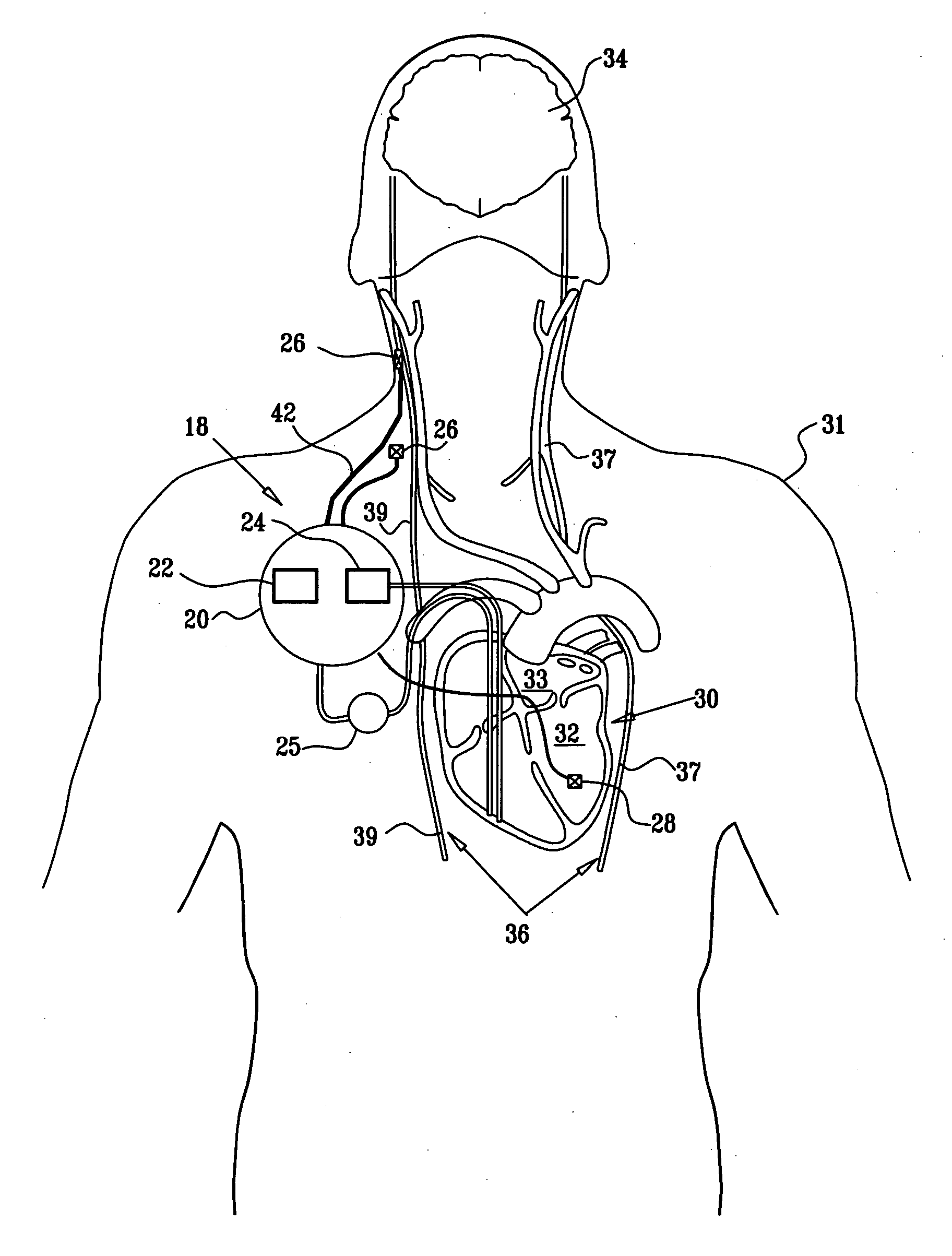
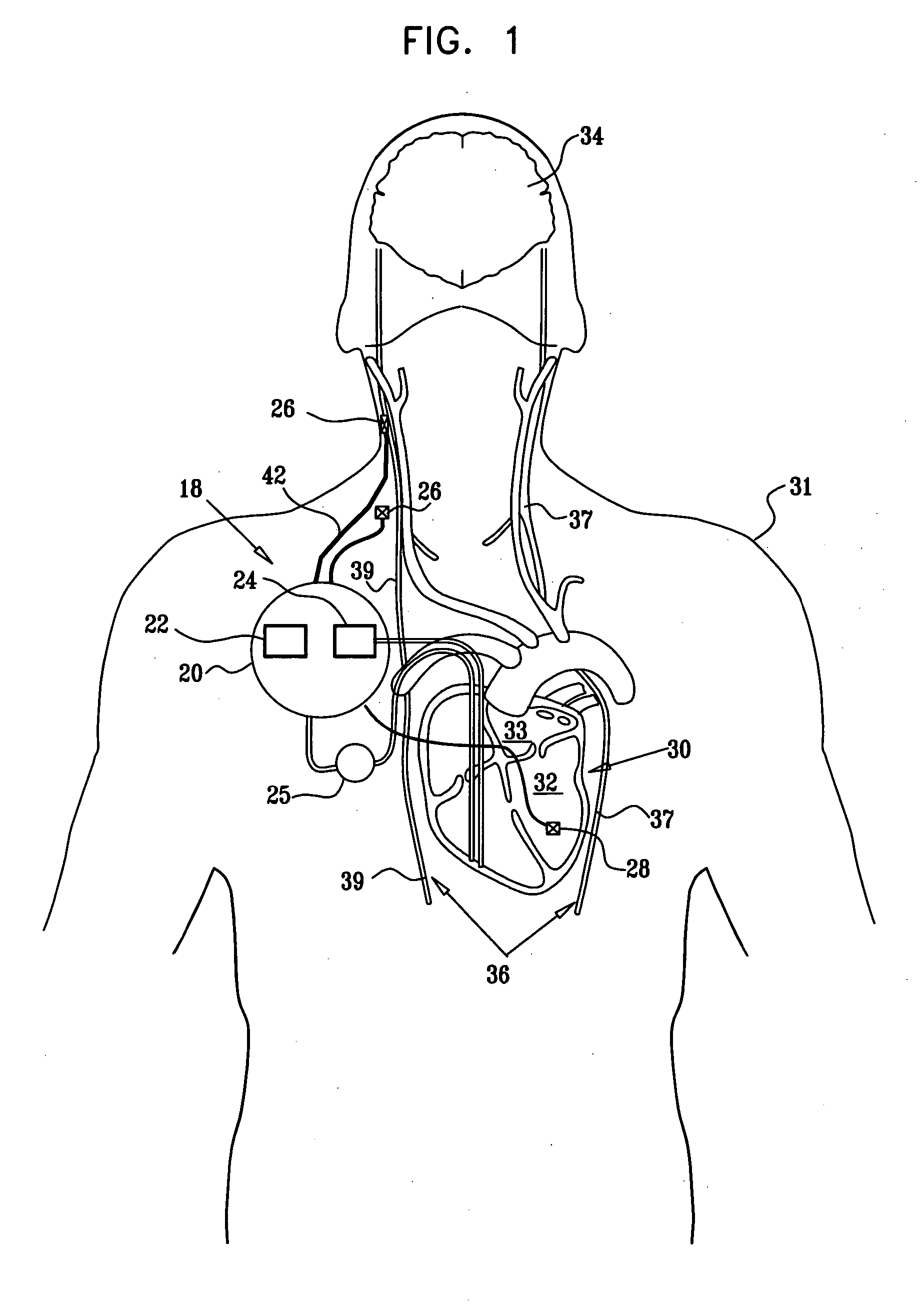
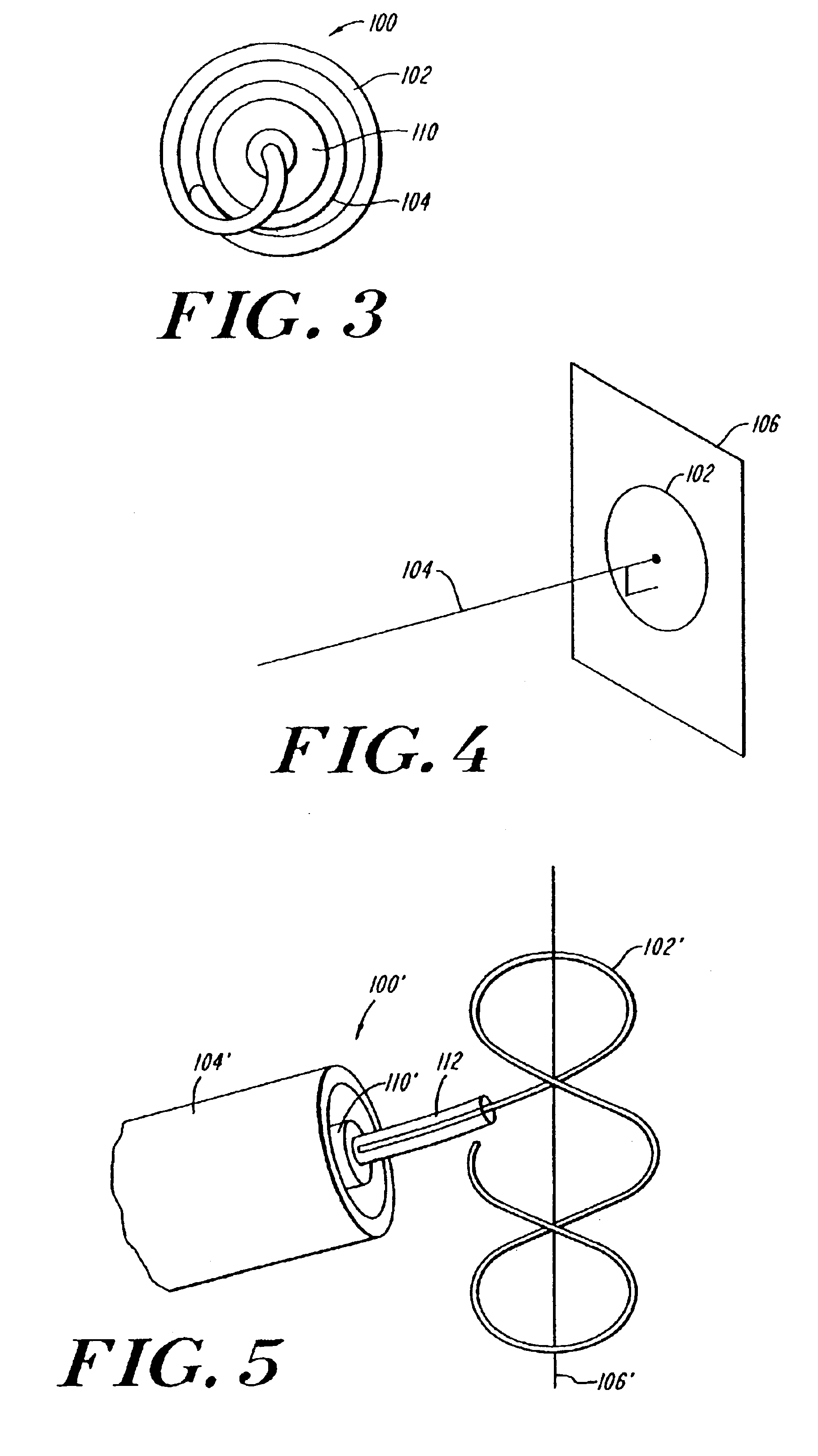
Patents
Literature
319 results about "Pulmonary vein" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
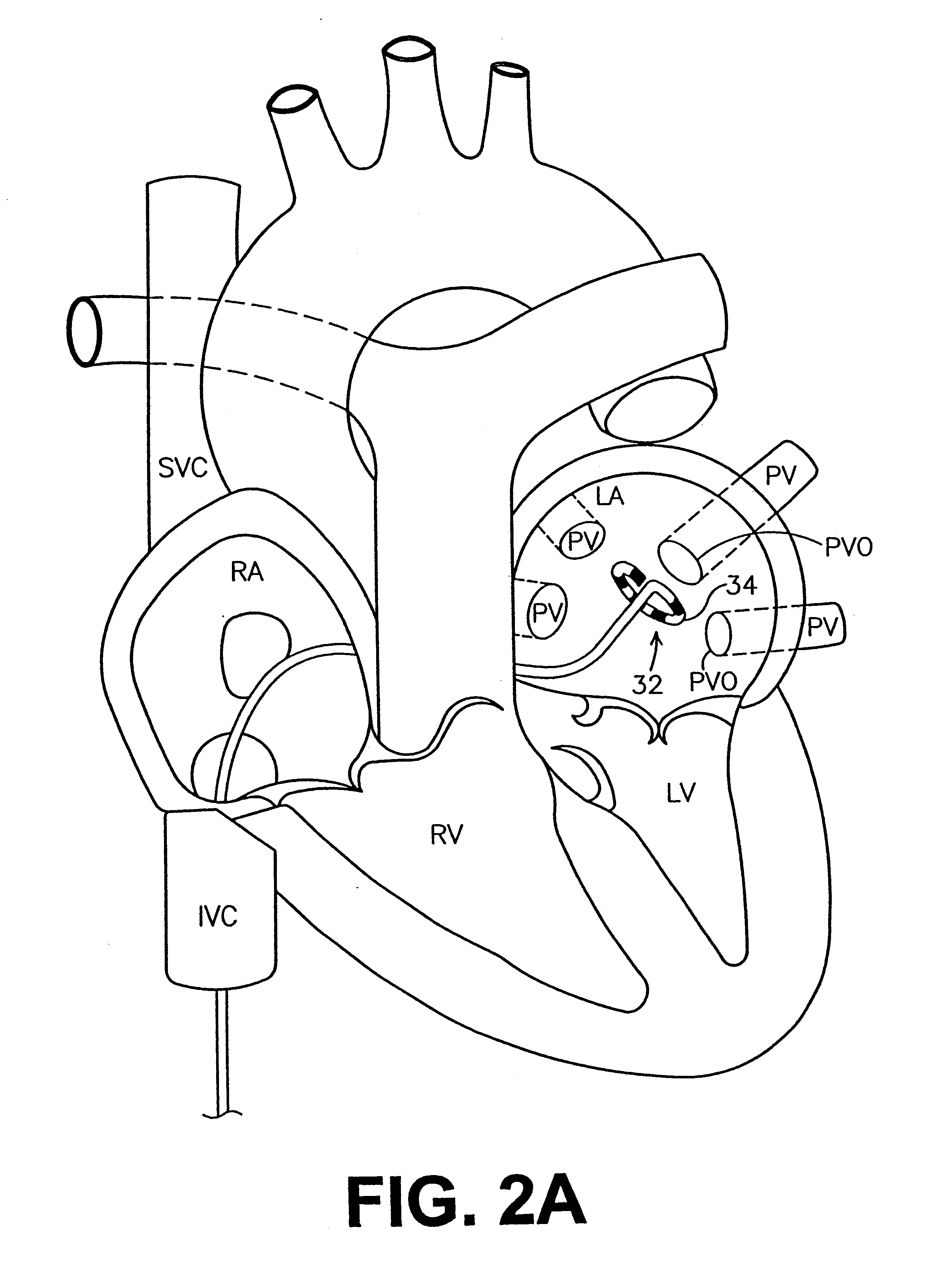
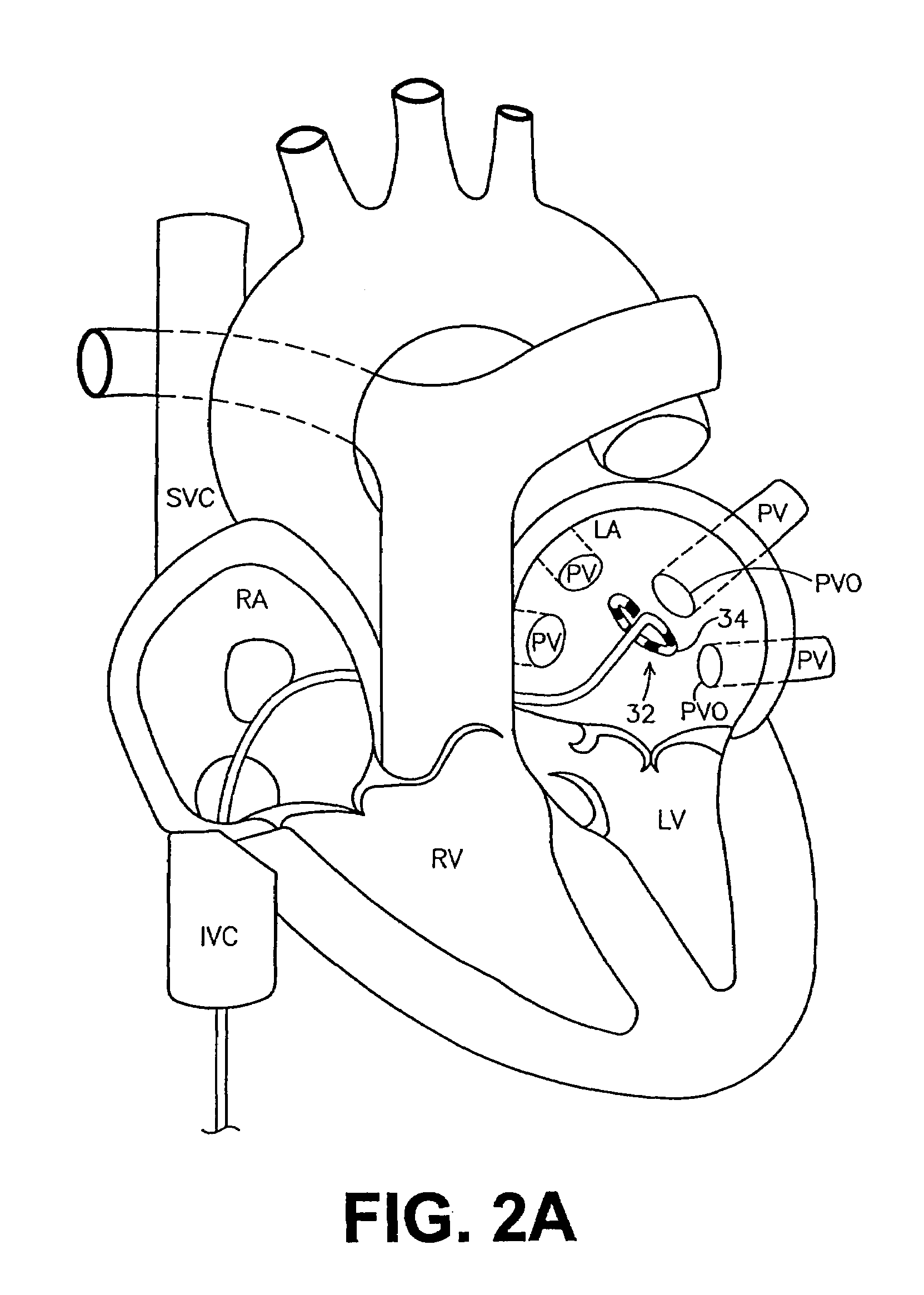
The pulmonary veins are the veins that transfer oxygenated blood from the lungs to the heart. The largest pulmonary veins are the four main pulmonary veins, two from each lung that drain into the left atrium of the heart. The pulmonary veins are part of the pulmonary circulation.
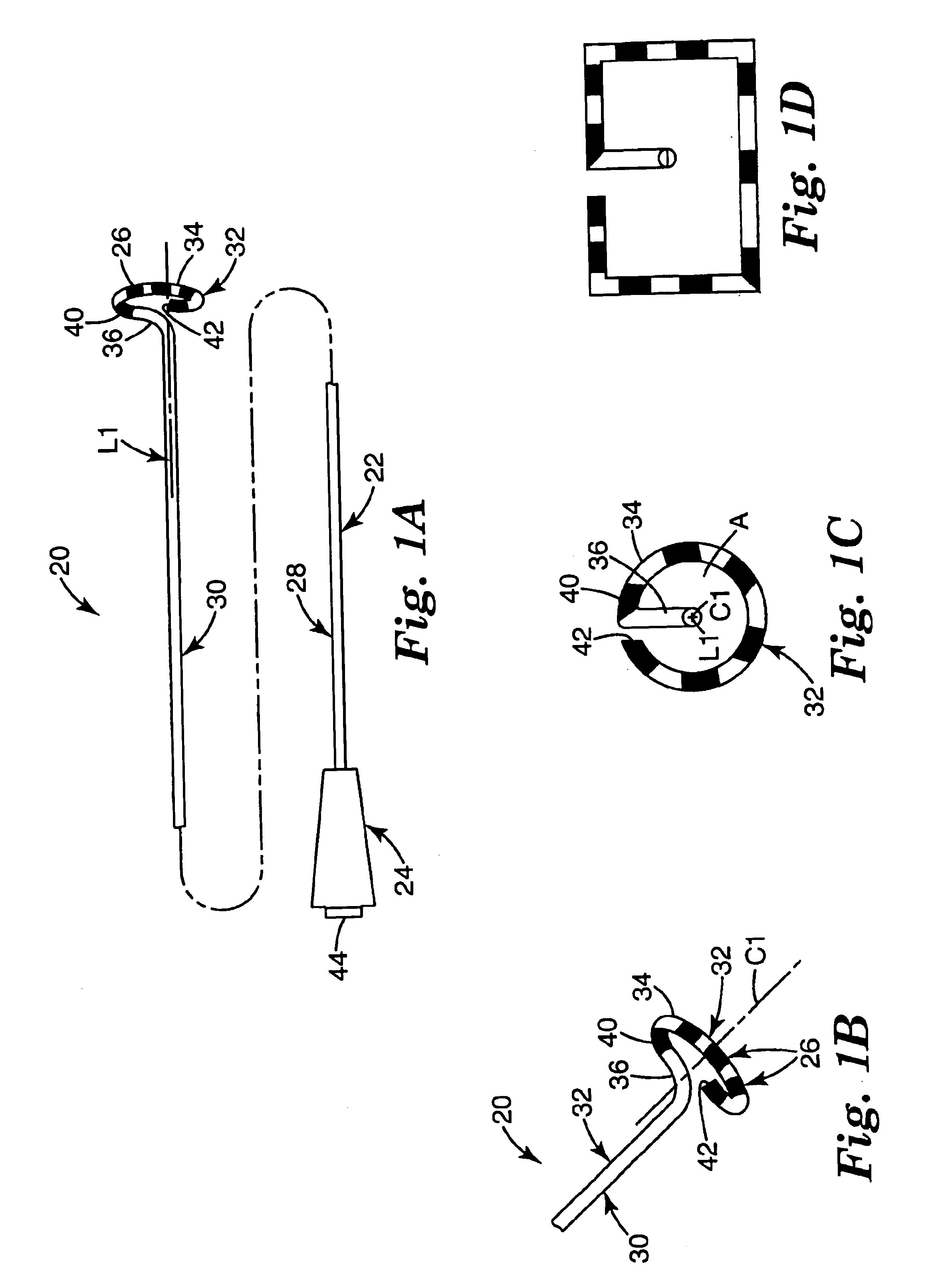
Positioning system and method for orienting an ablation element within a pulmonary vein ostium
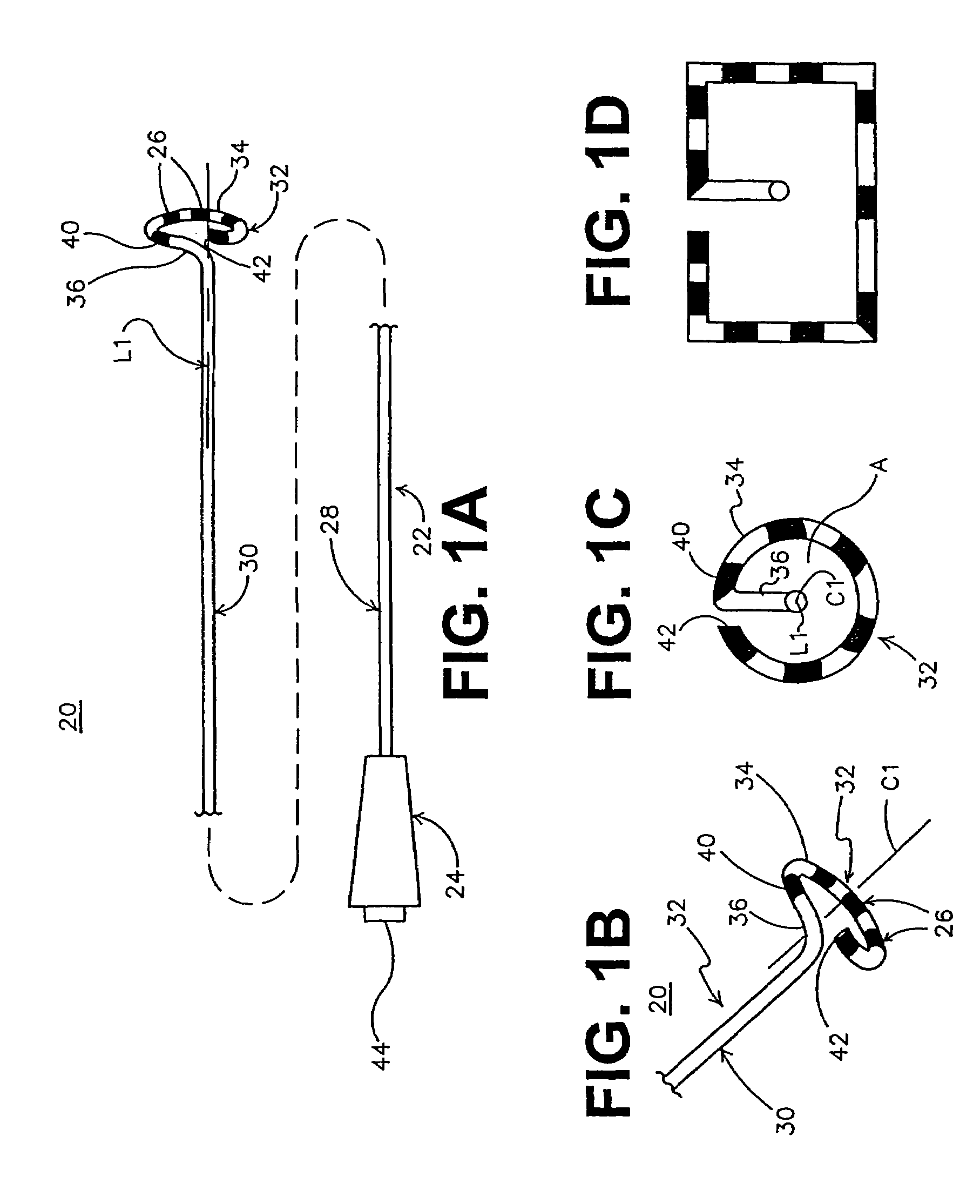
InactiveUS6514249B1Eliminates arrhythmogenic conductionPrevent atrial arrhythmiaUltrasound therapyElectrotherapySurgical operationVein
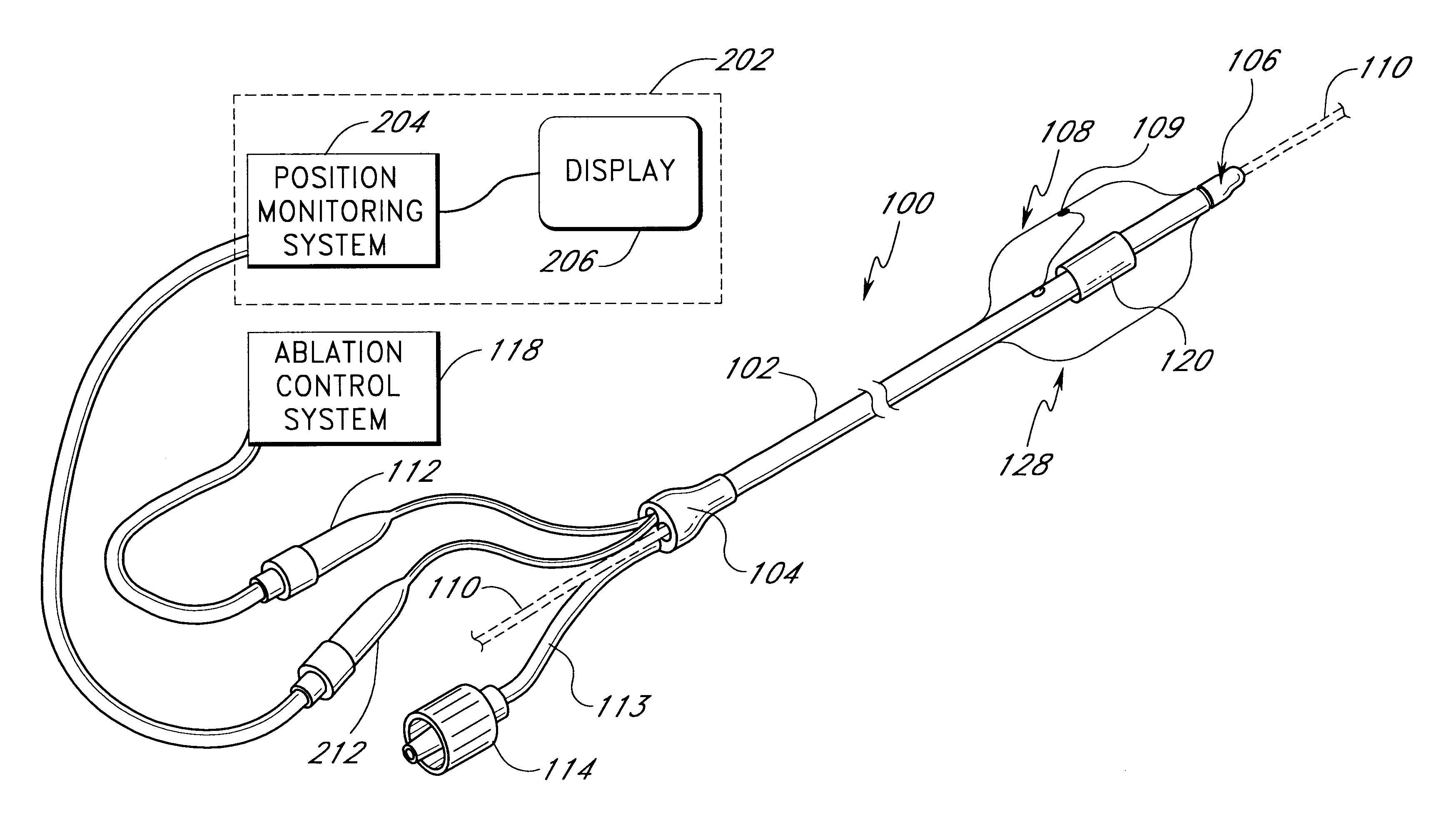
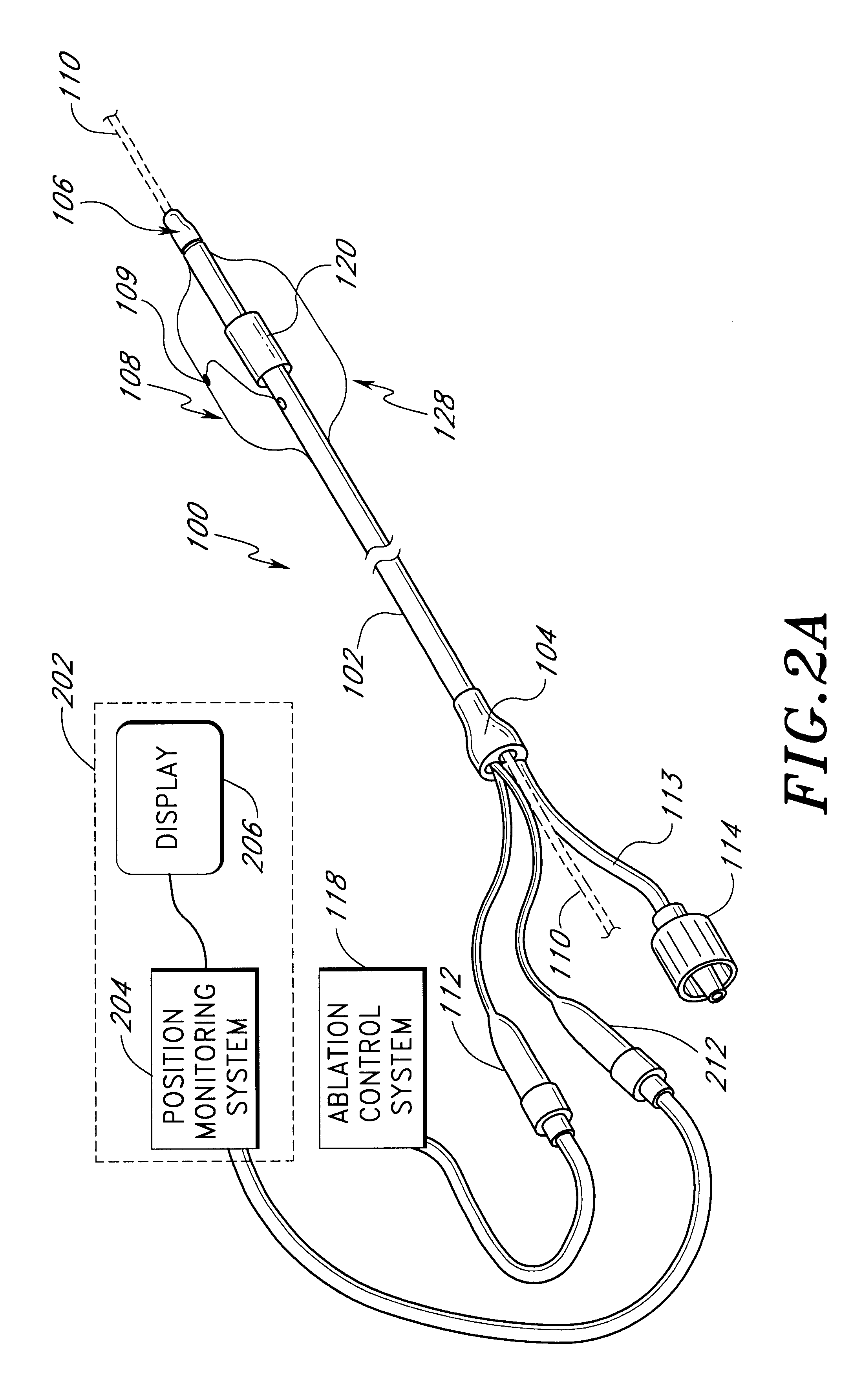
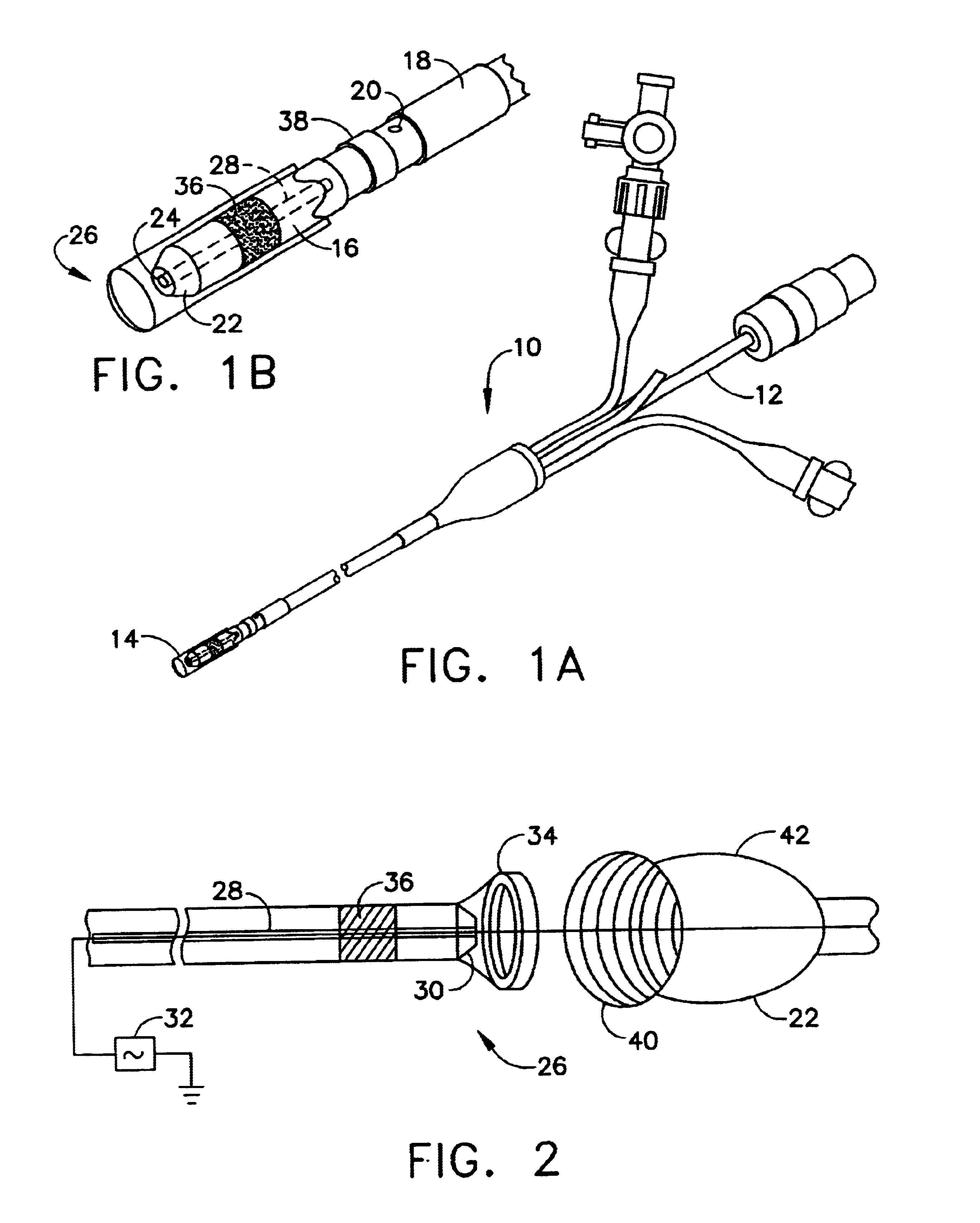
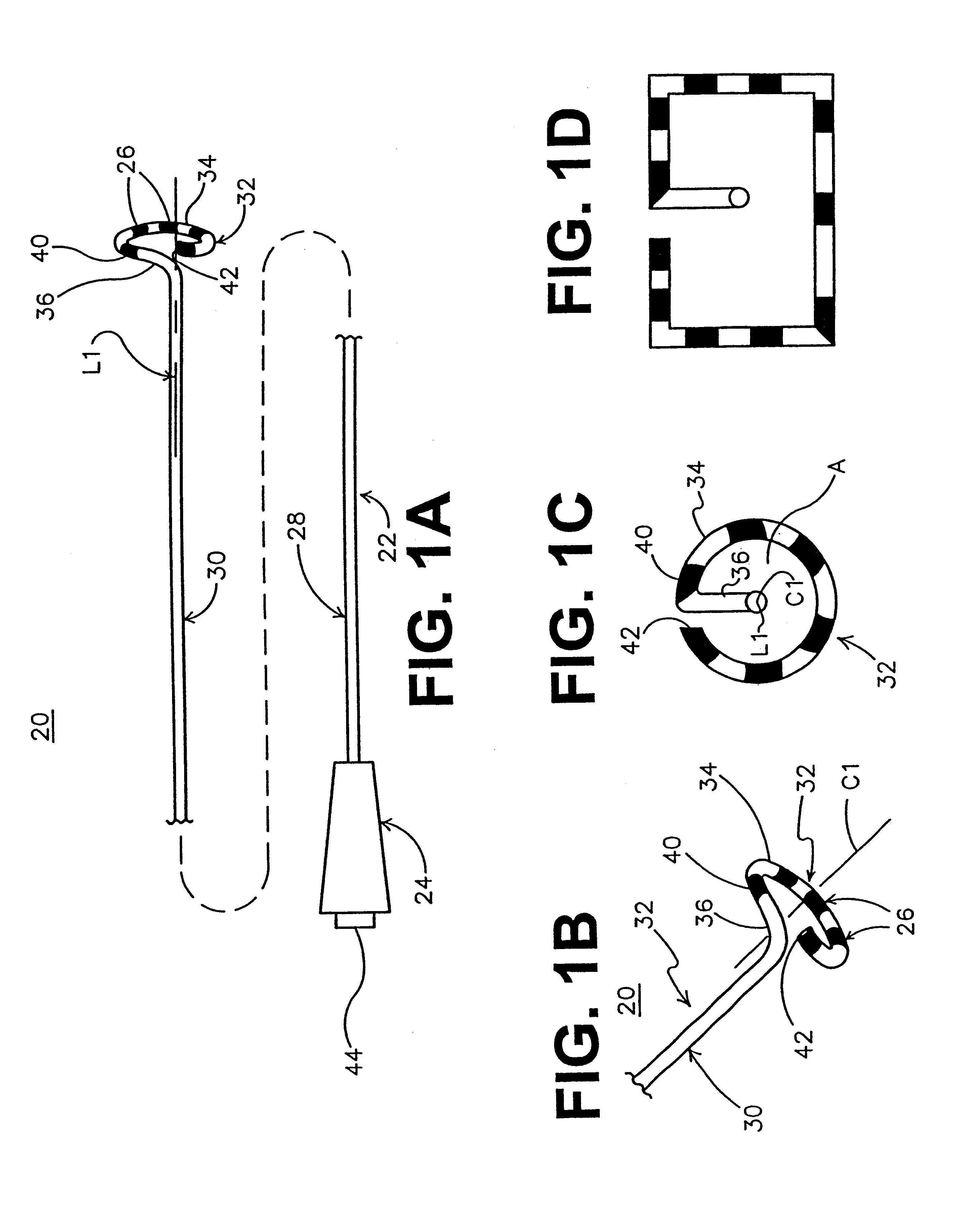
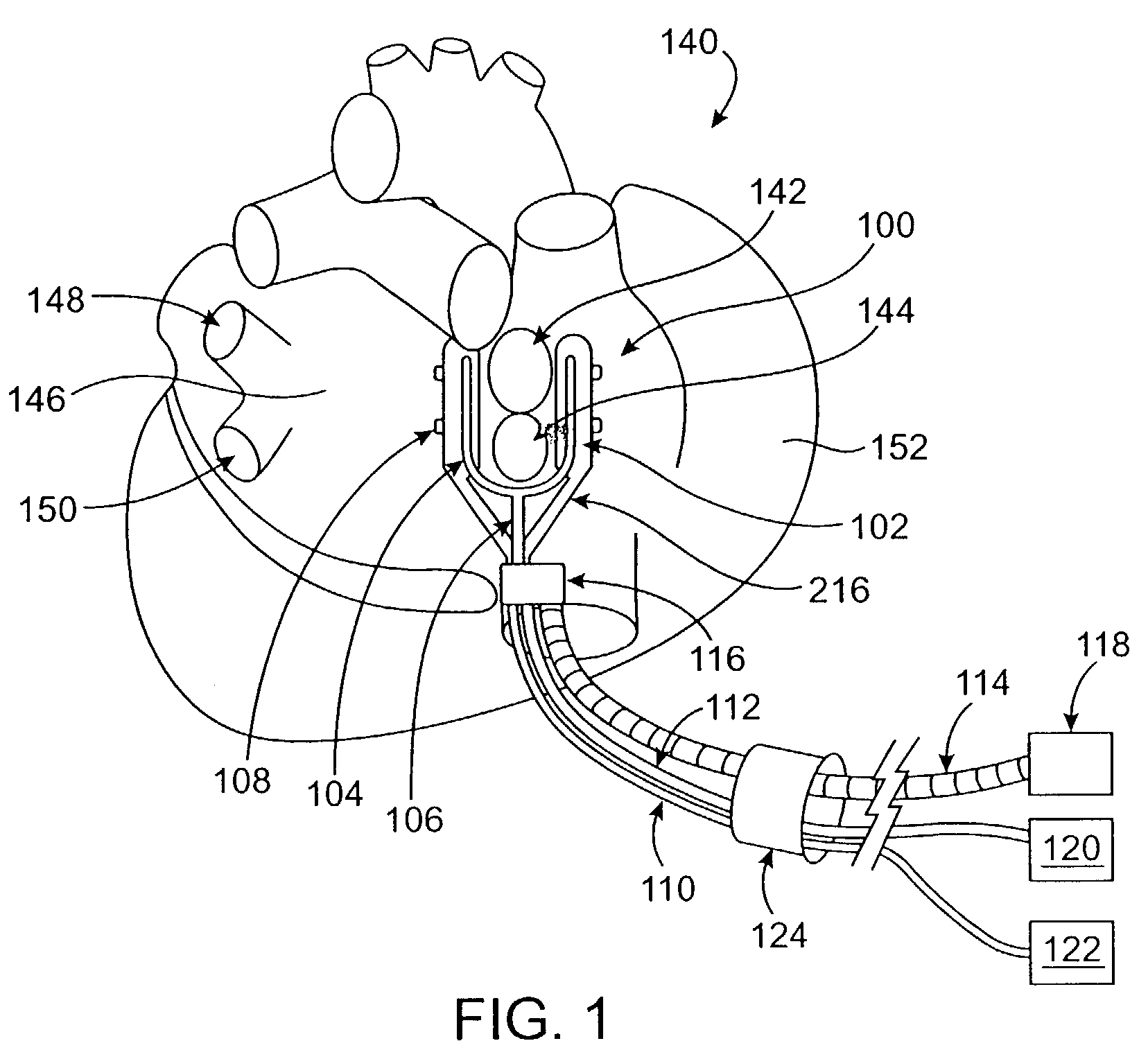
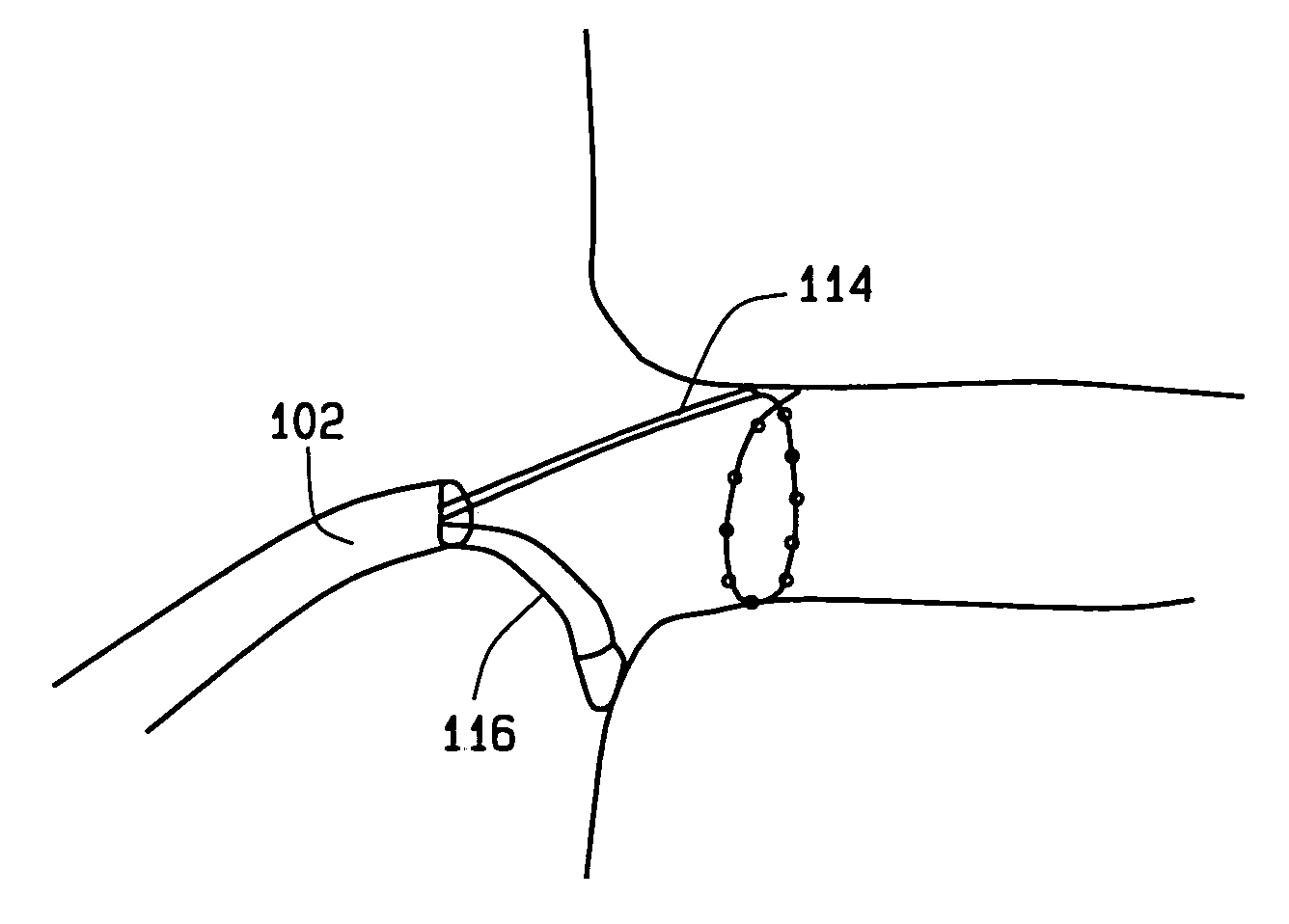
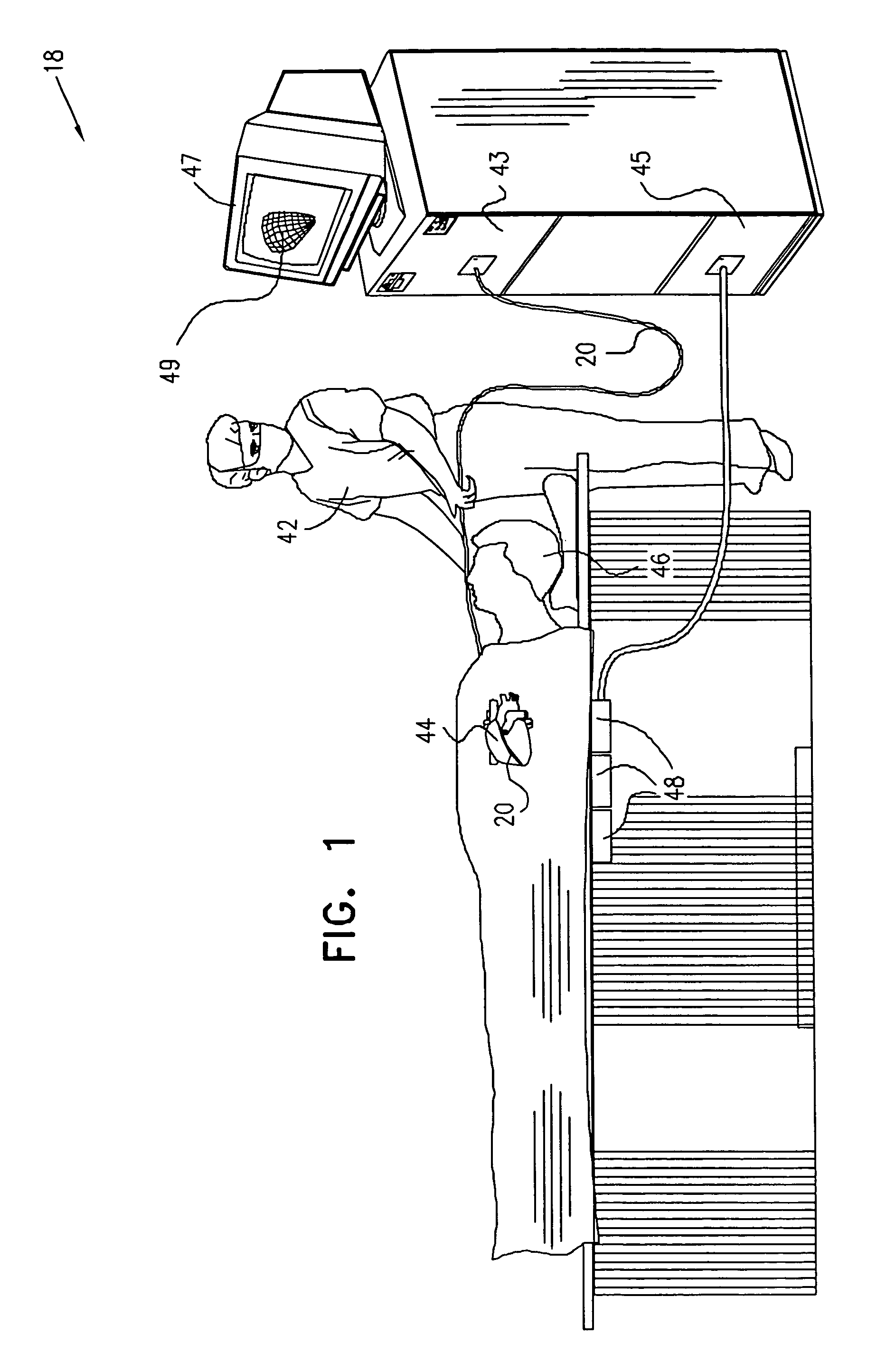
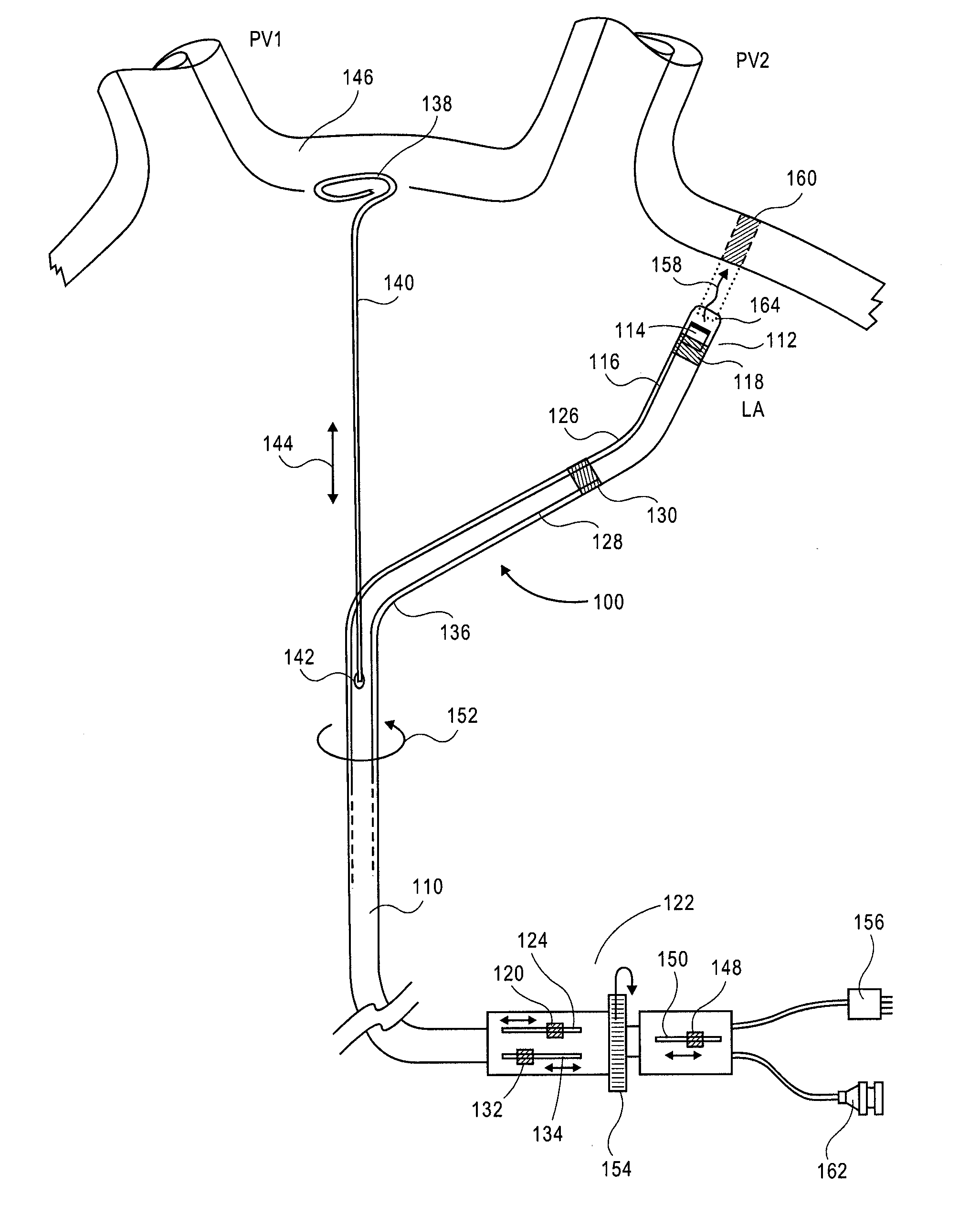
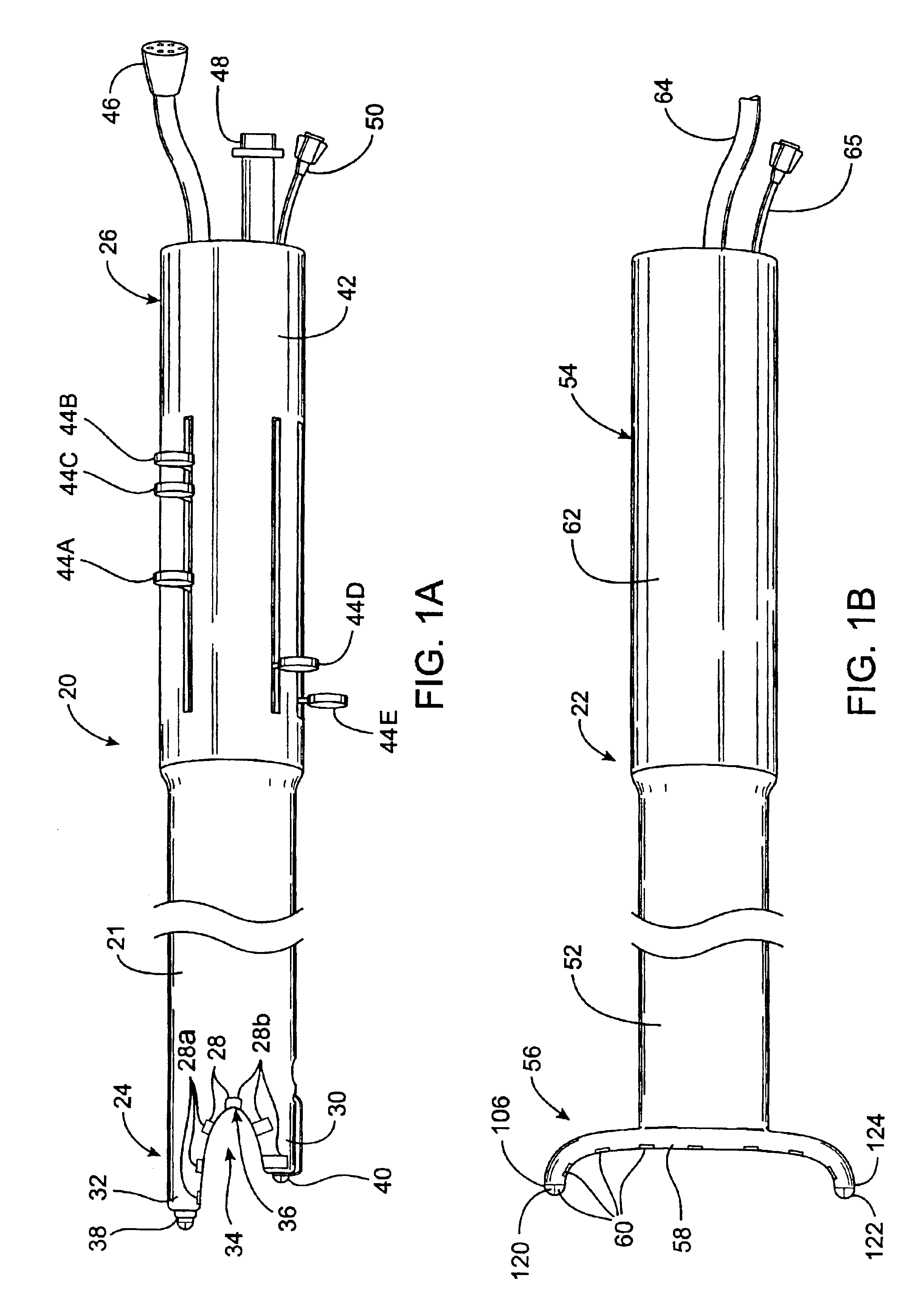
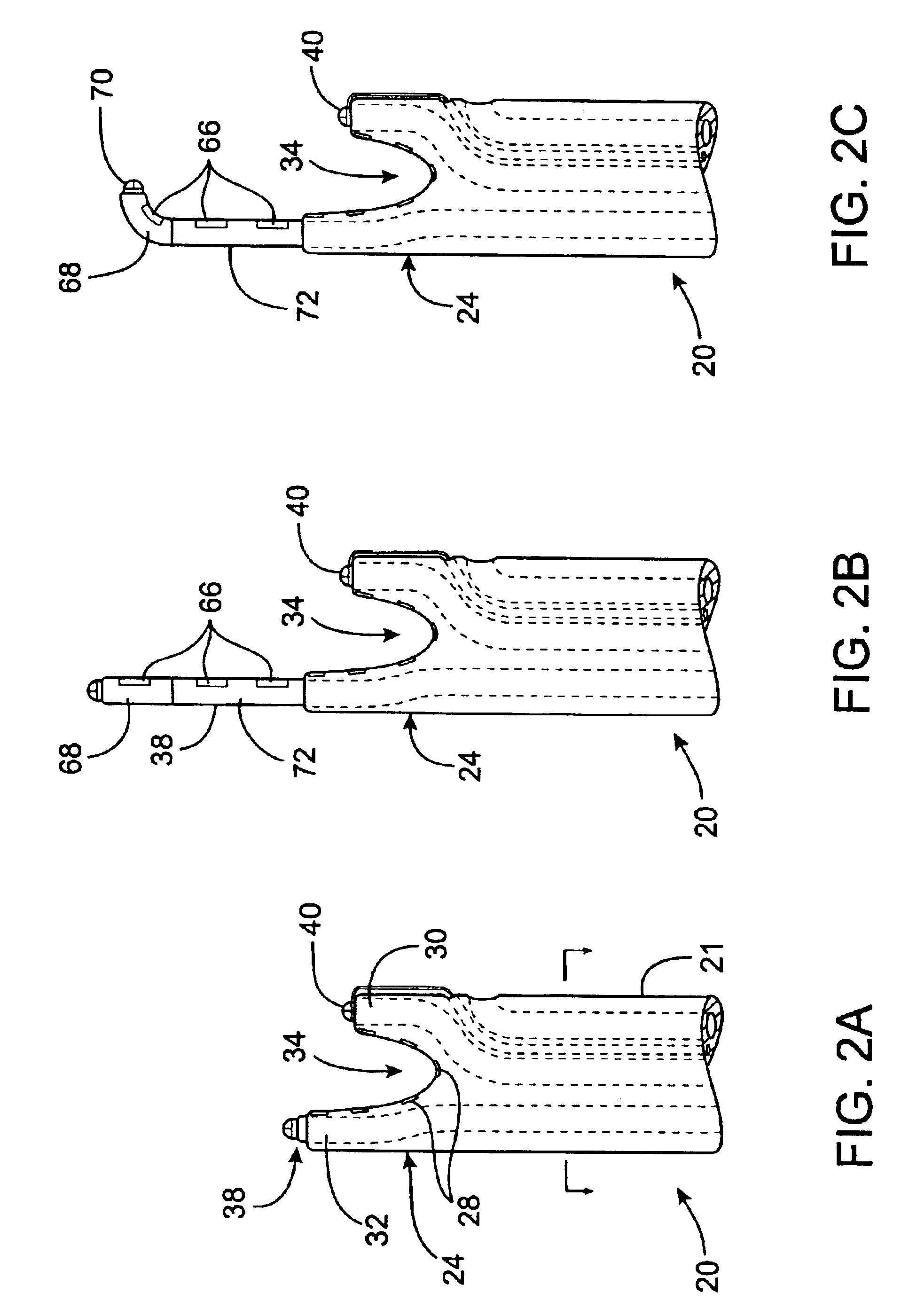
This invention relates to a surgical device and method. More particularly, it relates to a tissue ablation device assembly and method using a circumferential ablation member in combination with a position monitoring assembly in order to position the circumferential ablation member along a circumferential region of tissue at a location where a pulmonary vein extends from a left atrium.
Owner:ATRIONIX
Laser pulmonary vein isolation
A catheter introduction apparatus provides an optical assembly for emission of laser light energy. In one application, the catheter and the optical assembly are introduced percutaneously, and transseptally advanced to the ostium of a pulmonary vein. An anchoring balloon is expanded to position a mirror near the ostium of the pulmonary vein, such that light energy is reflected and directed circumferentially around the ostium of the pulmonary vein when a laser light source is energized. A circumferential ablation lesion is thereby produced, which effectively blocks electrical propagation between the pulmonary vein and the left atrium.
Owner:BIOSENSE
Ablation catheter
Devices, systems and methods are disclosed for the mapping of electrical signals and the ablation of tissue. Embodiments include an ablation catheter that has an array of ablation elements attached to a deployable carrier assembly. The carrier assembly can be transformed from a compact, linear configuration to a helical configuration, such as to map and ablate pulmonary vein ostia.
Owner:MEDTRONIC ABLATION FRONTIERS
Loop ablation apparatus and method
Embodiments of the invention provide an ablation apparatus for ablating target tissue adjacent pulmonary veins of a patient. The ablation apparatus can include a tube capable of being advanced around the pulmonary veins to form a loop. The tube can receive or include electrodes to ablate target tissue. Some embodiments provide a loop ablation device, which may include a cannula and two or more electrode rods carrying two or more bipolar electrodes. The electrode rods can be advanced through the distal ends toward the proximal ends of the loop and toward the target tissue. The bipolar electrodes can receive energy to ablate the target tissue. The bipolar electrodes may be surrounded by the liquid within the cannula while ablating the target tissue. The loop ablation device can further include a rotating grasping mechanism coupled to the electrode rods.
Owner:MEDTRONIC INC
Ablation catheter and method for isolating a pulmonary vein
Owner:MEDTRONIC INC
Cardiac treatment devices and methods
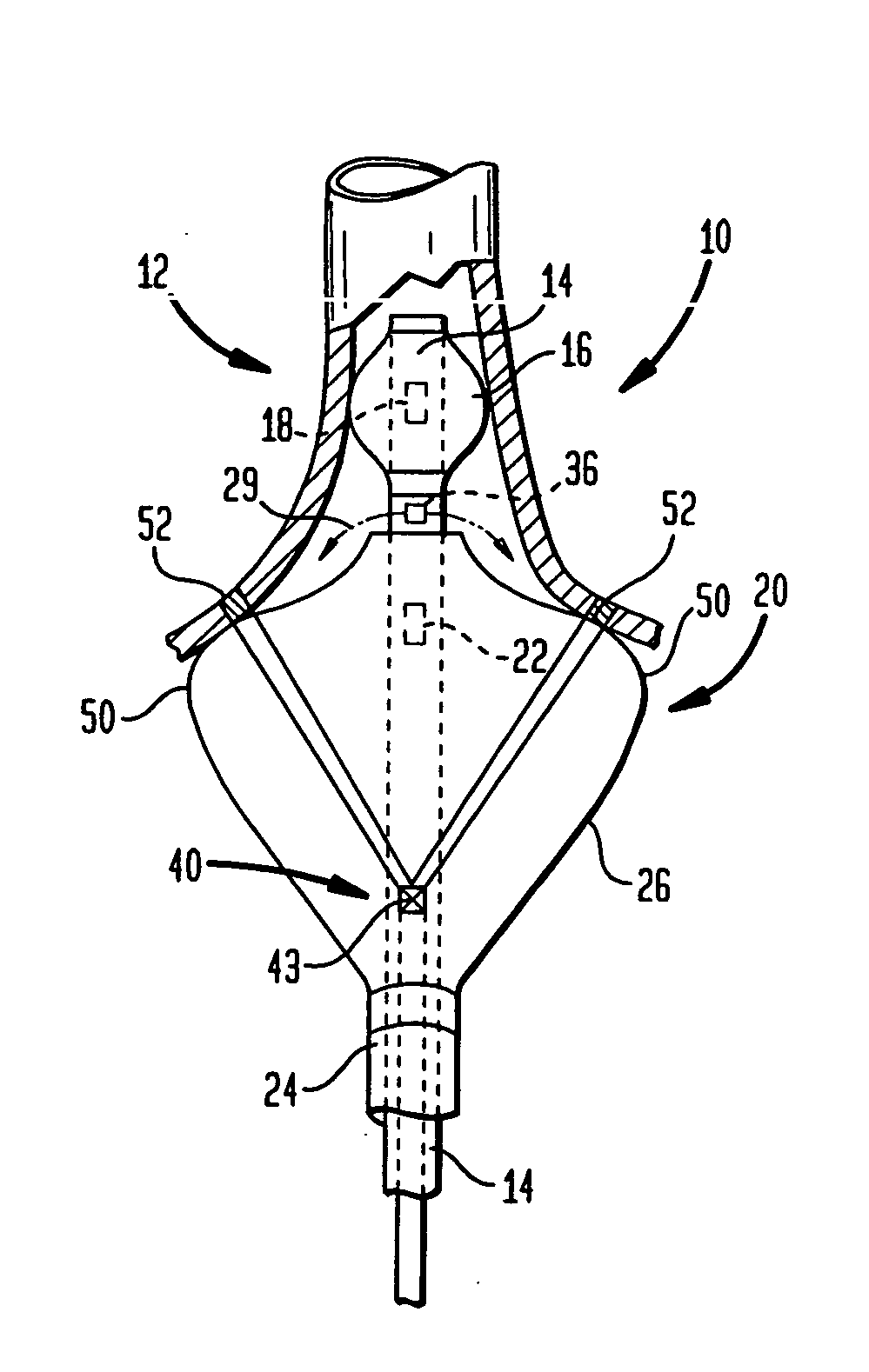
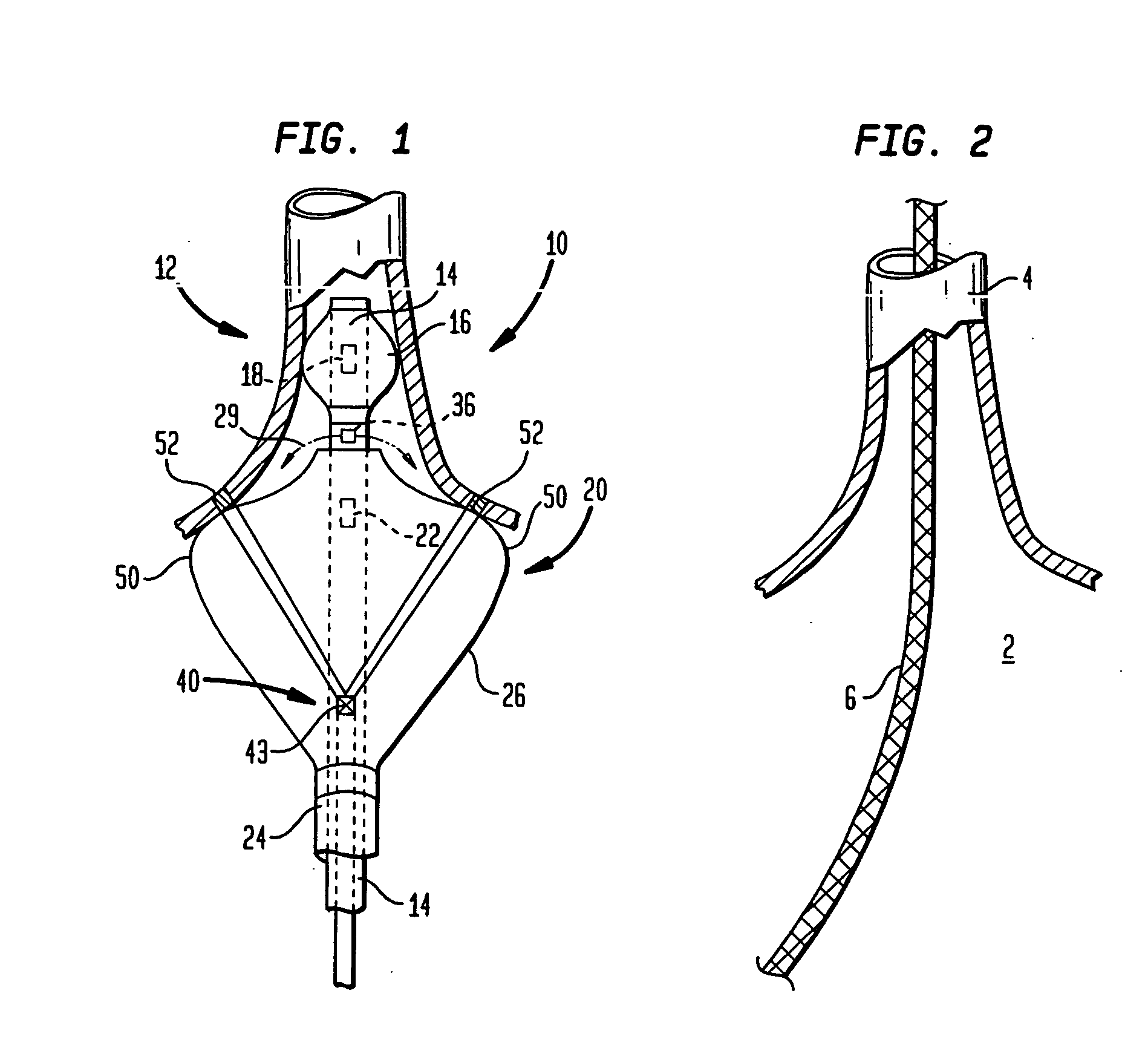
Devices and methods provide for ablation of cardiac tissue for treating cardiac arrhythmias such as atrial fibrillation. Although the devices and methods are often be used to ablate epicardial tissue in the vicinity of at least one pulmonary vein, various embodiments may be used to ablate other cardiac tissues in other locations on a heart. Devices generally include at least one tissue contacting member for contacting epicardial tissue and securing the ablation device to the epicardial tissue, and at least one ablation member for ablating the tissue. Various embodiments include features, such as suction apertures, which enable the device to attach to the epicardial surface with sufficient strength to allow the tissue to be stabilized via the device. For example, some embodiments may be used to stabilize a beating heart to enable a beating heart ablation procedure. Many of the devices may be introduced into a patient via minimally invasive introducer devices and the like. Although devices and methods of the invention may be used to ablate epicardial tissue to treat atrial fibrillation, they may also be used in veterinary or research contexts, to treat various heart conditions other than atrial fibrillation and / or to ablate cardiac tissue other than the epicardium.
Owner:ESTECH ENDOSCOPIC TECH +1
Ablation catheter
Devices, systems and methods are disclosed for the mapping of electrical signals and the ablation of tissue. Embodiments include an ablation catheter that has an array of ablation elements attached to a deployable carrier assembly. The carrier assembly can be transformed from a compact, linear configuration to a helical configuration, such as to map and ablate pulmonary vein ostia.
Owner:MEDTRONIC ABLATION FRONTIERS
Techniques for applying, configuring, and coordinating nerve fiber stimulation
ActiveUS20050267542A1Decreased heart rateEliminate side effectsSpinal electrodesHeart stimulatorsCardiac arrhythmiaCarotid sinus
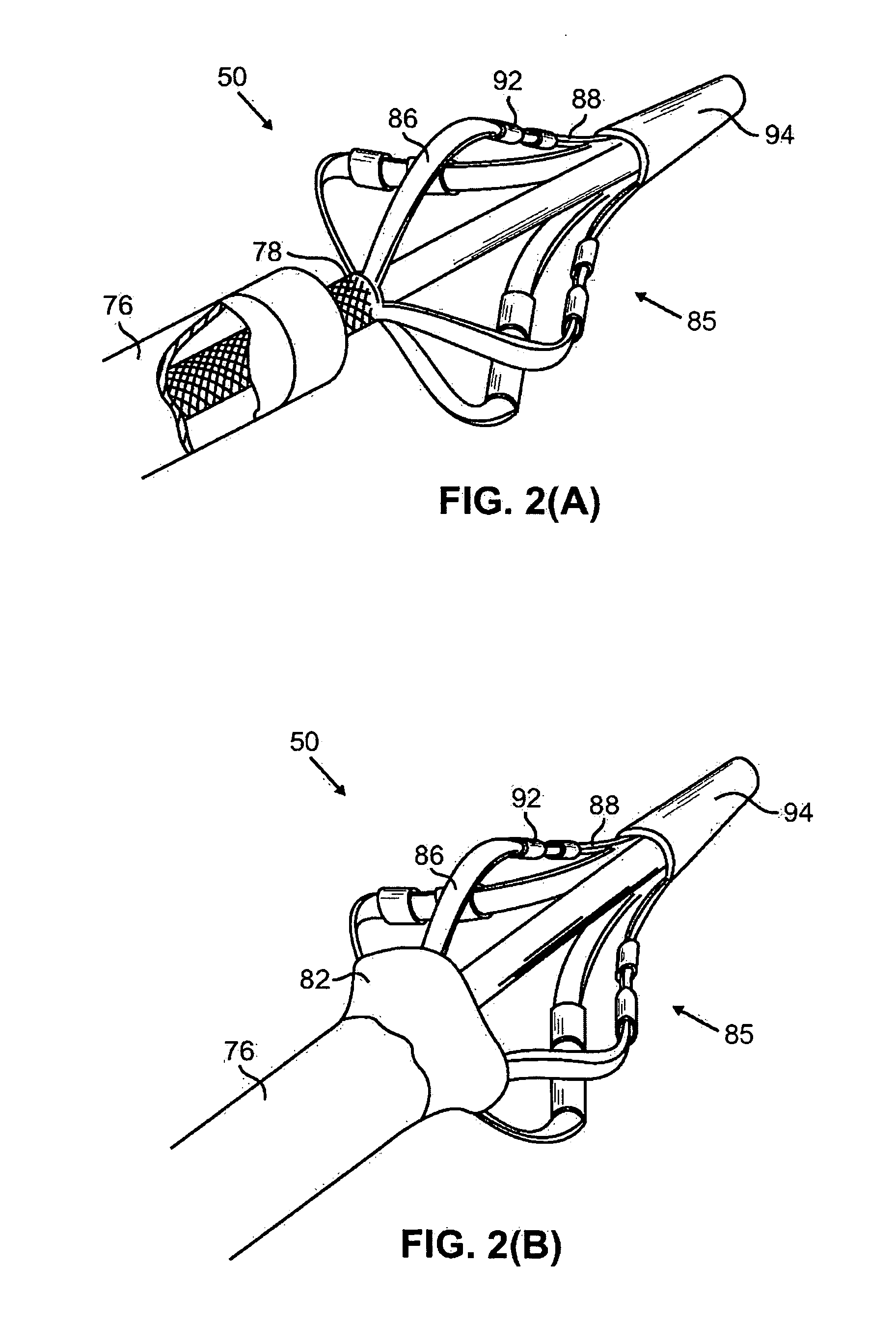
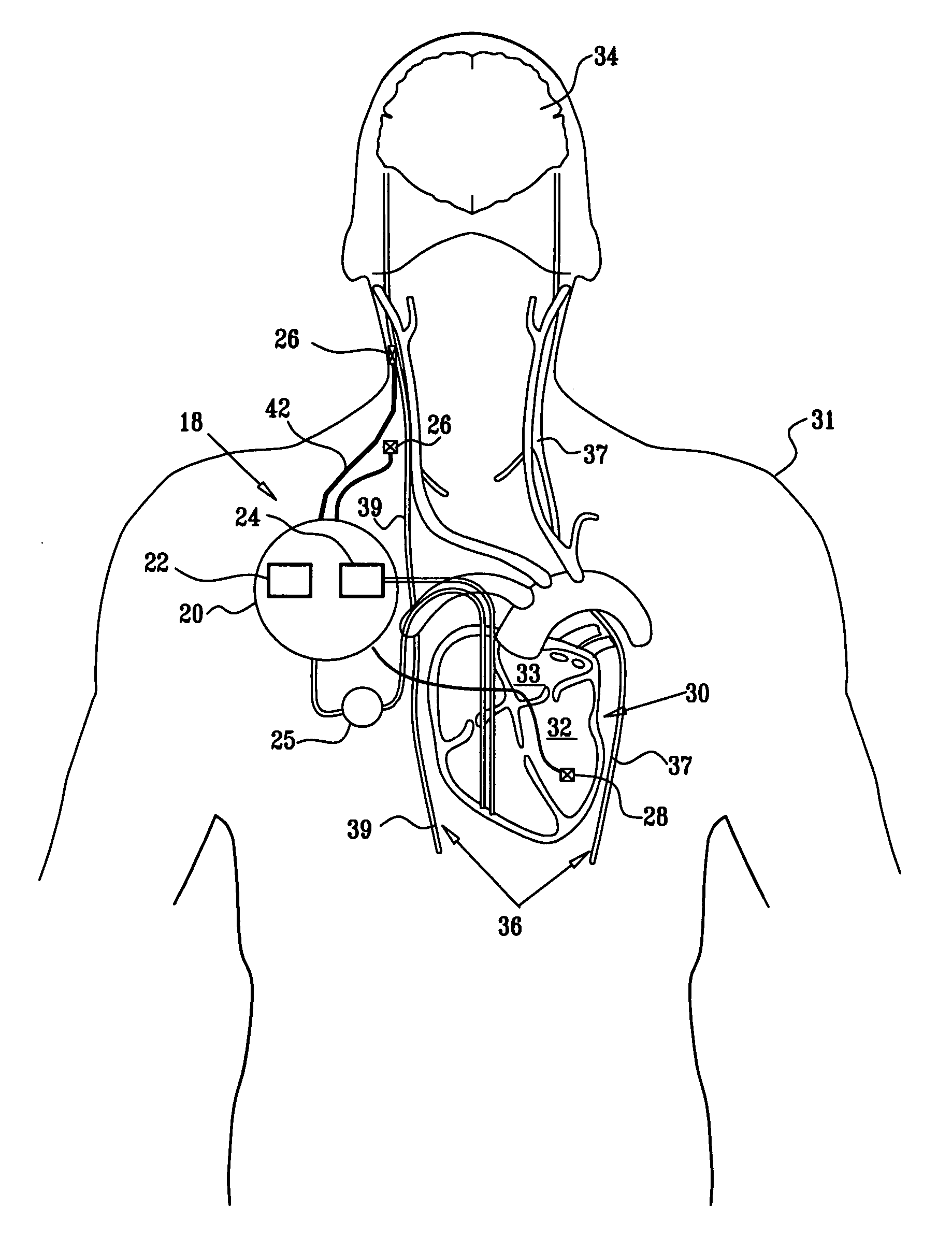
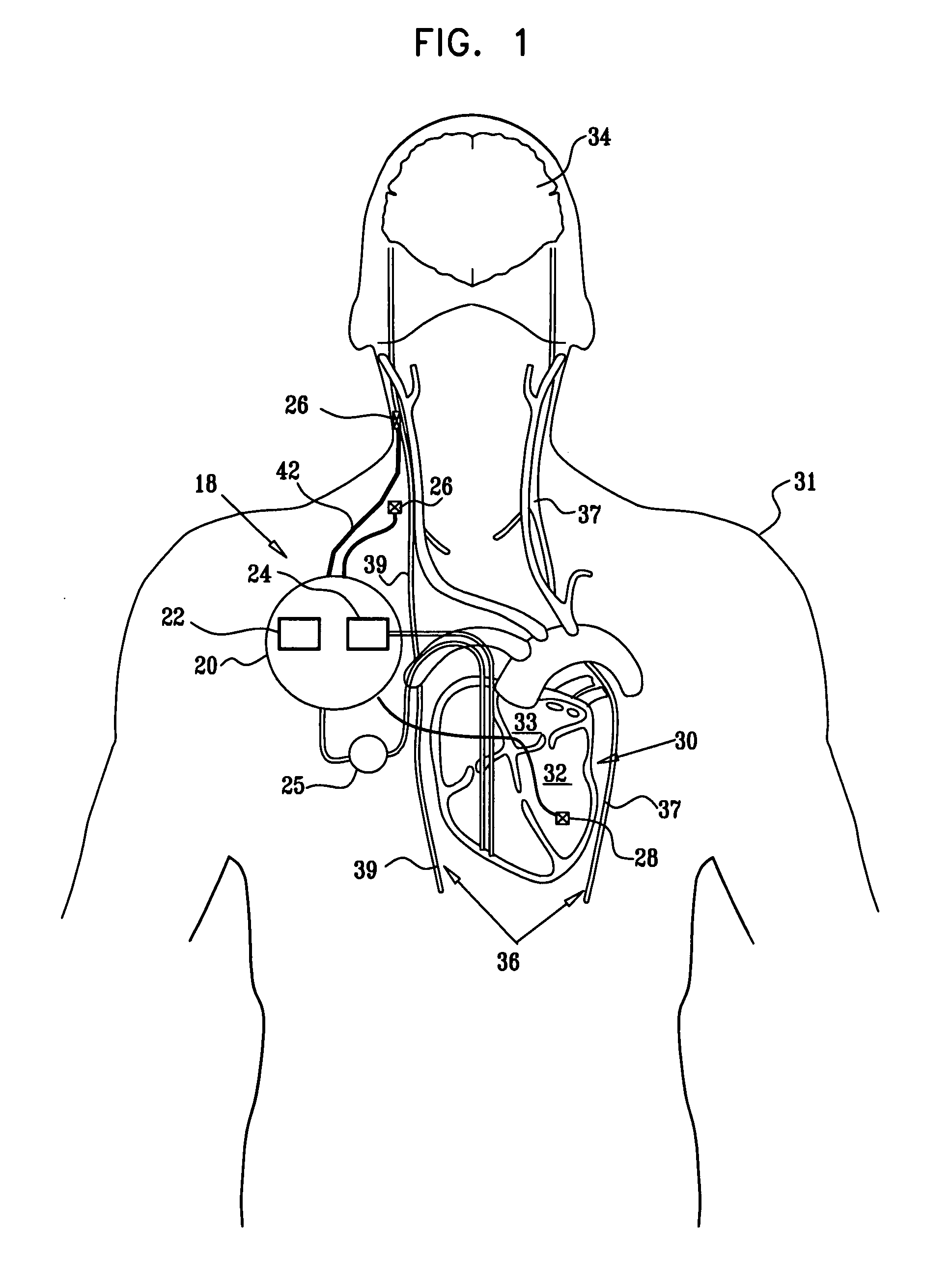
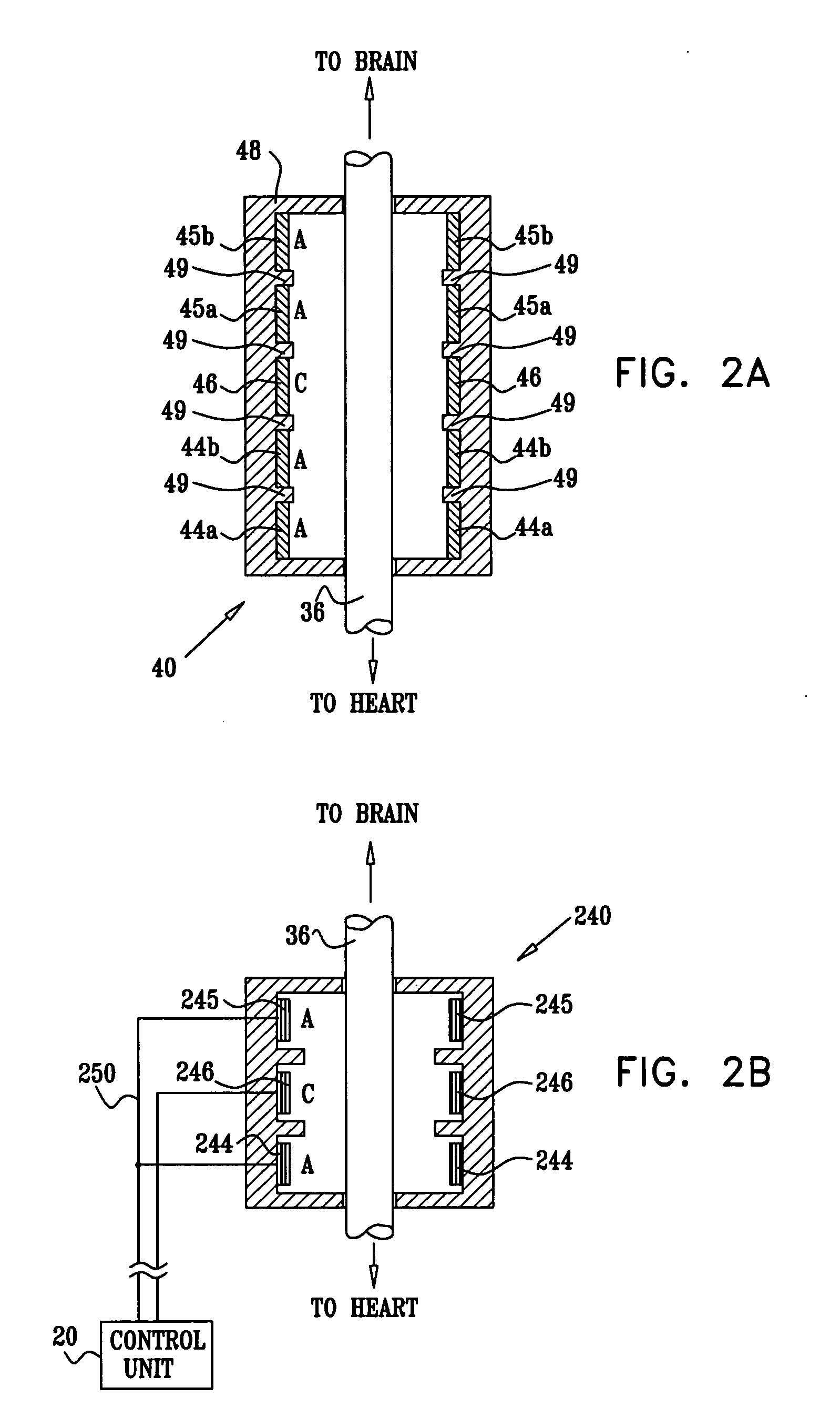
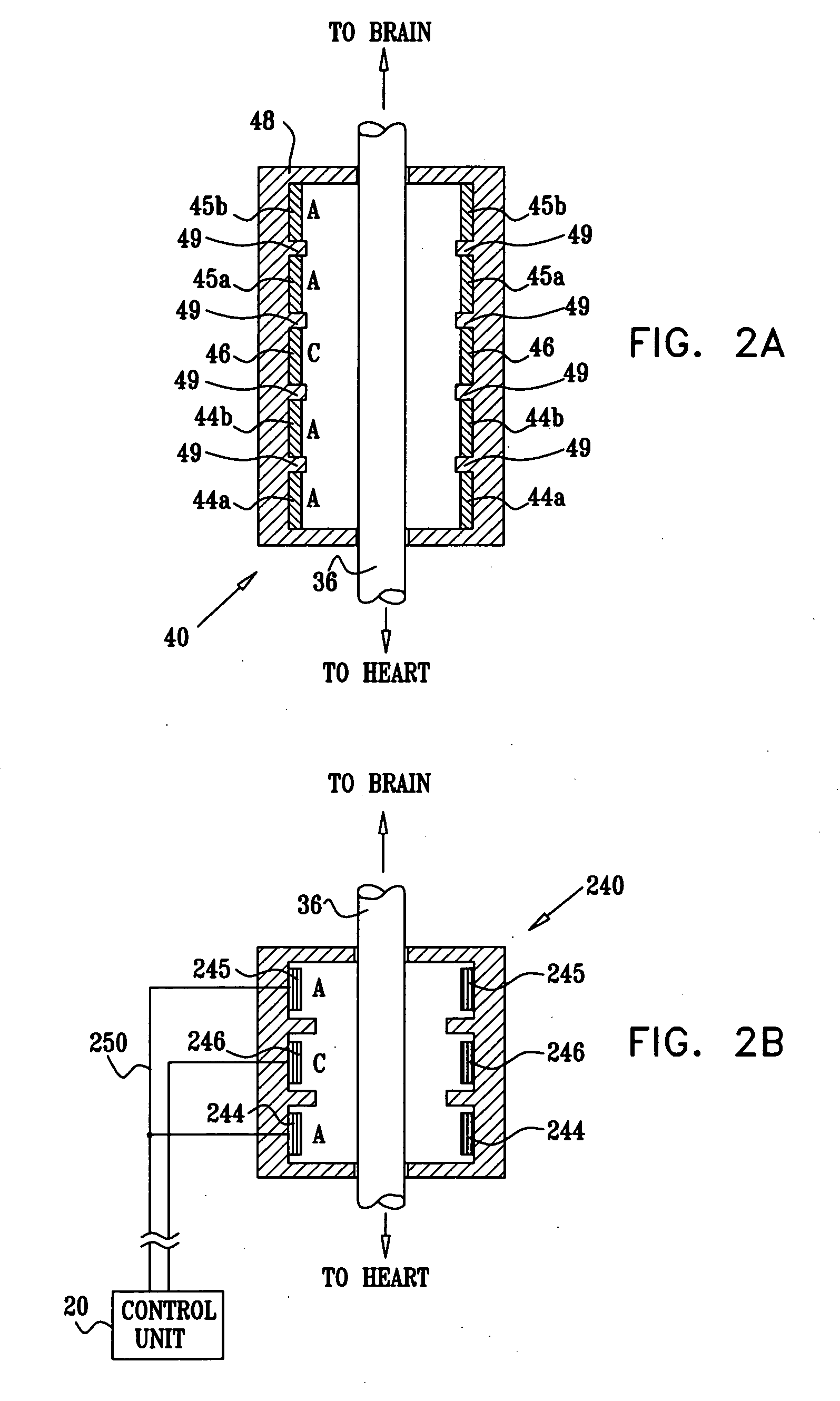
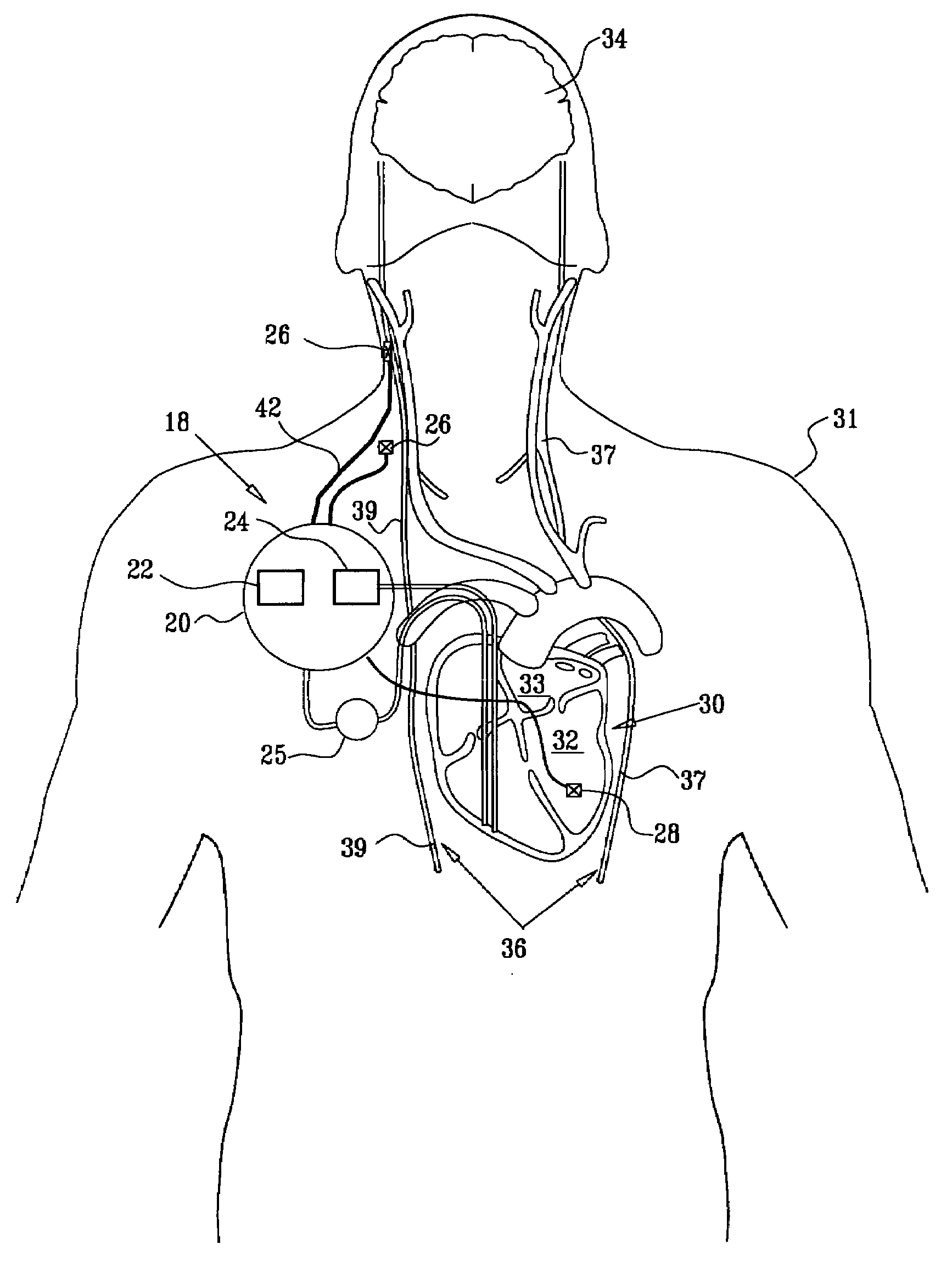
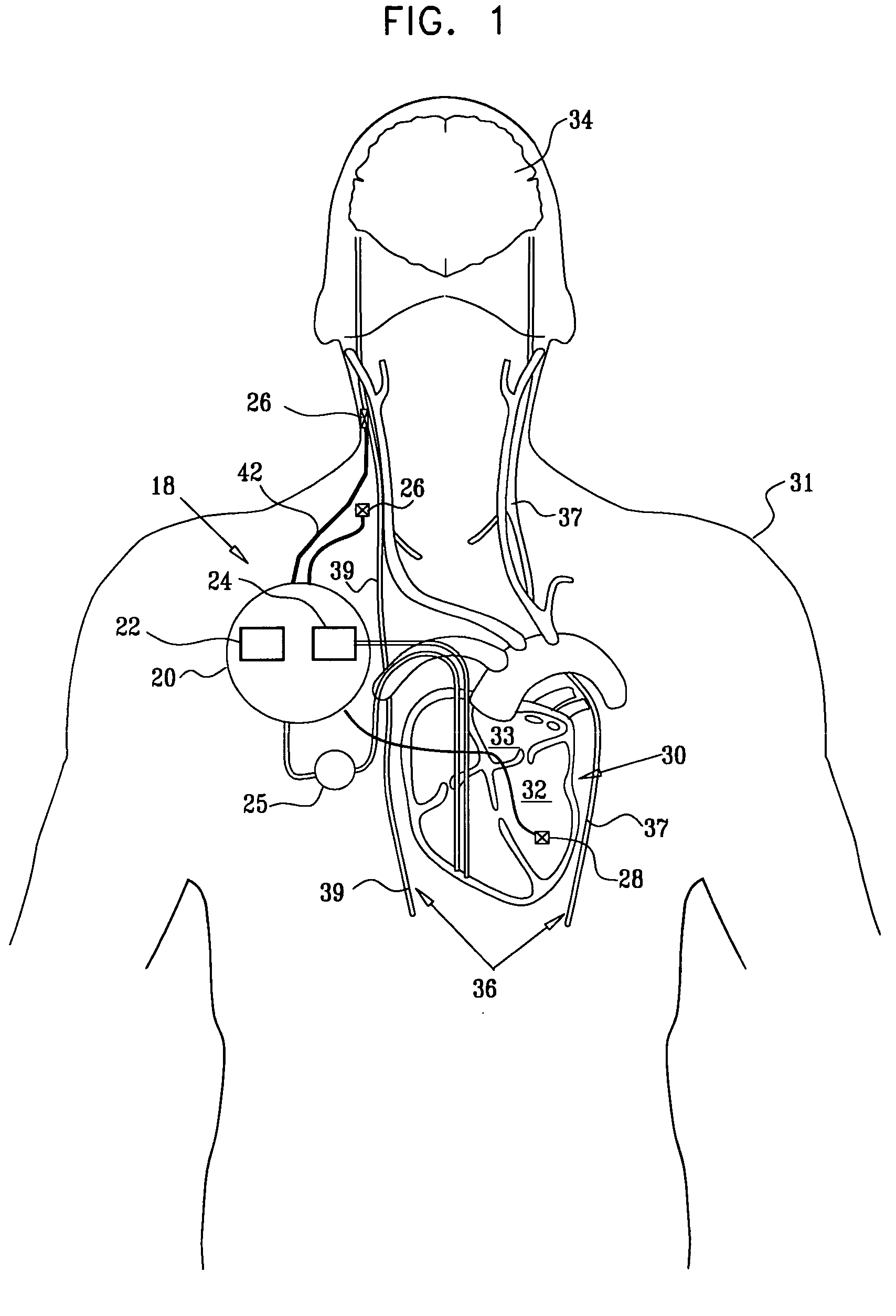
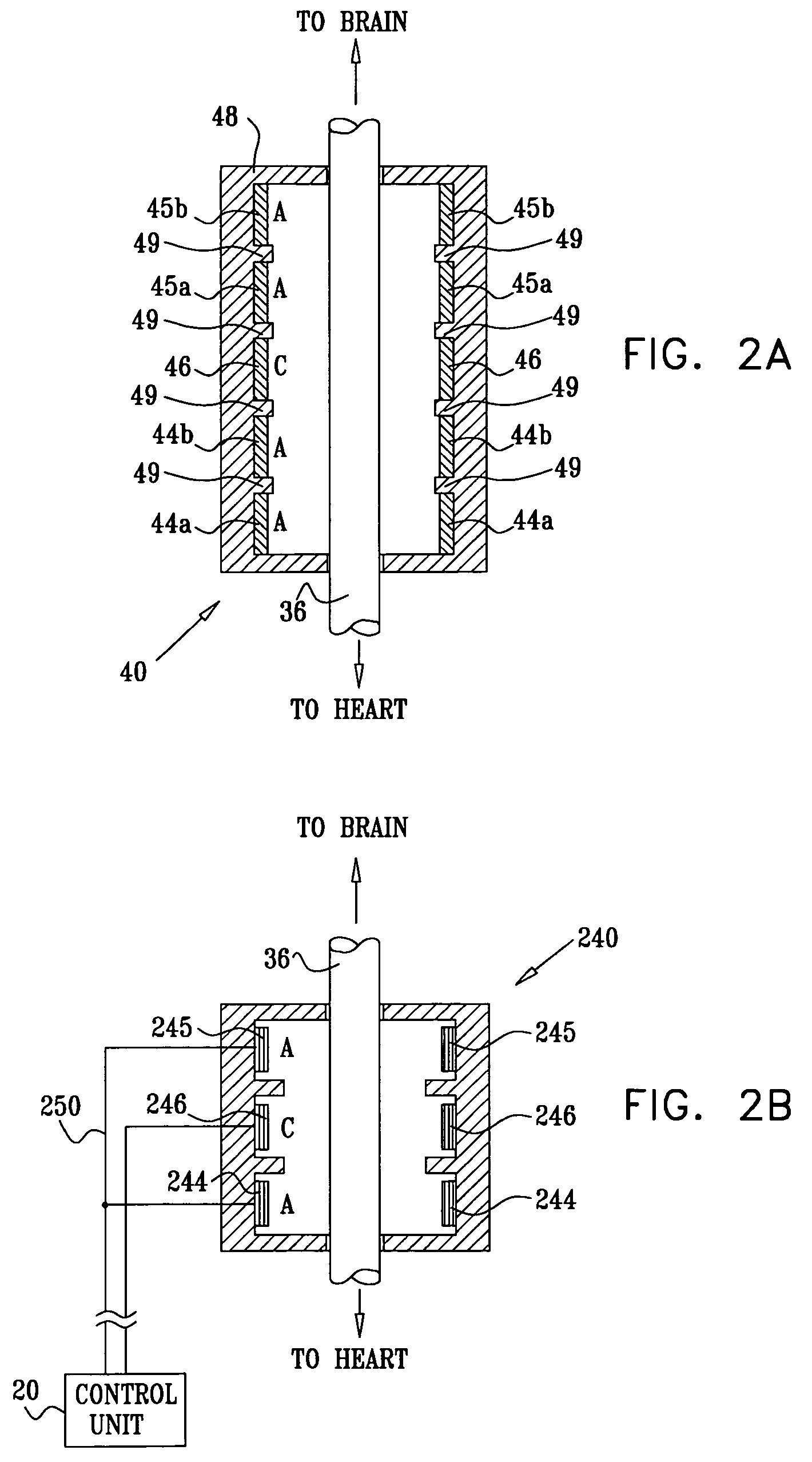
Apparatus is provided including an implantable sensor, adapted to sense an electrical parameter of a heart of a subject, and a first control unit, adapted to apply pulses to the heart responsively to the sensed parameter, the pulses selected from the list consisting of: pacing pulses and anti-arrhythmic energy. The apparatus further includes an electrode device, adapted to be coupled to a site of the subject selected from the list consisting of: a vagus nerve of the subject, an epicardial fat pad of the subject, a pulmonary vein of the subject, a carotid artery of the subject, a carotid sinus of the subject, a coronary sinus of the subject, a vena cava vein of the subject, a right ventricle of the subject, and a jugular vein of the subject; and a second control unit, adapted to drive the electrode device to apply to the site a current that increases parasympathetic tone of the subject and affects a heart rate of the subject. The first and second control units are not under common control. At least one of the control units is adapted to coordinate an aspect of its operation with an aspect of operation of the other control unit. Other embodiments are also described.
Owner:MEDTRONIC INC
Magnetically assisted pulmonary vein isolation
InactiveUS7008418B2Quickly and easily navigatedFacilitate fastElectrotherapyDiagnosticsVeinDistal portion
A method for forming an ablation pattern to electrically isolate a vessel having an ostium from a chamber formed within a patient for treatment of cardiac arrhythmia. The distal portion of a catheter is navigated to a chamber. An anchor member is deployed from the distal end of the catheter into the chamber, and the distal end is navigated into the ostium of the vessel, and temporarily secured in the vessel. An ablation member is deployed from the distal end of the catheter into the chamber and successively navigated into contact with tissue adjacent the ostium and ablating the tissue in contact with the ablation member to form a line of ablation. At least one of the navigating the distal portion of the catheter, navigating the anchor member and navigating the ablation member is performed by applying an external magnetic field to orient the device being navigated.
Owner:STEREOTAXIS
Tissue ablation device assembly and method for electrically isolating a pulmonary vein ostium from an atrial wall
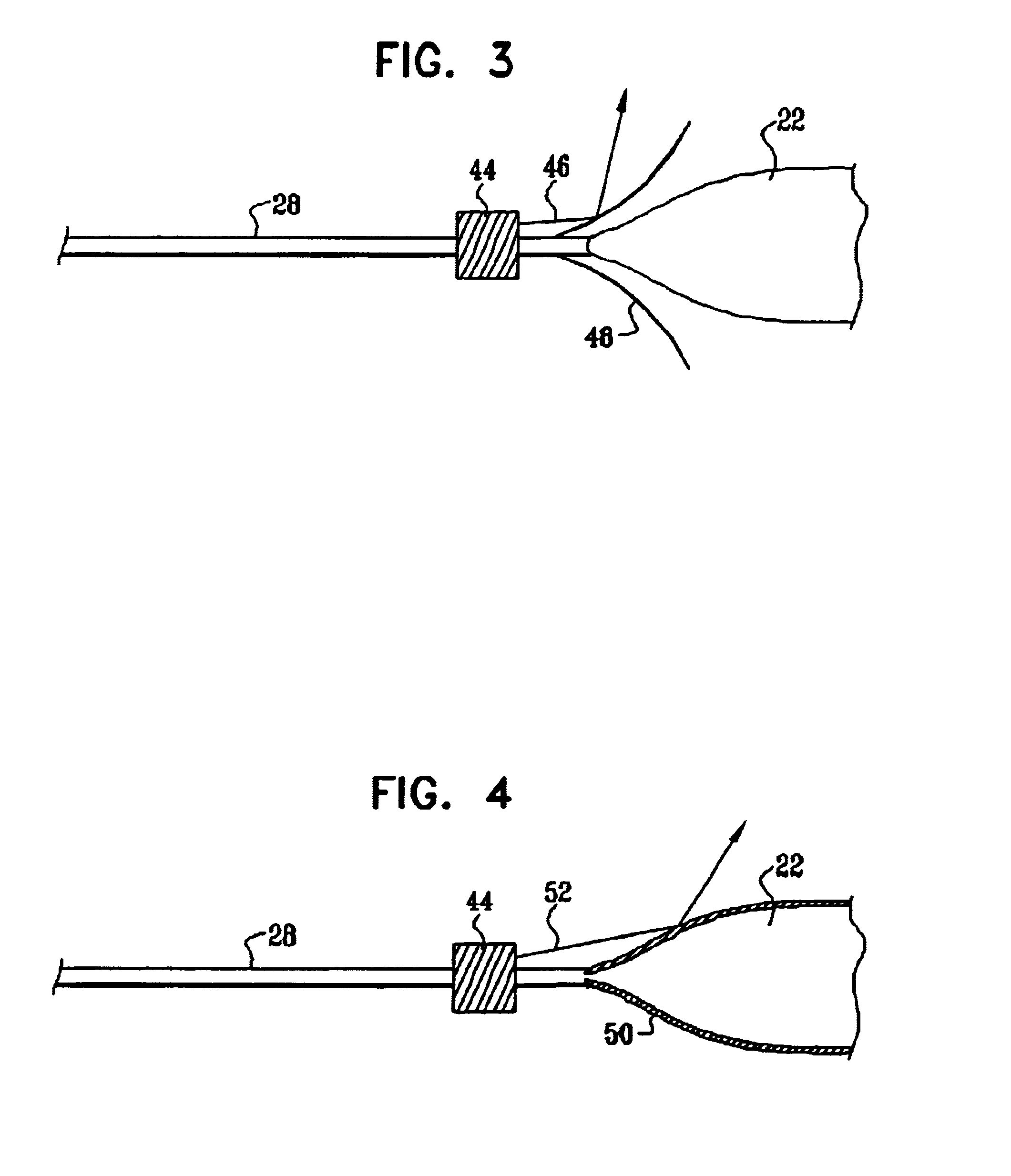
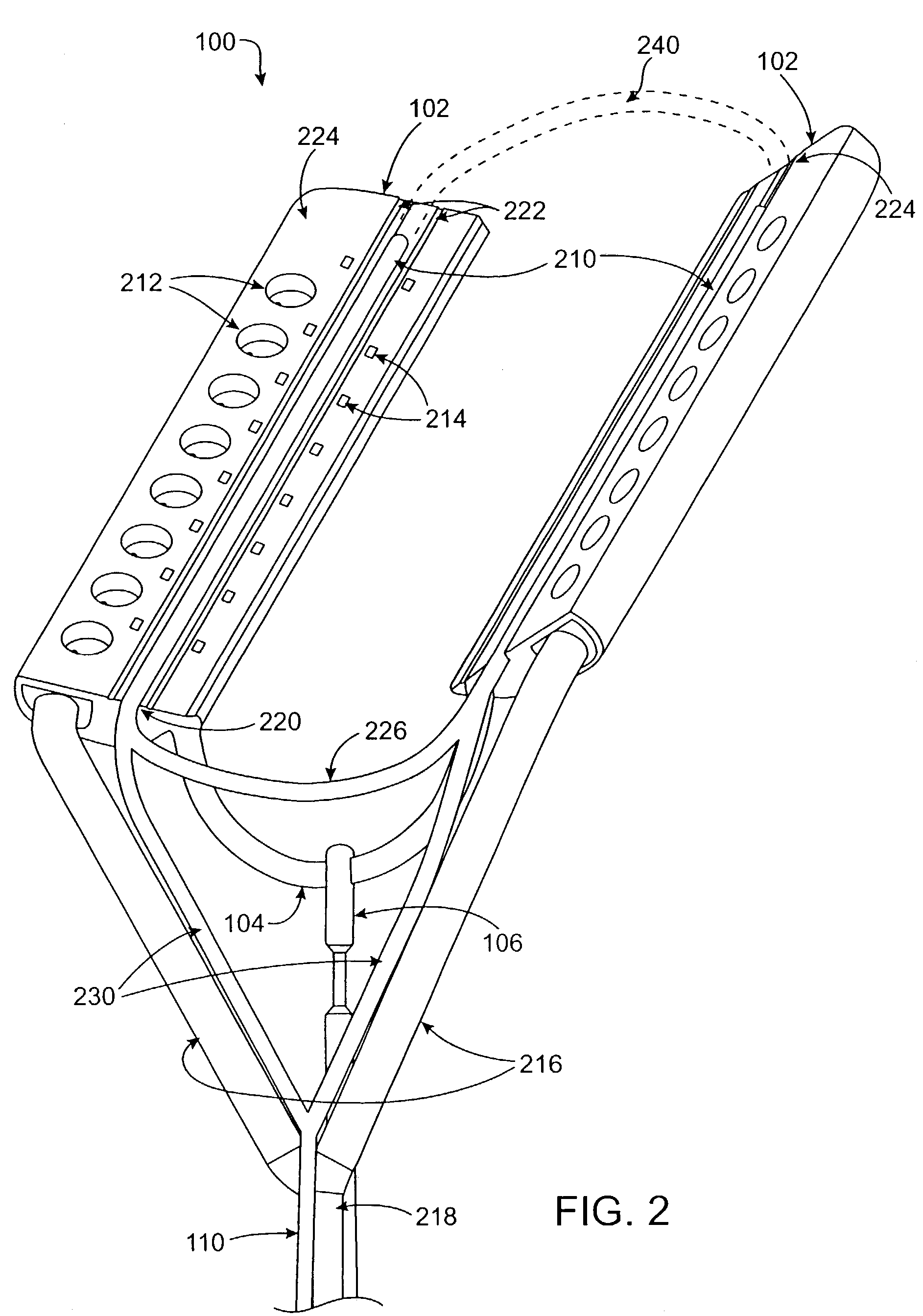
This invention is related to a tissue ablation system and method that treats atrial arrhythmia by ablating a circumferential region of tissue at a location where a pulmonary vein extends from an atrium. The system includes a circumferential ablation member with an ablation element and also includes a delivery assembly for delivering the ablation member to the location. The circumferential ablation member is generally adjustable between different configurations to allow both the delivery through a delivery sheath into the atrium and the ablative coupling between the ablation element and the circumferential region of tissue.
Owner:ATRIONIX
Ablation catheter and method for isolating a pulmonary vein
A catheter assembly and method for treatment of cardiac arrhythmia, for example, atrial fibrillation, by electrically isolating a vessel, such as a pulmonary vein, from a chamber, such as the left atrium. The catheter assembly includes a catheter body and at least one electrode. The catheter body includes a proximal portion, an intermediate portion and a distal portion. The intermediate portion extends from the proximal portion and defines a longitudinal axis. The distal portion extends from the intermediate portion and forms a substantially closed loop transverse to the longitudinal axis. The at least one electrode is disposed along the loop. With this configuration, the loop is axially directed into contact with the chamber wall about the vessel ostium. Upon energization, the electrode ablates a continuous lesion pattern about the vessel ostium, thereby electrically isolating the vessel from the chamber.
Owner:MEDTRONIC INC
Tissue ablation device assembly and method of electrically isolating a pulmonary vein ostium from an atrial wall
This invention is related to a tissue ablation system and method that treats atrial arrhythmia by ablating a circumferential region of tissue at a location where a pulmonary vein extends from an atrium. The system includes a circumferential ablation member with an ablation element and also includes a delivery assembly for delivering the ablation member to the location. The circumferential ablation member is generally adjustable between different configurations to allow both the delivery through a delivery sheath into the atrium and the ablative coupling between the ablation element and the circumferential region of tissue.
Owner:ATRIONIX
Tissue ablation device assembly and method for electrically isolating a pulmonary vein ostium from an atrial wall
This invention is related to a tissue ablation system and method that treats atrial arrhythmia by ablating a circumferential region of tissue at a location where a pulmonary vein extends from an atrium. The system includes a circumferential ablation member with an ablation element and also includes a delivery assembly for delivering the ablation member to the location. The circumferential ablation member is generally adjustable between different configurations to allow both the delivery through a delivery sheath into the atrium and the ablative coupling between the ablation element and the circumferential region of tissue.
Owner:ATRIONIX
Ablation catheter assembly and method for isolating a pulmonary vein
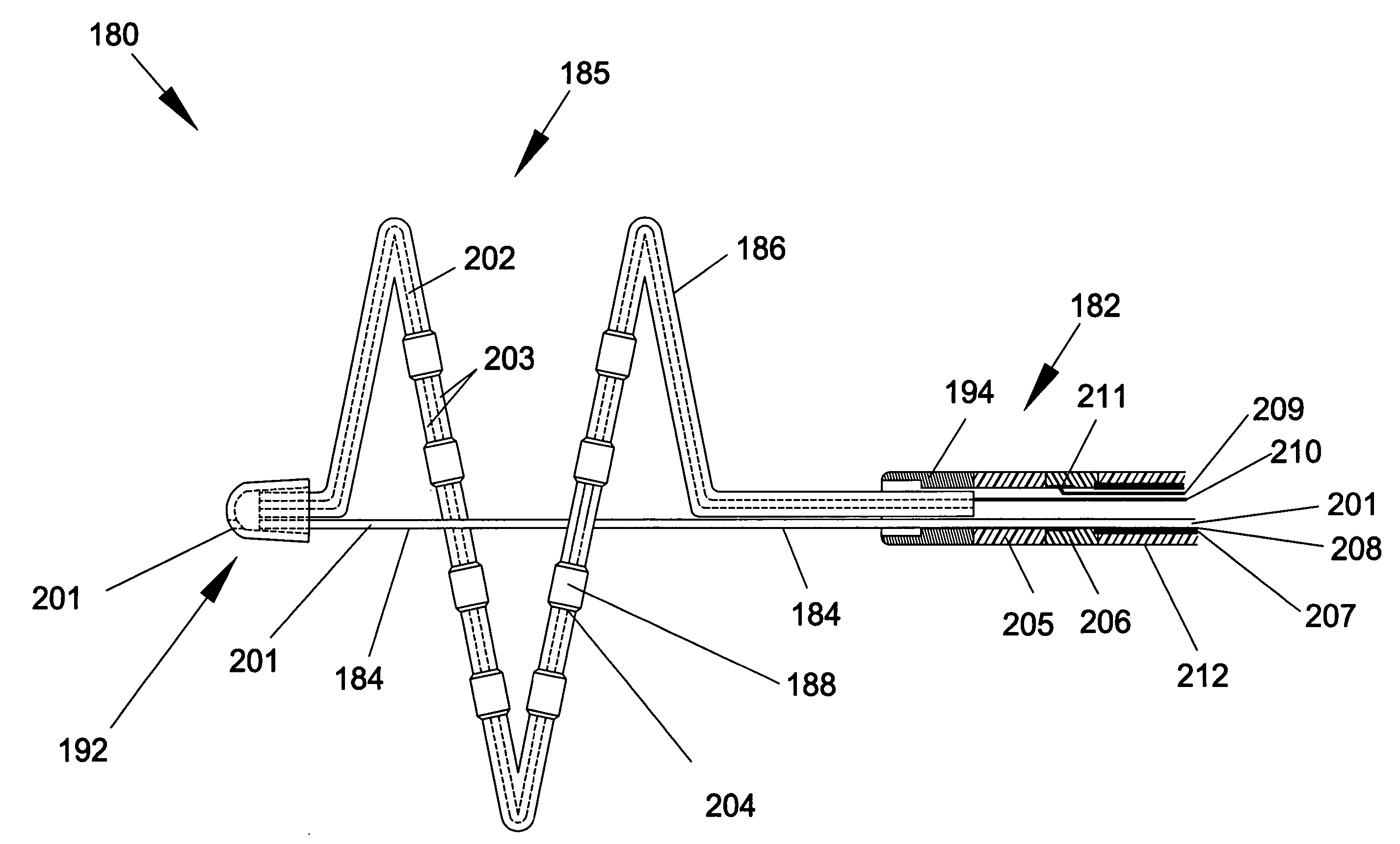
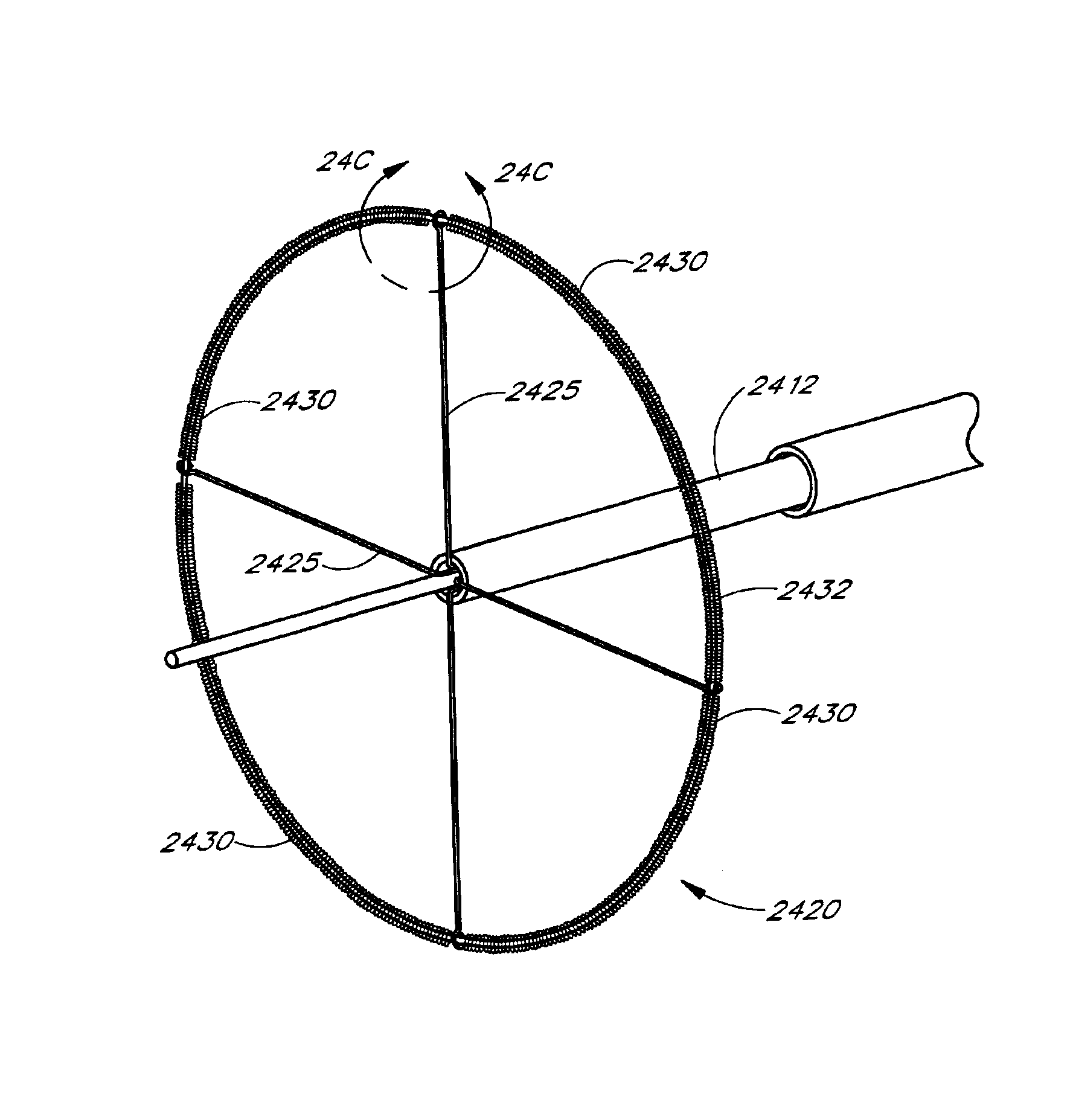
A catheter body includes a proximal portion, an intermediate portion extending from the proximal portion and defining a longitudinal axis, and a distal portion extending from the intermediate portion and terminated by a distal end of the catheter body; the distal portion forms a coil about a central loop axis, which is substantially parallel to the longitudinal axis. A first lumen extends through the proximal and intermediate portions of the catheter body and is terminated at an opening proximal to an ablation section, which is formed along the coil.
Owner:MEDTRONIC INC
Coaxial catheter instruments for ablation with radiant energy
InactiveUS20050038419A9Rapid and effective photoablationLess timeStentsUltrasound therapyCoaxial catheterTarget tissue
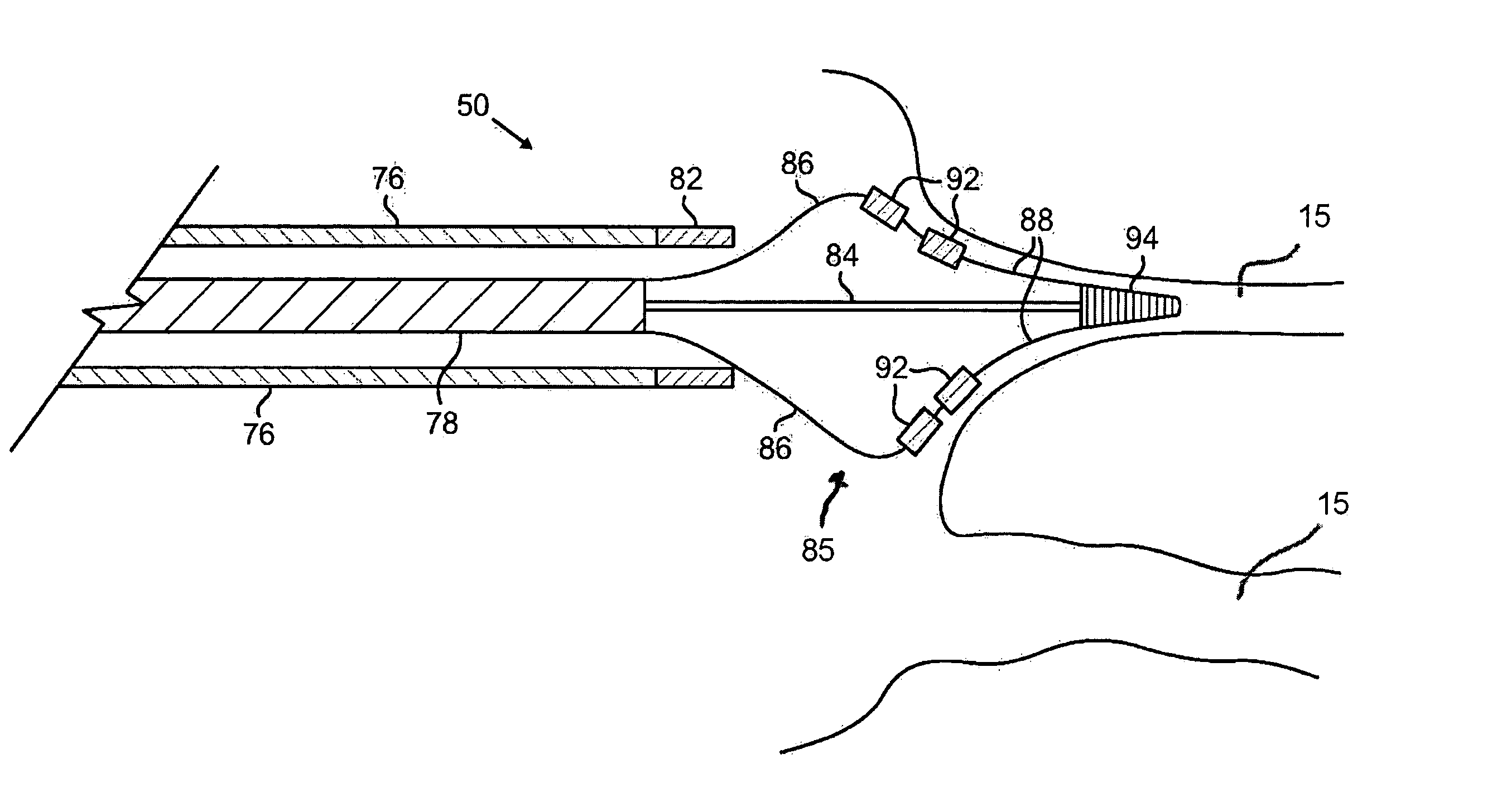
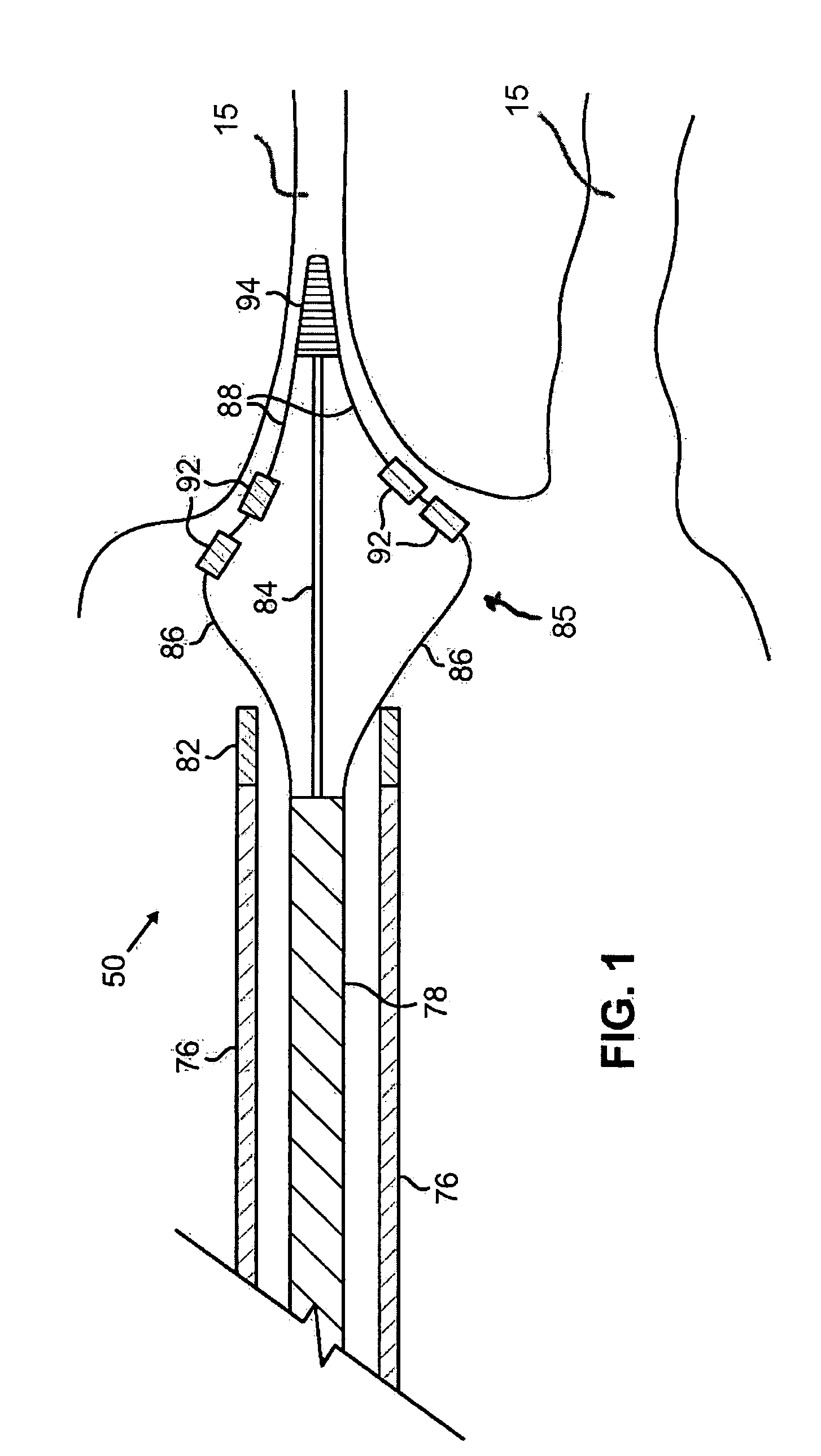
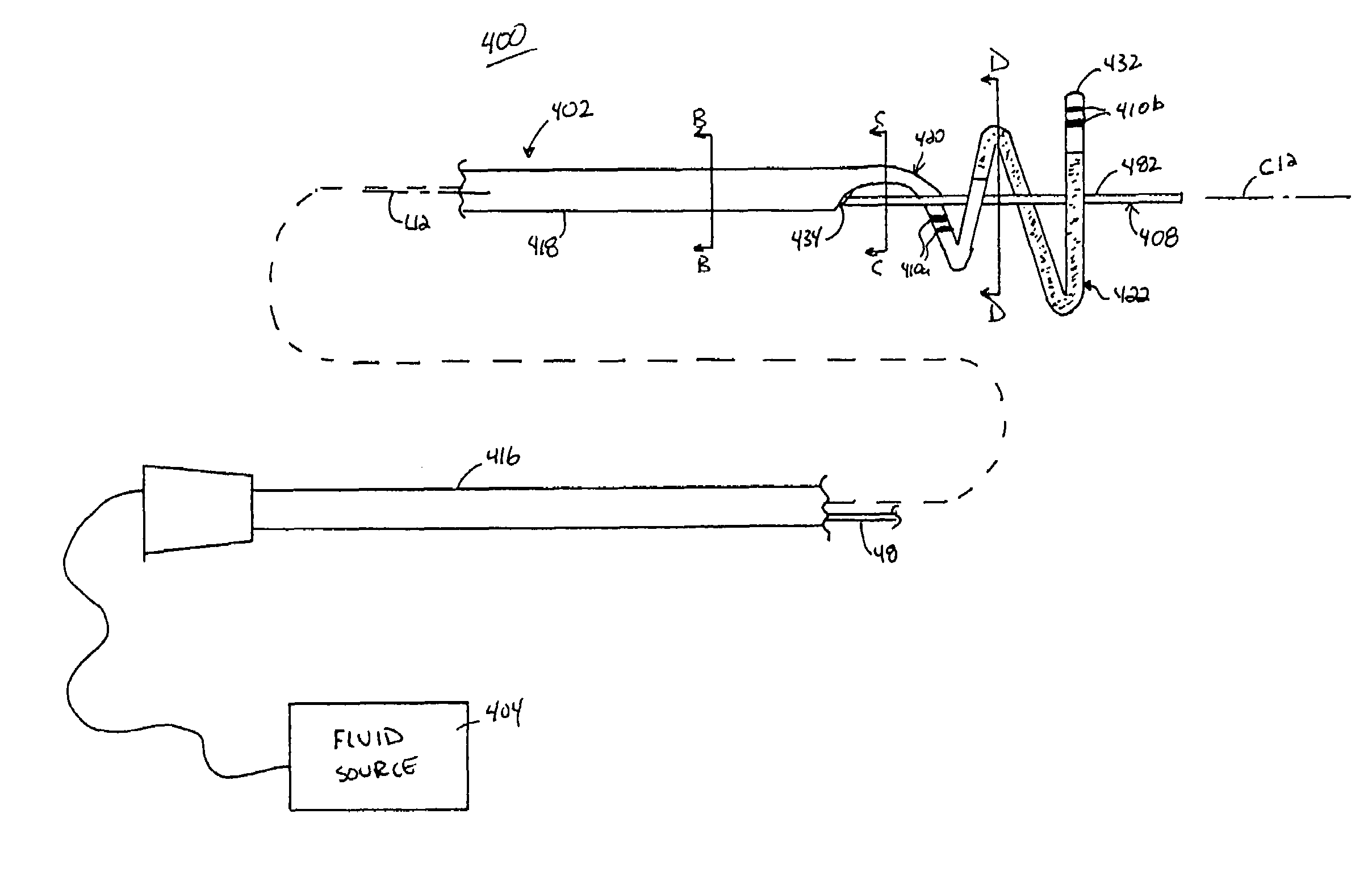
Ablation methods and instruments are disclosed for creating lesions in tissue, especially cardiac tissue for treatment of arrhythmias and the like. Percutaneous ablation instruments in the form of coaxial catheter bodies are disclosed having at least one central lumen therein and having one or more balloon structures at the distal end region of the instrument. The instruments include an energy emitting element which is independently positionable within the lumen of the instrument and adapted to project radiant energy through a transmissive region of a projection balloon to a target tissue site. The instrument can optionally include at least one expandable anchor balloon disposed about, or incorporated into an inner catheter body designed to be slid over a guidewire. This anchor balloon can serve to position the device within a lumen, such as a pulmonary vein. A projection balloon structure is also disclosed that can be slid over the first (anchor balloon) catheter body and inflated within the heart, to define a staging from which to project radiant energy. An ablative fluid can also be employed outside of the instrument (e.g., between the balloon and the target region) to ensure efficient transmission of the radiant energy when the instrument is deployed. In another aspect of the invention, generally applicable to a wide range of cardiac ablation instruments, mechanisms are disclosed for determining whether the instrument has been properly seated within the heart, e.g., whether the device is in contact with a pulmonary vein and / or the atrial surface, in order to form a lesion by heating, cooling or projecting energy. This contact-sensing feature can be implemented by an illumination source situated within the instrument and an optical detector that monitors the level of reflected light. Measurements of the reflected light (or wavelengths of the reflected light) can thus be used to determine whether contact has been achieved and whether such contact is continuous over a desired ablation path.
Owner:CARDIOFOCUS INC
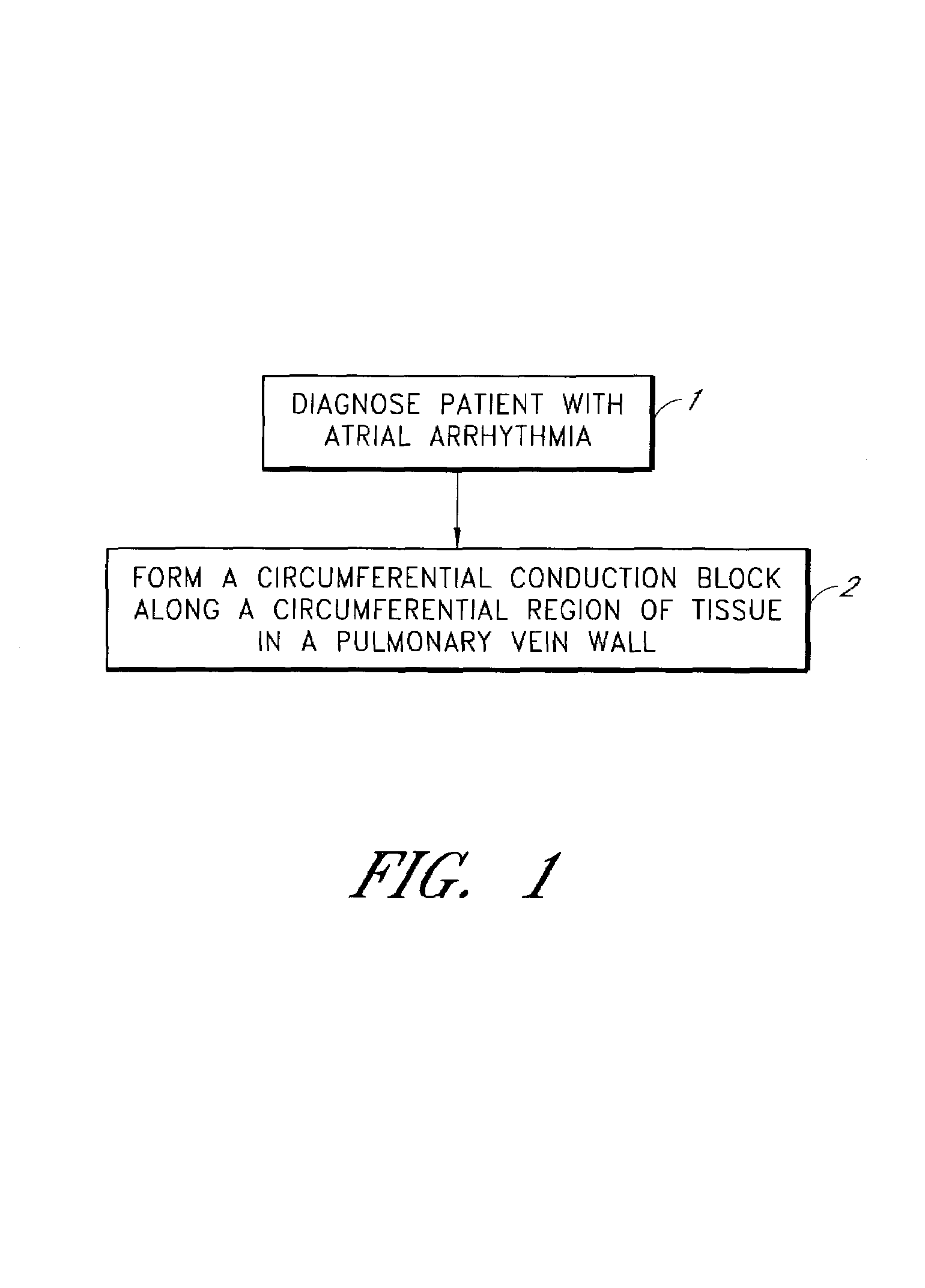
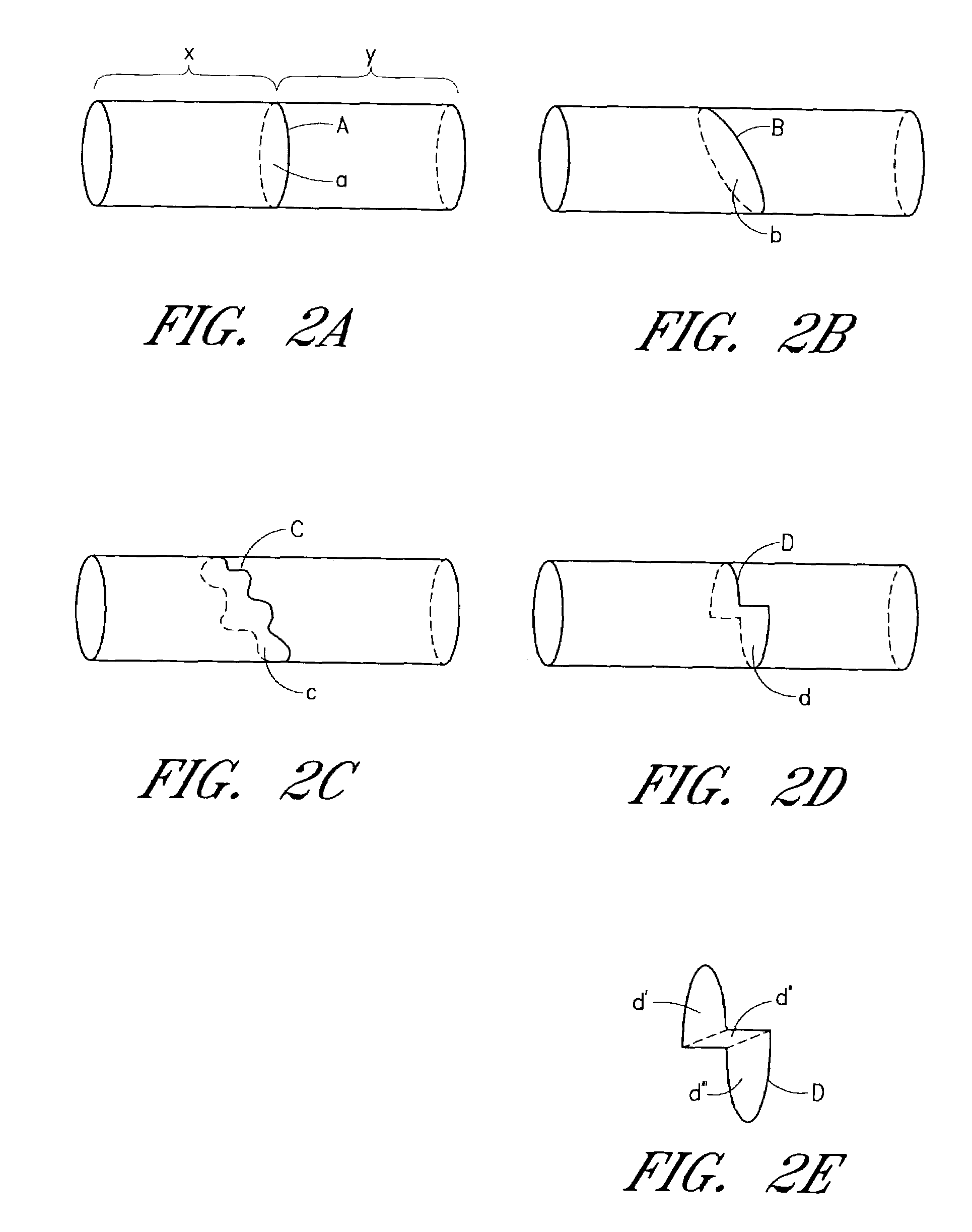
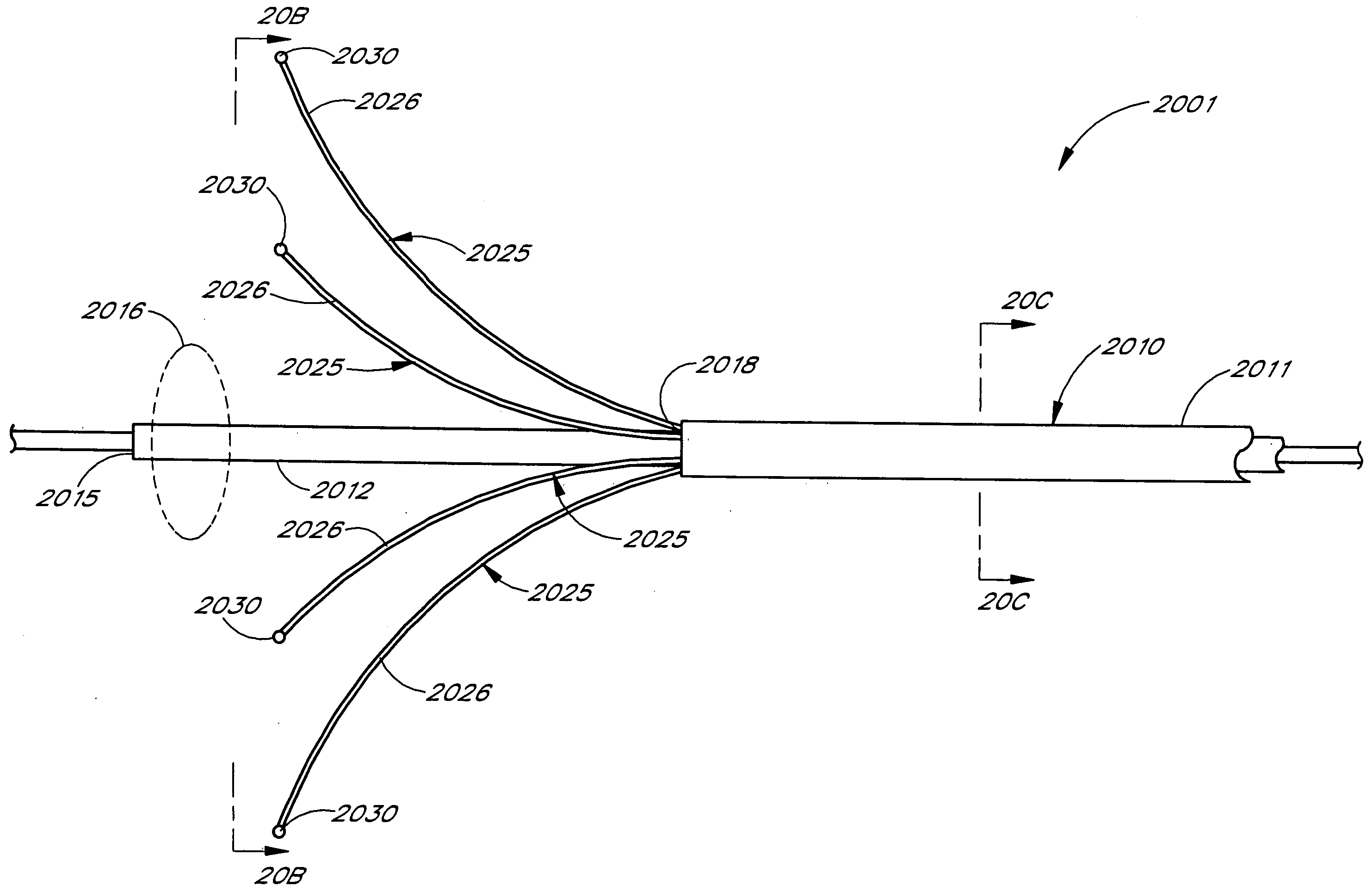
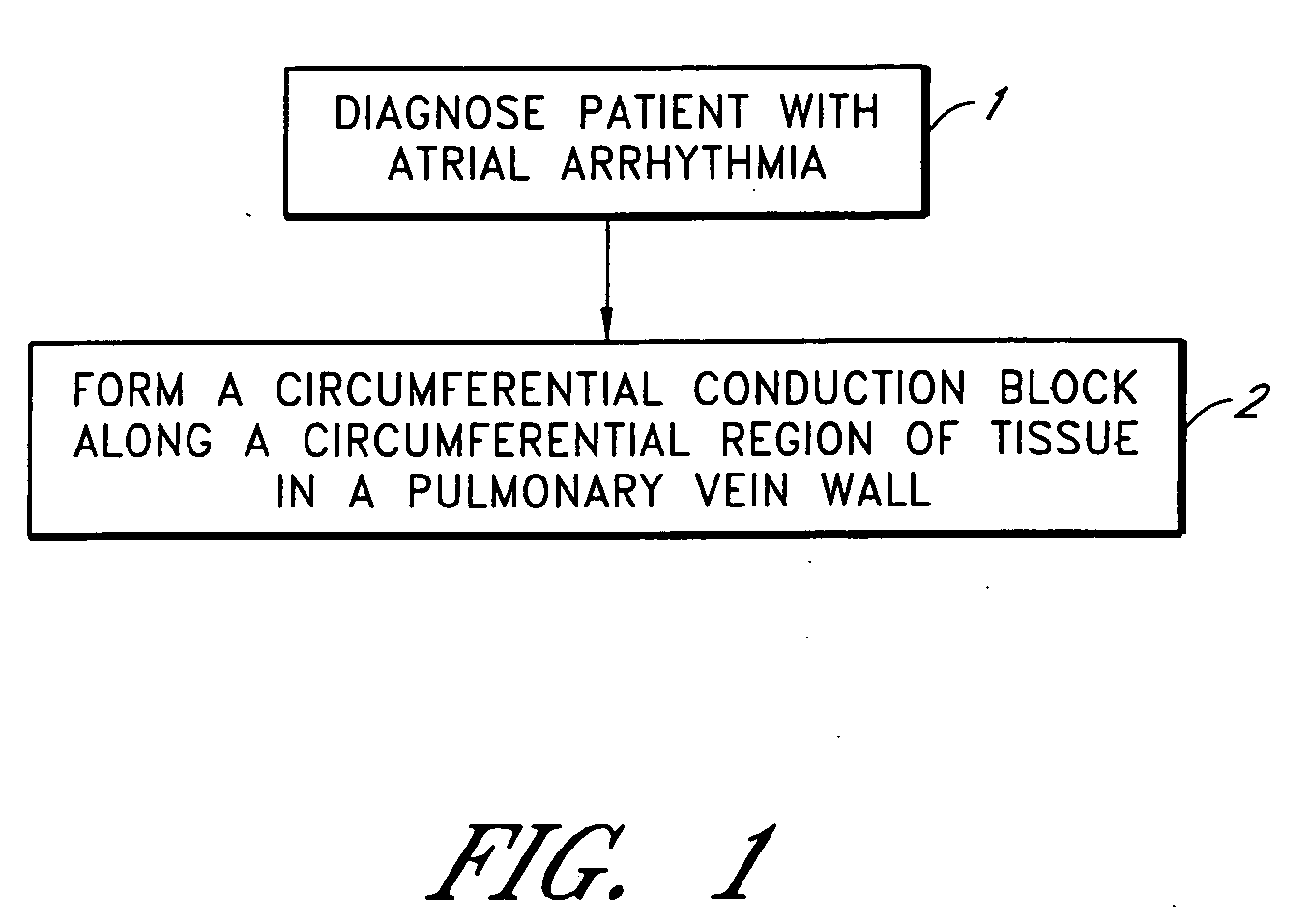
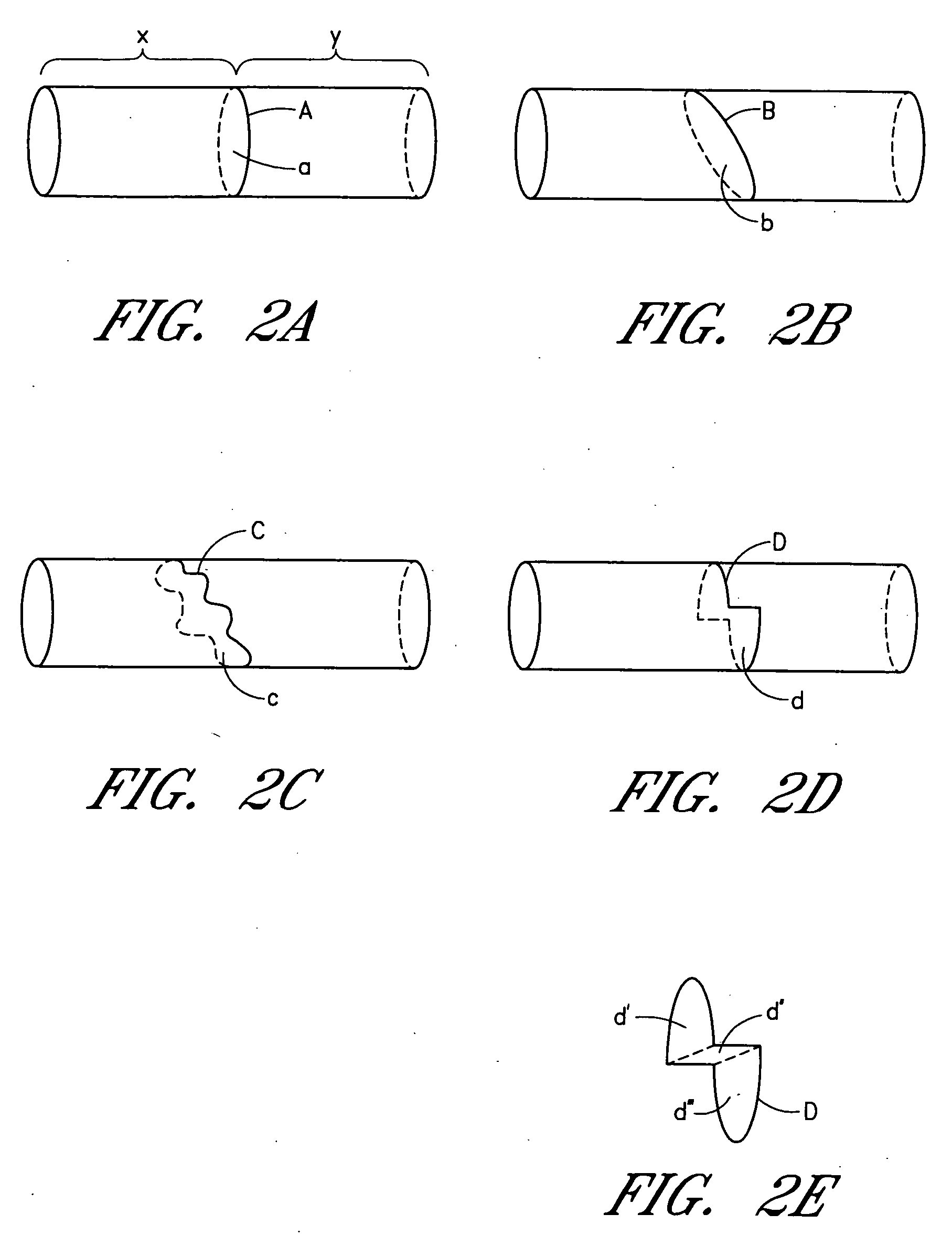
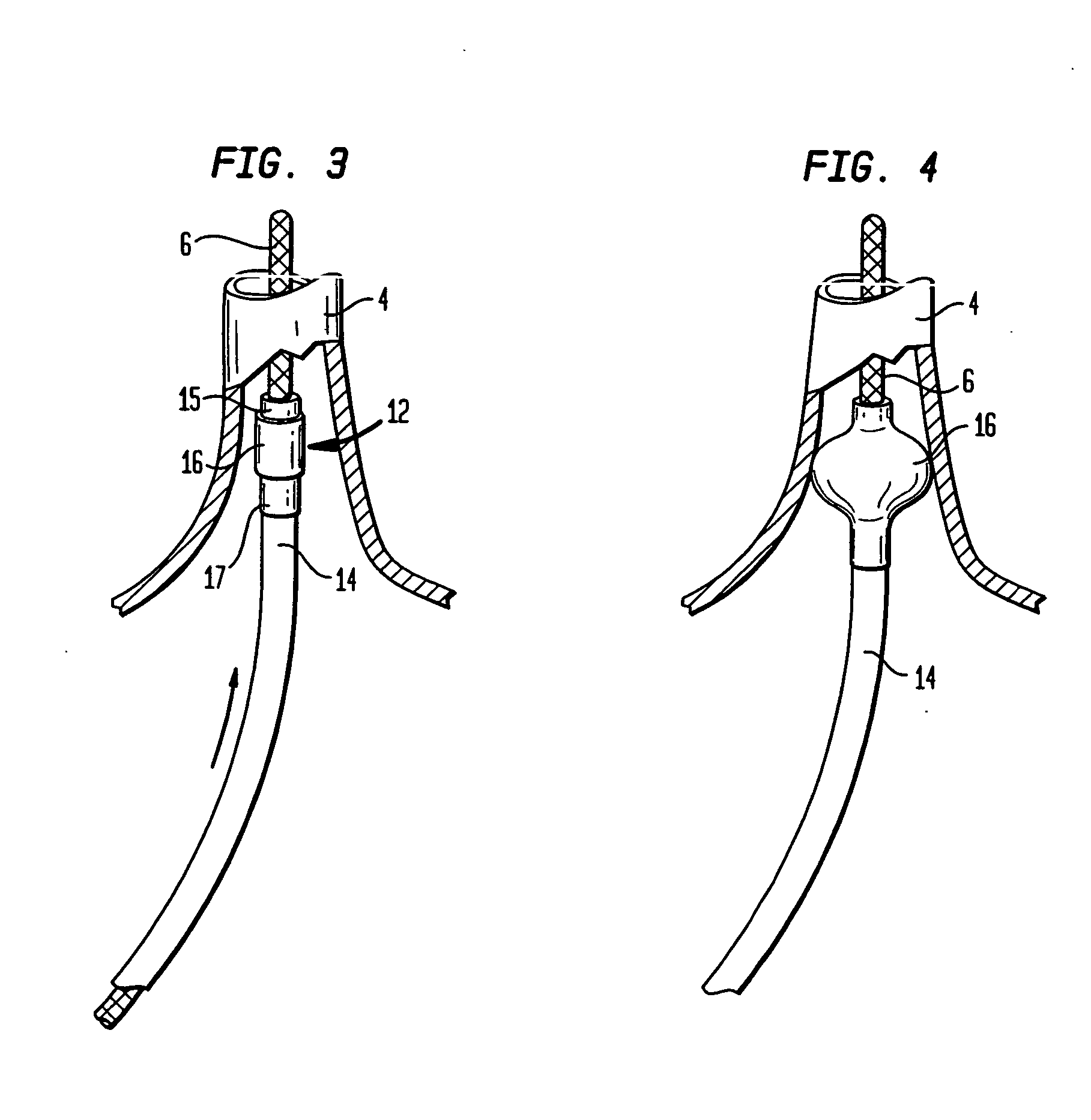
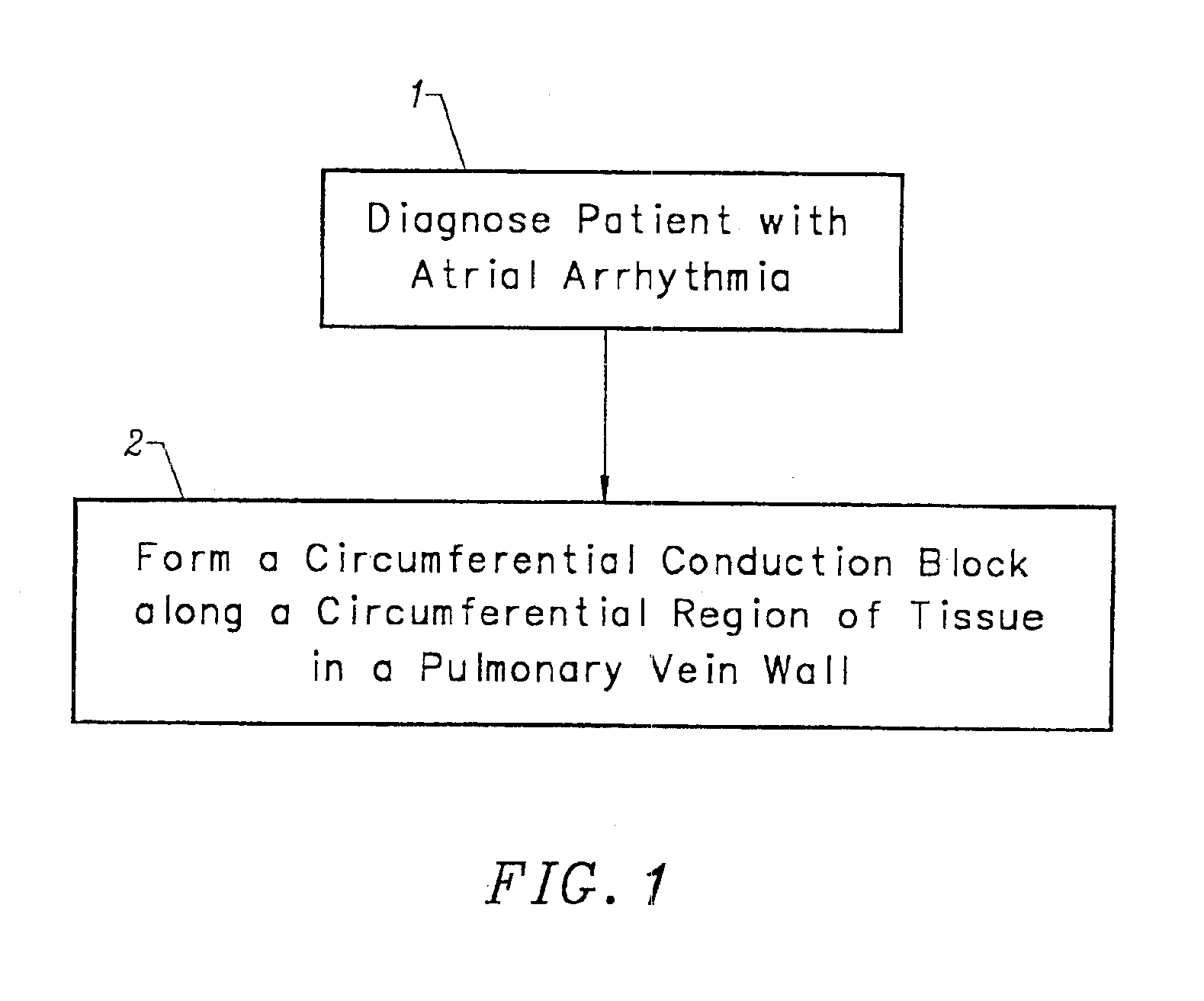
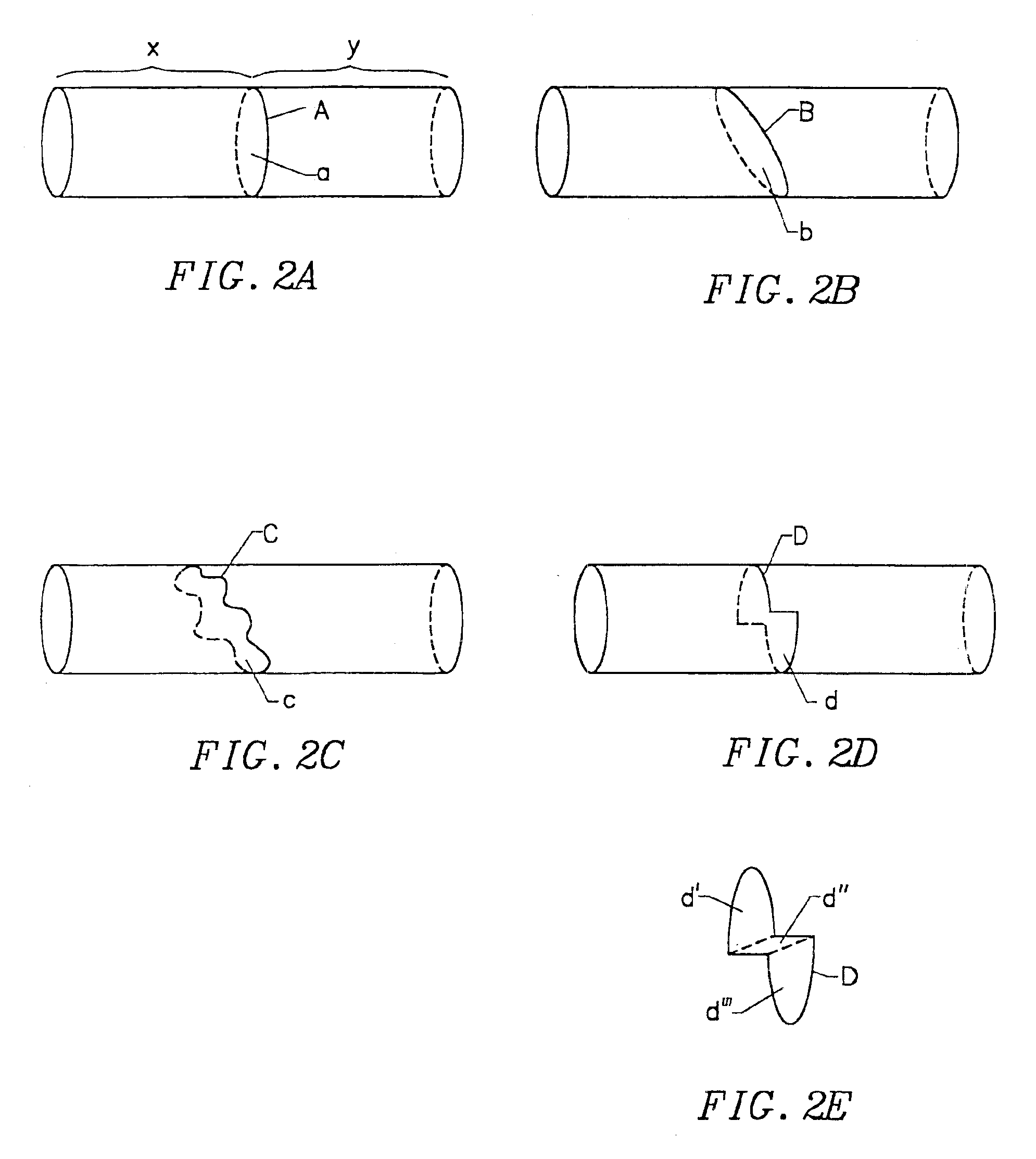
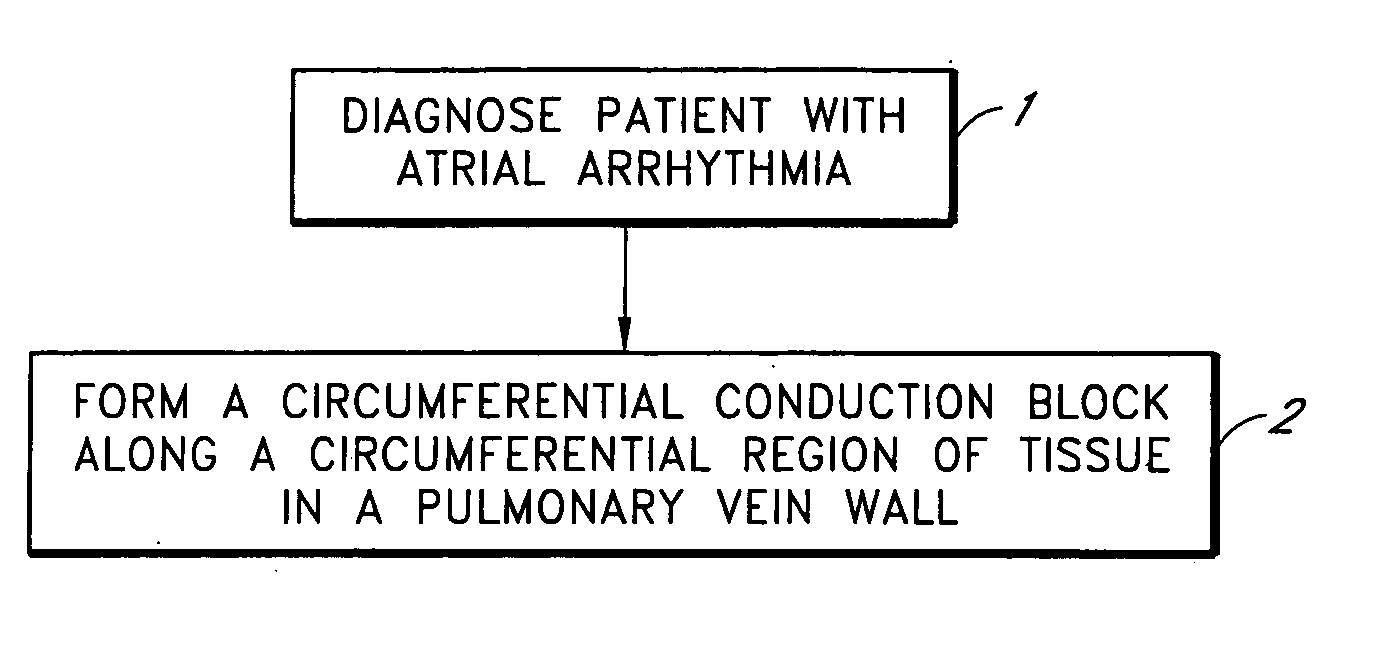
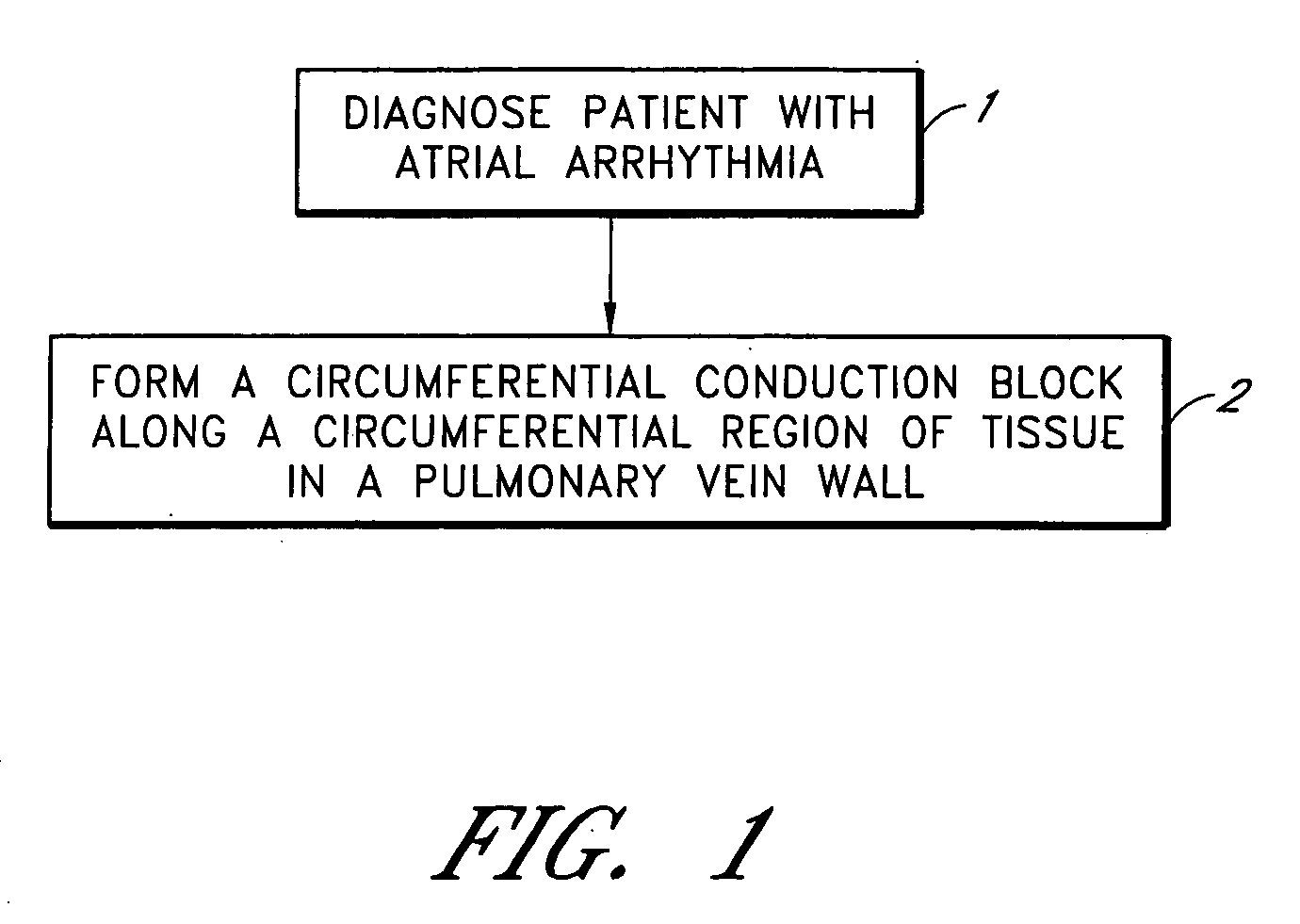
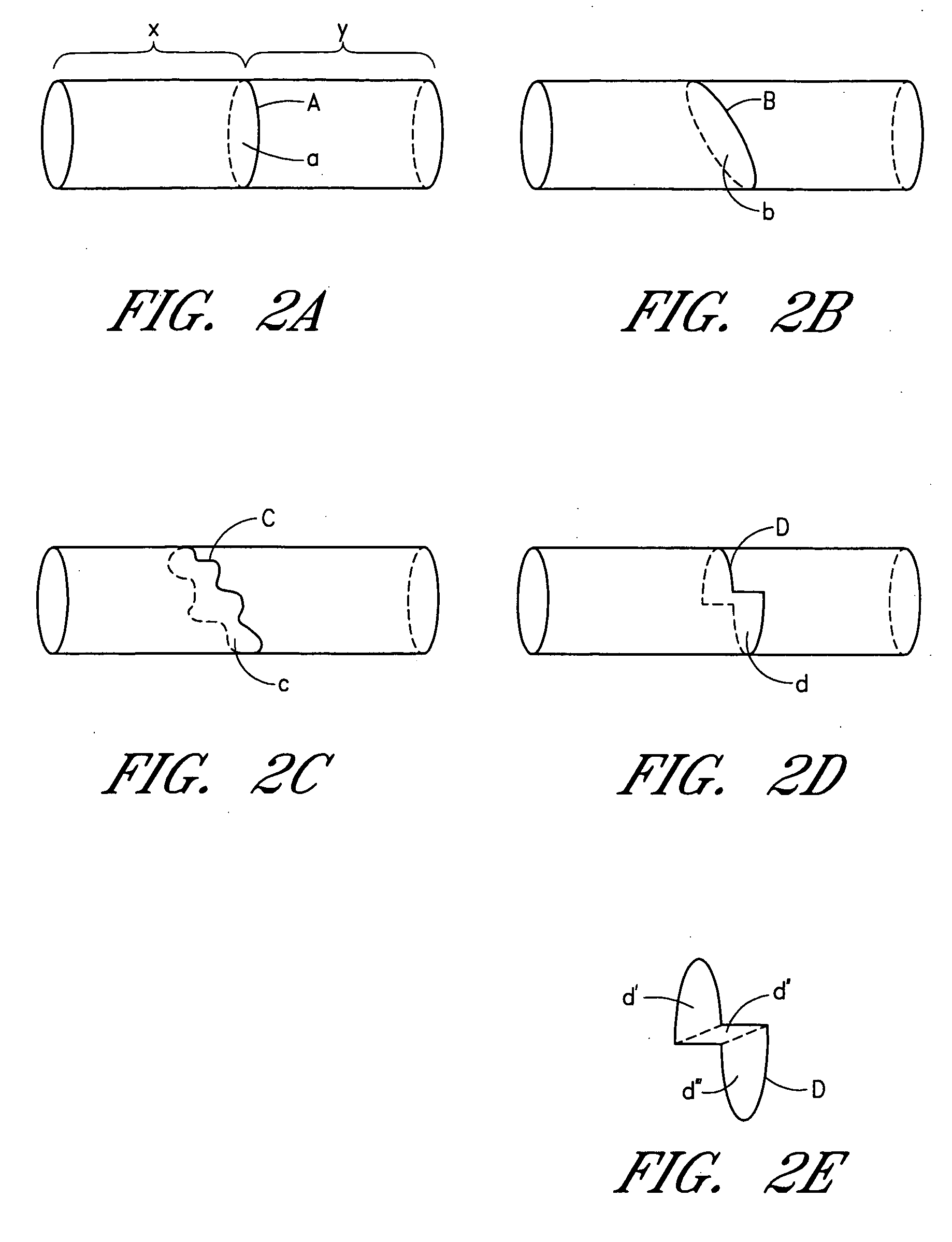
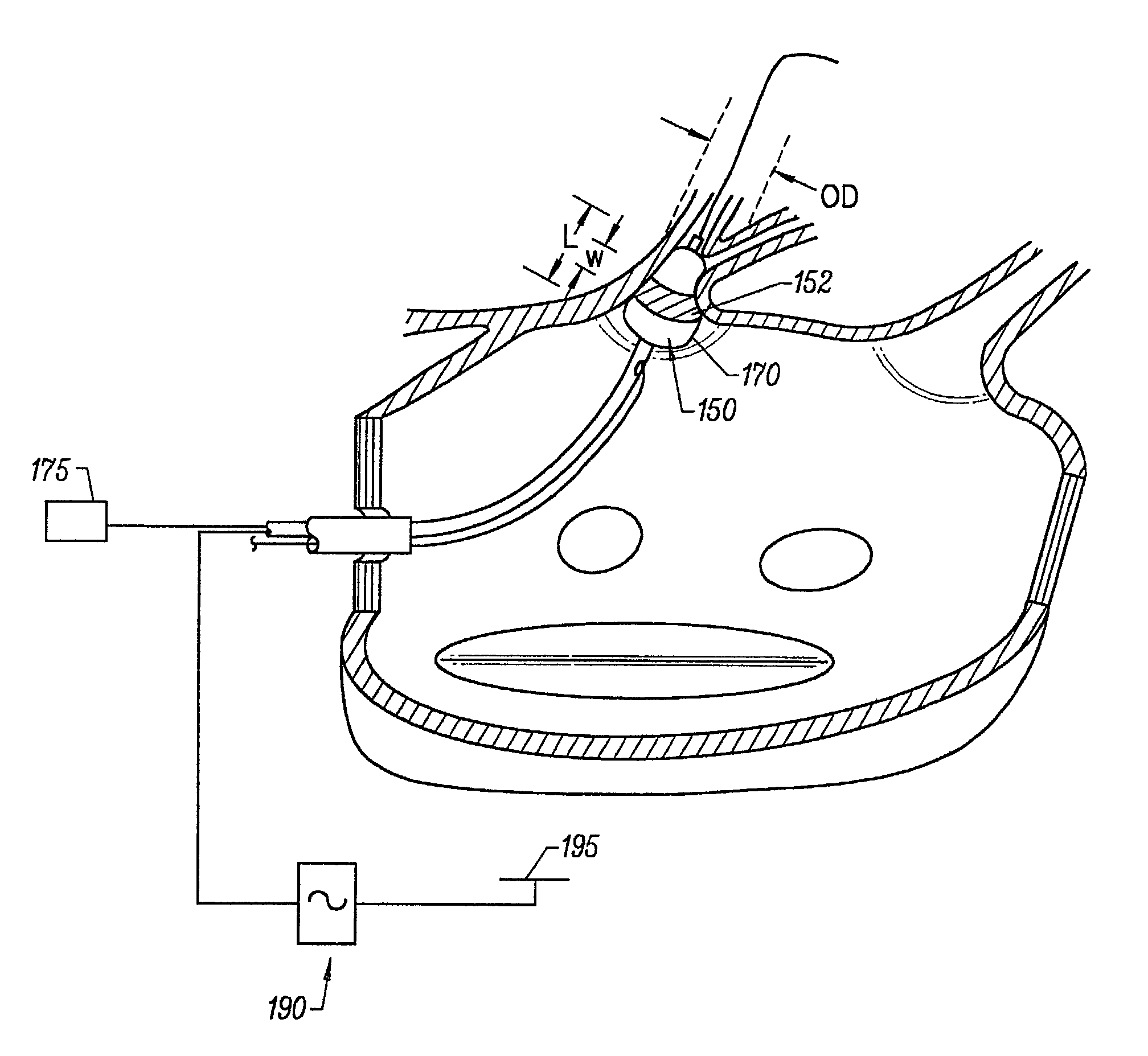
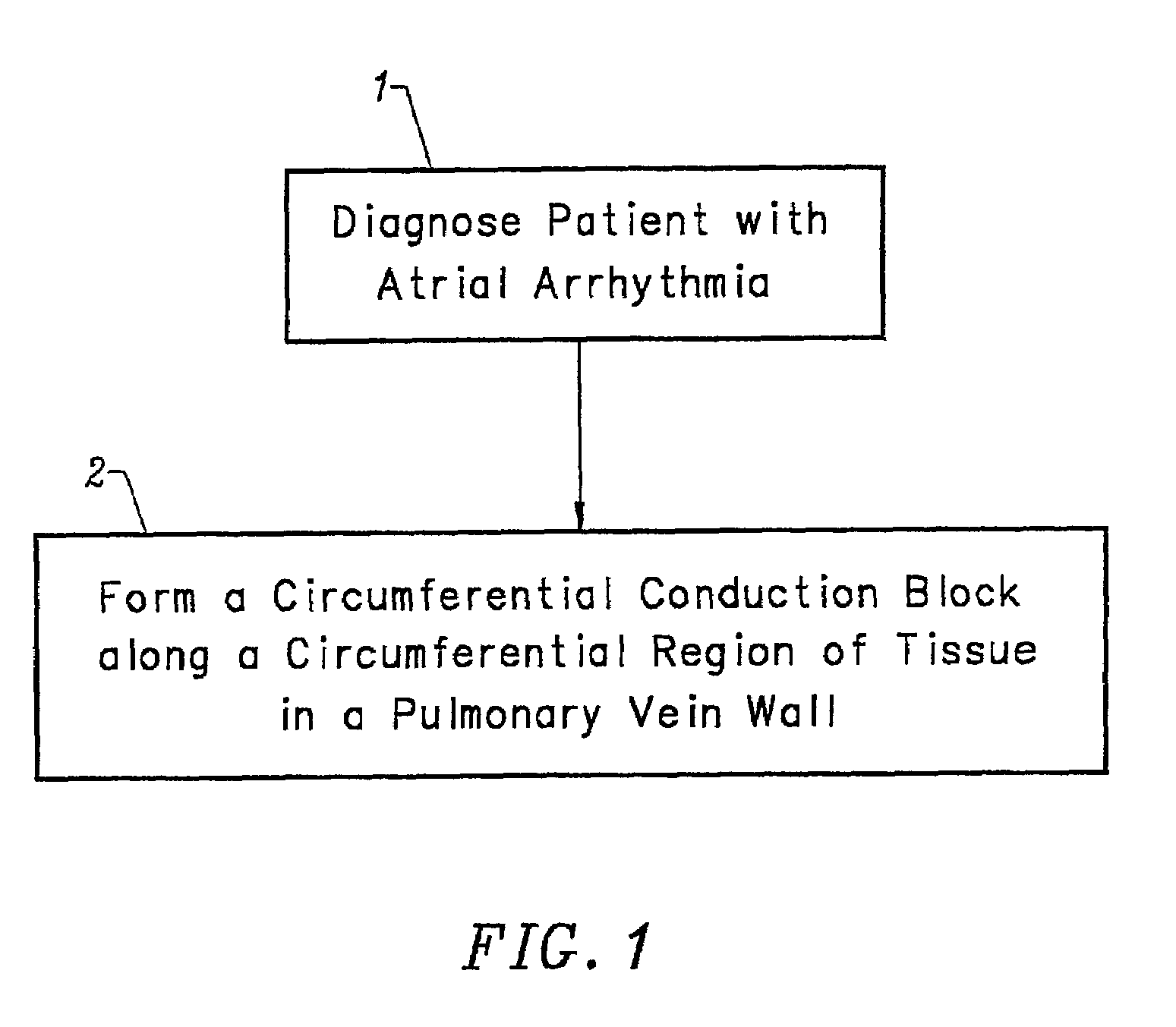
Device and method for forming a circumferential conduction block in a pulmonary vein
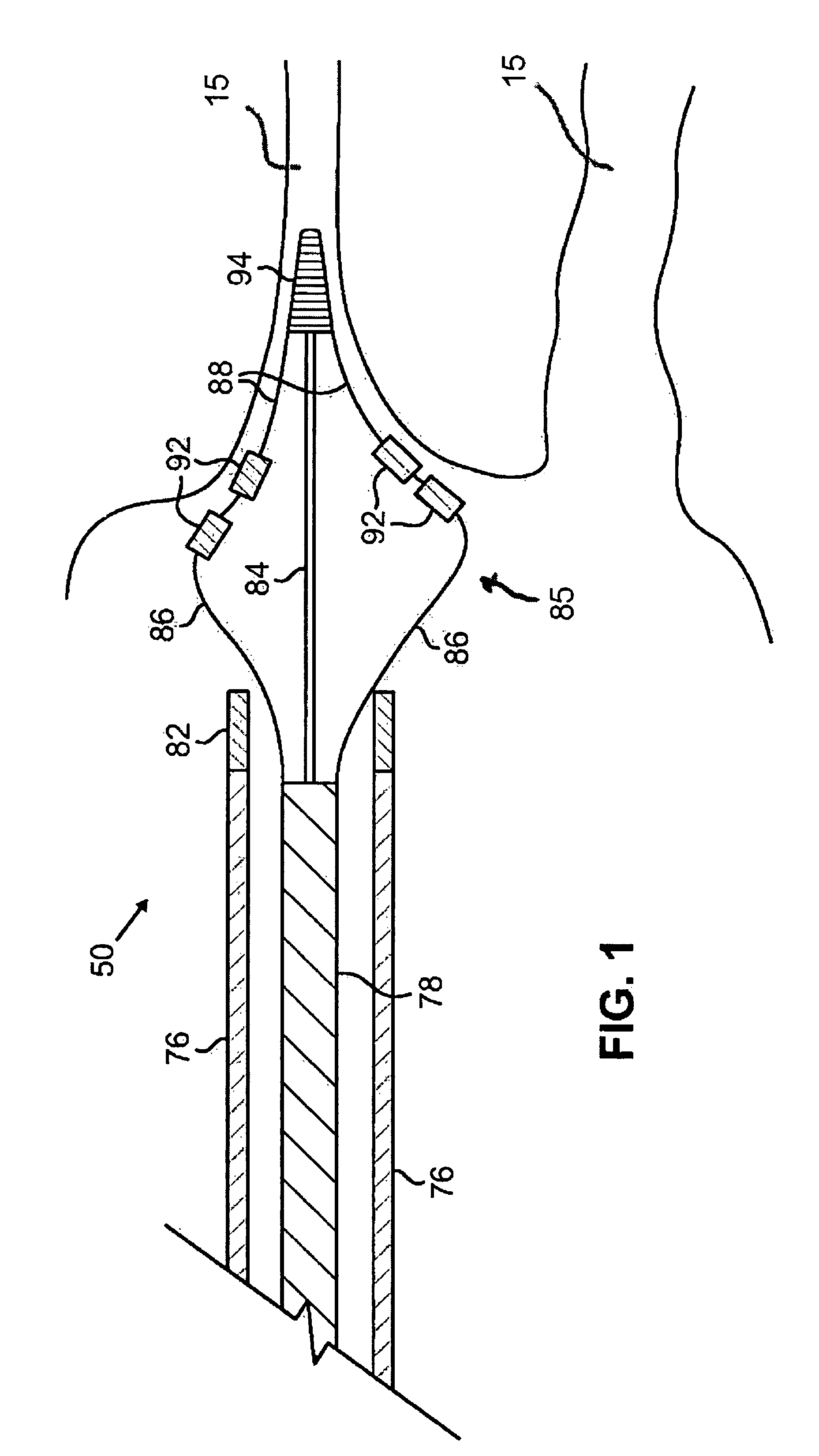
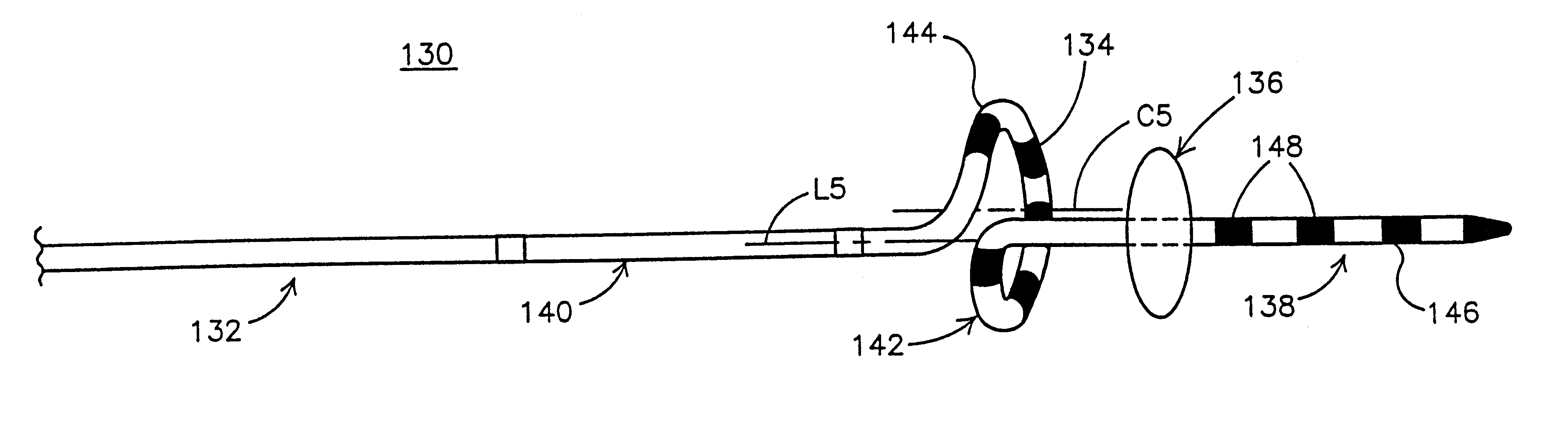
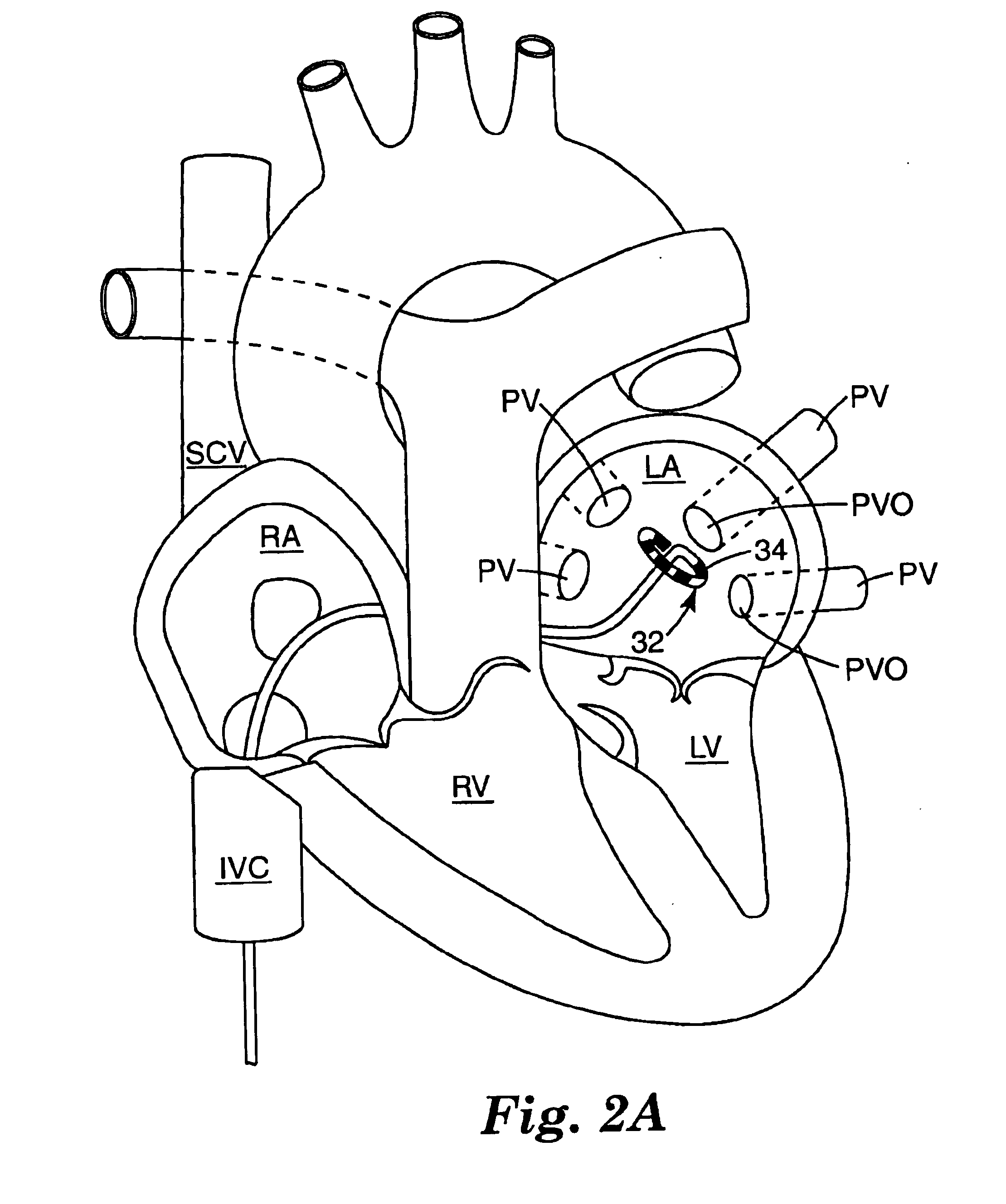
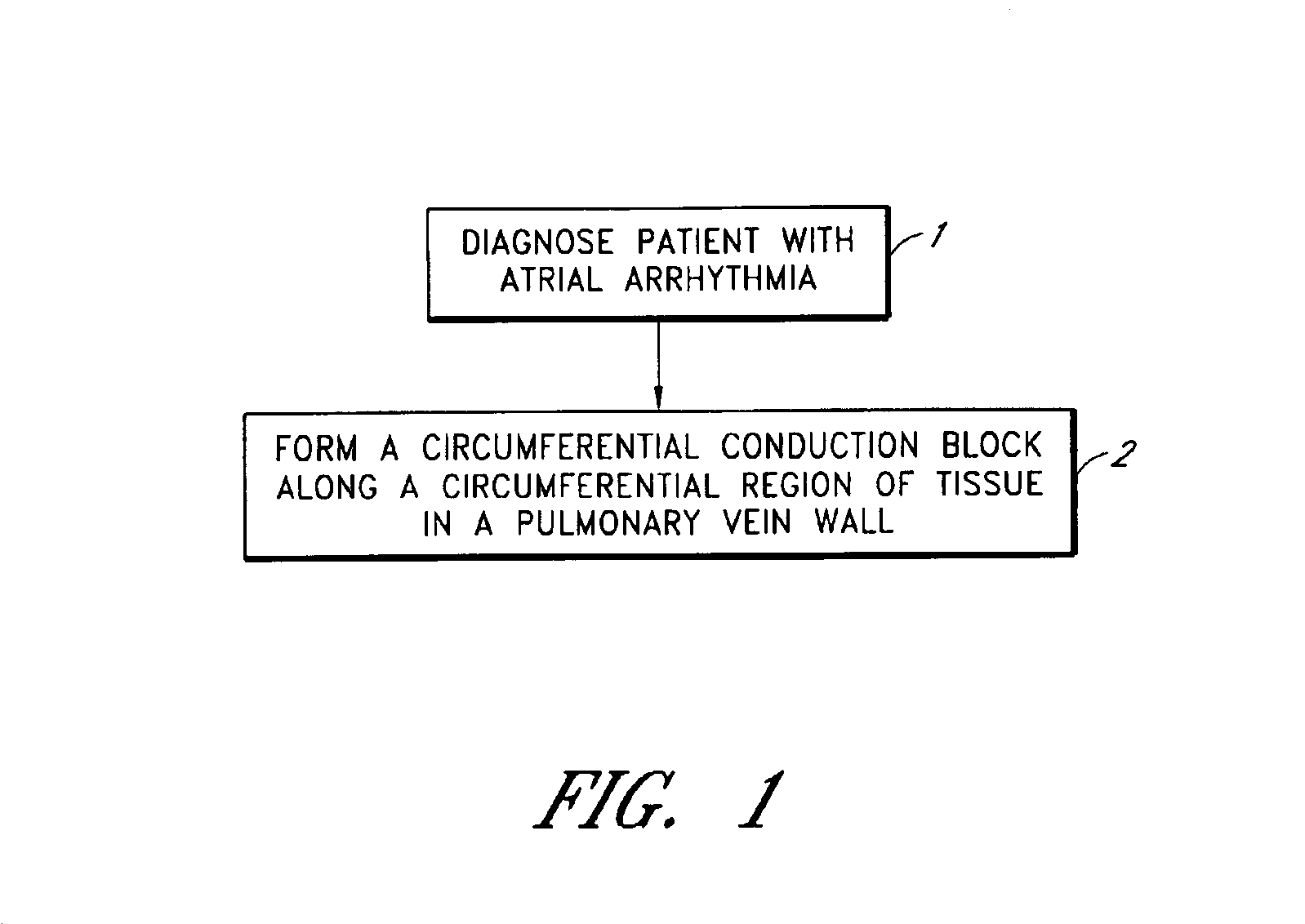
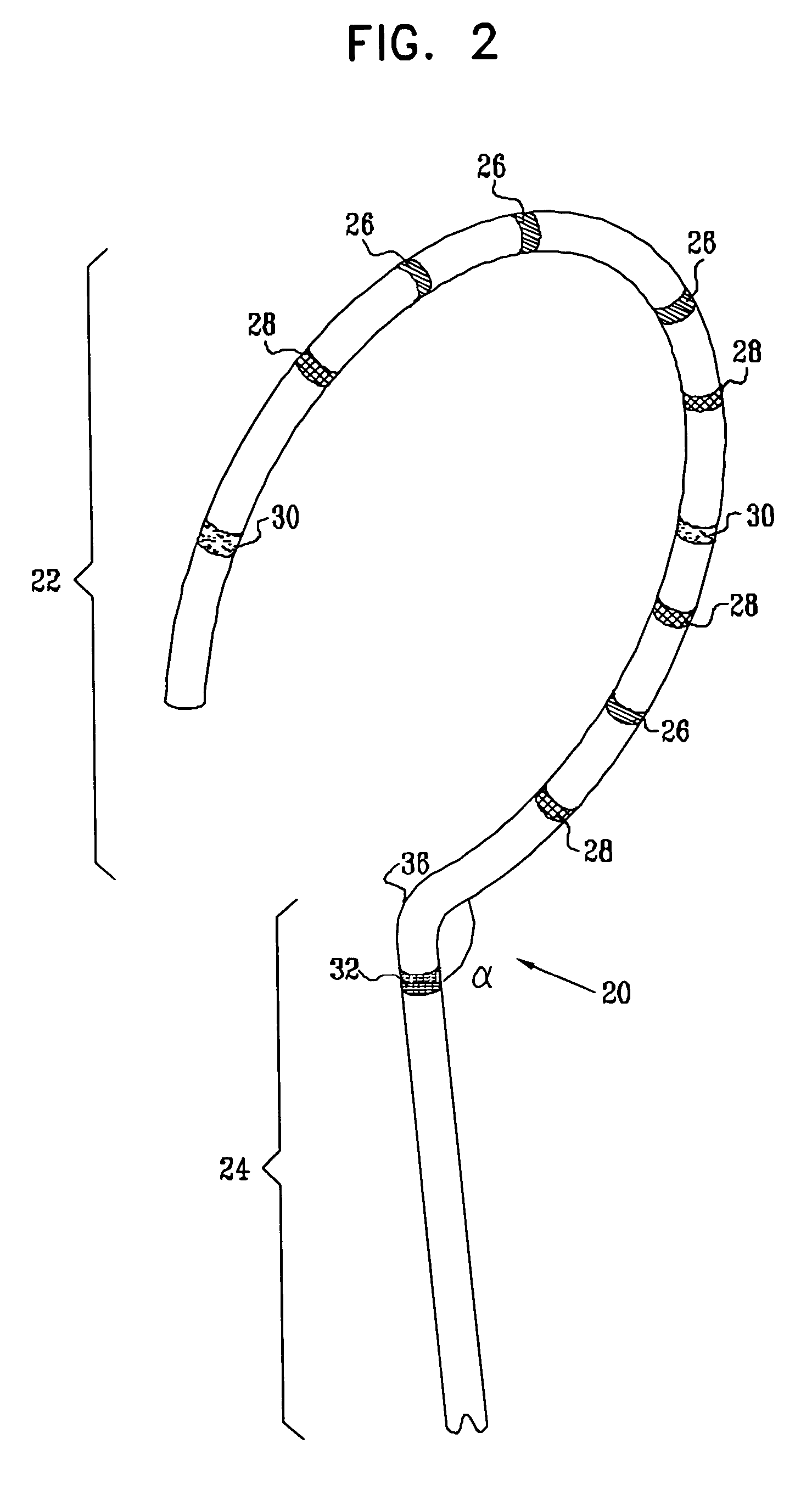
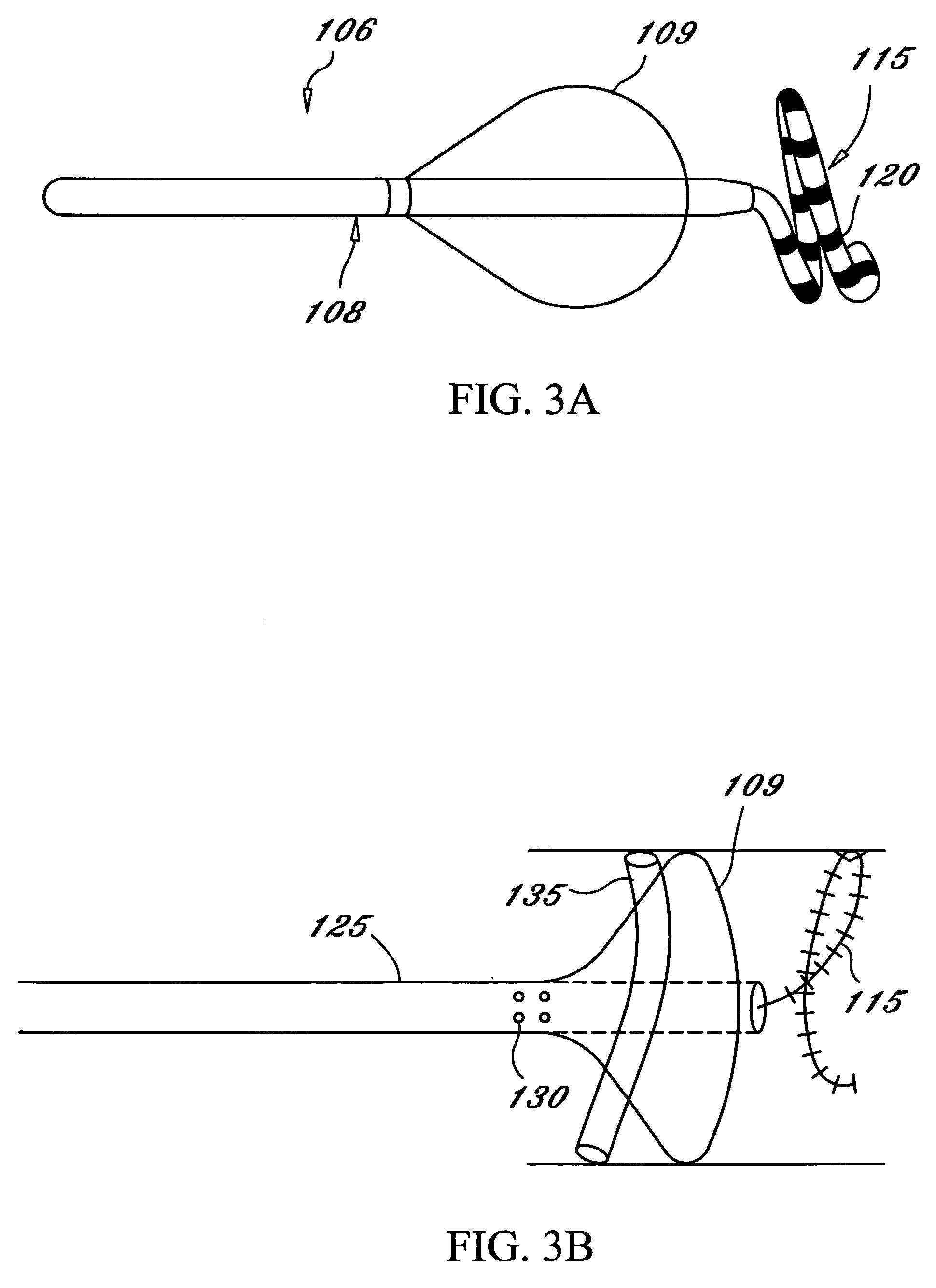
This invention is a method for treating a patient diagnosed with atrial arrhythmia by forming a circumferential conduction block along a circumferential path of tissue in a pulmonary vein wall that circumscribes the pulmonary vein lumen and transects the electrical conductivity of the pulmonary vein such that conduction is blocked along the longitudinal axis of the vein wall and into the left atrial wall. The method is performed to treat a patient with a focal arrythmogenic origin along the pulmonary vein wall by either ablating the focal origin or by isolating the focal origin from the atrial wall with the circumferential conduction block. The circumferential conduction block is also formed in a pulmonary vein in order to bridge the adjacent ends of two linear lesions, wherein each linear lesion is formed to extend between the pulmonary vein and another adjacent pulmonary vein in a less-invasive “maze”-type procedure. A circumferential ablation element in a circumferential ablation device assembly is used in a percutaneous translumenal catheter technique in order to form the circumferential conduction block in the pulmonary vein wall.
Owner:RGT UNIV OF CALIFORNIA
Method and device for performing cooling or cryo-therapies, for, e.g., angioplasty with reduced restenosis or pulmonary vein cell necrosis to inhibit atrial fibrillation employing tissue protection
InactiveUS7001378B2Minimize and inhibit bio-chemical eventRobust designCatheterSurgical instruments for heatingPercent Diameter StenosisPercutaneous angioplasty
An enhanced method and device are provided to treat atrial fibrillation or inhibit or reduce restenosis following angioplasty or stent placement. A balloon-tipped catheter is disposed in the area treated or opened through balloon angioplasty immediately following angioplasty. The balloon, which can have a dual balloon structure, may be delivered through a guiding catheter and over a guidewire already in place. A fluid such as a perfluorocarbon flows into the balloon to freeze the tissue adjacent the balloon, this cooling being associated with reduction of restenosis. A similar catheter may be used to reduce atrial fibrillation by inserting and inflating the balloon such that an exterior surface of the balloon contacts at least a partial circumference of the portion of the pulmonary vein adjacent the left atrium. In another embodiment, blood perfusion is performed simultaneously. In another embodiment, tissue contacted by the cryoablation catheter, undesired to be ablated, is protected against damage by a separate heating step.
Owner:ZOLL CIRCULATION
Pulmonary vein valve implant
InactiveUS20050273160A1Reduced likelihoodReduce severityStentsBronchiPulmonary vasculatureAtrial cavity
The present invention involves placing a valve between the left atrium and the lung to prevent regurgitant flow from increasing the pulmonary pressures, which may lead to pulmonary edema and congestion. Mitral stenosis or poor synchronization of the mitral valve may add additional pressures to the left atrium thus raising the pulmonary pressures and leading to congestion in the lung vasculature. By blocking the additional pressures from the mitral regurgitant flow from reaching the pulmonary circulation, the left atrium may act as a sealed vessel to allow additional aortic output. The valve placement can be intralumenal or attached to the ostium of the atrium. The device can be placed via the vascular conduits or through a surgical procedure into the pulmonary circulation. One or more devices may be placed in each of the four pulmonary veins. Additionally only one, two, three or all four veins may be implanted with the valve as desired by the physician.
Owner:DIRECT FLOW MEDICAL INC
Parasympathetic pacing therapy during and following a medical procedure, clinical trauma or pathology
ActiveUS20060206155A1Increase parasympathetic toneReduced responseSpinal electrodesHeart stimulatorsParasympathetic ganglionPathology diagnosis
A treatment method is provided, including identifying a subject as one who is selected to undergo an interventional medical procedure, and, in response to the identifying, reducing a likelihood of a potential adverse effect of the procedure by applying an electrical current to a parasympathetic site of the subject selected from the group consisting of: a vagus nerve of the subject, an epicardial fat pad of the subject, a pulmonary vein of the subject, a carotid artery of the subject, a carotid sinus of the subject, a coronary sinus of the subject, a vena cava vein of the subject, a jugular vein of the subject, a right ventricle of the subject, a parasympathetic ganglion of the subject, and a parasympathetic nerve of the subject.
Owner:MEDTRONIC INC
Techniques for reducing pain associated with nerve stimulation
Apparatus is provided including an electrode device and a control unit. The electrode device is configured to be coupled to a site of a subject selected from the group consisting of: a vagus nerve, an epicardial fat pad, a pulmonary vein, a carotid artery, a carotid sinus, a coronary sinus, a vena cava vein, a right ventricle, a right atrium, and a jugular vein. The control unit is configured to drive the electrode device to apply to the site a current in at least first and second bursts, the first burst including a plurality of pulses, and the second burst including at least one pulse, and set (a) a pulse repetition interval (PRI) of the first burst to be on average at least 20 ms, (b) an interburst interval between initiation of the first burst and initiation of the second burst to be less than 10 seconds, (c) an interburst gap between a conclusion of the first burst and the initiation of the second burst to have a duration greater than the average PRI, and (d) a burst duration of the first burst to be less than a percentage of the interburst interval between, the percentage being less than 67%. Other embodiments are also described.
Owner:MEDTRONIC INC
Method and device for performing cooling- or cryo-therapies for, e.g., angioplasty with reduced restenosis or pulmonary vein cell necrosis to inhibit atrial fibrillation employing tissue protection
InactiveUS6905494B2Minimize and inhibit biochemical eventRobust designCatheterSurgical instruments for heatingPercent Diameter StenosisPercutaneous angioplasty
An enhanced method and device are provided to treat atrial fibrillation or inhibit or reduce restenosis following angioplasty or stent placement. A balloon-tipped catheter is disposed in the area treated or opened through balloon angioplasty immediately following angioplasty. The balloon, which can have a dual balloon structure, may be delivered through a guiding catheter and over a guidewire already in place. A fluid such as a perfluorocarbon flows into the balloon to freeze the tissue adjacent the balloon, this cooling being associated with reduction of restenosis. A similar catheter may be used to reduce atrial fibrillation by inserting and inflating the balloon such that an exterior surface of the balloon contacts at least a partial circumference of the portion of the pulmonary vein adjacent the left atrium. In another embodiment, blood perfusion is performed simultaneously. In another embodiment, tissue contacted by the cryoablation catheter, undesired to be ablated, is protected against damage by a separate heating step.
Owner:ZOLL CIRCULATION
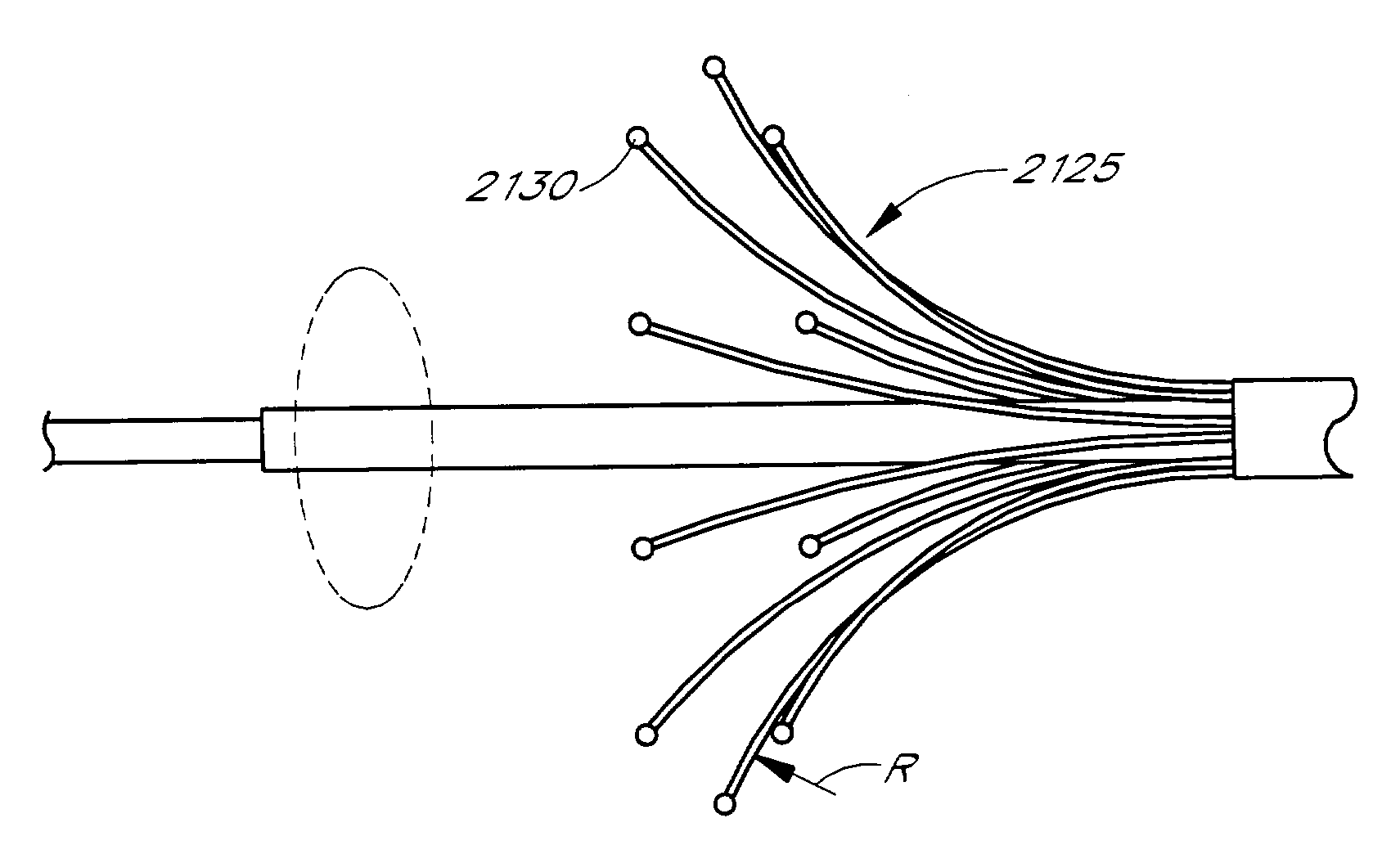
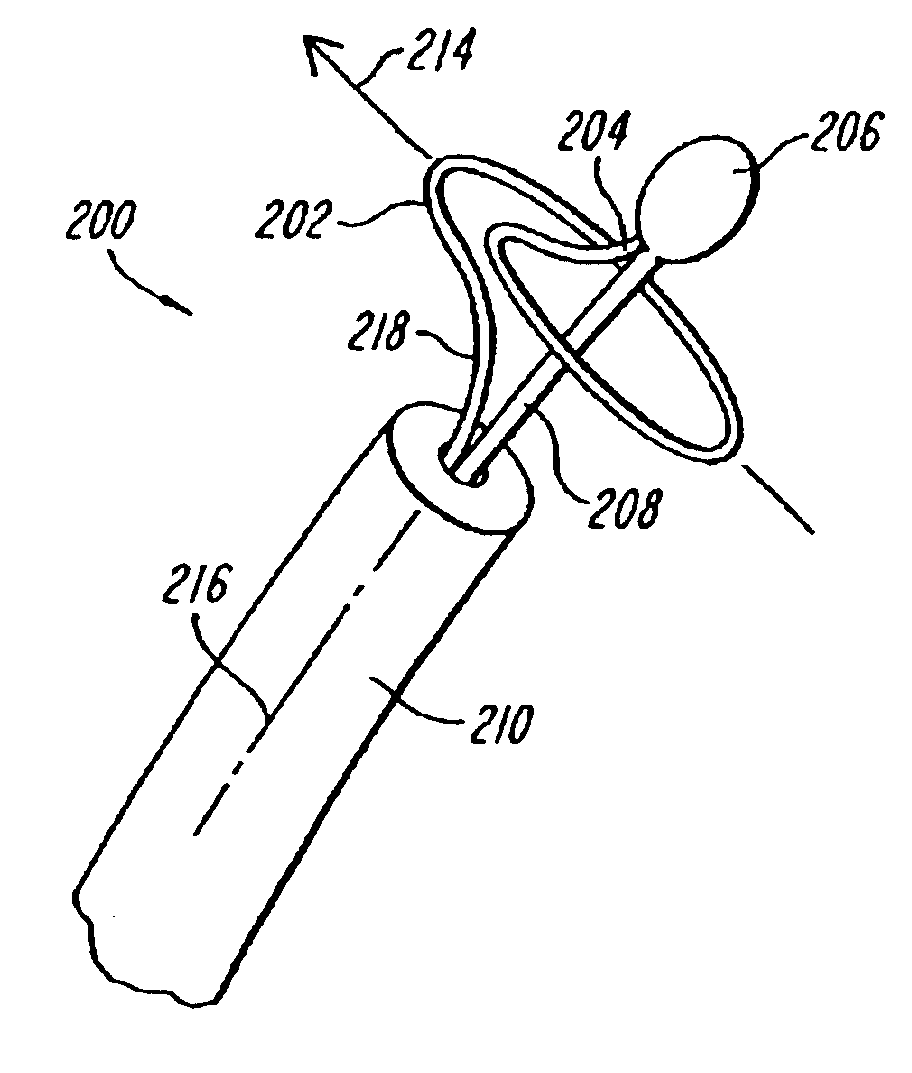
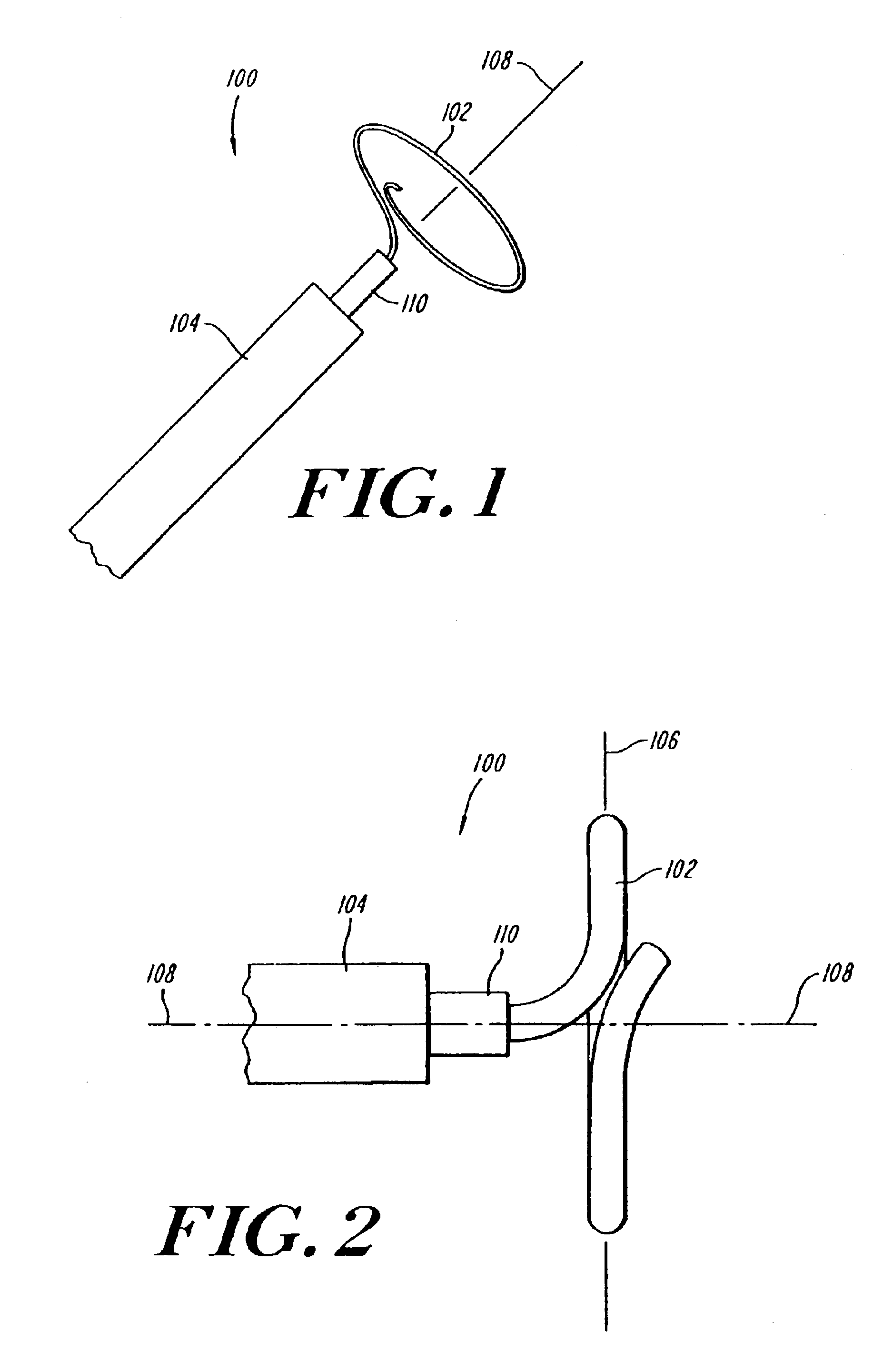
Lasso for pulmonary vein mapping and ablation
InactiveUS6973339B2Improve accuracyShorten the timeInternal electrodesDiagnostic recording/measuringVeinMedicine
Owner:BIOSENSE WEBSTER INC
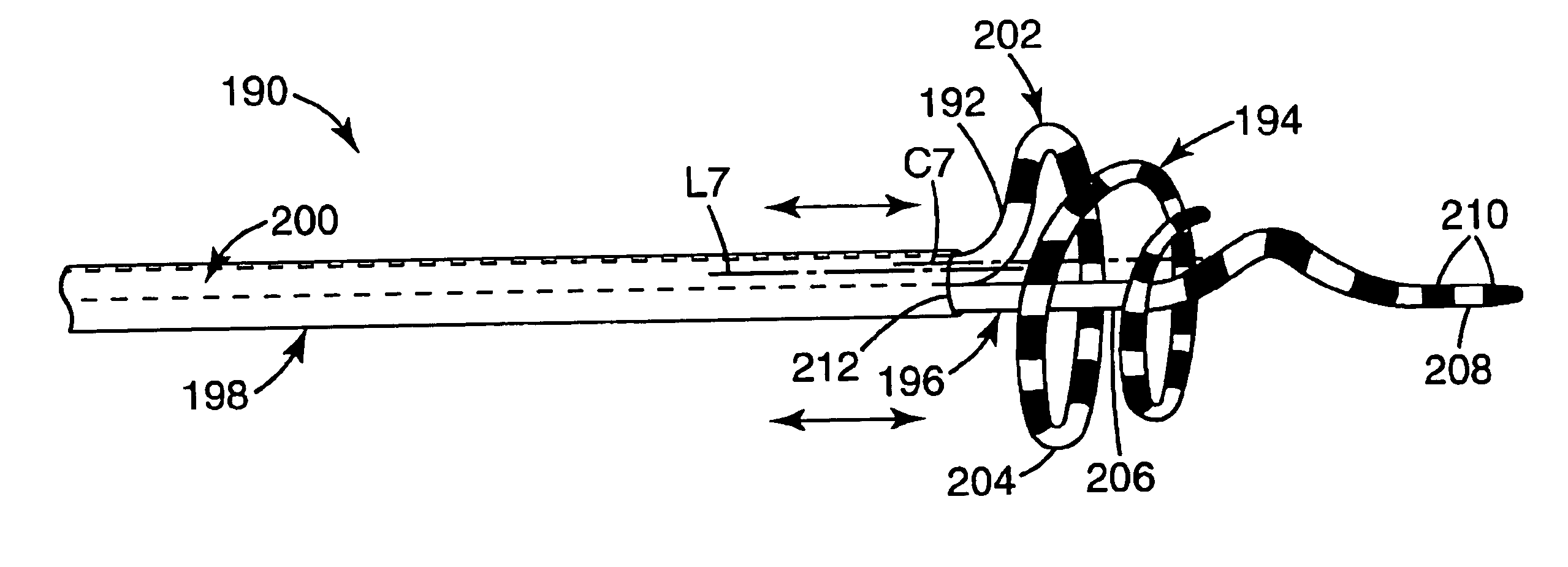
Coiled ablation catheter system
InactiveUS6960206B2Facilitate formation of lesionPromote formationStentsSurgical instruments for heatingVeinCardiac Ablation
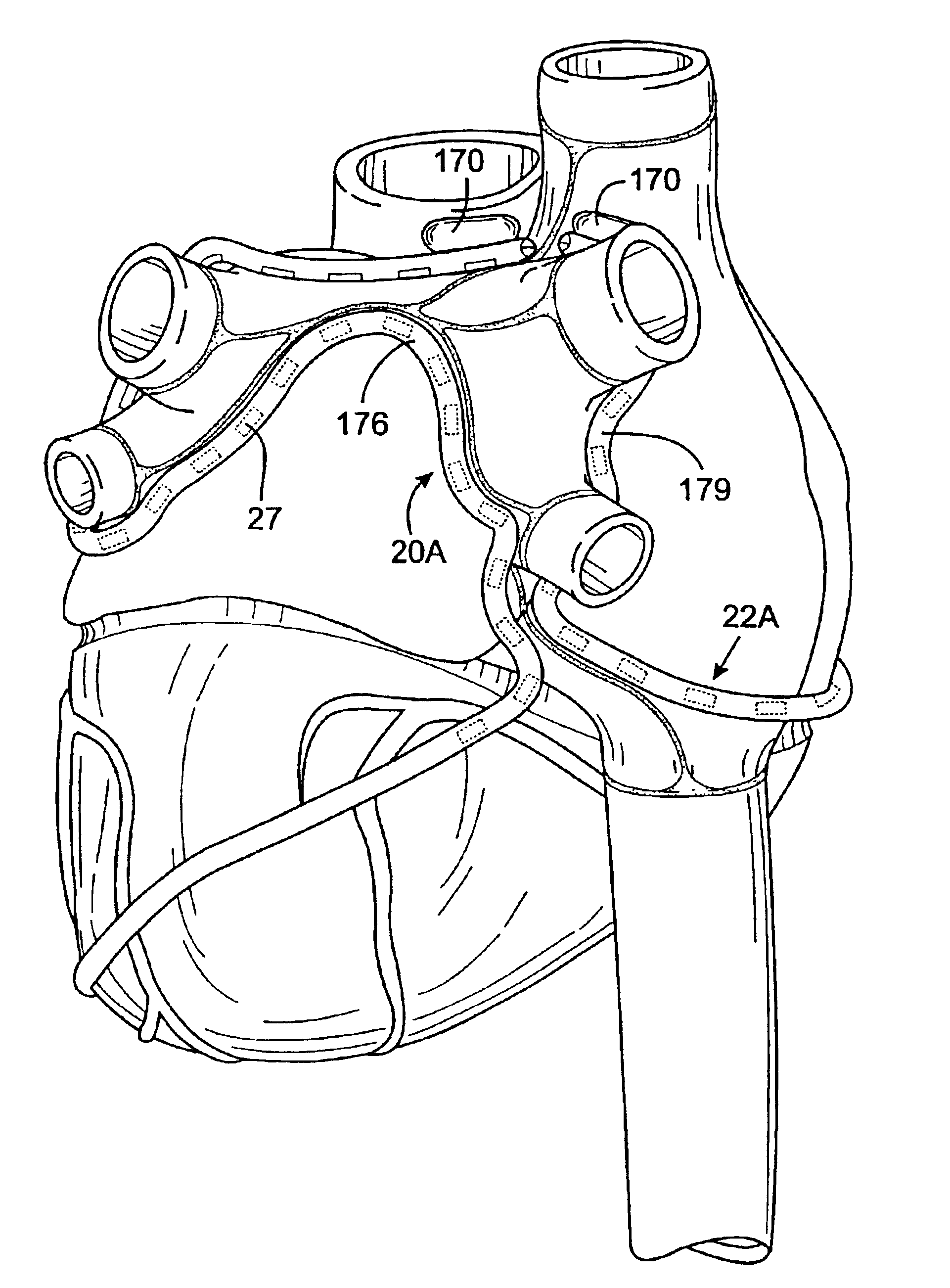
A cardiac ablation catheter includes a coil-like ablating element that is deployable from an elongate, flexible sheath. The ablating element, while in the deployed position, has a shape with at least one revolution oriented in a plane that is orthogonal to a longitudinal axis of the sheath. The catheter system is well-suited to ablate a circumferential region of tissue about a pulmonary vein or the posterior wall of the left atrium proximate the pulmonary vein os. The treated tissue region electrically isolates the atria from the pulmonary vein.
Owner:THE GENERAL HOSPITAL CORP
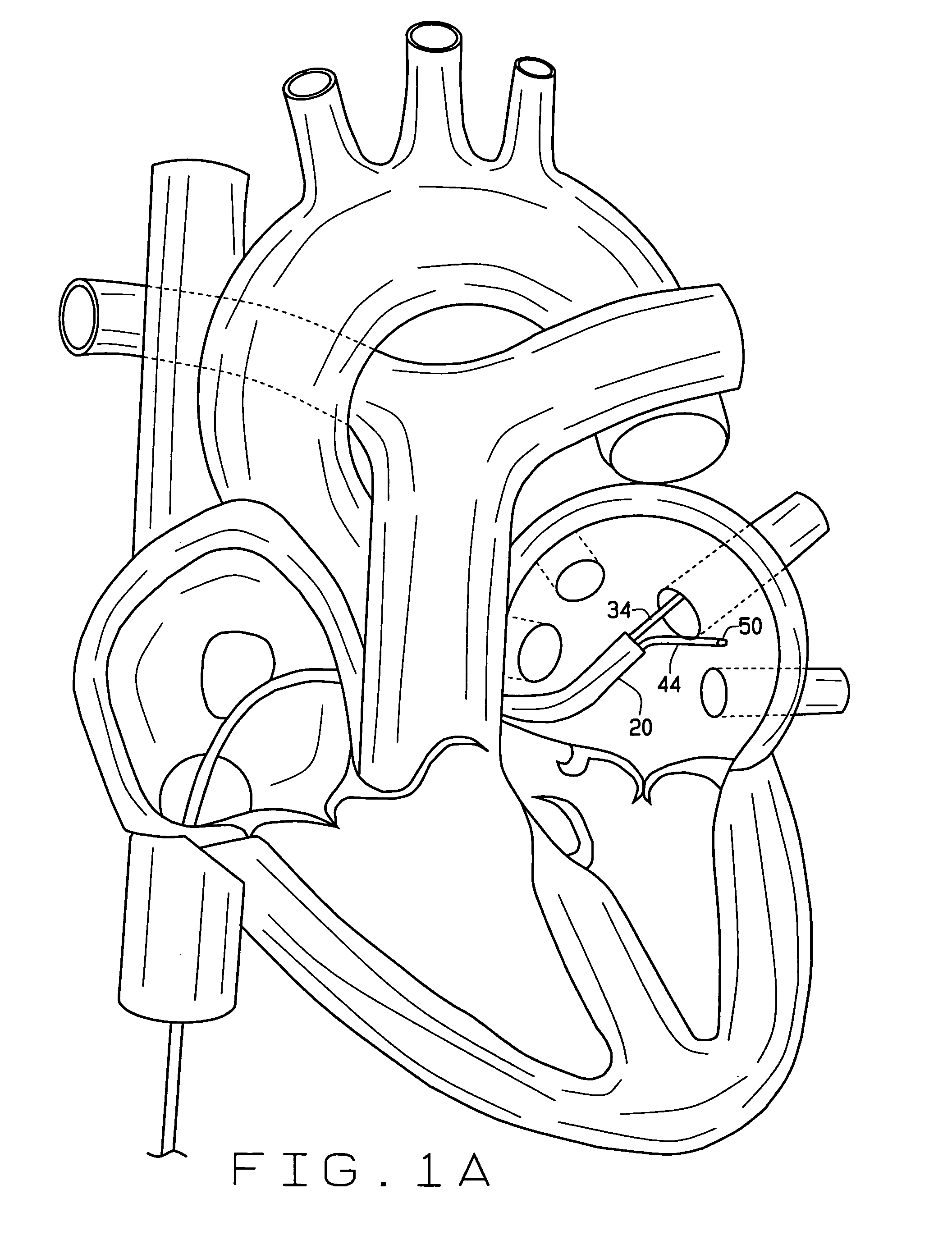
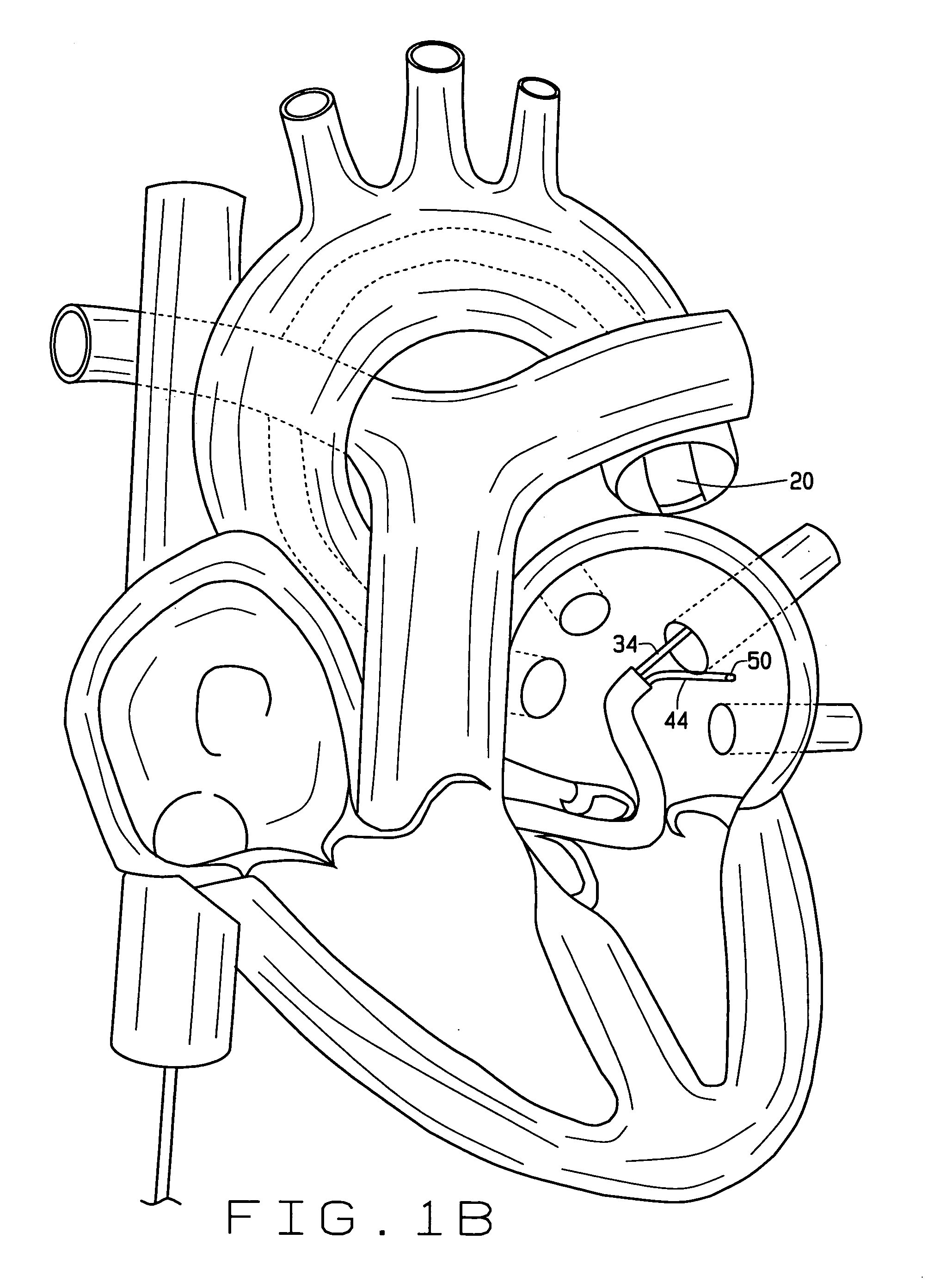
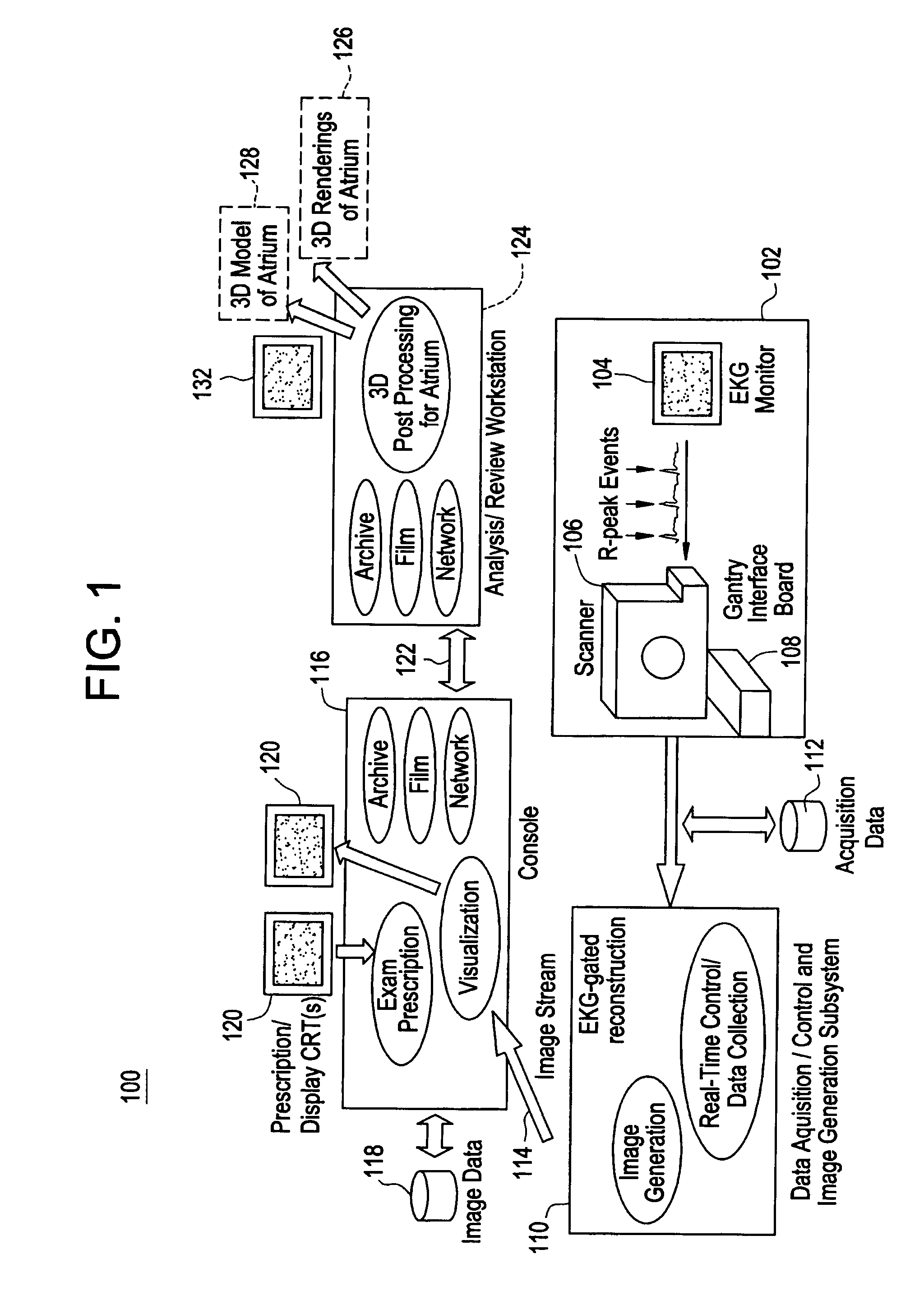
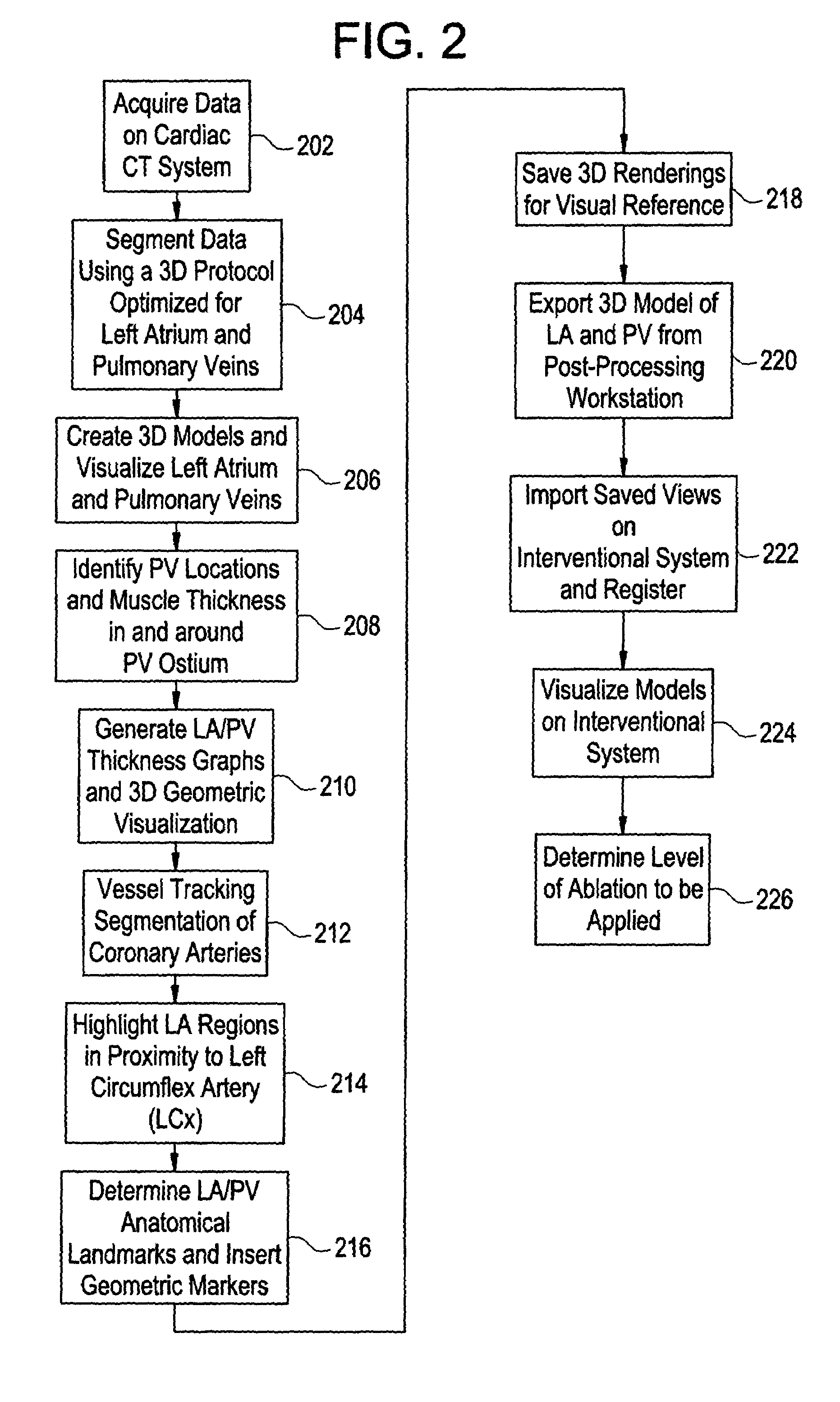
Cardiac CT system and method for planning atrial fibrillation intervention
A method for planning atrial fibrillation (AF) intervention for a patient includes obtaining acquisition data from a medical imaging system, and generating a 3D model of the left atrium and pulmonary veins of the patient. One or more left atrial anatomical landmarks are identified on the 3D model, and saved views of the 3D model are registered on an interventional system. One or more of the registered saved views are visualized with the interventional system.
Owner:GE MEDICAL SYST GLOBAL TECH CO LLC +1
Method for Ablating Body Tissue
A cardiac ablation method including the following steps: inserting a treatment catheter into an atrium of a heart, the treatment catheter including an ultrasound emitter; positioning the ultrasound emitter to face heart tissue within the left atrium outside of a pulmonary vein; emitting ultrasound energy from the ultrasound emitter while rotating the ultrasound emitter about a rotation axis; and ablating heart tissue with the ultrasound energy to form a lesion outside of a pulmonary vein.
Owner:AURIS HEALTH INC
Cardiac ablation devices
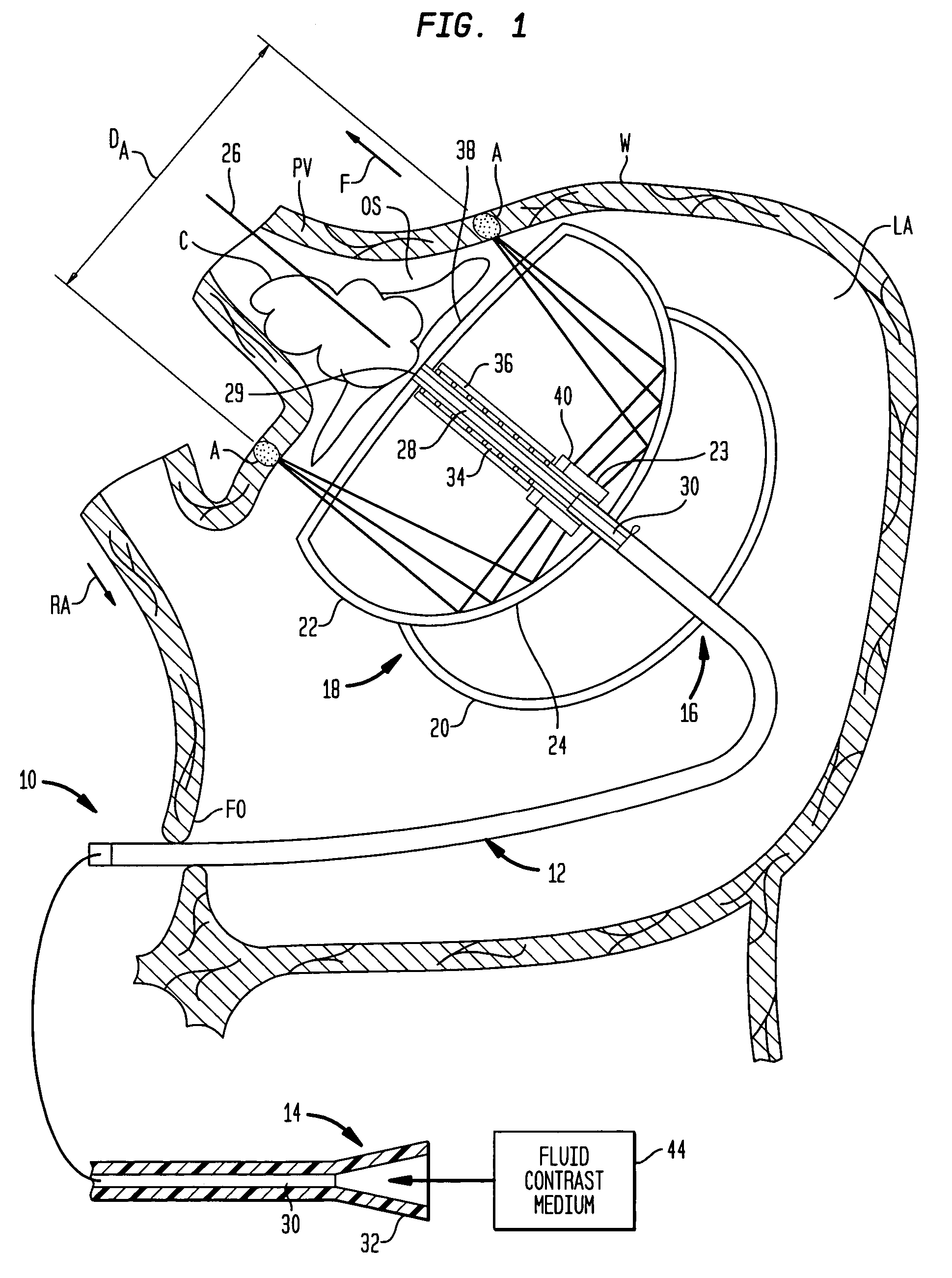
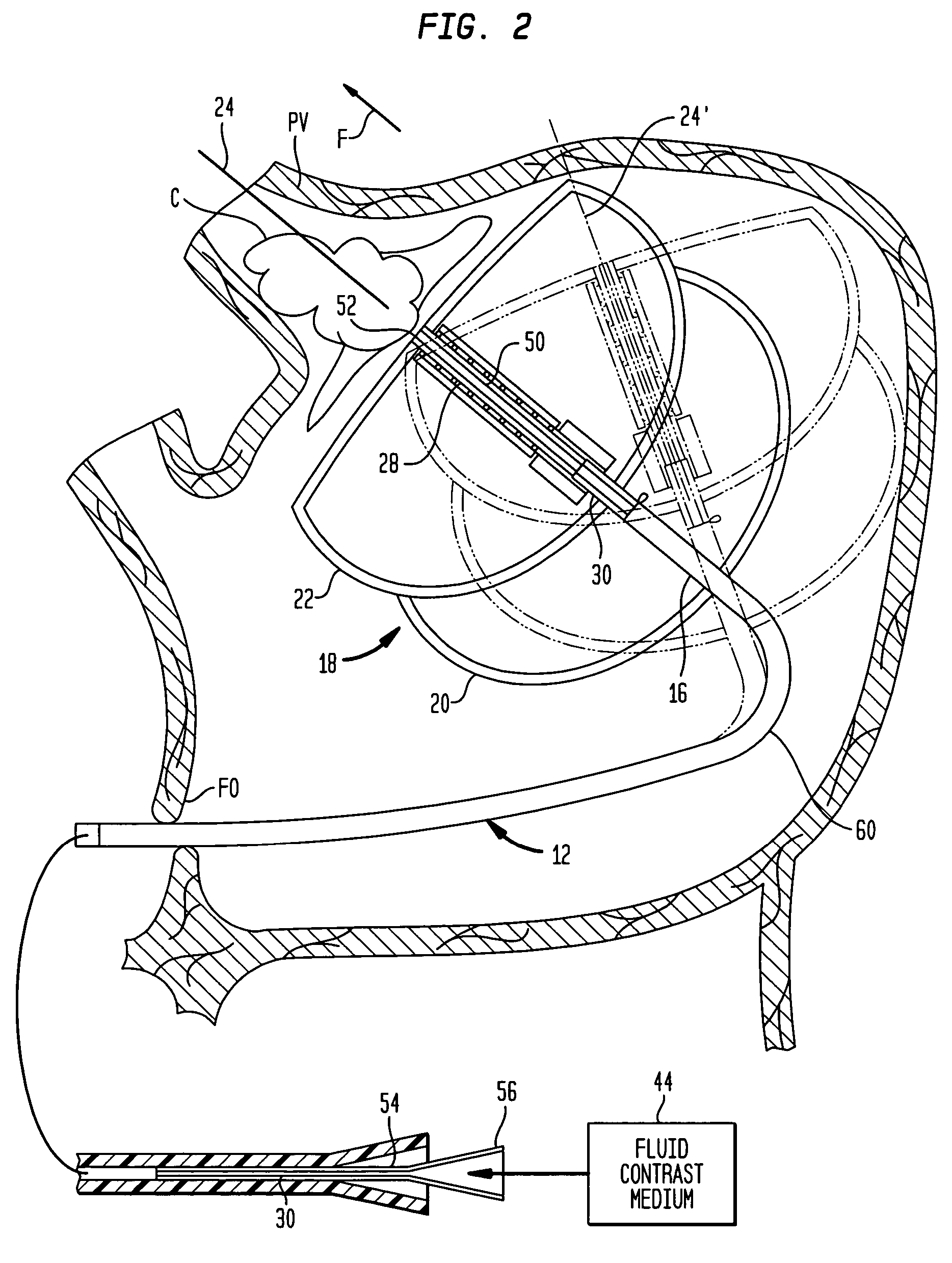
A cardiac ablation device treats atrial fibrillation by directing and focusing ultrasonic waves into a ring-like ablation region (A). The device desirably is steerable and can be moved between a normal disposition, in which the ablation region lies parallel to the wall of the heart for ablating a loop-like lesion, and a canted disposition, in which the ring-like focal region is tilted relative to the wall of the heart, to ablate only a short, substantially linear lesion. The ablation device desirably includes a balloon reflector structure (18, 1310) and an ultrasonic emitter assembly (23, 1326), and can be steered and positioned without reference to engagement between the device and the pulmonary vein or ostium. A contrast medium (C) can be injected through the ablation device to facilitate imaging, so that the device can be positioned based on observation of the images.
Owner:BOSTON SCI SCIMED INC
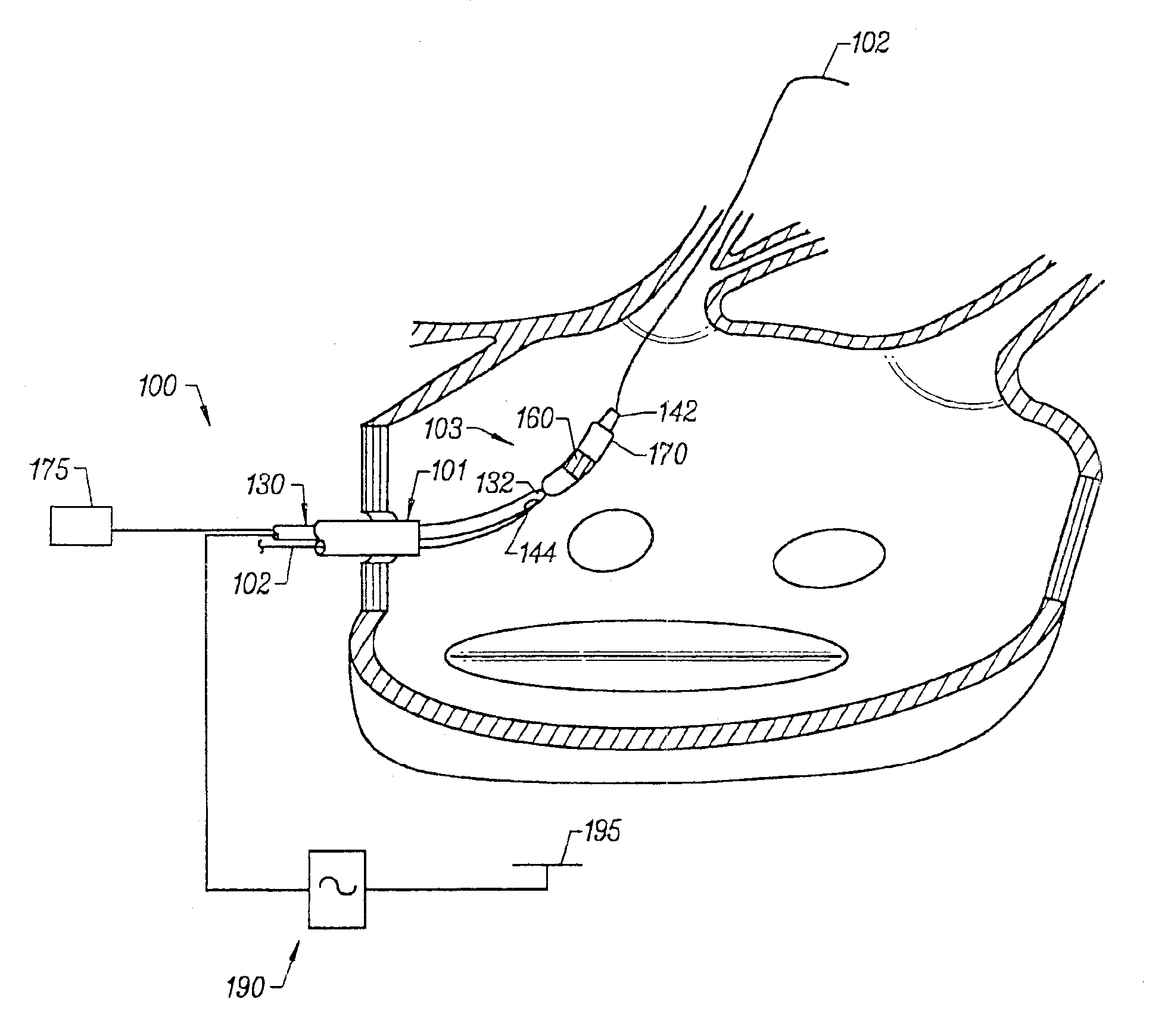
Tissue ablation system including guidewire with sensing element
A tissue ablation system for ablating human tissue wherein sensing and ablation procedures are performed and controlled independently. A sensing wire is positioned distally to the ablation region and is adapted to pass thorough the ablation device such that it may move with or independently of the ablation device without obstructing the surface tissue interface of the ablation energy. The ablation device can ablate a substantial portion of a circumferential region of tissue, for example at or near the location where the pulmonary vein extends from the atrium. The tissue ablation system comprises an ablation device comprised of an elongated catheter with a proximal region and a distal region and an ablation element located proximate the distal region of the catheter. A sensing device having an elongated body with a proximal portion and a distal portion is adapted to be positioned within a vessel at or near a vessel ostium, wherein the sensing device is adapted to be slidably received within a lumen of the ablation device. The sensing device, a guide wire for example, may be shaped in various configurations to allow sensing device such as electrodes disposed thereon to contact the vessel wall near the ablation region. In this fashion, the sensing and ablation procedures are de-coupled such that the sensing device does not interfere or obstruct the ablation member's interface with the tissue.
Owner:MEDTRONIC CRYOCATH LP
Apparatus and method for diagnosis and therapy of electrophysiological disease
InactiveUS6949095B2Improve visualizationQuick to useCannulasSurgical needlesDiseaseEpicardial ablation
Owner:ST JUDE MEDICAL ATRIAL FIBRILLATION DIV
Tissue ablation device assembly and method for electrically isolating a pulmonary vein ostium from an atrial wall
This invention is related to a tissue ablation system and method that treats atrial arrhythmia by ablating a circumferential region of tissue at a location where a pulmonary vein extends from an atrium. The system includes a circumferential ablation member with an ablation element and also includes a delivery assembly for delivering the ablation member to the location. The circumferential ablation member is generally adjustable between different configurations to allow both the delivery through a delivery sheath into the atrium and the ablative coupling between the ablation element and the circumferential region of tissue.
Owner:ATRIONIX
Device and method for forming a circumferential conduction block in a pulmonary vein
This invention is a method for treating a patient diagnosed with atrial arrhythmia by forming a circumferential conduction block along a circumferential path of tissue in a pulmonary vein wall that circumscribes the pulmonary vein lumen and transects the electrical conductivity of the pulmonary vein such that conduction is blocked along the longitudinal axis of the vein wall and into the left atrial wall. The method is performed to treat a patient with a focal arrythmogenic origin along the pulmonary vein wall by either ablating the focal origin or by isolating the focal origin from the atrial wall with the circumferential conduction block. The circumferential conduction block is also formed in a pulmonary vein in order to bridge the adjacent ends of two linear lesions, wherein each linear lesion is formed to extend between the pulmonary vein and another adjacent pulmonary vein in a less-invasive “maze”-type procedure. A circumferential ablation element in a circumferential ablation device assembly is used in a percutaneous translumenal catheter technique in order to form the circumferential conduction block in the pulmonary vein wall.
Owner:RGT UNIV OF CALIFORNIA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com