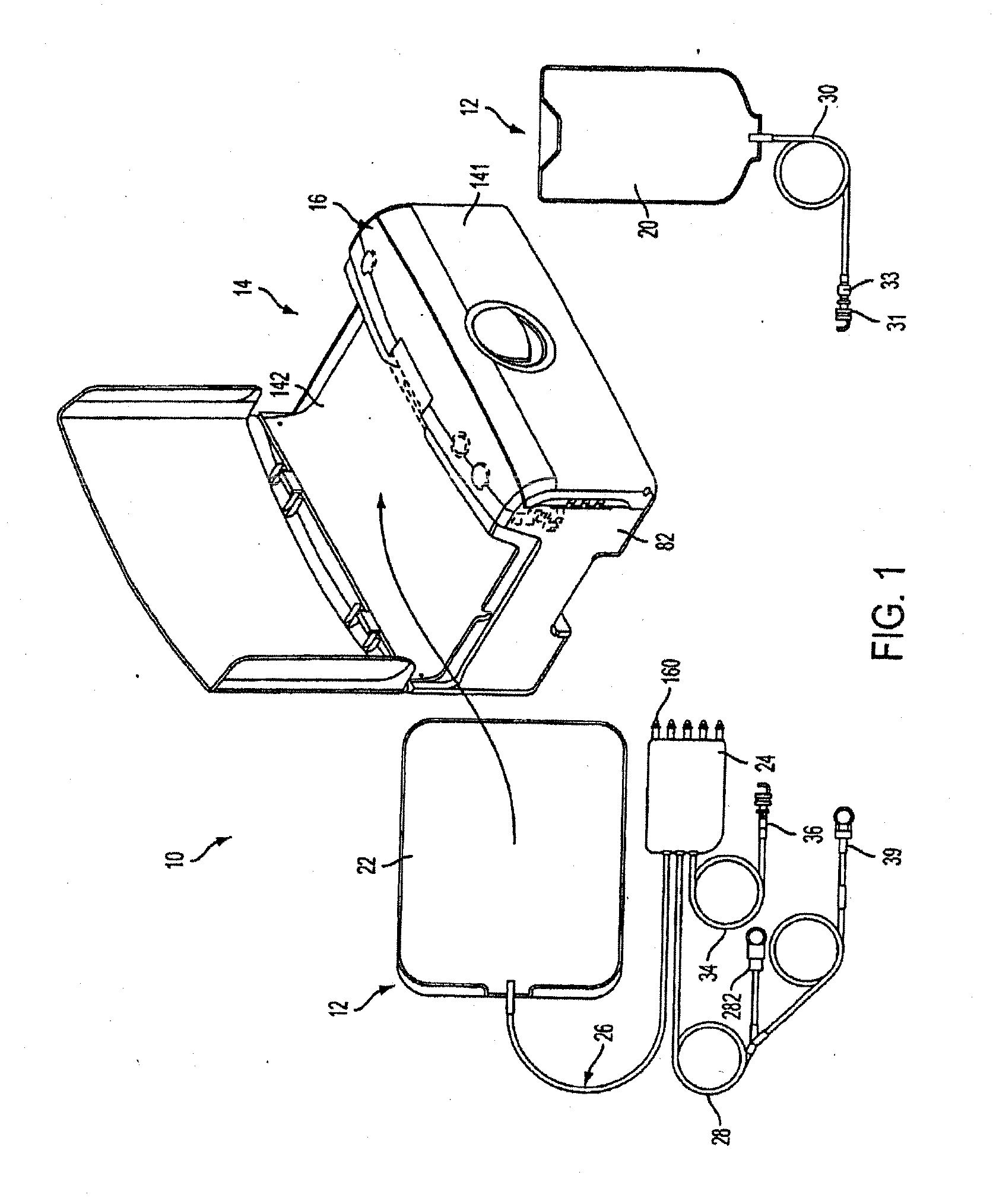
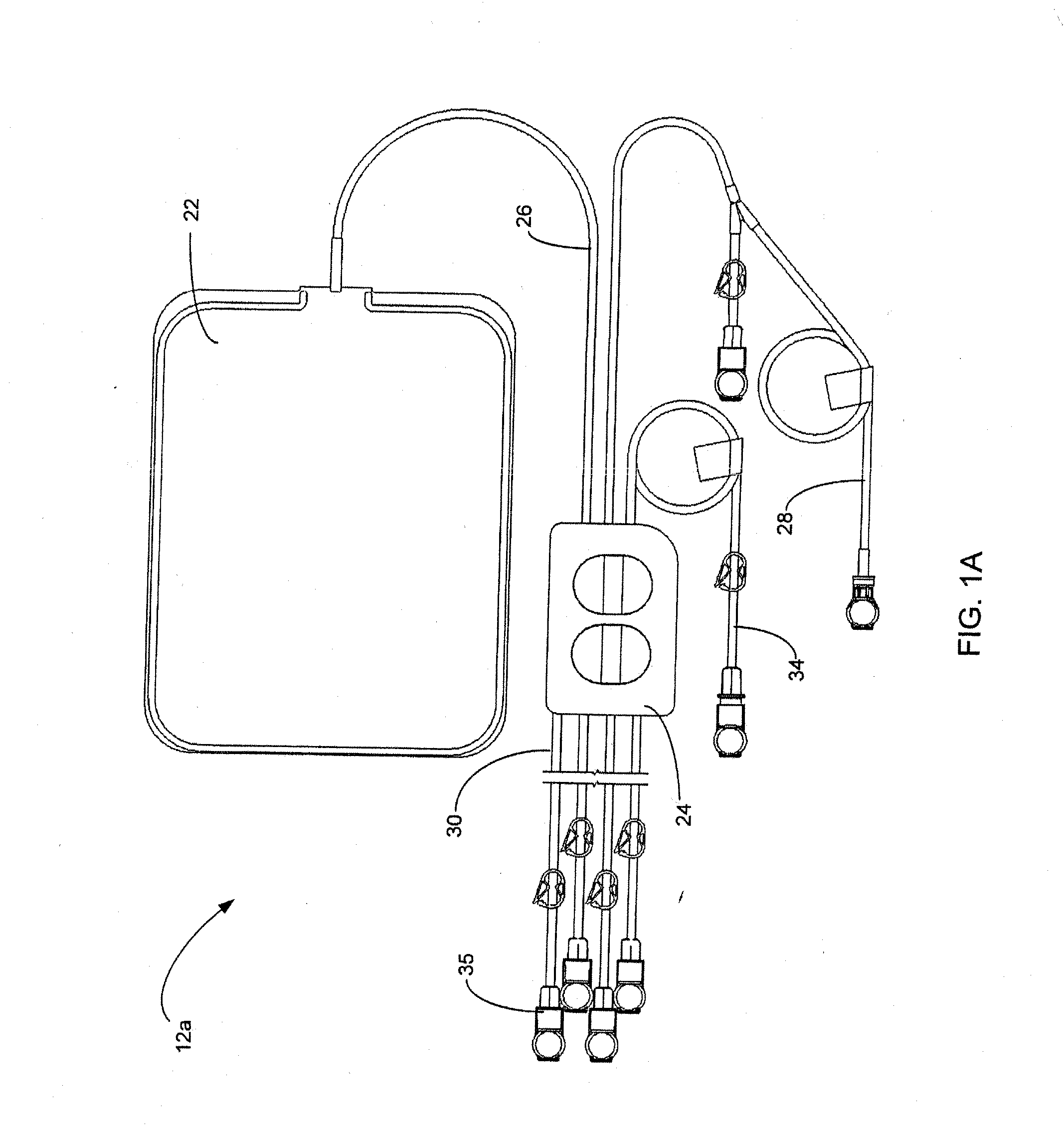
Disposable medical fluid unit having rigid frame
ActiveUS7175606B2Strengthen the systemImprove methodMedical devicesPressure infusionPeritoneal dialysisBiomedical engineering
A method, system and apparatus for performing peritoneal dialysis are provided. To this end, in part, a frame of a disposable unit for a medical fluid system is provided. The frame includes a body having a pair of opposing sides and defining a space between the sides, and a number of membranes sealed to the body along the opposing sides. The body and the membranes form at least one medical fluid pathway in the space between the opposing sides, and the opposing sides are bowed.
Owner:BAXTER HEALTHCARE SA +1
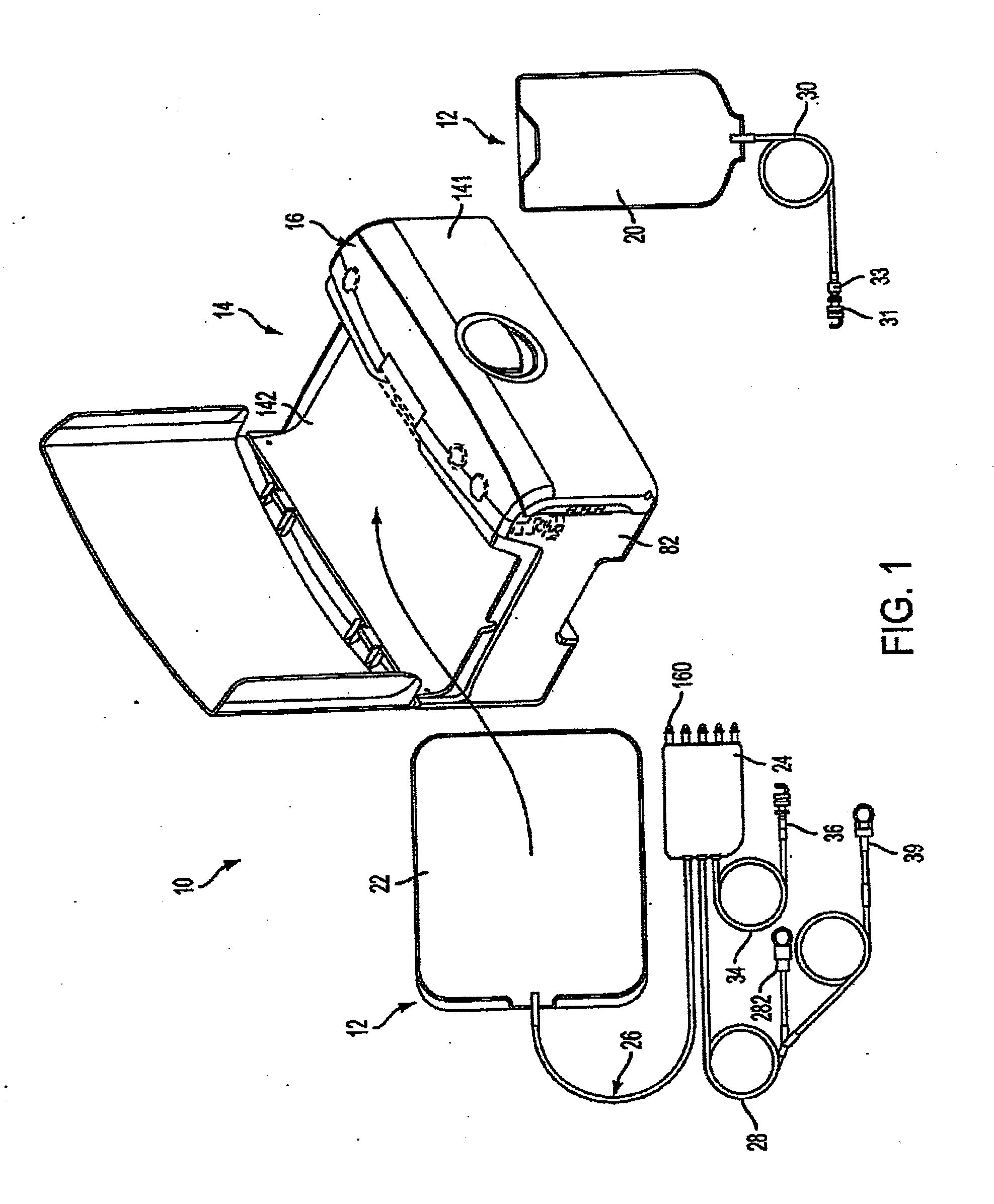
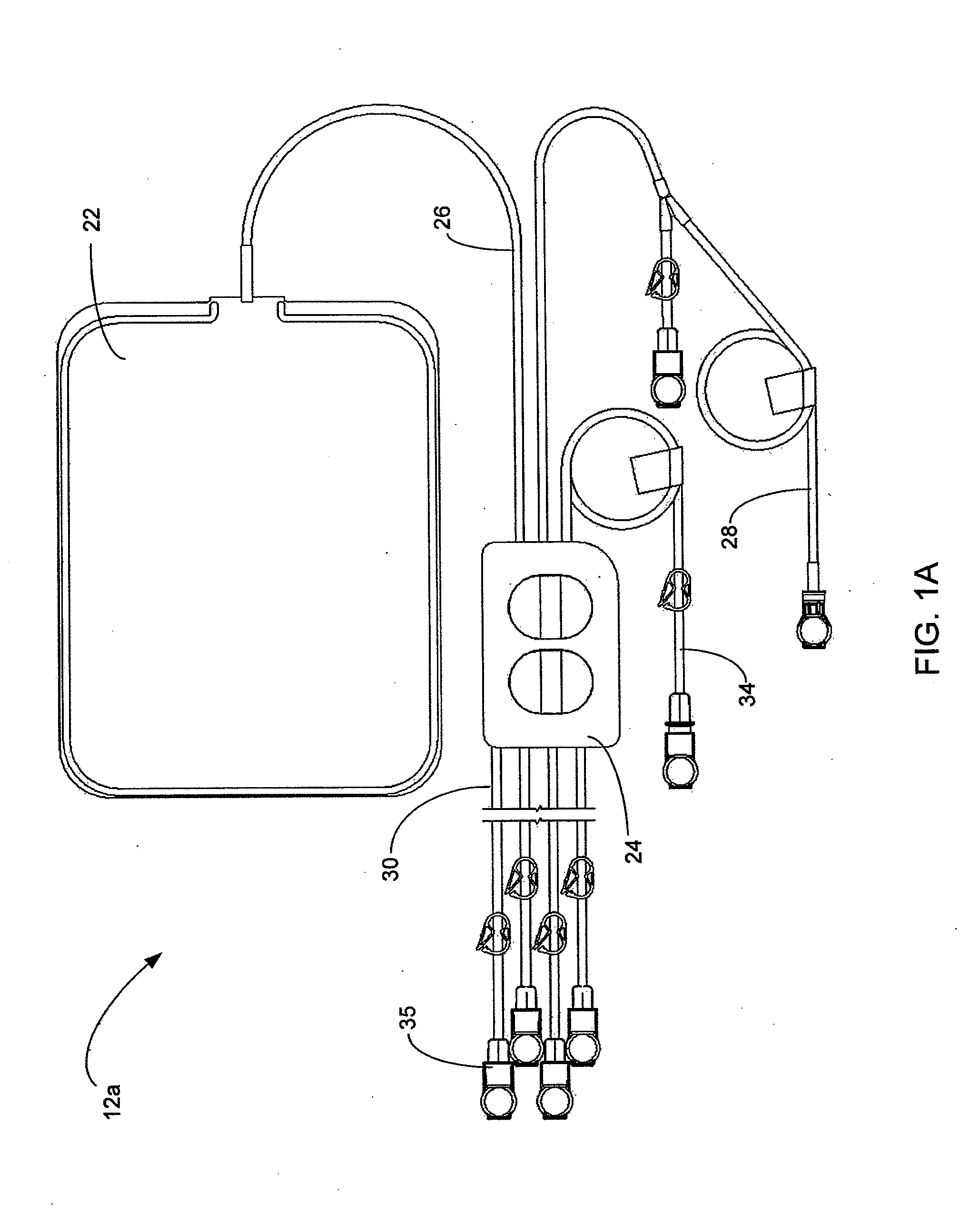
Systems and methods for performing peritoneal dialysis
ActiveUS7867214B2Strengthen the systemImprove methodSolvent extractionIon-exchanger regenerationMetabolic wasteSorbent
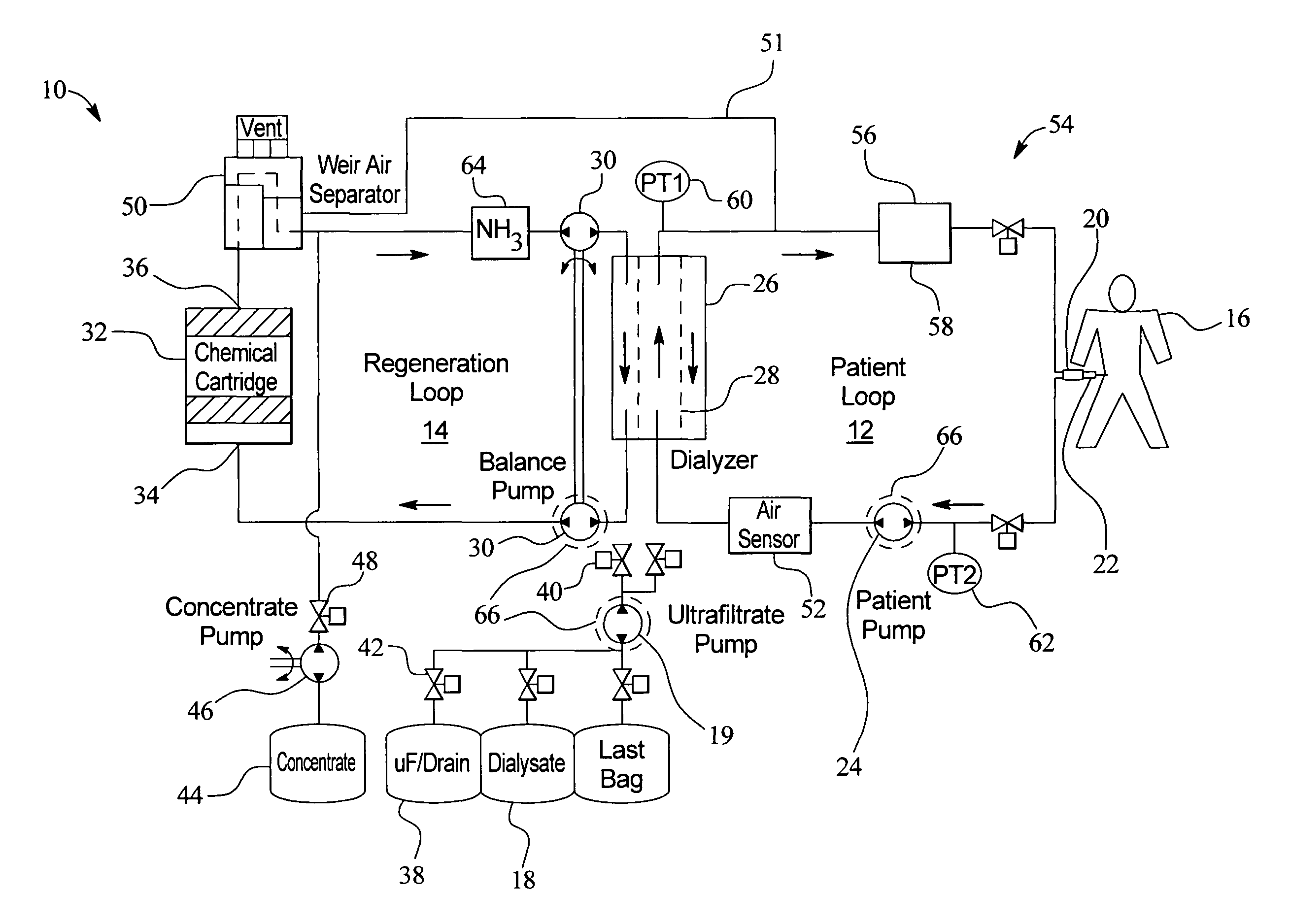
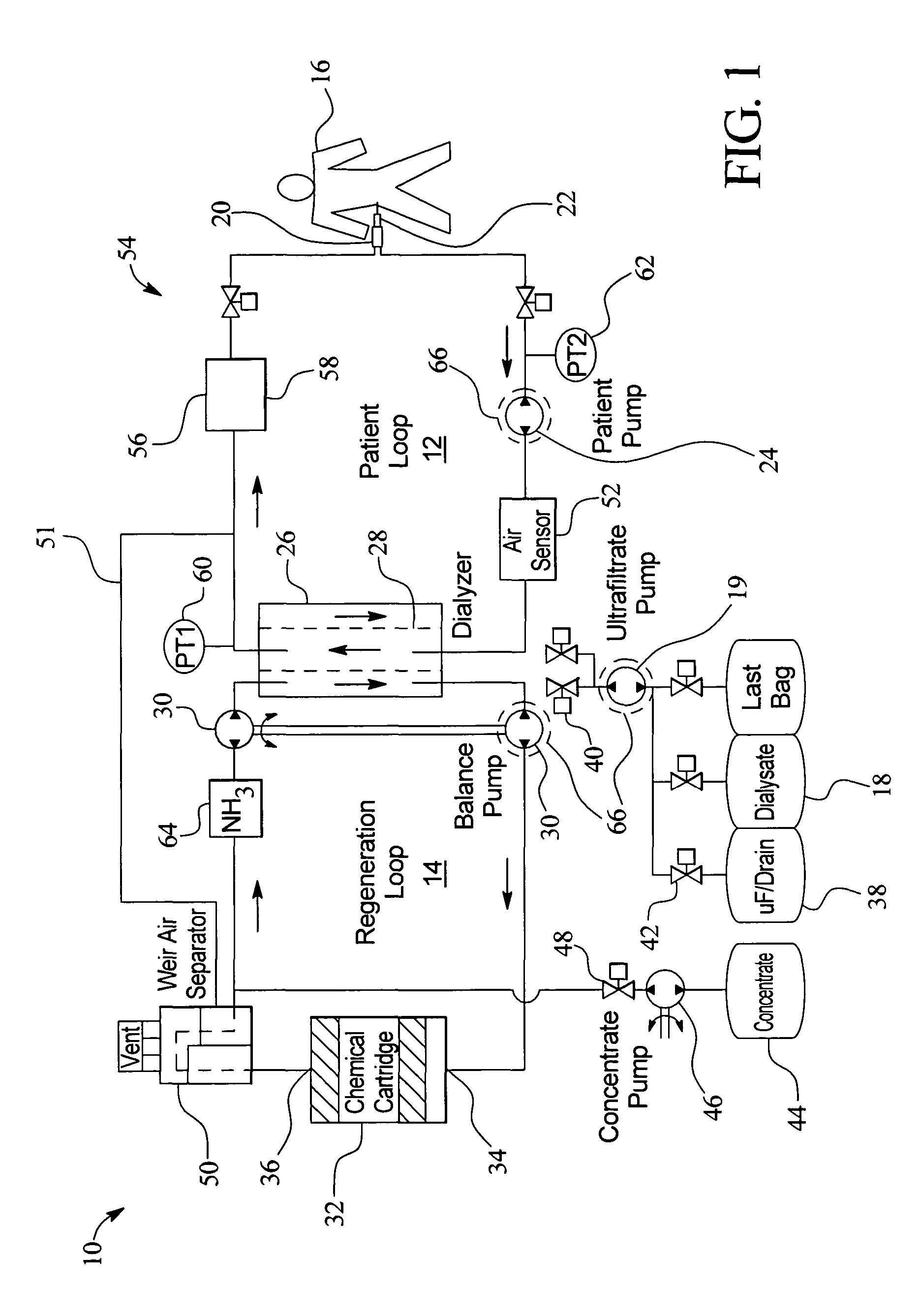
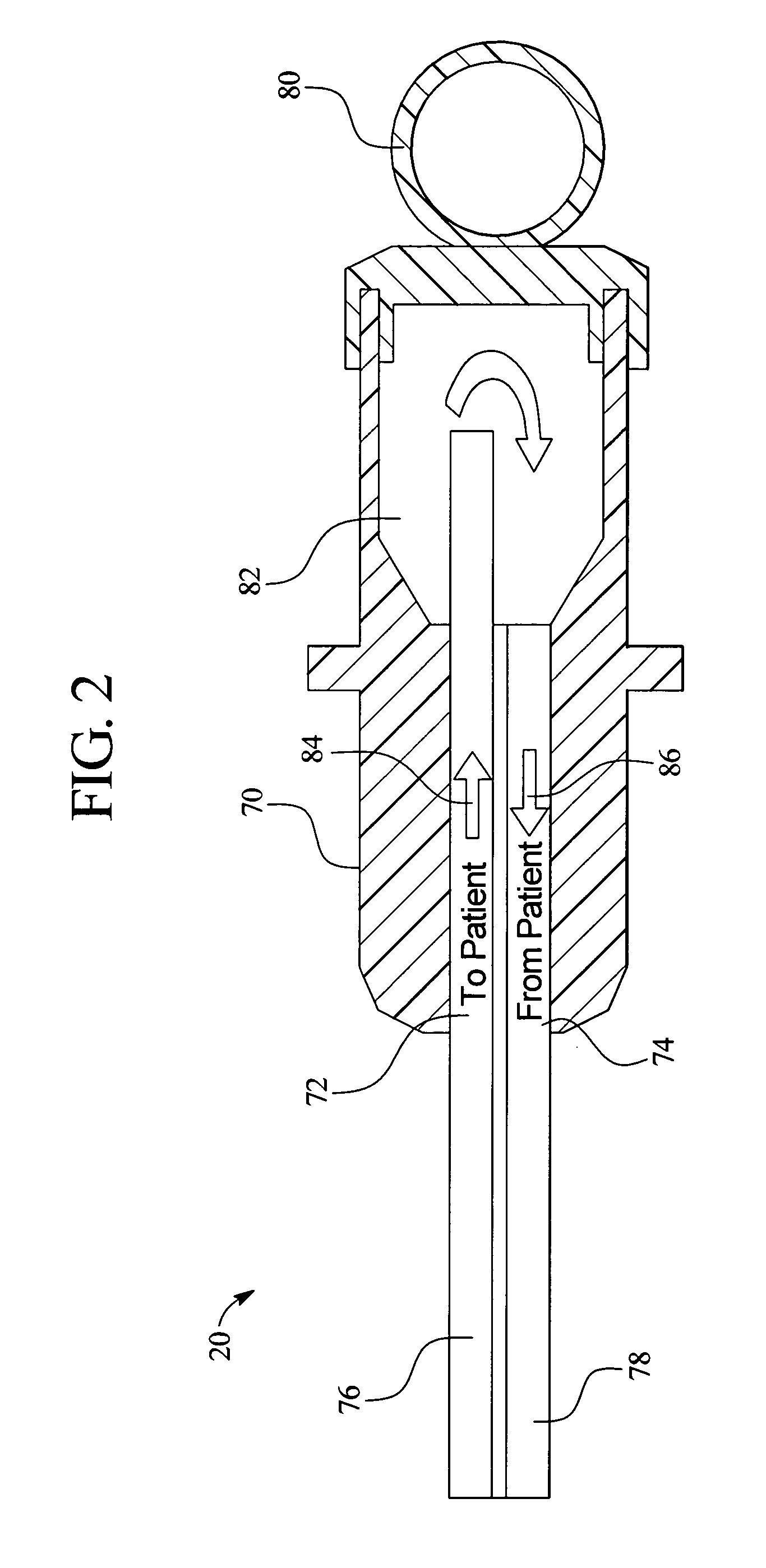
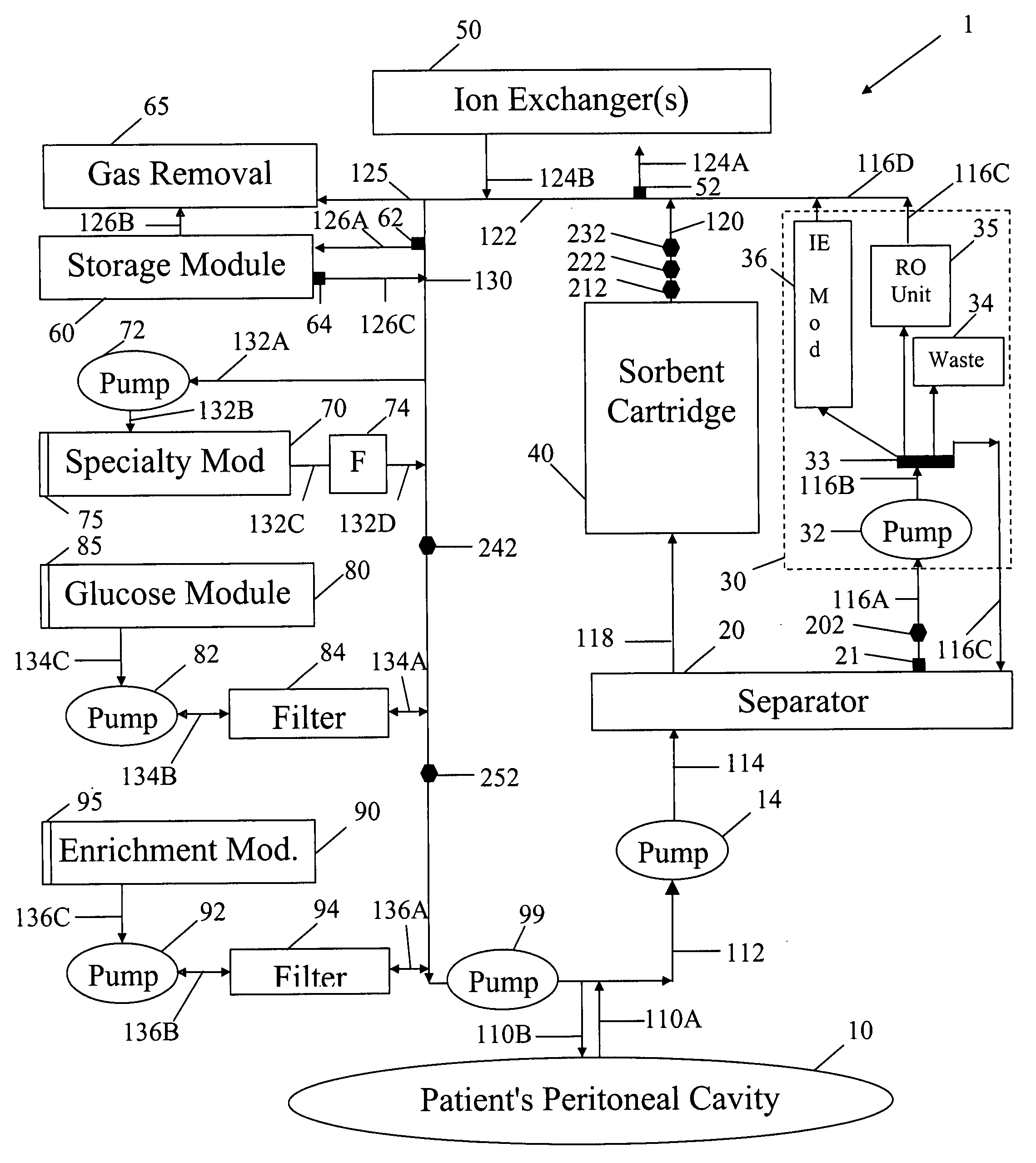
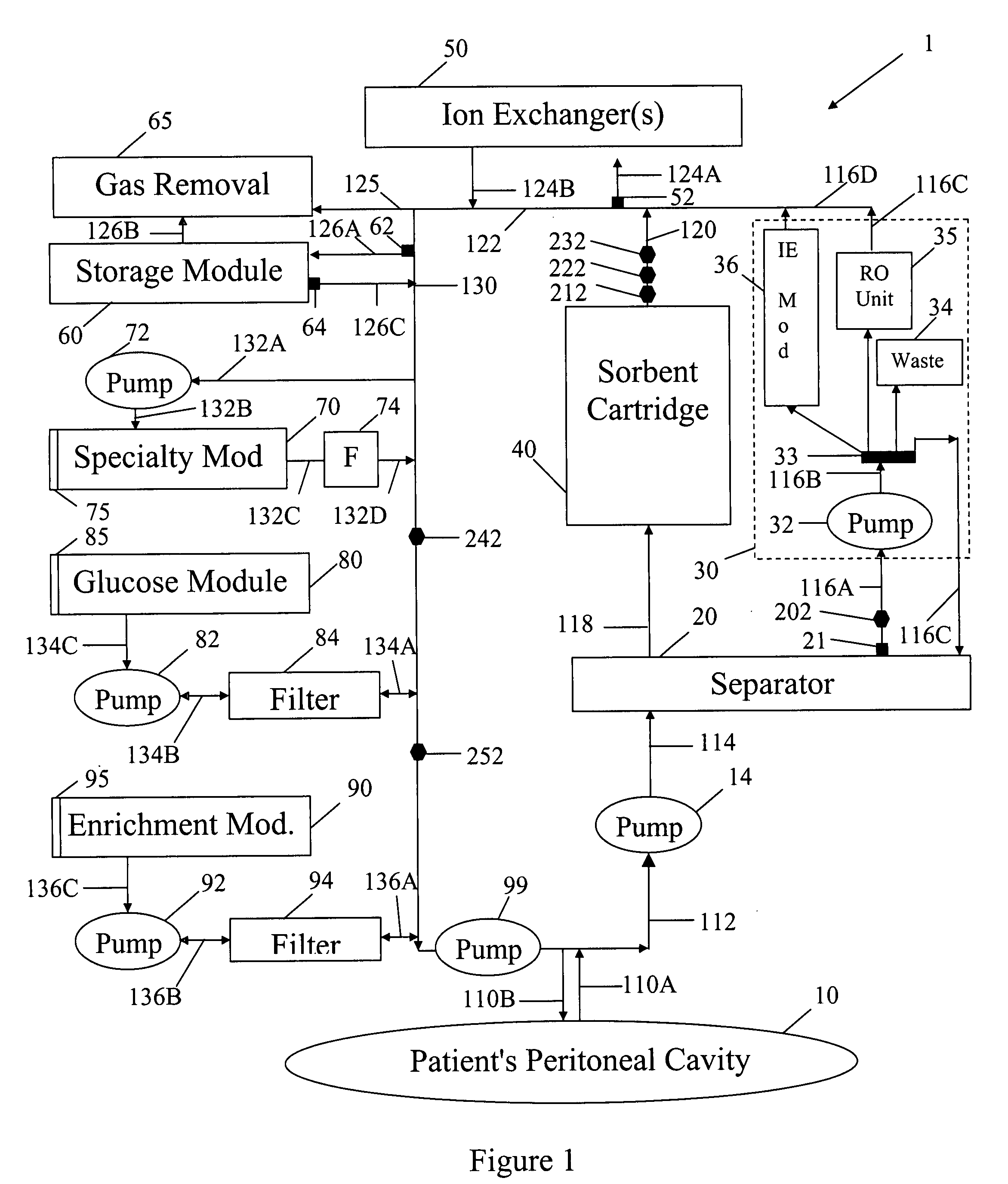
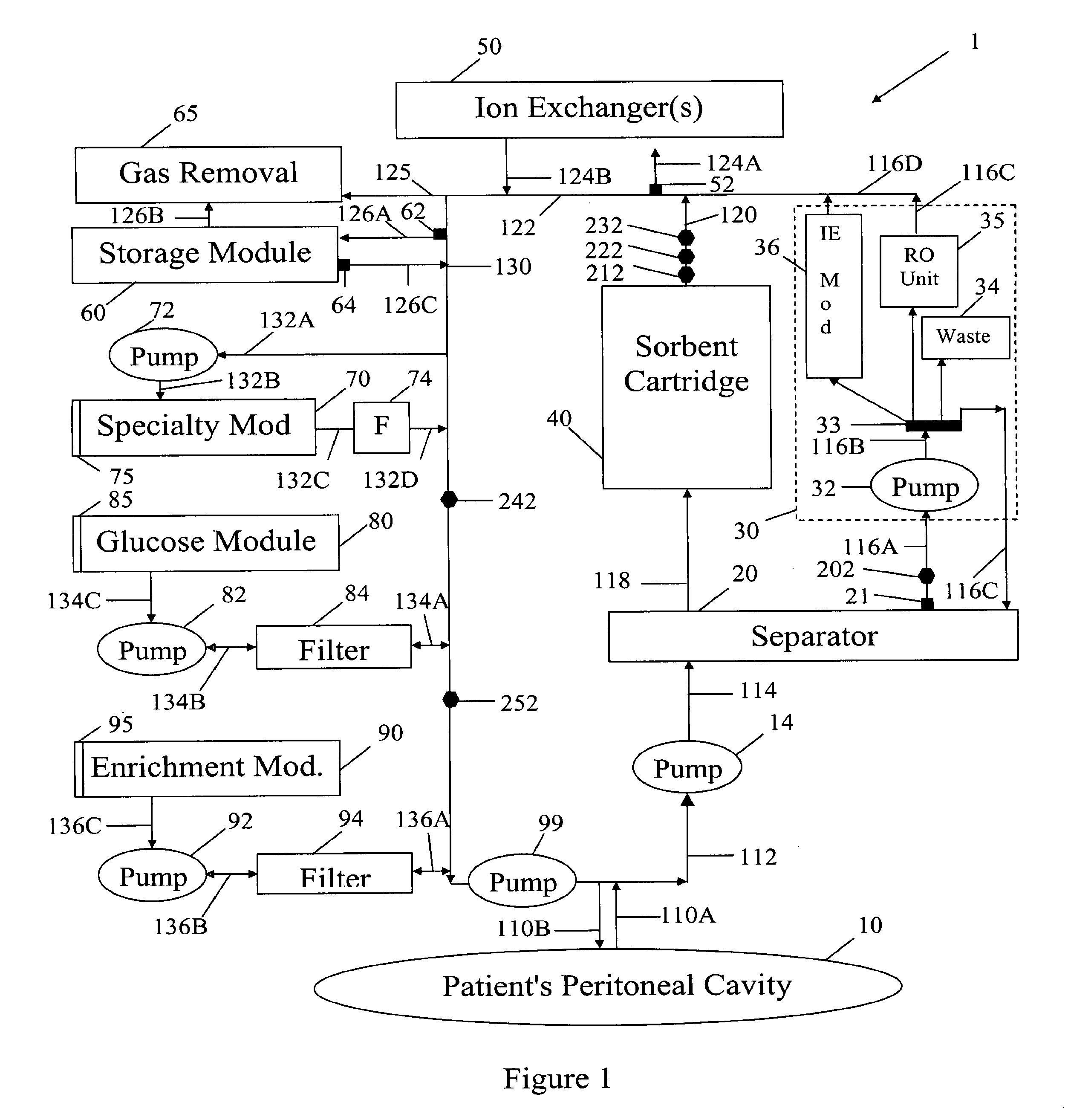
In a peritoneal dialysis embodiment of the present invention, spent dialysate from the patient's peritoneal cavity passes, along a patient loop, through a dialyzer having a membrane that separates waste components from the spent dialysate, wherein the patient loop returns fresh dialysate to the patient's peritoneal cavity. The waste components are carried away in a second regeneration loop to a regeneration unit or sorbent cartridge, which absorbs the waste components. The regeneration unit removes undesirable components in the dialysate that were removed from the patient loop by the dialyzer, for example, excess water (ultrafiltrate or UF), toxins and metabolic wastes. Desirable components can be added to the dialysate by the system, such as glucose and electrolytes. The additives assist in maintaining the proper osmotic gradients in the patient to perform dialysis and provide the necessary compounds to the patient.
Owner:BAXTER INT INC
Multimodal dialysis system
ActiveUS20120273354A1Rate of fluid is decreasedReduce probabilitySludge treatmentIon-exchanger regenerationClinical settingsHaemodialysis machine
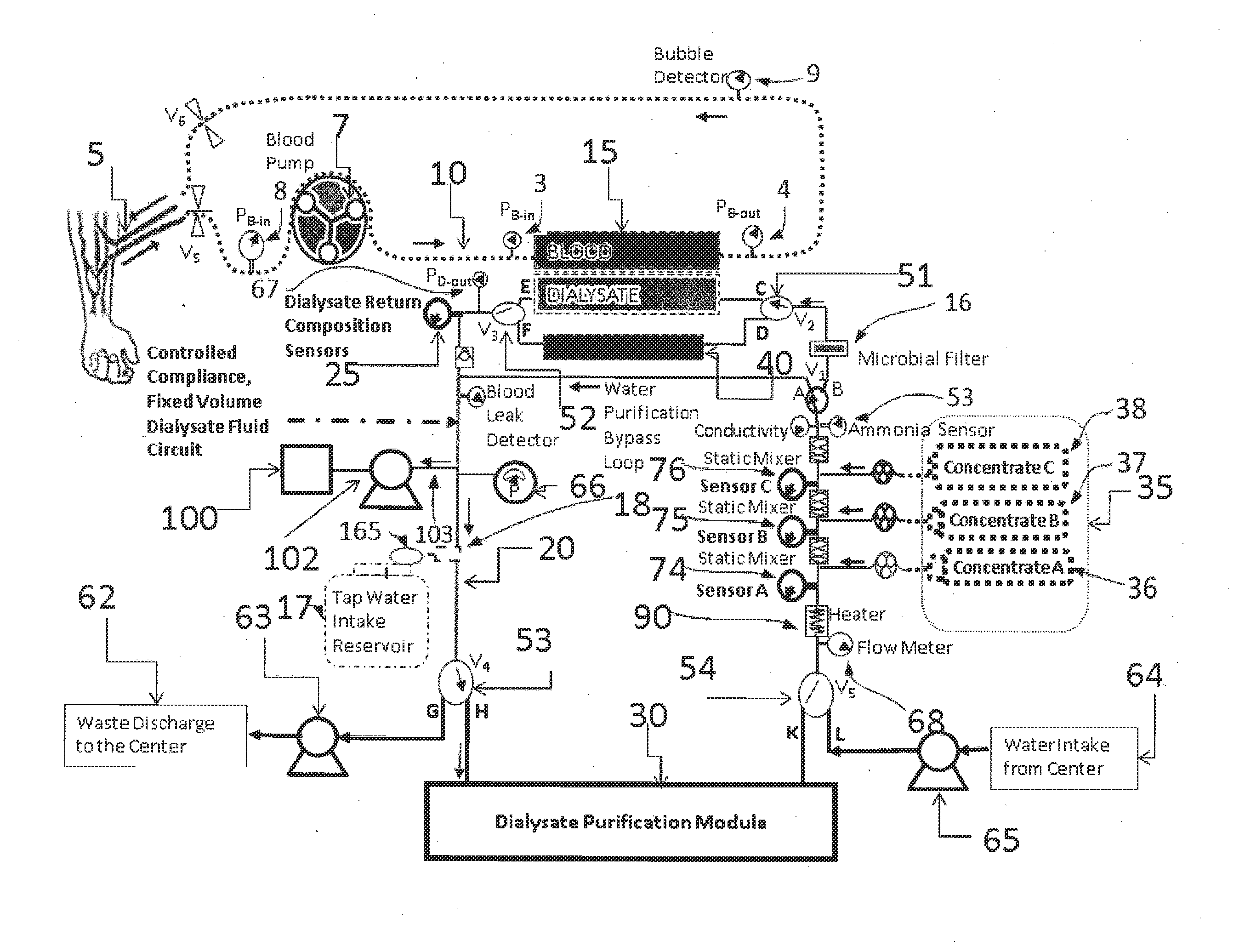
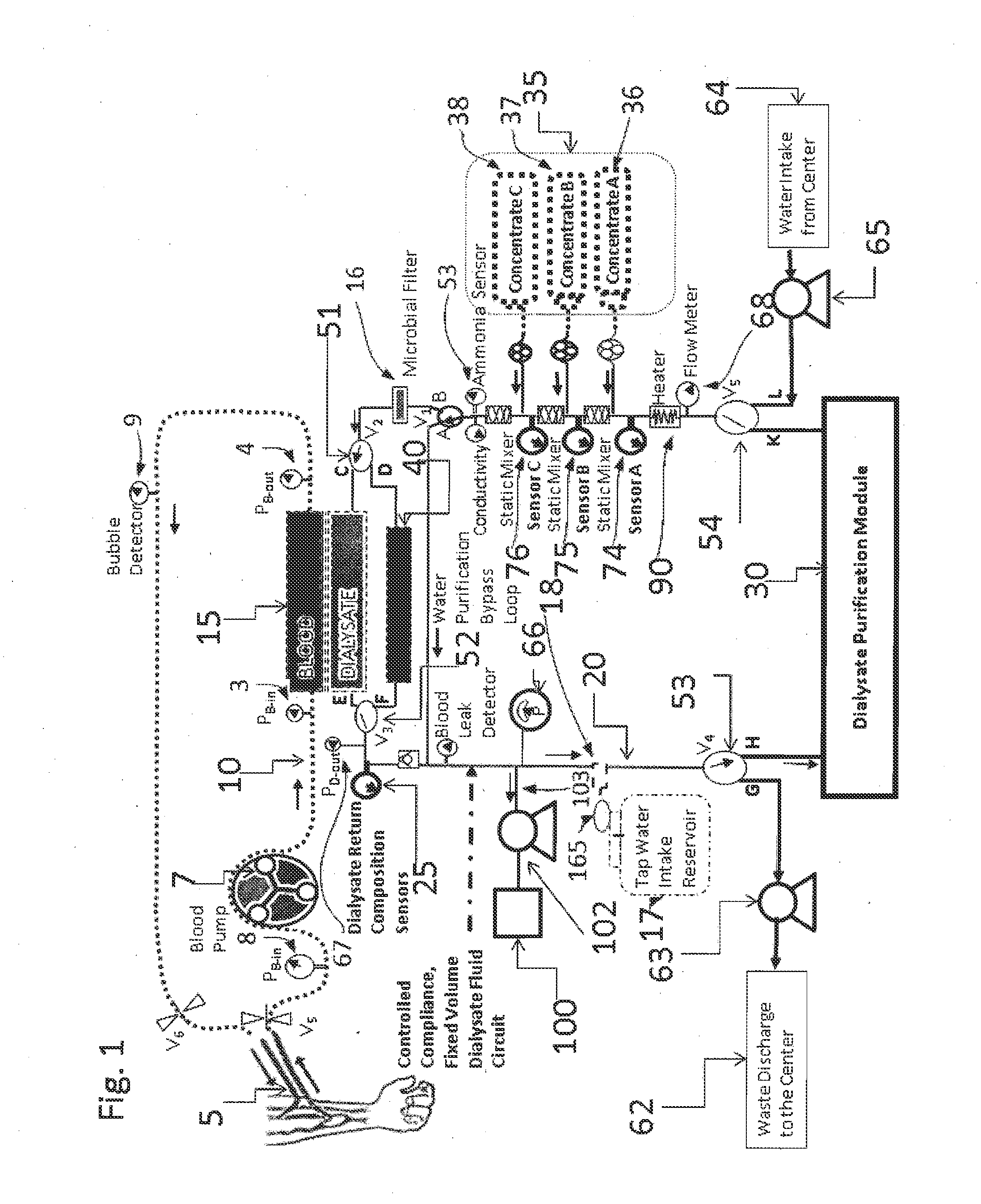
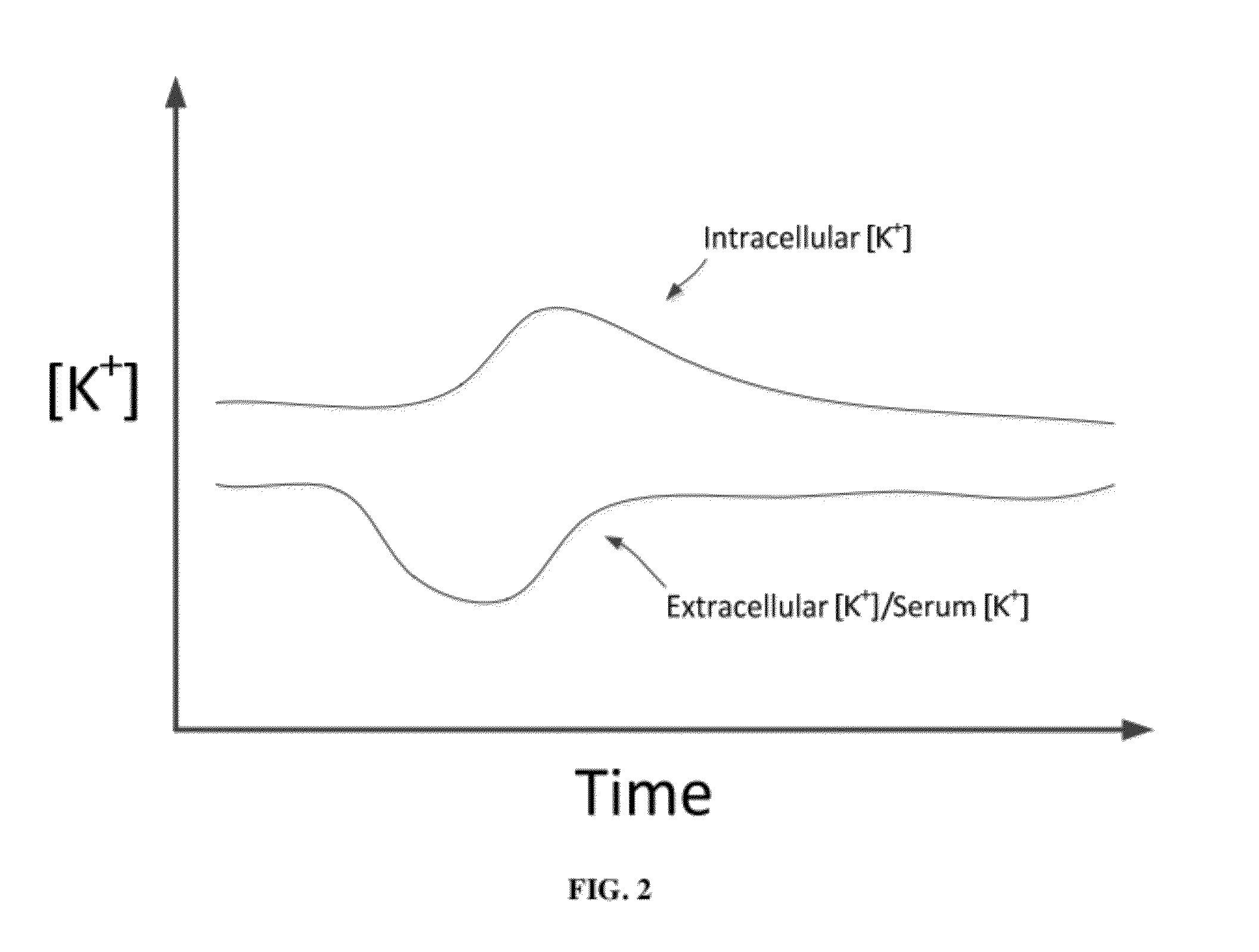
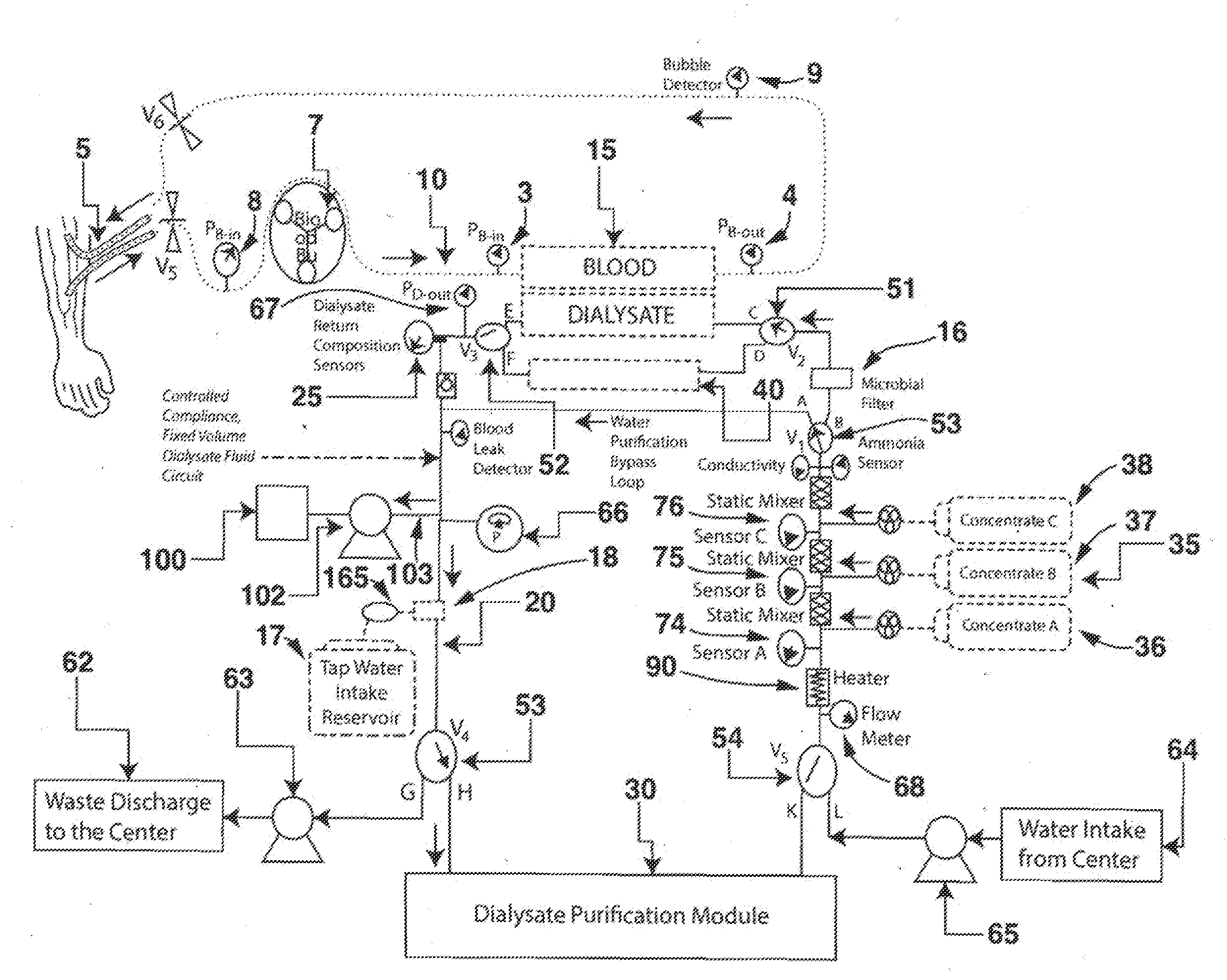
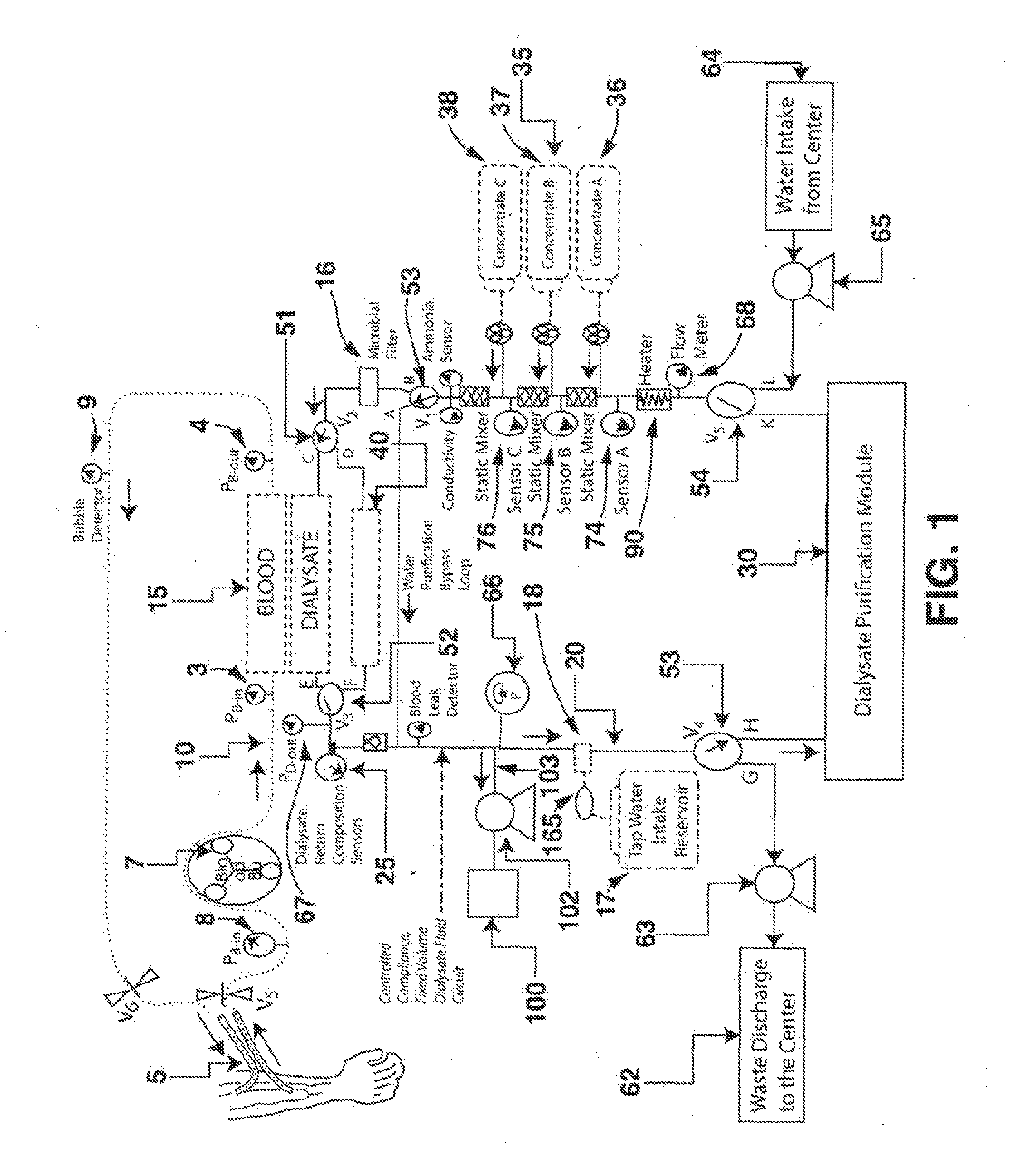
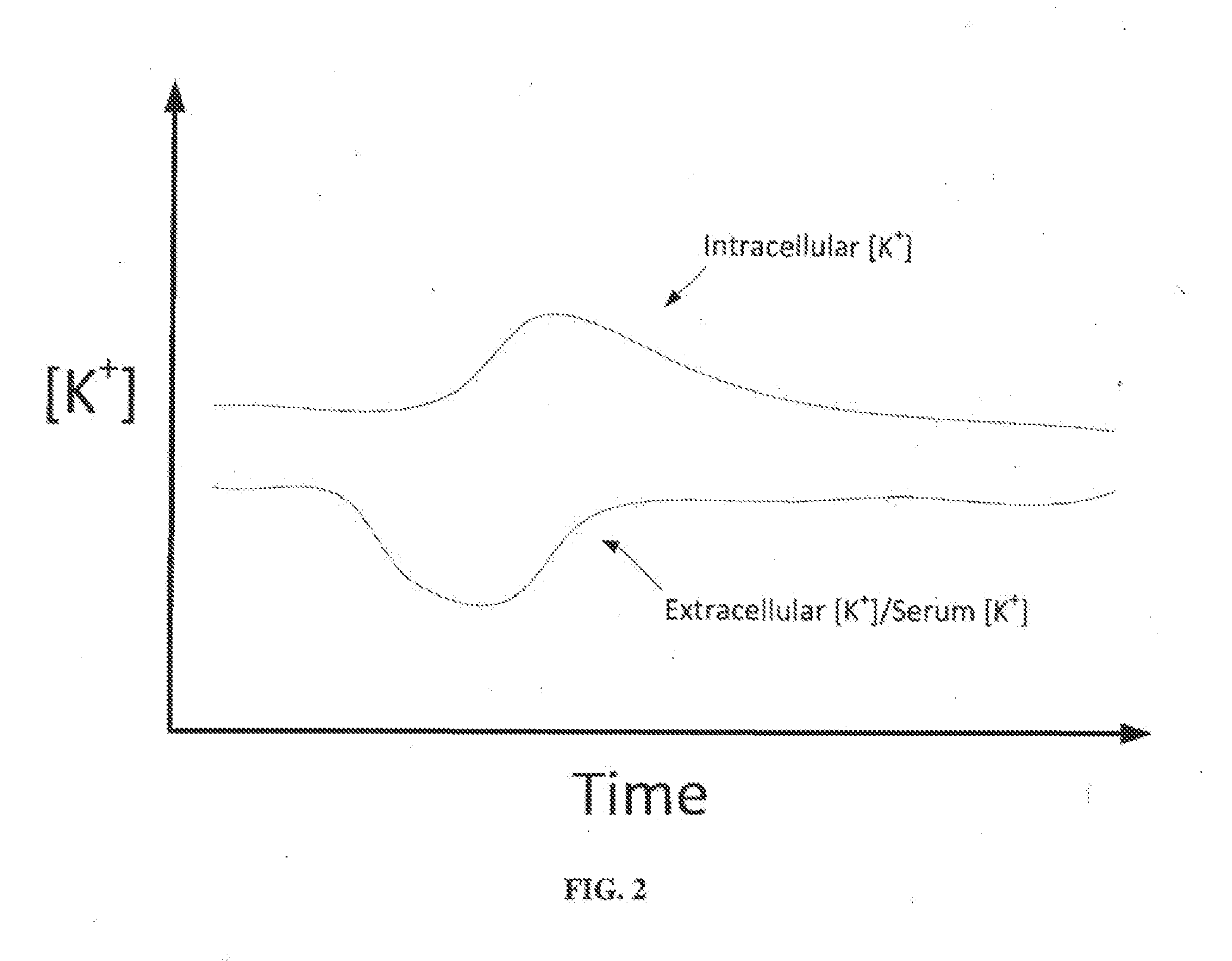
A dialysis device for operation in multiple modes and for maintaining a known gradient of potassium ion or other electrolyte between the blood of a patient and a dialysate fluid is described. The dialysis device is capable of being used for hemodialysis or peritoneal dialysis, and the dialysis device is capable of operation with a dialysate purification unit outside of a clinical setting or with a supply of water that can be supplied in a clinical setting. The dialysis device has a composition sensor containing a potassium-sensitive electrode for measuring a potassium ion concentration in one or more of the patient's blood and the dialysate fluid and an infusate pump operated to adjust a potassium ion concentration in the dialysate fluid based at least in part on data from the composition sensor.
Owner:MOZARC MEDICAL US LLC
Apparatus and methods for facilitating treatment of tissue via improved delivery of energy based and non-energy based modalities
InactiveUS20050020901A1Avoid excessive forceAccurately advancedEndoscopesCatheterThoracic structureAutomatic control
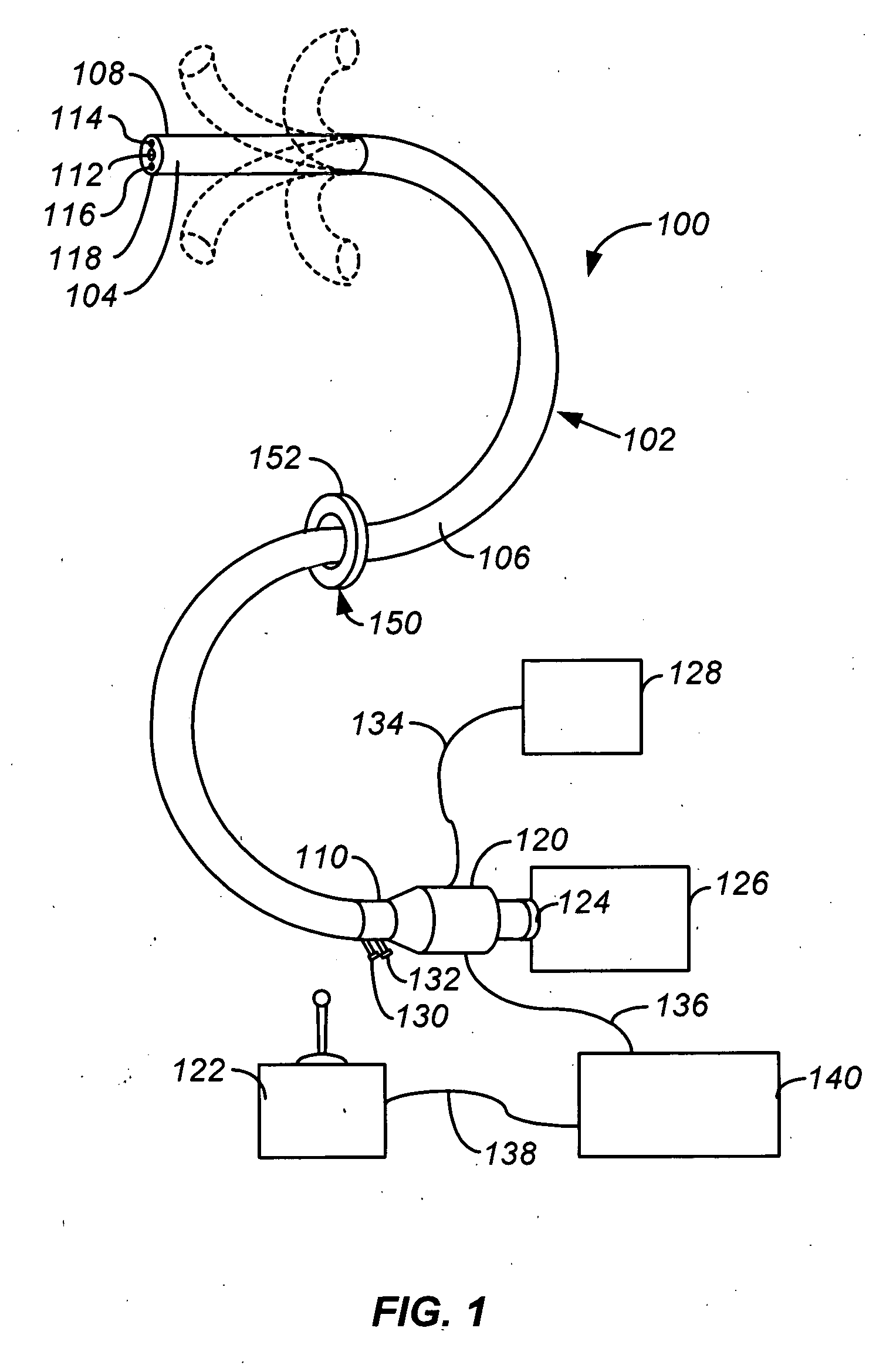
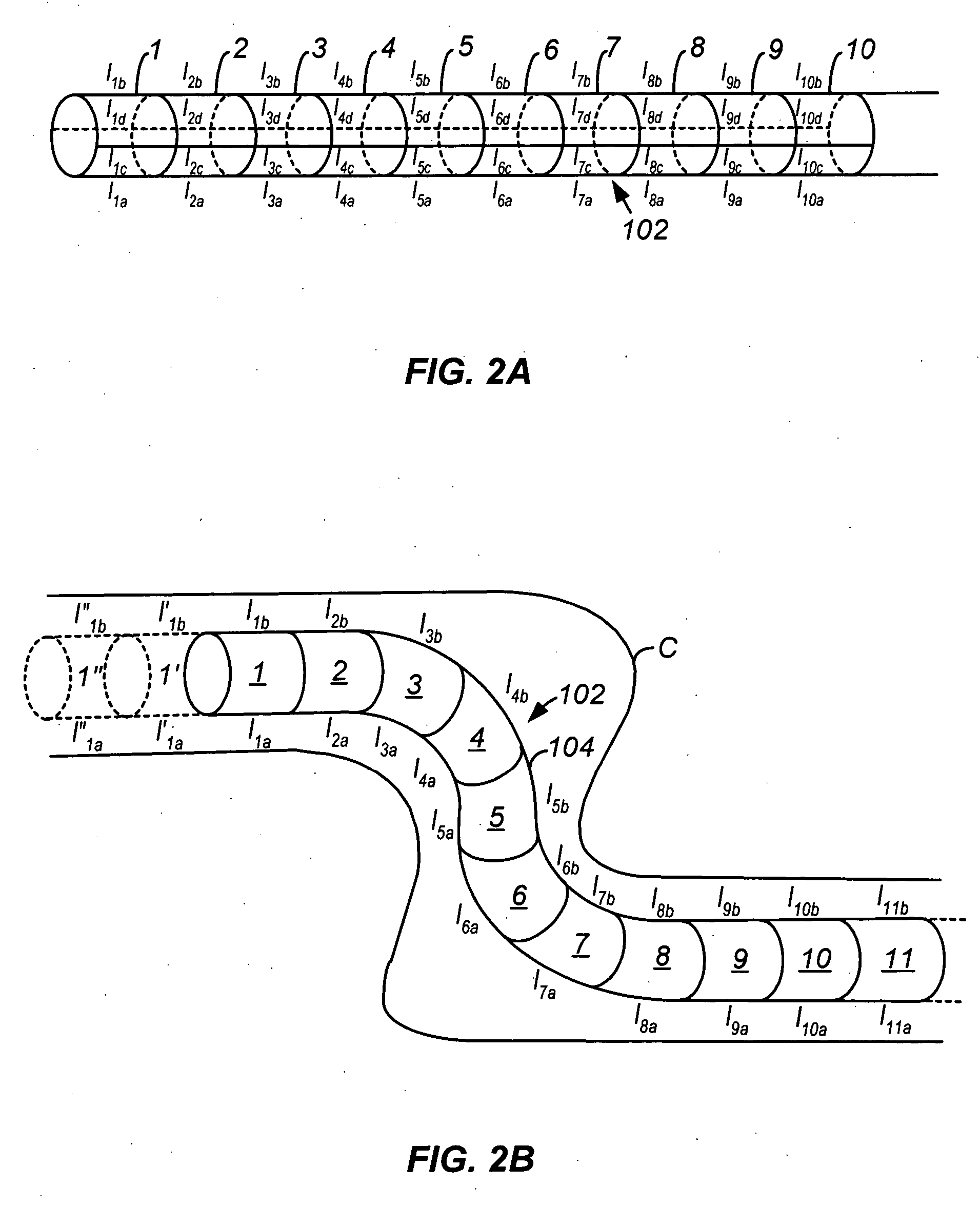
Methods and apparatus for accessing and treating regions of the body are disclosed herein. Using an endoscopic device having an automatically controllable proximal portion and a selectively steerable distal portion, the device generally may be advanced into the body through an opening. The distal portion is selectively steered to assume a selected curve along a desired path within the body which avoids contact with tissue while the proximal portion is automatically controlled to assume the selected curve of the distal portion. The endoscopic device can then be used for accessing various regions of the body which are typically difficult to access and treat through conventional surgical techniques because the device is unconstrained by “straight-line” requirements. Various applications can include accessing regions of the brain, thoracic cavity, including regions within the heart, peritoneal cavity, etc., which are difficult to reach using conventional surgical procedures.
Owner:INTUITIVE SURGICAL OPERATIONS INC
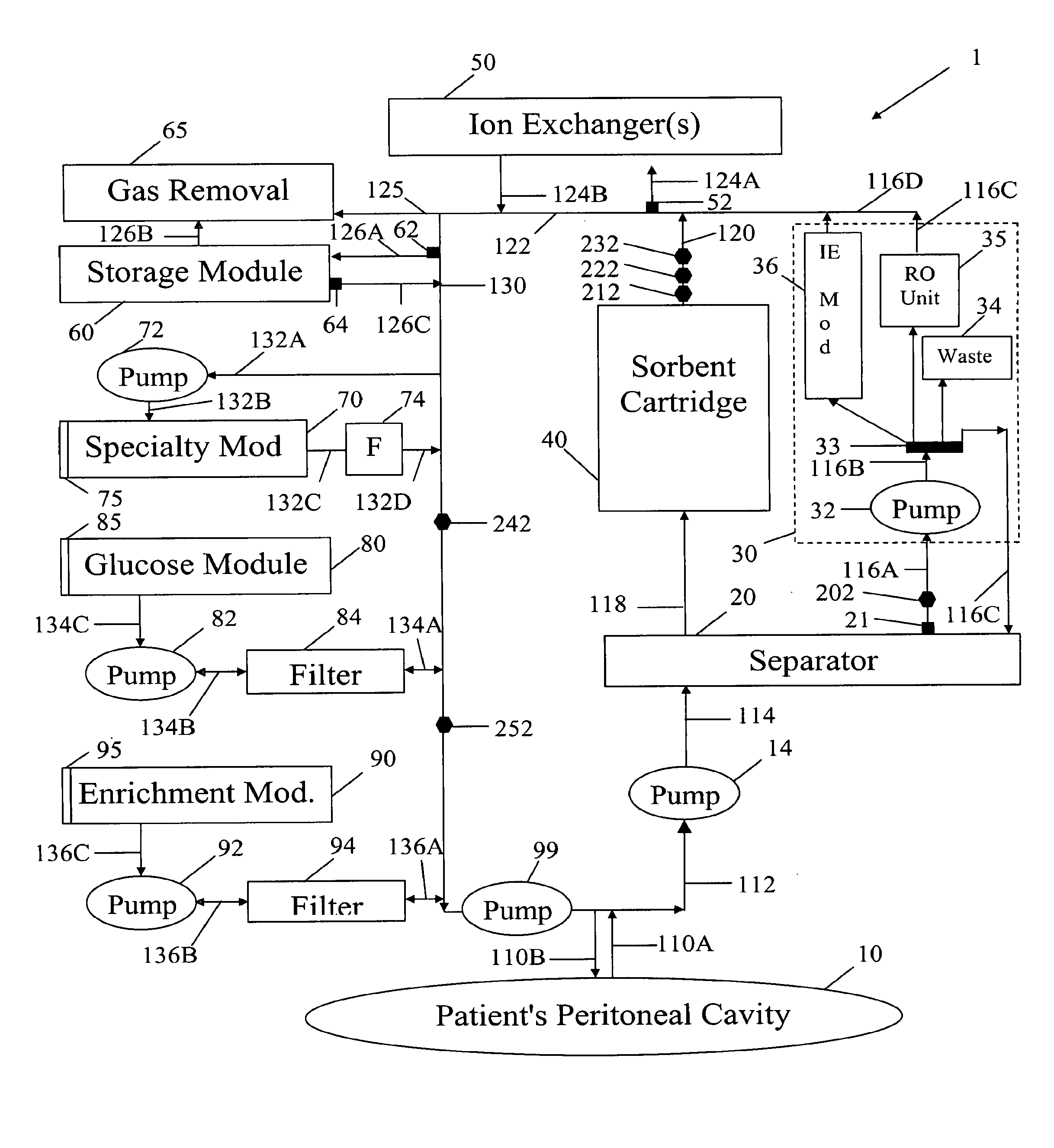
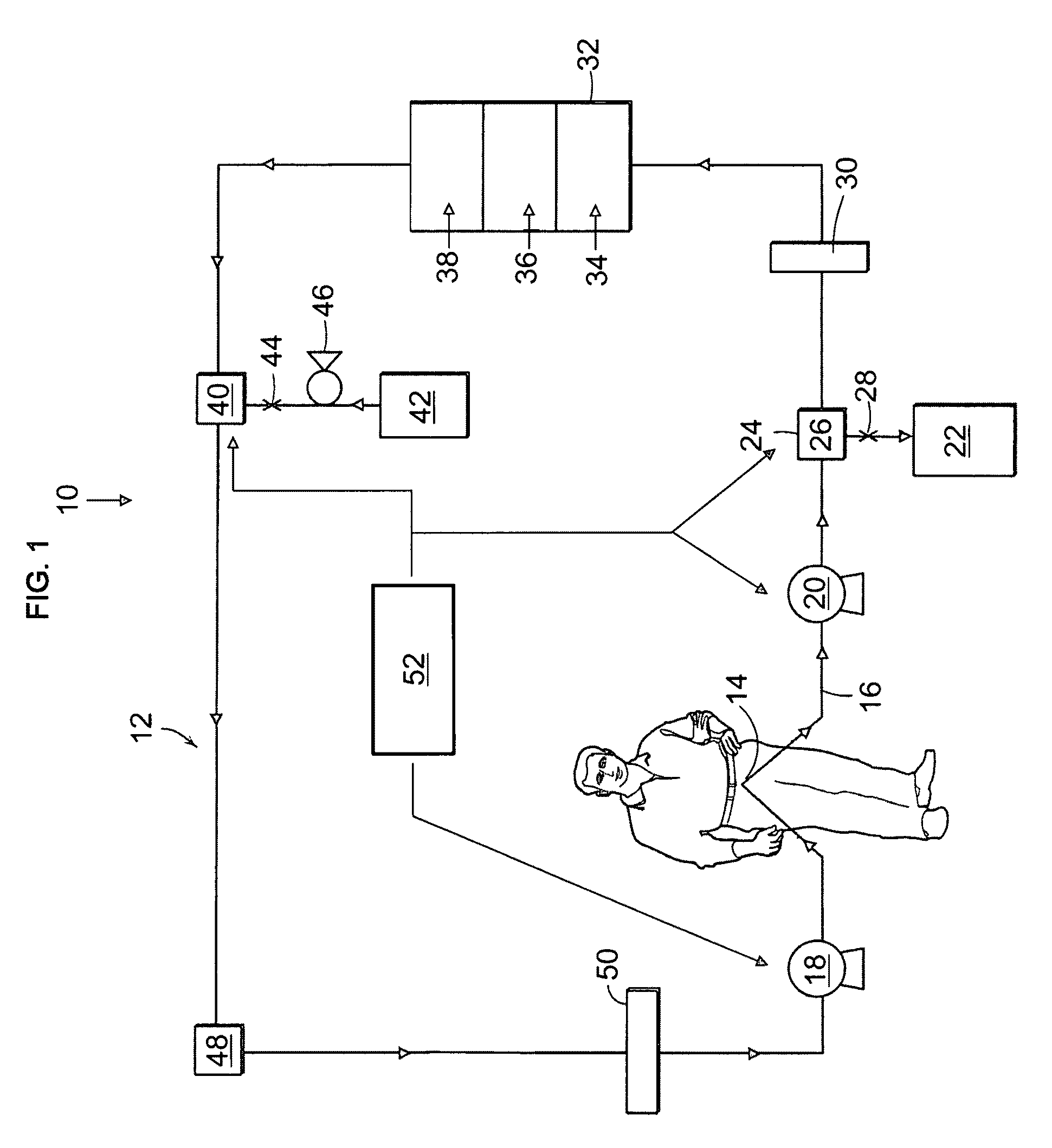
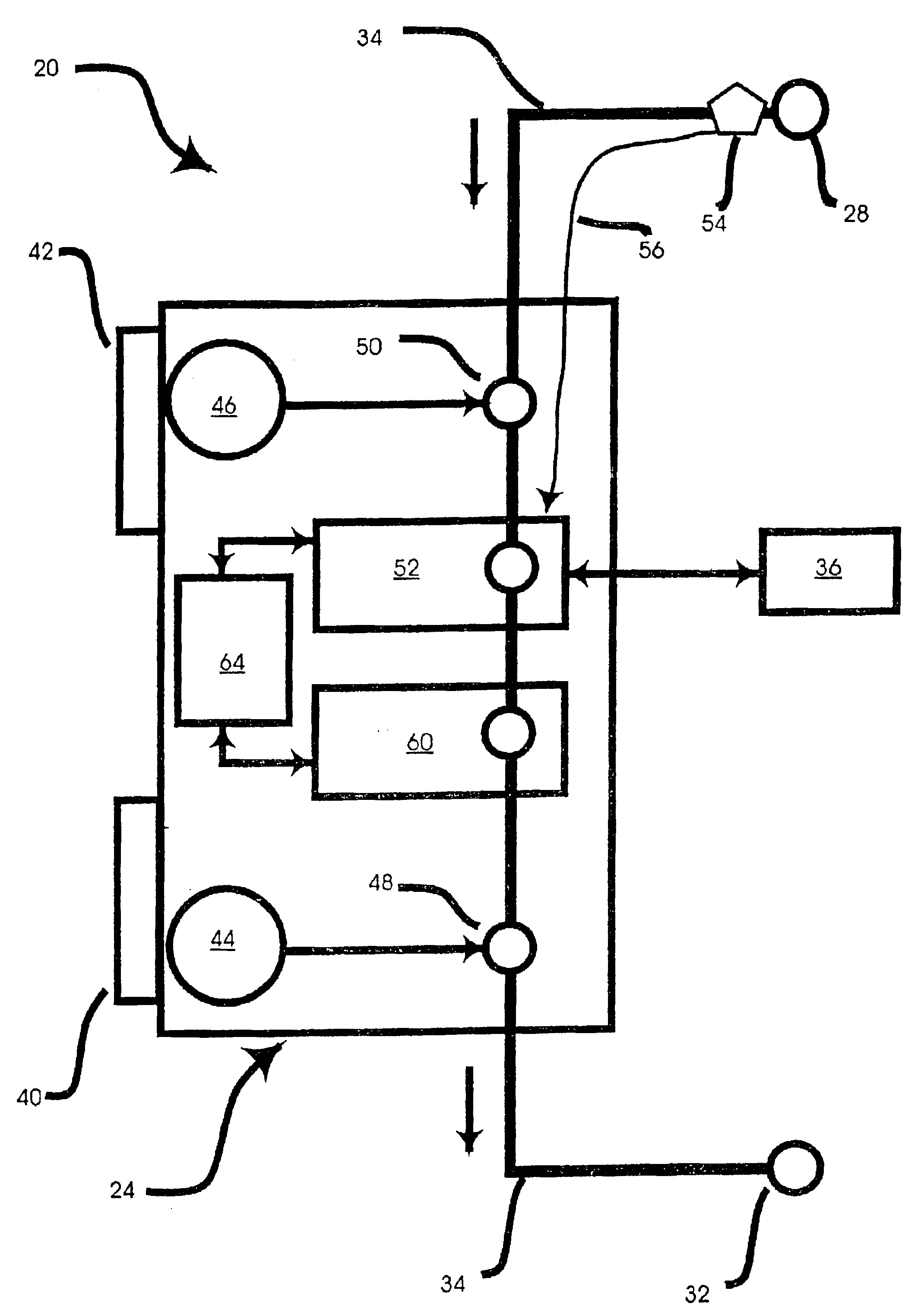
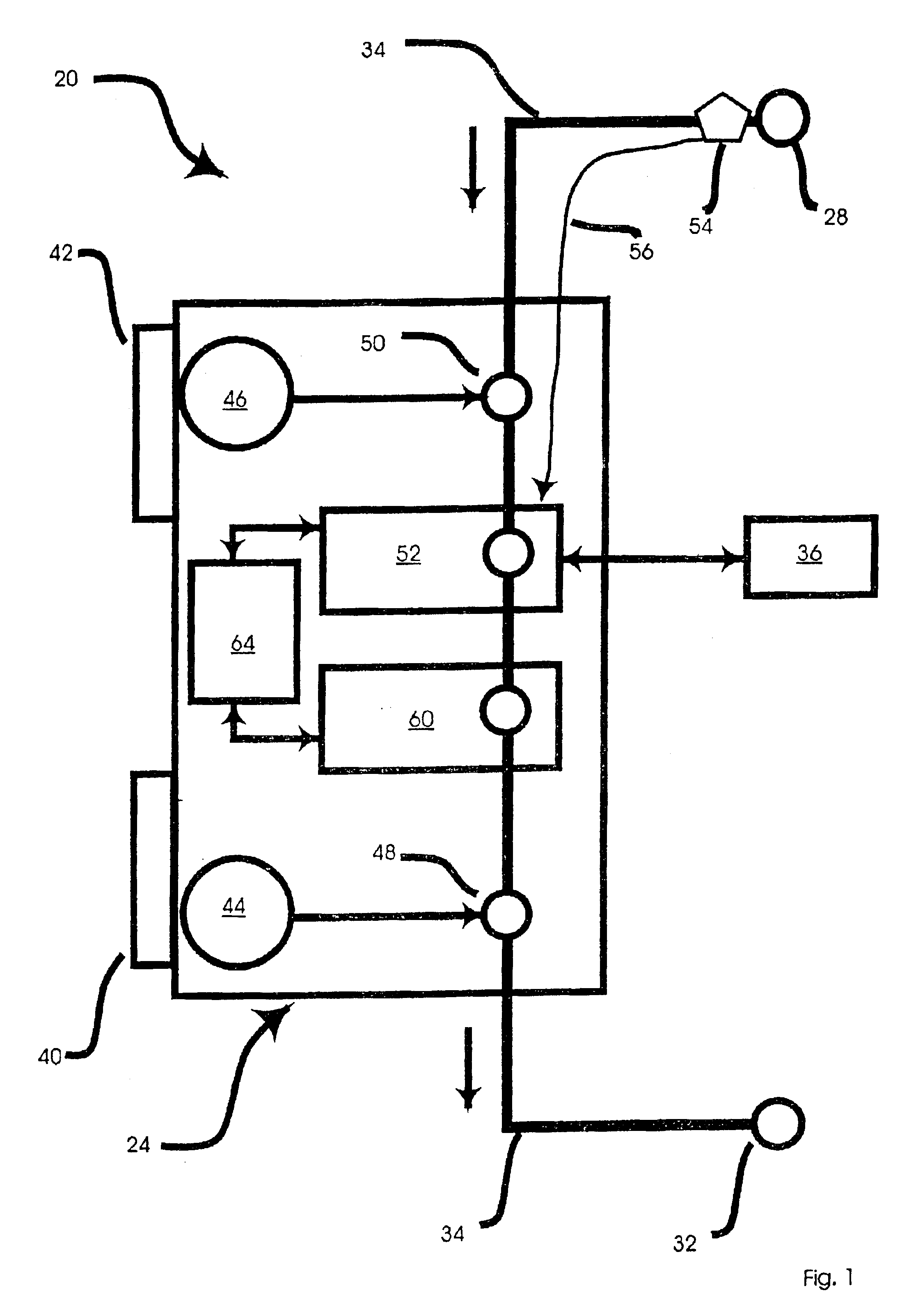
Peritoneal dialysis methods and apparatus
ActiveUS20070179431A1Increase percentageImprove wear resistancePeritoneal dialysisProtein compositionPeritoneal fluid
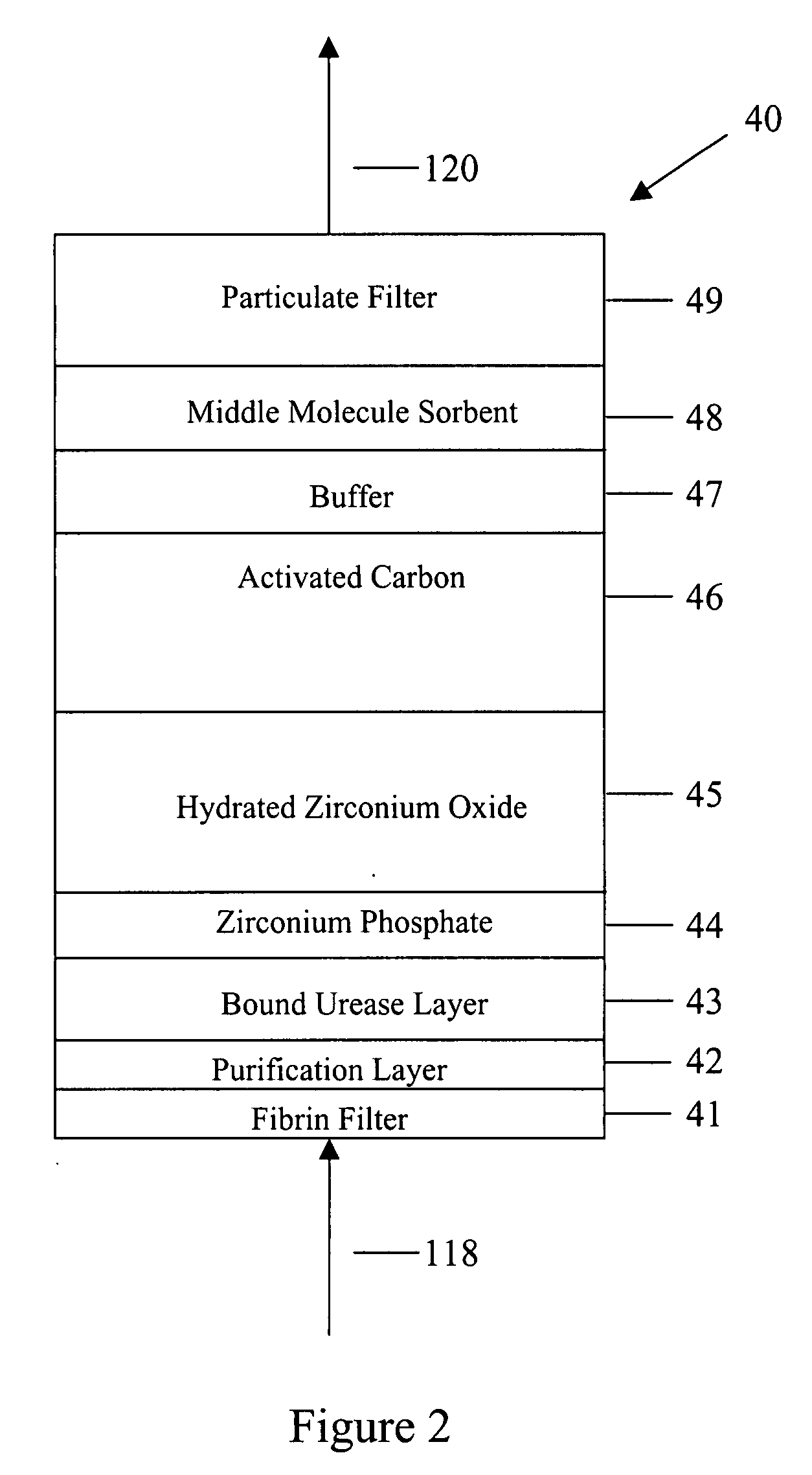
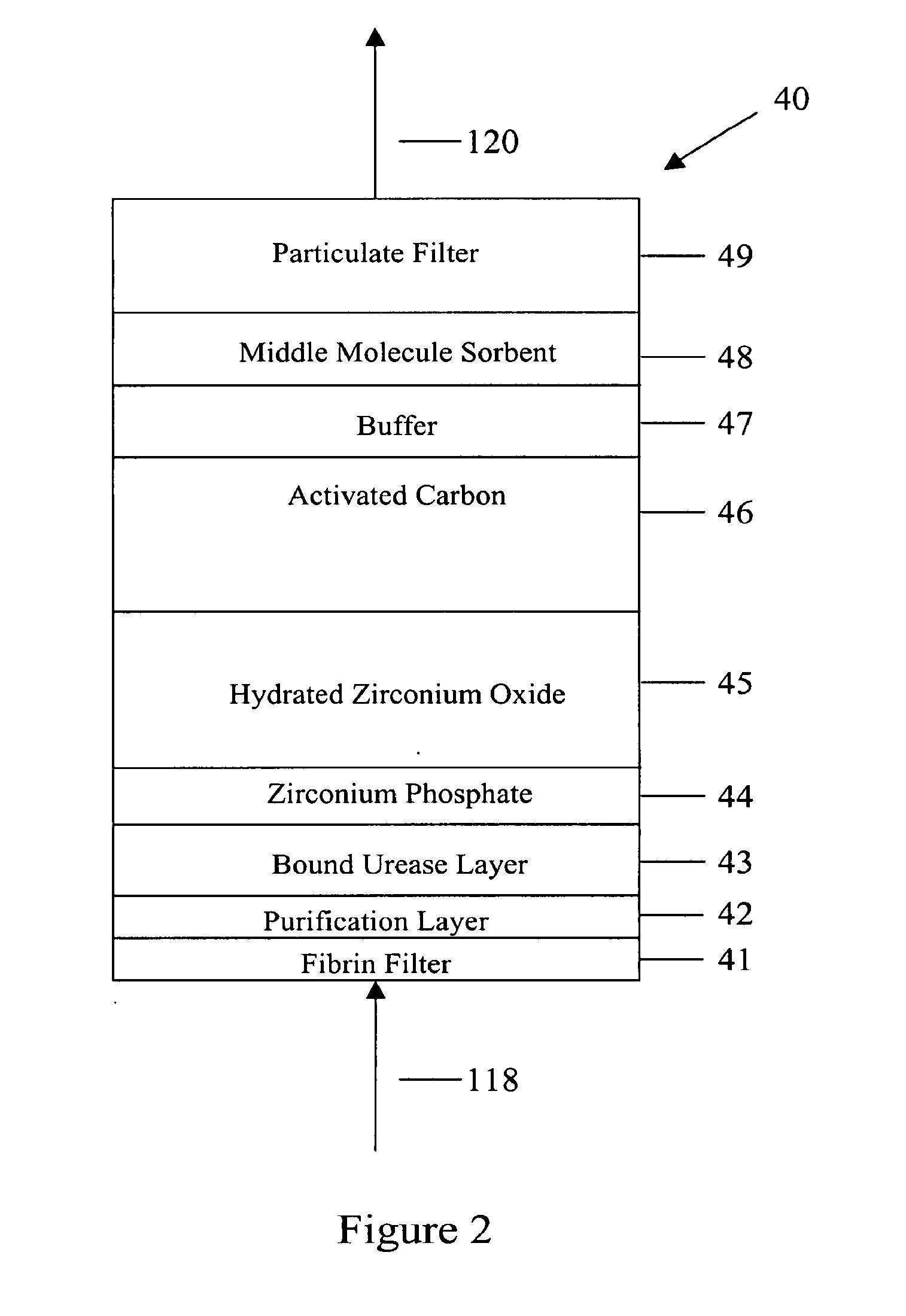
A peritoneal-based (“bloodless”) artificial kidney processes peritoneal fluid without need for additional fluids (“waterless”). Fluid is separated into a protein-rich stream and a protein-free stream. The protein-rich stream is regenerated using a sorbent assembly, and its protein composition can be modified by removal of selected protein(s) (“dialysate-pheresis”). It is then reconstituted with additives and returned into the peritoneal cavity, thereby reducing protein-loss and providing oncotic-pressure for ultrafiltration. The protein-free stream is used to produce free water, and an alkaline or acid fluid for optimization of the composition of the regenerated stream. The unused protein-free stream can be used to “reverse flush” the separator to maintain its patency and the excess discarded for fluid-balance regulation. Compared to prior art, immobilization of urease allows more protein rich fluid to be regenerated and re-circulated into the peritoneal cavity for toxin removal and allows practicable development of portable and wearable artificial kidneys.
Owner:RGT UNIV OF CALIFORNIA +1
Fluid pumping systems, devices and methods
ActiveUS20080175719A1Reduces shear on the fluidReduce hemolysisThermometer detailsFlexible member pumpsHemolysisPeritoneal fluid
Embodiments of the present invention relate generally to certain types of reciprocating positive-displacement pumps (which may be referred to hereinafter as “pods,”“pump pods,” or “pod pumps”) used to pump fluids, such as a biological fluid (e.g., blood or peritoneal fluid), a therapeutic fluid (e.g., a medication solution), or a surfactant fluid. The pumps may be configured specifically to impart low shear forces and low turbulence on the fluid as the fluid is pumped from an inlet to an outlet. Such pumps may be particularly useful in pumping fluids that may be damaged by such shear forces (e.g., blood, and particularly heated blood, which is prone to hemolysis) or turbulence (e.g., surfactants or other fluids that may foam or otherwise be damaged or become unstable in the presence of turbulence).
Owner:DEKA PROD LLP
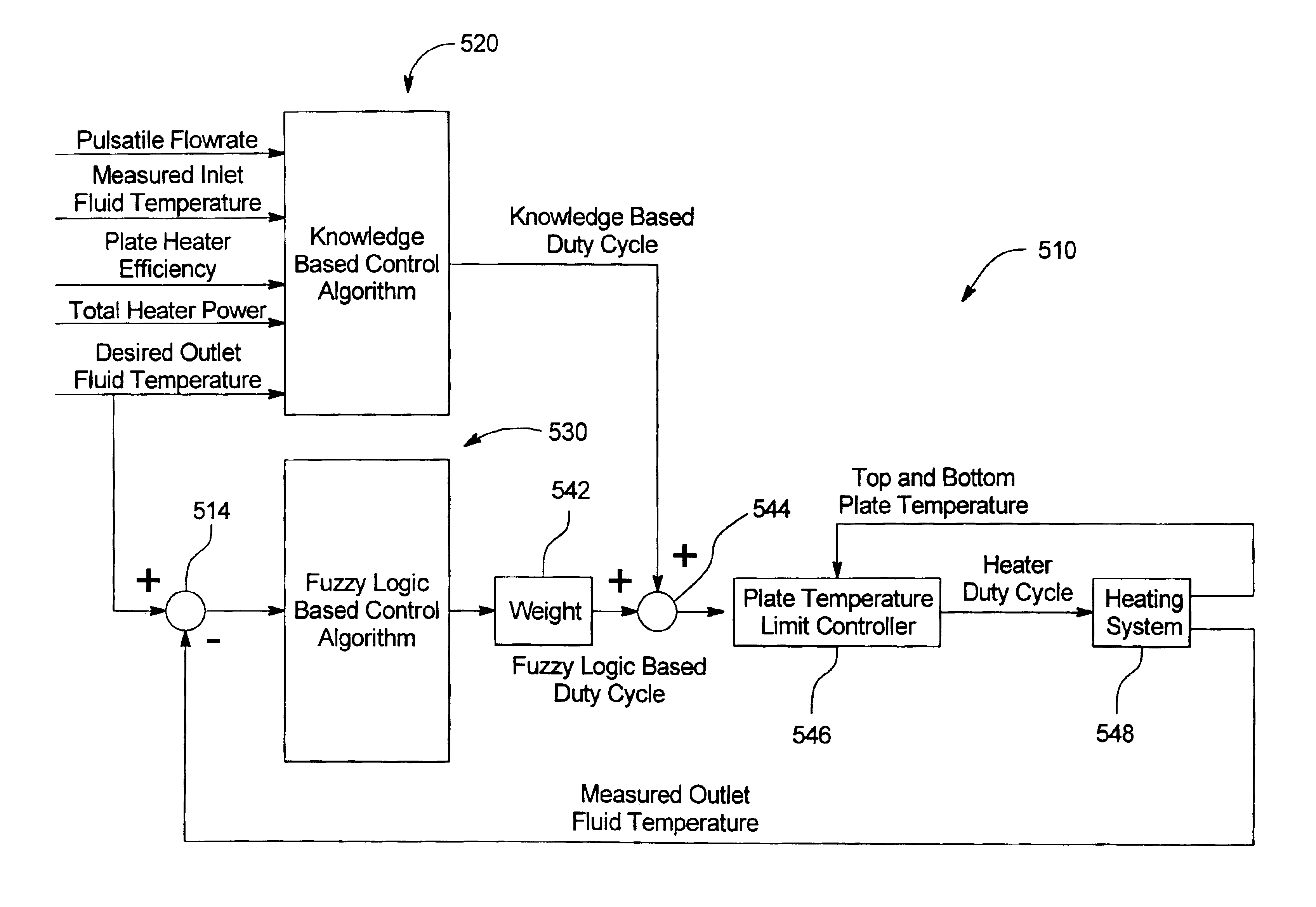
Method and apparatus for controlling a medical fluid heater
A method, system and apparatus for performing peritoneal dialysis are provided. To this end, in part, a method of controlling a medical fluid heater is provided. The method includes the steps of determining a first heater control output based on a number of measured inputs for the heater and at least one mathematical relationship between at least two of the measured inputs, determining a second heater control output based on at least one fuzzy logic membership function and at least one fuzzy logic rule, and determining a third heater control output based on the first and second outputs and using the third heater control output to control the heater.
Owner:BAXTER INT INC +1
Portable Peritoneal Dialysis System
ActiveUS20100114012A1Comfortable to wearComfortable to carryMedical devicesDialysisSimple Organic CompoundsMetabolite
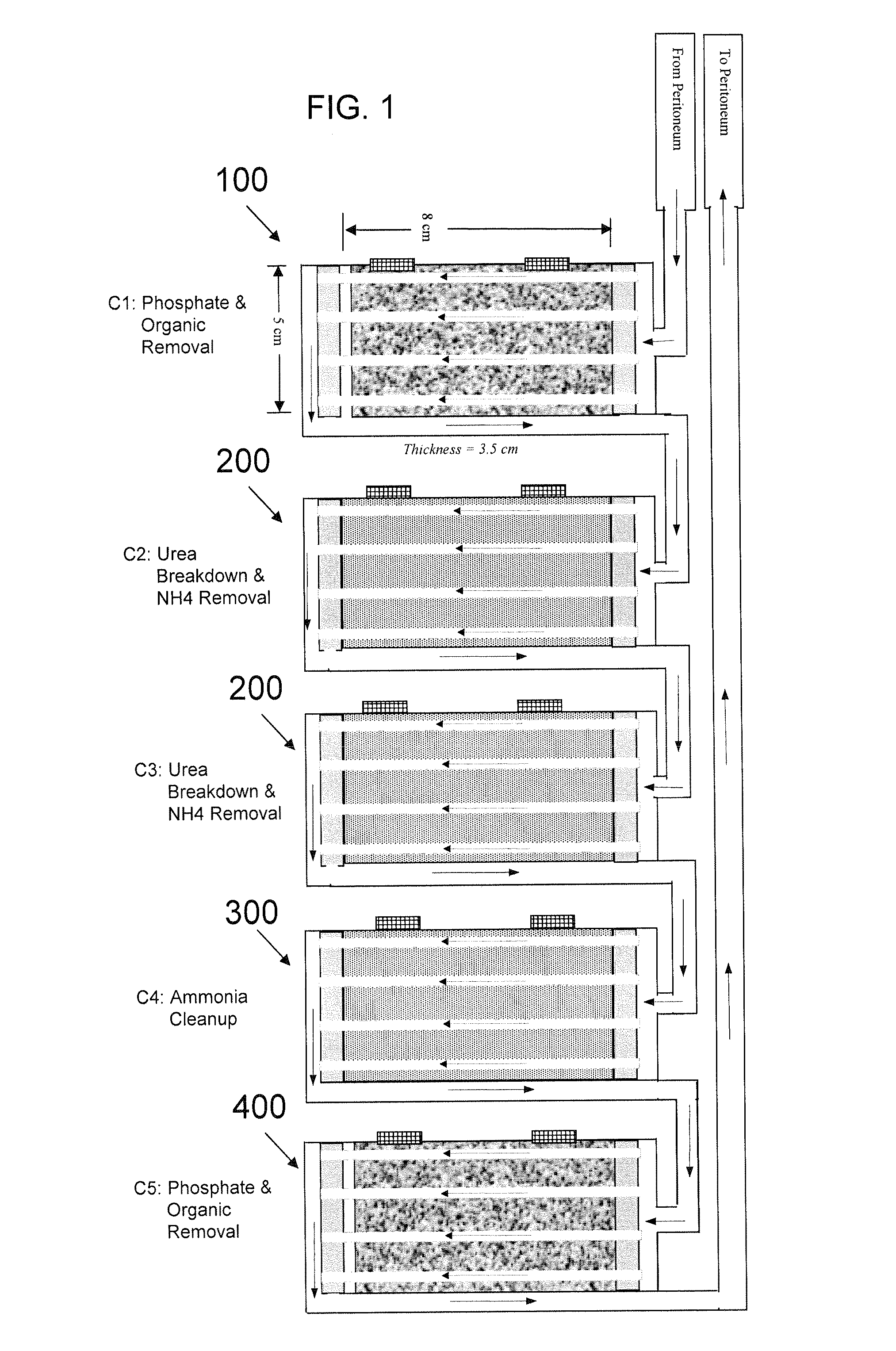
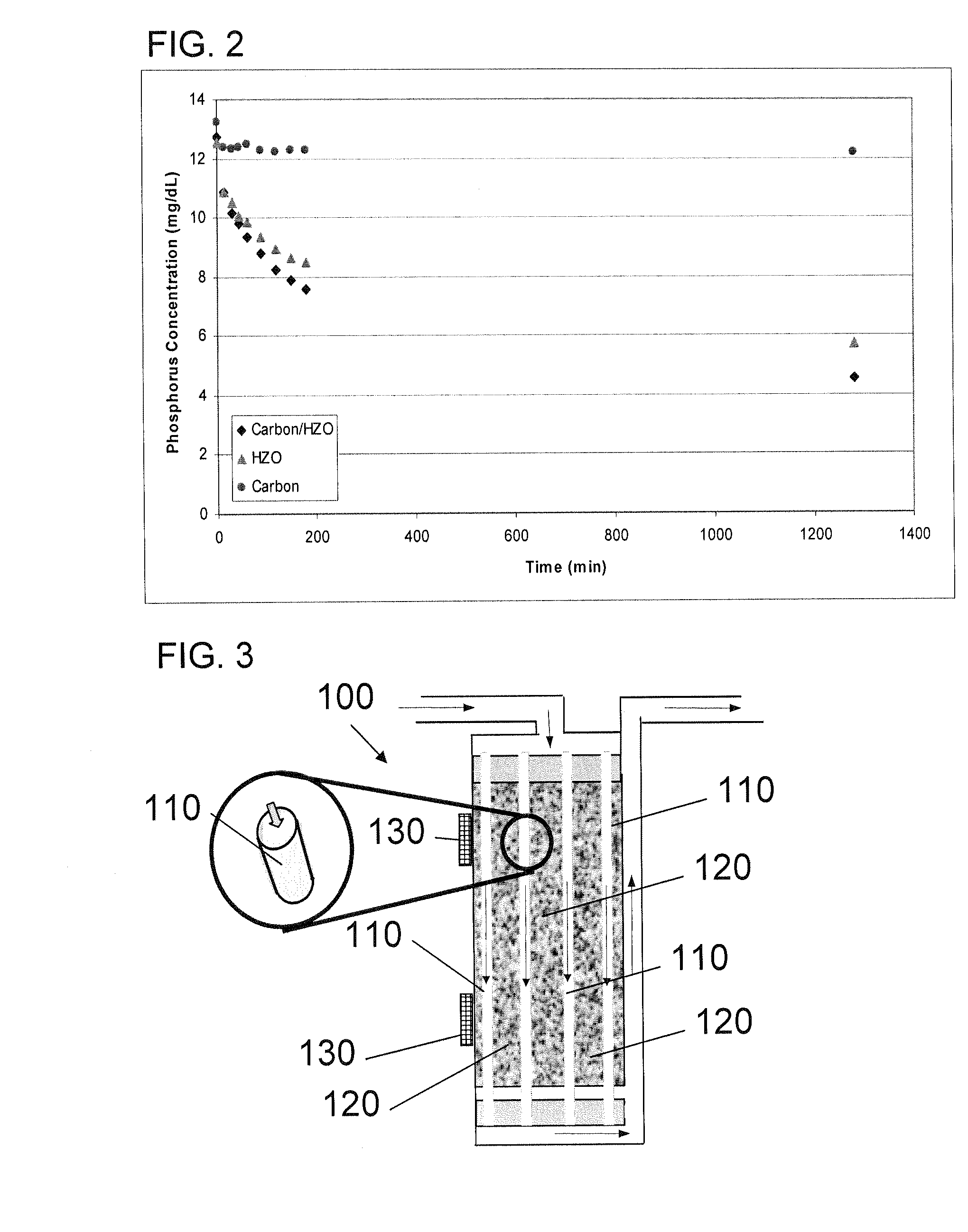
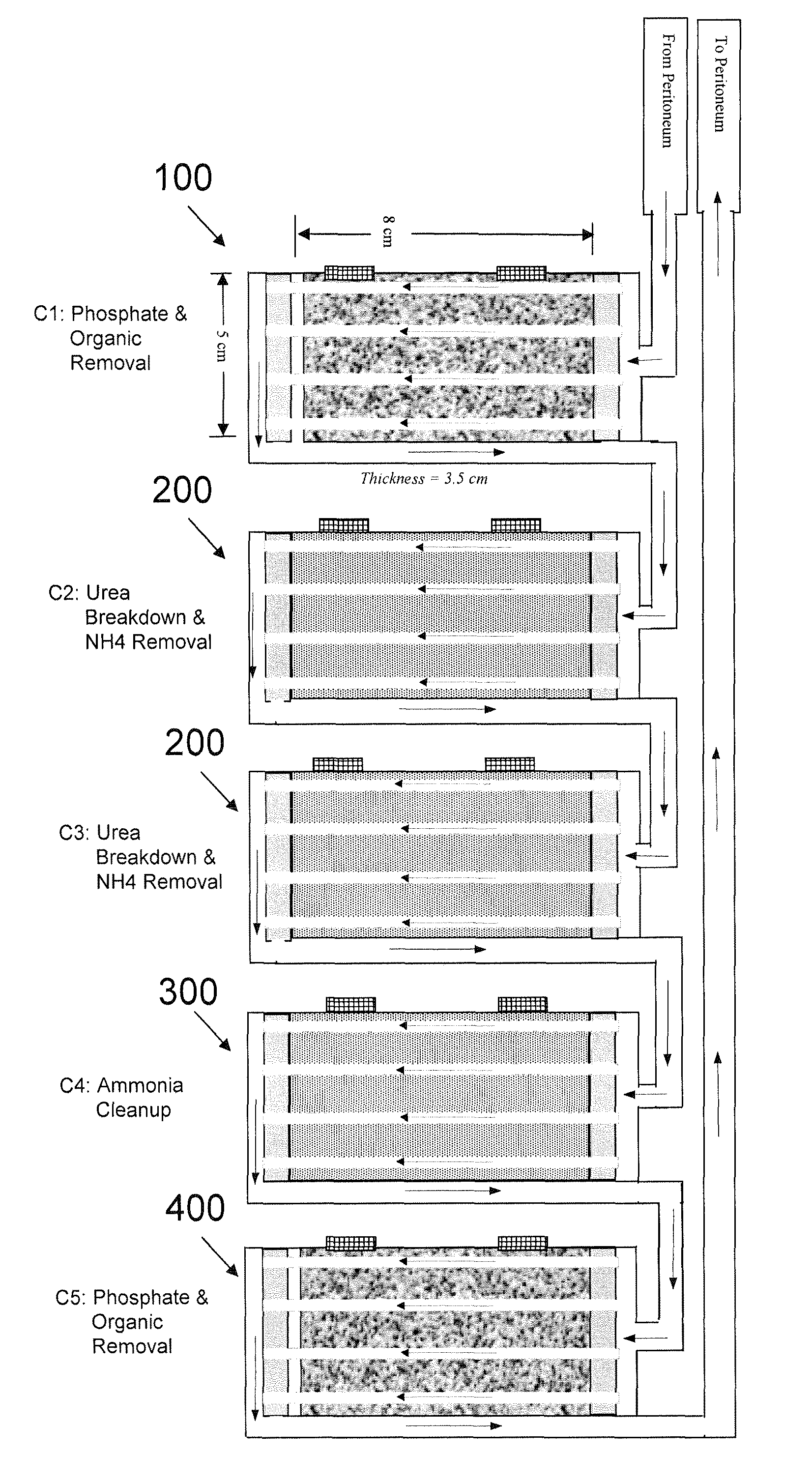
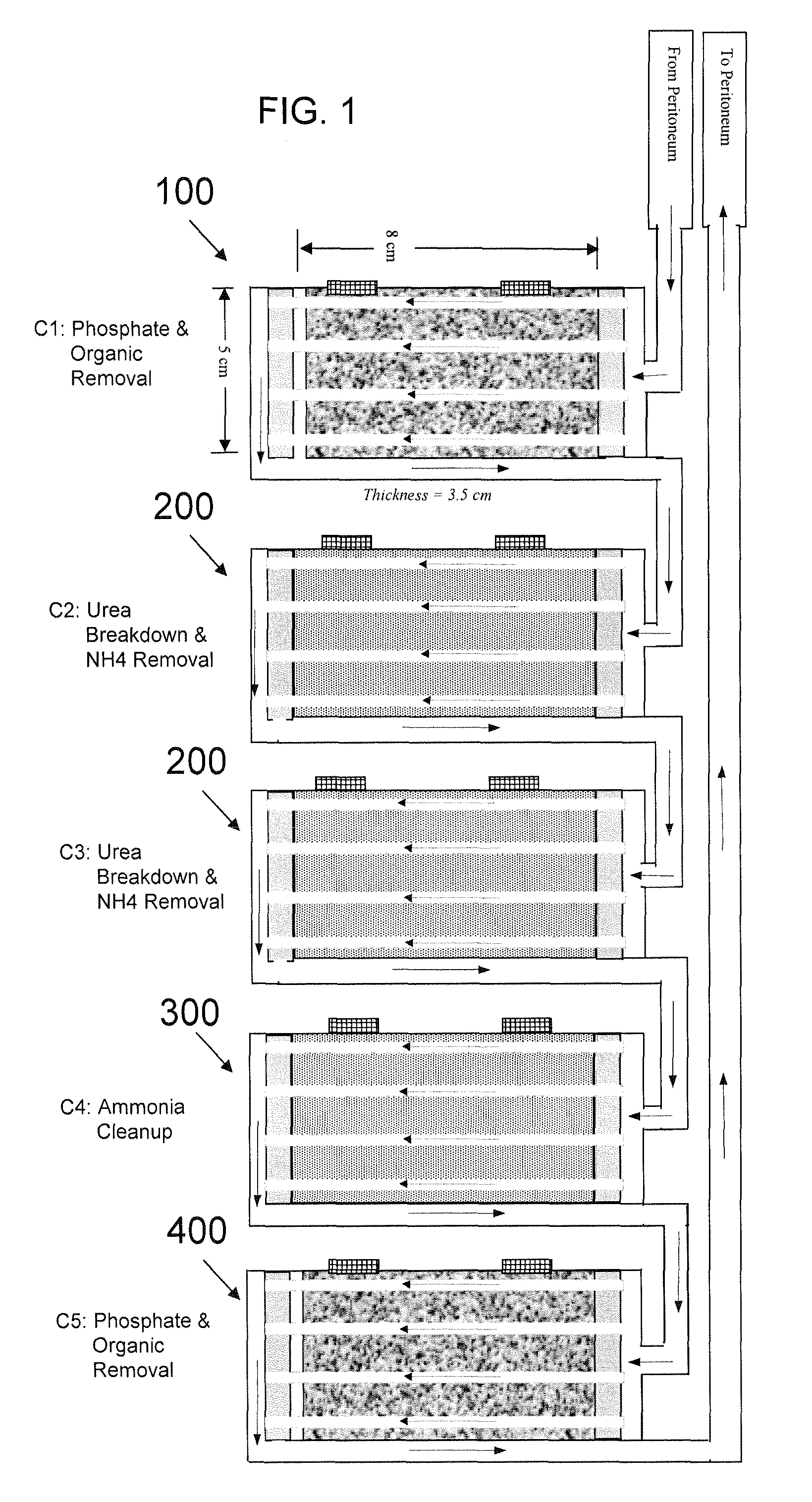
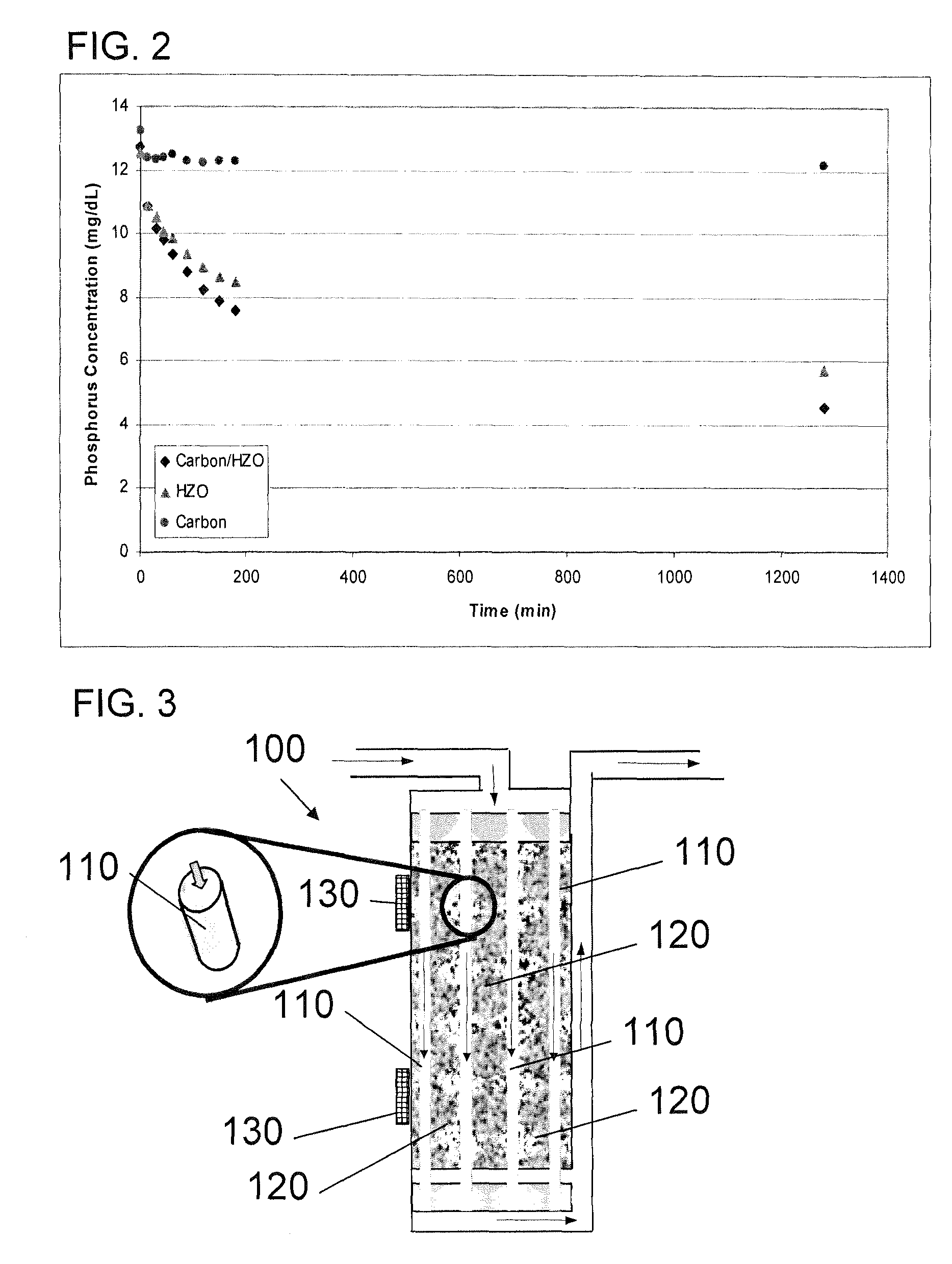
A portable peritoneal dialysis system for a patient includes an inlet port for providing inflow to the patient's peritoneal cavity, an outlet port for providing outflow from the patient's peritoneal cavity, and a volume of dialysate for flow into and out of the patient's peritoneal cavity, thereby removing from the dialysate uremic waste metabolites that have diffused into the dialysate. The portable peritoneal dialysis system also includes a closed liquid flow loop, including a pump, for flowing the dialysate into and out of the patient's peritoneal cavity, and an organic- and phosphate-removing stage, including at least one replaceable cartridge in the closed liquid flow loop, the cartridge containing material for removing organic compounds and phosphate from dialysate removed from the patient's peritoneal cavity. The portable peritoneal dialysis system further includes a urea- and ammonia-removing stage, including at least one replaceable cartridge in the closed liquid flow loop, the cartridge containing material for removing urea and ammonia from dialysate removed from the patient's peritoneal cavity, the material being packed around semi-permeable hollow fibers with interior fiber walls that reject cations, thereby retaining cations in the dialysate.
Owner:FRESENIUS MEDICAL CARE HLDG INC
Automatic exchanger for peritoneal dialysis
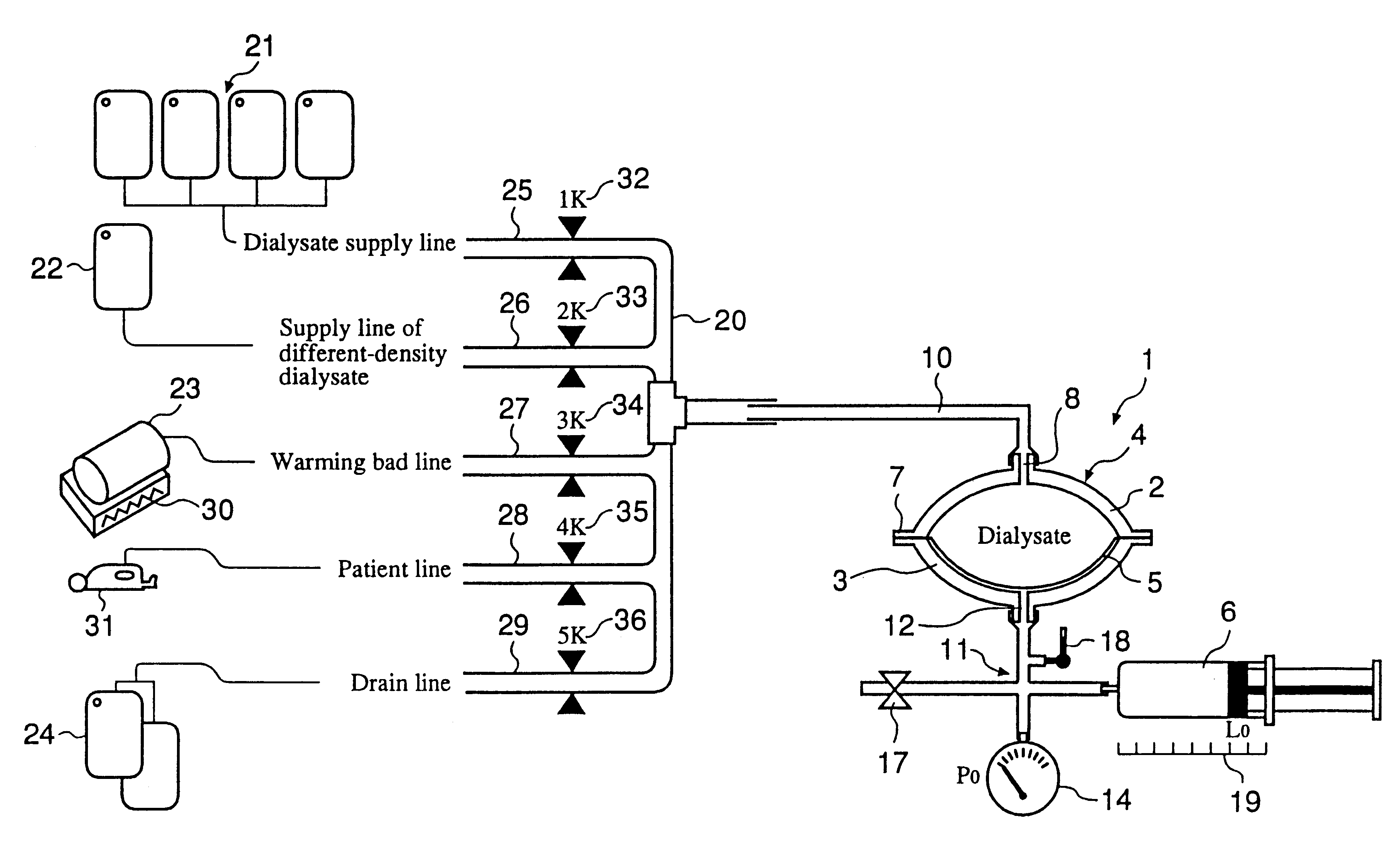
InactiveUS6293921B1Avoid misuseMinimize the possibilityPeritoneal dialysisTube connectorsPeritoneal dialysis fluidEngineering
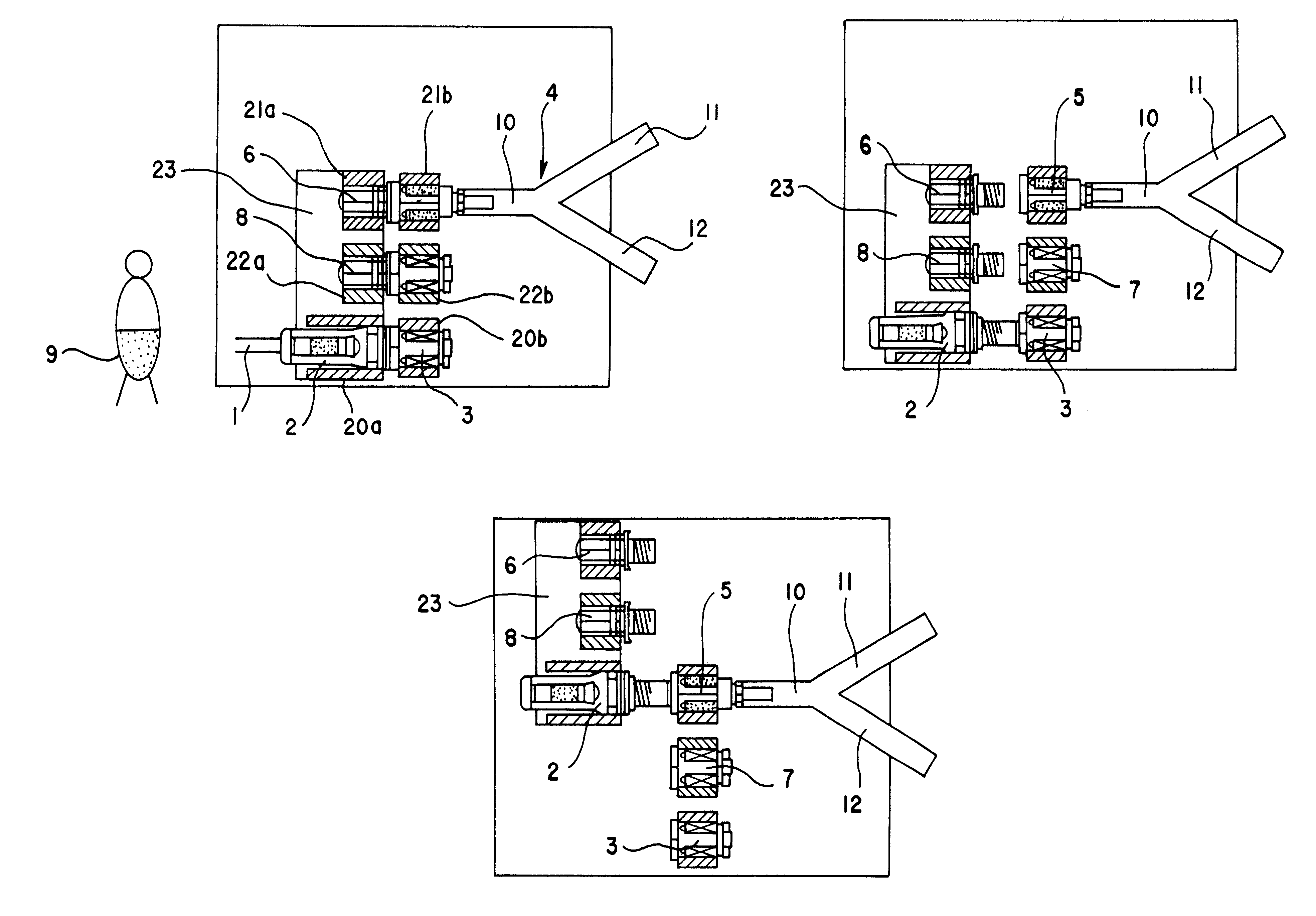
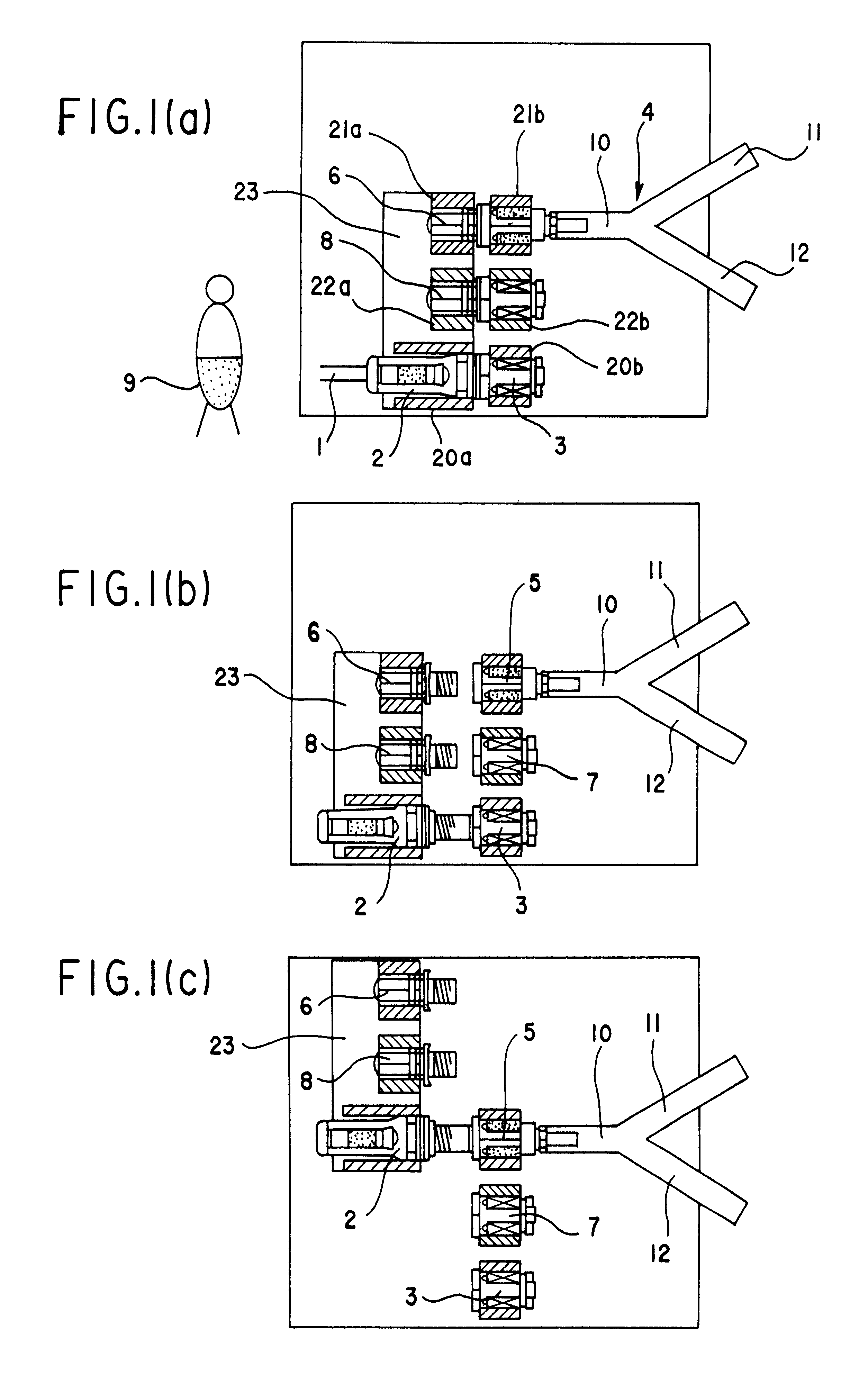
An automatic exchanger apparatus for peritoneal dialysis fluids is provided having a dialysis fluid bag and a drained fluid bag and arranged for connecting and disconnecting between the end of a peritoneal dialysis circuit equipped with a branching point and the end of a tube extending from a patient to drain the waste dialysis fluid from the cavity of the patient and fill the peritoneal cavity of the patient with a fresh peritoneal dialysis fluid for exchange, and in particular comprises: means A, B, and C for carrying out respectively a step (A) of connecting the end of the patient side tube to the end of the peritoneal dialysis circuit, a step (B) of delivering and draining the waste fluid, and a step (C) of, disconnecting the two ends and connecting the end of the patient side tube to its shut-off member, arranged for carrying out their respective steps (A) and (C) automatically; and a controlling means for controlling the respectively means to execute their respectively steps in a sequence. The apparatus is simple in the construction while carrying out, with much ease, the connection and disconnection of the tubes and the exchange of the fluids including a waste and a fresh supply one, hence avoiding operational errors of the operator and minimizing the possibility of infection and contamination.
Owner:JMS CO LTD
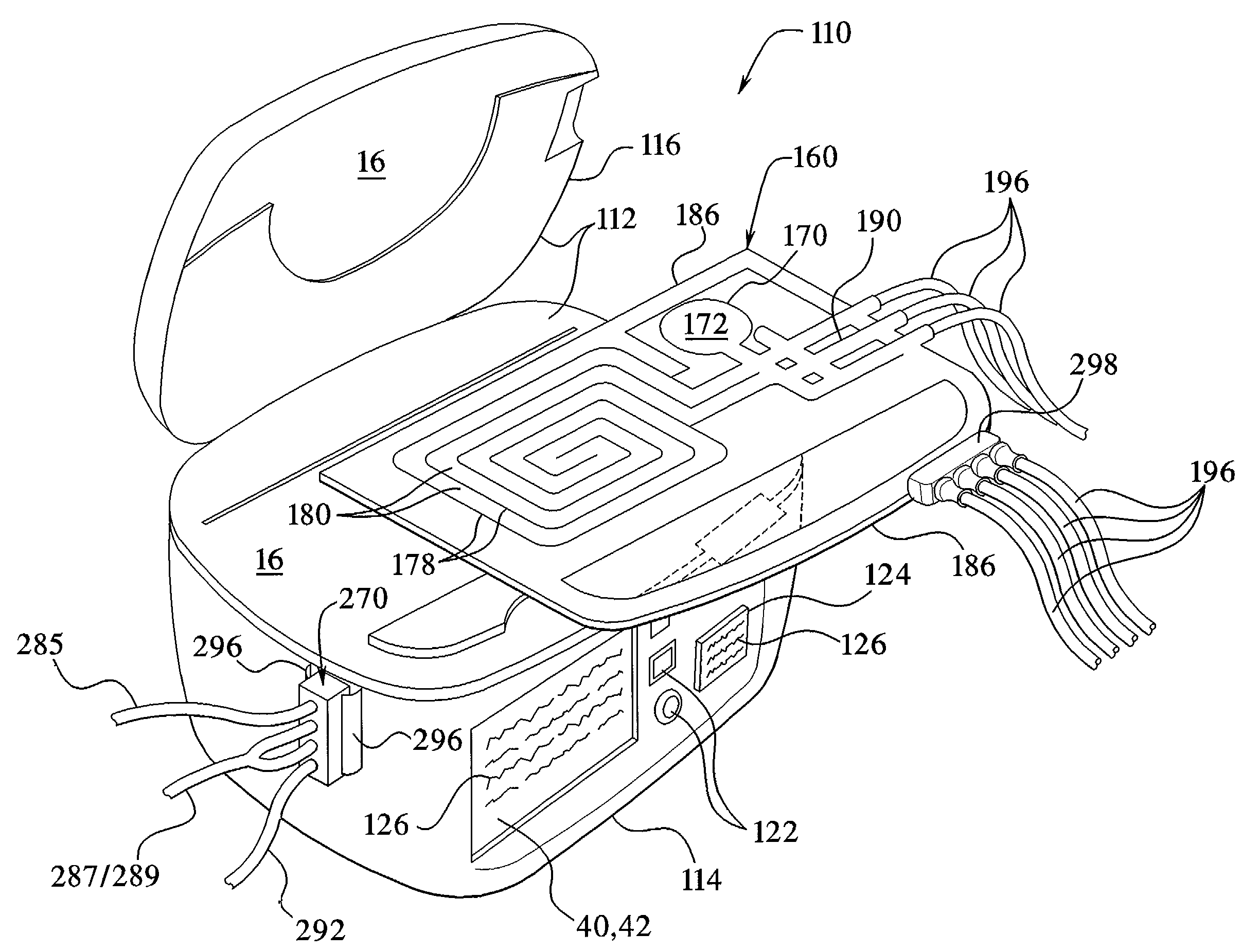
Medical treatment system and methods using a plurality of fluid lines
ActiveUS20130184638A1Reduce trafficIncrease distanceMedical devicesOptical detectionControl systemPeritoneal cavity
A medical treatment system, such as peritoneal dialysis system, may include control and other features to enhance patient comfort and ease of use. For example, a peritoneal dialysis system may include a control system that can adjust the volume of fluid infused into the peritoneal cavity to prevent the intraperitoneal fluid volume from exceeding a pre-determined amount. The control system can adjust by adding one or more therapy cycles, allowing for fill volumes during each cycle to be reduced. The control system may continue to allow the fluid to drain from the peritoneal cavity as completely as possible before starting the next therapy cycle. The control system may also adjust the dwell time of fluid within the peritoneal cavity during therapy cycles in order to complete a therapy within a scheduled time period. The cycler may also be configured to have a heater control system that monitors both the temperature of a heating tray and the temperature of a bag of dialysis fluid in order to bring the temperature of the dialysis fluid rapidly to a specified temperature, with minimal temperature overshoot.
Owner:DEKA PROD LLP
Medical treatment system and methods using a plurality of fluid lines
ActiveUS20120123322A1Prevent slight movementReduce noiseBeam/ray focussing/reflecting arrangementsInvestigating moving fluids/granular solidsPatient comfortIntensive care medicine
A medical treatment system, such as peritoneal dialysis system, may include control and other features to enhance patient comfort and ease of use. For example, a peritoneal dialysis system may include patient line state detector for detecting whether a patient line is primed before it is to be connected to the patient. The patient line state detector can also the ability to detect whether a patient line has been properly mounted for priming. Both patient line presence / absence and fill state can be determined using an optical system, e.g., one that employs a single optical sensor.
Owner:DEKA PROD LLP
Peritoneal Dialysis Methods and Apparatus
ActiveUS20100217181A1Increase percentageImprove wear resistancePeritoneal dialysisProtein compositionPeritoneal fluid
A peritoneal-based (“bloodless”) artificial kidney processes peritoneal fluid without need for additional fluids (“waterless”). Fluid is separated into a protein-rich stream and a protein-free stream. The protein-rich stream is regenerated using a sorbent assembly, and its protein composition can be modified by removal of selected protein(s) (“dialysate-pheresis”). It is then reconstituted with additives and returned into the peritoneal cavity, thereby reducing protein-loss and providing oncotic-pressure for ultrafiltration. The protein-free stream is used to produce free water, and an alkaline or acid fluid for optimization of the composition of the regenerated stream. The unused protein-free stream can be used to “reverse flush” the separator to maintain its patency and the excess discarded for fluid-balance regulation. Compared to prior art, immobilization of urease allows more protein rich fluid to be regenerated and re-circulated into the peritoneal cavity for toxin removal and allows practicable development of portable and wearable artificial kidneys.
Owner:RGT UNIV OF CALIFORNIA +1
Method and device for minimally invasive implantation of biomaterial
A minimally invasive method of placing a delivery device substantially adjacent to vascular tissue and a device for use with such a method are disclosed. The delivery device may be a flexible biological construct with a flexible tethering means. The delivery device may be percutaneously inserted near vascular tissue such as, for example, peritoneal tissue. When the delivery device has been inserted, the tether may be used to pull the delivery device toward the vascular tissue and secure the device thereto. Contact between the front surface of the delivery device and the vascular tissue may be maintained by making and keeping the tether substantially taut. The delivery device may serve accomplish sustained delivery of active agents.
Owner:ETHICON ENDO SURGERY INC
Multimodal dialysis system
A dialysis device for operation in multiple modes and for maintaining a known gradient of potassium ion or other electrolyte between the blood of a patient and a dialysate fluid is described. The dialysis device is capable of being used for hemodialysis or peritoneal dialysis, and the dialysis device is capable of operation with a dialysate purification unit outside of a clinical setting or with a supply of water that can be supplied in a clinical setting. The dialysis device has a composition sensor containing a potassium-sensitive electrode for measuring a potassium ion concentration in one or more of the patient's blood and the dialysate fluid and an infusate pump operated to adjust a potassium ion concentration in the dialysate fluid based at least in part on data from the composition sensor.
Owner:MOZARC MEDICAL US LLC
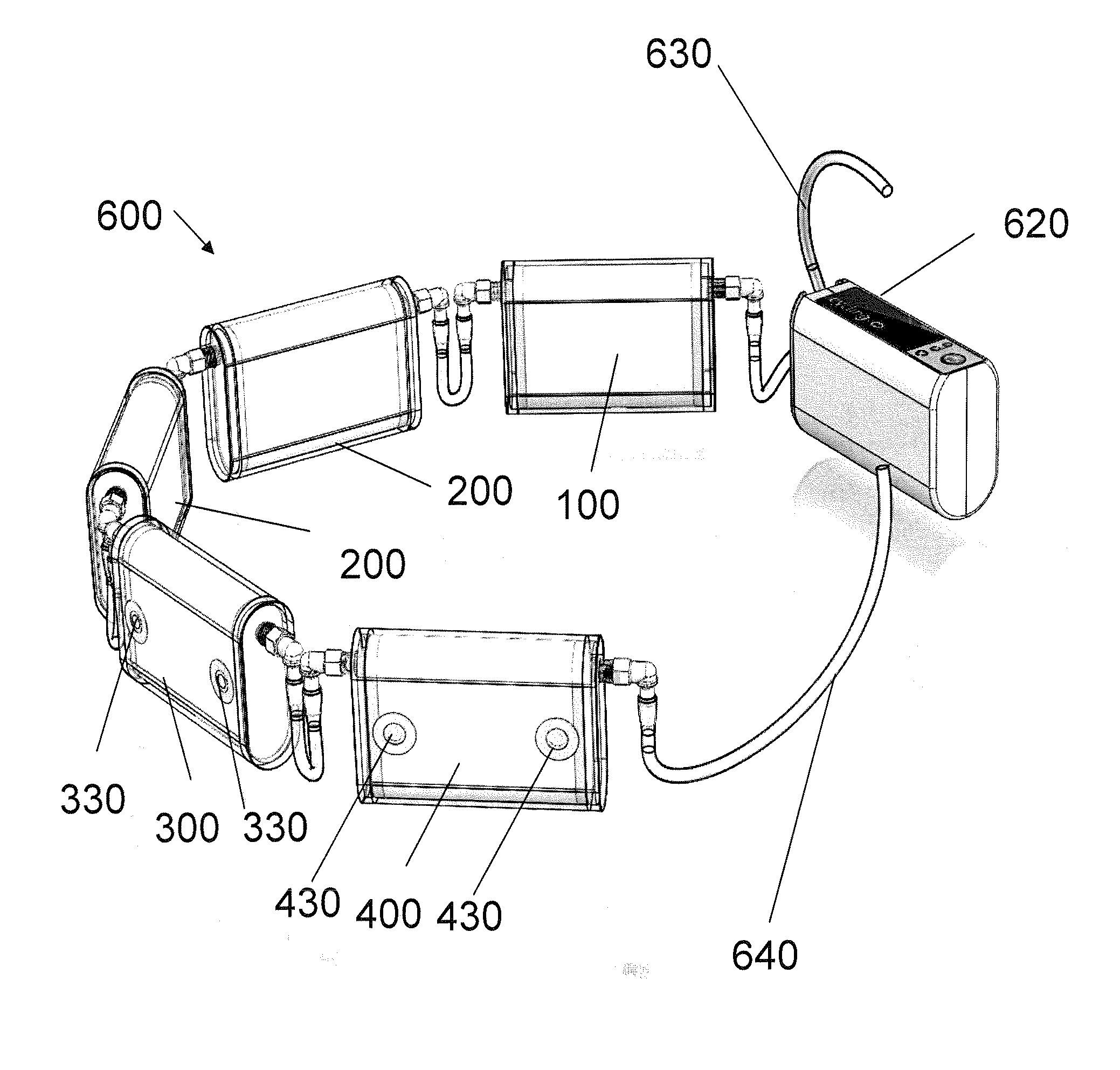
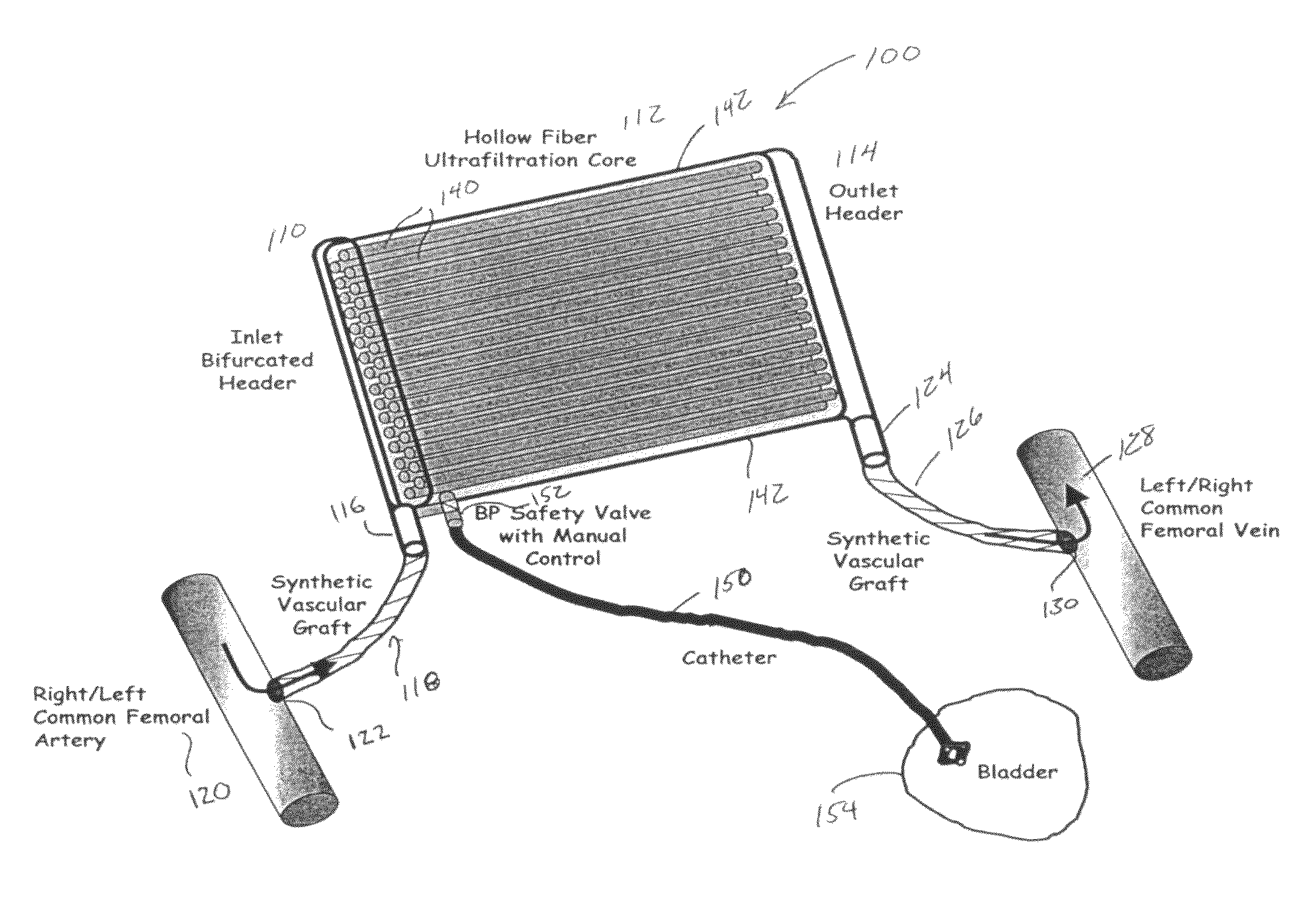
Artificial kidney dialysis system
ActiveUS20080051696A1Cumbersome to wearImprove the quality of lifeMedical devicesPeritoneal dialysisNephrosisMetabolite
The present disclosure relates to a wearable dialysis system and method for removing uremic waste metabolites and fluid from a patient suffering from renal disease. Uremic waste metabolites can be removed by a wearable peritoneal dialysis device that regenerates the peritoneal dialysis solution without removing positively charged, essential ions from the solution and, consequently, the patient. Fluids can be removed from the blood of the patient by an implantable fluid removing device. Fluids are delivered to the bladder and preferably removed from the body of the patient through urination. The wearable dialysis system may be operated continuously or semi-continuously and be comfortably adapted to the body of the patient while allowing the patient to perform normal activities.
Owner:FRESENIUS MEDICAL CARE HLDG INC
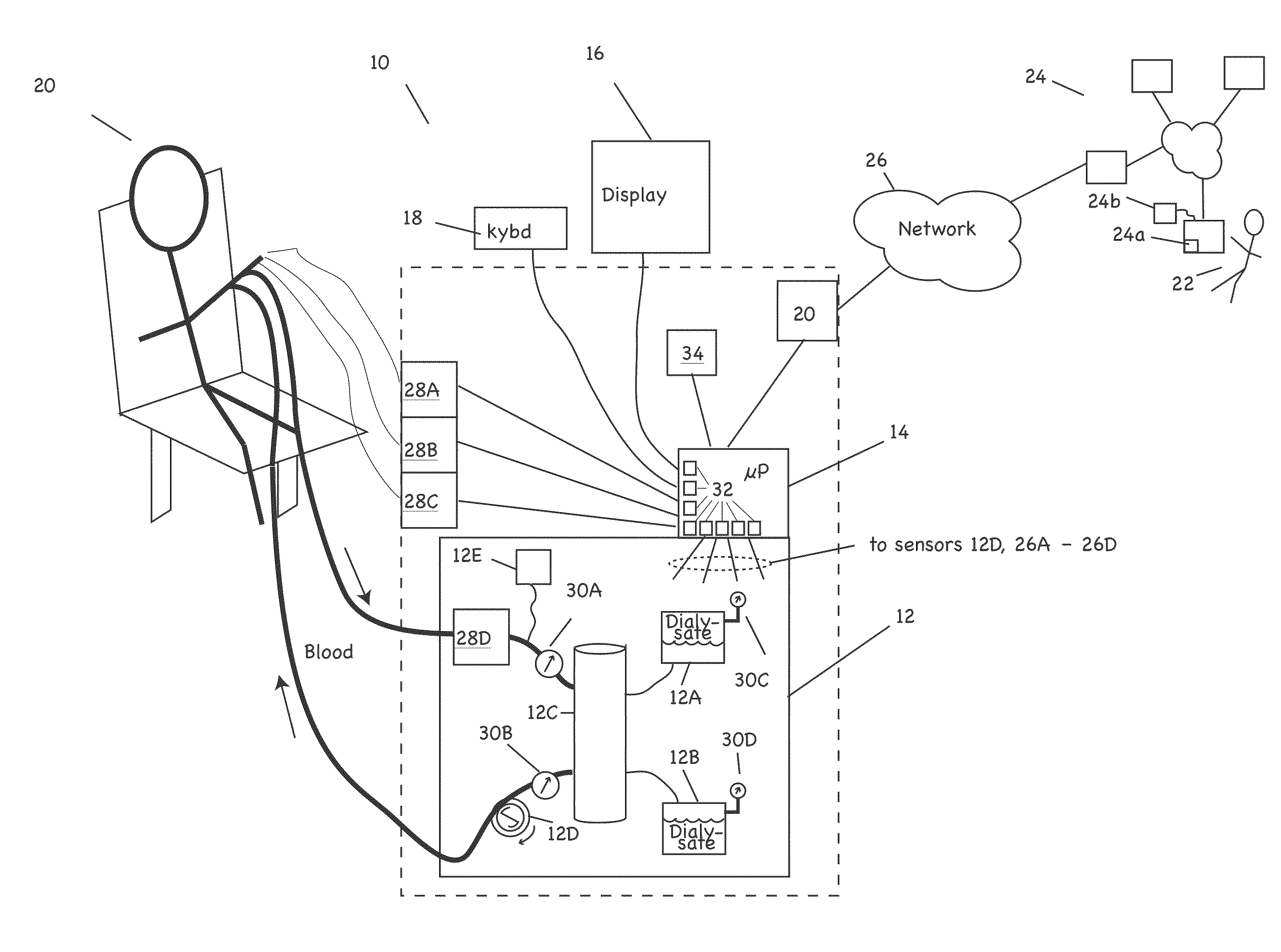
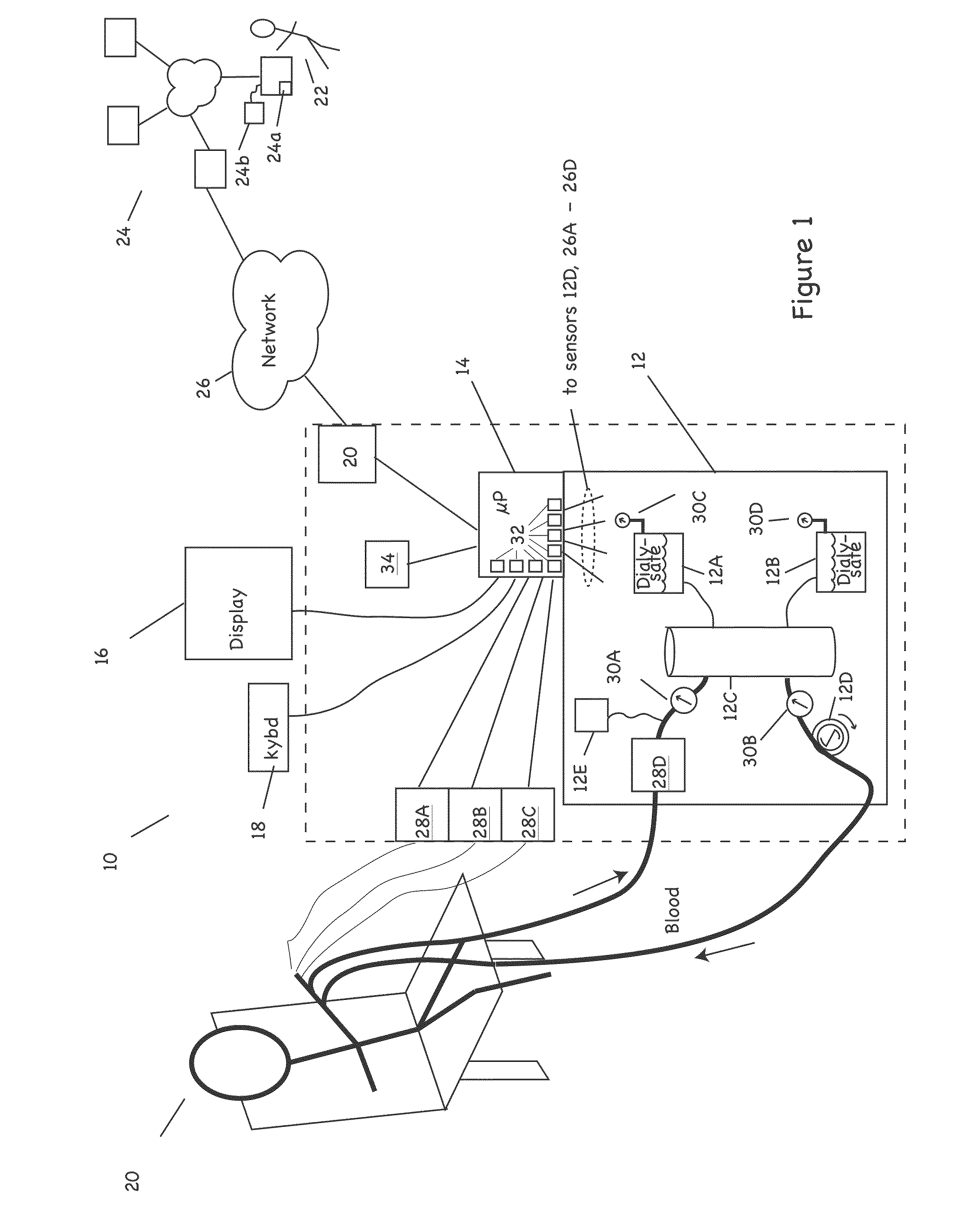
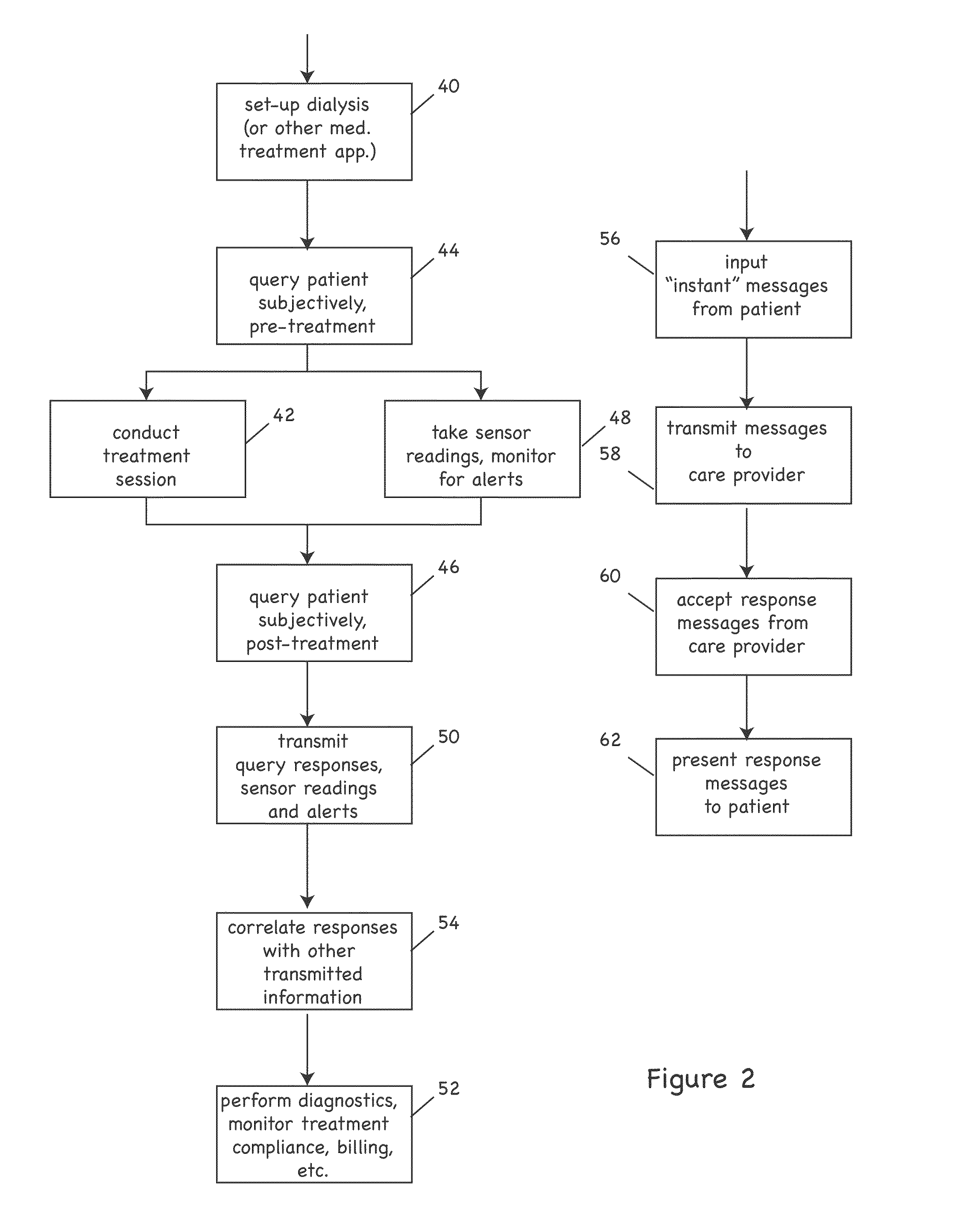
Patient treatment and monitoring systems and methods
ActiveUS20110105979A1Facilitating regulatory complianceMechanical/radiation/invasive therapiesMedical devicesMedical equipmentTherapeutic Devices
The invention provides apparatus and methods for home or other remote delivery of health care that gather subjective and objective measures of patient health and treatment, analyzing them (e.g., correlating them with one another and / or with norms) and reporting them to aid in on-going patient diagnosis and treatment (both on acute and chronic bases), as well as to aid physicians, nurses and other caregivers in decision support, monitoring treatment compliance, facilitating regulatory compliance, billing, and so forth. Thus, for example, in some aspects a health care delivery device comprising a medical treatment apparatus, such as a home hemodialysis or home peritoneal dialysis unit, that is coupled to a processor. The processor generates patient queries in connection with treatments rendered by the dialysis equipment (or other treatment apparatus). The queries are directed, at least in part, to subjective topics, such as the state of the patient's mental health and well being, quality of life, degrees of pain, views on success of therapy, and so forth. They are presented on an LCD screen or other output device coupled to the processor to elicit responses on a keyboard or other input device, also coupled to the processor.
Owner:FRESENIUS MEDICAL CARE HLDG INC
Portable apparatus for peritoneal dialysis therapy
A portable peritoneal dialysis apparatus having (1) a hinged door for enclosing a disposable cassette that seals tightly shut using air pressure; (2) accurate pressure sensing of pressures applied to the patient through an enclosure in the disposable cassette; (3) two pumps that can operate separately or in tandem actuated by two separate stepper motors; and (4) a touch screen user interface where indicia of the operating mode is always visible along with indicia for the other possible operating modes and the mode can be changed by touching one of these indicia.
Owner:FRESENIUS MEDICAL CARE HLDG INC
Tumor targeting drug-loaded particles
A composition for delivering a tumor therapeutic agent to a patient includes a fast-release formulation of a tumor apoptosis inducing agent, a slow-release formulation of a tumor therapeutic agent, and a pharmaceutically acceptable carrier. An apoptosis-inducing agent in a pharmaceutically acceptable carrier may be administered before or concomitantly therewith. Nanoparticles or microparticles (e.g., cross-linked gelatin) of the therapeutic agent (e.g., paclitaxel) also may be used. The nanoparticles or microparticles may be coated with a bioadhesive coating. Microspheres that agglomerate to block the entrance of the lymphatic ducts of the bladder to retard clearance of the microparticles through the lymphatic system also may be employed. This invention also uses drug-loaded gelatin and poly(lactide-co-glycolide) (PLGA) nanoparticles and microparticles to target drug delivery to tumors in the peritoneal cavity, bladder tissues, and kidneys.
Owner:AU JESSIE L S +1
Dialysis solution for peritoneal dialysis
InactiveUS6284140B1Easy to degradeDwell timeBiocideSolvent extractionHydroxyethyl starchUltrafiltration
The present invention relates to dialysis solutions for peritoneal dialysis, containing hydroxyethyl starch as the osmotically-active substance, electrolytes and, optionally, conventional additives, where the hydroxyethyl starch has a molecular weight Mw in the range from 10,000 to 150,000, a substitution MS in the range from 0.10 to 0.40, a substitution DS in the range from 0.09 to 0.35 and a substitution ratio C2 / C6>=8. With this peritoneal dialysis solution it is possible, with an outstanding ultrafiltration, to maintain a longer dwell time, for example the dialysis solution can be utilized for a period of 12 hours in the CAPD without replacement. In addition, the inventive dialysis solution is also particularly advantageous for patients with residual kidney function. The resorption of the osmotically active substance is clearly diminished and even after a dwell time of 12 hours it amounts to a maximum of 60-70%.
Owner:FRESENIUS AG
Peritoneal dialysis method
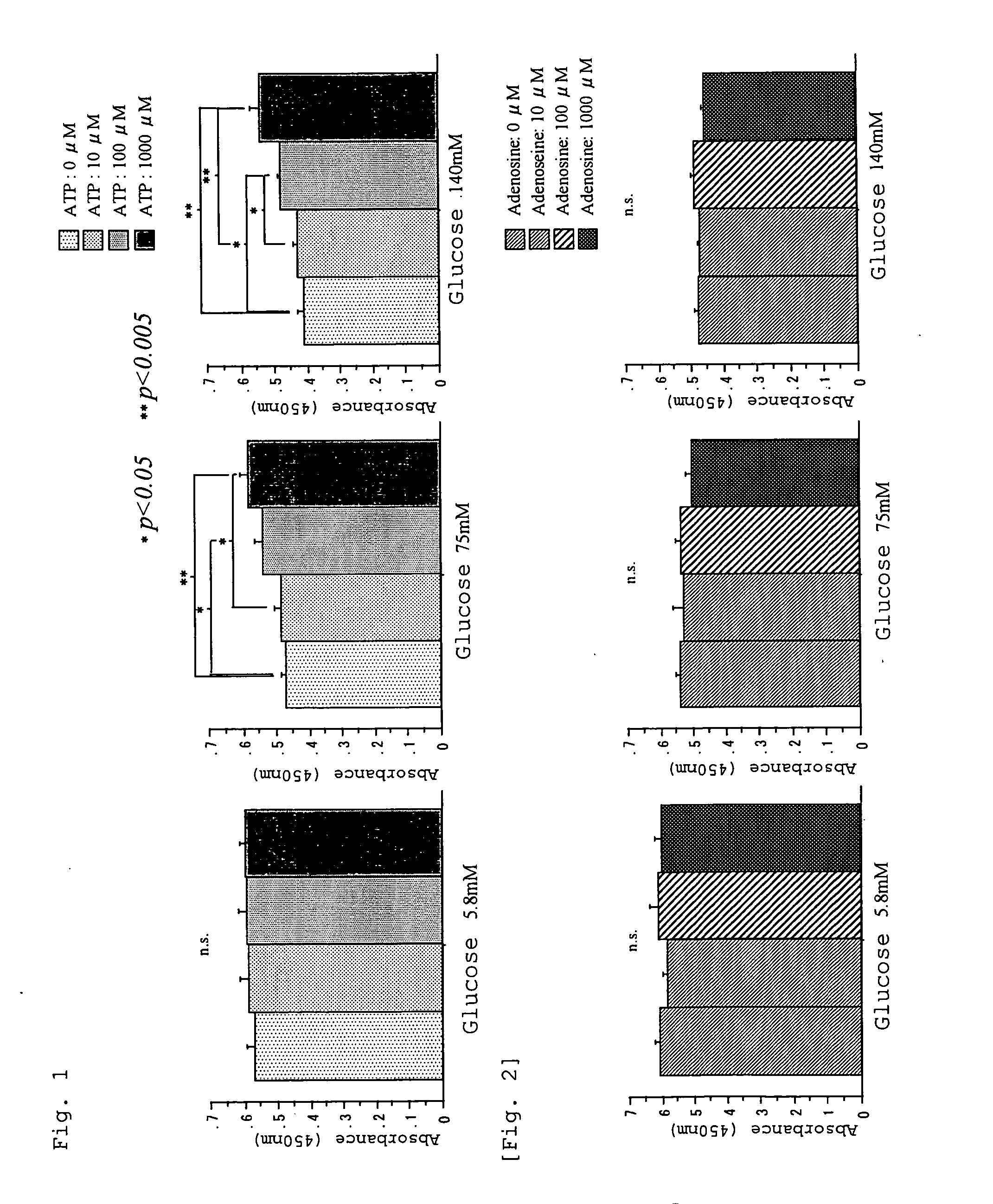
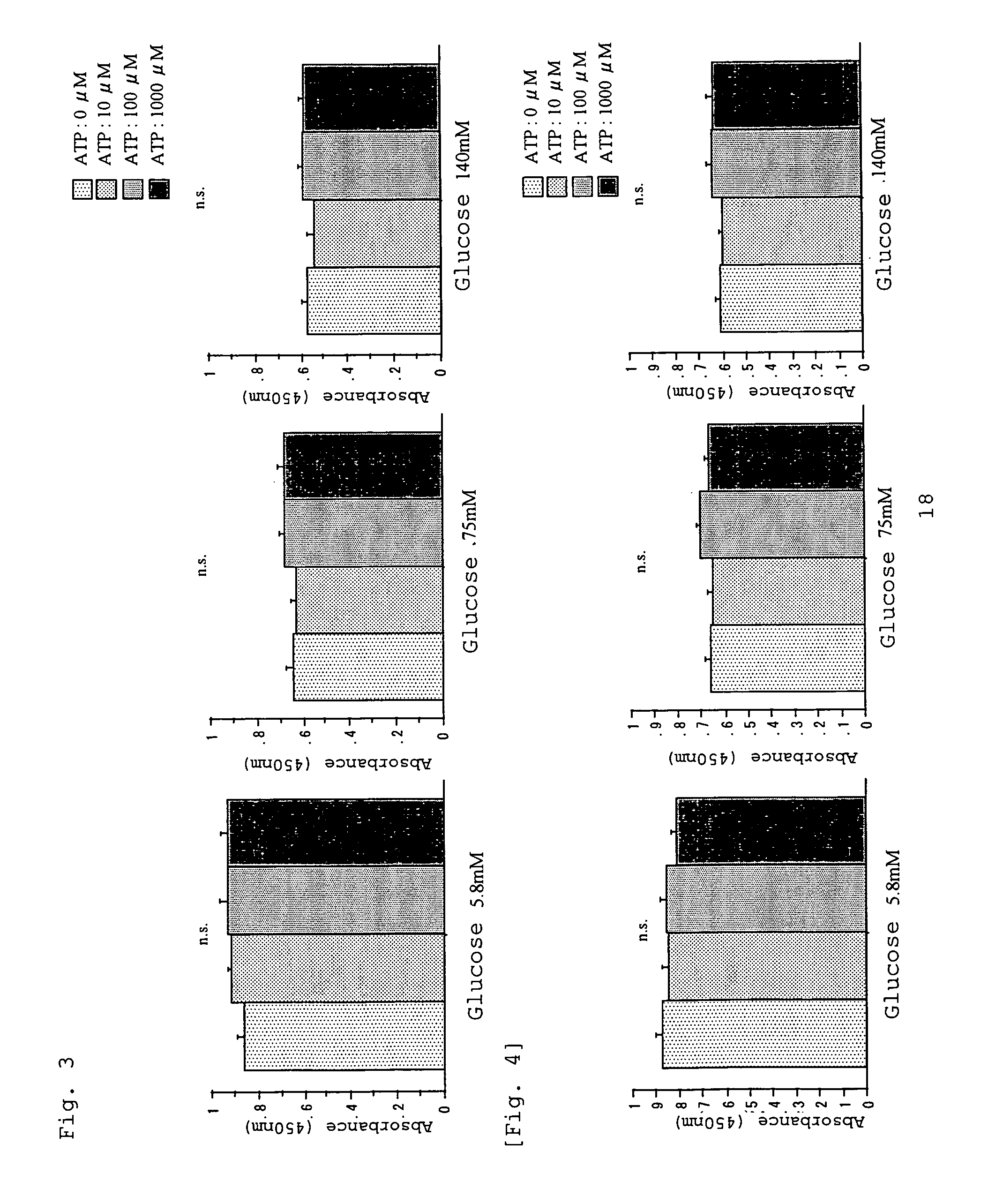
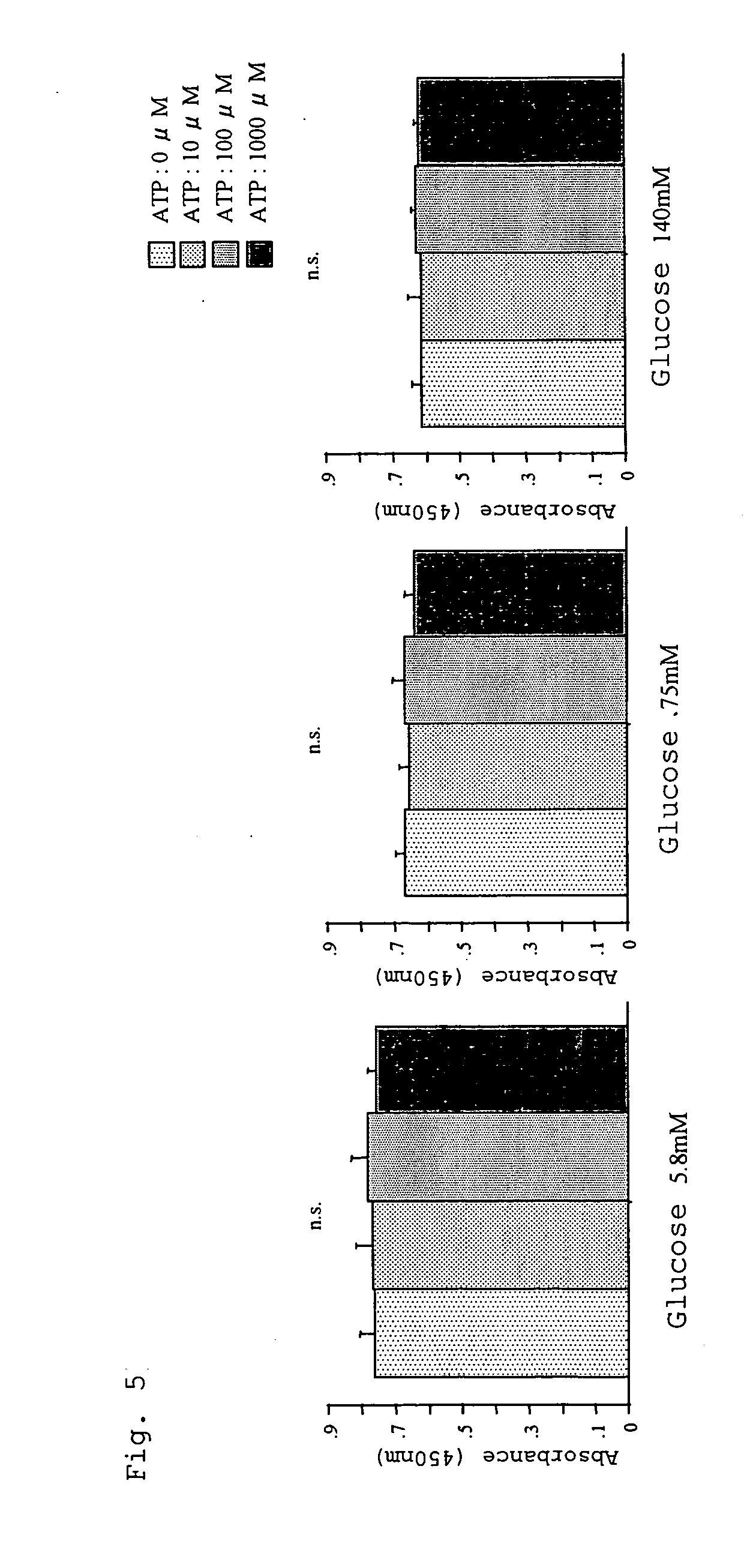
InactiveUS20060019925A1Less peritoneal injuryAvoid excessive injuryBiocideAntipyreticIntensive care medicineDialysis fluid
A peritoneal dialysate containing adenosine triphosphate or a salt thereof, and a peritoneal dialysis method using the dialysate. The peritoneal dialysate is safe and causes less peritoneal injuries even when employed in peritoneal dialysis over a long period of time.
Owner:KOWA CO LTD
Peritoneal dialysis optimized using a patient hand-held scanning device
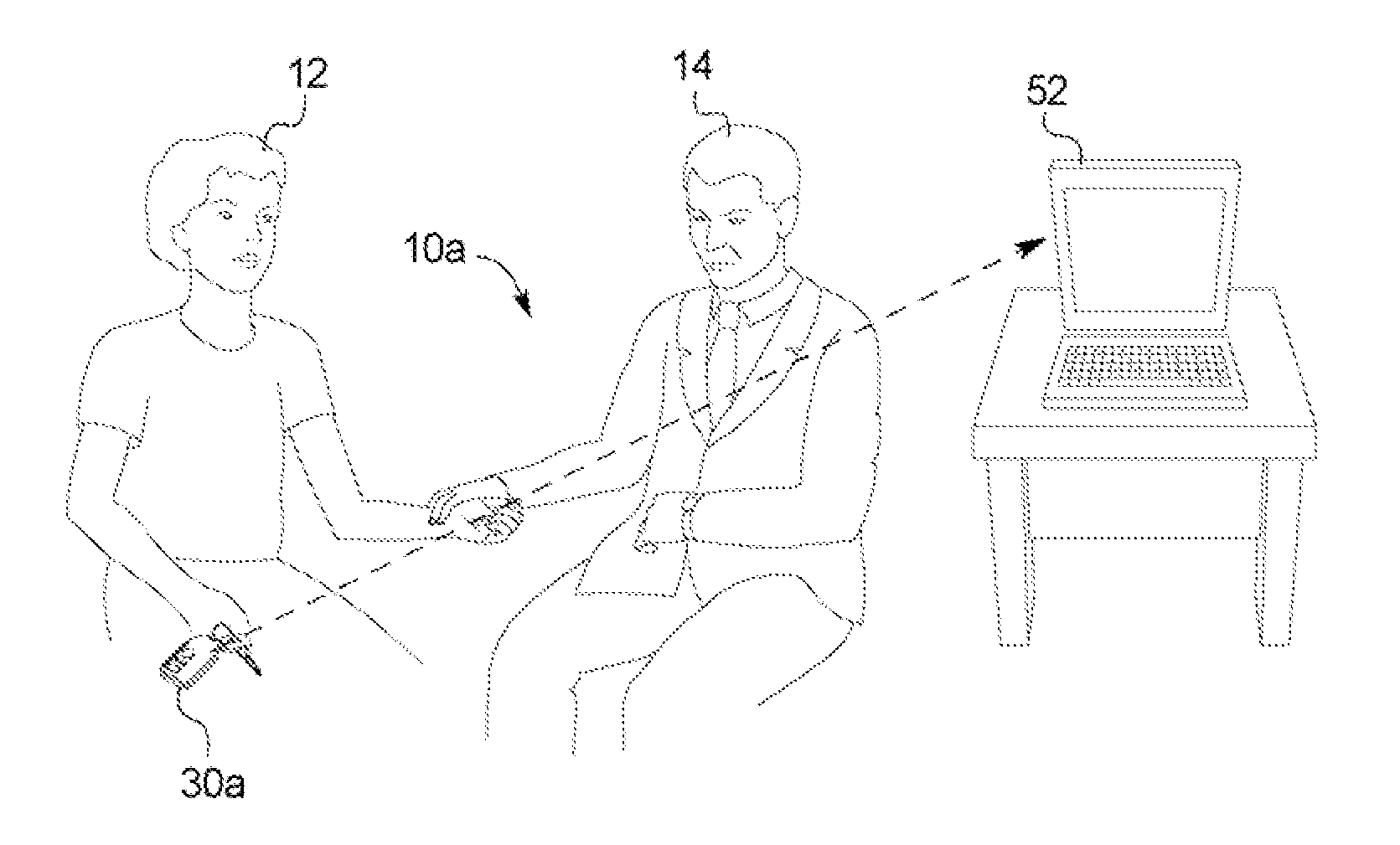
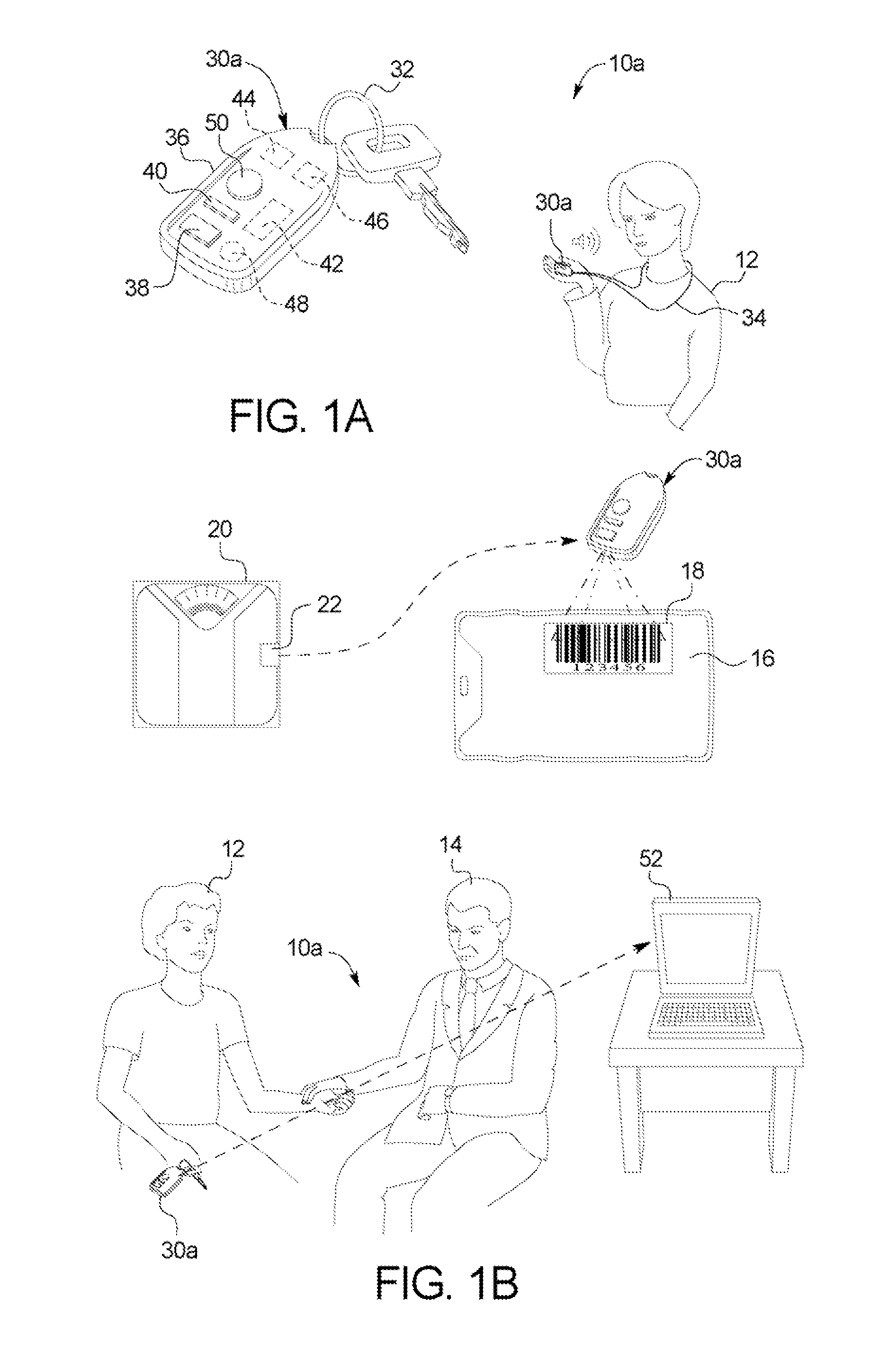
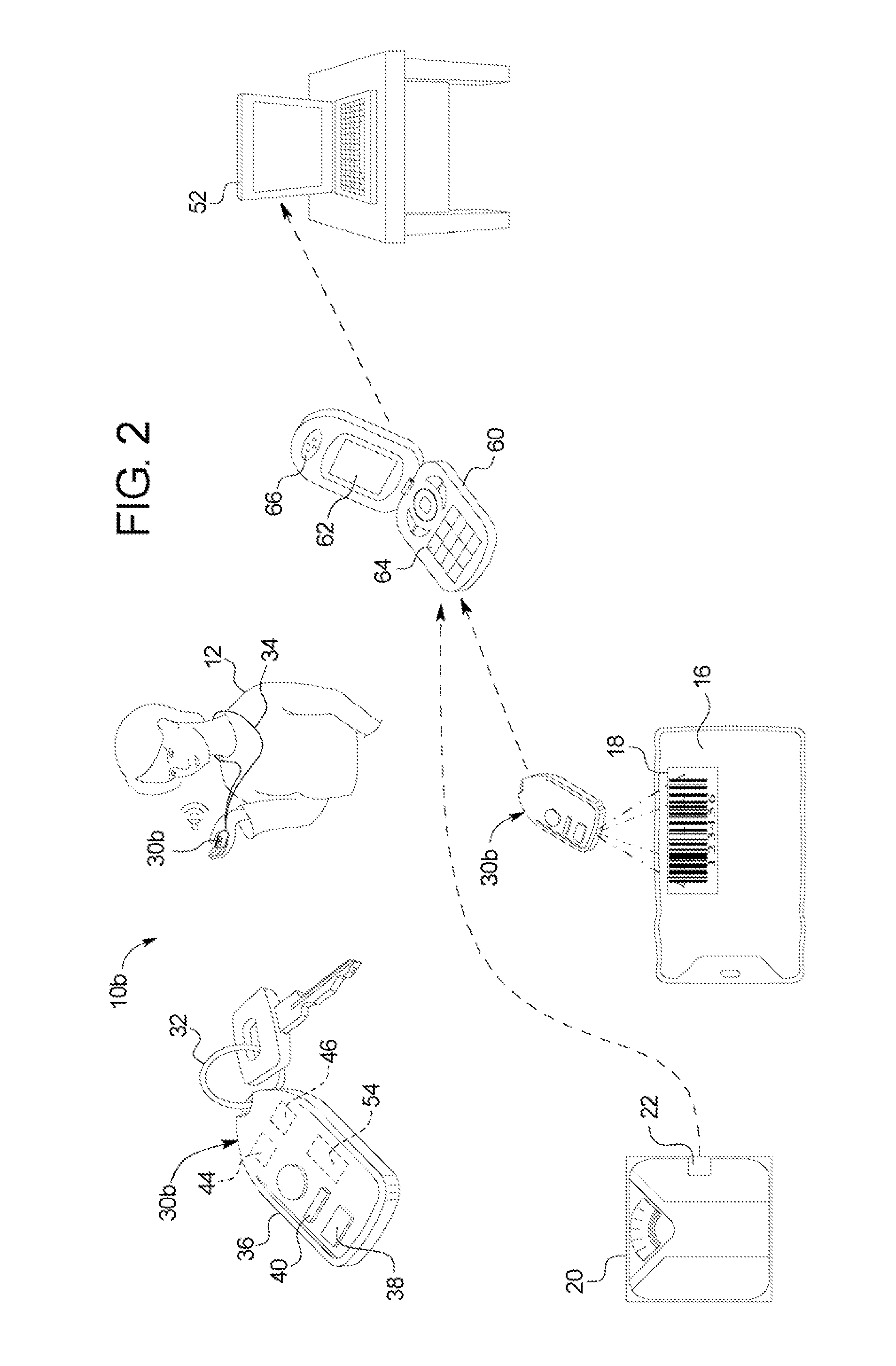
ActiveUS20110093294A1Optimize therapyConvenient treatmentData processing applicationsDigital data processing detailsData transmissionDialysis fluid
A dialysis system includes: a portable device configured to be carried by a patient and to read a marking displayed on a dialysis fluid container, the device obtaining data concerning at least one of a dialysis fluid type and a dialysis fluid volume from the marking; the device defined dialysate dwell time, alert patients for the next exchange and further configured to transfer the data to a computer; and wherein the computer is configured to use the data to track therapy progress of the product.
Owner:BAXTER INT INC +1
Medical treatment system and methods using a plurality of fluid lines
ActiveUS20130165847A1Prevent slight movementReduce noiseFlexible member pumpsPipe heating/coolingControl systemPeritoneal cavity
A medical treatment system, such as peritoneal dialysis system, may include control and other features to enhance patient comfort and ease of use. For example, a peritoneal dialysis system may include a control system that can adjust the volume of fluid infused into the peritoneal cavity to prevent the intraperitoneal fluid volume from exceeding a pre-determined amount. The control system can adjust by adding one or more therapy cycles, allowing for fill volumes during each cycle to be reduced. The control system may continue to allow the fluid to drain from the peritoneal cavity as completely as possible before starting the next therapy cycle. The control system may also adjust the dwell time of fluid within the peritoneal cavity during therapy cycles in order to complete a therapy within a scheduled time period. The cycler may also be configured to have a heater control system that monitors both the temperature of a heating tray and the temperature of a bag of dialysis fluid in order to bring the temperature of the dialysis fluid rapidly to a specified temperature, with minimal temperature overshoot.
Owner:DEKA PROD LLP
Artificial kidney dialysis system
The present disclosure relates to a wearable dialysis system and method for removing uremic waste metabolites and fluid from a patient suffering from renal disease. Uremic waste metabolites can be removed by a wearable peritoneal dialysis device that regenerates the peritoneal dialysis solution without removing positively charged, essential ions from the solution and, consequently, the patient. Fluids can be removed from the blood of the patient by an implantable fluid removing device. Fluids are delivered to the bladder and preferably removed from the body of the patient through urination. The wearable dialysis system may be operated continuously or semi-continuously and be comfortably adapted to the body of the patient while allowing the patient to perform normal activities.
Owner:FRESENIUS MEDICAL CARE HLDG INC
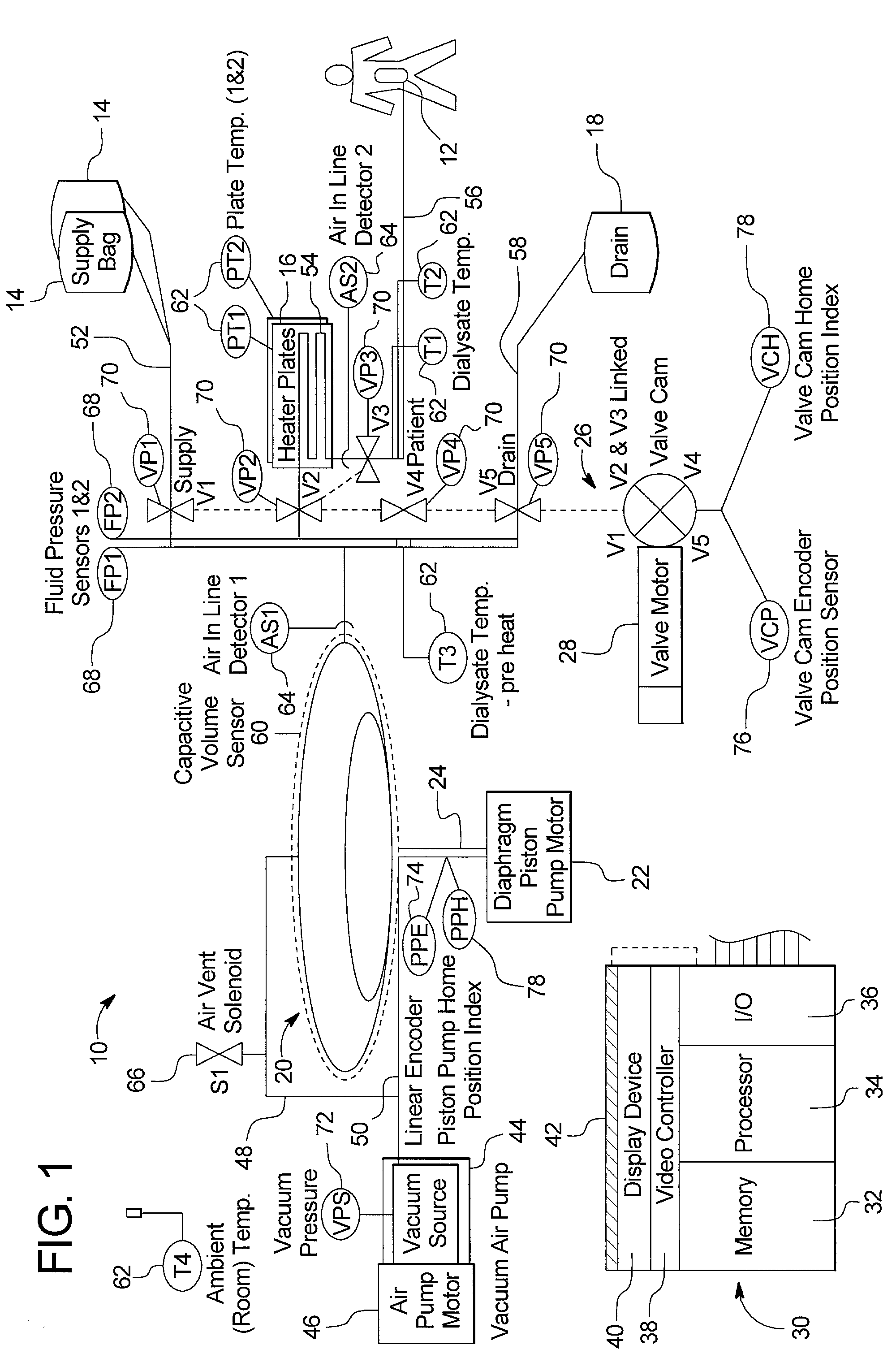
Automated solution injection-discharge system and automated peritoneal dialysis system
InactiveUS6491658B1Easy to controlOperational securityPeritoneal dialysisIntensive care medicineDialysis fluid
The automated solution injection-discharge system is used as an APDS system to supply and discharge a dialysate and a patient's drain. And an automated solution injection-discharge system provides free of contamination and operation mistakes, and can accurately control the injected dialysate volume and the discharged dwell solution volume even when a patient does not maintain a fixed posture while replacing the solution.
Owner:JMS CO LTD
Portable peritoneal dialysis system
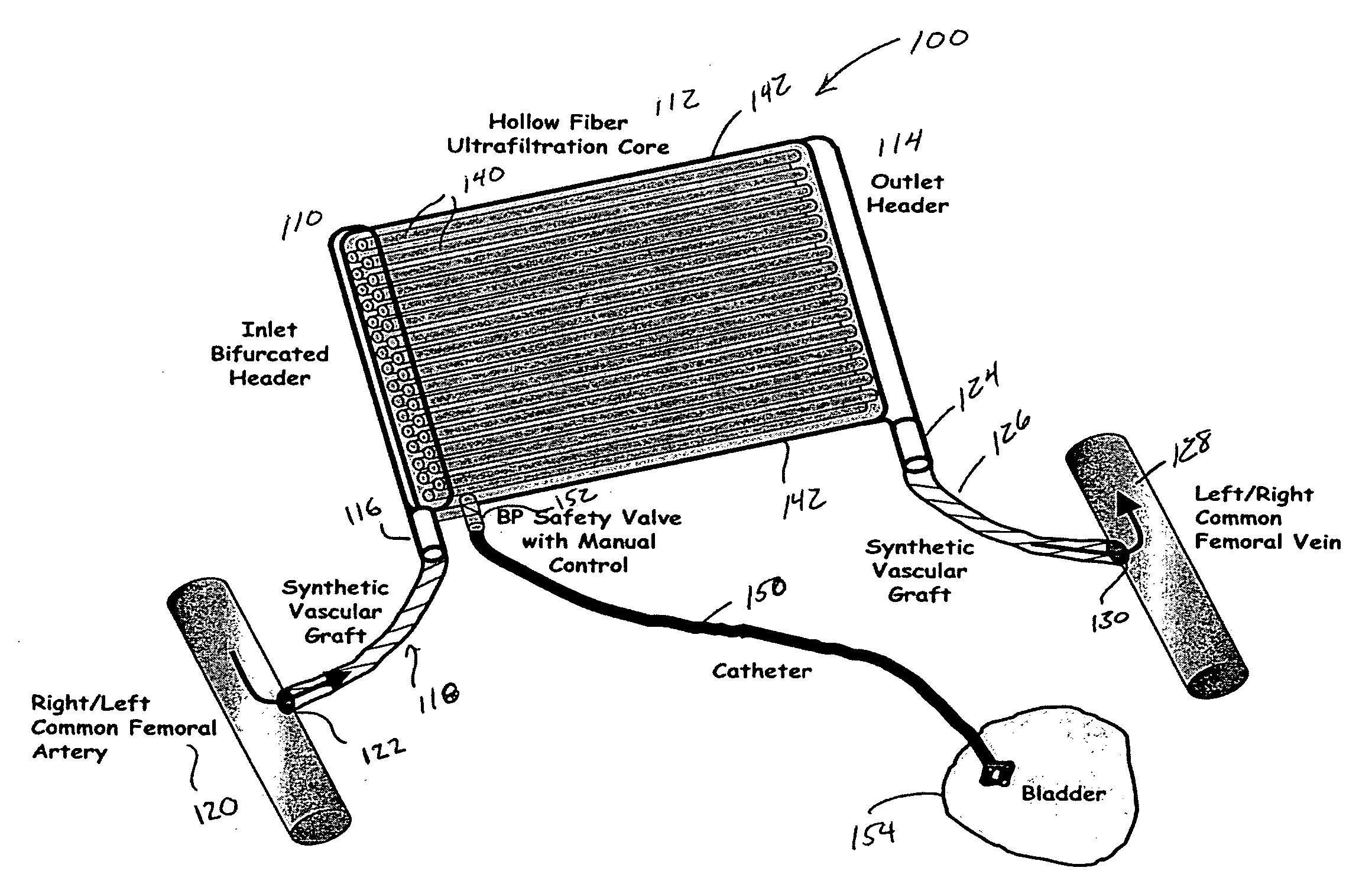
ActiveUS8777892B2Comfortably wornComfortable to carrySolvent extractionMedical devicesHollow fibreFiber
A portable peritoneal dialysis system for a patient includes an inlet port for providing inflow to the patient's peritoneal cavity, an outlet port for providing outflow from the patient's peritoneal cavity, and a volume of dialysate for flow into and out of the patient's peritoneal cavity, thereby removing from the dialysate uremic waste metabolites that have diffused into the dialysate. The portable peritoneal dialysis system also includes a closed liquid flow loop, including a pump, for flowing the dialysate into and out of the patient's peritoneal cavity, and an organic- and phosphate-removing stage, including at least one replaceable cartridge in the closed liquid flow loop, the cartridge containing material for removing organic compounds and phosphate from dialysate removed from the patient's peritoneal cavity. The portable peritoneal dialysis system further includes a urea- and ammonia-removing stage, including at least one replaceable cartridge in the closed liquid flow loop, the cartridge containing material for removing urea and ammonia from dialysate removed from the patient's peritoneal cavity, the material being packed around semi-permeable hollow fibers with interior fiber walls that reject cations, thereby retaining cations in the dialysate.
Owner:FRESENIUS MEDICAL CARE HLDG INC
Drain and fill logic for automated peritoneal dialysis
ActiveUS20100191181A1Limit low drain alarmLimitMedical devicesDialysisContinuous cycling peritoneal dialysisDraining tube
A system and method for automatically adjusting a Continuous Cycling Peritoneal Dialysis (“CCPD”) therapy to minimize the potential for excess intra-peritoneal volume. The adjustments are made at the end of the drain, just prior to the next fill. The adjustments short the next fill, if necessary, to limit the intra-peritoneal volume, add a cycle, if necessary, to use all of the available dialysis solution and will average the remaining dwell time to maximize the therapeutic benefit of the therapy in the allotted time. In another embodiment, a tidal therapy using trended patient UF data is provided.
Owner:BAXTER HEALTHCARE SA +1
Shunt
A shunt for draining cerebral spinal fluid from the brain is provided. In an embodiment, the shunt includes a master control unit that is located in the abdomen, which interconnects a ventricular catheter and a second catheter, typically located in the peritoneal cavity. In a specific embodiment, the master control unit includes a variety of ‘smart’ features including at least one access port to allow the injection of solutions for the prevention or removal of blockages in the catheter, and / or antibiotics. The access port can have other uses, such as allowing a point of access for physical navigation of a catheter or the like within the shunt, thereby providing another option for breaking-up blockages, and / or allowing an access point for repairing the shunt's components. Additionally, the master control unit includes a diagnostic unit that transmits, either wirelessly or through a wired connection via the access port, diagnostic information about the status of the patient and / or the shunt.
Owner:COWAN JOHN A +3
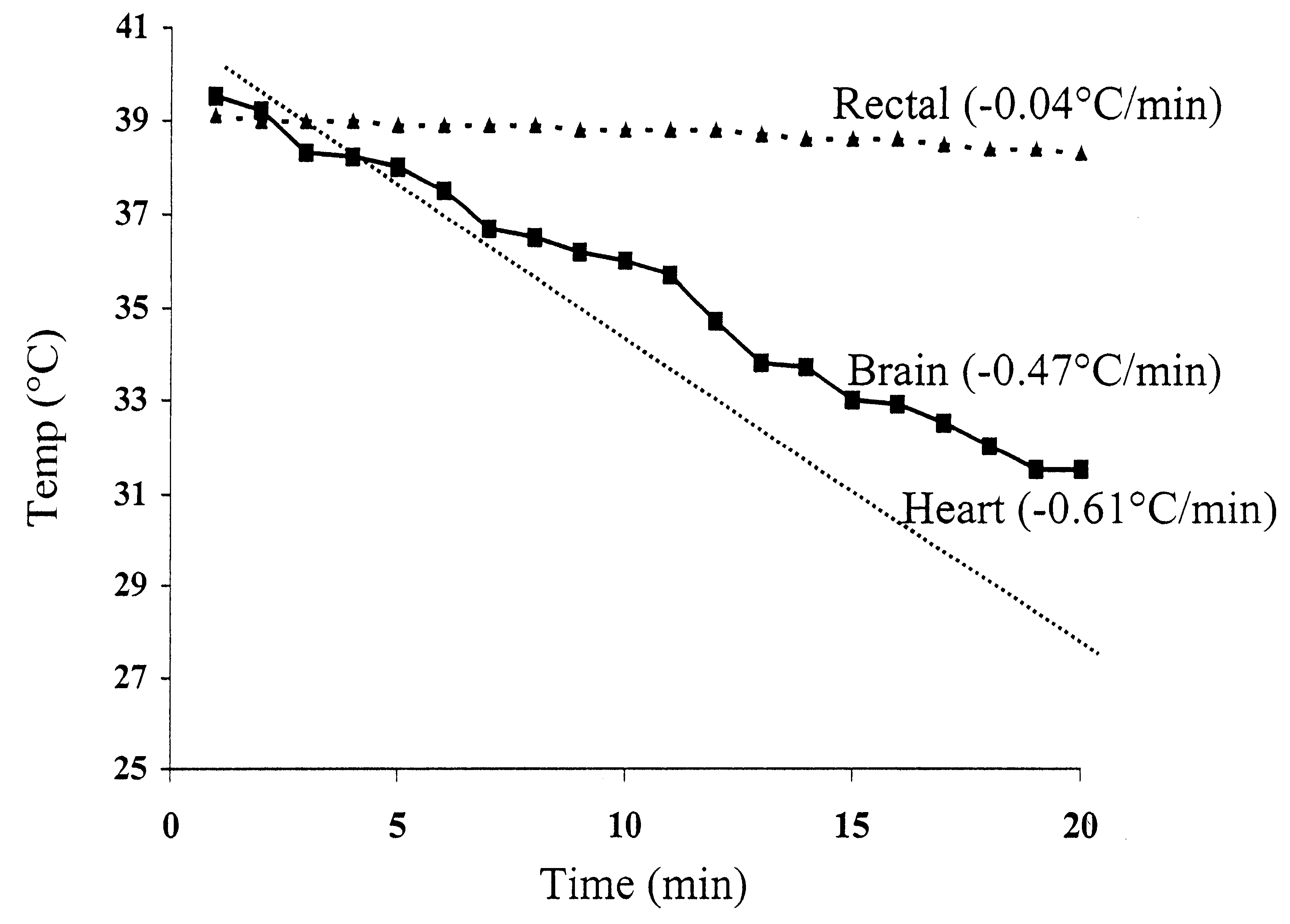
Method for inducing hypothermia
InactiveUS6962601B2Simple methodImprove the protective effectOther chemical processesLighting and heating apparatusParticulatesSlurry
Systems for phase-change particulate slurry cooling equipment and methods to induce hypothermia in a patient through internal and external cooling are provided. Subcutaneous, intravascular, intraperitoneal, gastrointestinal, and lung methods of cooling are carried out using saline ice slurries or other phase-change slurries compatible with human tissue. Perfluorocarbon slurries or other slurry types compatible with human tissue are used for pulmonary cooling. And traditional external cooling methods are improved by utilizing phase-change slurry materials in cooling caps and torso blankets.
Owner:UNIVERSITY OF CHICAGO +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com