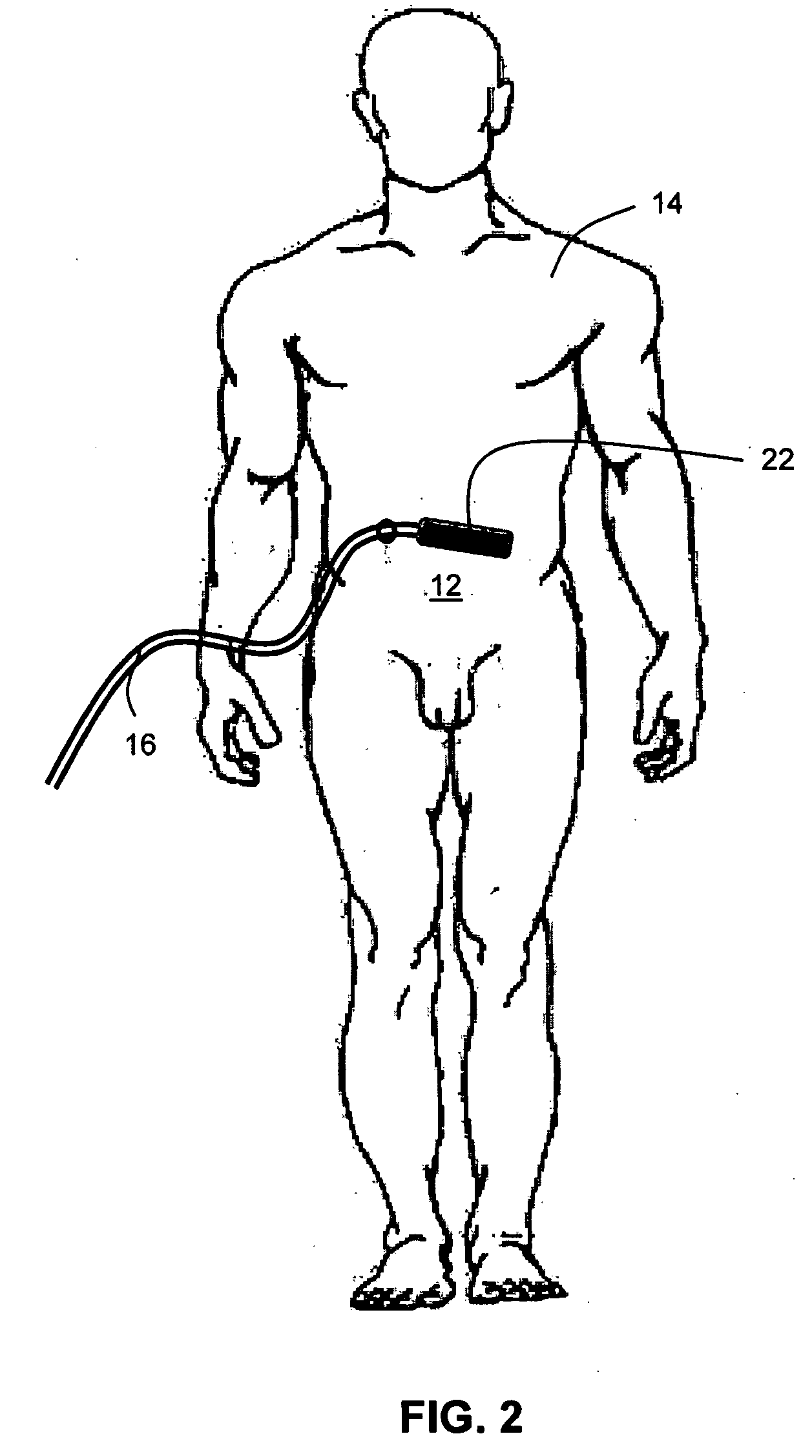
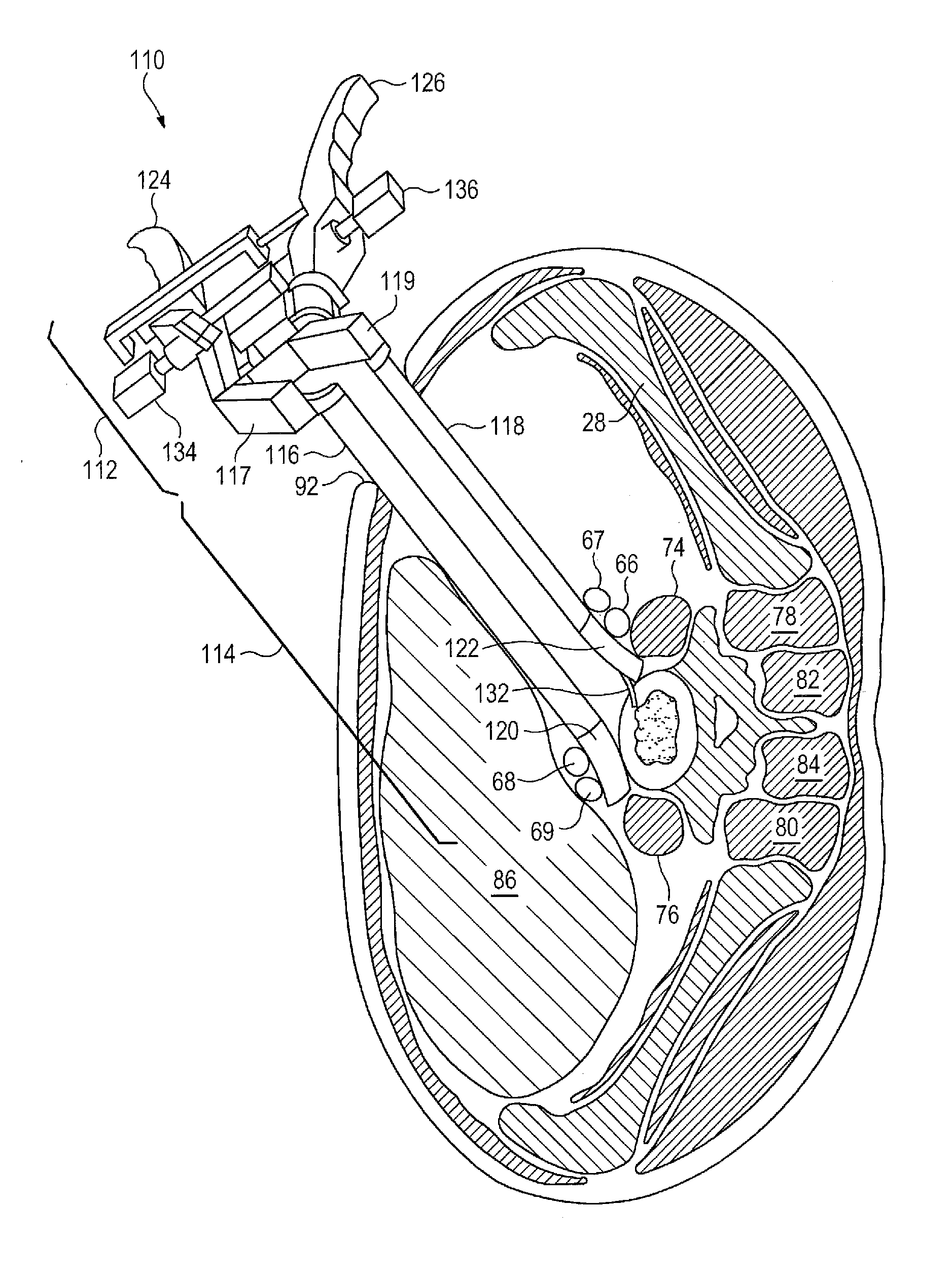
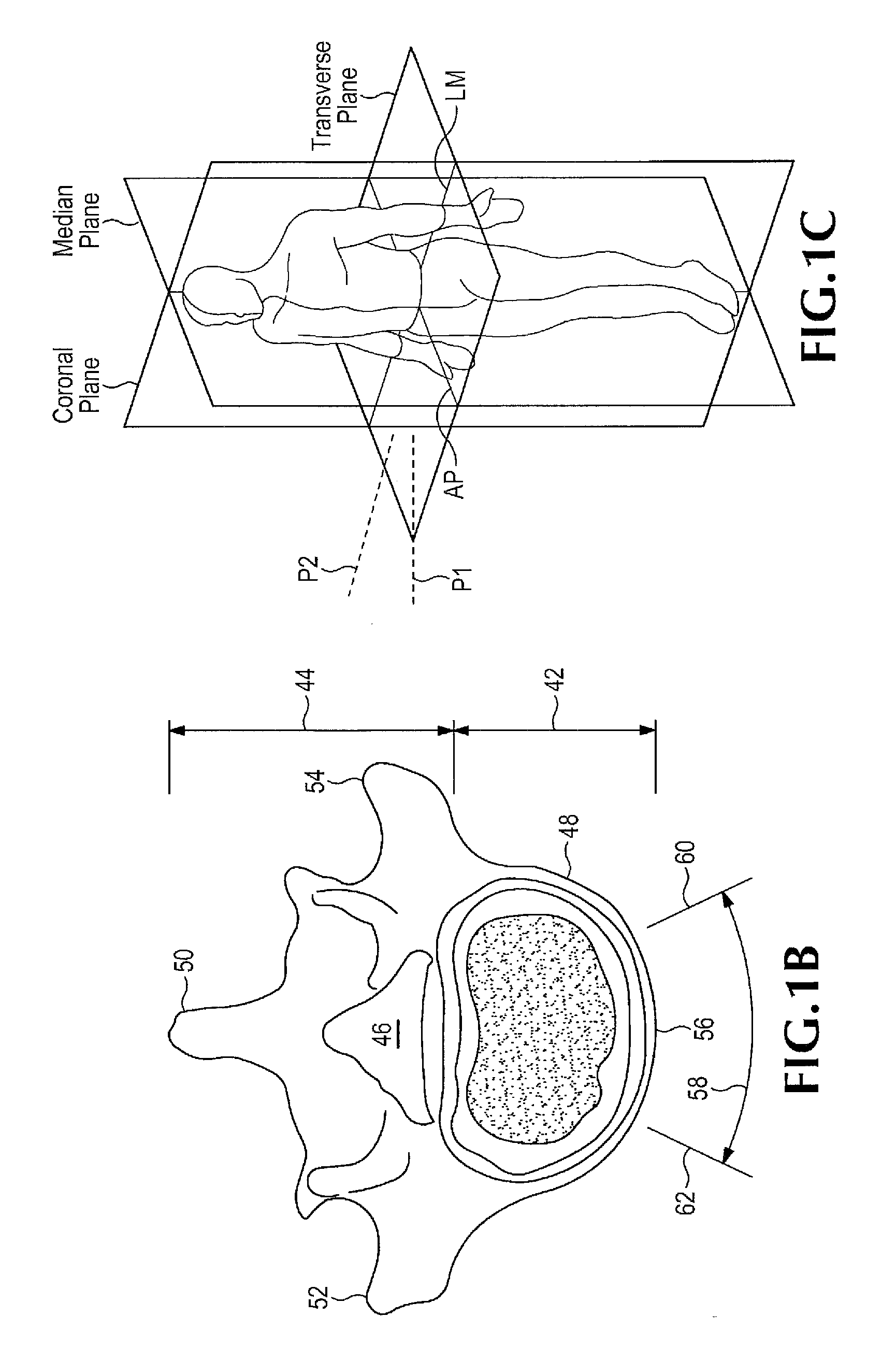
Patents
Literature
101 results about "Hemoperitoneum" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Hemoperitoneum (sometimes also hematoperitoneum) is the presence of blood in the peritoneal cavity. The blood accumulates in the space between the inner lining of the abdominal wall and the internal abdominal organs. Hemoperitoneum is generally classified as a surgical emergency; in most cases, urgent laparotomy is needed to identify and control the source of the bleeding. In selected cases, careful observation may be permissible. The abdominal cavity is highly distensible and may easily hold greater than five liters of blood, or more than the entire circulating blood volume for an average-sized individual. Therefore, large-scale or rapid blood loss into the abdomen will reliably induce hemorrhagic shock and, if untreated, may rapidly lead to death.
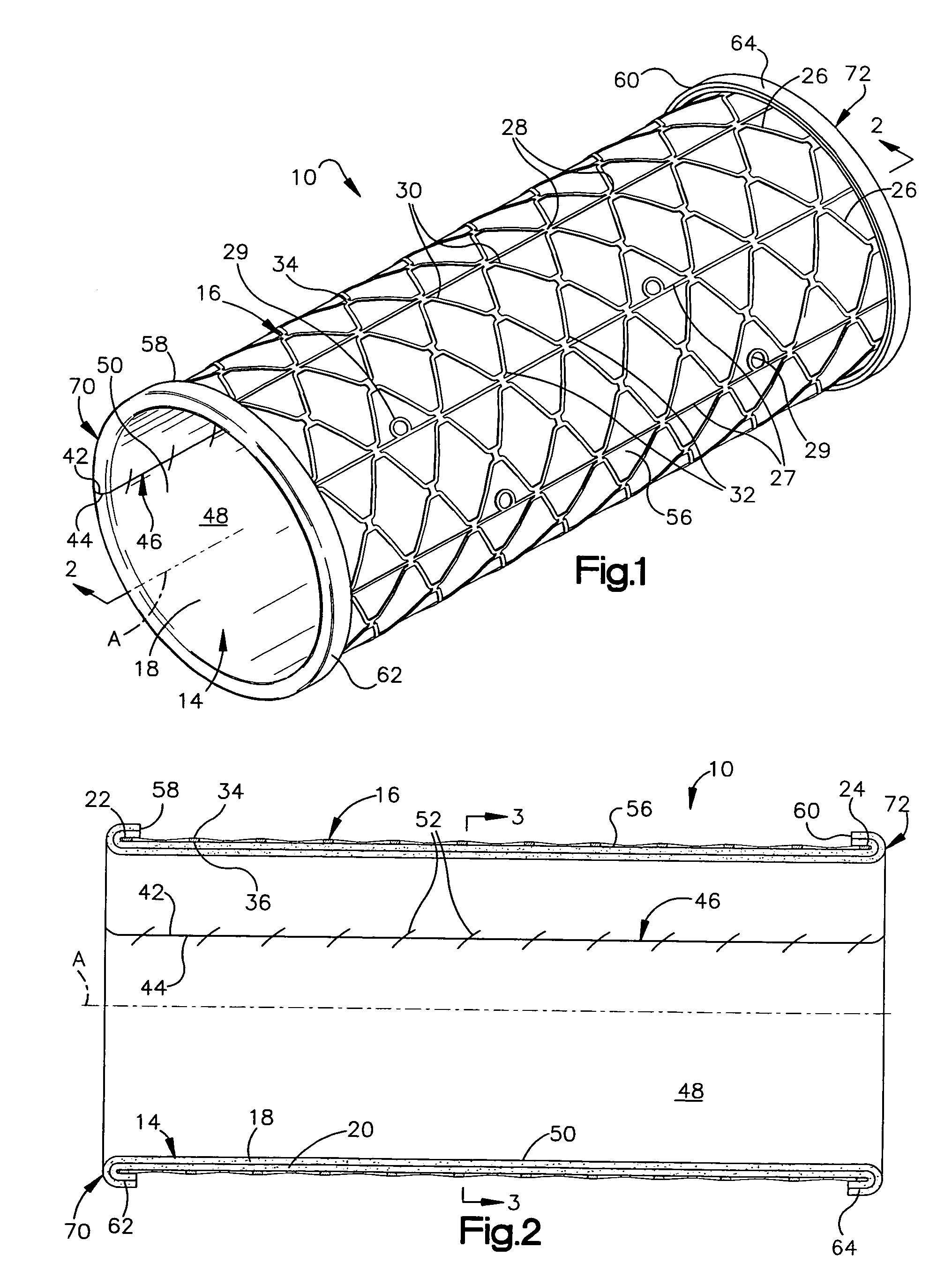
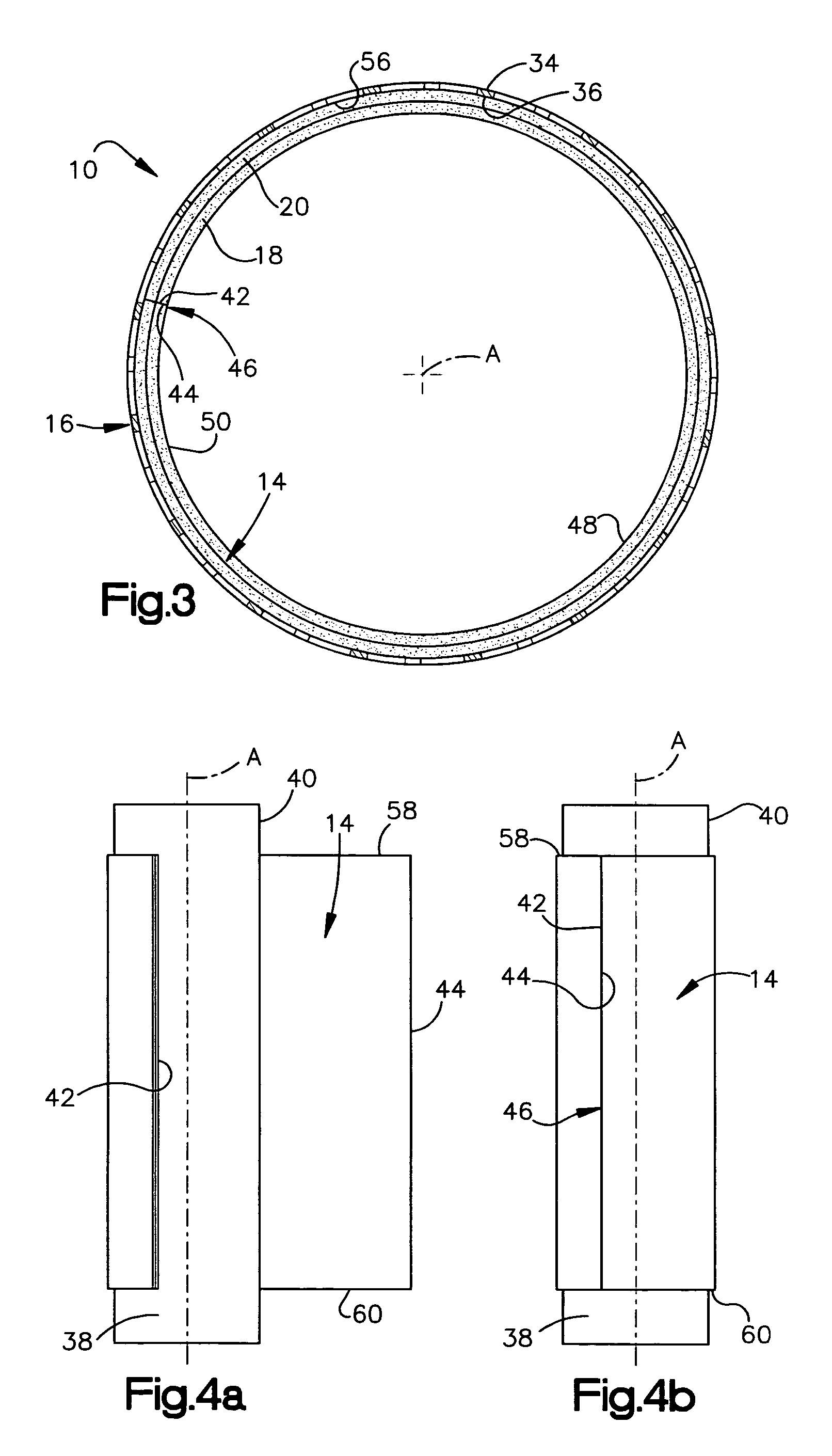
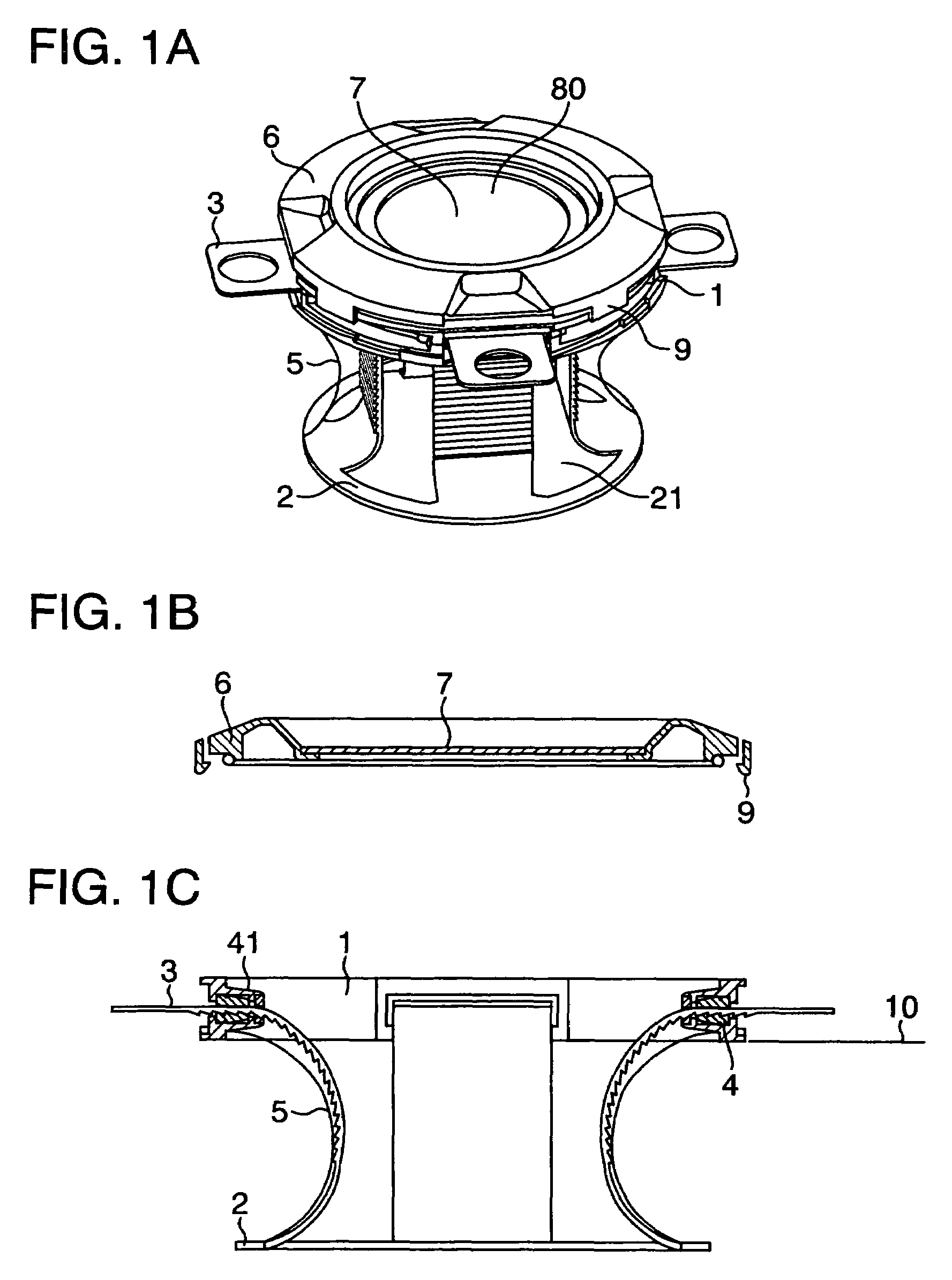
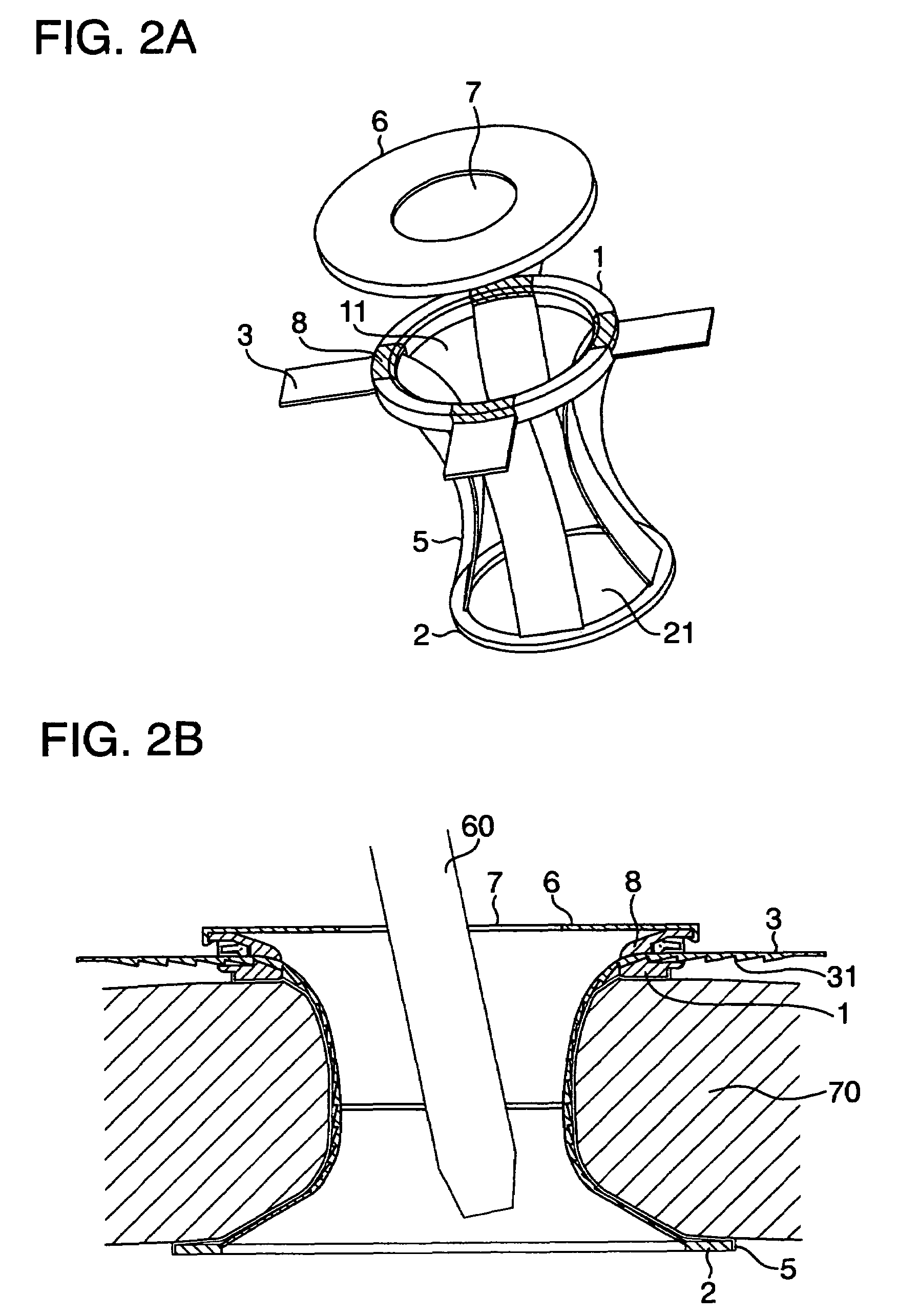
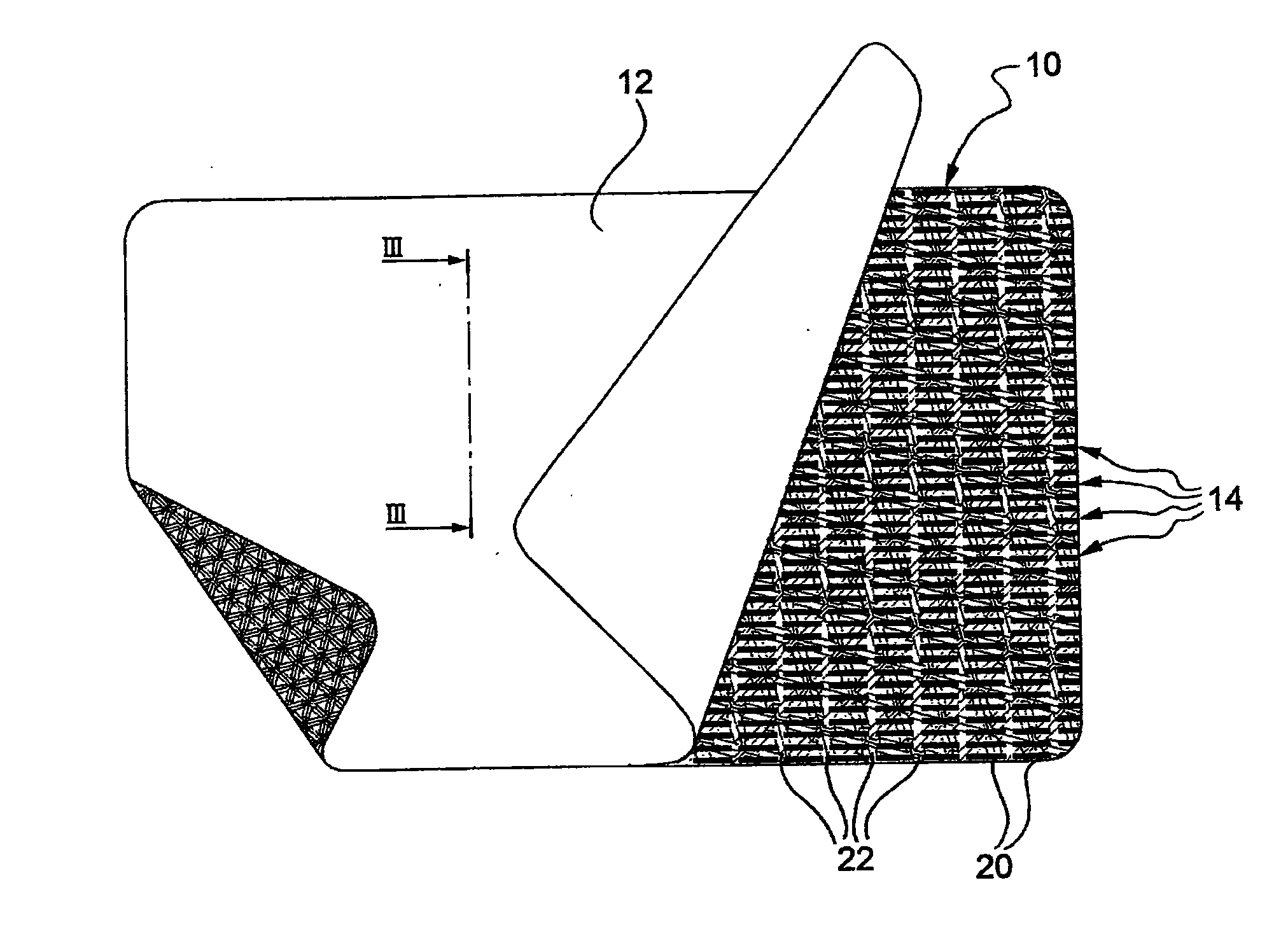
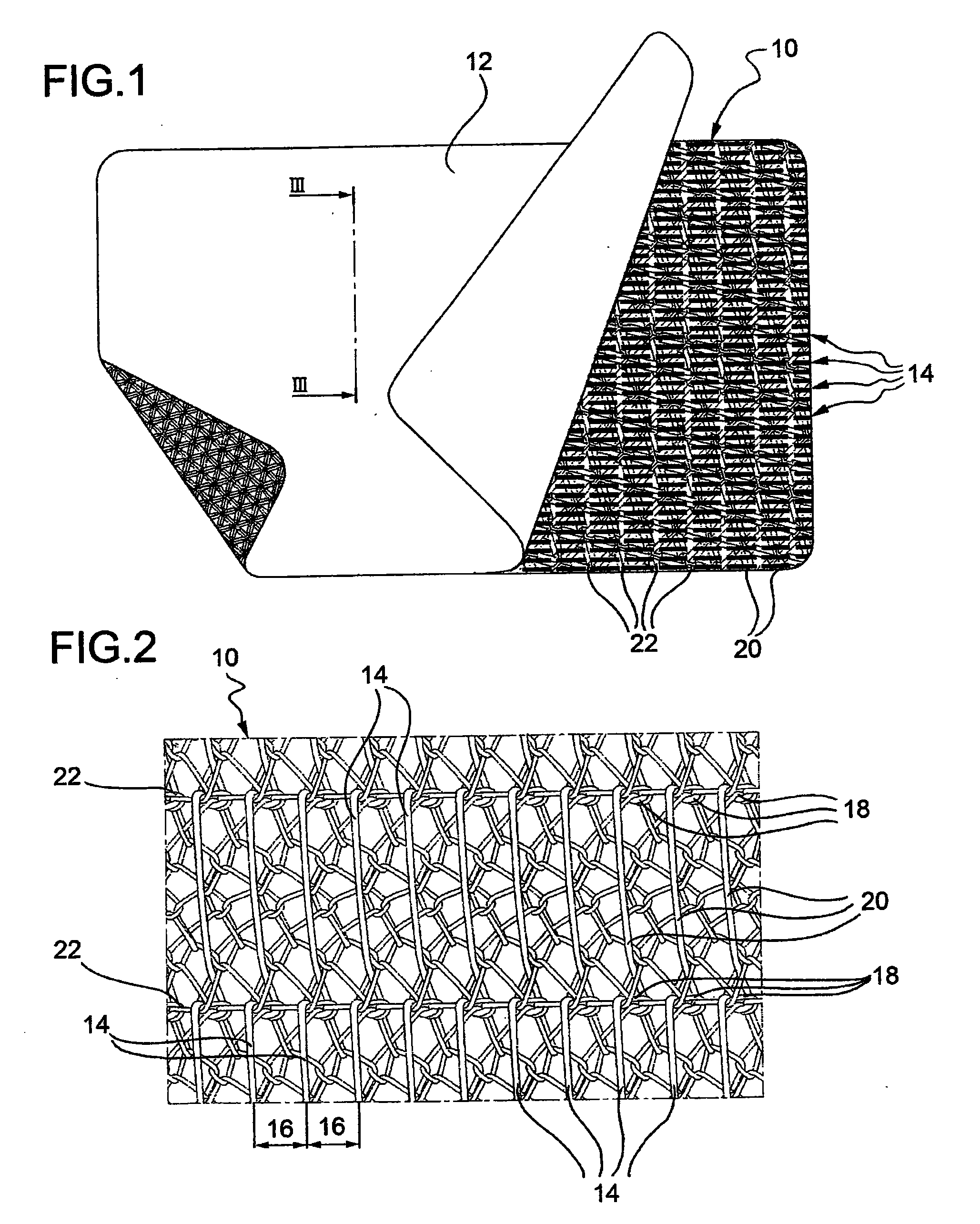
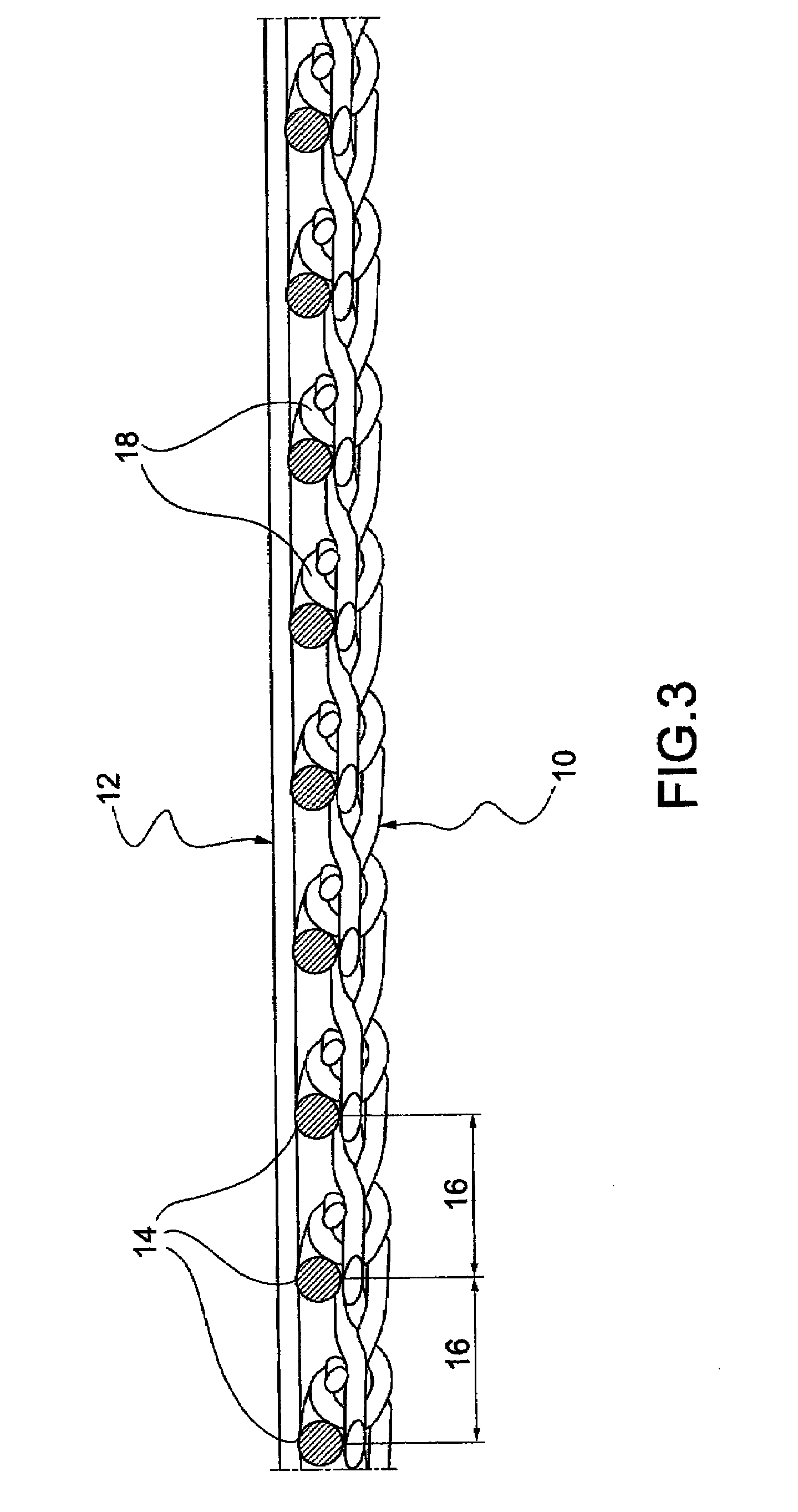
Electrosurgical instrument
InactiveUS7278994B2Lower impedanceReduced effectivenessCannulasDiagnosticsGynecologyPeritoneal cavity
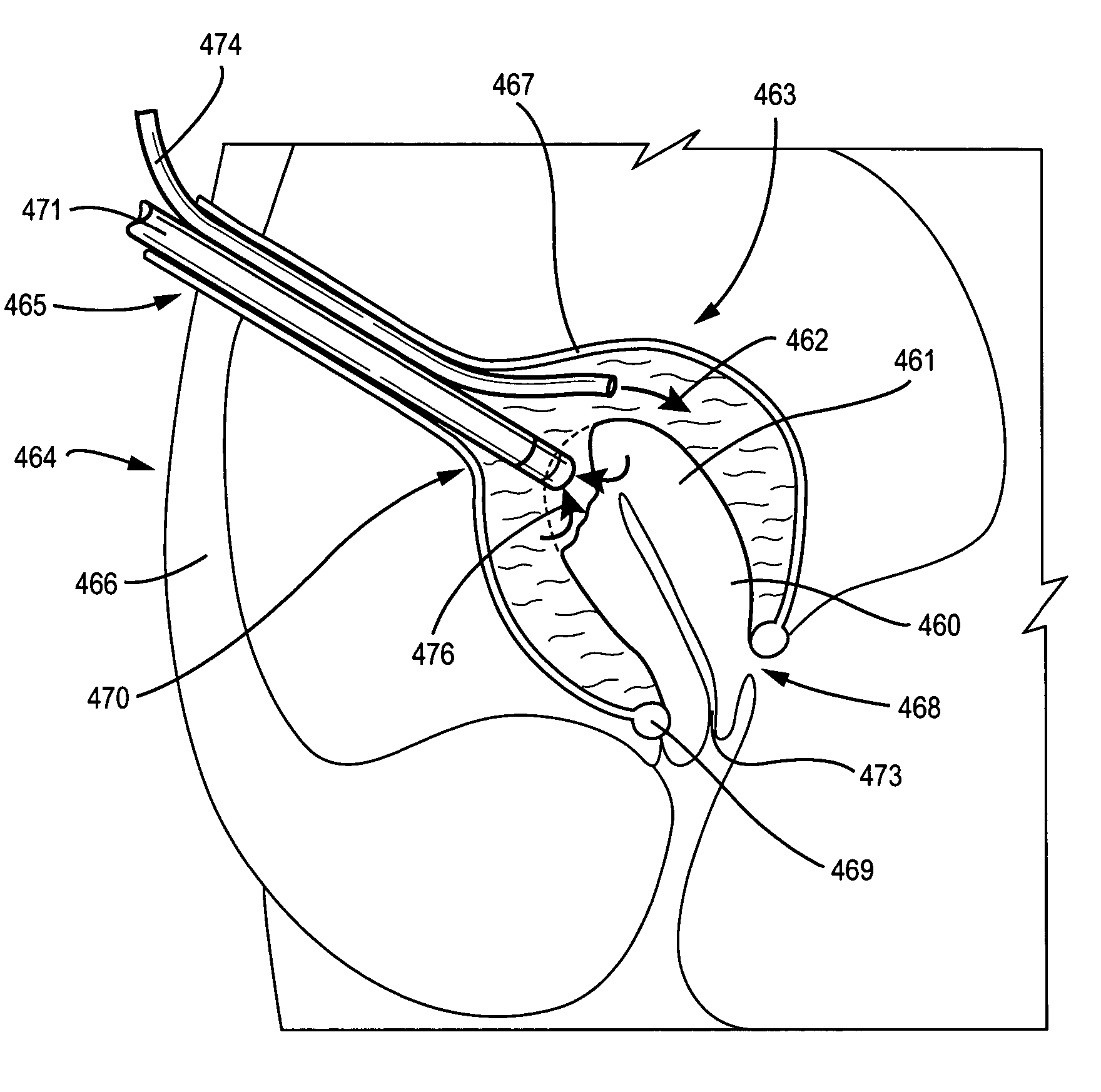
A system and method are disclosed for removing a uterus using a fluid enclosure inserted in the peritoneal cavity of a patient so as to enclose the uterus. The fluid enclosure includes a distal open end surrounded by an adjustable loop, that can be tightened, a first proximal opening for inserting an electrosurgical instrument into the fluid enclosure, and a second proximal opening for inserting an endoscope. The loop is either a resilient band extending around the edge of the distal open end or a drawstring type of arrangement that can be tightened and released. The fluid enclosure is partially inserted into the peritoneal cavity of a patient in a deflated condition and then manipulated within the peritoneal cavity over the body and fundus of the uterus to the level of the uterocervical junction. The loop is tightened around the uterocervical junction, after which the enclosure is inflated using a conductive fluid. The loop forms a pressure seal against the uterocervical junction to contain the conductive fluid used to fill the fluid enclosure. Endoscopically inserted into the fluid enclosure is an electrosurgical instrument that is manipulated to vaporize and morcellate the fundus and body of the uterus. The fundus and body tissue that is vaporized and morcellated is then removed from the fluid enclosure through the shaft of the instrument, which includes a hollow interior that is connected to a suction pump The fundus and body are removed after the uterus has been disconnected from the tissue surrounding uterus.
Owner:GYRUS MEDICAL LTD
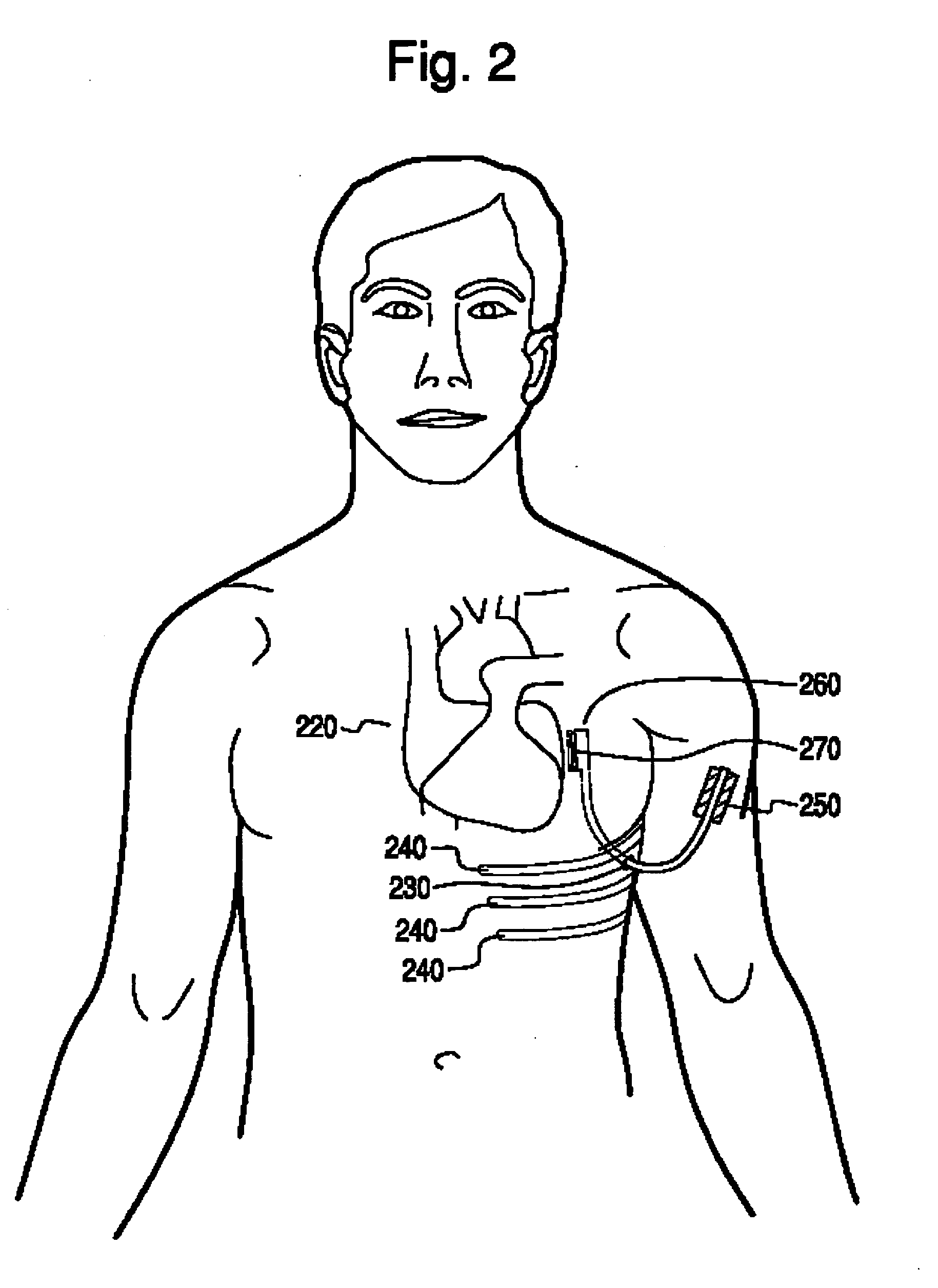
Prosthetic cardiac value and method for making same
A prosthetic valve for replacing a cardiac valve includes an expandable support member and at least two valve leaflets made of a first layer of biological material selected from peritoneal tissue, pleural tissue or pericardial tissue. A second layer of biological material is attached to the support member. The second layer is also made from peritoneal tissue, pleural tissue or pericardial tissue. The second layer includes a radially inwardly facing surface that defines a conduit for directing blood flow. The valve leaflets extend across the conduit to permit unidirectional flow of blood through the conduit. Methods for making and implanting the prosthetic valve are also provided.
Owner:THE CLEVELAND CLINIC FOUND
Methods and systems for ultrasound imaging of the heart from the pericardium
InactiveUS20050203410A1Low costOptimize locationSurgeryHeart/pulse rate measurement devicesUltrasound imagingThoracic structure
A peritoneal ultrasound imager includes an elongated body less than about 20 inches in length that is adapted to be inserted through a cannula into or near the pericardium space, and an ultrasound transducer array at one end of the body that is suitable for ultrasound echocardiography. The cannula and ultrasound imager may be of a single piece construction. A method for imaging the heart includes introducing a cannula into the wall of a patient's chest, inserting the elongated body into the cannula, moving the inserted elongated body to a position near the heart, and imaging the heart with ultrasound echo.
Owner:EP MEDSYST
Surgical access system and related methods
ActiveUS20100130827A1Increase the number ofStructural damageElectrotherapySurgeryDistractionSurgical operation
A surgical access system including a tissue distraction assembly and a tissue retraction assembly, both of which may be equipped with one or more electrodes for use in detecting the existence of (and optionally the distance and / or direction to) neural structures before, during, and after the establishment of an operative corridor to a surgical target site. Some embodiments of the surgical access system may be particularly suited for establishing an operative corridor to a surgical target site in the spine. Such an operative corridor may be established through the retroperitoneal space and the psoas muscle during a direct lateral, retroperitoneal approach to the spine.
Owner:NUVASIVE
Implantable Device For Obesity Prevention
InactiveUS20080208240A1Avoid displacementExpansion is limitedSurgeryDilatorsMinimally invasive proceduresObesity prevention
A device for controlling the expansion of a hollow internal organ, comprising an inflatable balloon, which is inserted in an uninflated state into the desired position close to the organ using minimally invasive procedures. After insertion, the balloon is inflated to its required size and shape. The invention is useful for restricting the expansion of the stomach during meals, thus inducing a feeling of satiety and preventing over-eating. Inflation may be performed through a catheter connected to a readily accessible inflation port. In the case of the gastric embodiment, the balloon may be positioned pro-peritoneally, such that the procedure is surgically simple. One or more sensors located close to the organ to be controlled, may monitor a physiological effect relating to the organ, and the output of the sensor used to control the level of inflation of the balloon in order to correct the condition being monitored.
Owner:STIMPLANT
Medical treating instrument
InactiveUS7297106B2Easy to switchReduce the overall heightCannulasDiagnosticsSurgical treatmentForceps
There is provided a surgical treatment instrument in which the mode can be easily switched between a treatment under a pneumoperitoneum condition and a treatment outside the peritoneal cavity, and forceps can be manipulated under the pneumoperitoneum condition, and when taking out a cancer-affected part, an incision in the abdominal wall is protected, and the incision is free from infection. The surgical treatment instrument includes a tubular portion which has a first fixing member provided at a near end-side open portion thereof, and also has a second fixing member provided at a remote end-side open portion thereof. At least two tension belts are mounted on the second fixing member, and fixing means for adjusting the length of the tension belts to position the first fixing member is provided at the first fixing member.
Owner:SUMITOMO BAKELITE CO LTD
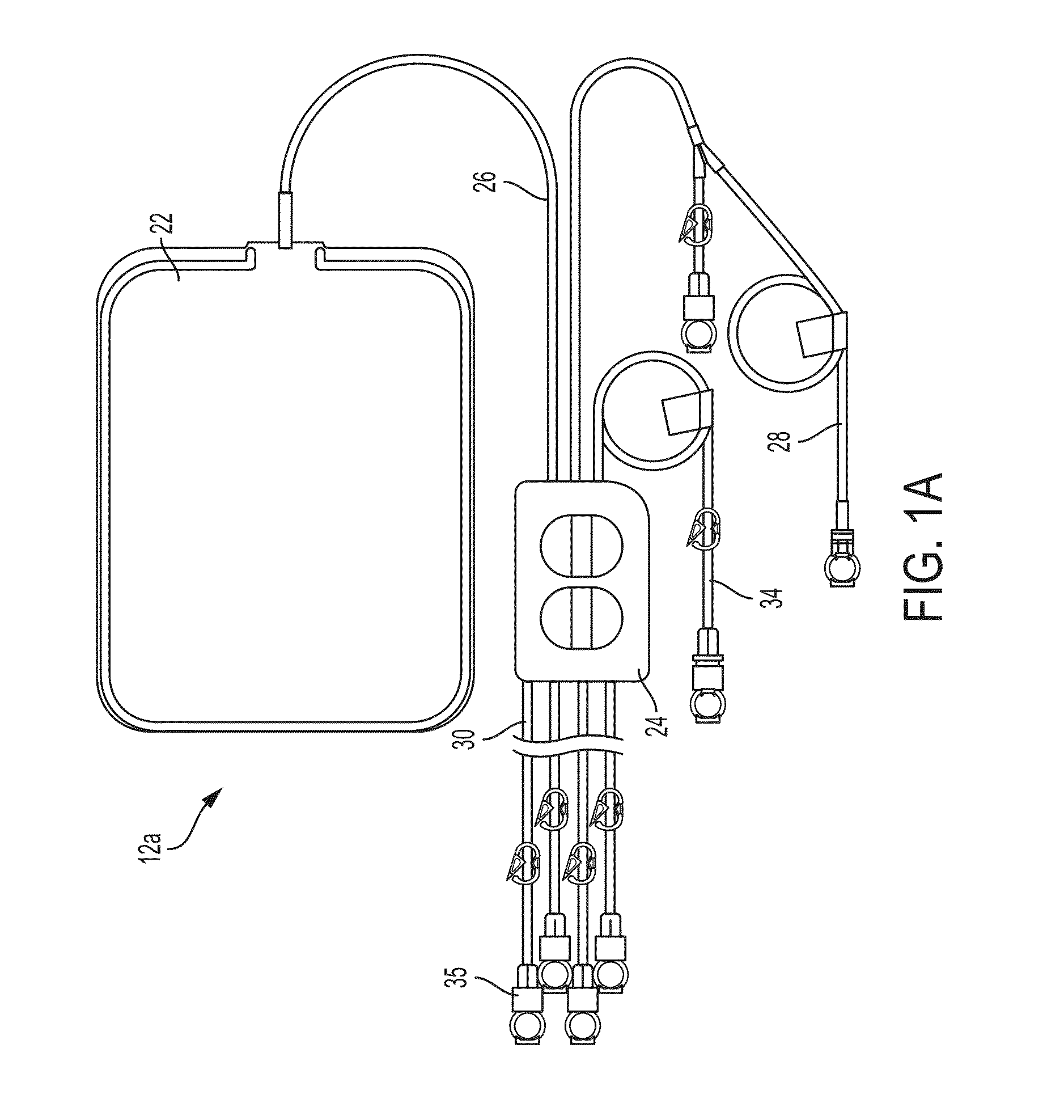
Hypothermia Devices and Methods
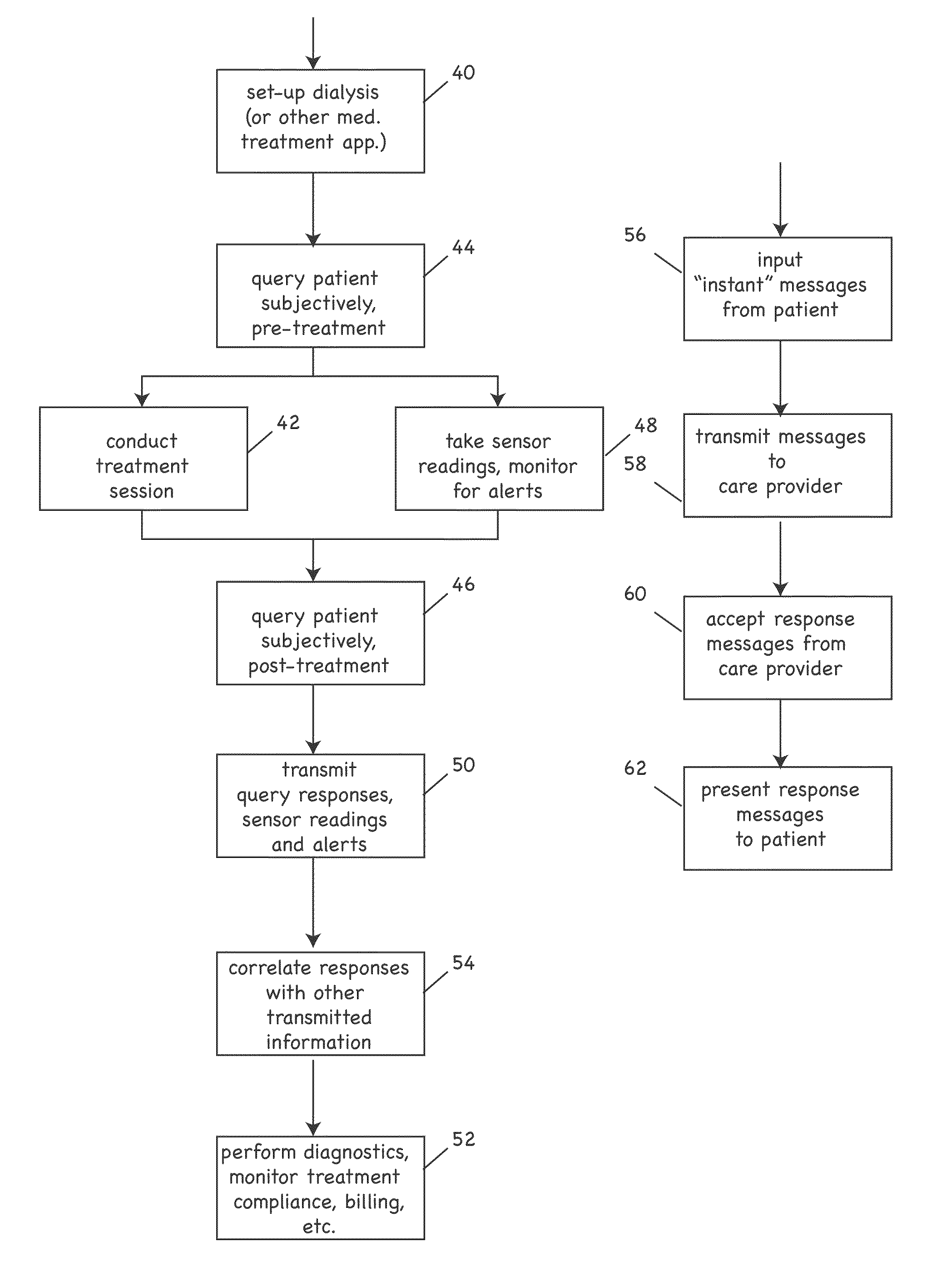
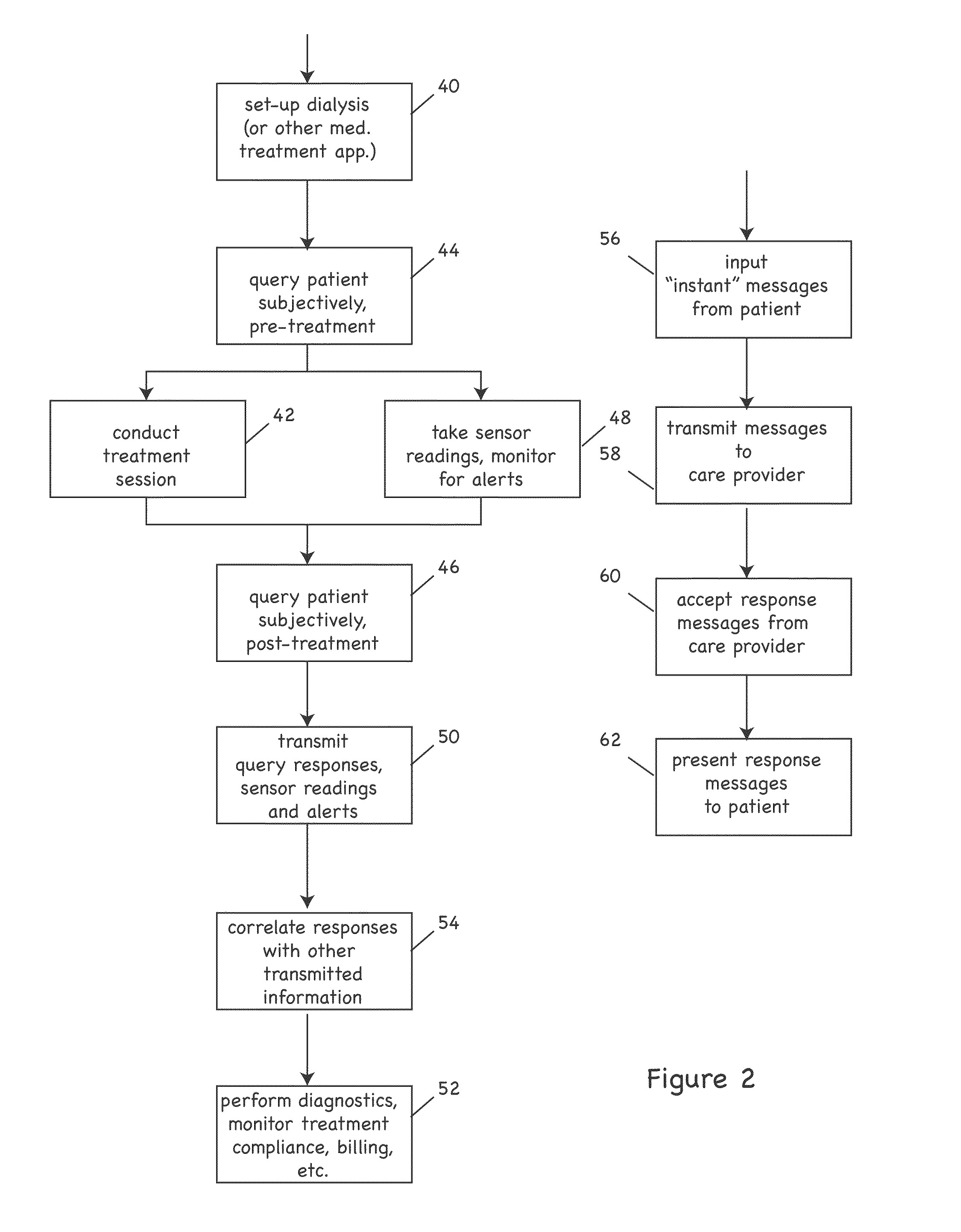
ActiveUS20090076573A1Reduce the amount requiredLower body temperatureMedical devicesTherapeutic coolingPeritoneal cavityFeedback control
A method of providing hypothermia to a patient including the steps of inserting a fluid delivery member into a peritoneal cavity of the patient; delivering hypothermia fluid from a fluid source into the peritoneal cavity through the delivery member; and limiting fluid pressure within the peritoneal cavity without providing feedback control to the fluid source. The invention also provides an apparatus for practicing the method.
Owner:THERANOVA LLC
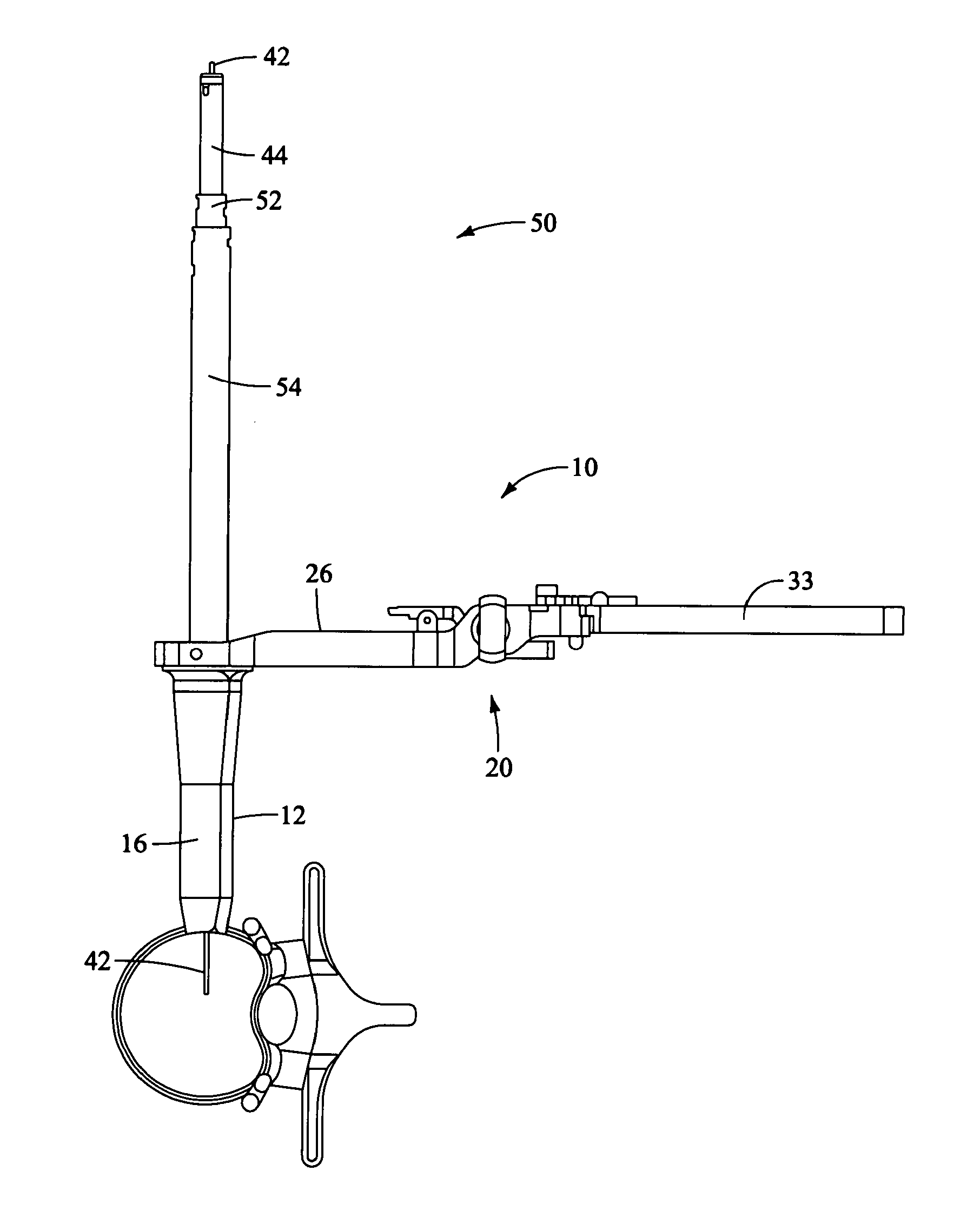
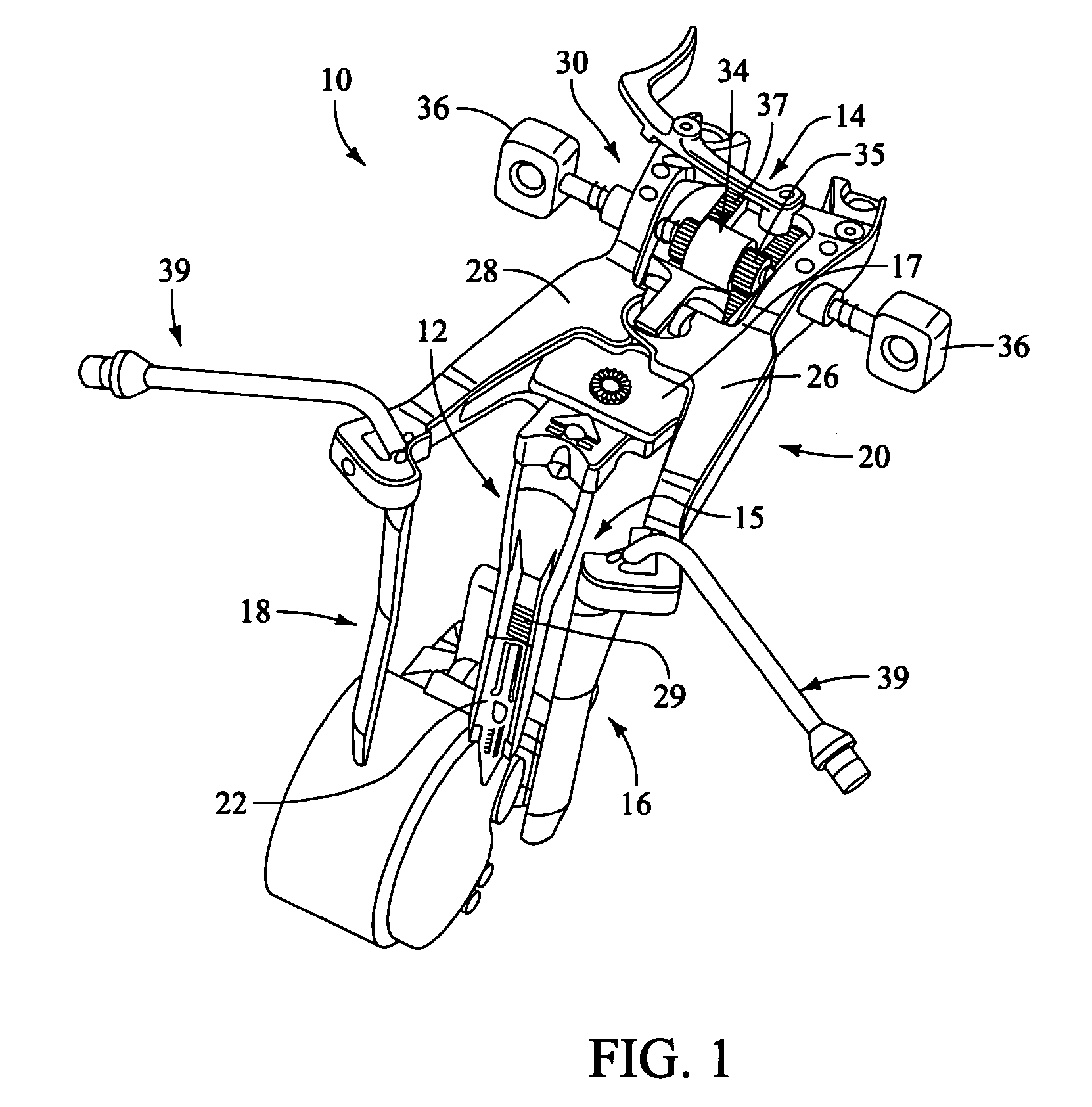
Retractor for use during retroperitoneal lateral insertion of spinal implants
InactiveUS20120010472A1Realize distributionMinimize traumaSurgerySpinal implantsSpinal columnIntervertebral spaces
A method is disclosed for introducing a spinal disc implant into an intervertebral space of a subject. The subject is placed in a lateral position, and the anterior face of the spinal disc intervertebral space is accessed, between the L5 and S1 vertebrae, from an anterior and lateral retroperitoneal approach. An operative corridor to the anterior face of the spinal disc space is established by introducing a retractor instrument anterolaterally to the spinal disc space between the anterior superior iliac spine and the anterior inferior iliac spine. The damaged spinal disc contents are removed from the intervertebral space through the operative corridor, and the implant is advanced into the intervertebral space at an oblique angle and pivoted to position the implant substantially laterally within the intervertebral space. Elongated retractor and insertion instruments, as well as a modified disc implant, are also disclosed for carrying out the method.
Owner:SPANN SCOTT
Minimally-invasive retroperitoneal lateral approach for spinal surgery
InactiveUS20120035730A1Realize distributionMinimize traumaSurgerySpinal implantsSpinal columnIntervertebral spaces
A method is disclosed for introducing a spinal disc implant into an intervertebral space of a subject. The subject is placed in a lateral position, and the anterior face of the spinal disc intervertebral space is accessed, between the L5 and S1 vertebrae, from an anterior and lateral retroperitoneal approach. An operative corridor to the anterior face of the spinal disc space is established by introducing a retractor instrument anterolaterally to the spinal disc space between the anterior superior iliac spine and the anterior inferior iliac spine. The damaged spinal disc contents are removed from the intervertebral space through the operative corridor, and the implant is advanced into the intervertebral space at an oblique angle and pivoted to position the implant substantially laterally within the intervertebral space. Elongated retractor and insertion instruments, as well as a modified disc implant, are also disclosed for carrying out the method.
Owner:PANTHEON SPINAL
Automated solution injection-discharge system and automated peritoneal dialysis system
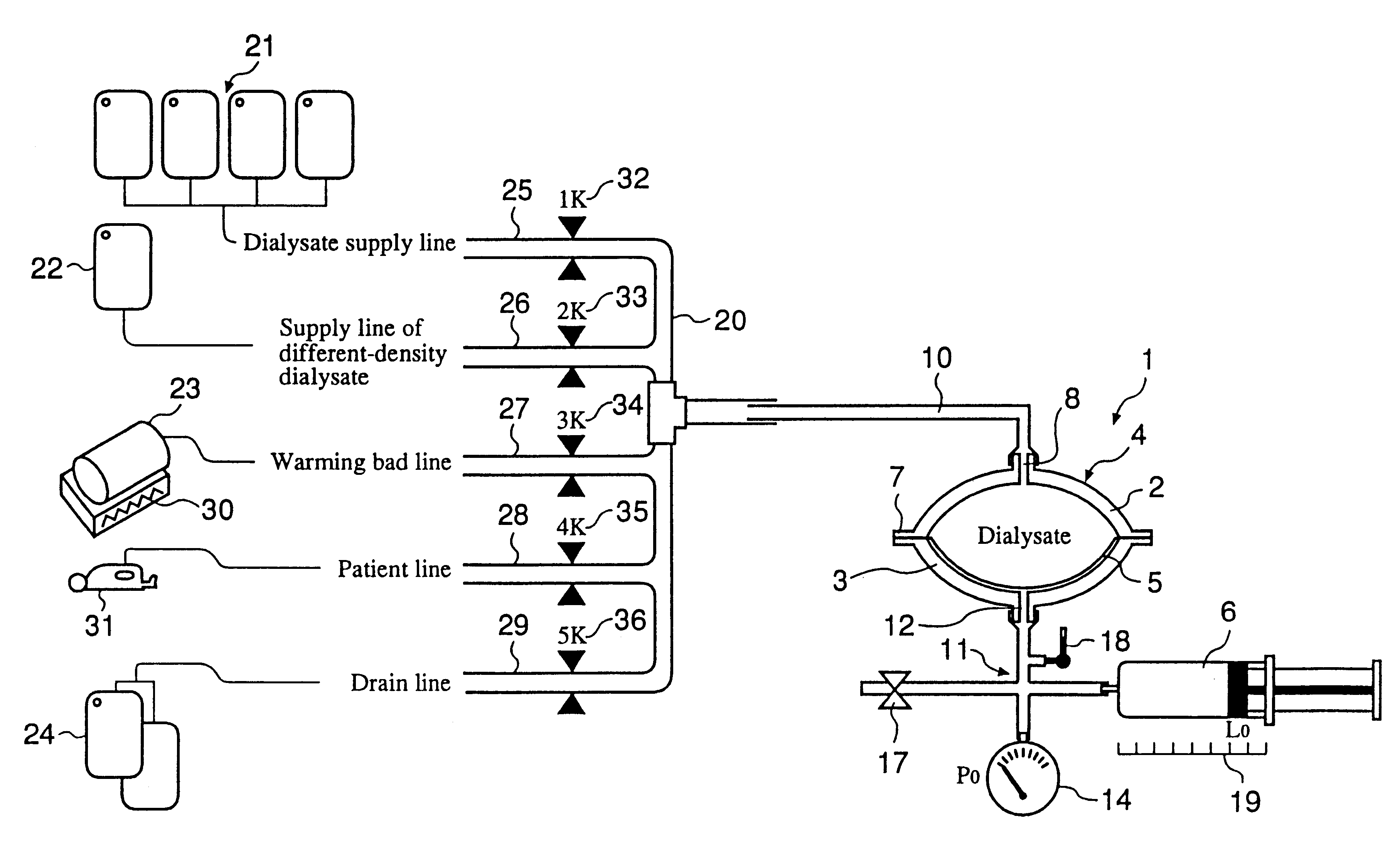
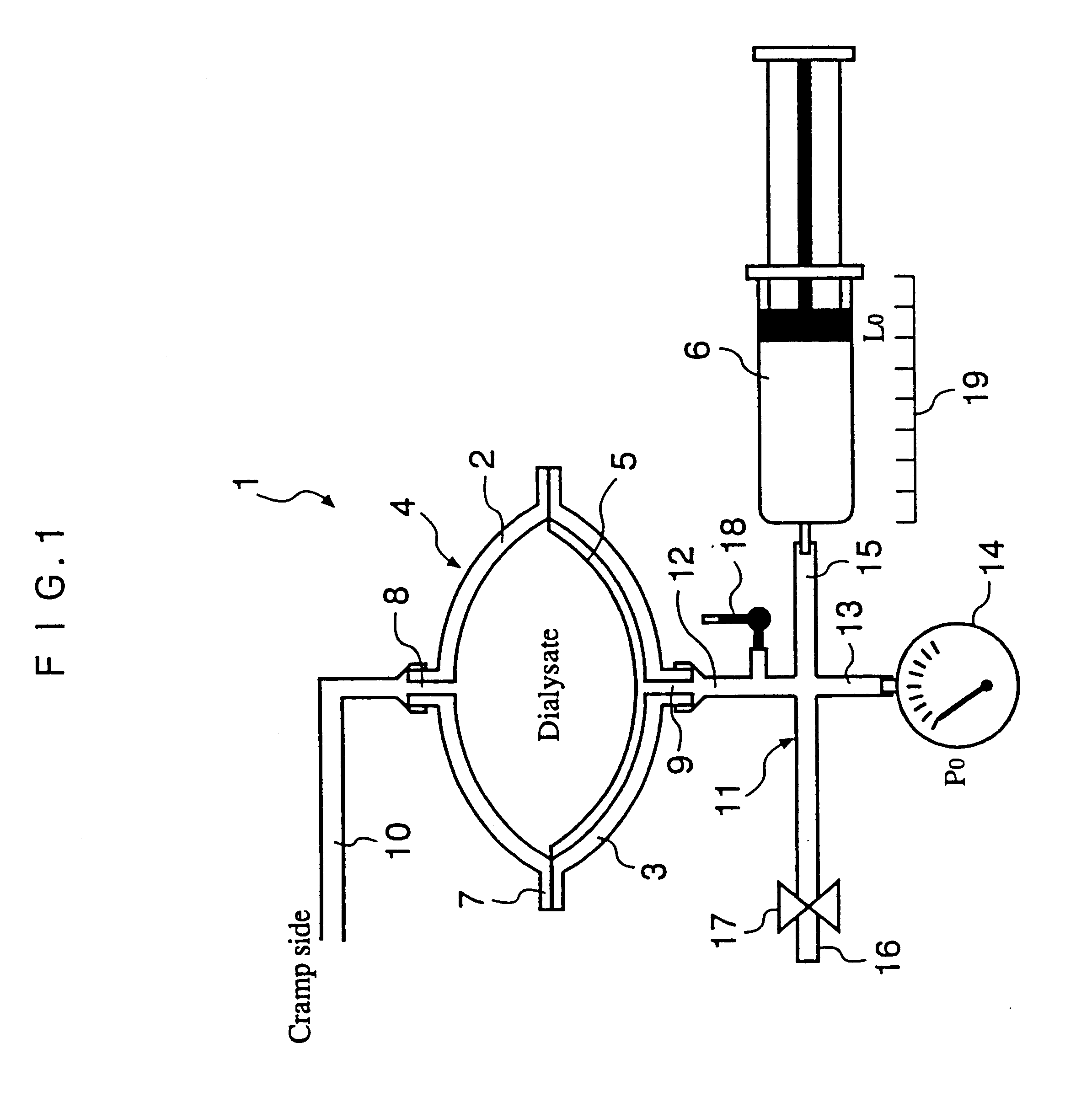
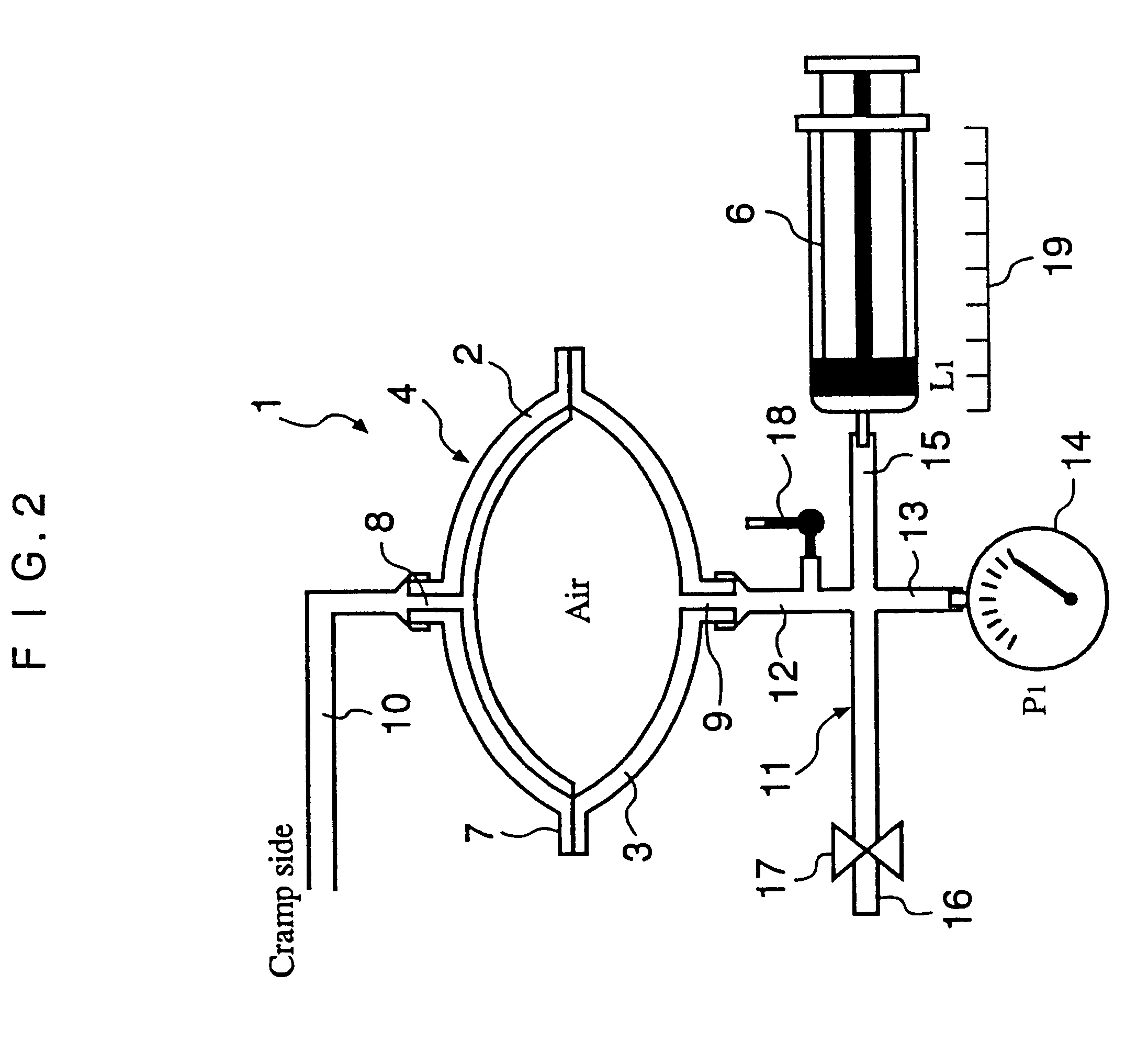
InactiveUS6491658B1Easy to controlOperational securityPeritoneal dialysisIntensive care medicineDialysis fluid
The automated solution injection-discharge system is used as an APDS system to supply and discharge a dialysate and a patient's drain. And an automated solution injection-discharge system provides free of contamination and operation mistakes, and can accurately control the injected dialysate volume and the discharged dwell solution volume even when a patient does not maintain a fixed posture while replacing the solution.
Owner:JMS CO LTD
Medical Treatment System and Methods Using a Plurality of Fluid Lines
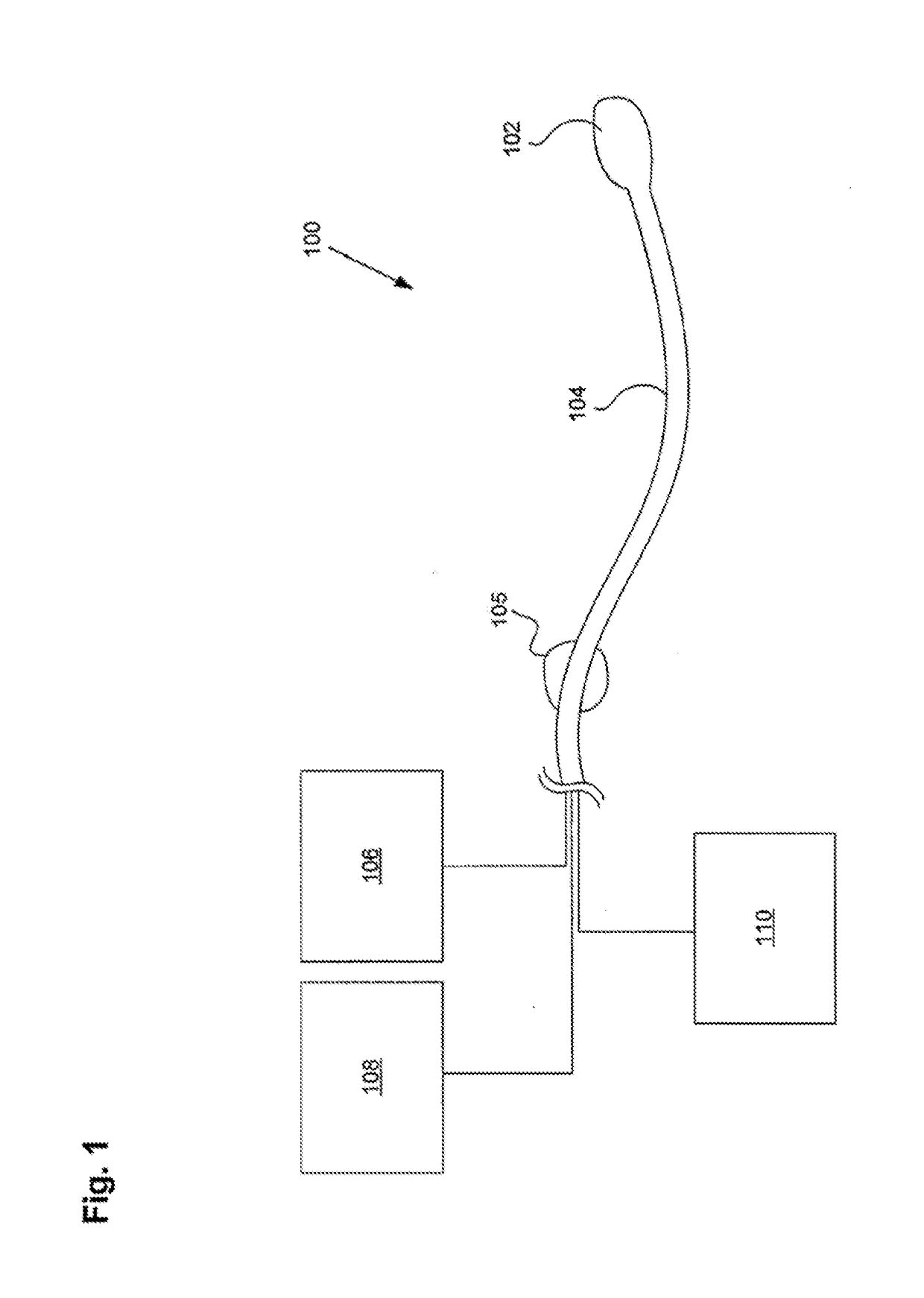
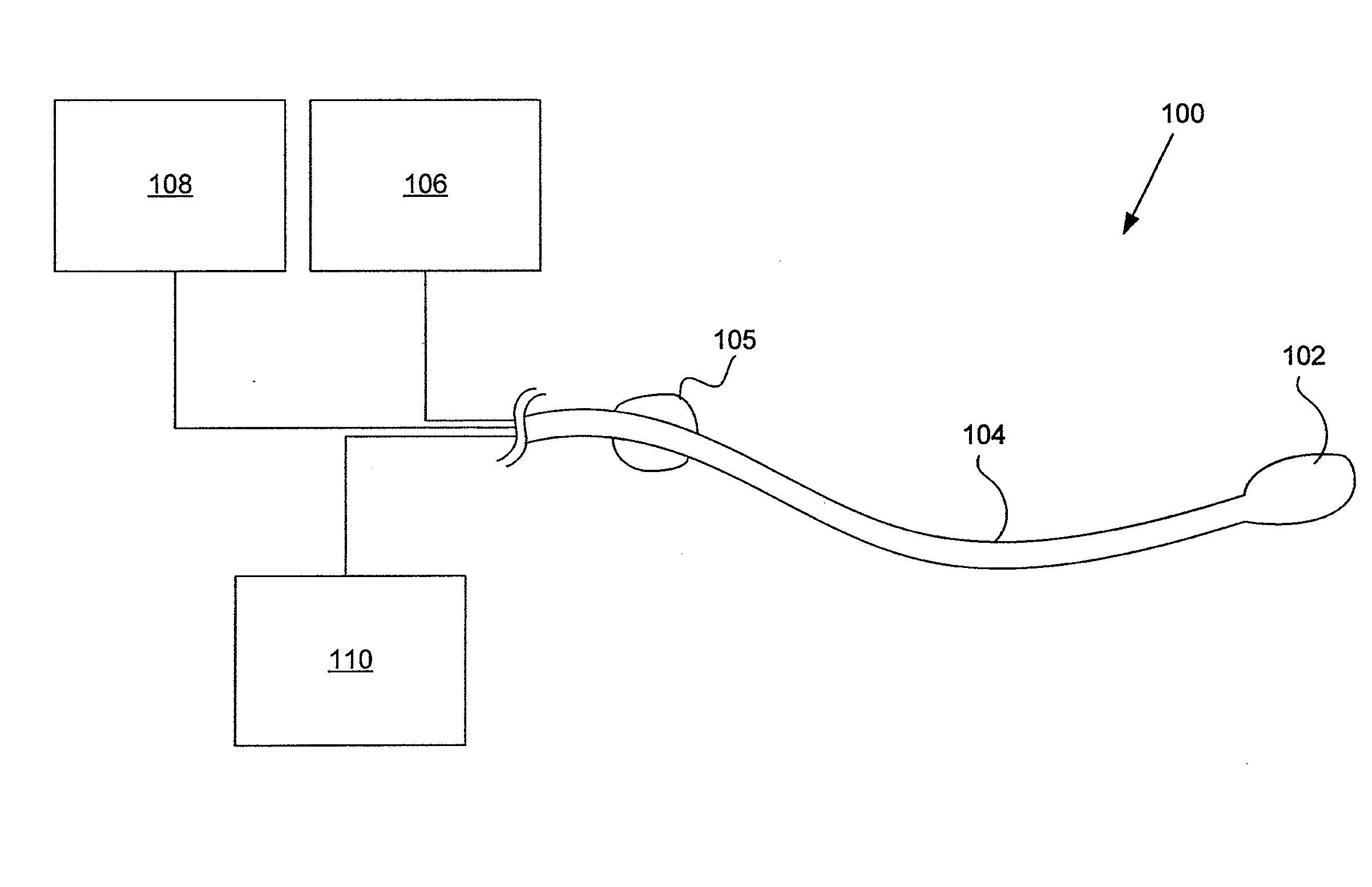
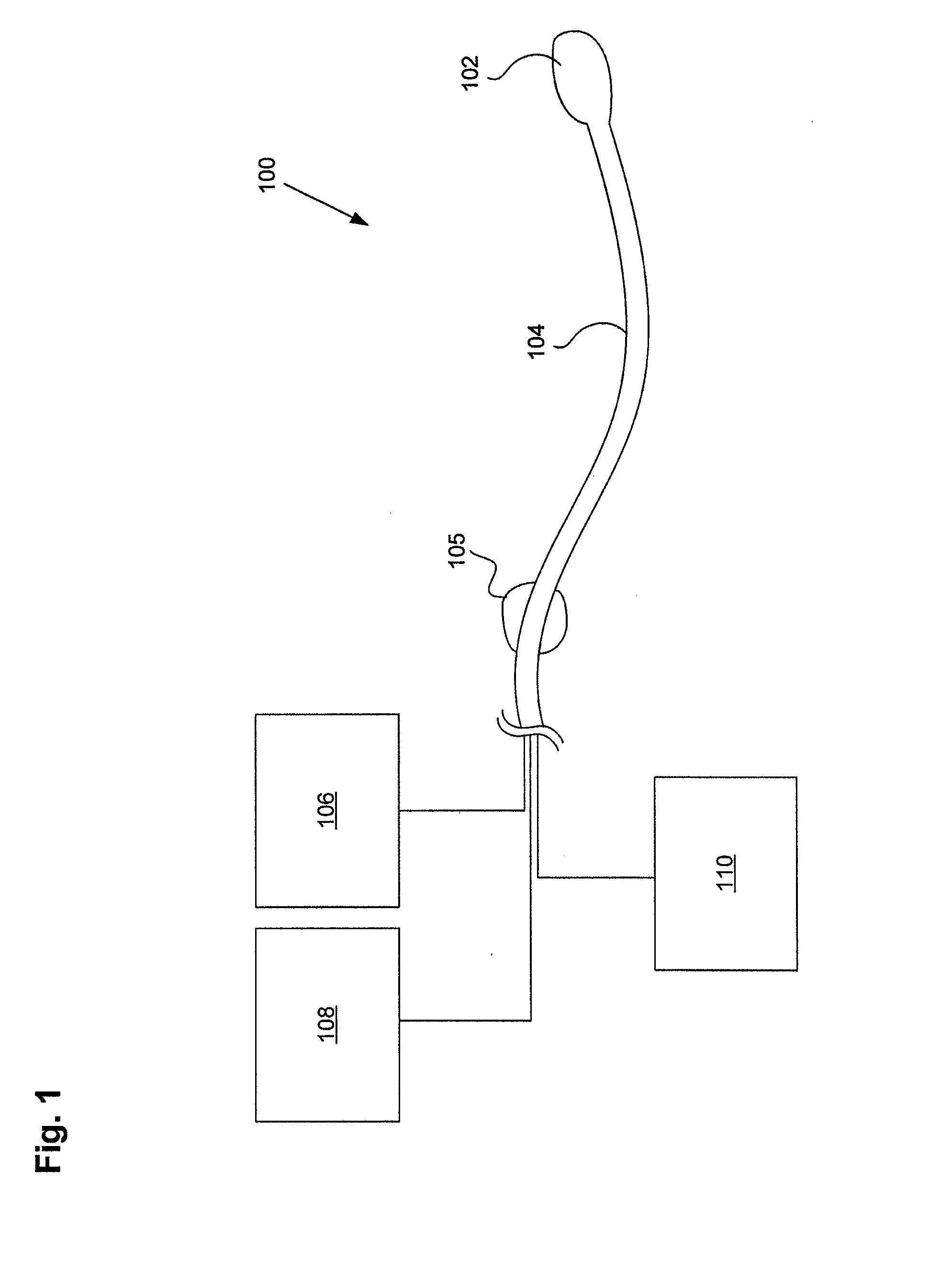
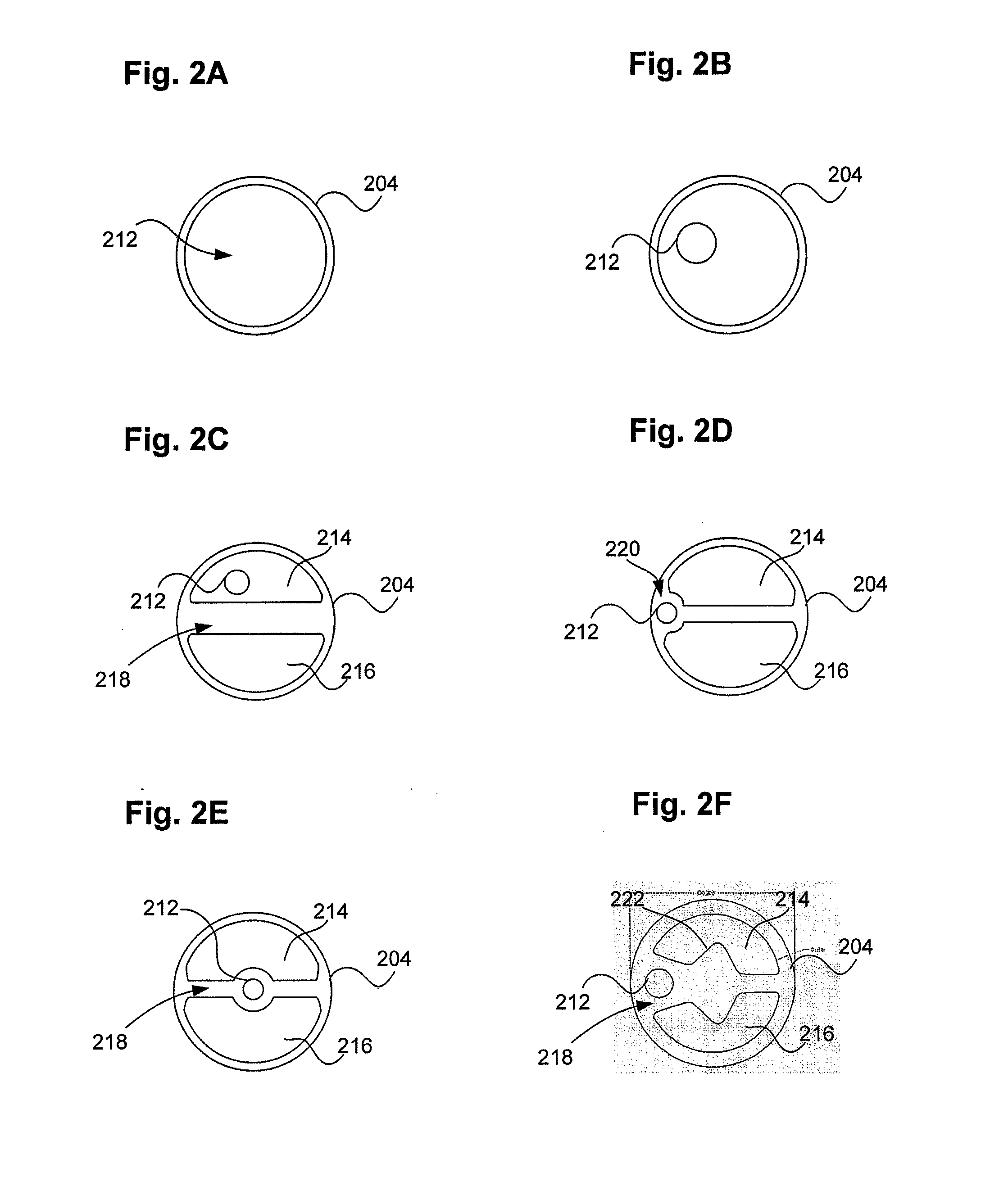
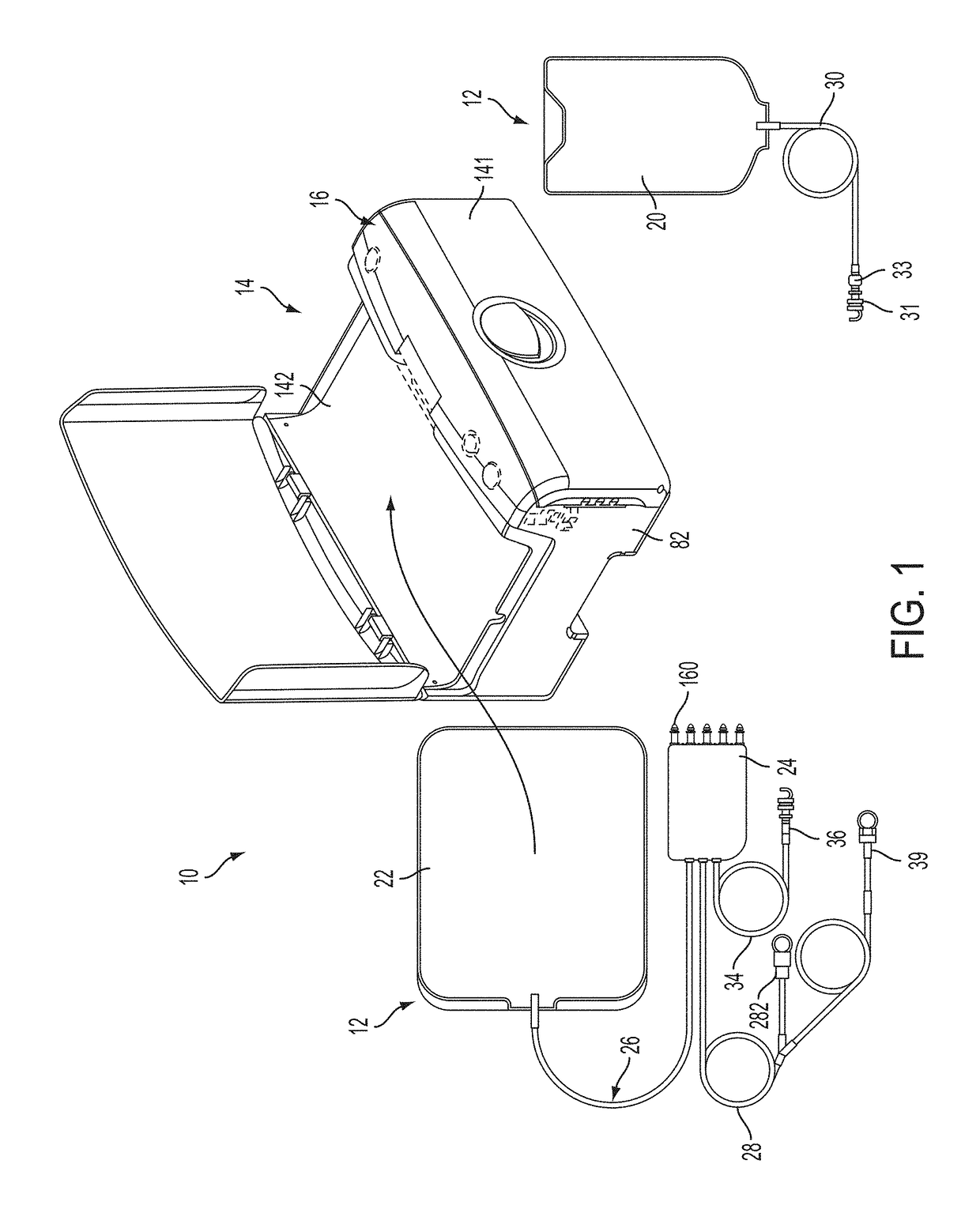
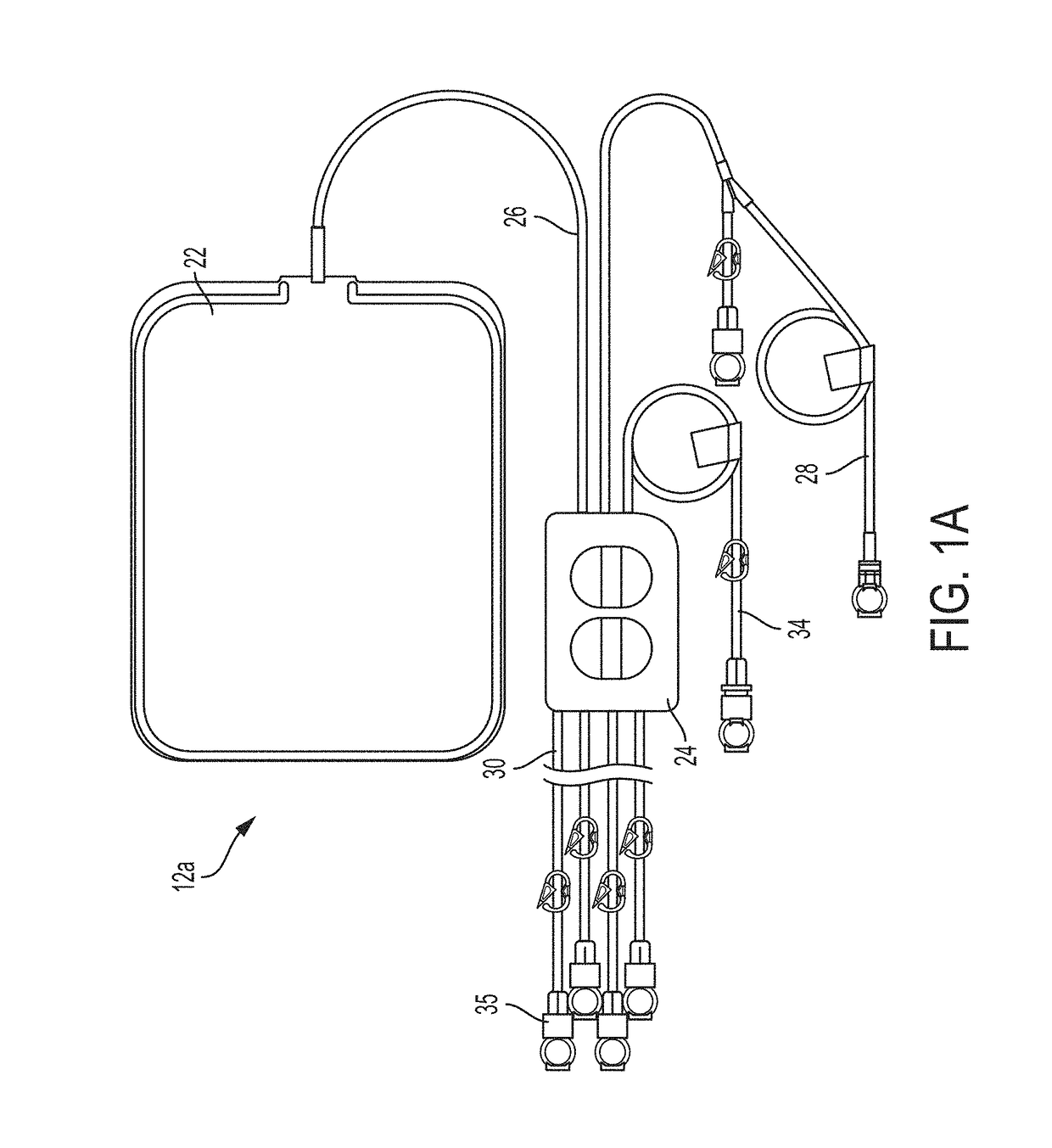
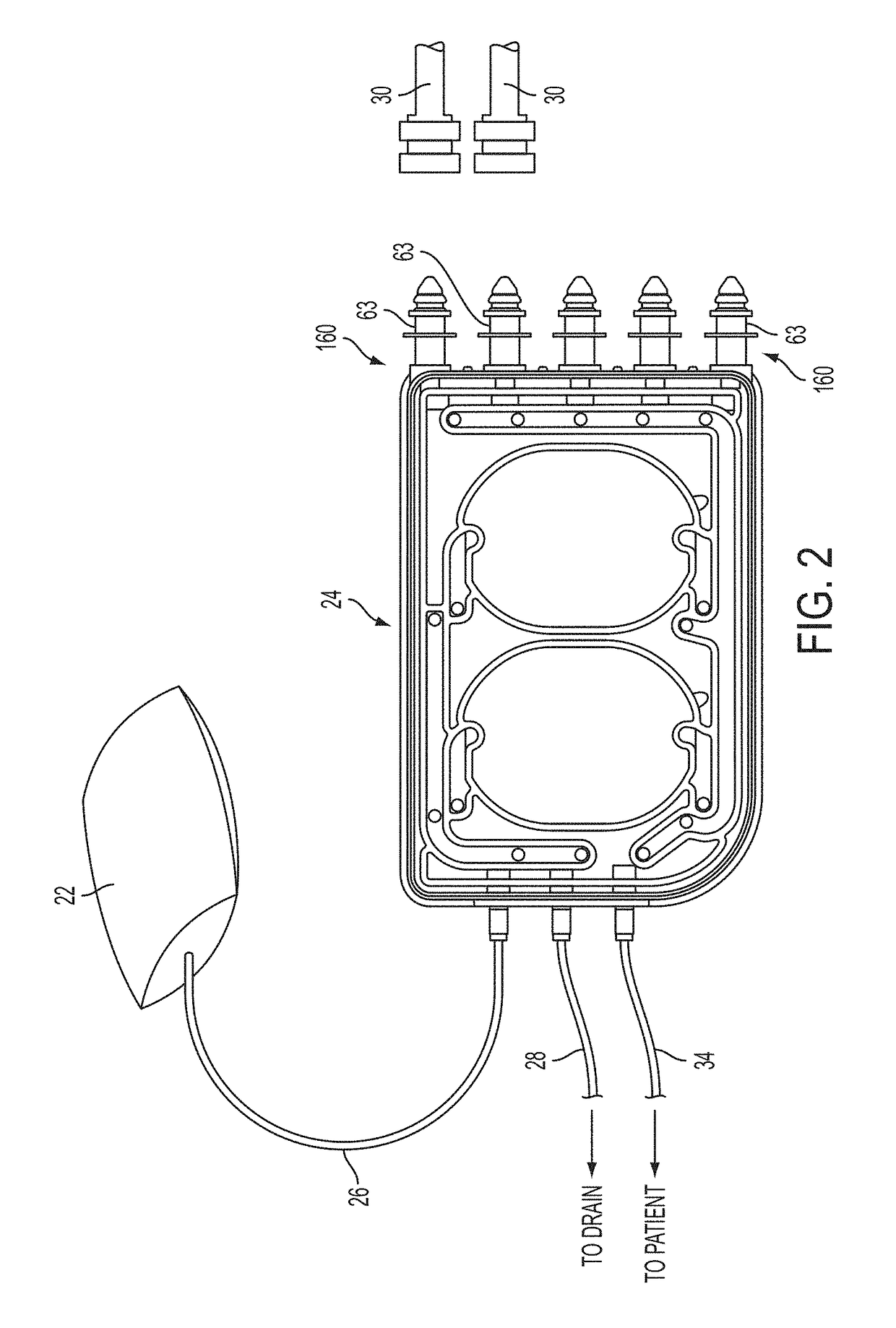
ActiveUS20160101227A1Reduce touchReduce leakage currentMedical devicesOptical detectionPeritoneal dialysisHemoperitoneum
Improvements in fluid volume measurement systems are disclosed for a pneumatically actuated diaphragm pump in general, and a peritoneal dialysis cycler using a pump cassette in particular. Pump fluid volume measurements are based on pressure measurements in a pump control chamber and a reference chamber in a two-chamber model, with different sections of the apparatus being modeled using a combination of adiabatic, isothermal and polytropic processes. Real time or instantaneous fluid flow measurements in a pump chamber of a diaphragm pump are also disclosed, in this case using a one-chamber ideal gas model and using a high speed processor to obtain and process pump control chamber pressures during fluid flow into or out of the pump chamber. Improved heater control circuitry is also disclosed, to provide added or redundant safety measures, or to reduce current leakage from a heater element during pulse width modulation control of the heater. Improvements are also disclosed in the application of negative pressure during a drain phase in peritoneal dialysis therapy, and to control the amount of intraperitoneal fluid accumulation during a therapy. Improvements in efficiency are also disclosed in the movement of fluid into and out of a two-pump cassette and heater bag of a peritoneal dialysis cycler, and in the synchronization of the operation of two or more pumps in a peritoneal dialysis cycler or other fluid handling devices using a multi-pump arrangement.
Owner:DEKA PROD LLP
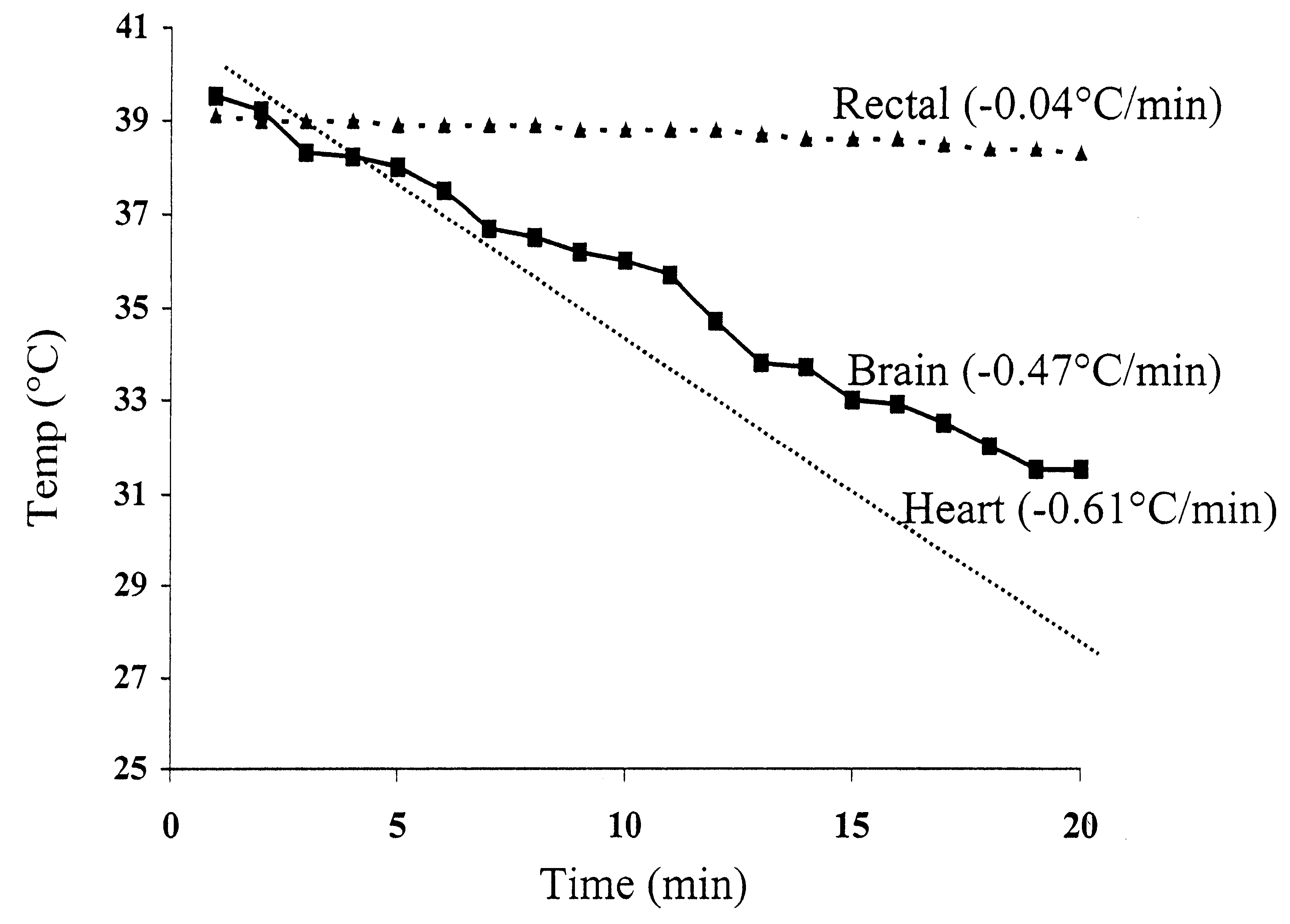
Method for inducing hypothermia
InactiveUS6962601B2Simple methodImprove the protective effectOther chemical processesLighting and heating apparatusParticulatesSlurry
Systems for phase-change particulate slurry cooling equipment and methods to induce hypothermia in a patient through internal and external cooling are provided. Subcutaneous, intravascular, intraperitoneal, gastrointestinal, and lung methods of cooling are carried out using saline ice slurries or other phase-change slurries compatible with human tissue. Perfluorocarbon slurries or other slurry types compatible with human tissue are used for pulmonary cooling. And traditional external cooling methods are improved by utilizing phase-change slurry materials in cooling caps and torso blankets.
Owner:UNIVERSITY OF CHICAGO +1
Method of retroperitoneal lateral insertion of spinal implants
ActiveUS20120010715A1Realize distributionMinimize traumaSurgerySpinal implantsSpinal columnIntervertebral spaces
A method is disclosed for introducing a spinal disc implant into an intervertebral space of a subject. The subject is placed in a lateral position, and the anterior face of the spinal disc intervertebral space is accessed, between the L5 and S1 vertebrae, from an anterior and lateral retroperitoneal approach. An operative corridor to the anterior face of the spinal disc space is established by introducing a retractor instrument anterolaterally to the spinal disc space between the anterior superior iliac spine and the anterior inferior iliac spine. The damaged spinal disc contents are removed from the intervertebral space through the operative corridor, and the implant is advanced into the intervertebral space at an oblique angle and pivoted to position the implant substantially laterally within the intervertebral space. Elongated retractor and insertion instruments, as well as a modified disc implant, are also disclosed for carrying out the method.
Owner:PANTHEON SPINAL
Expandable catheter system for peri-ostial injection and muscle and nerve fiber ablation
ActiveUS20160235464A1Improve control and treatmentTime efficient and safeElectrocardiographySurgical needlesCapital equipmentLeft atrium
At the present time, physicians often treat patients with atrial fibrillation (AF) using radiofrequency (RF) catheter systems to ablate conducting tissue in the wall of the Left Atrium of the heart around the ostium of the pulmonary veins. These systems are expensive and take time consuming to use. The present invention circular ablation system CAS includes a multiplicity of expandable needles that can be expanded around a central axis and positioned to inject a fluid like ethanol to ablate conductive tissue in a ring around the ostium of a pulmonary vein quickly and without the need for expensive capital equipment. The expansion of the needles is accomplished by self-expanding or balloon expandable structures. The invention includes centering means so that the needles will be situated in a pattern surrounding the outside of the ostium of a vein. Also included are members that limit the distance of penetration of the needles into the wall of the left atrium, or the aortic wall. The present invention also has an important application to ablate tissue around the ostium of one or both renal arteries, for the ablation of the sympathetic nerve fibers and / or other afferent or efferent nerves going to or from each kidney in order to treat hypertension.
Owner:ABLATIVE SOLUTIONS INC
Methods for producing high concentration lyophilized pharmaceutical formulations
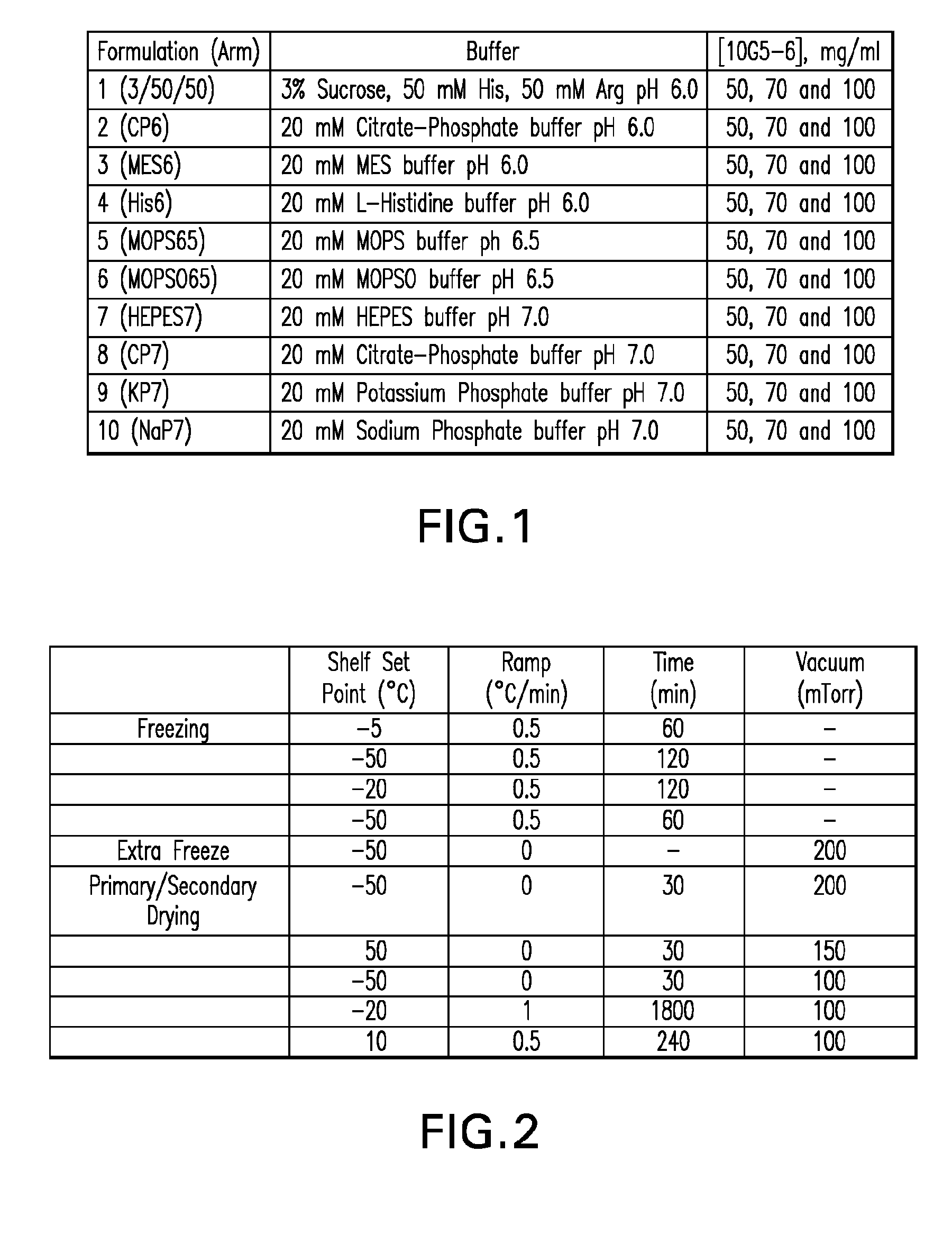
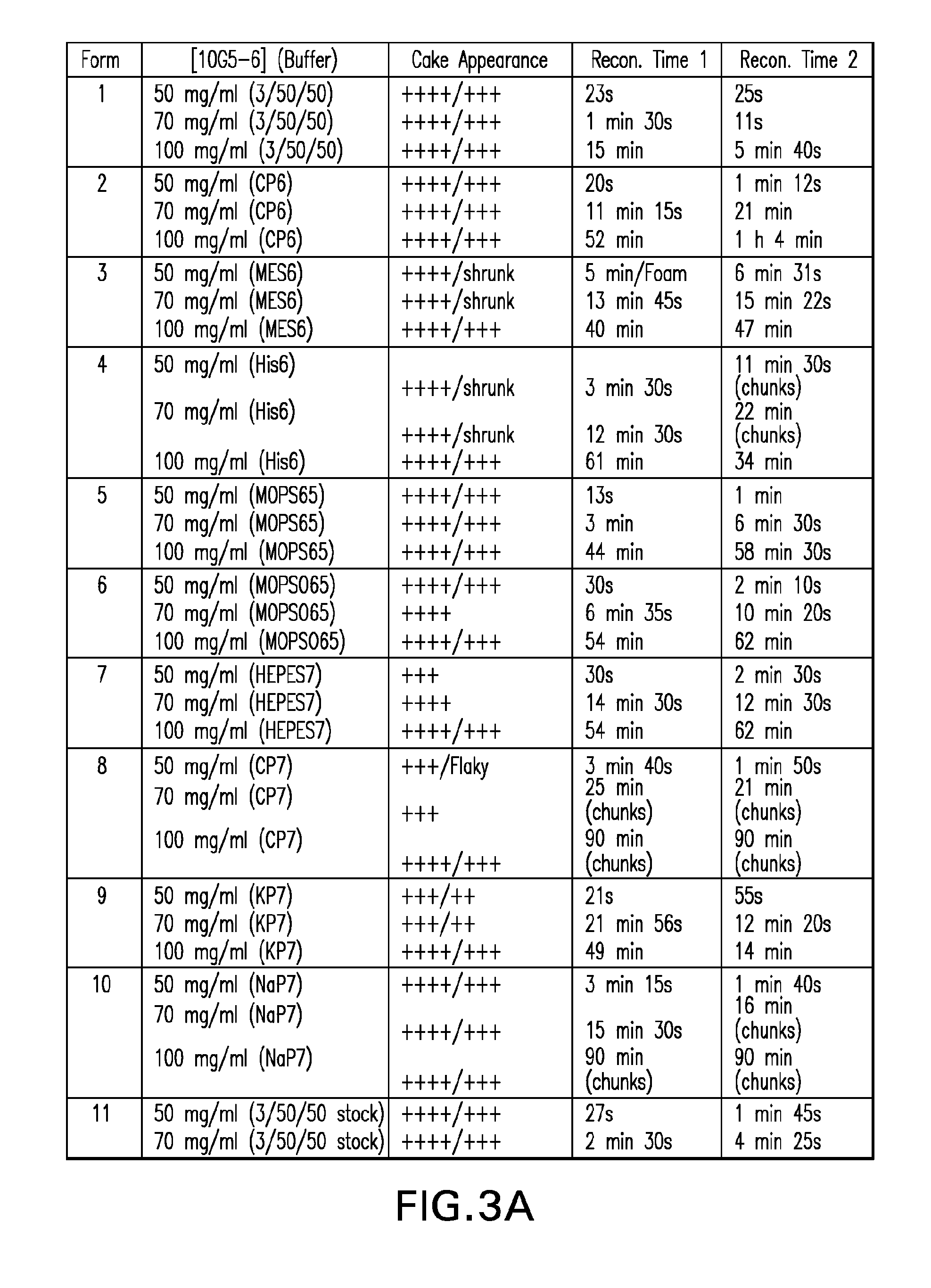
InactiveUS20120121580A1Contribute to physical structure and uniformity and stabilityPeptide/protein ingredientsImmunoglobulins against animals/humansHigh concentrationTherapeutic protein
The present invention relates to methods of producing lyophilized pharmaceutical compositions comprising a high concentration of therapeutic protein or antibody prior to lyophilization, wherein the lyophilized formulation can be reconstituted with a diluent in about 15 minutes or less. The invention also relates to the high concentration lyophilized formulations produced by the methods described herein. The lyophilized formulations produced by the methods of the invention are stable and are suitable for veterinary and human medical use and are suitable for modes of administration including oral, pulmonary and parenteral, such as intravenous, intramuscular, intraperitoneal, or subcutaneous injection. Also provided by the invention are high concentration pharmaceutical compositions that have long term stability and can be reconstituted, following lyophilization, in a short period of time, preferably 15 minutes or less.
Owner:MERCK SHARP & DOHME CORP
Method and Apparatus for Performing Retro Peritoneal Dissection
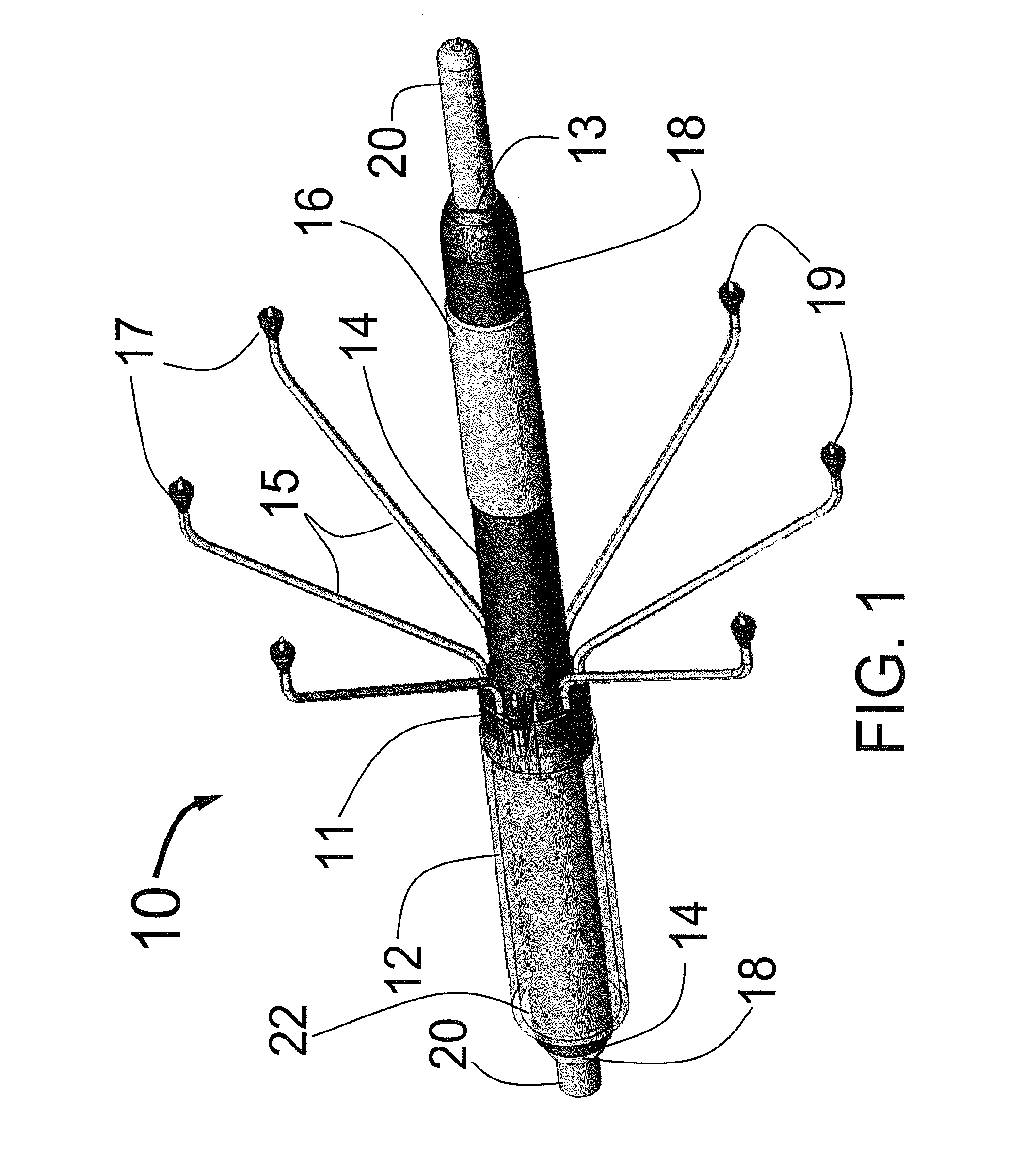
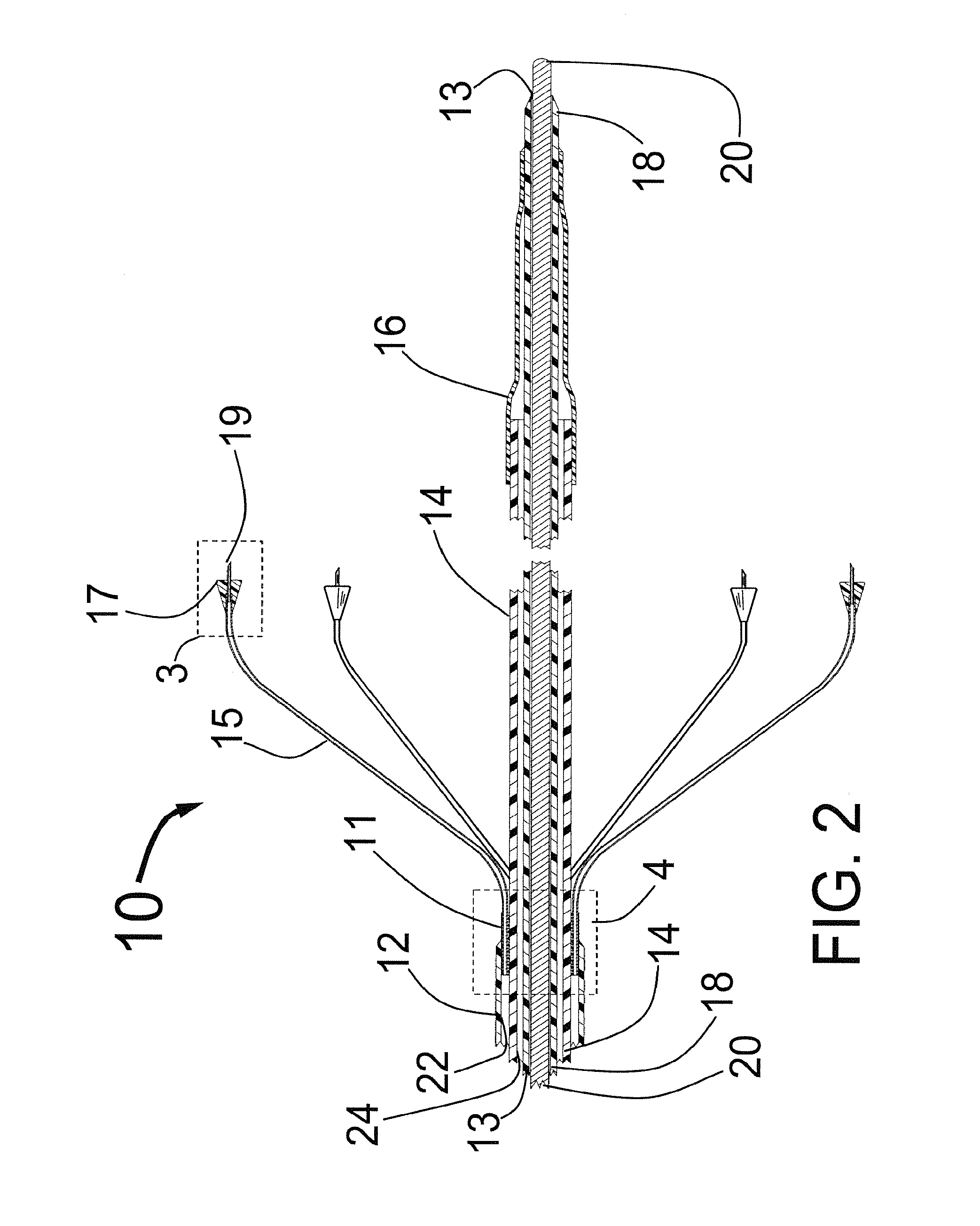
The foregoing application describes a system and method of performing a minimally invasive surgical operation. More specifically, the invention involves the use of disposable cannula and slender dilators of variable lengths, which incorporate a source of illumination to carry light to a surgical site and video capabilities for capturing and displaying images from aCMOS or CCD camera device. According to one embodiment, fiber optics run semi-circumferentially or along walls of the cannula / dilator and terminate at about a centimeter from the distal end of the cannula / dilator, thereby preventing illumination from “bottoming out” at the floor of the incision. According to one alternate embodiment, the light fibers may be fashioned in an annulus around one or more camera chips to provide illumination and video of the surgical site. In still another embodiment, the light fibers may be replaced by light emitting diodes in a more remote light source or alternatively at the distal-tip of the CMOS or CCD camera device.
Owner:KLEINER JEFFREY B
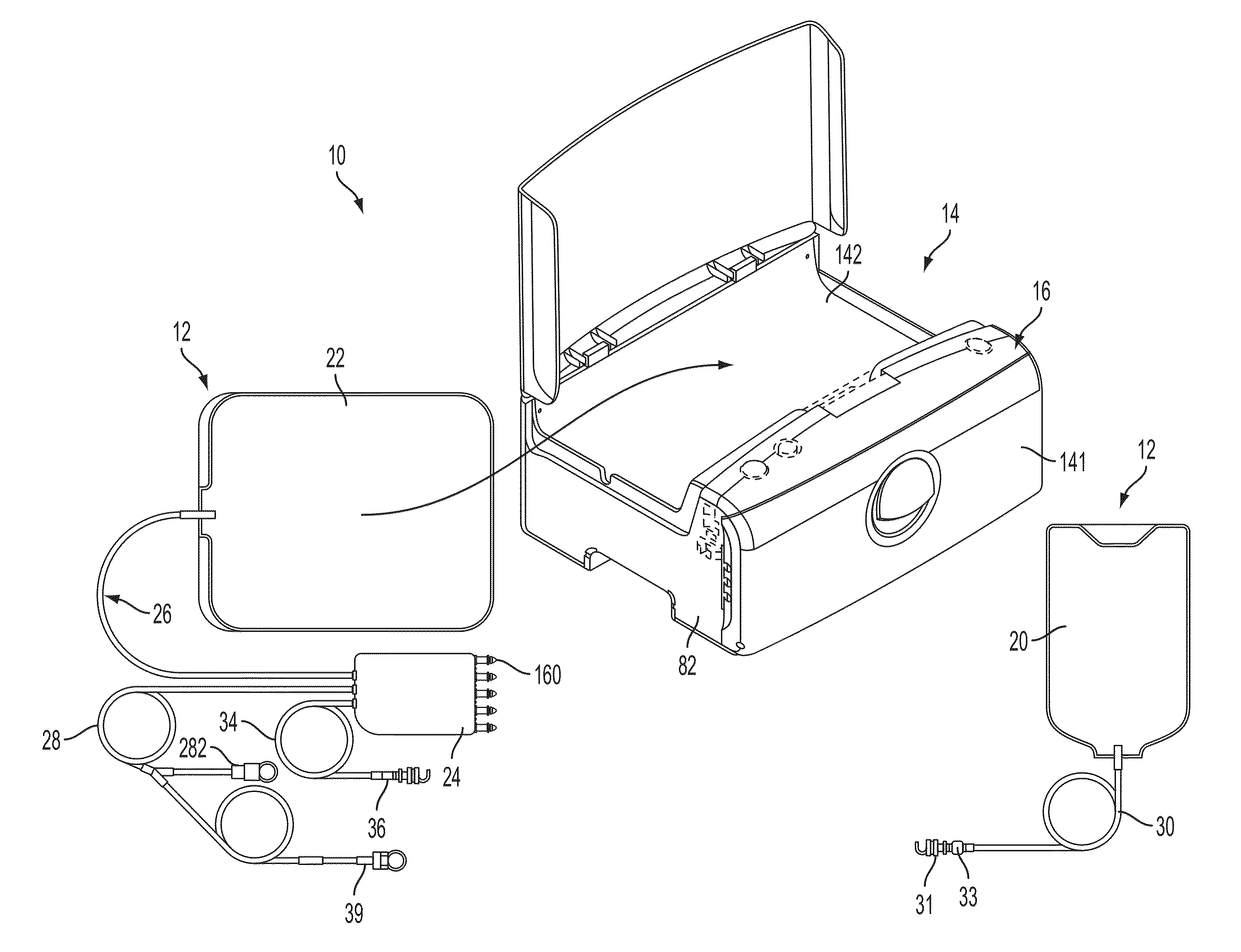
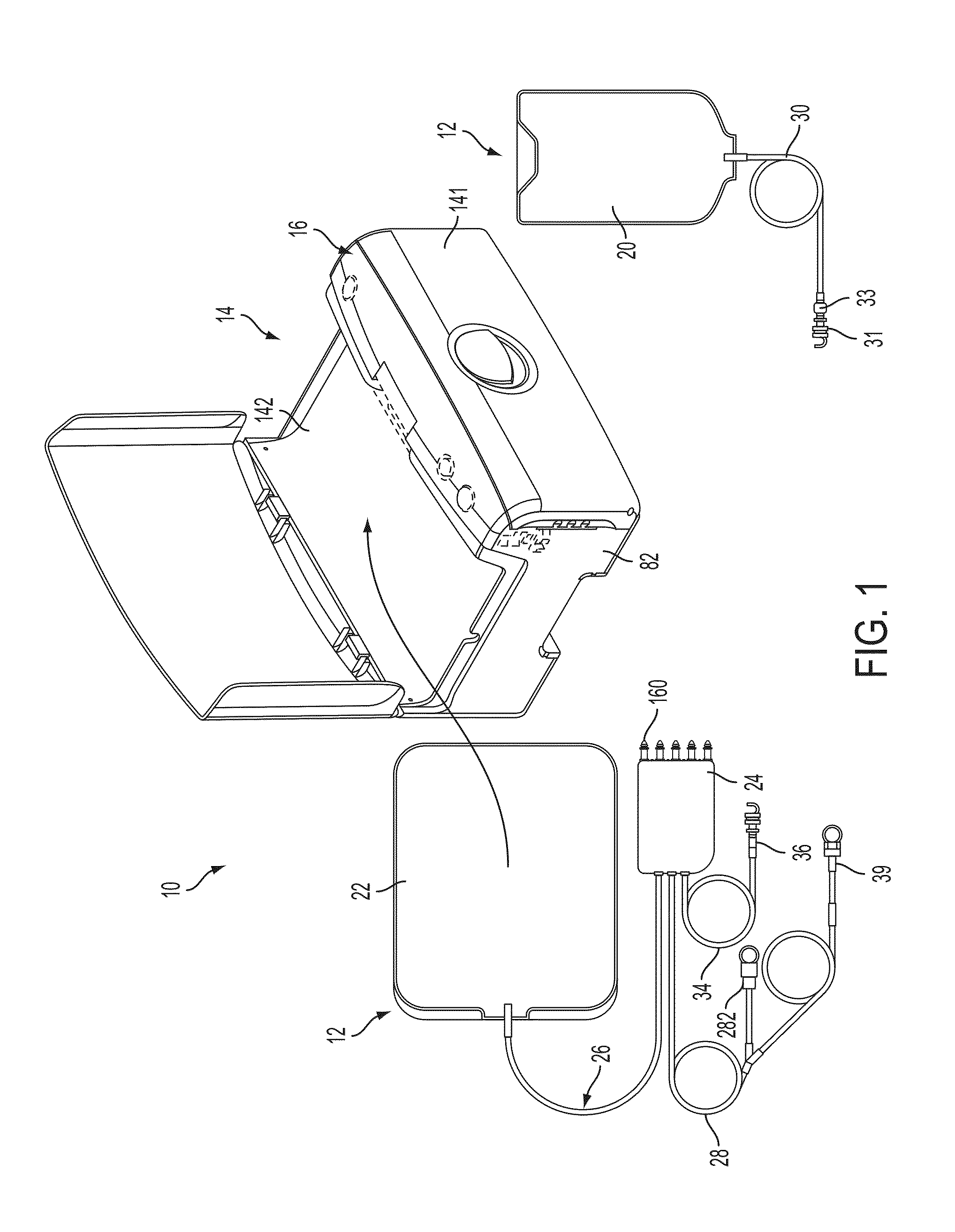
Patient treatment and monitoring systems and methods
ActiveUS8632485B2Facilitating regulatory complianceMechanical/radiation/invasive therapiesSolvent extractionLife qualityTreatment success
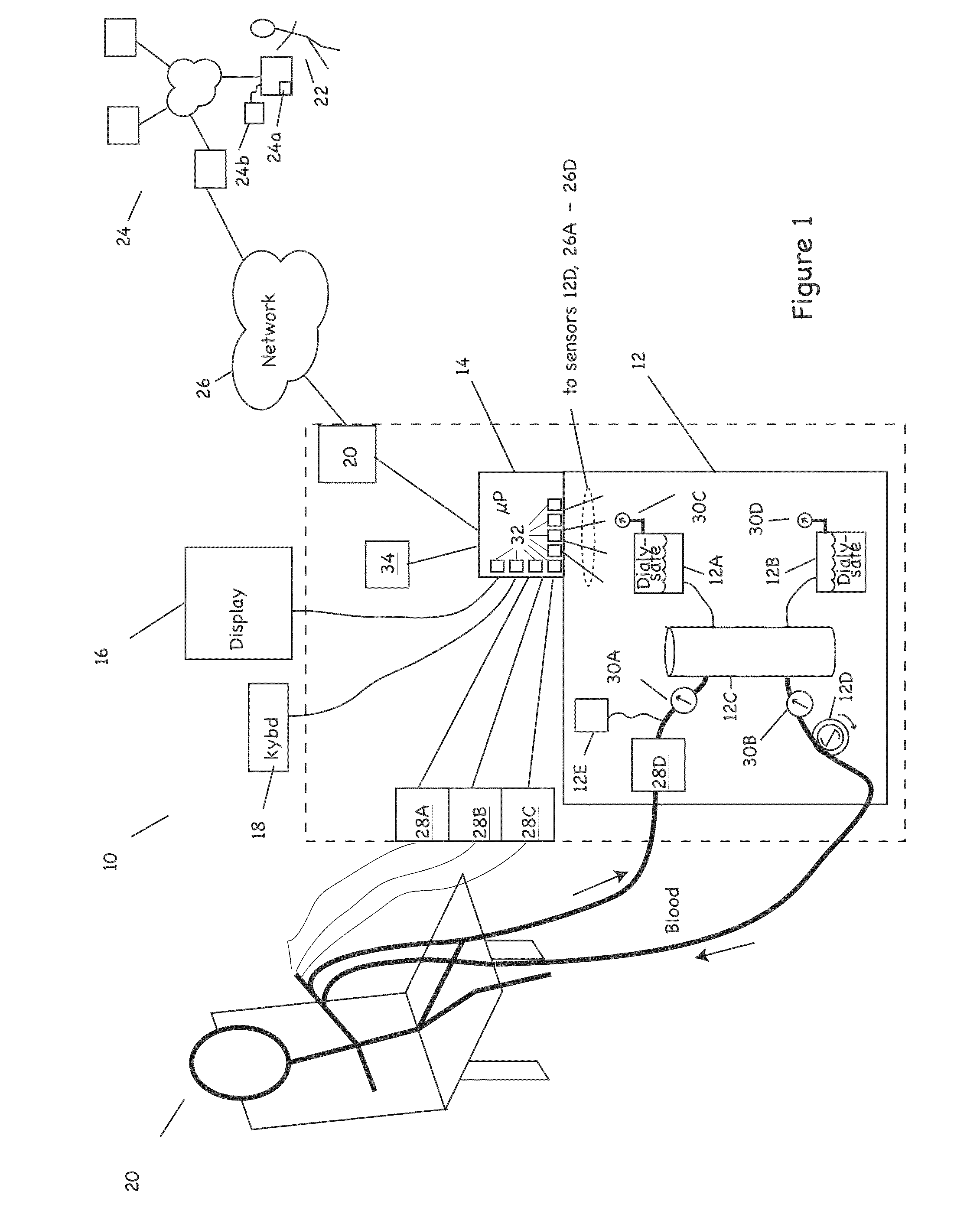
The invention provides apparatus and methods for home or other remote delivery of health care that gather subjective and objective measures of patient health and treatment, analyzing them (e.g., correlating them with one another and / or with norms) and reporting them to aid in on-going patient diagnosis and treatment (both on acute and chronic bases), as well as to aid physicians, nurses and other caregivers in decision support, monitoring treatment compliance, facilitating regulatory compliance, billing, and so forth. Thus, for example, in some aspects a health care delivery device comprising a medical treatment apparatus, such as a home hemodialysis or home peritoneal dialysis unit, that is coupled to a processor. The processor generates patient queries in connection with treatments rendered by the dialysis equipment (or other treatment apparatus). The queries are directed, at least in part, to subjective topics, such as the state of the patient's mental health and well being, quality of life, degrees of pain, views on success of therapy, and so forth. They are presented on an LCD screen or other output device coupled to the processor to elicit responses on a keyboard or other input device, also coupled to the processor.
Owner:FRESENIUS MEDICAL CARE HLDG INC
Embeddable multipurpose external hernia remedying slice
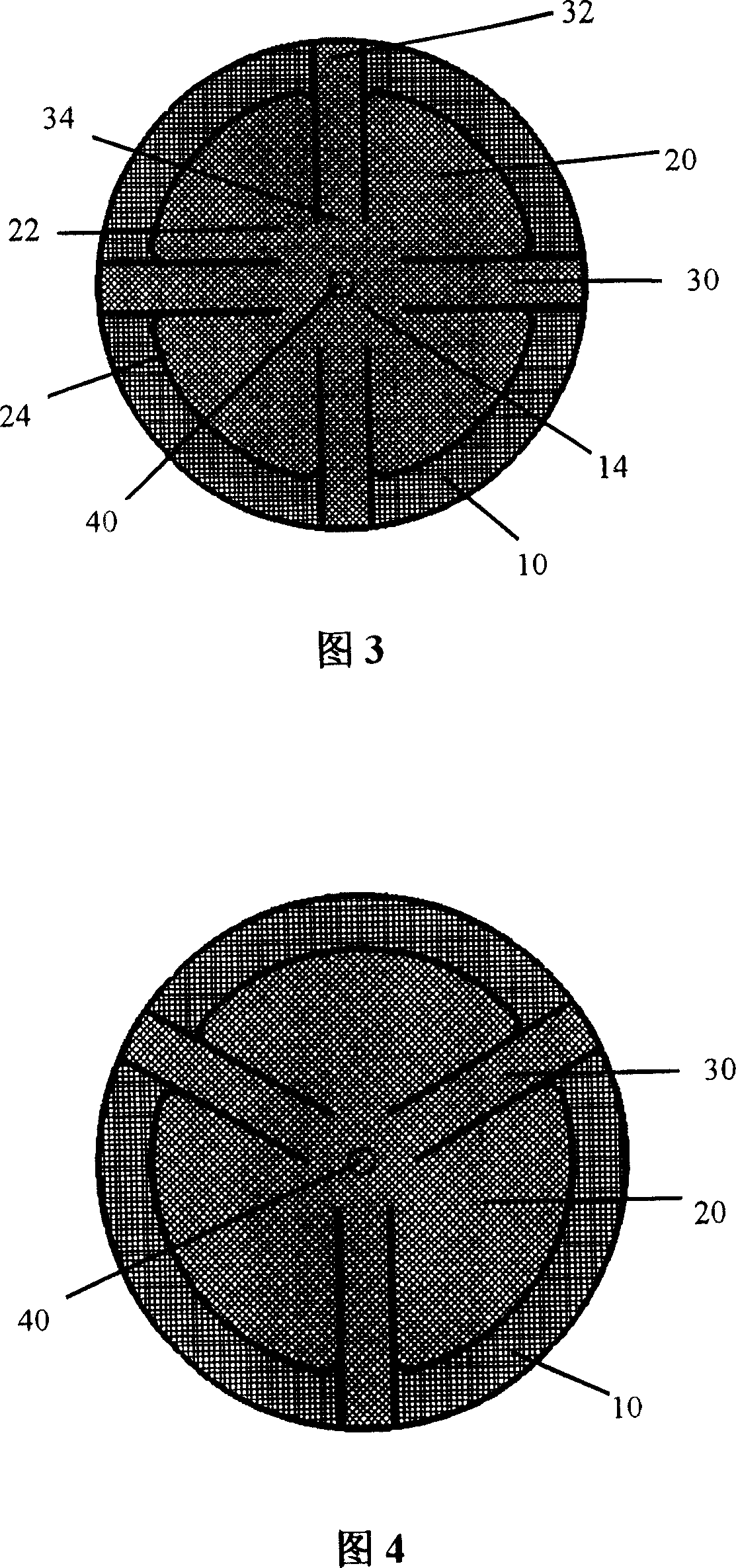
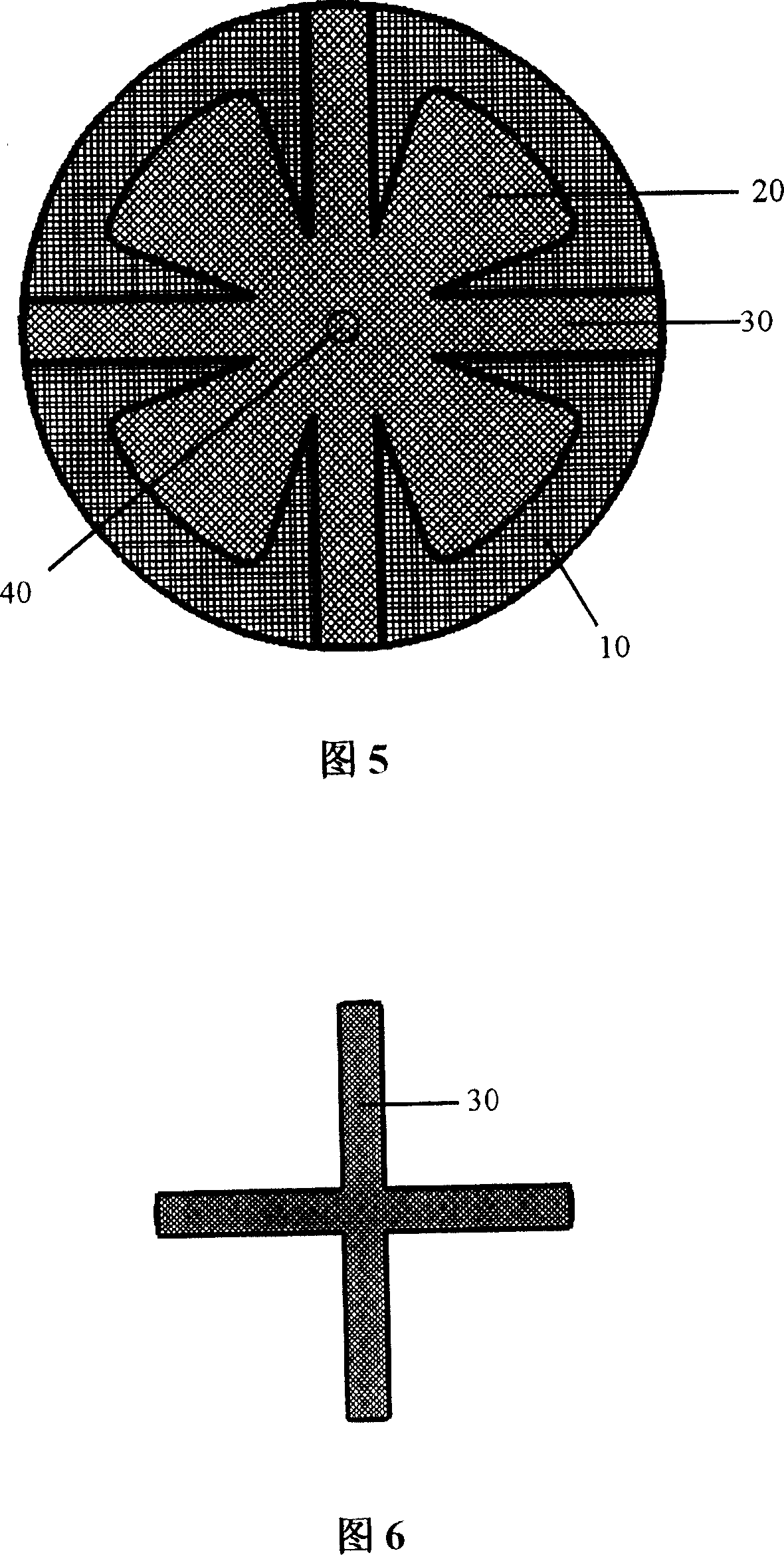
The present invention provides a multi-purpose repair patch used for hernia repair. The repair patch includes: a substrate which is formed by implantable mesh knitted fabrics, wherein, a plurality of small holes are arranged in thereof; and a plurality of petals which are formed by implantable mesh knitted fabrics, wherein, the petals are arranged on the upper surface of the substrate and are arranged around the center of the substrate, the distal ends of the pedals are free, and the proximal ends are fixed in a central region of the substrate. The repair patch can further include a plurality of reinforced ribs which are arranged on the upper surface of the substrate in a preferential implementation mode. The repair patch of the invention can not only adopt the embolization method, but can also adopt the preperitoneal space method for hernia repair, which has good clinical effect.
Owner:TRANSEASY MEDICAL TECH
Method and apparatus for anchoring cardiovascular implants
InactiveUS20090030435A1Reduce risk of migrationReduce intrusionSuture equipmentsStentsVascular implantTherapeutic Devices
Methods, devices and systems facilitate retention of a variety of therapeutic devices. Devices generally include an anchoring element, which has been designed to promote fibrotic ingrowth, and an anchored device, which has been designed to firmly engage the complementary region of the anchoring element. The anchoring element may be placed in a minimally invasive procedure temporally separated from the deployment of the anchored device. Once enough time has passed to ensure appropriate fixation of the anchoring element by tissue and cellular ingrowth at the site of placement, the anchored device may then be deployed during which it firmly engages the complementary region of the anchoring element. In this manner, a firm attachment to the implantation site may be made with a minimum of required hardware. Some embodiments are delivered through a delivery tube or catheter and while some embodiments may require laparoscopy or open surgery for one or more of the placement procedures. Some embodiments anchor devices within the cardiovascular tree while others may anchor devices within the gastrointestinal, peritoneal, pleural, pulmonary, urogynecologic, nasopharyngeal or dermatologic regions of the body. An alternative embodiment provides for the placement of the anchoring element and anchored device simultaneously, but allows for their removal separately. This embodiment allows the device, which may be placed only temporarily and be designed to be removed, to experience significant fibrotic ingrowth, but then to be easily detached from the ingrowth-anchored region to allow for simple and quick device removal.
Owner:THERANOVA LLC
Method of retroperitoneal lateral insertion of spinal implants
ActiveUS9451940B2Realize distributionMinimize traumaSurgeryJoint implantsSpinal columnIntervertebral spaces
A method is disclosed for introducing a spinal disc implant into an intervertebral space of a subject. The subject is placed in a lateral position, and the anterior face of the spinal disc intervertebral space is accessed, between the L5 and S1 vertebrae, from an anterior and lateral retroperitoneal approach. An operative corridor to the anterior face of the spinal disc space is established by introducing a retractor instrument anterolaterally to the spinal disc space between the anterior superior iliac spine and the anterior inferior iliac spine. The damaged spinal disc contents are removed from the intervertebral space through the operative corridor, and the implant is advanced into the intervertebral space at an oblique angle and pivoted to position the implant substantially laterally within the intervertebral space. Elongated retractor and insertion instruments, as well as a modified disc implant, are also disclosed for carrying out the method.
Owner:PANTHEON SPINAL
Method for inducing hypothermia
InactiveUS7422601B2Rapid and safeStart fastLighting and heating apparatusIce productionParticulatesSlurry
Systems for phase-change particulate slurry cooling equipment and methods to induce hypothermia in a patient through internal and external cooling are provided. Subcutaneous, intravascular, intraperitoneal, gastrointestinal, and lung methods of cooling are carried out using saline ice slurries or other phase-change slurries compatible with human tissue. Perfluorocarbon slurries or other slurry types compatible with human tissue are used for pulmonary cooling. And traditional external cooling methods are improved by utilizing phase-change slurry materials in cooling caps and torso blankets.
Owner:UNIVERSITY OF CHICAGO
Method and apparatus for locating and tracking persons
The invention relates to a method and apparatus for locating and tracking persons by use of an implantable device. The described invention is an implant able device composed of biocompatible materials in all areas where contact with organic tissue occurs. The gross anatomic siting of the device includes any limb, the torso, including back and perineum, the neck, and the head. The surgical anatomic siting of the device includes: (1) Supramuscular: for example, deep to the epidermis, dermis, and subcutaneous fat, on or attached to muscle and / or muscle fascia. Such a location is currently used for implantation of commercially available buried intravenous access ports, which are positioned on, and attached to, the pectoralis major muscle fascia; (2) Intramuscular: for example, within or between the muscles of a limb; (3) Submuscular: for example, deep to a large muscle. Such a location is currently used for implantation of commercially available artificial urethral and anal sphincter reservoirs, which are positioned deep to the rectus abdominus muscles, within the pre-peritoneal Space of Retzius; (4) Intraluminal: for example, within the lumen of an organ which has a naturally occurring orifice. Such a location is currently used for implantation of commercially available ingested video endoscopy capsule devices, i.e. gastrointestinal tract lumen, and intrauterine contraceptive devices, i.e. uterus lumen; and (5) Intracavitary: for example, intrathoracic or intraperitoneal. Such an intraperitoneal location is currently used for implantation of commercially available intraperitoneal dialysis catheters.
Owner:PERSEPHONE
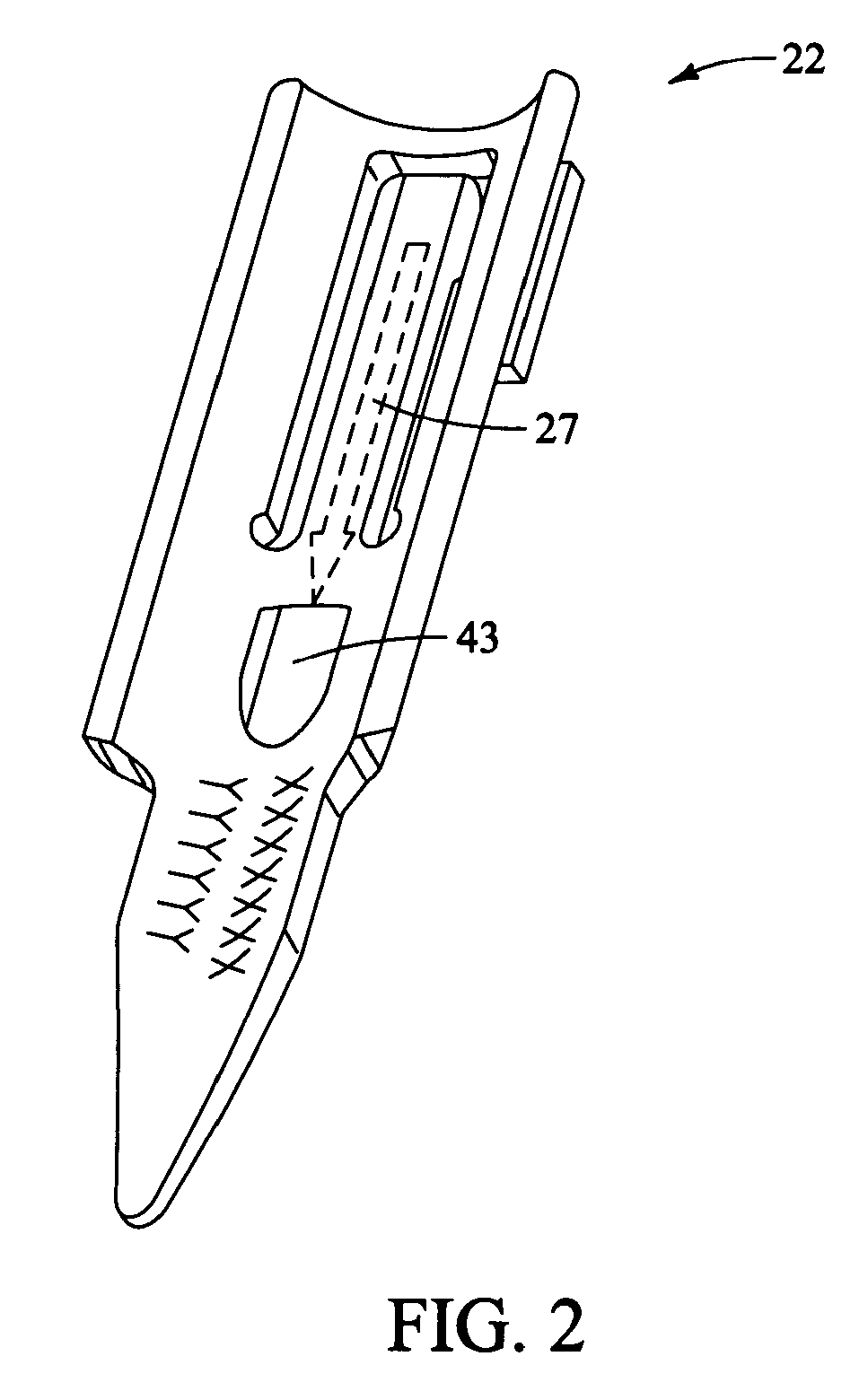
Stapling device for closure of deep tissue
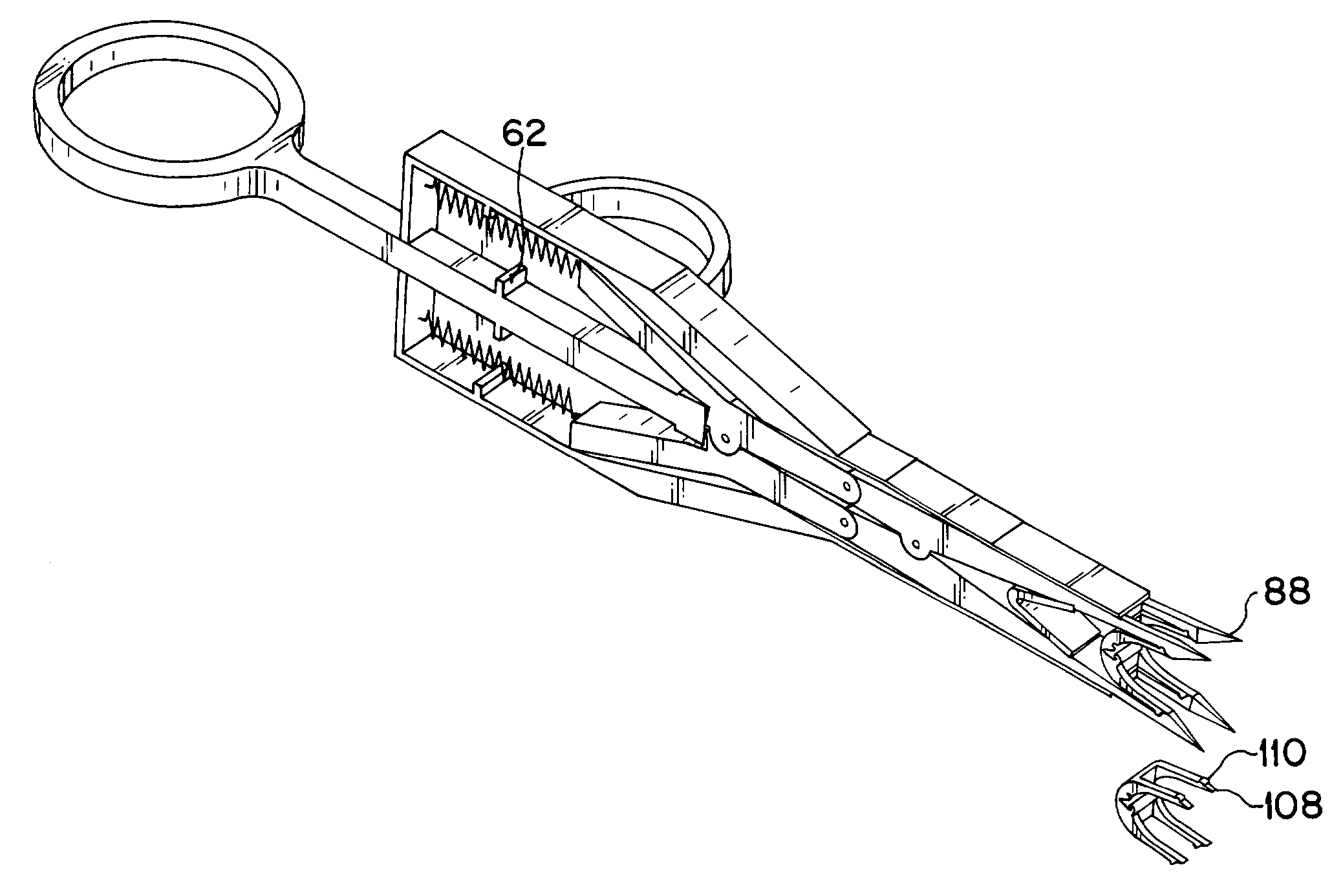
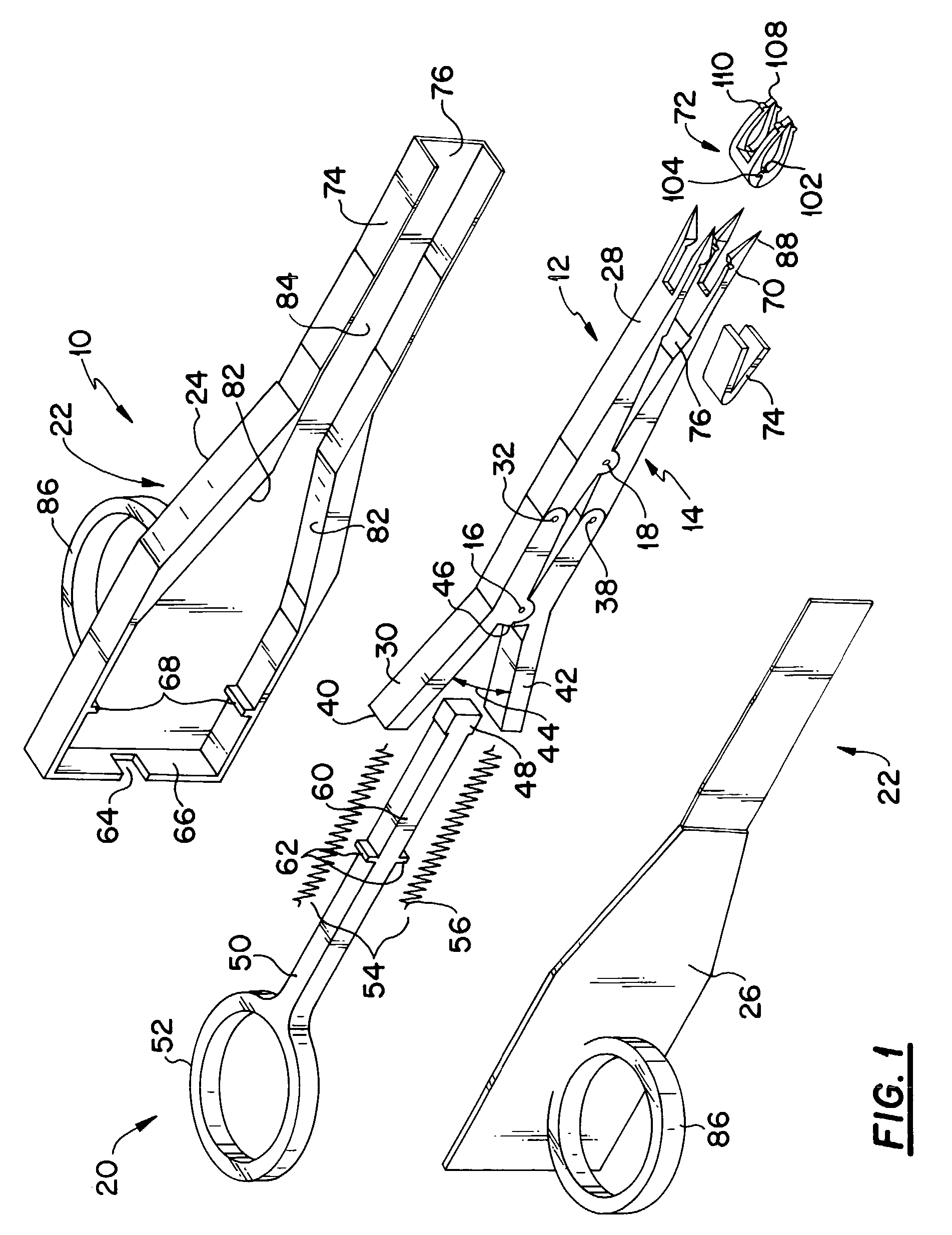
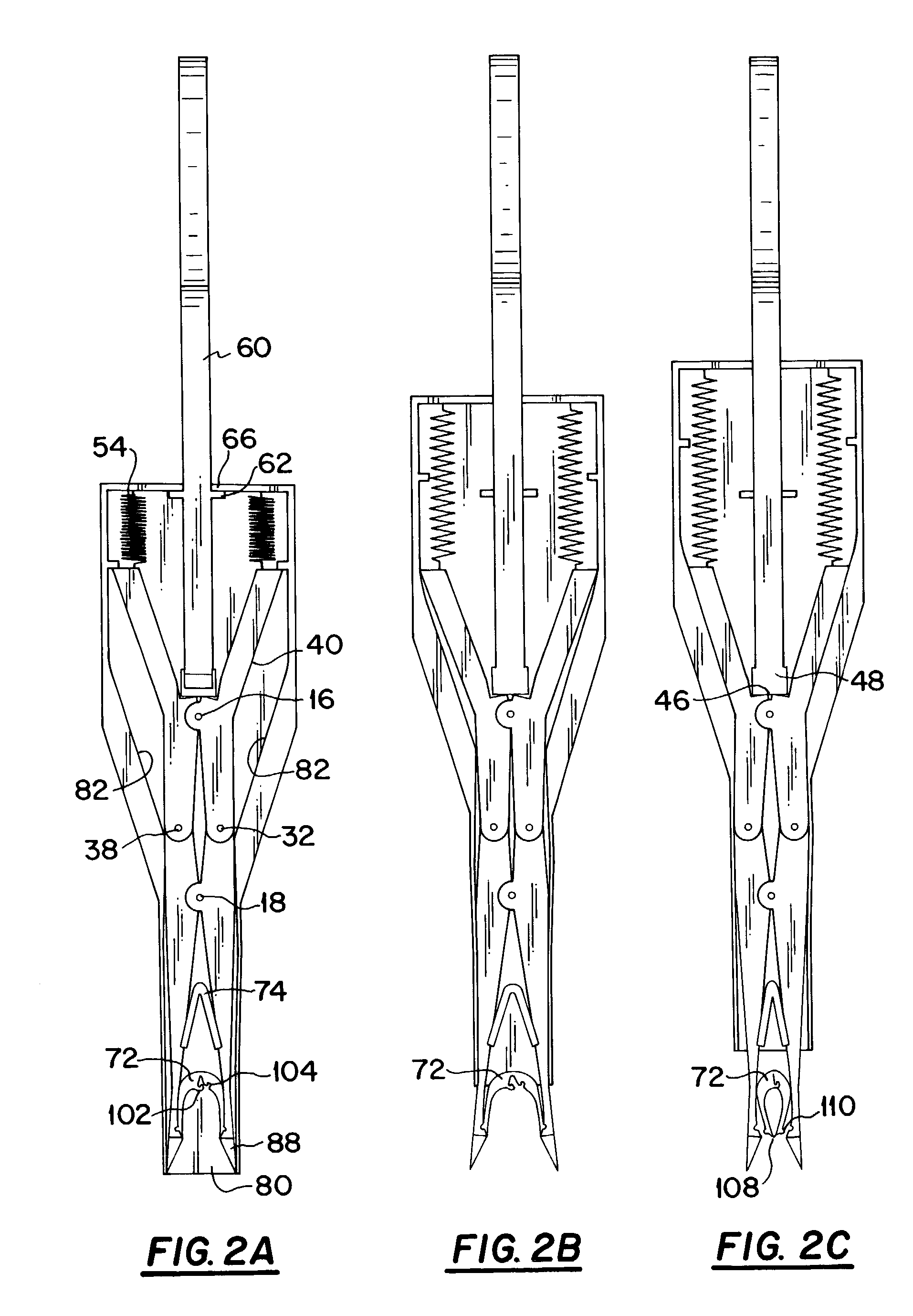
A mechanical stapling device for fastening deep tissue during the closing of peritoneal side of a stab wound, which is associated with a laparoscopic surgical procedure, is provided. Also provided is a unique staple for use with the stapling device of the present invention. A method of using the device and staple of the present invention is also provided.
Owner:GE SURGICAL
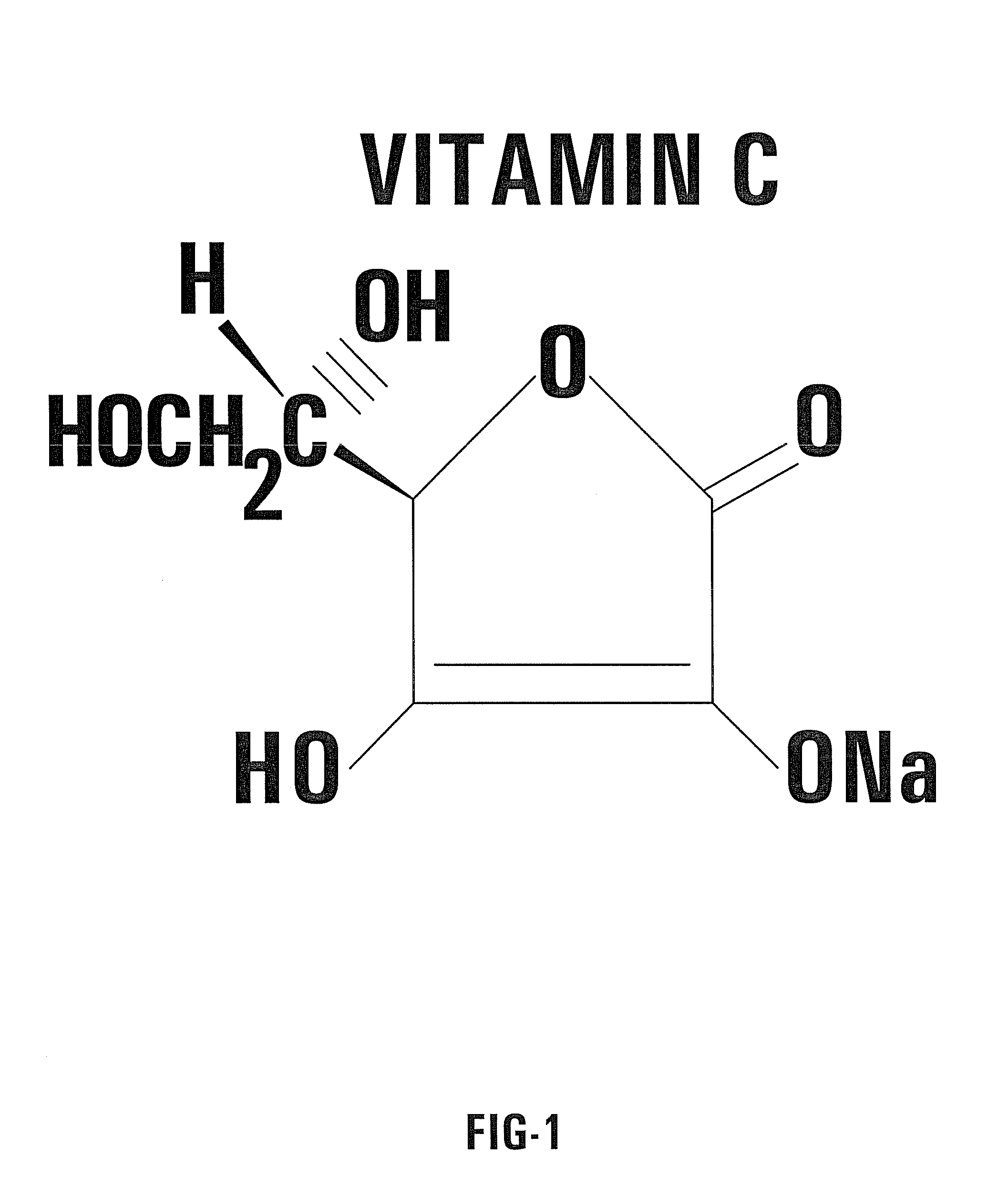
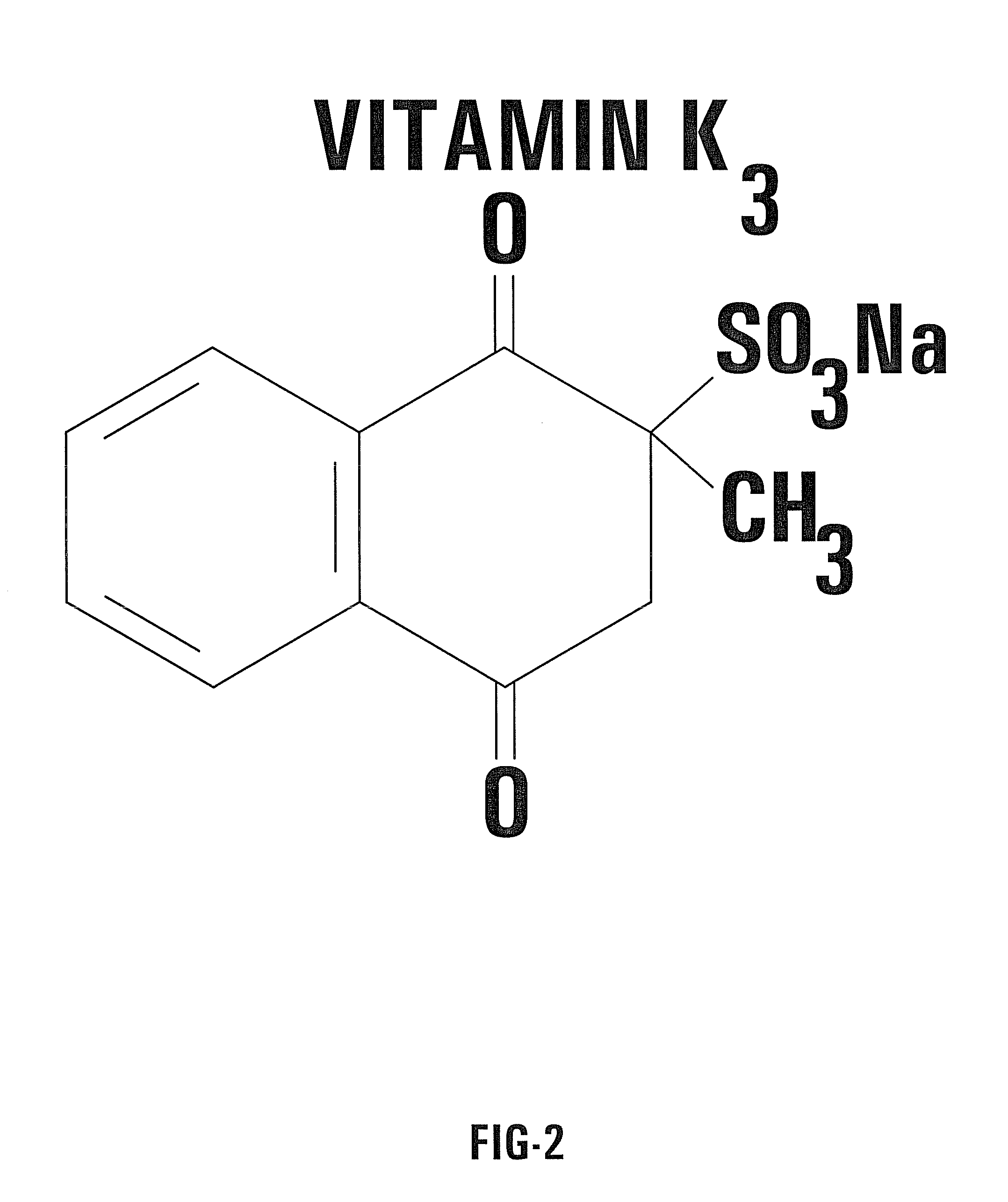
Nontoxic potentiation/sensitization of cancer therapy by supplementary treatment with combined vitamins C and K3
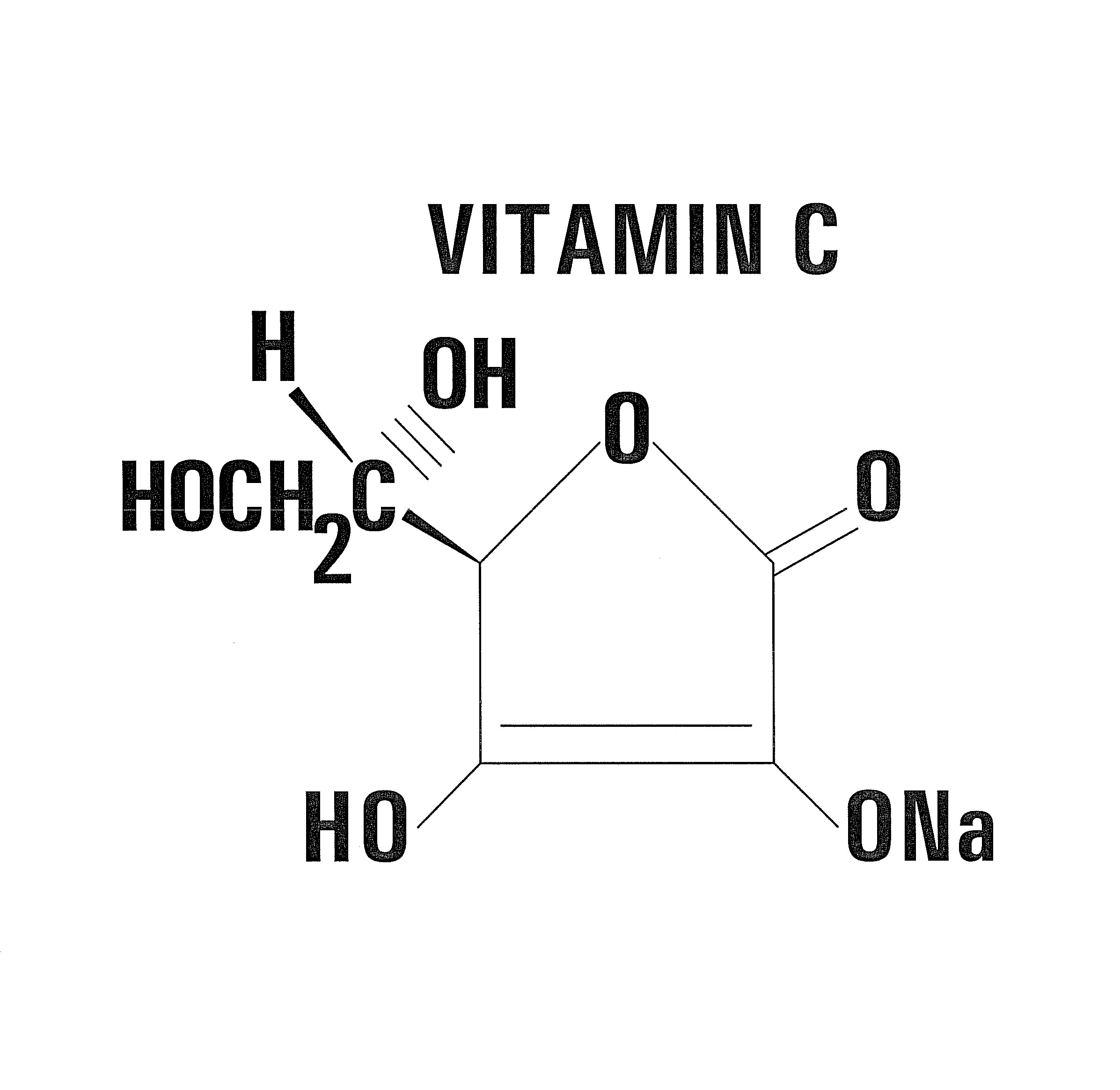
A combination of Vitamin C and a quinone used as a supplemental treatment for a cancer patient. The combination may be administered before, during and after the patient undergoes a conventional cancer treatment protocol. The combination may be administered orally, intravenously, or intraperitoneally. Oral administration may be in the form of capsules containing a predetermined ratio of Vitamin C to Vitamin K3. The supplemental treatment is effective to inhibit metastases of cancer cells and inhibit tumor growth. The ratio of Vitamin C to Vitamin K3 is in the range of about 50 to 1 to about 250 to 1. A method for evaluating the effectiveness of the supplemental treatment includes monitoring the patient's serum DNase activity throughout the course of treatment.
Owner:IC MEDTECH CORP
Method for inducing hypothermia
InactiveUS20090125087A1Rapid and safeStart fastLighting and heating apparatusIce productionParticulatesSlurry
Systems for phase-change particulate slurry cooling equipment and methods to induce hypothermia in a patient through internal and external cooling are provided. Subcutaneous, intravascular, intraperitoneal, gastrointestinal, and lung methods of cooling are carried out using saline ice slurries or other phase-change slurries compatible with human tissue. Perfluorocarbon slurries or other slurry types compatible with human tissue are used for pulmonary cooling. And traditional external cooling methods are improved by utilizing phase-change slurry materials in cooling caps and torso blankets.
Owner:UNIVERSITY OF CHICAGO
Implantable prosthesis
InactiveUS20130178875A1Easy maintenanceGreat ability can be customizedPharmaceutical containersPretreated surfacesThree dimensional shapeMirror image
An implantable prosthesis can have a three-dimensional shape that is invertible, so as to assume either a right configuration or a left configuration, which can be substantially mirror images of each other, so as to eliminate the need for separately manufacturing a left prosthesis and a right prosthesis. An implantable prosthesis can be preformed to independently assume a contoured three-dimensional shape that more adequately fits the extraperitoneal laparoscopic inguinal space, while simultaneously maintaining a relatively large area for fixation of the prostheses (e.g., through suturing or integration with the surrounding tissue). An implantable prosthesis can have a three-dimensional contoured shape that is formed from a single piece of continuous material, such as a mesh, and can possess substantially uniform rigidity. An implantable prosthesis may be trimmed, cut, or altered at an outer perimeter with no detrimental effect on its ability to independently maintain a predetermined three-dimensional contoured shape.
Owner:ATRIUM MEDICAL
Double layer surgical prosthesis to repair soft tissue
ActiveUS20090276057A1Less thicknessSmall dimensionProsthesisWound clampsIntraperitoneal routeProsthesis
A prosthesis for the treatment of hernias and / or laparoceles via an intraperitoneal route, having a mesh of filaments of non-resorbable and biocompatible polymer material having interstices permitting tissue growth and a sheet of polymer material having barrier properties and low adhesion to sensitive organs and tissues. The sheet is superimposed upon and joined to the mesh so as to form a stratified structure. In particular, the sheet is joined to mesh through a plurality of filaments located alongside each other at a spacing of not more than 5 mm. Each filament has a plurality of attachment sites to the mesh which are not more than 15 mm apart, and each length of filament between two successive attachment sites projects from the surface of the mesh facing sheet and is fused to the sheet.
Owner:HERNIAMESH
Method and apparatus for pressure measurement
Methods and apparatus for measuring pressure in a patient are provided which may include any number of features. One feature is a pressure measurement system comprising a pressure source, a compliant bladder, a catheter in communication with the pressure source, pressure sensors, and a controller configured to determine a pressure within the compliant bladder. The pressure measurement system can inflate the compliant bladder with gas or air to determine a pressure within a patient. In one embodiment, the pressure measurement system measures pressure within a peritoneal cavity.
Owner:POTRERO MEDICAL
Method and apparatus for pressure measurement
Methods and apparatus for measuring pressure in a patient are provided which may include any number of features. One feature is a pressure measurement system comprising a pressure source, a compliant bladder, a catheter in communication with the pressure source, pressure sensors, and a controller configured to determine a pressure within the compliant bladder. The pressure measurement system can inflate the compliant bladder with gas or air to determine a pressure within a patient. In one embodiment, the pressure measurement system measures pressure within a peritoneal cavity.
Owner:POTRERO MEDICAL
Medical treatment system and methods using a plurality of fluid lines
ActiveUS10058694B2Reduce touchReduce leakage currentControl devicesBlood pumpsPeritoneal dialysisHemoperitoneum
Improvements in fluid volume measurement systems are disclosed for a pneumatically actuated diaphragm pump in general, and a peritoneal dialysis cycler using a pump cassette in particular. Pump fluid volume measurements are based on pressure measurements in a pump control chamber and a reference chamber in a two-chamber model, with different sections of the apparatus being modeled using a combination of adiabatic, isothermal and polytropic processes. Real time or instantaneous fluid flow measurements in a pump chamber of a diaphragm pump are also disclosed, in this case using a one-chamber ideal gas model and using a high speed processor to obtain and process pump control chamber pressures during fluid flow into or out of the pump chamber. Improved heater control circuitry is also disclosed, to provide added or redundant safety measures, or to reduce current leakage from a heater element during pulse width modulation control of the heater. Improvements are also disclosed in the application of negative pressure during a drain phase in peritoneal dialysis therapy, and to control the amount of intraperitoneal fluid accumulation during a therapy. Improvements in efficiency are also disclosed in the movement of fluid into and out of a two-pump cassette and heater bag of a peritoneal dialysis cycler, and in the synchronization of the operation of two or more pumps in a peritoneal dialysis cycler or other fluid handling devices using a multi-pump arrangement.
Owner:DEKA PROD LLP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com