Patents
Literature
250 results about "Posterior wall" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
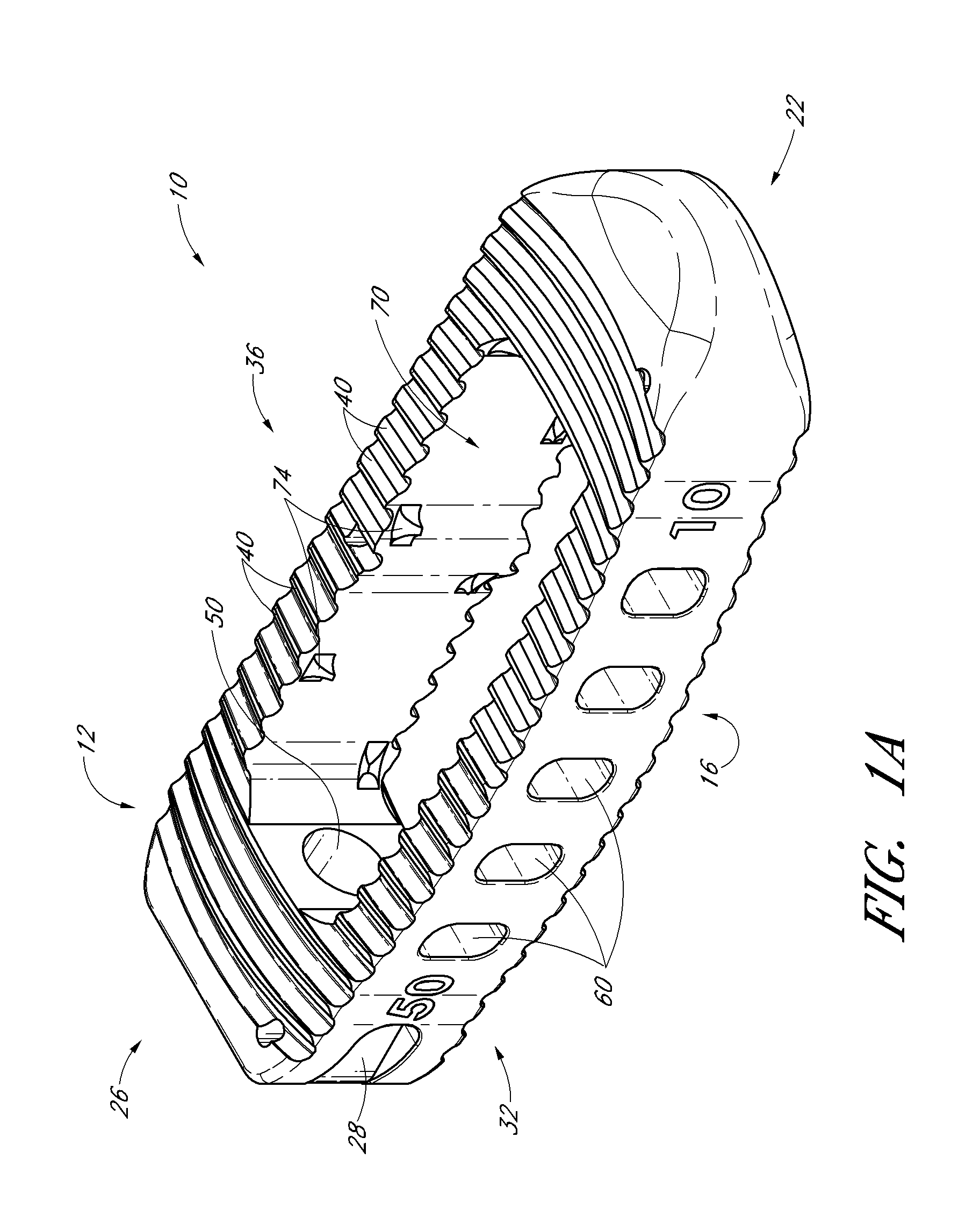
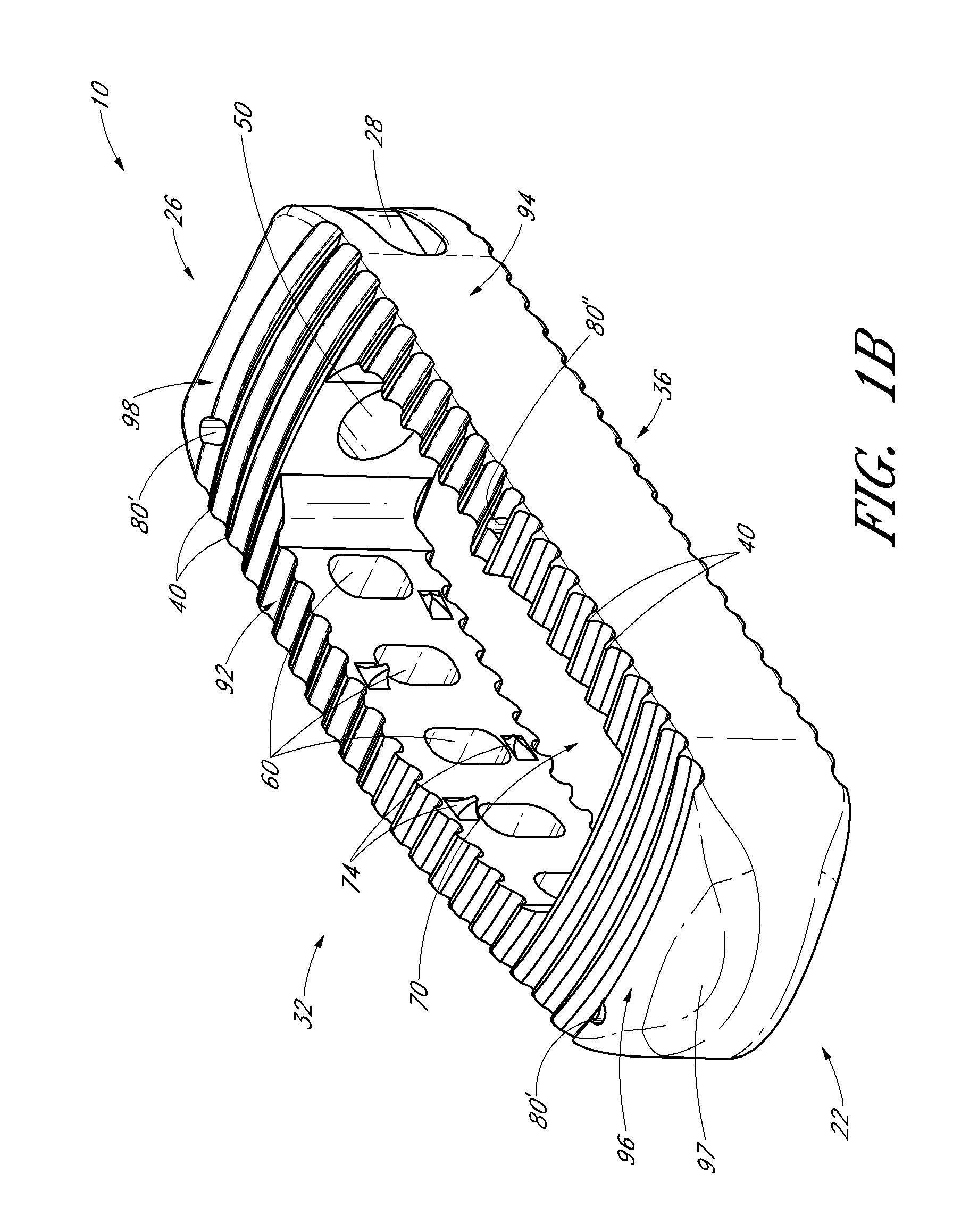
Intervertebral implants and graft delivery systems and methods
ActiveUS20110230970A1Reduce the likelihood of migrationBone implantJoint implantsFilling materialsSpinal implant
According to some embodiments, a method for promoting spinal fusion using a spinal implant comprises providing a spinal implant, wherein the spinal implant comprises an anterior wall, a posterior wall and two lateral walls configured to extend between the anterior wall and the posterior wall. In some embodiments, the spinal implant further comprises at least one internal chamber generally positioned between the anterior wall, the posterior wall and the two lateral walls, wherein the internal chamber being is adapted to receive at least one graft and / or other fill material. In some arrangements, the anterior wall of the spinal implant comprises at least one opening or hole that places the internal chamber in fluid communication with an exterior area or portion of the spinal implant. In one embodiment, at least one of the two lateral walls comprises an access port. The method additionally includes positioning the spinal implant between two adjacent vertebrae of a patient and directing at least one graft and / or other fill material into the internal chamber of the spinal implant through the access port. In some embodiments, at least a portion of the graft and / or other fill material delivered into the internal chamber is configured to exit through the one or more of the openings of the anterior wall.
Owner:SPINE HLDG LLC
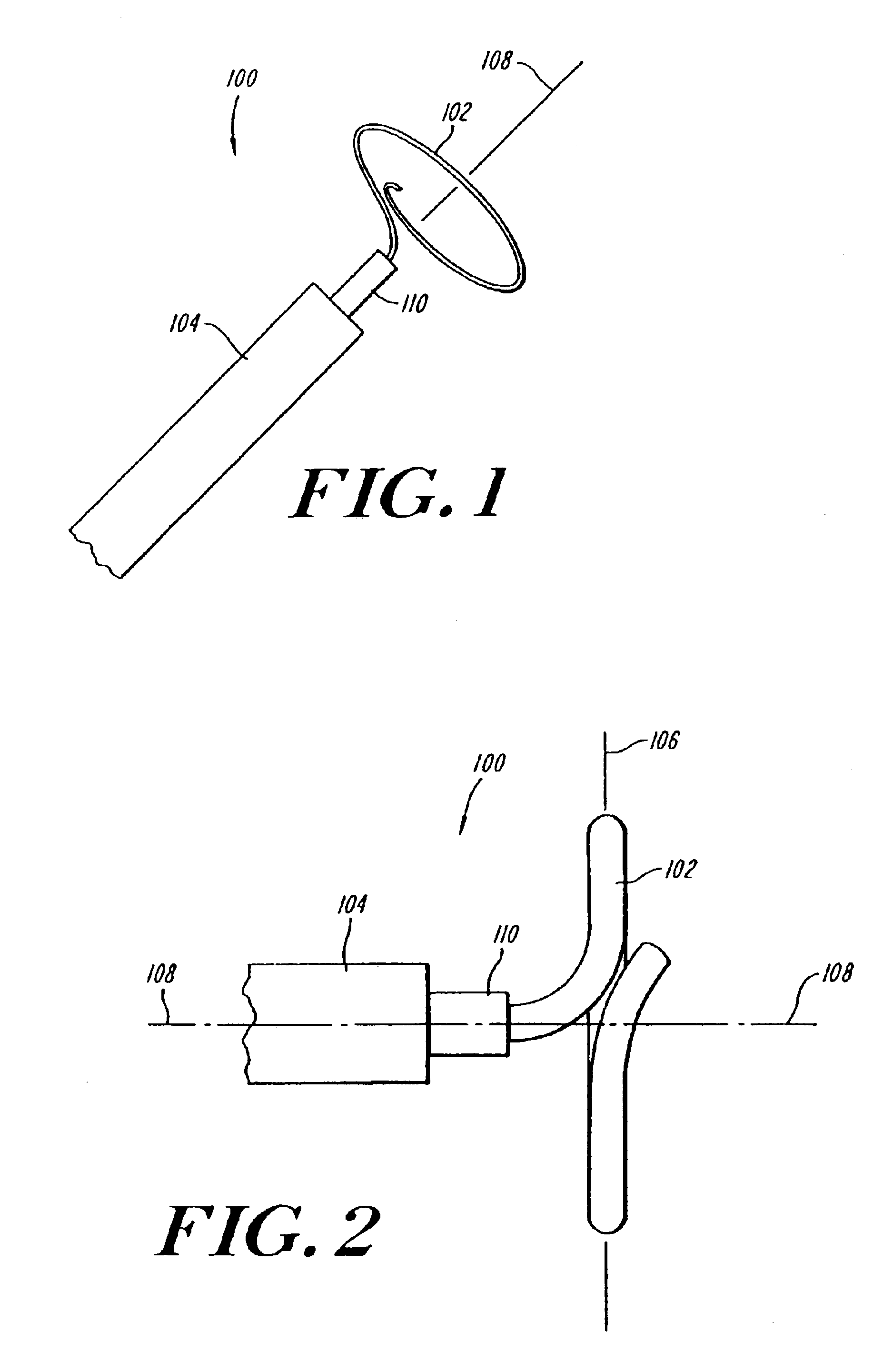
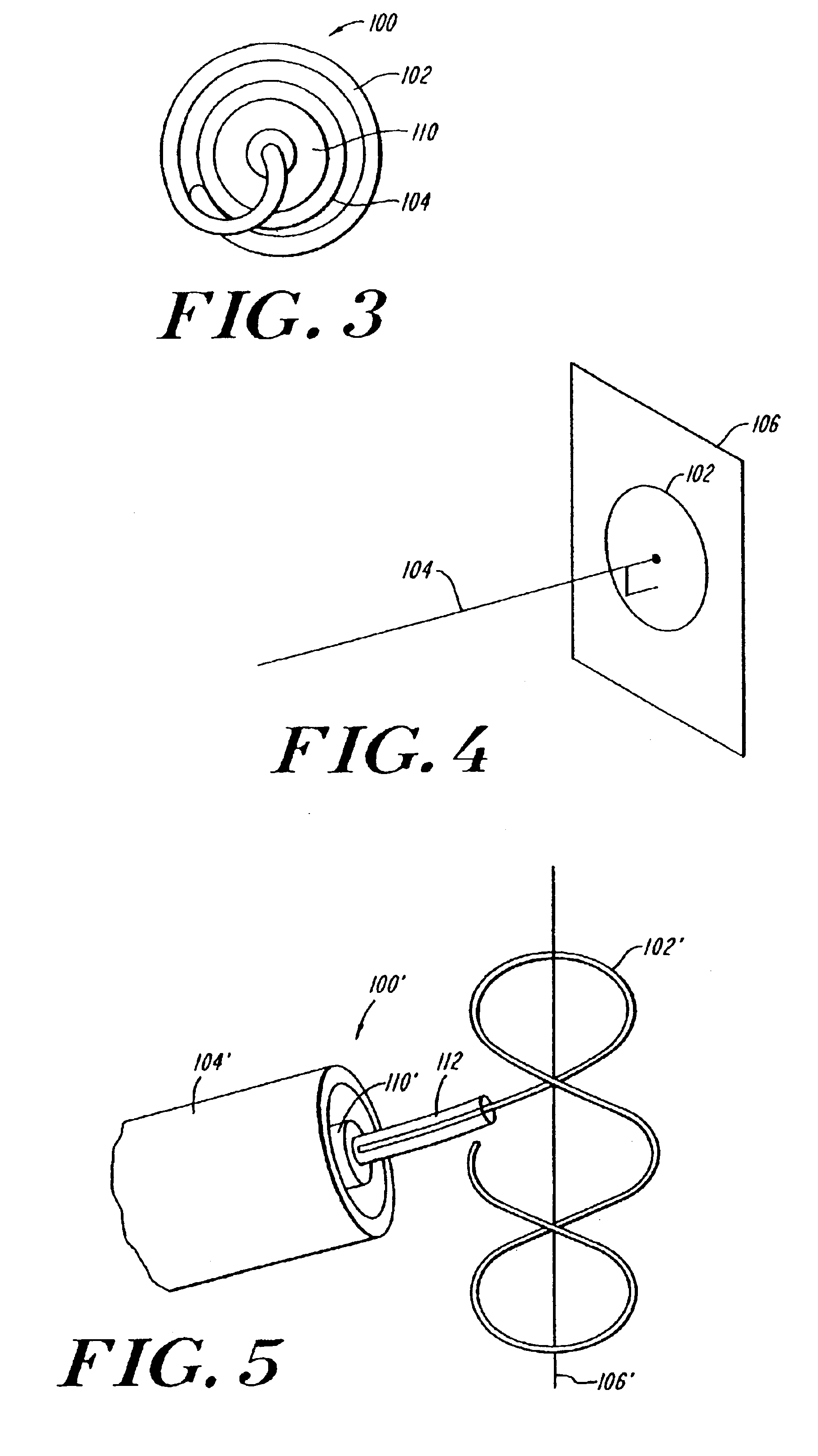
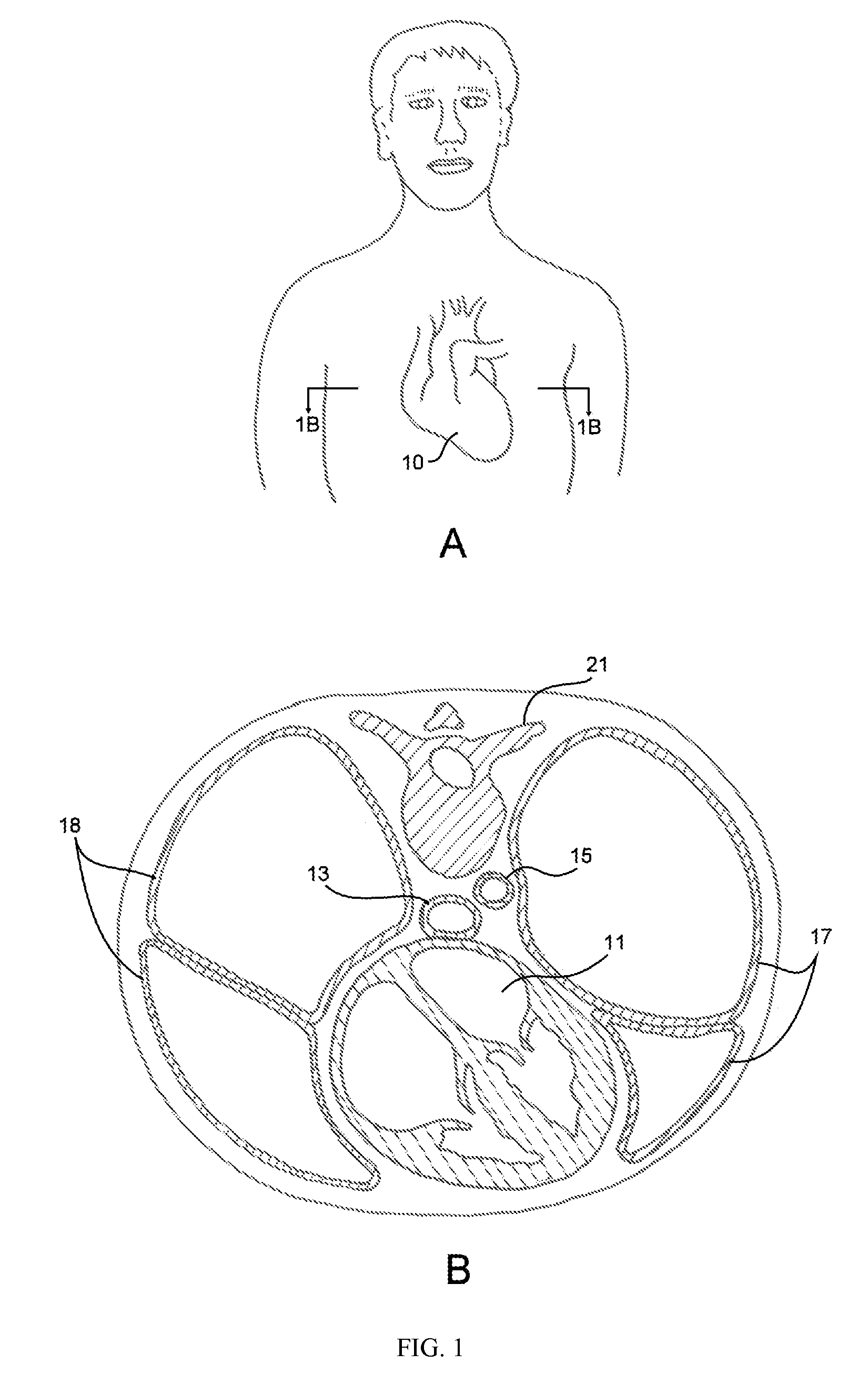
Coiled ablation catheter system
InactiveUS6960206B2Facilitate formation of lesionPromote formationStentsSurgical instruments for heatingVeinCardiac Ablation
A cardiac ablation catheter includes a coil-like ablating element that is deployable from an elongate, flexible sheath. The ablating element, while in the deployed position, has a shape with at least one revolution oriented in a plane that is orthogonal to a longitudinal axis of the sheath. The catheter system is well-suited to ablate a circumferential region of tissue about a pulmonary vein or the posterior wall of the left atrium proximate the pulmonary vein os. The treated tissue region electrically isolates the atria from the pulmonary vein.
Owner:THE GENERAL HOSPITAL CORP
Naturally contoured, preformed, three dimensional mesh device for breast implant support
ActiveUS20090082864A1Easy to deployInherent disadvantageMammary implantsWound clampsWrinkle skinBreast implant
A preformed, seamless, three-dimensional, anatomically contoured prosthetic device for reinforcing breast tissue and supporting a breast implant includes a flat back wall, a concave front wall and a curved transitional region between the flat back wall and the front wall defining a smoothly curved bottom periphery. A concave receiving space is defined by the back wall and the front wall for at least partially receiving and supporting the breast implant therein. The three-dimensional prosthetic device is free of wrinkles, creases, folds or seams, which may have otherwise caused potential tissue irritation, bacteria hosting, infection and palpability problems.
Owner:ETHICON INC
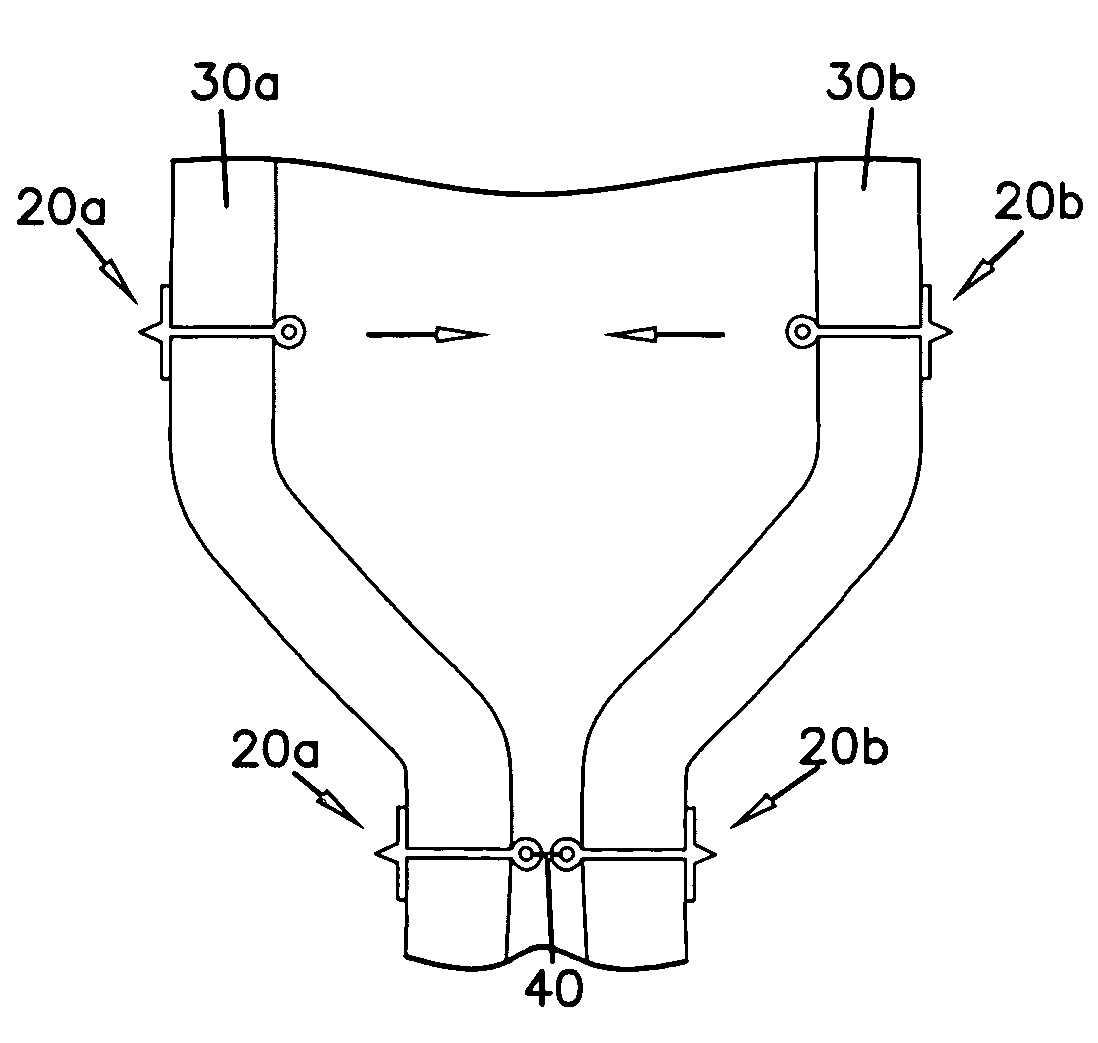
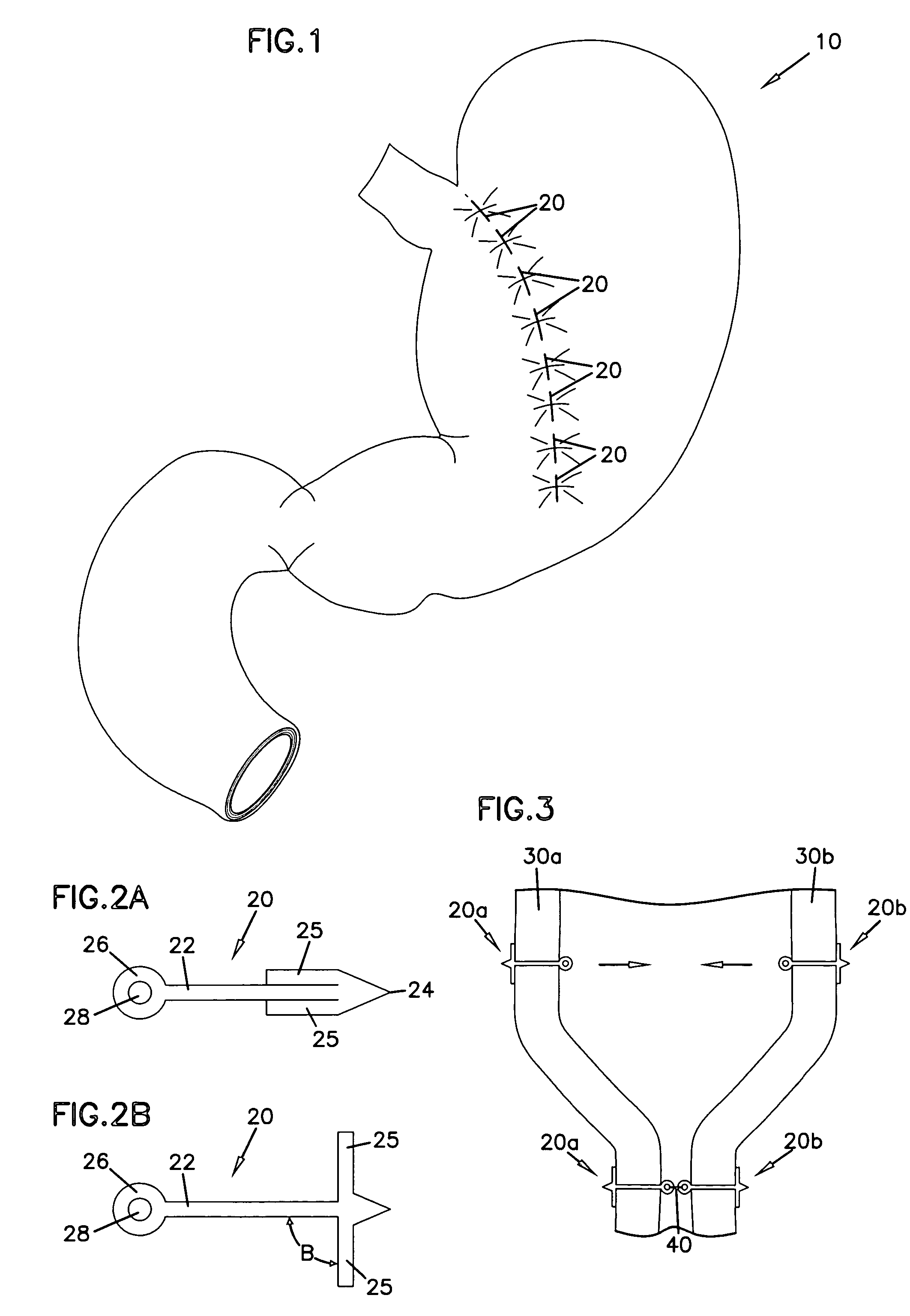
Gastric reshaping devices and methods
InactiveUS20060020277A1Reducing gastric volumeSuture equipmentsObesity treatmentStomach wallsAnterior wall
Gastric volume of a patient is reduced by deploying an endoscope into a stomach through the esophagus. A plurality of anterior anchors are affixed to the anterior wall of the stomach. The anterior anchors are distributed along an anterior line of the stomach wall beginning near the cardia region and extending toward the stomach exit. A plurality of posterior anchors are affixed to the posterior wall of the stomach. The posterior anchors are distributed along a posterior line of the stomach wall beginning near the cardia region and toward the stomach exit. The anchor line and the stomach wall are drawn towards the posterior line of the stomach wall to reduce gastric volume.
Owner:MAYO FOUND FOR MEDICAL EDUCATION & RES
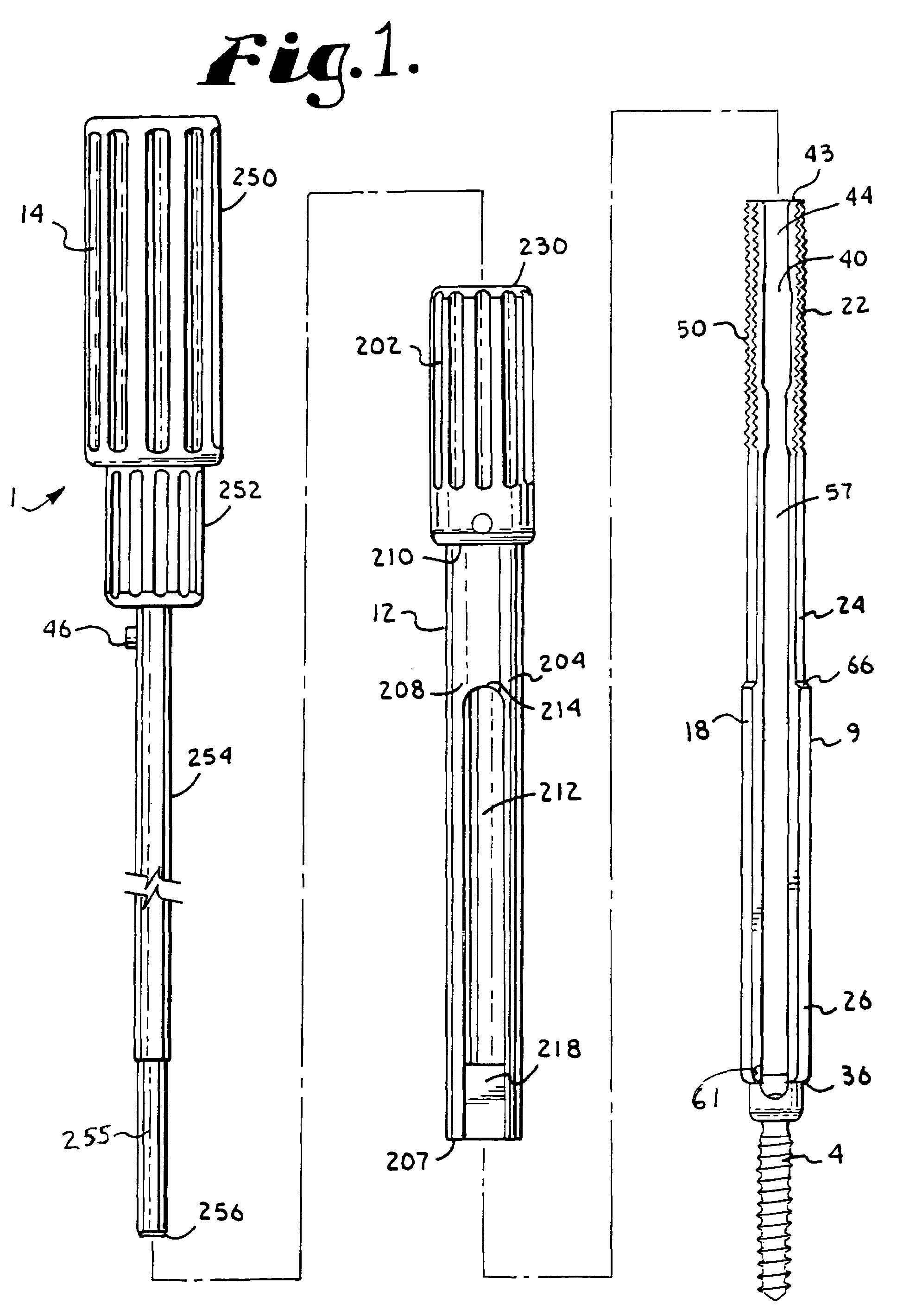
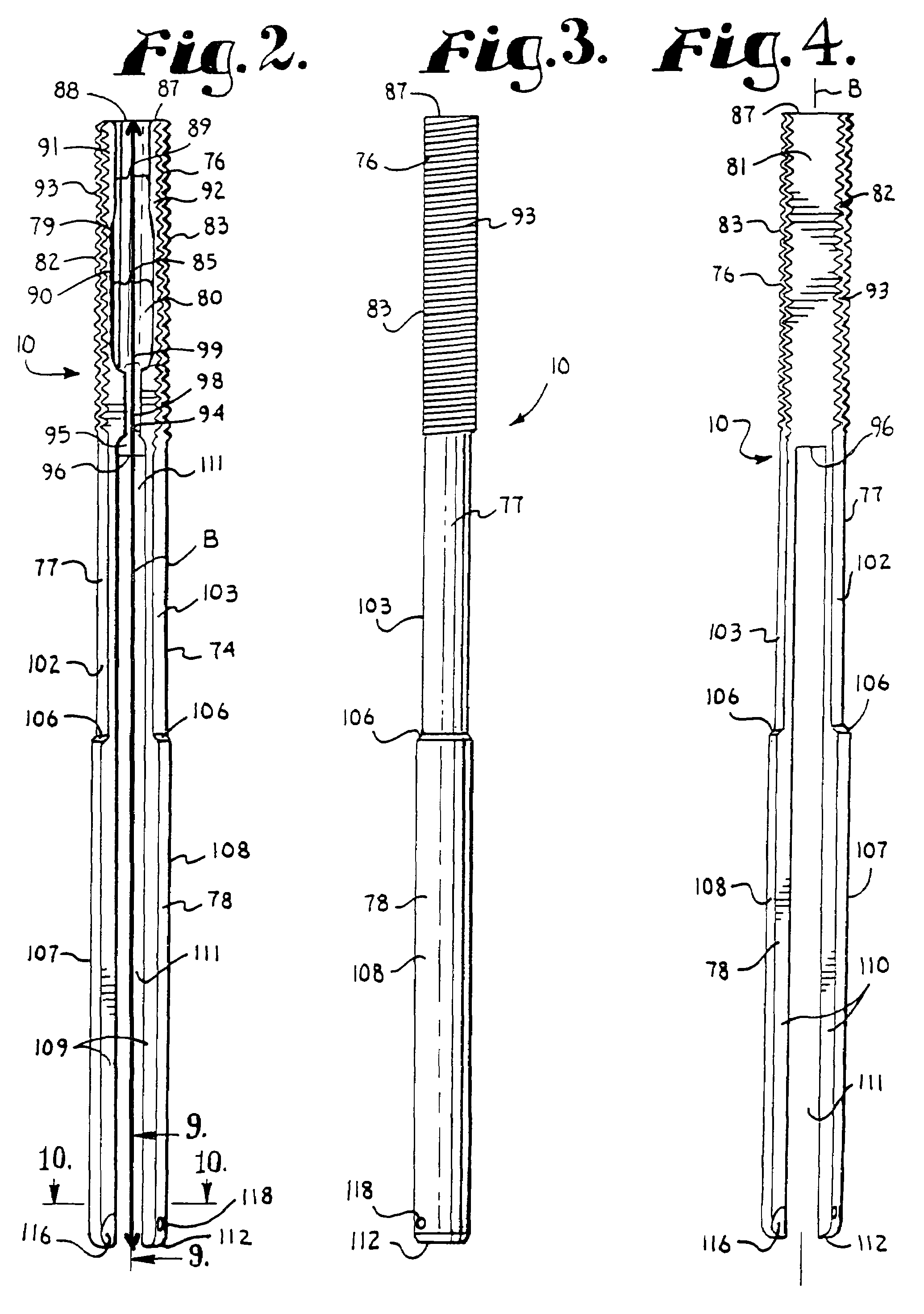
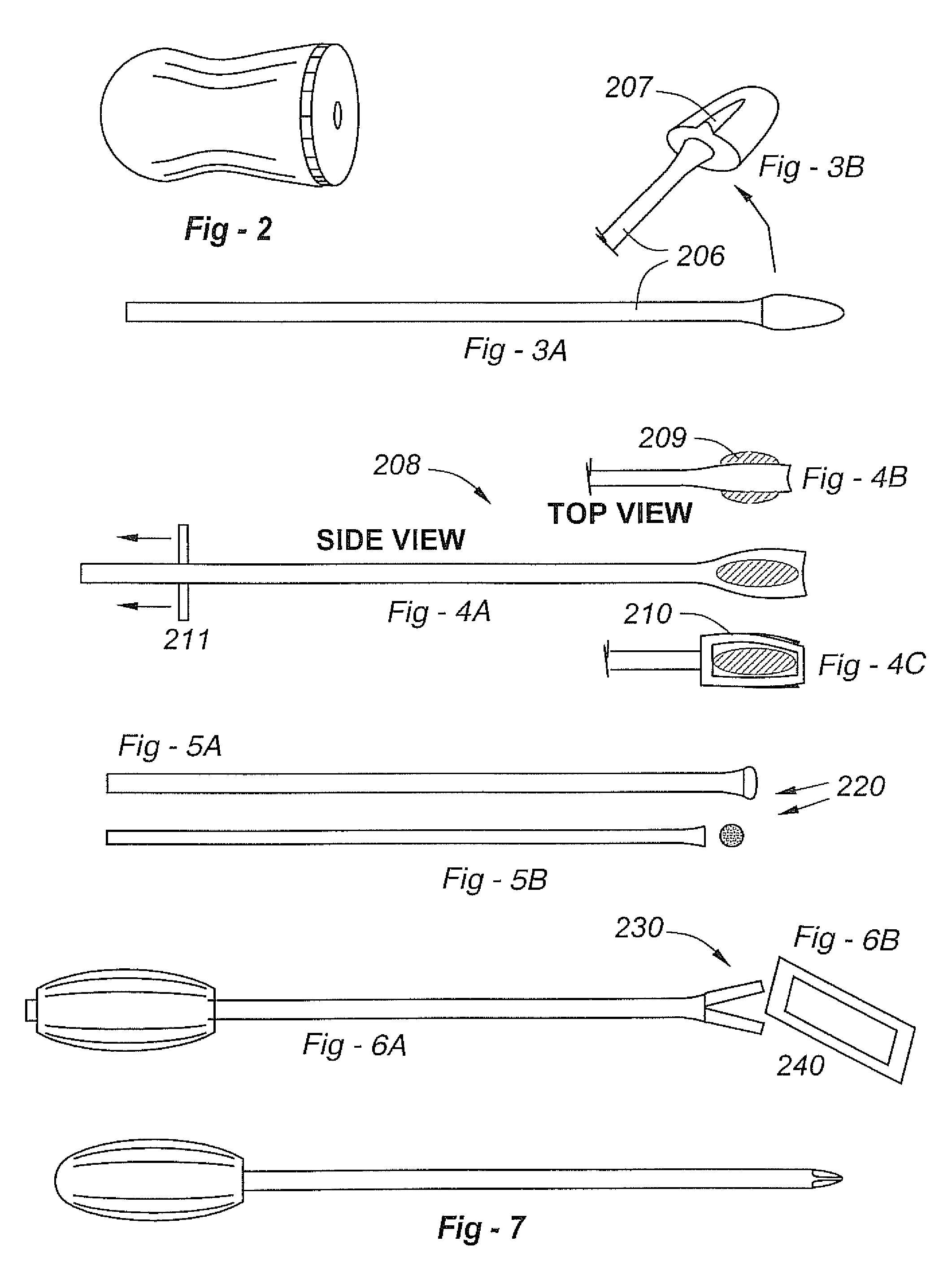
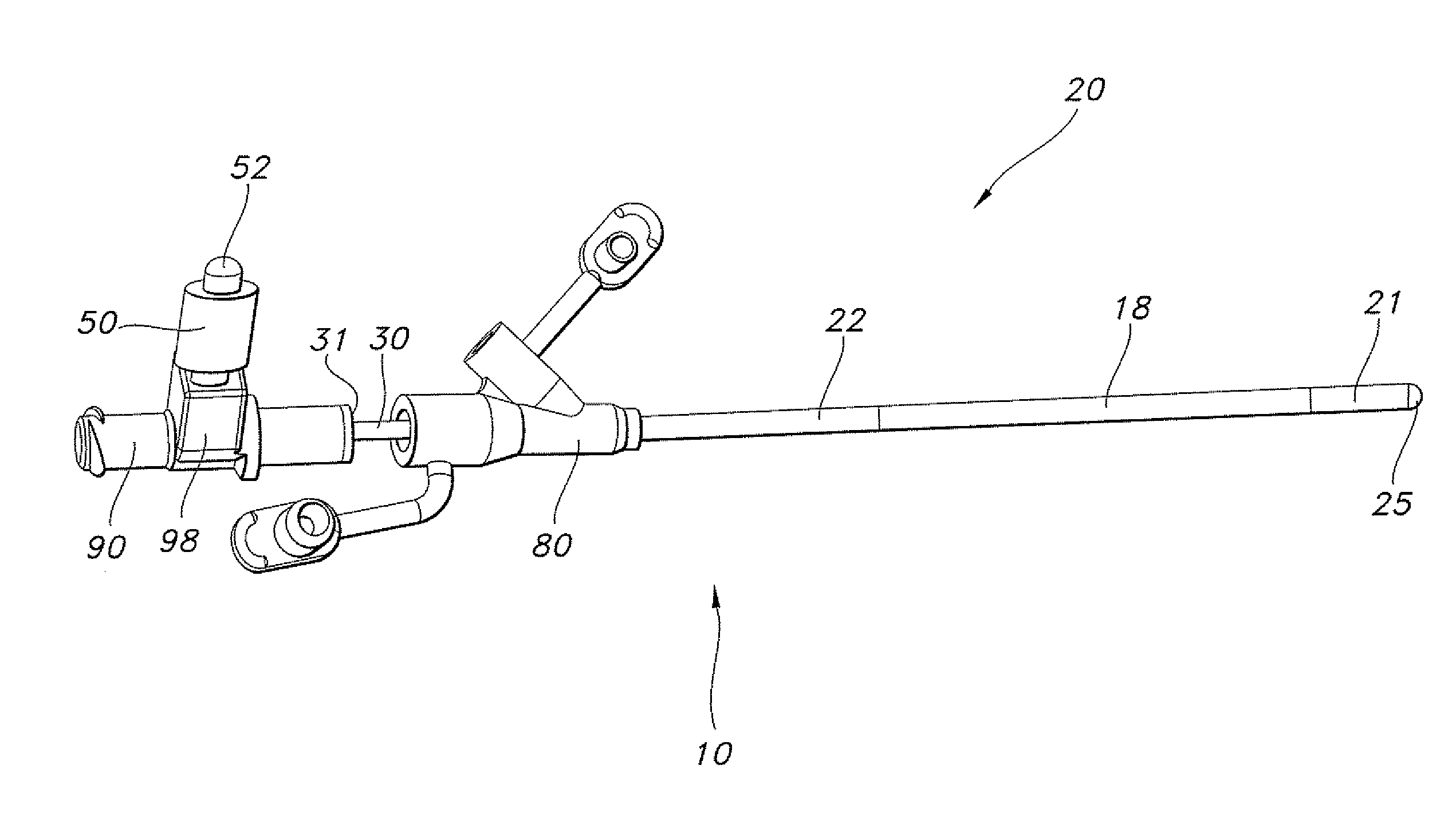
Spinal fixation tool set and method
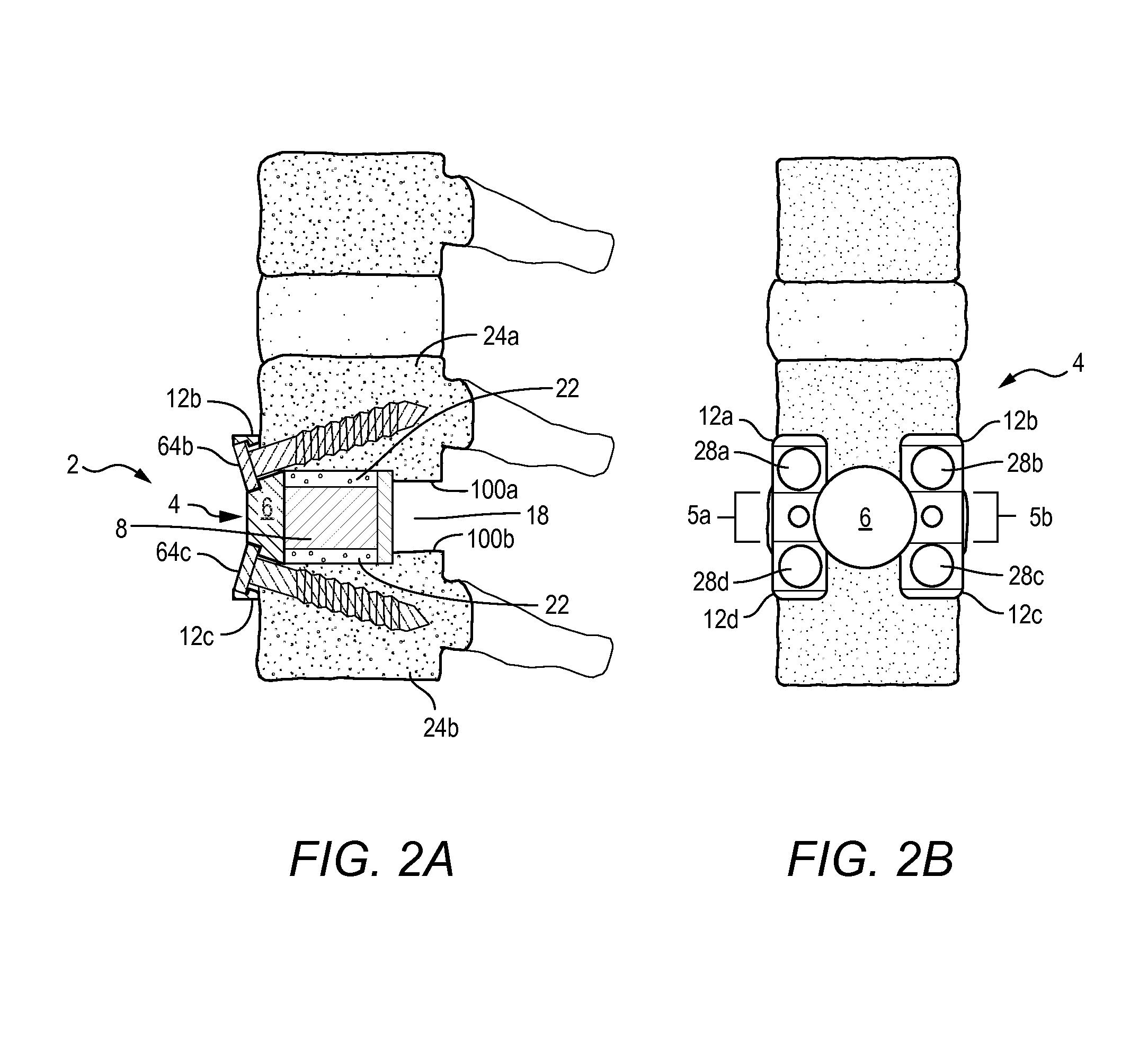
A spinal implant tool set includes end guide tools having flexible back wall flaps that receive opposite ends of the rod and intermediate guide tools that hold the rod in intermediate locations between the end guide tools. Both the end and intermediate guide tools include an attachment structure for operably connecting the guide tool to a bone screw. A multi-function installation tool and a bone screw driver each mate and cooperate with the guide tools. A method utilizing the tool set allows a surgeon to percutaneously implant the bone screws and the rod in the patient.
Owner:JACKSON
Device and method for esophageal cooling
InactiveUS20070055328A1Equally distributedDiagnosticsSurgical instrument detailsOesophageal injuryPreventing injury
The present invention includes a device and a method for preventing injury of the esophagus during thermal ablation of the left atrium. The device has an esophageal probe with a balloon tip for insertion into the esophagus of a patient. During usage, coolant passes into the esophageal probe and then fills its balloon. The coolant, when circulating through the balloon and an external cooling machine, protects the esophageal tissue in contact with the esophageal probe from thermal damage during ablation of the posterior wall of the left atrium of the heart, or other procedure.
Owner:MAYSE MARTIN L +1
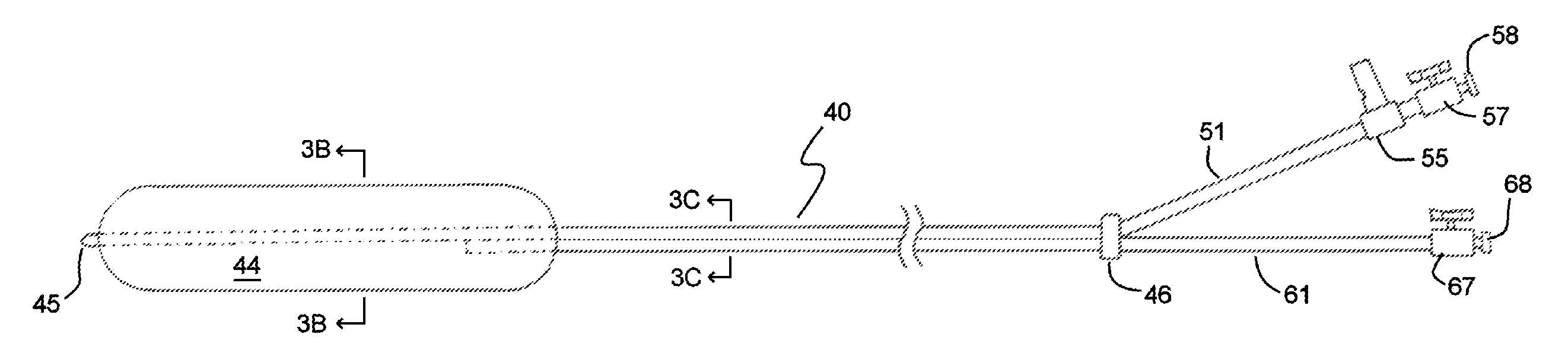
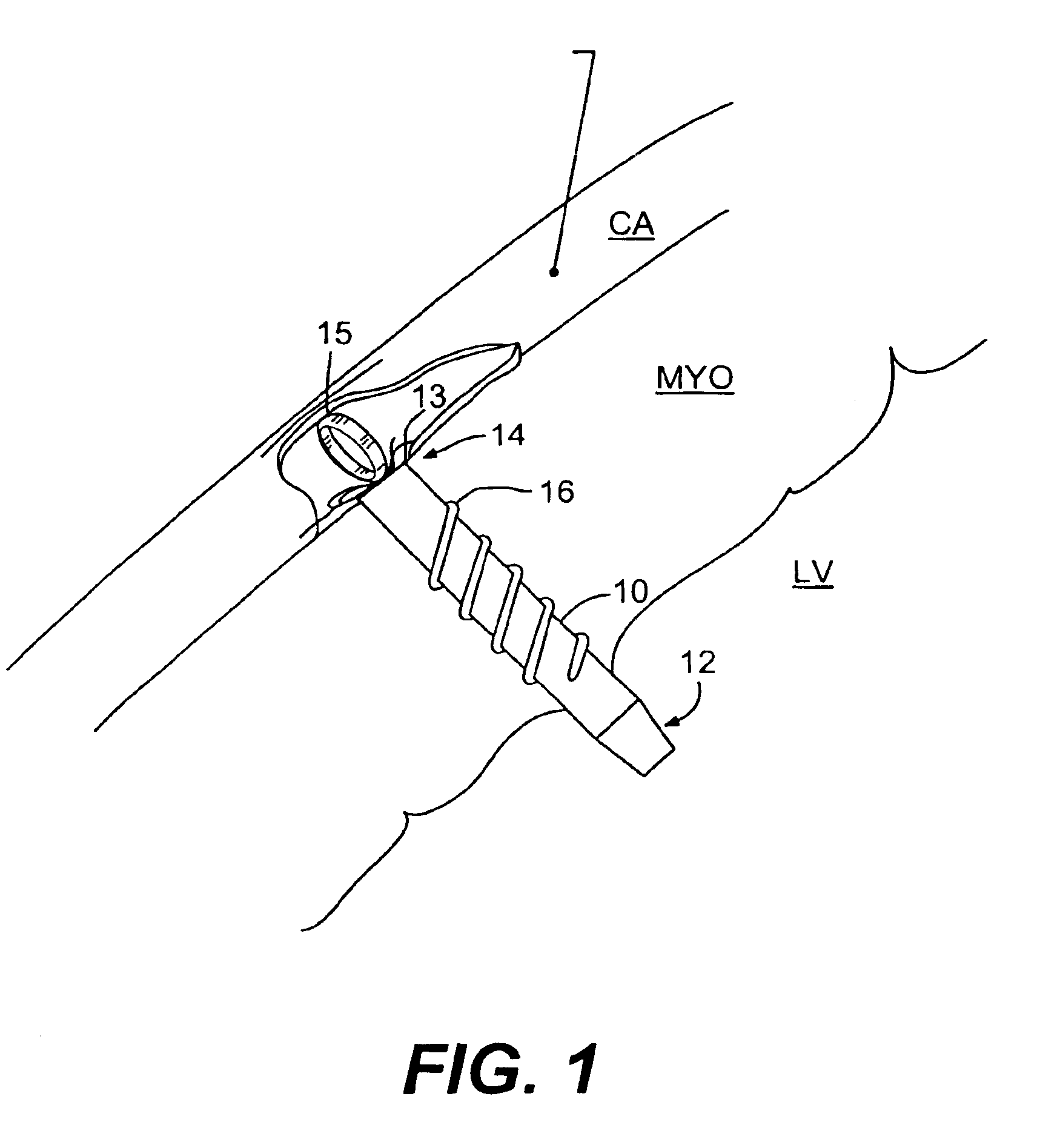
Methods and devices for delivering a ventricular stent
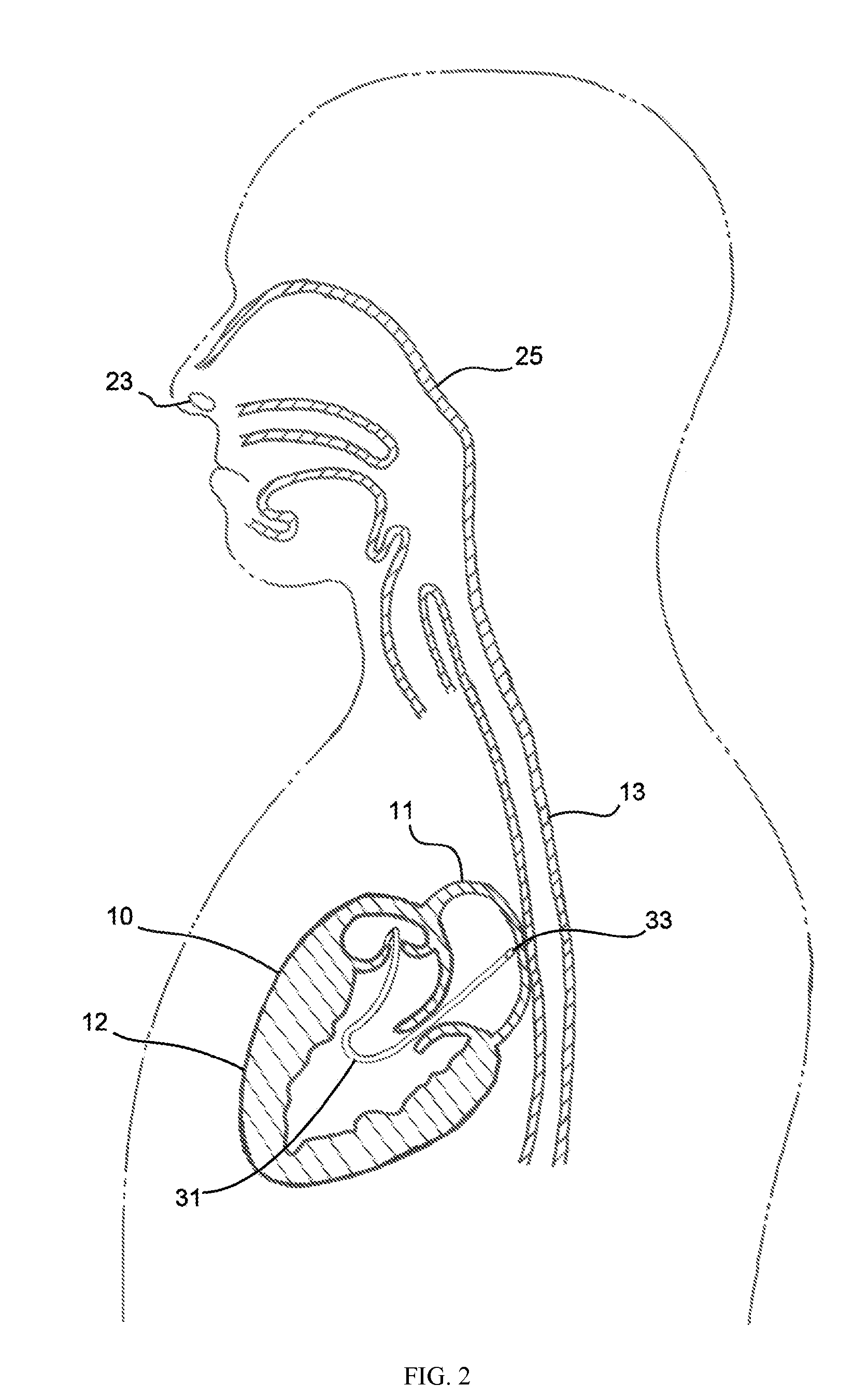
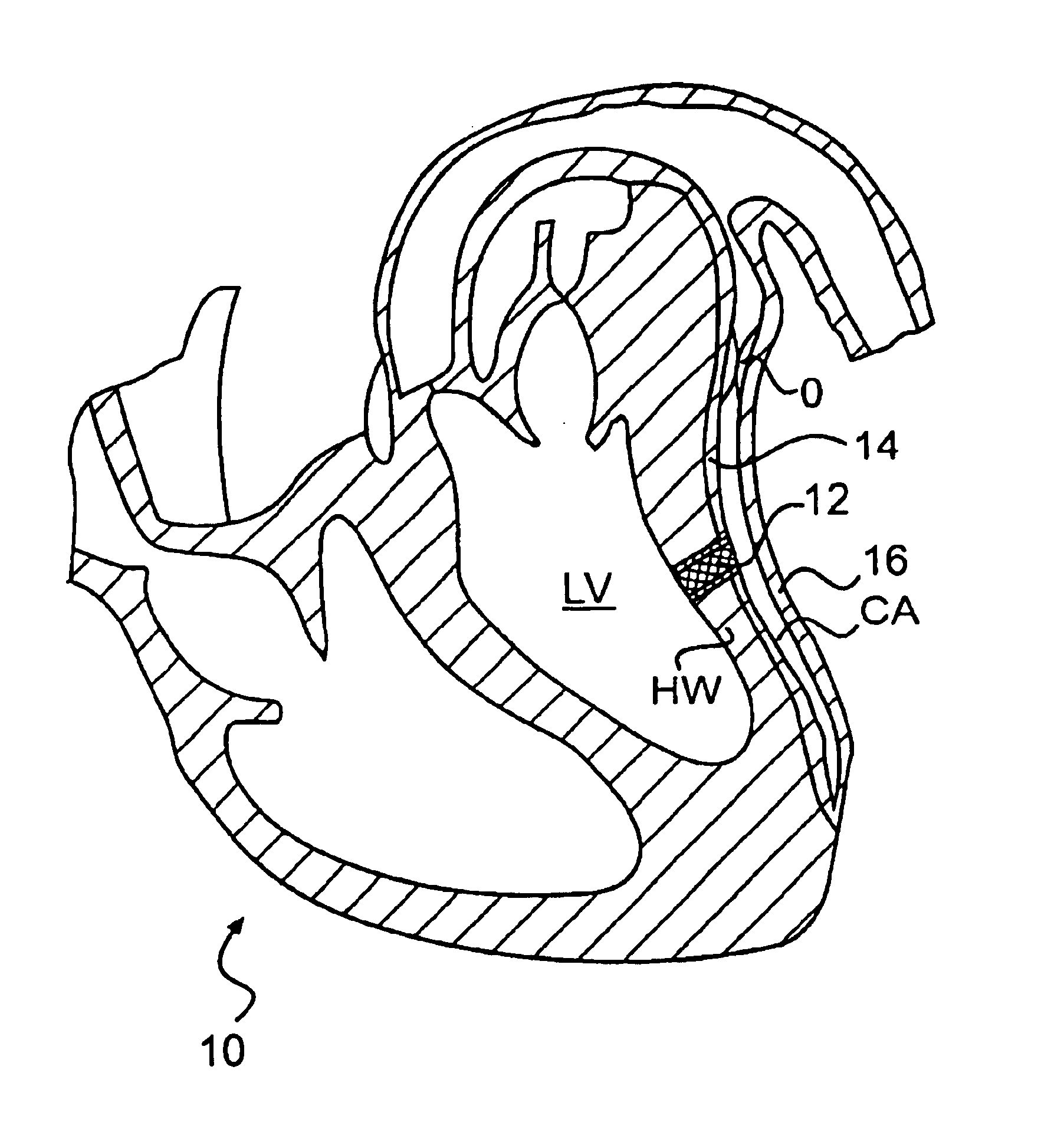
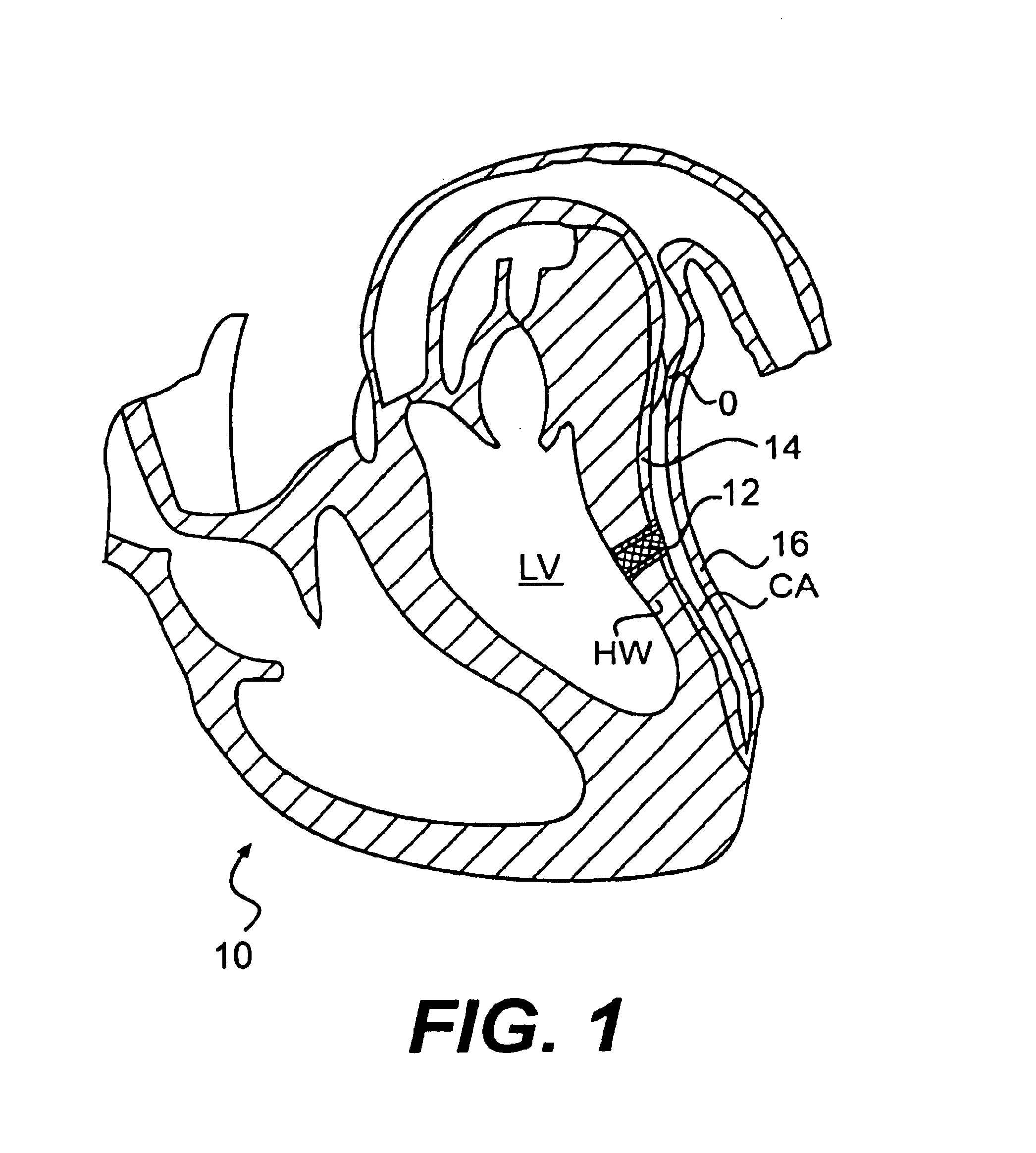
A method, and related tools for performing the method, of delivering a stent or other like device to the heart to connect the left ventricle to the coronary artery to thereby supply blood directly from the ventricle to the coronary artery may be used to bypass a total or partial occlusion of a coronary artery. The method may include placing a guide device and a dilation device through an anterior wall and a posterior wall of the coronary vessel and through a heart wall between the heart chamber and the coronary vessel. The dilation device may be used to form a passageway in the heart wall at a location defined by the guide device. The method may then include placing a stent within the passageway.
Owner:HORIZON TECH FUNDING CO LLC
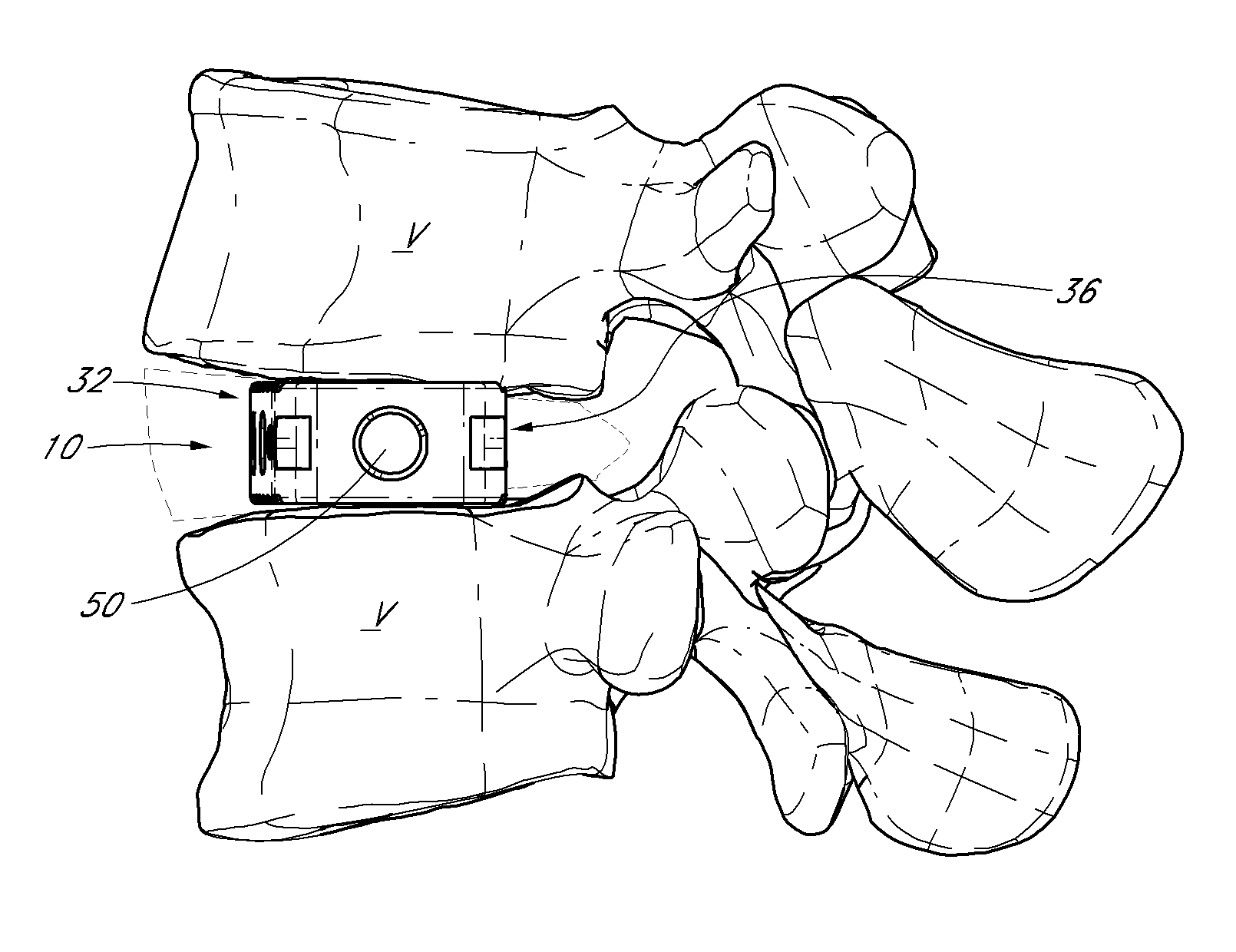
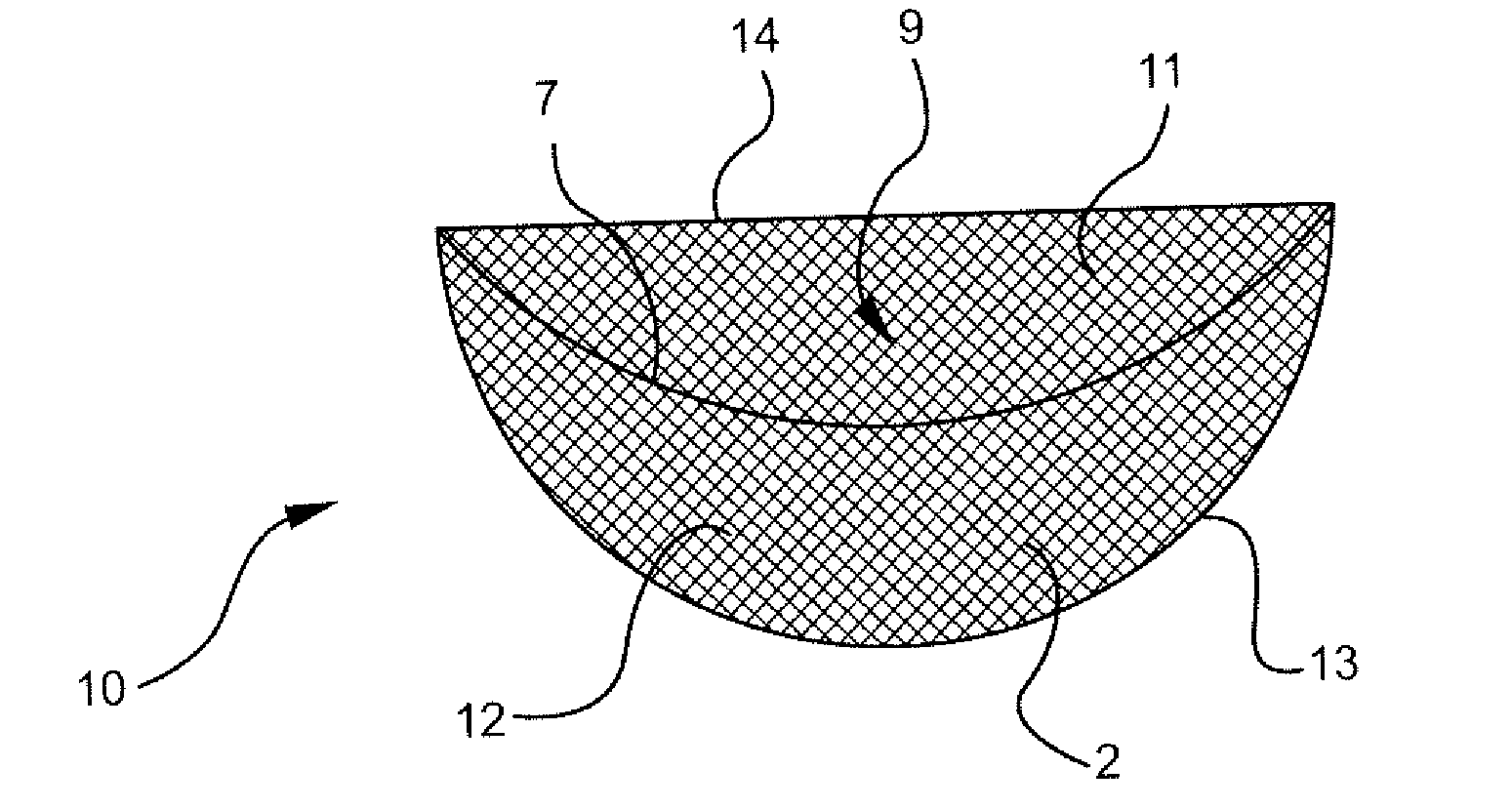
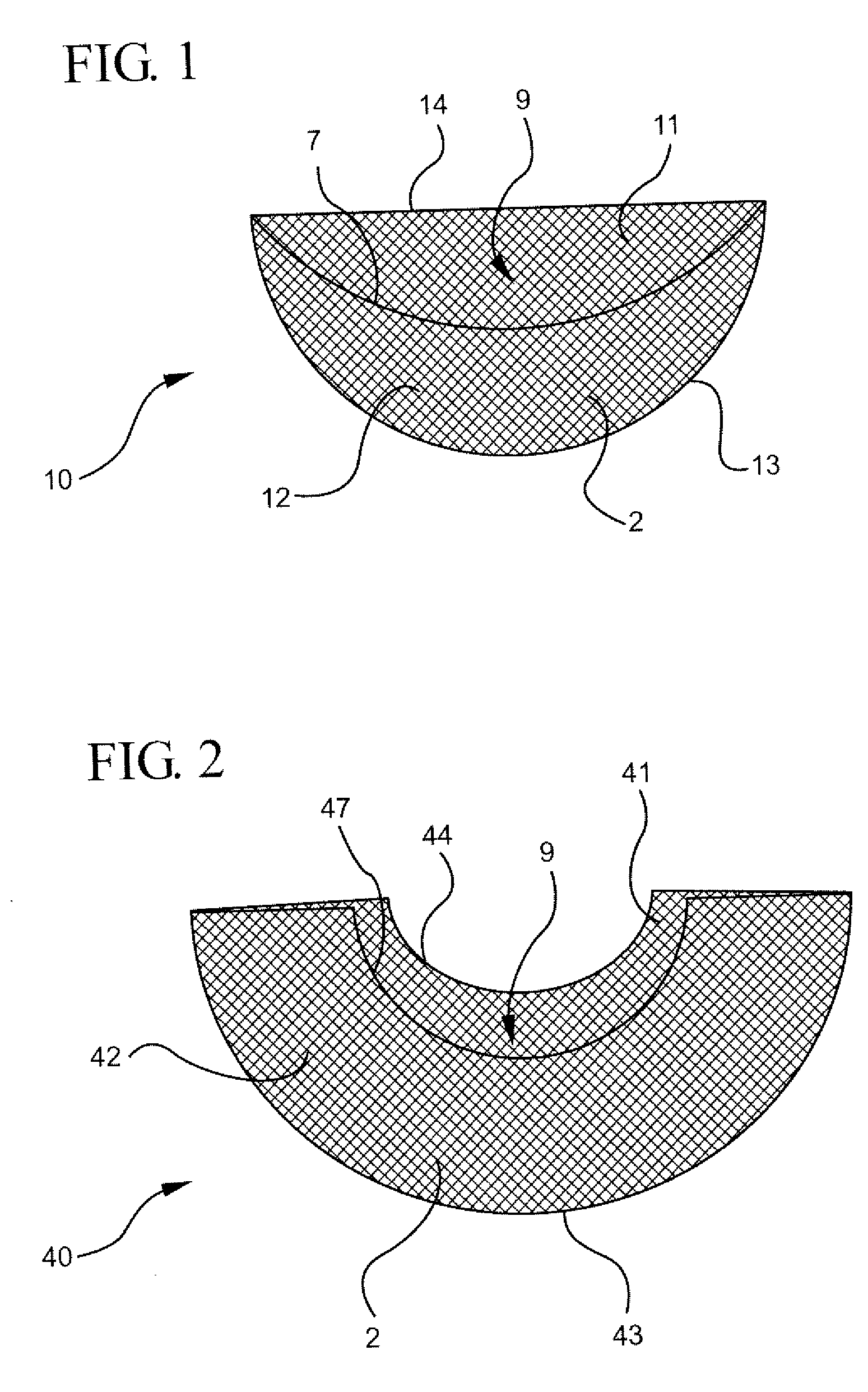
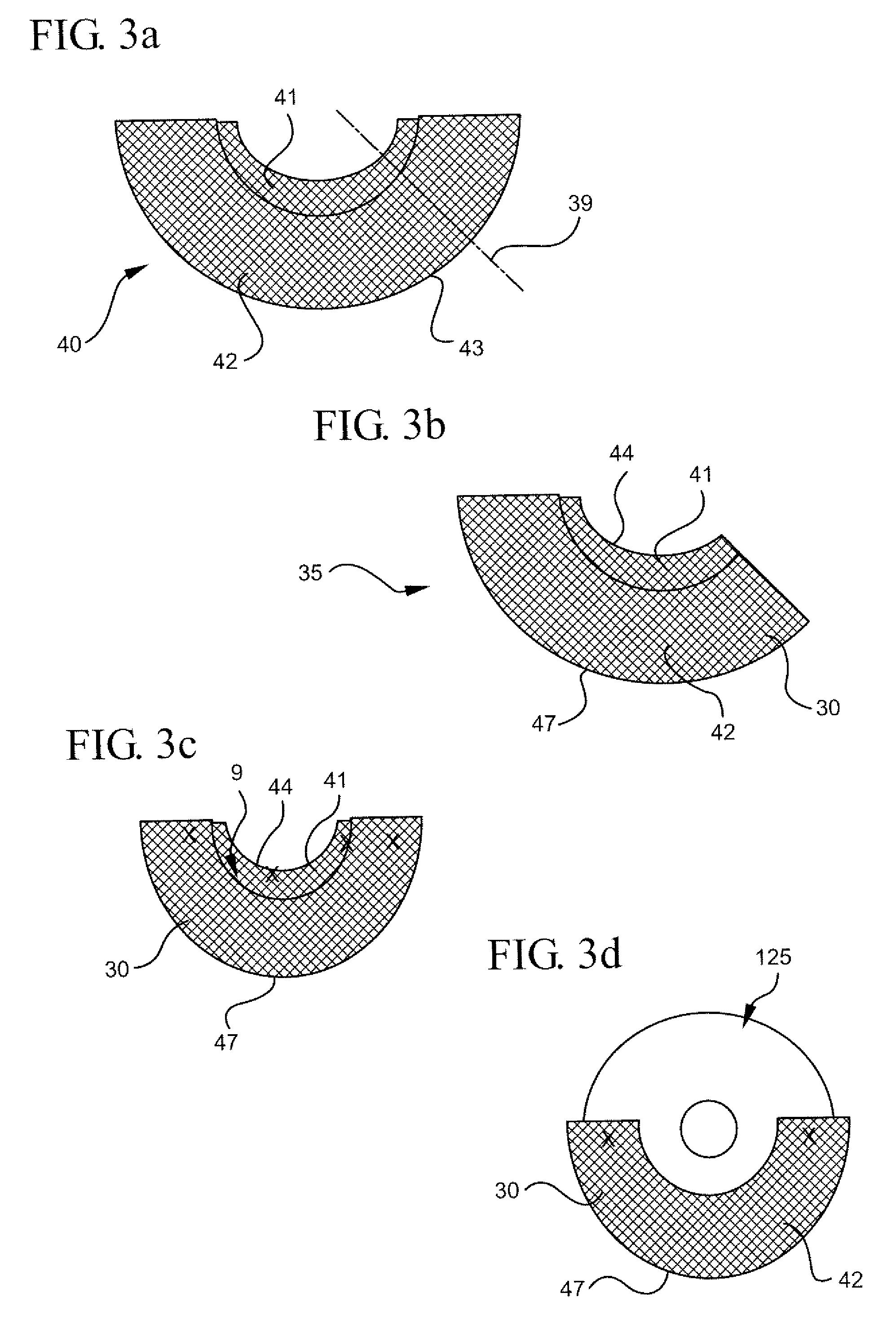
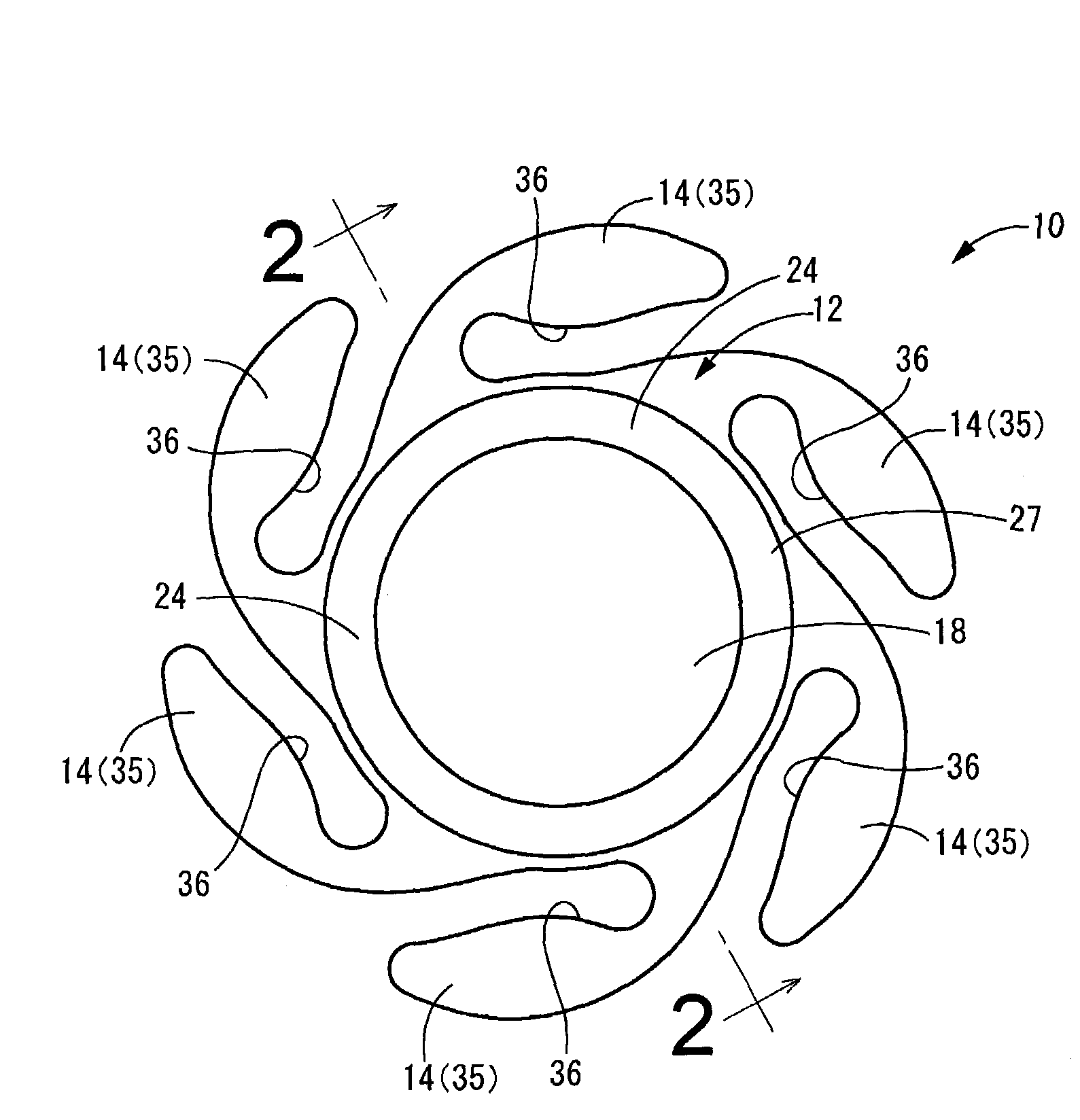
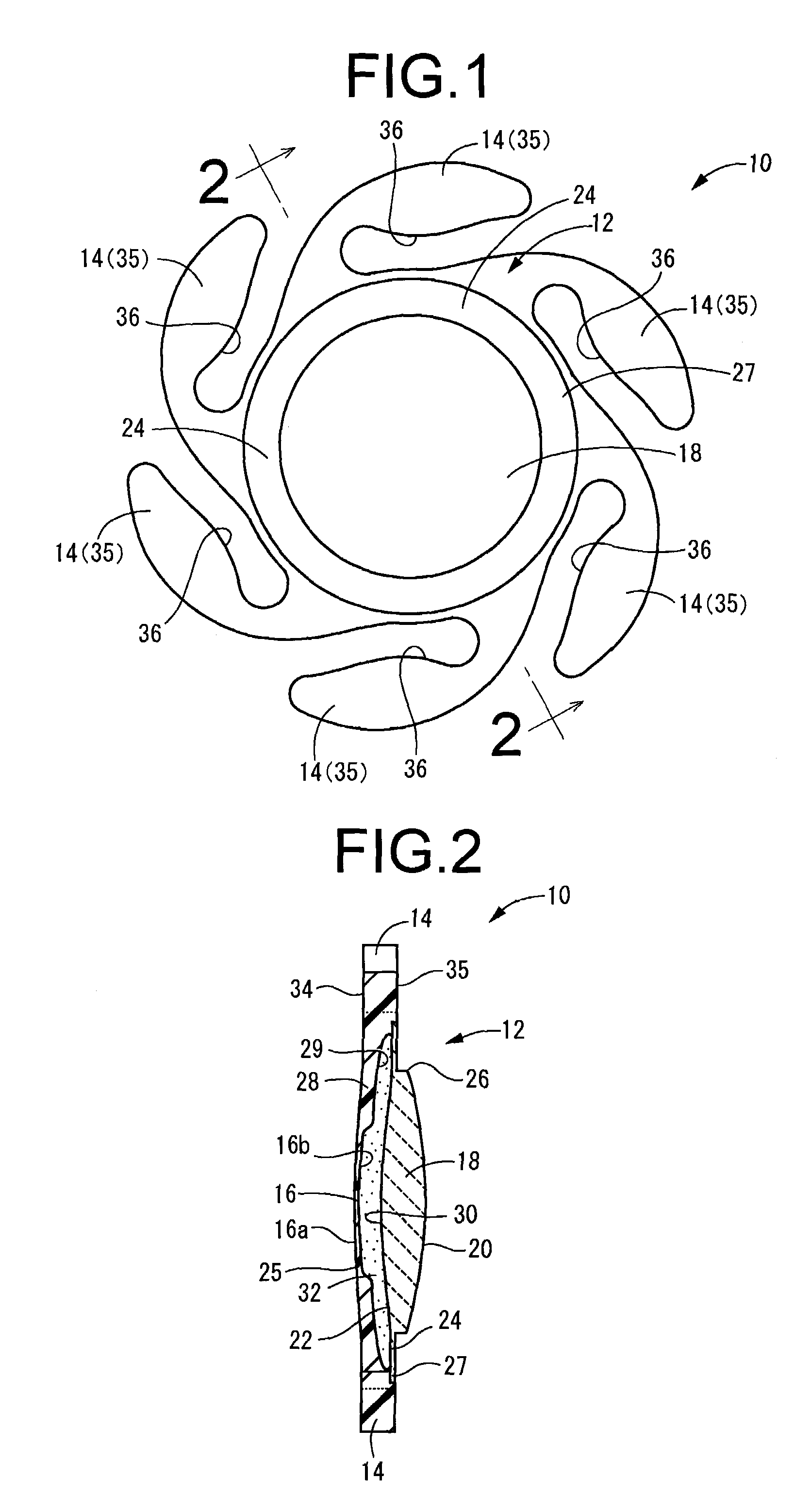
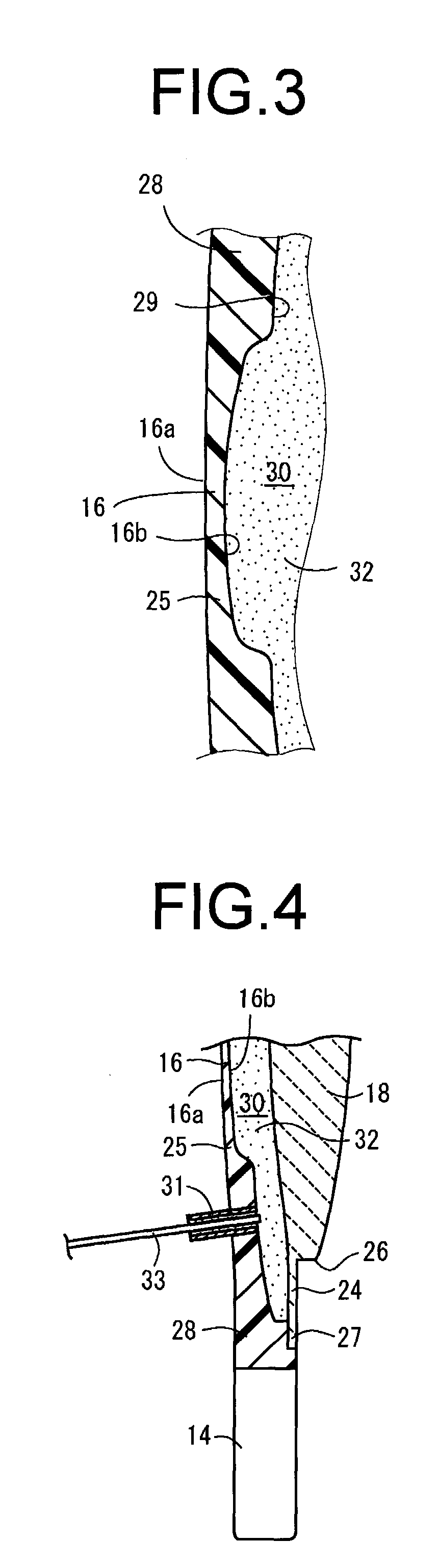
Intervertebral spacers
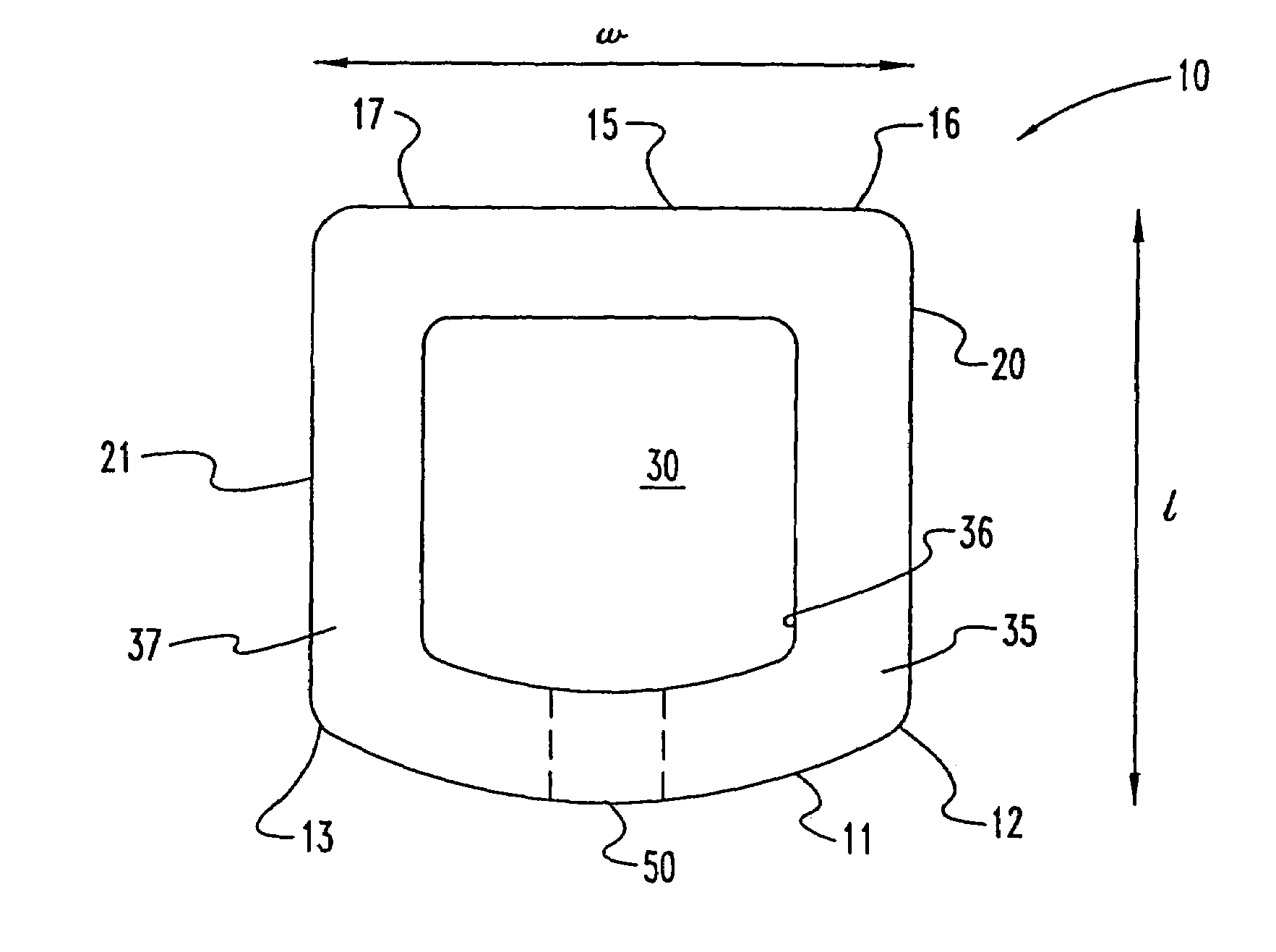
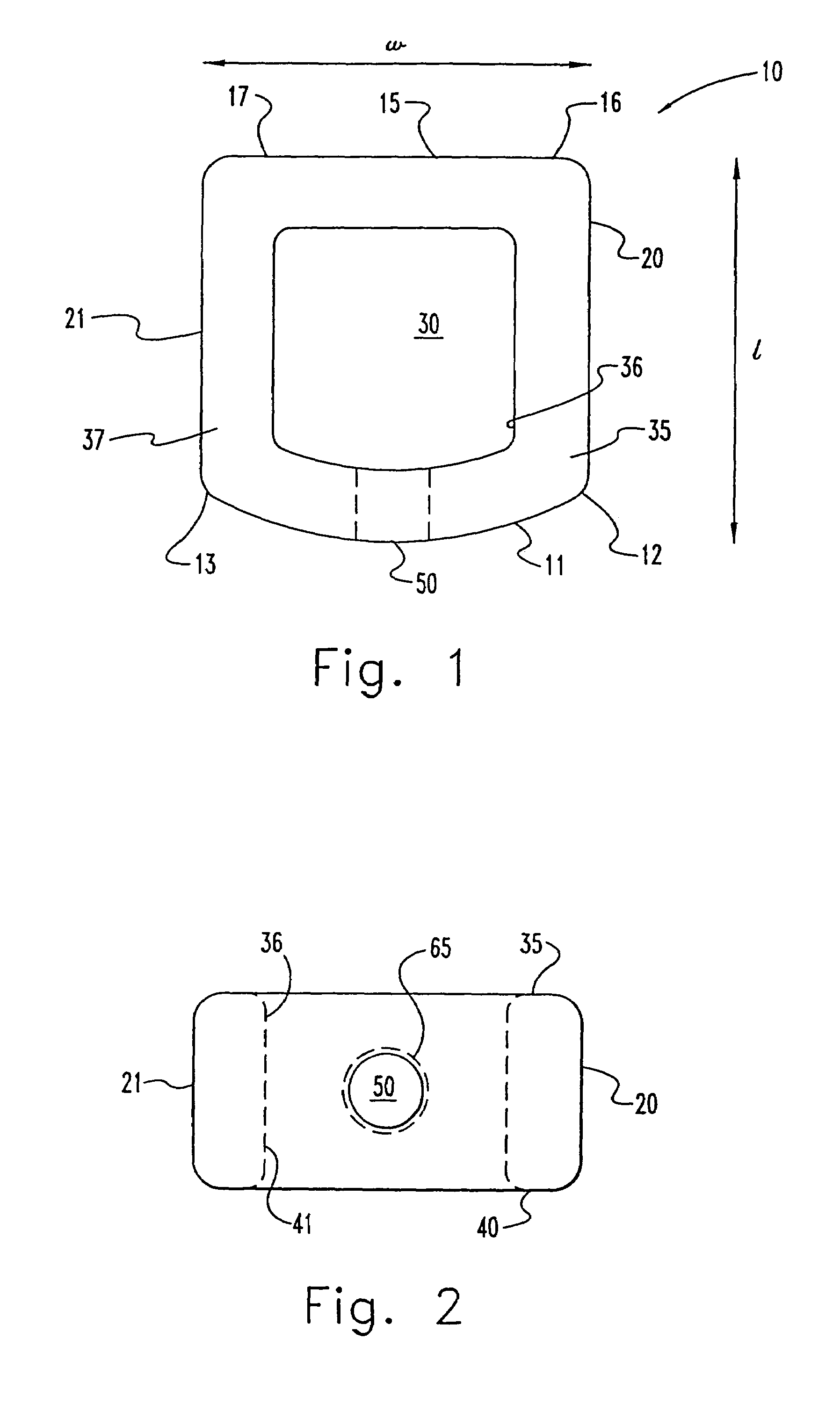
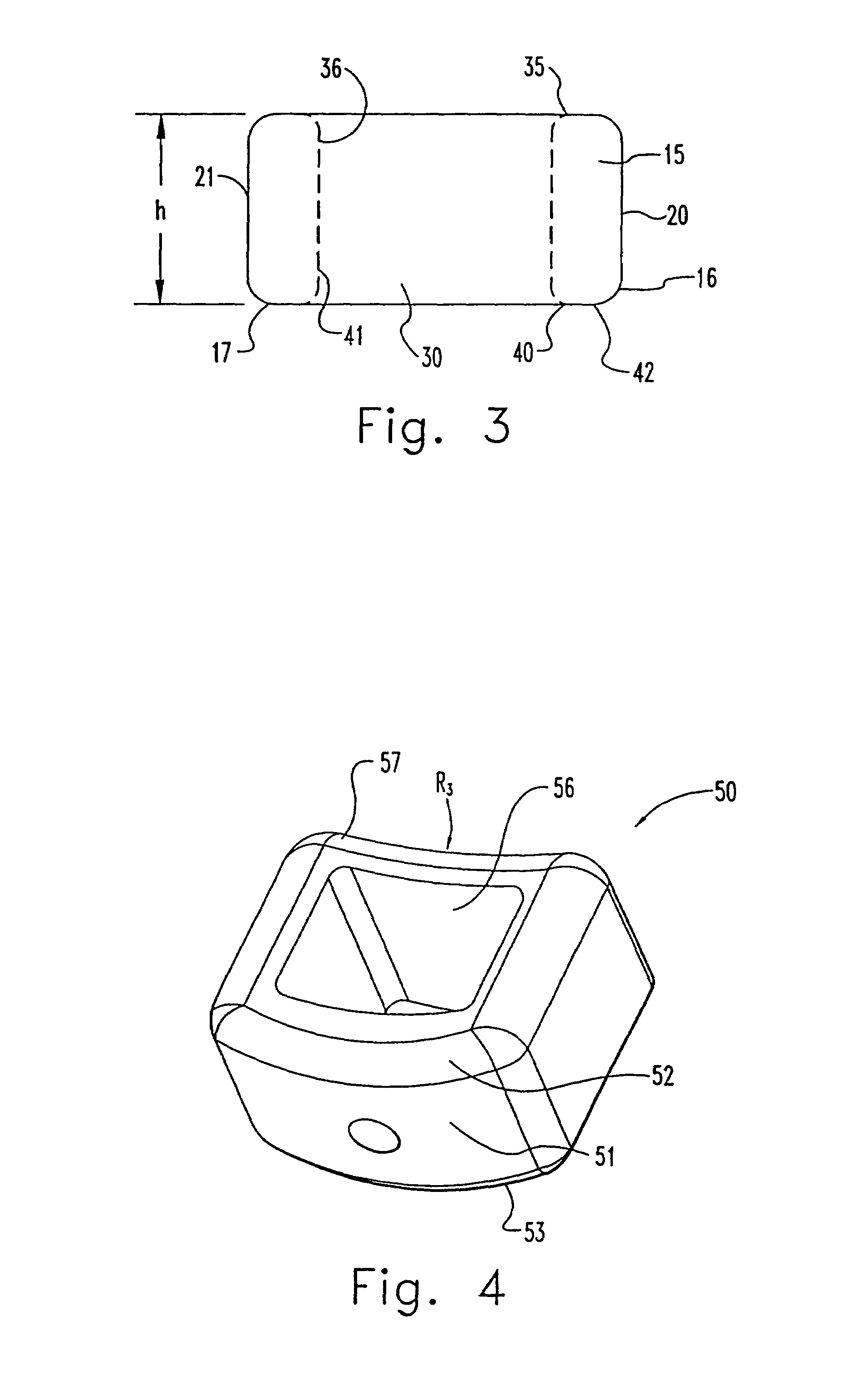
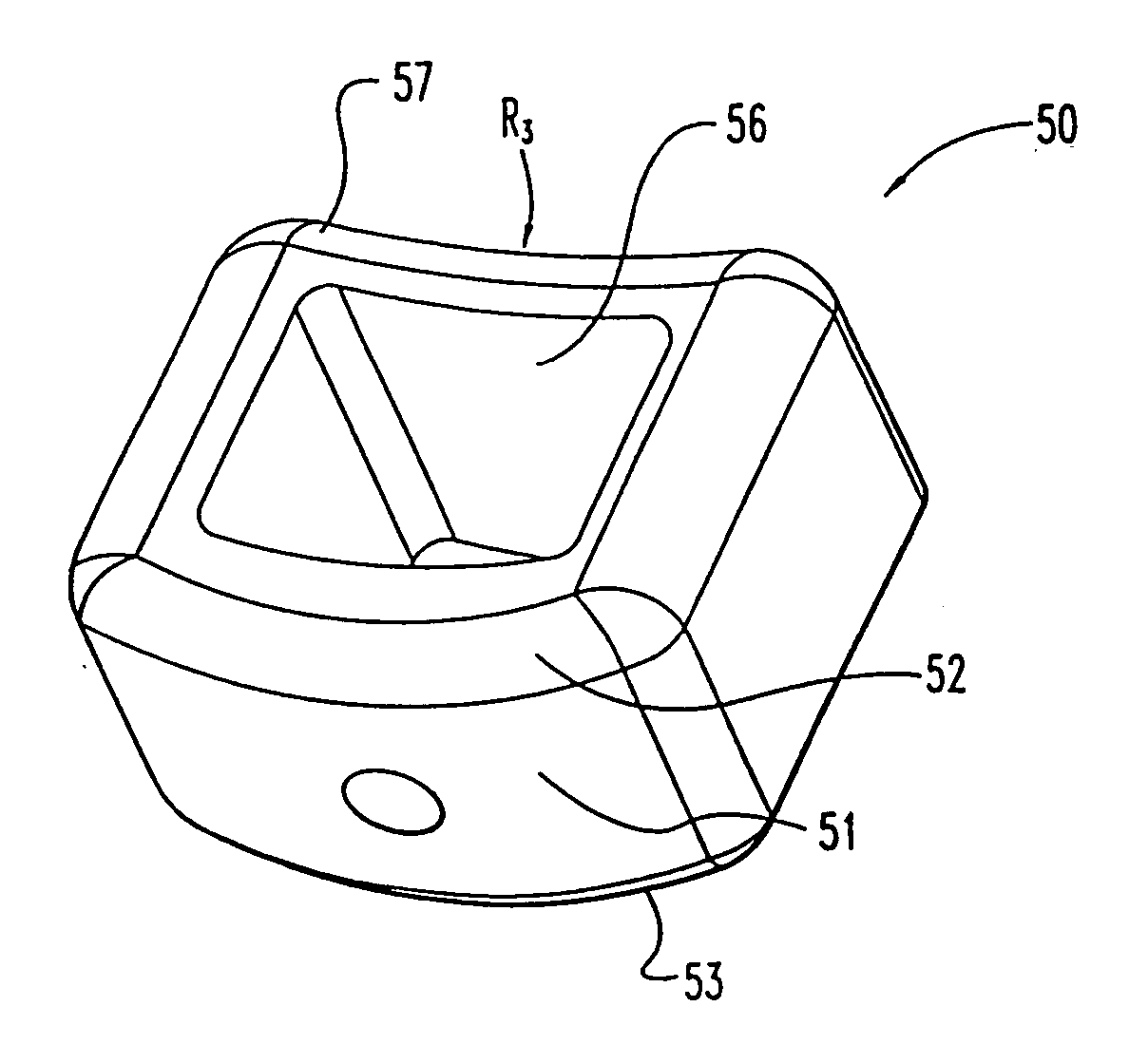
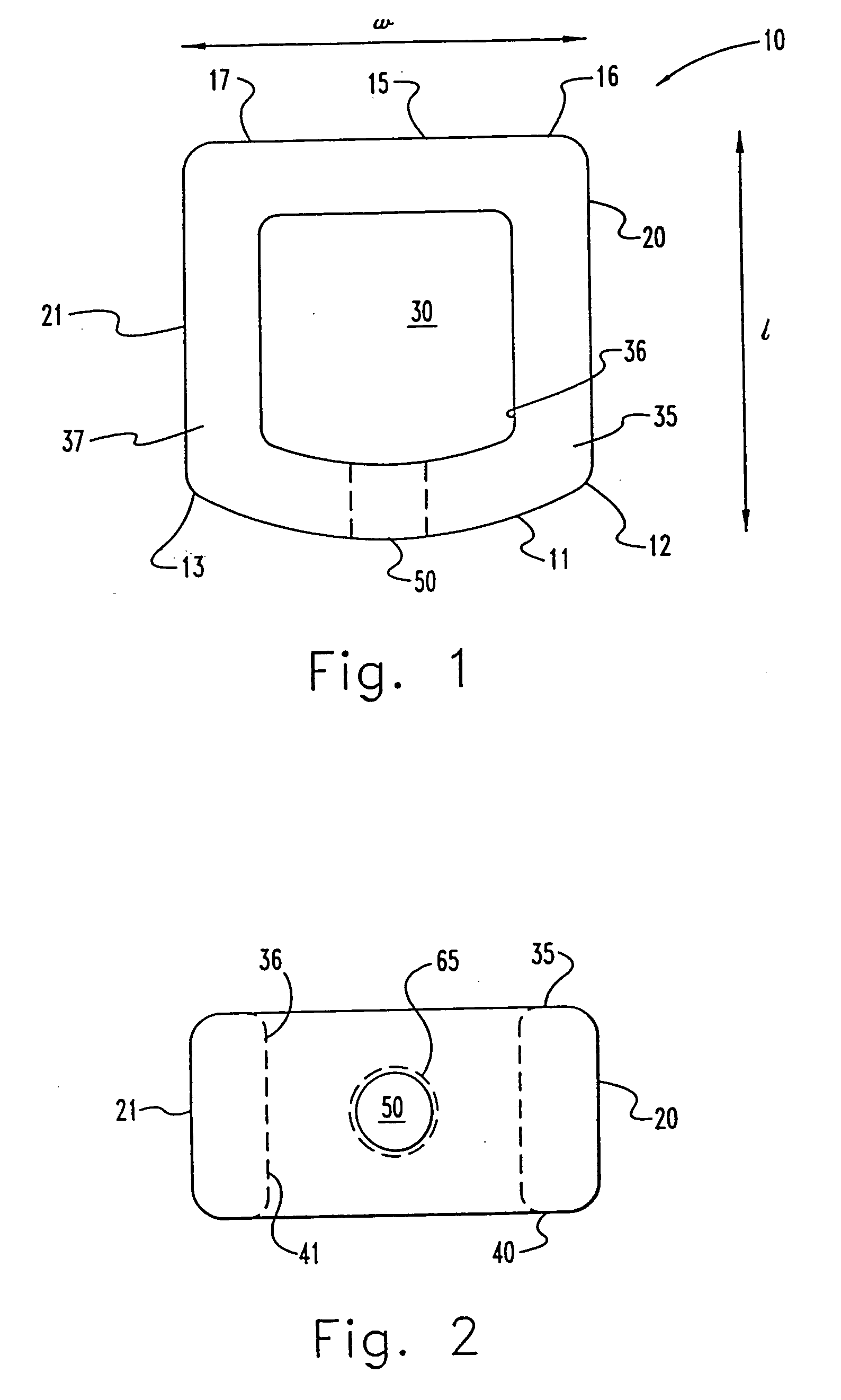
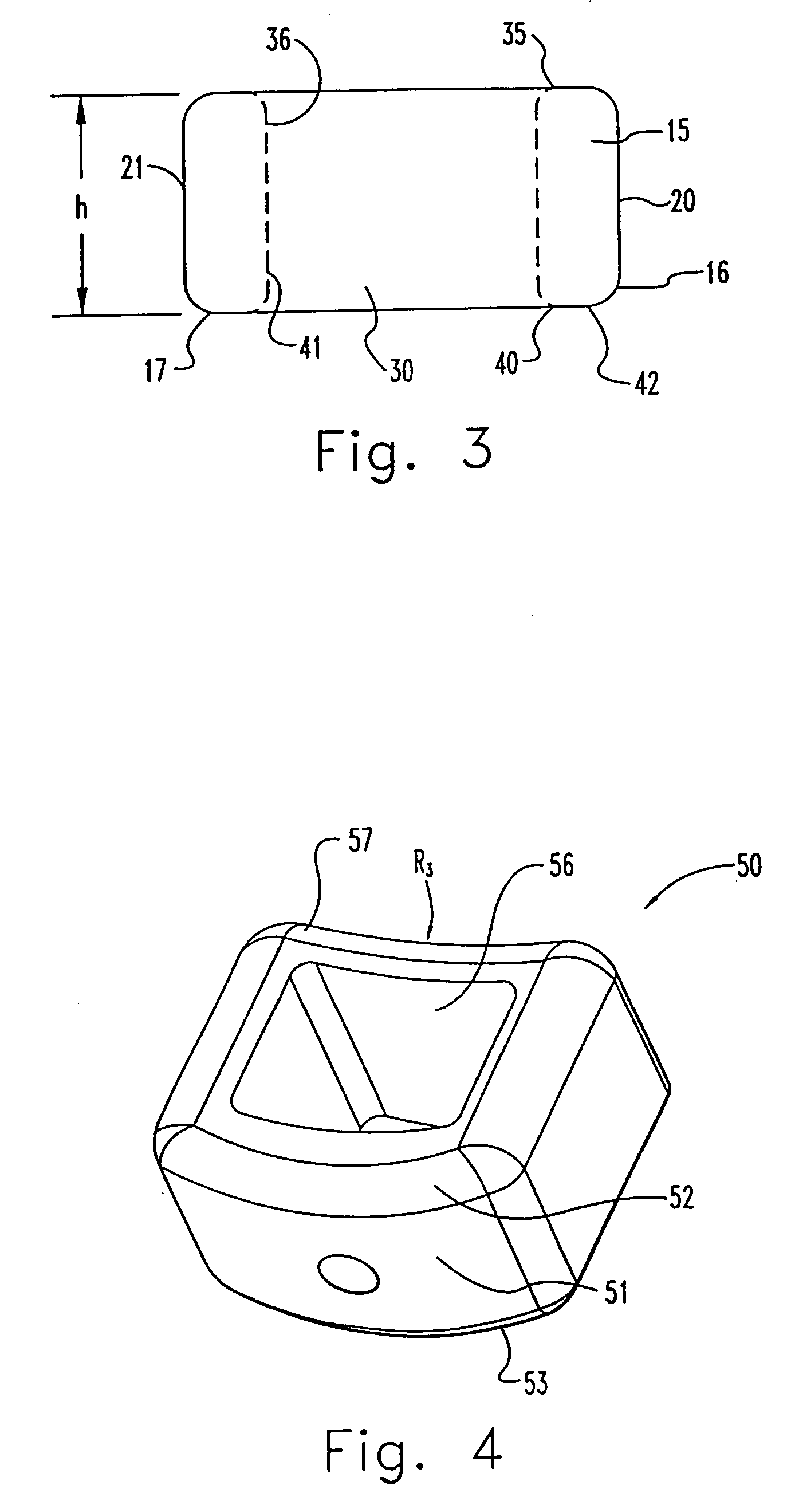
InactiveUS7311734B2Prevent significant subsidencePromoting bone ingrowthBone implantJoint implantsIntervertebral diskOrthodontics
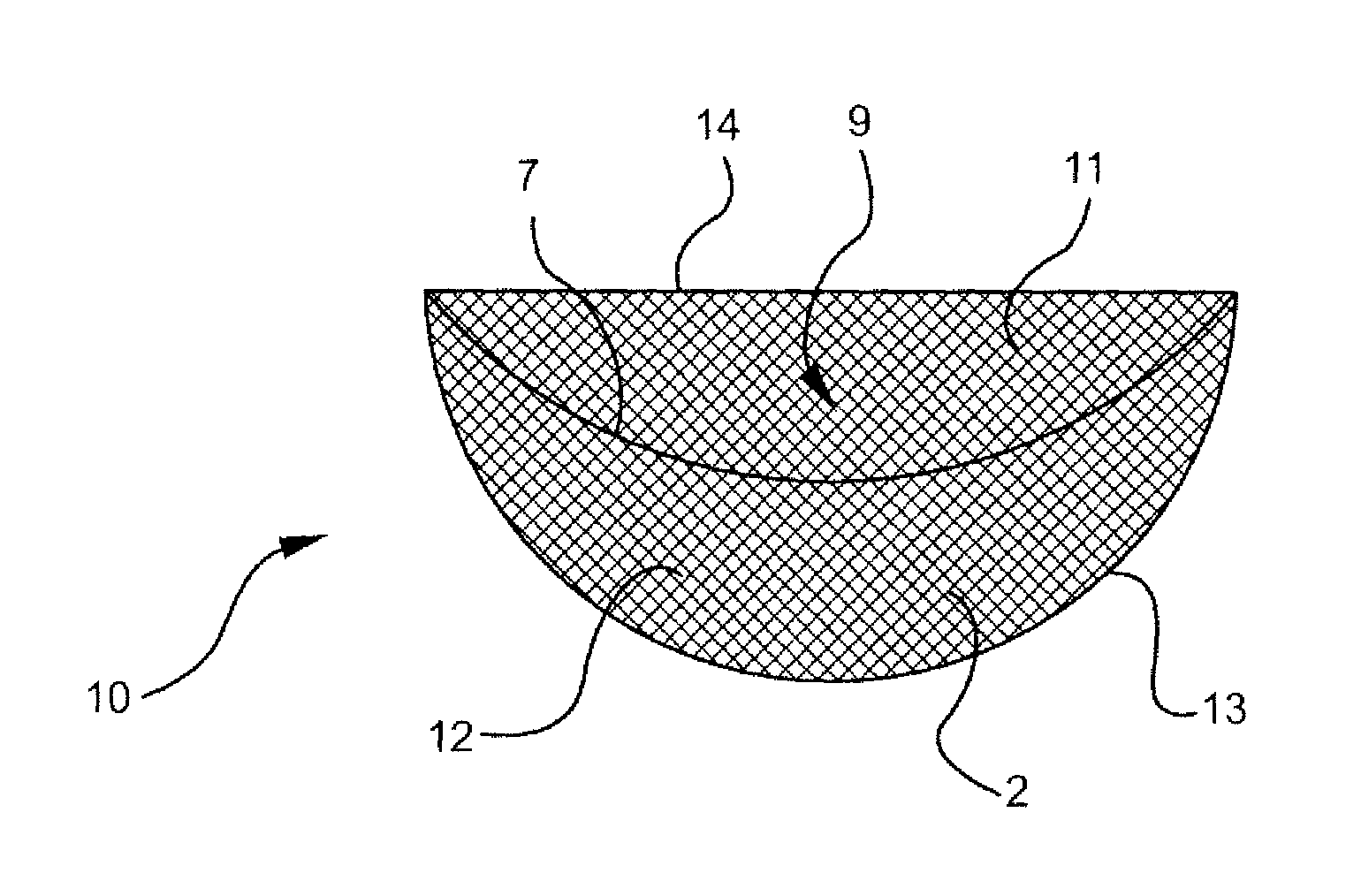
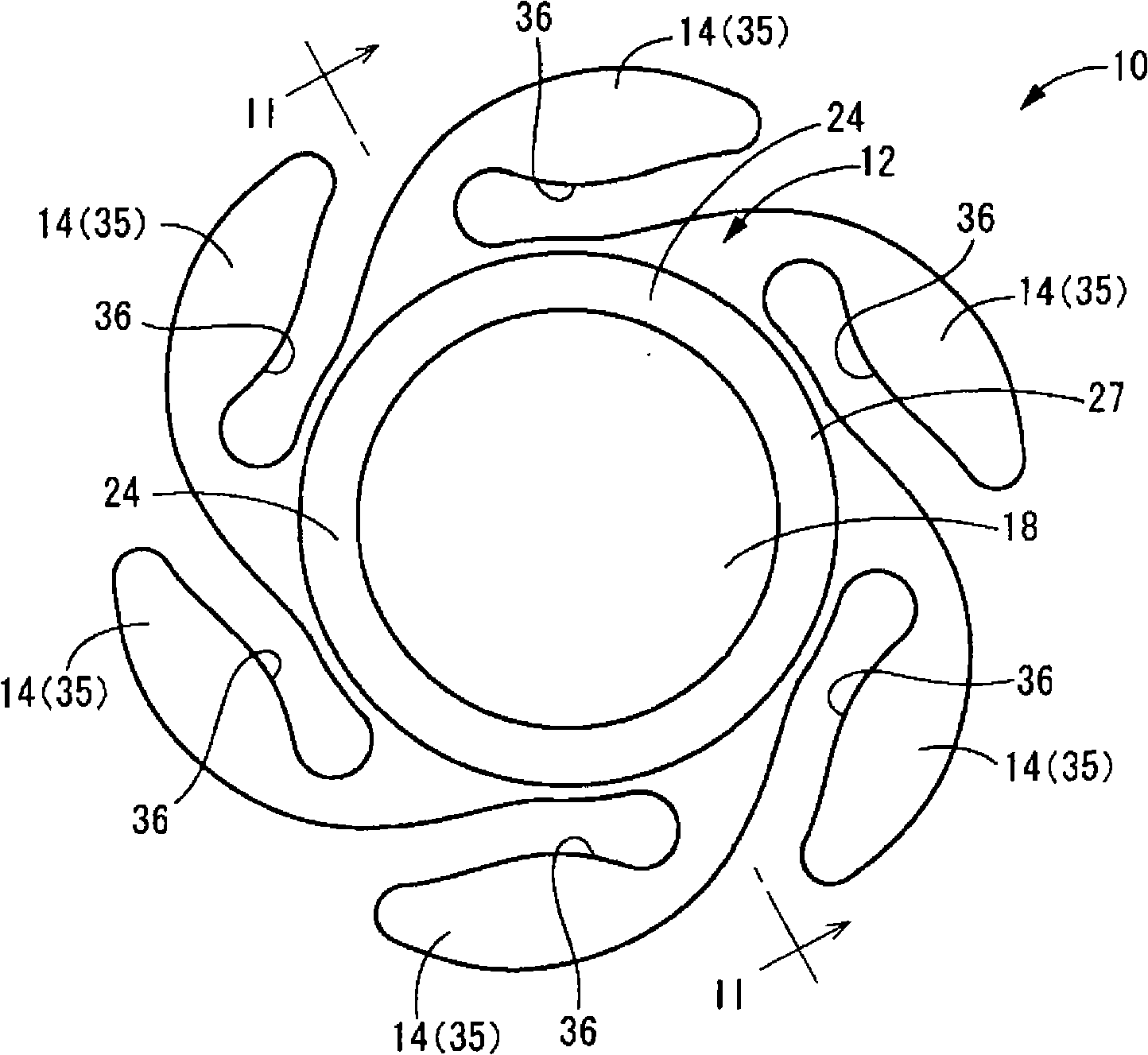
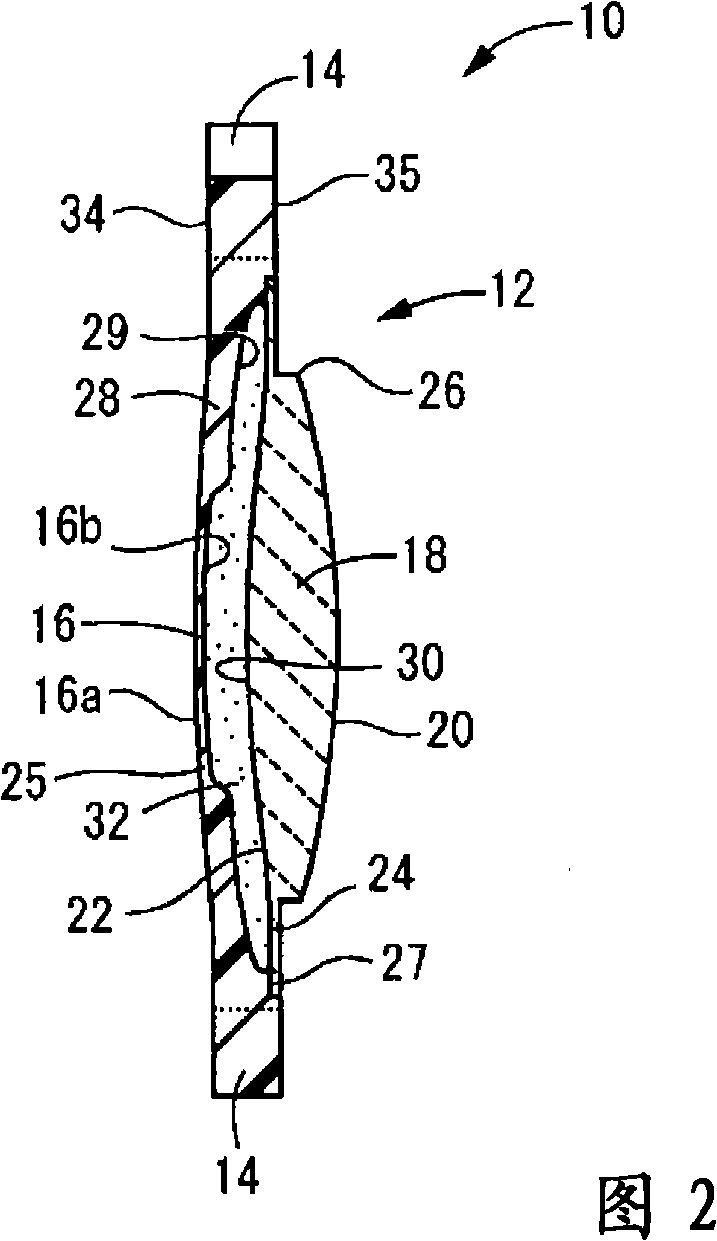
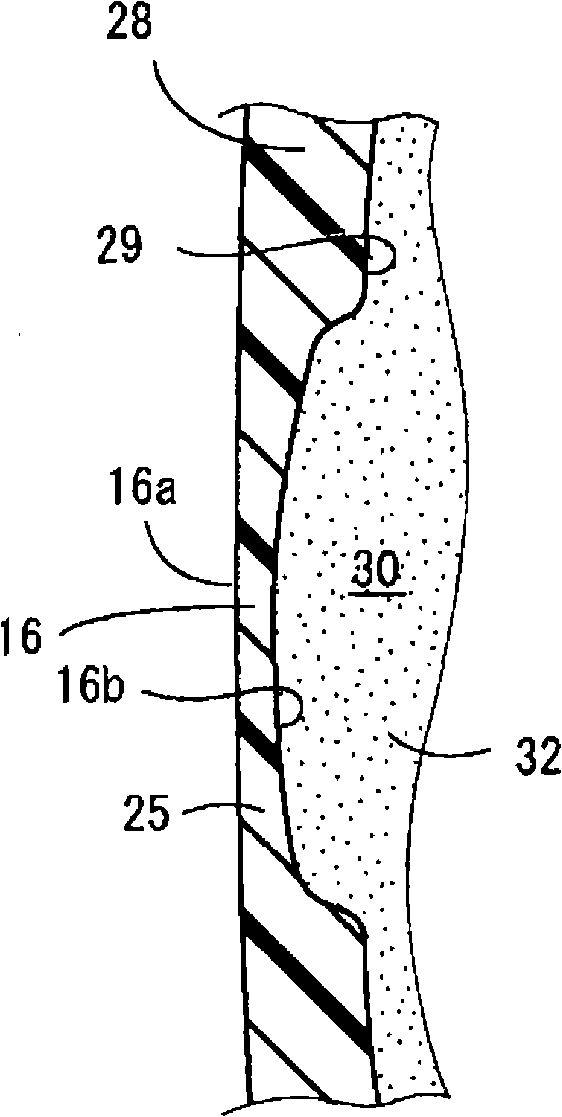
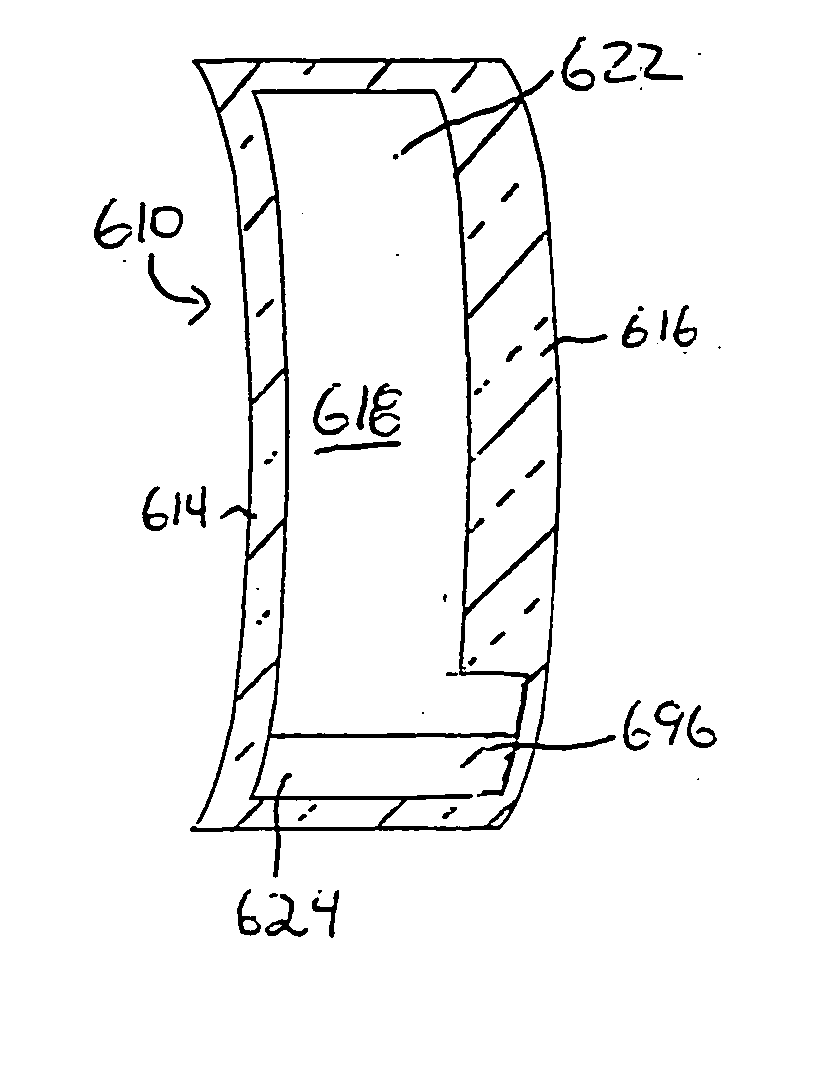
One embodiment of a hollow spinal spacer (10) includes a curved anterior wall (11) having opposite ends (12, 13), a posterior wall (15) having opposite ends (16, 17), two lateral walls (20, 21), each integrally connected between the opposite ends (12, 13, 16, 17) of the anterior (11) and posterior (15) walls to define a chamber (30). The walls (11, 15, 20, 21) include a superior face (35) and an inferior face (40). The superior face (35) defines a first opening (36) in communication with the chamber (30) and includes a first vertebral engaging surface (37). The inferior face (40) defines a second opening (41) in communication with the chamber (30) and includes a second vertebral engaging surface (42).
Owner:WARSAW ORTHOPEDIC INC
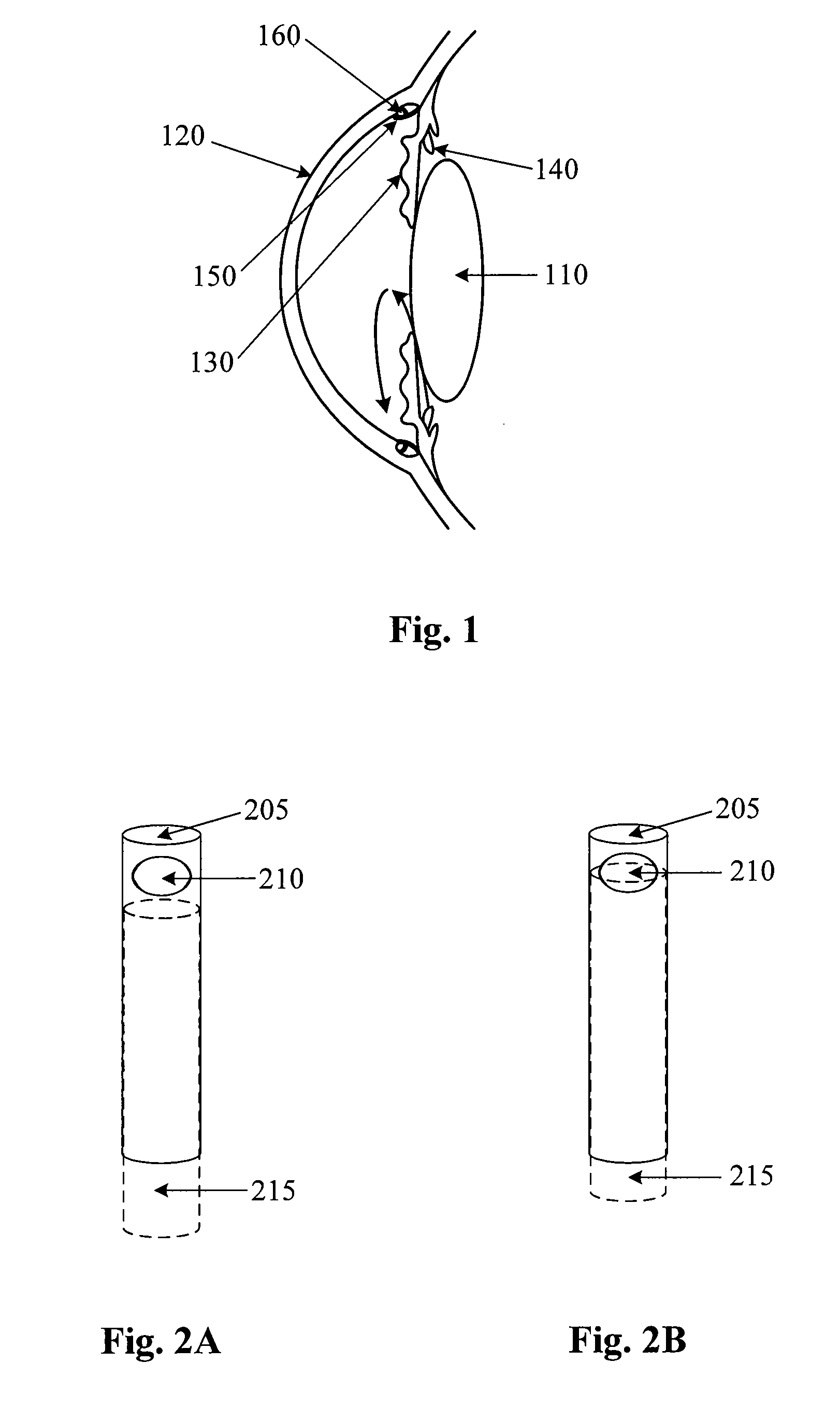
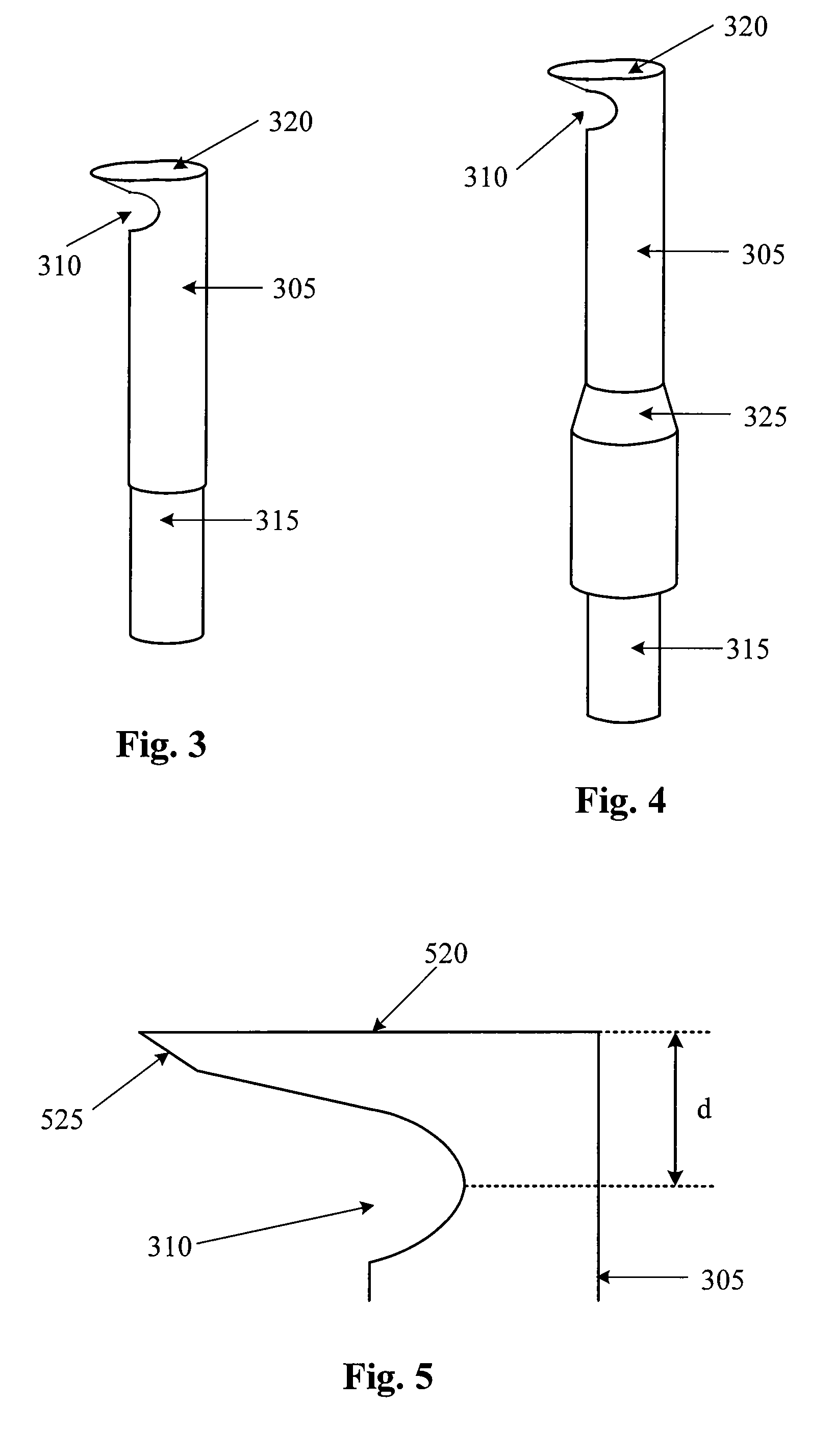
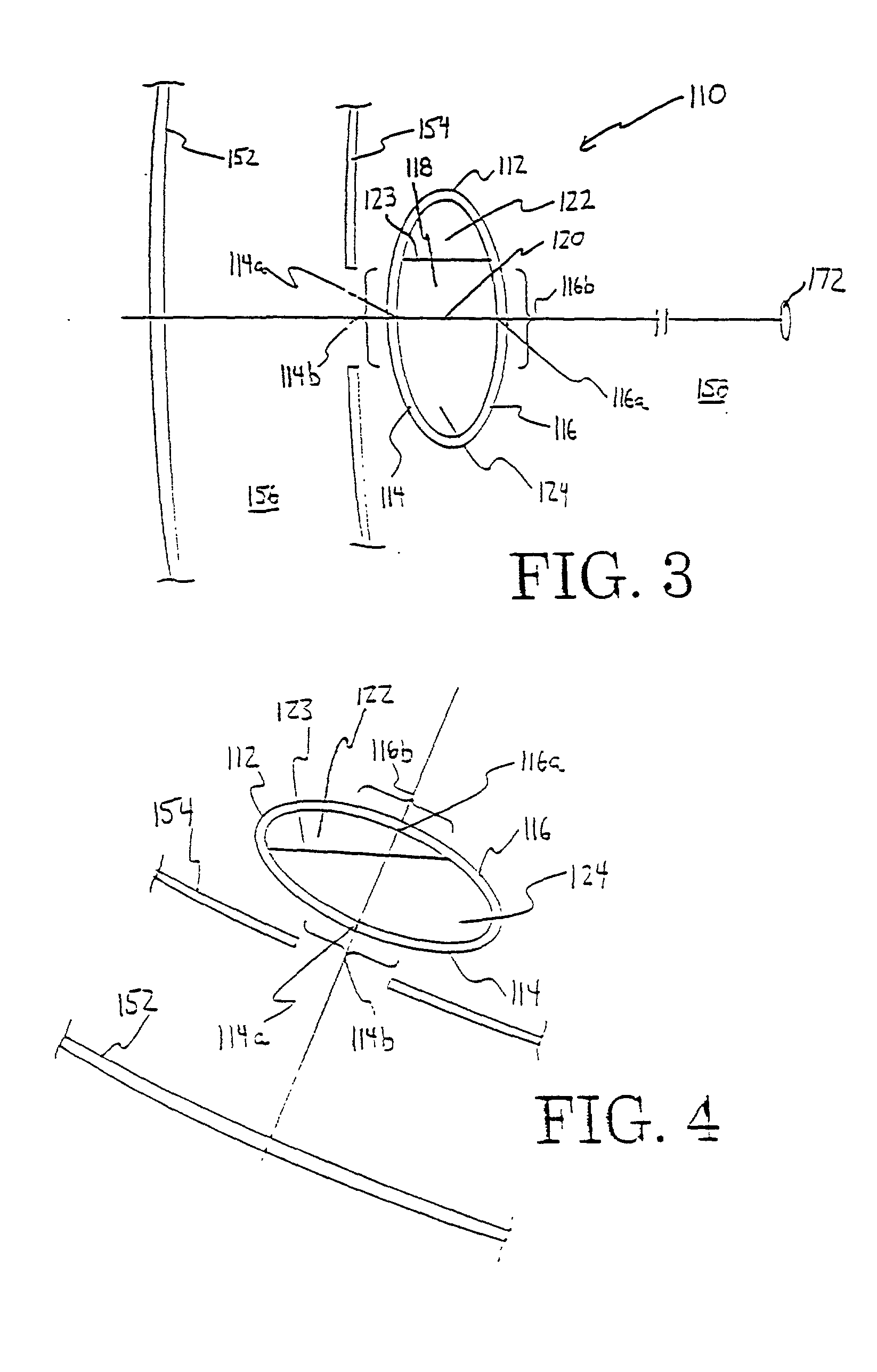
Small Gauge Mechanical Tissue Cutter/Aspirator Probe For Glaucoma Surgery
A small gauge mechanical tissue cutter / aspirator probe useful for removing the trabecular meshwork of a human eye has a generally cylindrical outer cannula, an inner cannula that reciprocates in the outer cannula, a port located near or at the distal end of the outer cannula on a side or tip of the outer cannula, and a guide with a distal surface located on the distal end of the outer cannula. A distance between the distal surface of the guide and the port is approximately equal to the distance between the back wall of Schlemm's canal and the trabecular meshwork.
Owner:ALCON RES LTD
Naturally contoured, preformed, three dimensional mesh device for breast implant support
ActiveUS7875074B2Shorten the construction periodOvercomes inherent disadvantageMammary implantsWound clampsWrinkle skinBreast implant
A preformed, seamless, three-dimensional, anatomically contoured prosthetic device for reinforcing breast tissue and supporting a breast implant includes a flat back wall, a concave front wall and a curved transitional region between the flat back wall and the front wall defining a smoothly curved bottom periphery. A concave receiving space is defined by the back wall and the front wall for at least partially receiving and supporting the breast implant therein. The three-dimensional prosthetic device is free of wrinkles, creases, folds or seams, which may have otherwise caused potential tissue irritation, bacteria hosting, infection and palpability problems.
Owner:ETHICON INC
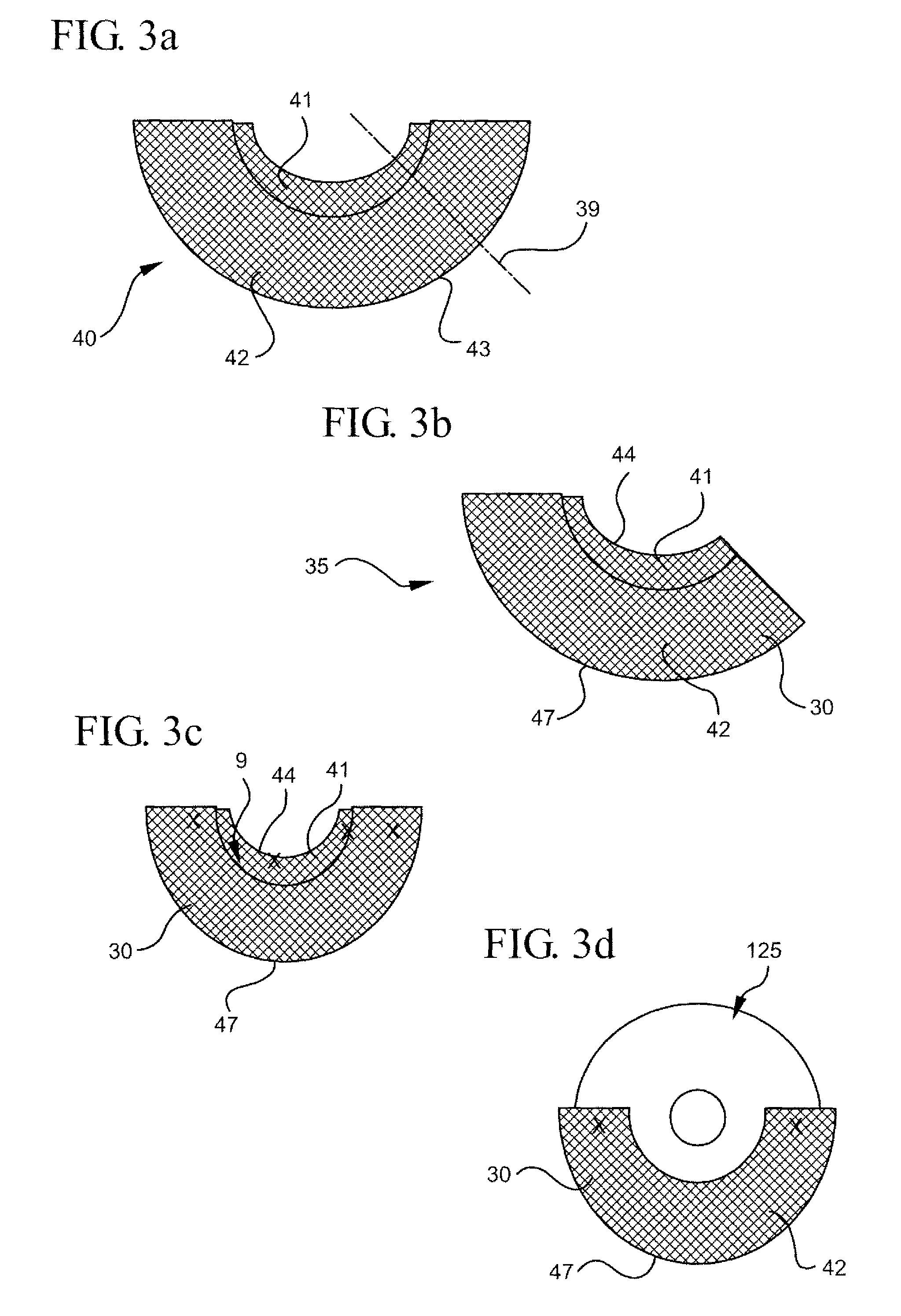
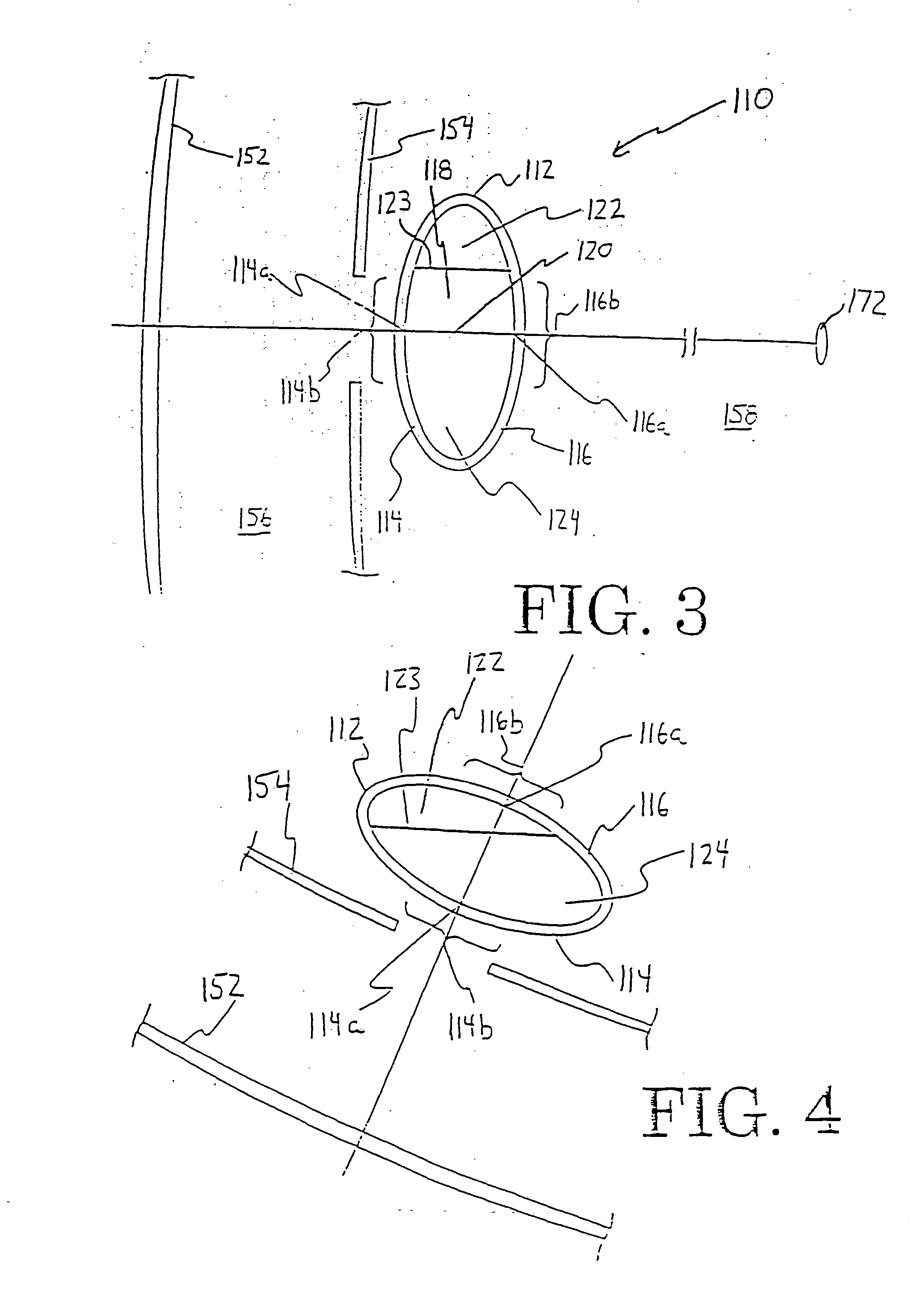
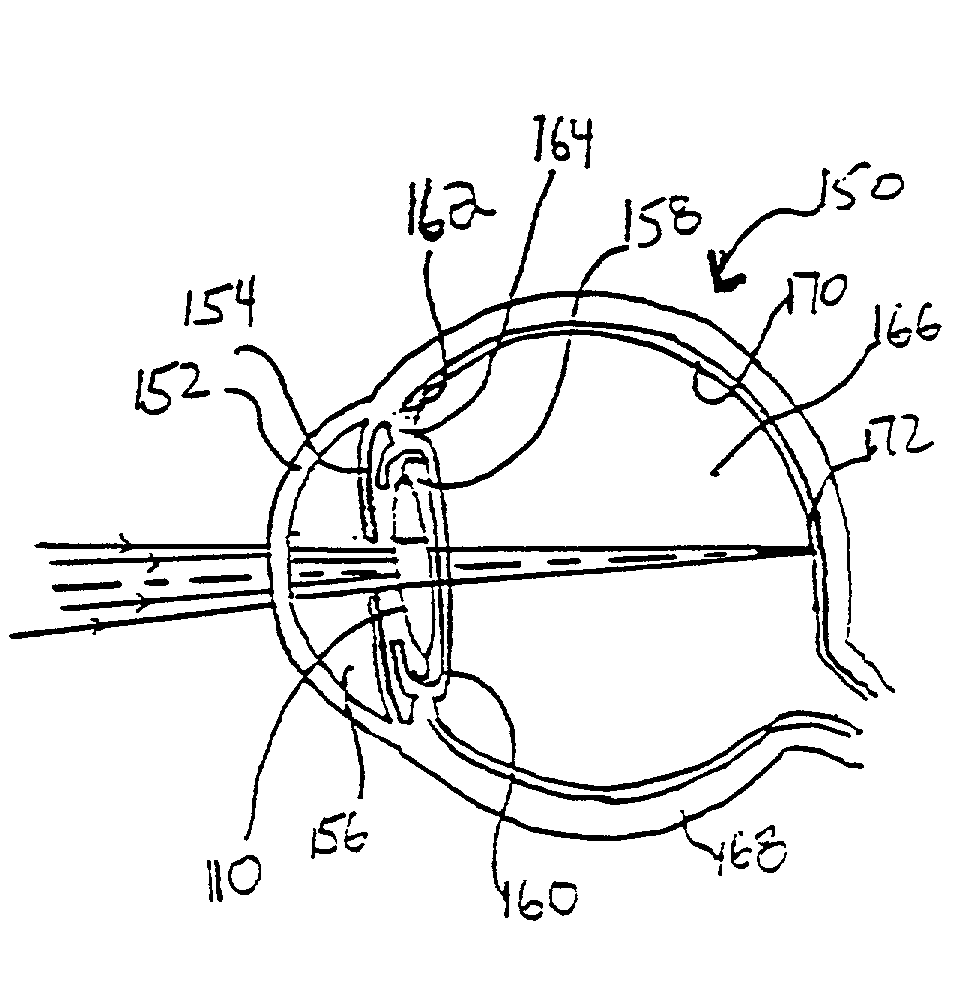
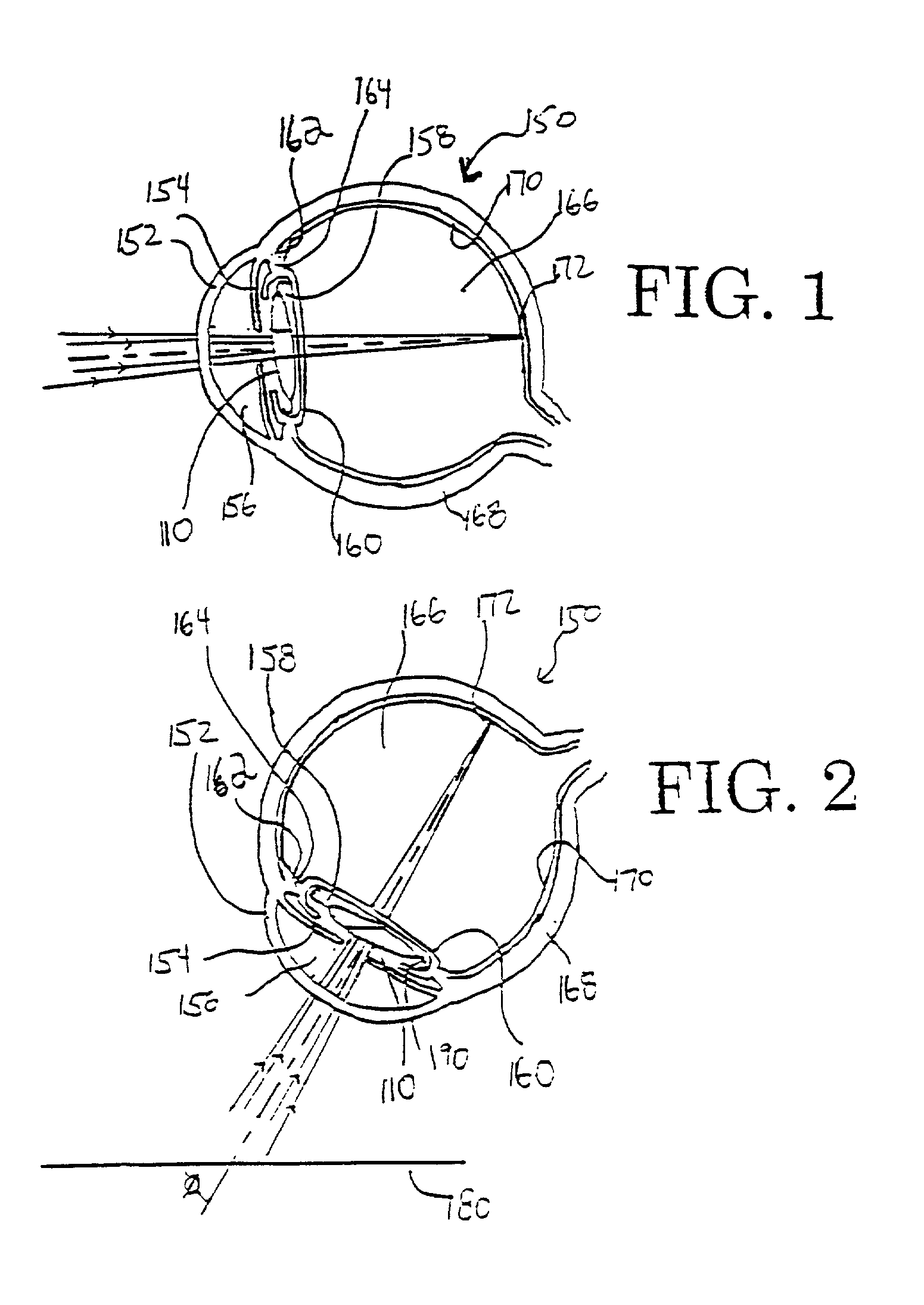
Multi-focal intraocular lens, and methods for making and using same
InactiveUS6855164B2Without significant disruptionRestores focus mechanismEye surgeryIntraocular lensOptical axisRefractive index
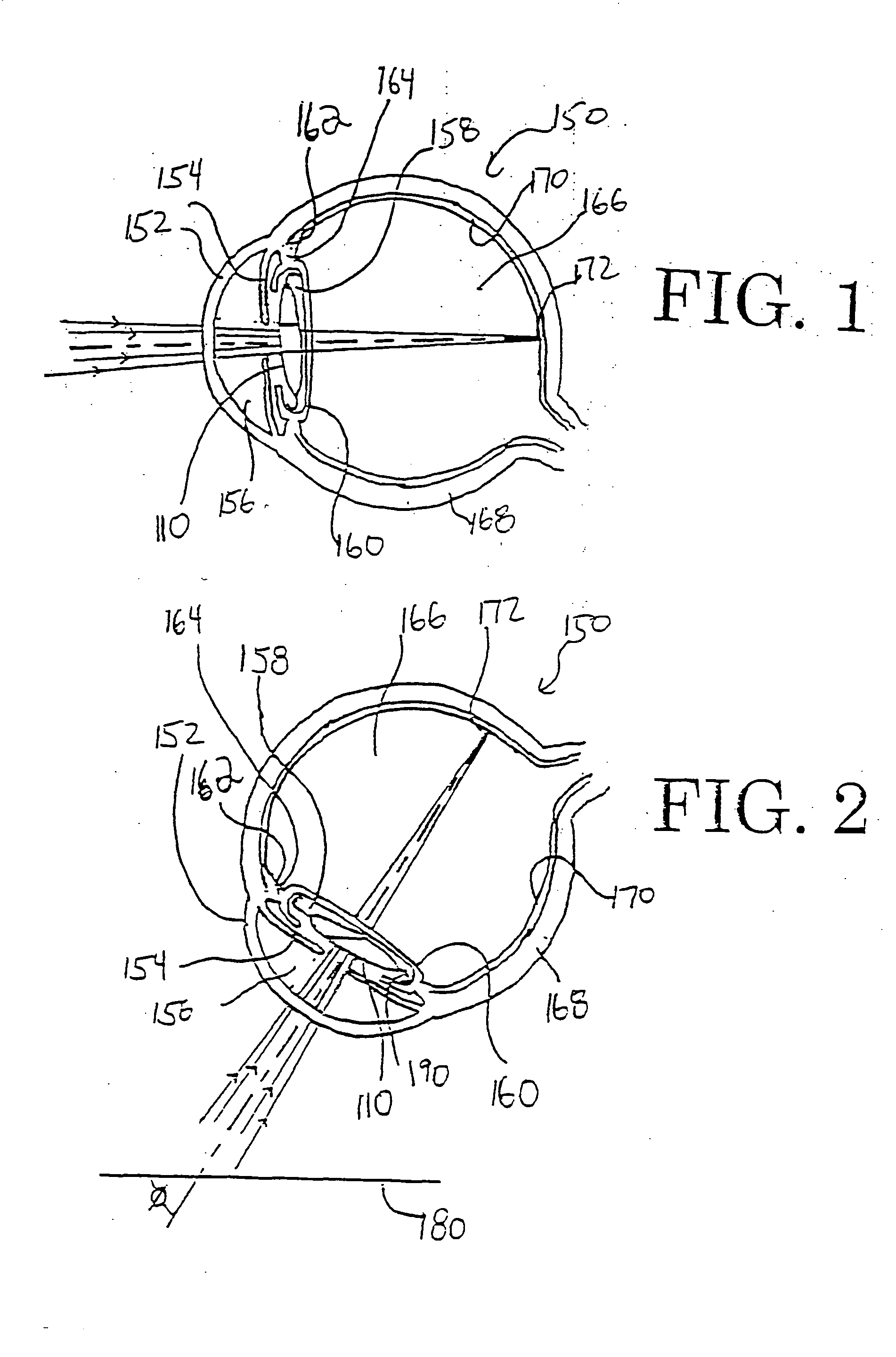
This intraocular lens includes an optic body having anterior and posterior walls, a chamber, and optically transmissive primary and secondary fluids, and method for making and using the same. The secondary fluid is substantially immiscible with the primary fluid and has a different density and a different refractive index than the primary fluid. The primary fluid is present in a sufficient amount that orienting optical body optical axis horizontally for far vision positions the optical axis through the primary fluid, thereby immersing the anterior and posterior optical centers in the primary fluid. The secondary fluid is contained in the optic body in a sufficient amount that orienting the optical axis at a range of effective downward angles relative to the horizontal for near vision positions the optical axis to extend through the primary fluid and the secondary fluid, thus changing the focus of the intraocular lens.
Owner:VISION SOLUTION TECH LLC
Intervertebral spacers
InactiveUS20080109083A1Prevent significant subsidencePromoting bone ingrowthBone implantSurgeryIntervertebral diskAnterior wall
One embodiment of a hollow spinal spacer (10) includes a curved anterior wall (11) having opposite ends (12, 13), a posterior wall (15) having opposite ends (16, 17), two lateral walls (20, 21), each integrally connected between the opposite ends (12, 13, 16, 17) of the anterior (11) and posterior (15) walls to define a chamber (30). The walls (11, 15, 20, 21) include a superior face (35) and an inferior face (40). The superior face (35) defines a first opening (36) in communication with the chamber (30) and includes a first vertebral engaging surface (37). The inferior face (40) defines a second opening (41) in communication with the chamber (30) and includes a second vertebral engaging surface (42).
Owner:WARSAW ORTHOPEDIC INC
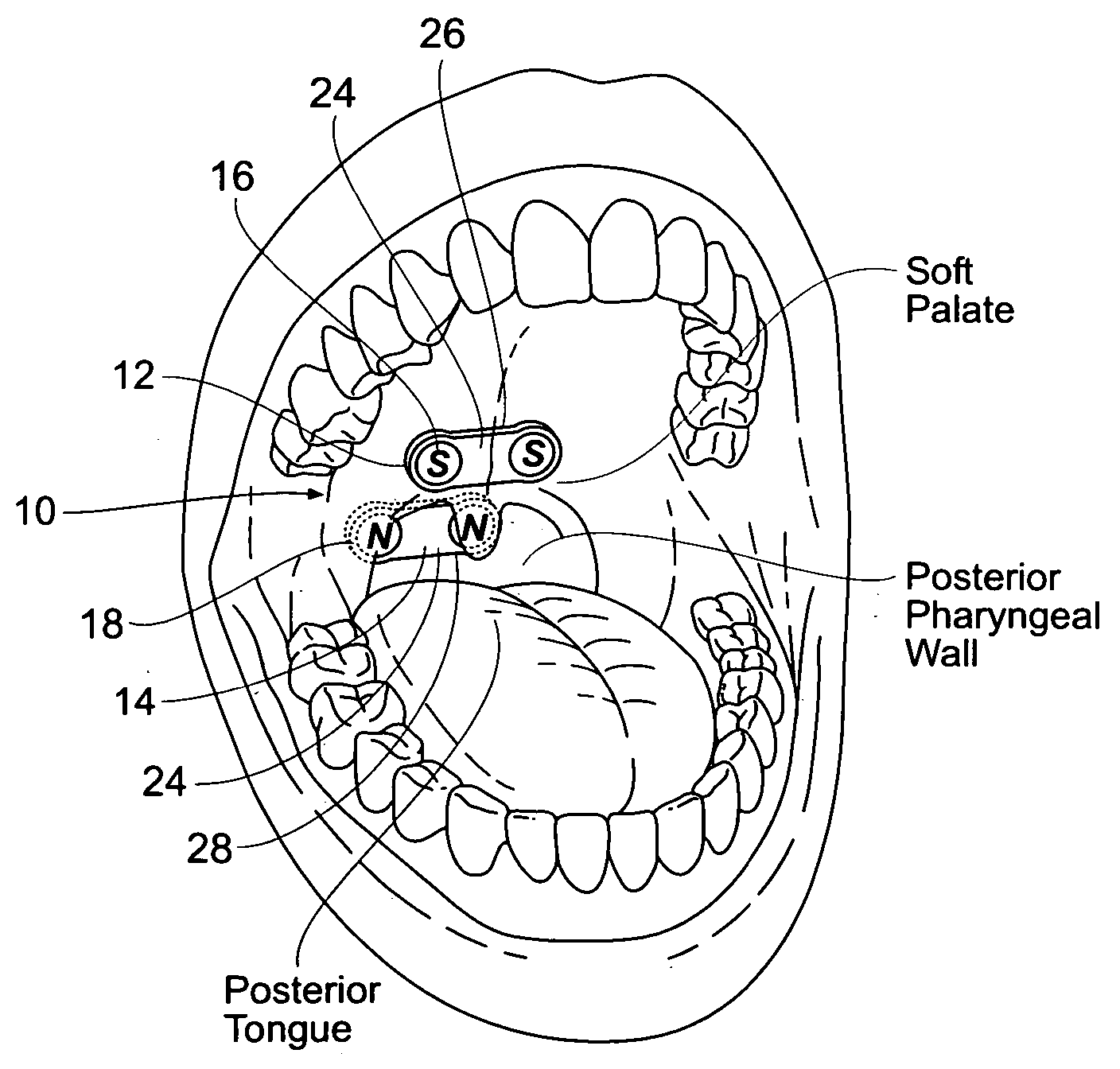
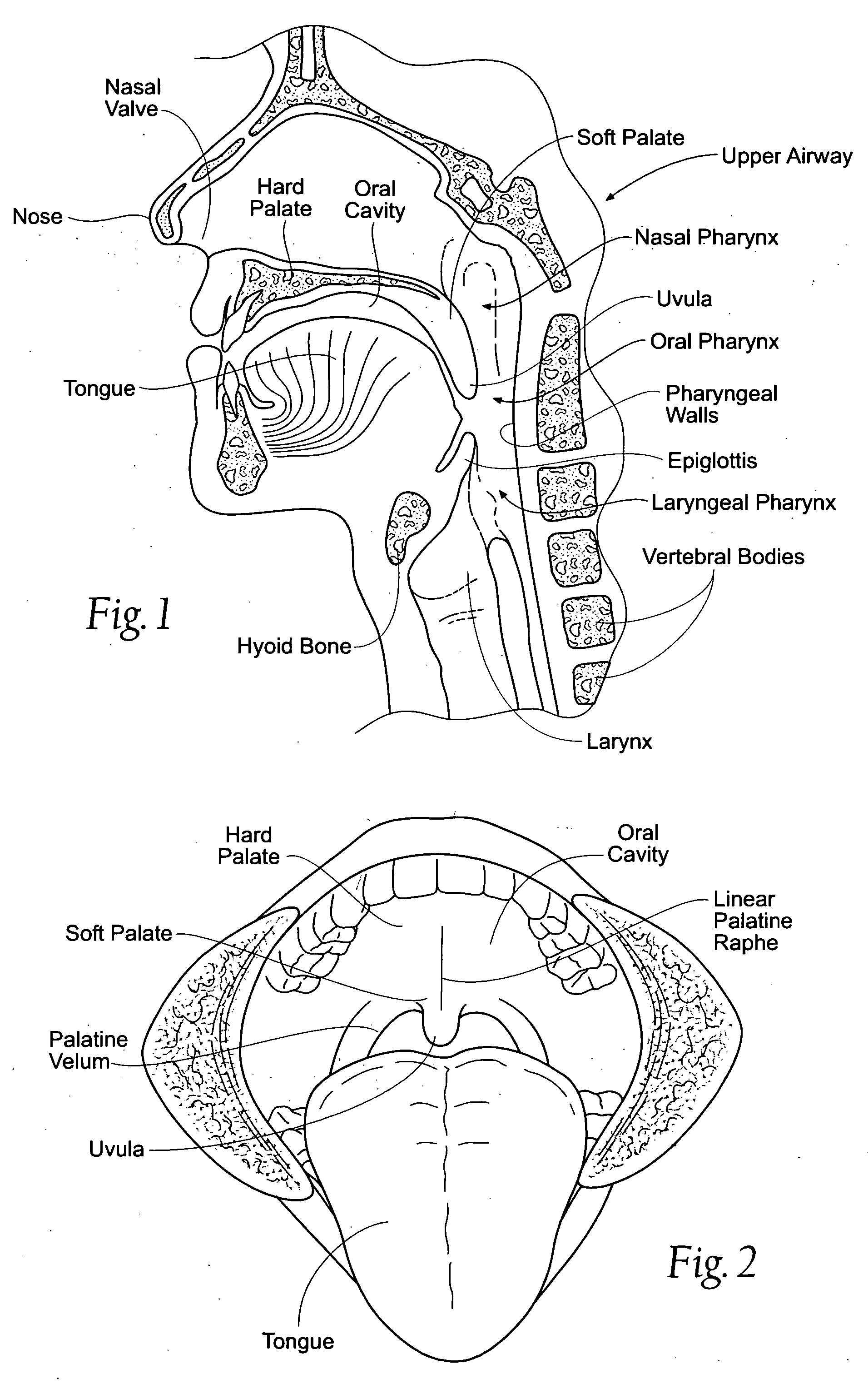
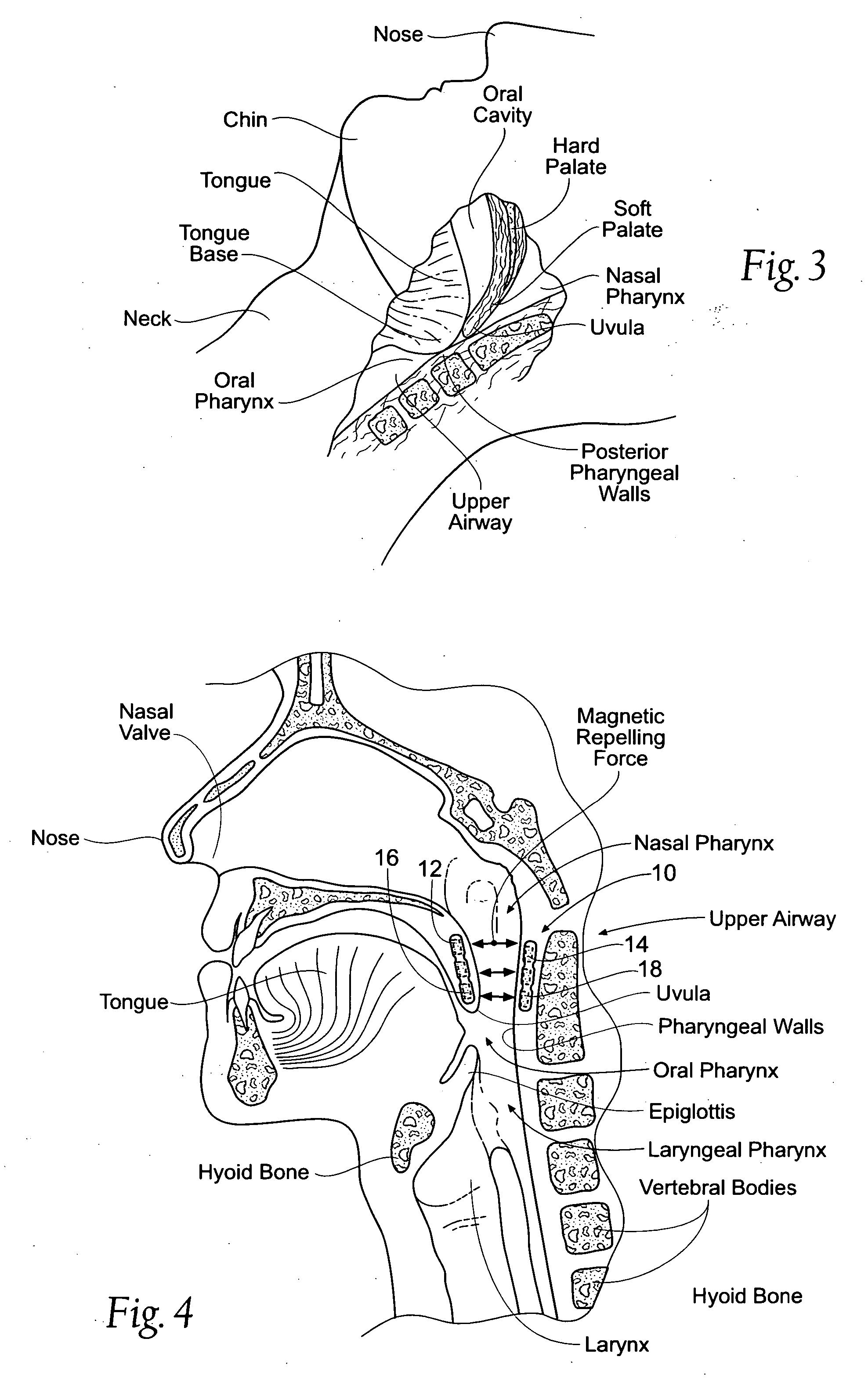
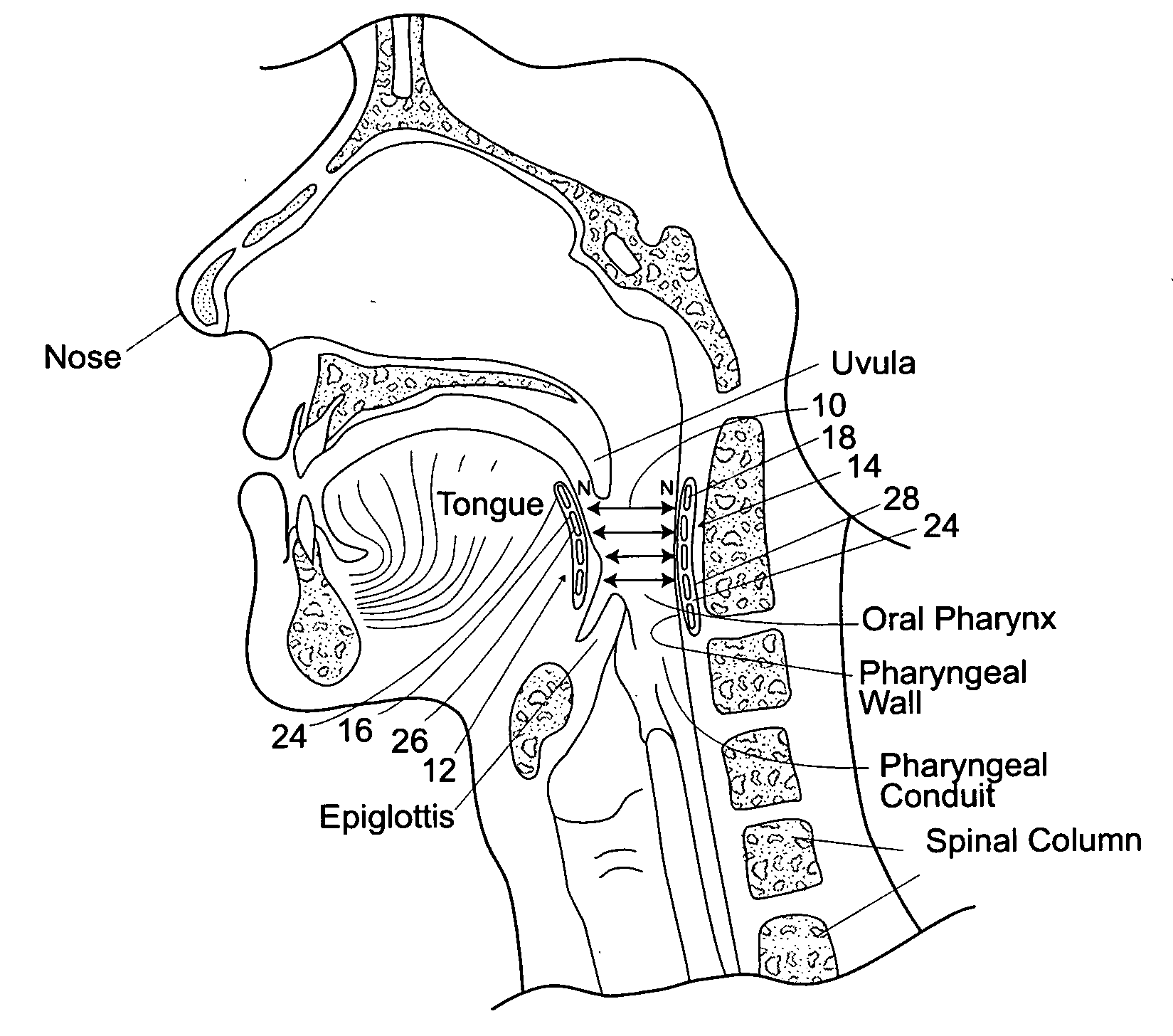
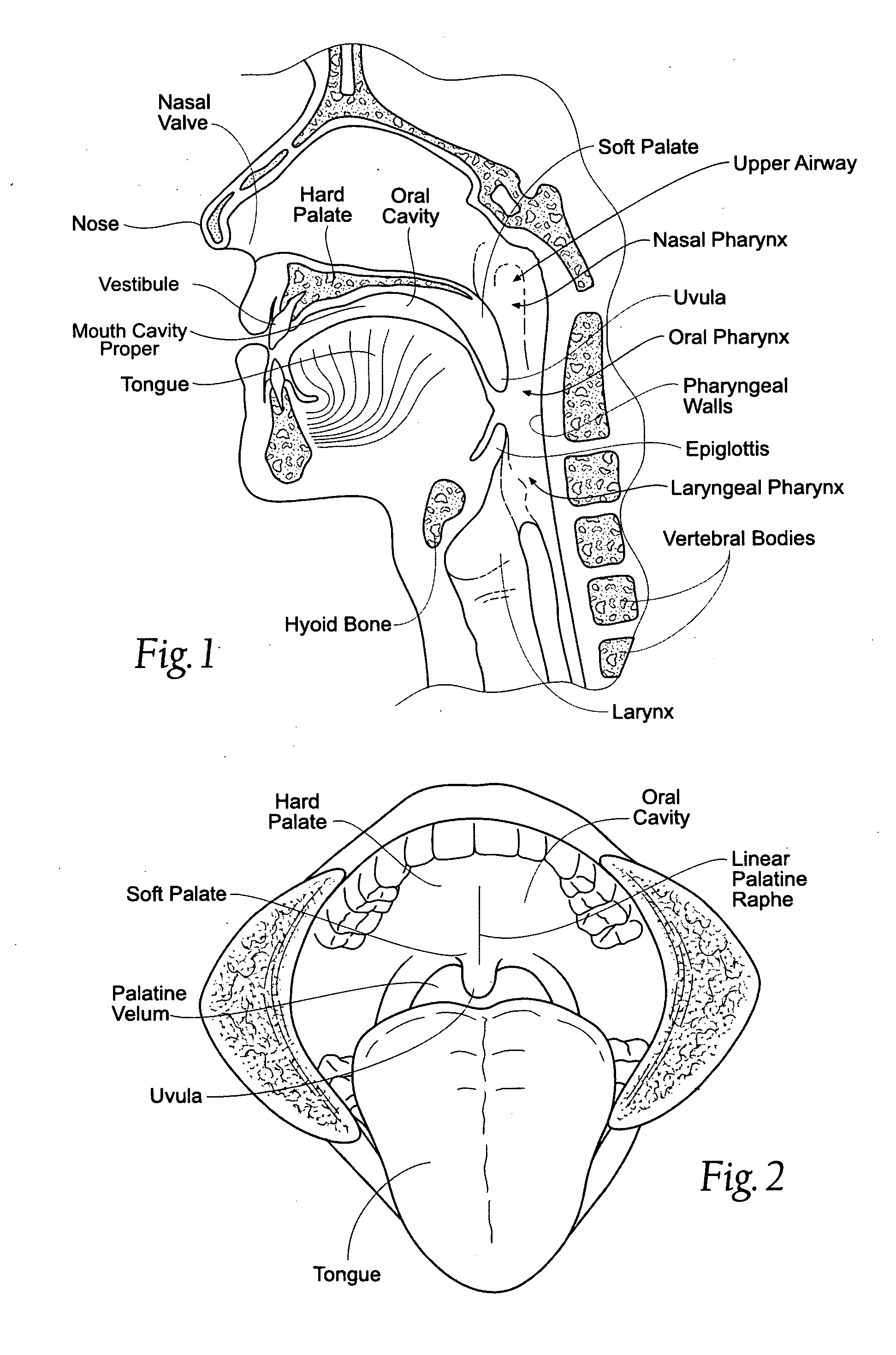
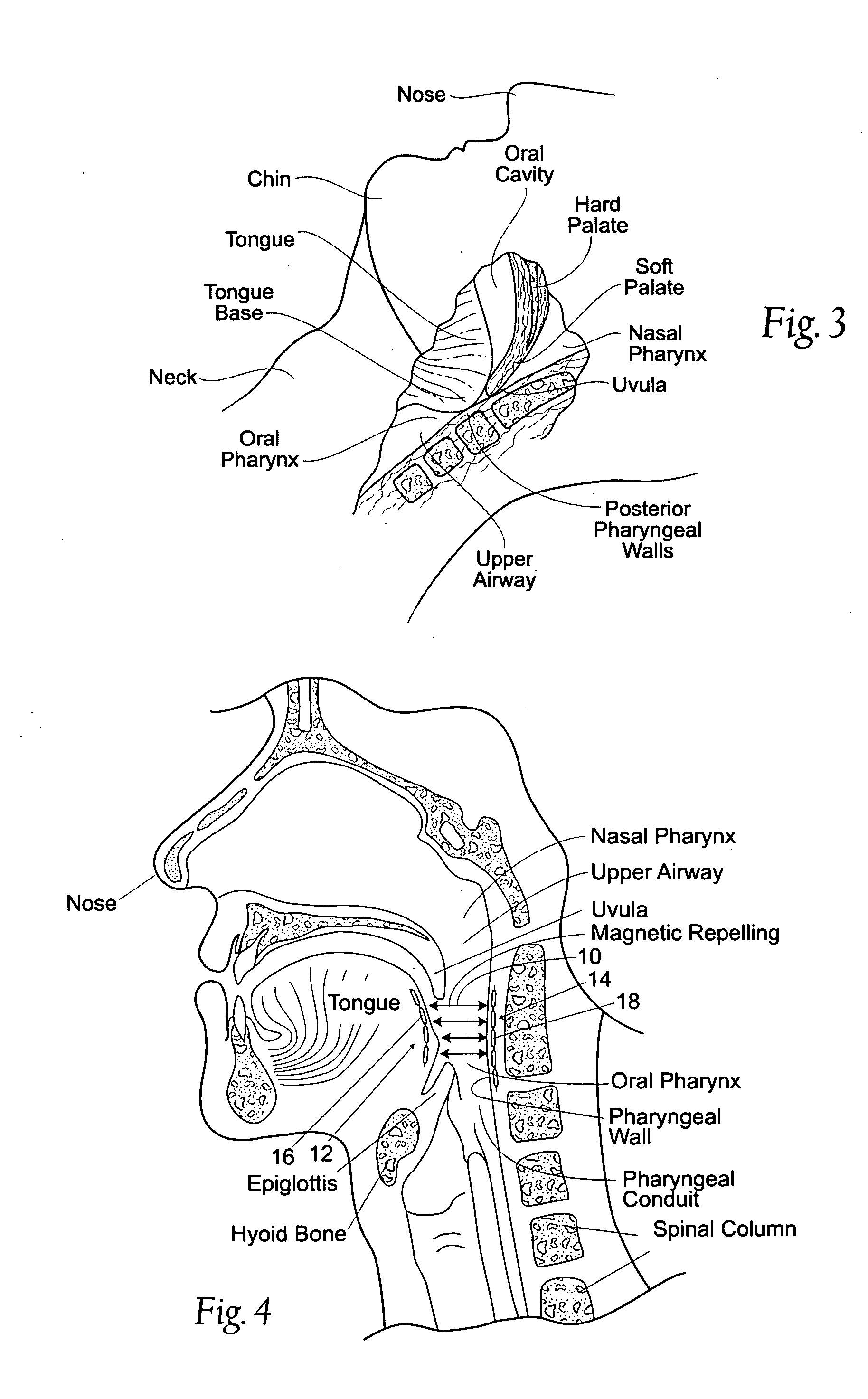
Devices, systems, and methods using magnetic force systems in or on soft palate tissue
InactiveUS20070256693A1Snoring preventionNon-surgical orthopedic devicesMagnetic tension forceMedicine
An implant system places magnetic structures in or on tissue that develop a magnetic repelling force between a soft palate a posterior pharyngeal wall. The magnetic repelling force has a magnitude F-mag, where F-mag=f (F-sep, F-nat), and where F-sep is a force required to separate soft palate tissue from pharyngeal wall tissue during sleep, and F-nat is a force exerted by native muscle on the soft palate during swallowing and / or drinking and / or speech. In another embodiment, a palate implant uses attractive magnetic force to keep the airway open.
Owner:KONINKLIJKE PHILIPS ELECTRONICS NV
Anatomic vertebral cage
ActiveUS7674297B2Precise positioningMinimize back-outBone implantJoint implantsSpinal levelEngineering
A spinal fusion system includes a cage with a fillable volume and removable locking gate, thereby enabling the fillable volume to be packed with graft, biologic or other materials prior to the gate being closed and locked. In the preferred embodiment, the locking gate is positioned anteriorally, though lateral, posterior, and combinations thereof are also possible. The cage is preferably radiolucent, being composed of a carbon fiber, but with one or more radiopaque markers to provide a certain degree of visualization. Some or all of the walls of the cage may include superior and / or inferior surface features to enhance positioning and / or minimize back-out, and the posterior wall may be indented to prevent neurocompression. The sidewalls of the cage may further include a recessed face with nipple indents and locking fasteners. According to a system aspect of the invention, multiple cages are provided, each being shaped differently for use at different spinal levels. For example, the cage may be larger and more trapezoidally-pronounced for the L5-S1 levels, or smaller and less trapezoidally pronounced for the T and L2 levels. The system may further including an implant introducer instrument geometrically matched to the cage, and the matched implant introducer instruments and cages may be color-coded to expedite the procedure.
Owner:CTL MEDICAL CORP
Bicuspid vascular valve and methods for making and implanting same
A vascular valve constructed from a biocompatible material that is designed to be surgically implanted in a patient's blood vessel, such as the right ventricular outflow tract. At the first end of the valve there is an orifice defined by at least two opposing free edges, and which can occupy either a first, closed position or a second, open position. At the second end of the valve there are at least two flexible members attachable to an anterior and a posterior wall of a patient's blood vessel. A length of the orifice between said at least two opposing free edges when the orifice is generally closed is equal to about 1.5 to 2 times the diameter of a patient's blood vessel. Optionally, the two flexible members to a stent or tubular graft. The valved stent or tubular graft can be inserted into a patient's blood vessel or heart.
Owner:QUINTESSENZA JAMES
Intraocular lens
An intraocular lens of a novel structure exhibiting an excellent focus adjusting power. A hollow capsule structure is filled with a transparent liquid-like or gel-like filler (32). Front wall of the capsule structure is composed of a flexible lens front film (16), and the rear wall of the capsule structure is composed of an optical lens (18) having a diameter larger than that of the flexible lens front film (16). Under a state inserted into and attached to a capsula lentis, the pressure variation of a corpus vitreum acts on the optical lens (18) to enable focal refraction power to be adjusted by utilizing swelling deformation of the lens front film (16).
Owner:KOWA CO LTD
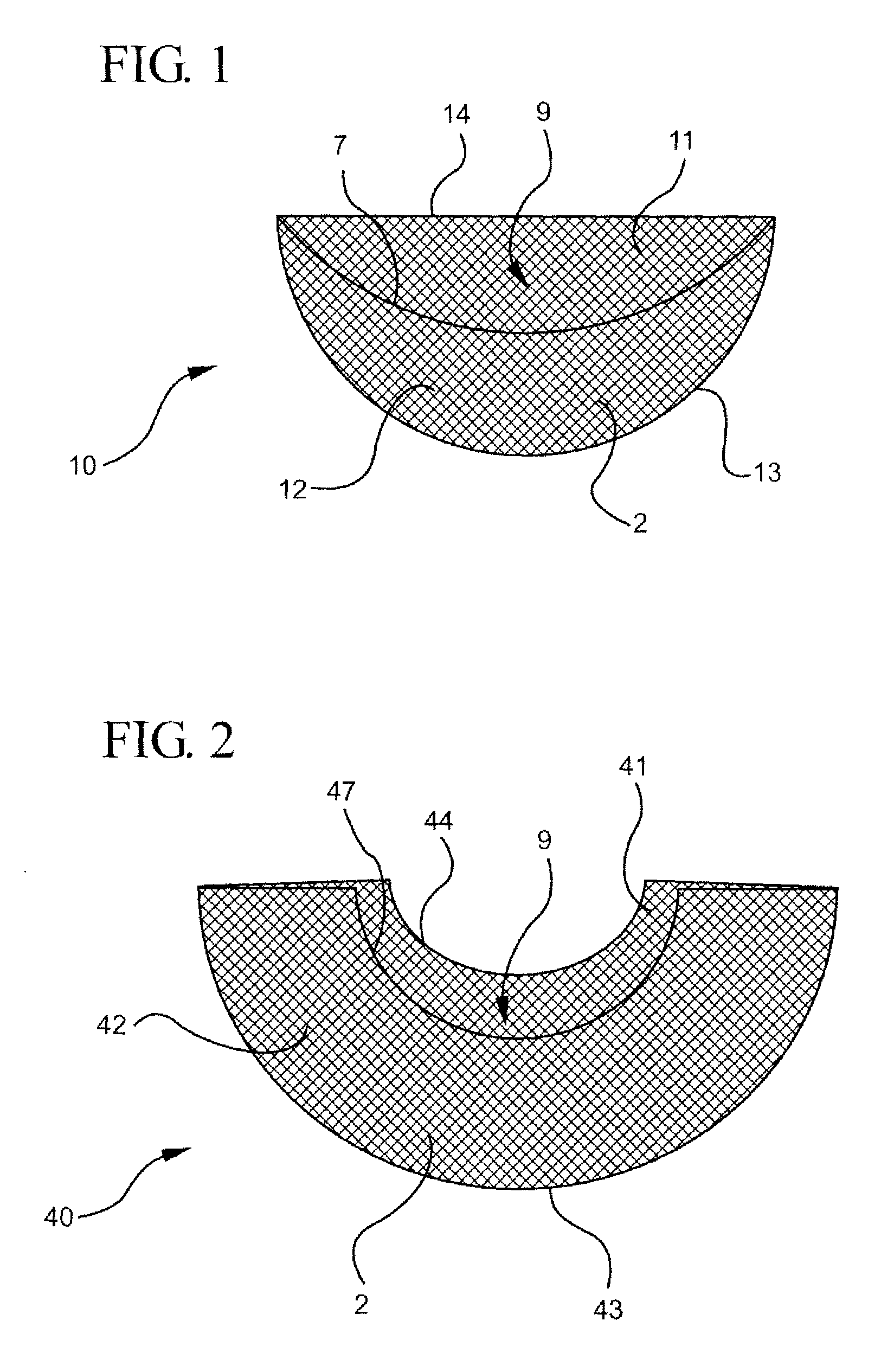
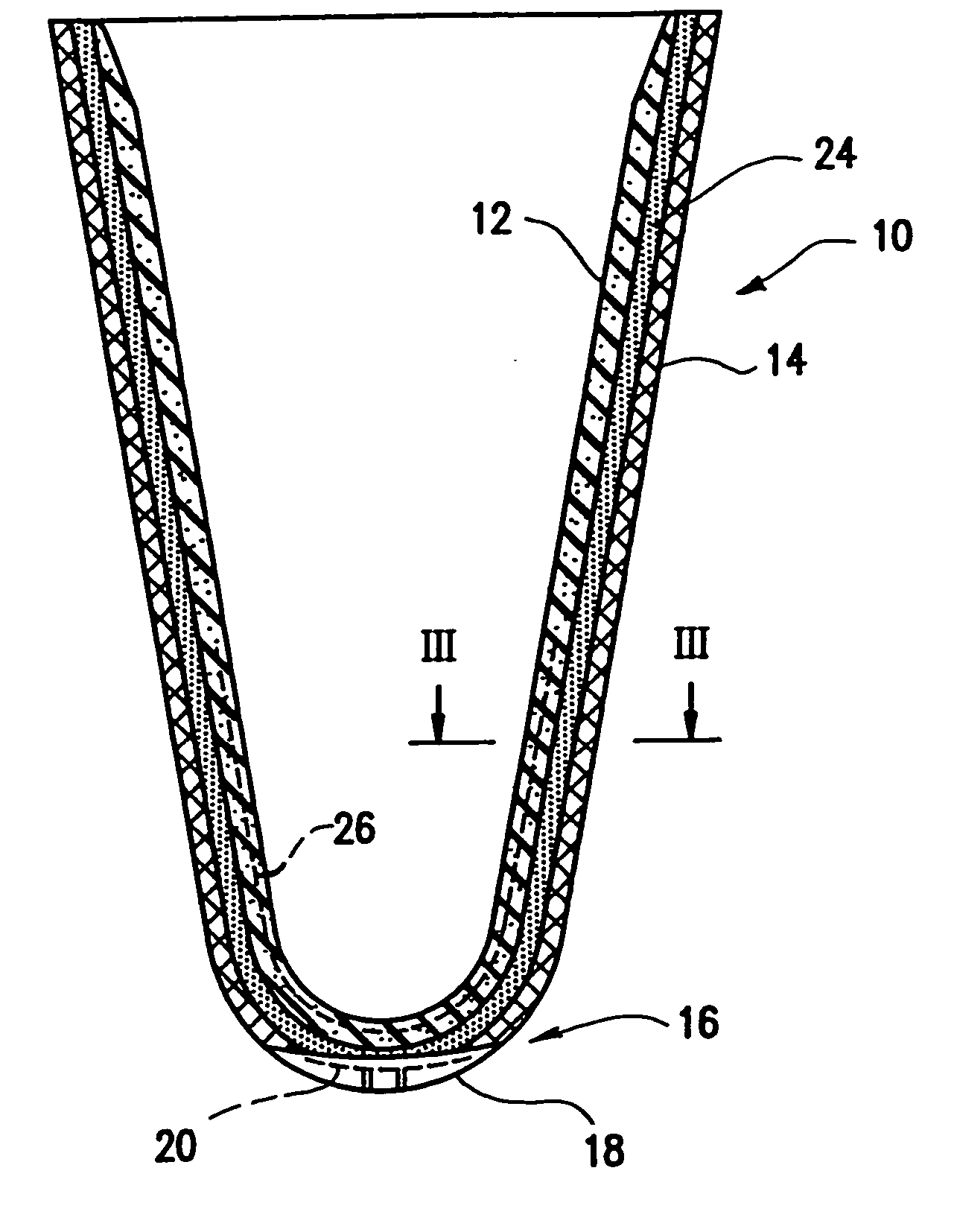
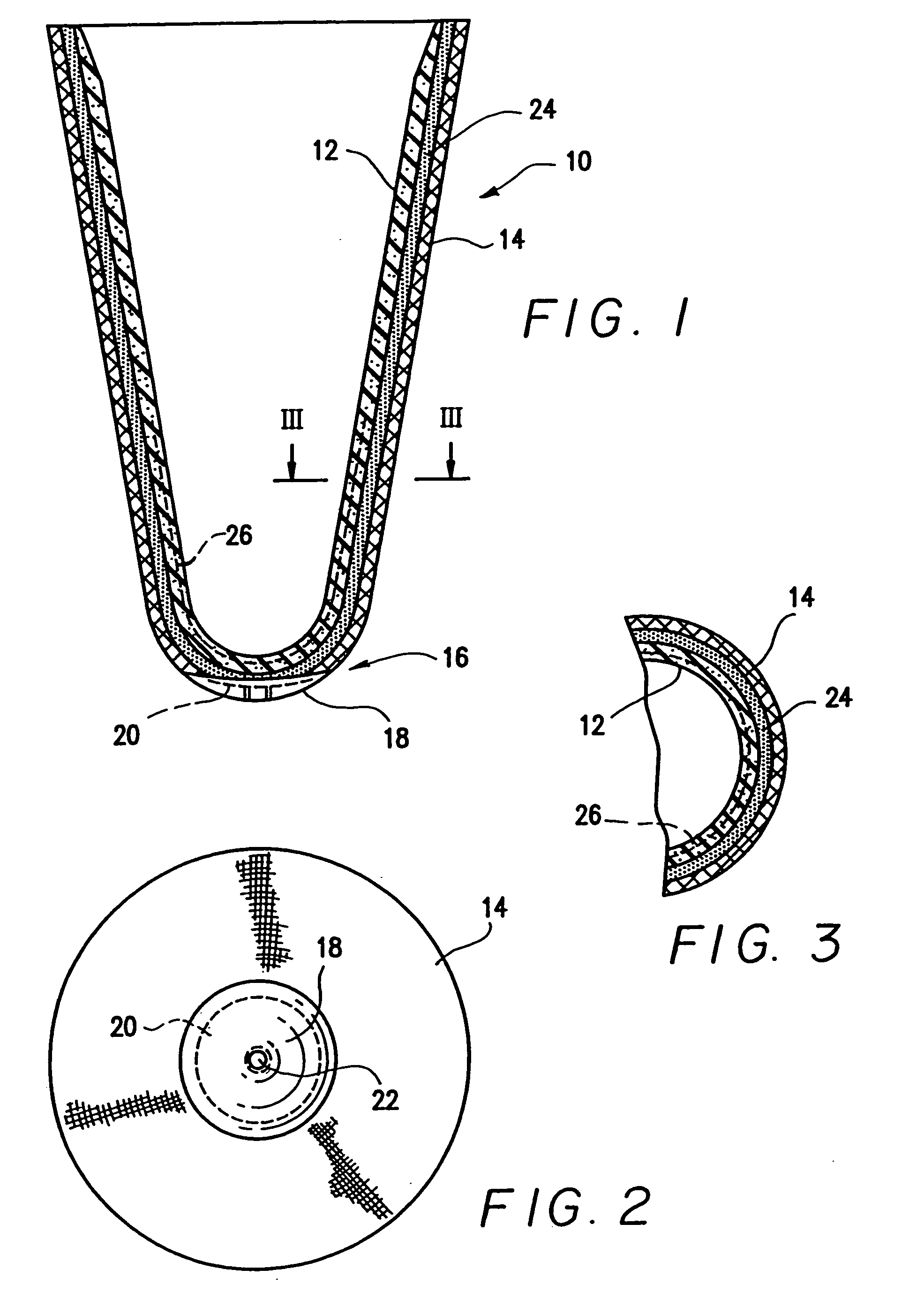
Anterior intervertebral spacer and integrated plate assembly and methods of use
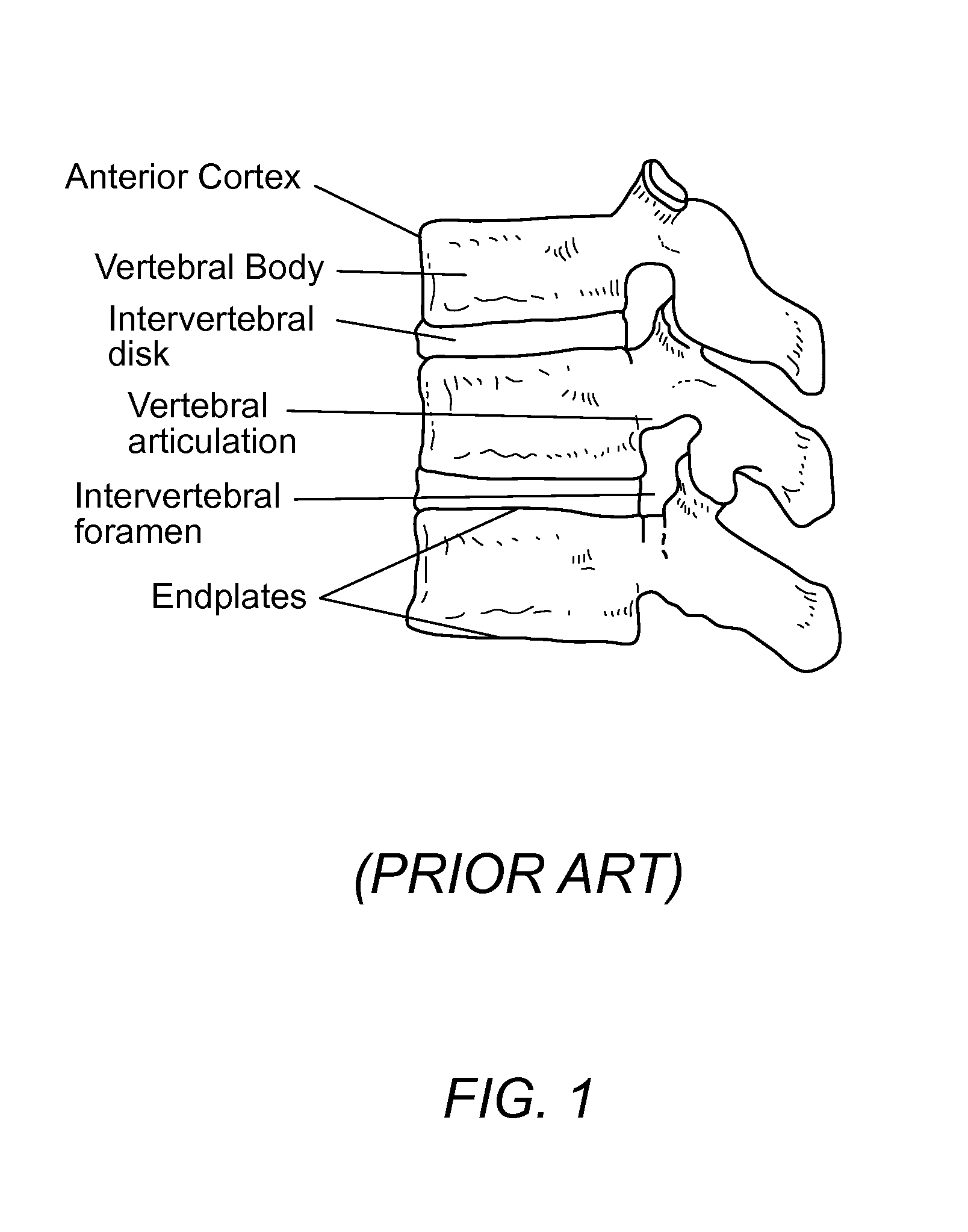
InactiveUS8932358B1Easy to fuseImprove stabilityBone implantSpinal implantsAnterior cortexIntervertebral spaces
A precisely size matched intervertebral plate and spacer assembly for ensuring a tight fit within a disc space to promote spinal fusion, comprising: a “U-shaped” spacer configured to fit within the intervertebral space; and, a matching countersunk low profile “H-shaped” anterior plate joined perpendicularly to the spacer. The plate further comprises: a plurality of anchor members configured to attach to the junctions of the anterior cortex faces and the endplates; and, channels individually traversing through the anchor members for inserting screws into the vertebral bodies' cortical bone. The spacer comprises a hollow three-sided U-shaped member, comprising two opposing parallel side walls, and a perpendicular posterior wall, while lacking a superior, inferior, and anterior wall. The exterior walls of the plate and spacer are planar, while the interior walls of the spacer are curved to house a precisely fitting cylindrical graft, or other insert such as DBM, bone dust, bone paste, bone dowel with direct contact to the endplates to promote fusion.
Owner:NEHLS DANIEL
Multi-focal intraocular lens, and methods for making and using same
InactiveUS20050071002A1Without significant disruptionRestores focus mechanismEye surgeryIntraocular lensLens crystallineOptical axis
An intraocular lens is provided that includes an optic body having anterior and posterior walls, a chamber, and optically transmissive primary and secondary fluids, and method for making and using the same. The secondary fluid is substantially immiscible with the primary fluid and has a different density and a different refractive index than the primary fluid. The primary fluid is present in a sufficient amount that orienting optical body optical axis horizontally for far vision positions the optical axis through the primary fluid, thereby immersing the anterior and posterior optical centers in the primary fluid. The secondary fluid is contained in the optic body in a sufficient amount that orienting the optical axis over a range of effective downward angles relative to the horizontal for near vision positions the optical axis to extend through the primary fluid and the secondary fluid, thus changing the focus of the intraocular lens.
Owner:VISION SOLUTION TECH LLC
Prosthetic liner
Prosthetic liner formed of a molded silicone elastomer material including an inner wall, a circular outer wall having radii of curvature centered along a first longitudinal axis of external symmetry extending longitudinally centrally within the liner, the inner wall including a circular curved inside anterior wall portion extending along a liner length and having first radii of curvature centered on a second longitudinal axis of anterior curvature extending longitudinally along the liner length and a circular curved inside posterior wall portion having a second radii of curvature centered on a third longitudinal axis of posterior curvature extending along said liner length. The first, second and third longitudinal axes lie in a common longitudinally and transversely extending plane bisecting the anterior and posterior wall portions, and the second and third axes are spaced apart a predetermined offset distance on opposed sides of the first axis to thereby define an anterior wall portion that is thicker along the liner length than the posterior portion.
Owner:KAUPTHING BANK
Intraocular lens
InactiveUS20090043384A1Efficient changeEffective focus adjusting powerIntraocular lensPosterior wallOptometry
An intraocular lens of novel structure exhibiting an excellent focus adjusting power. A hollow capsule structure is filled with a transparent liquid-like or gel-like filler (32). A front wall of the capsule structure is composed of a flexible lens front film (16), and a rear wall of the capsule structure is composed of an optical lens (18) having a diameter larger than that of the flexible lens front film (16). Under a state inserted into and attached to a capsula lentis, pressure variation of a corpus vitreum acts on the optical lens (18) to enable focal refraction power to be adjusted by utilizing swelling deformation of the lens front film (16).
Owner:KOWA CO LTD
Multi-focal intraocular lens, and methods for making and using same
InactiveUS20030093149A1Without significant disruptionRestores focus mechanismEye surgeryIntraocular lensOptical axisRefractive index
Owner:VISION SOLUTION TECH LLC
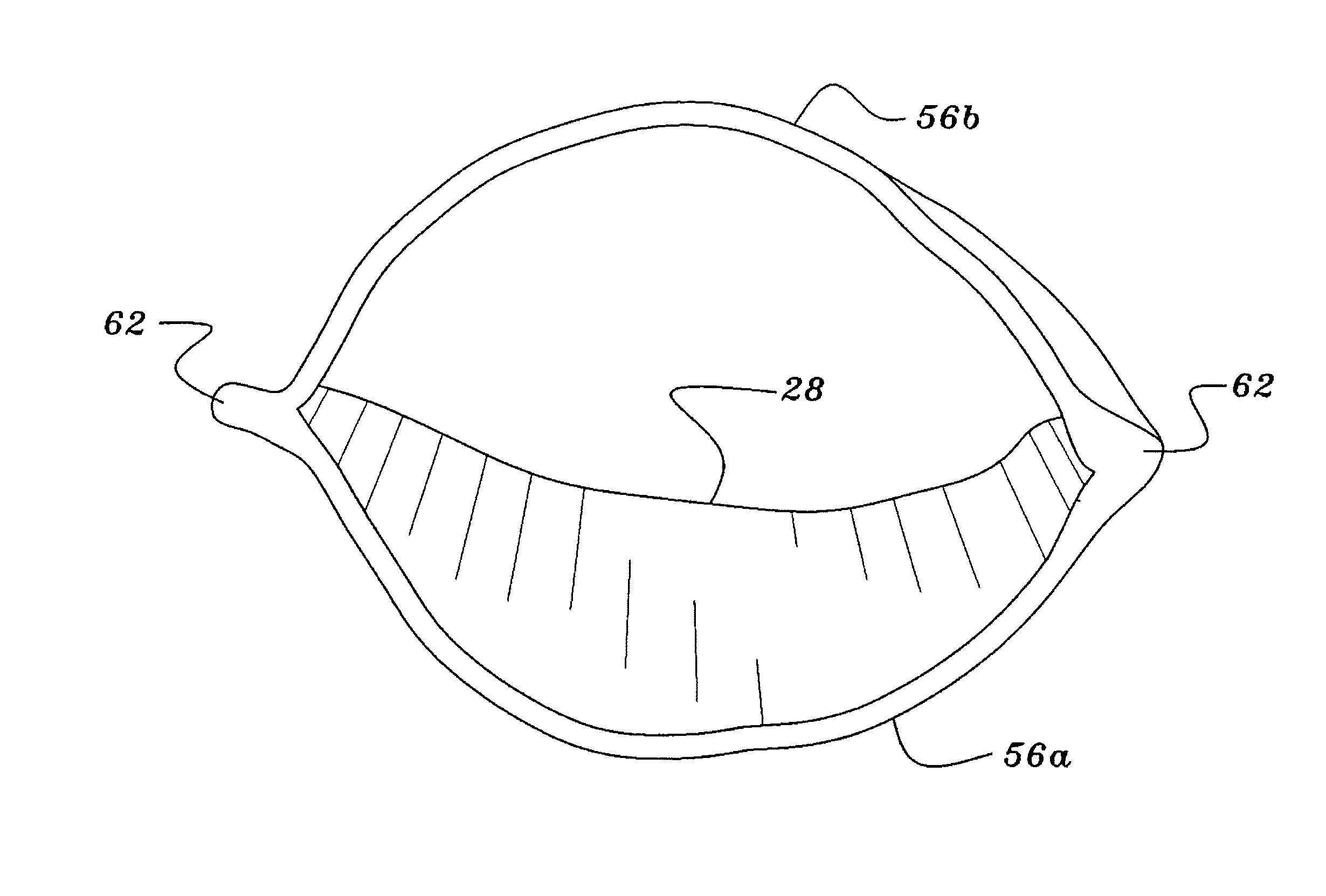
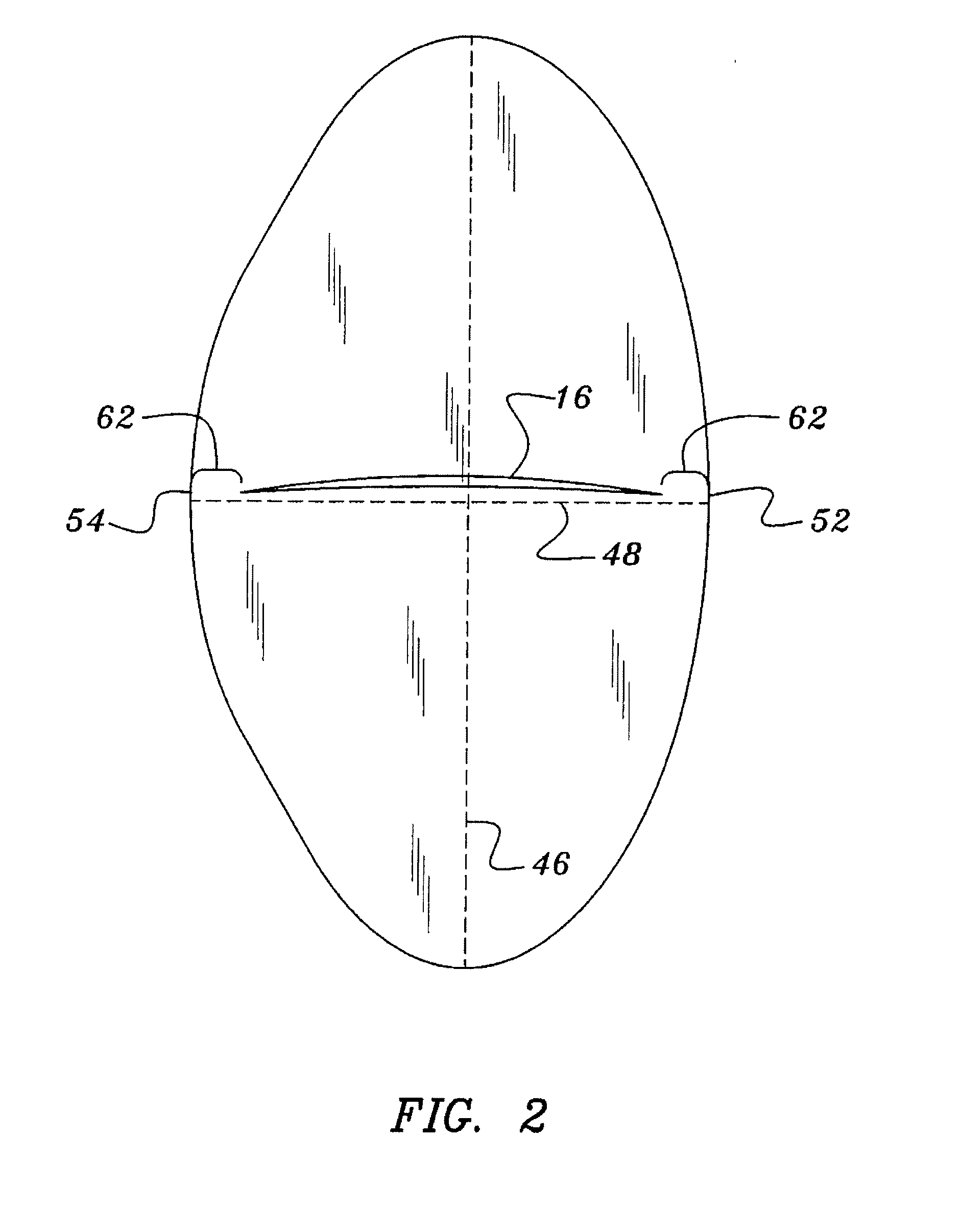
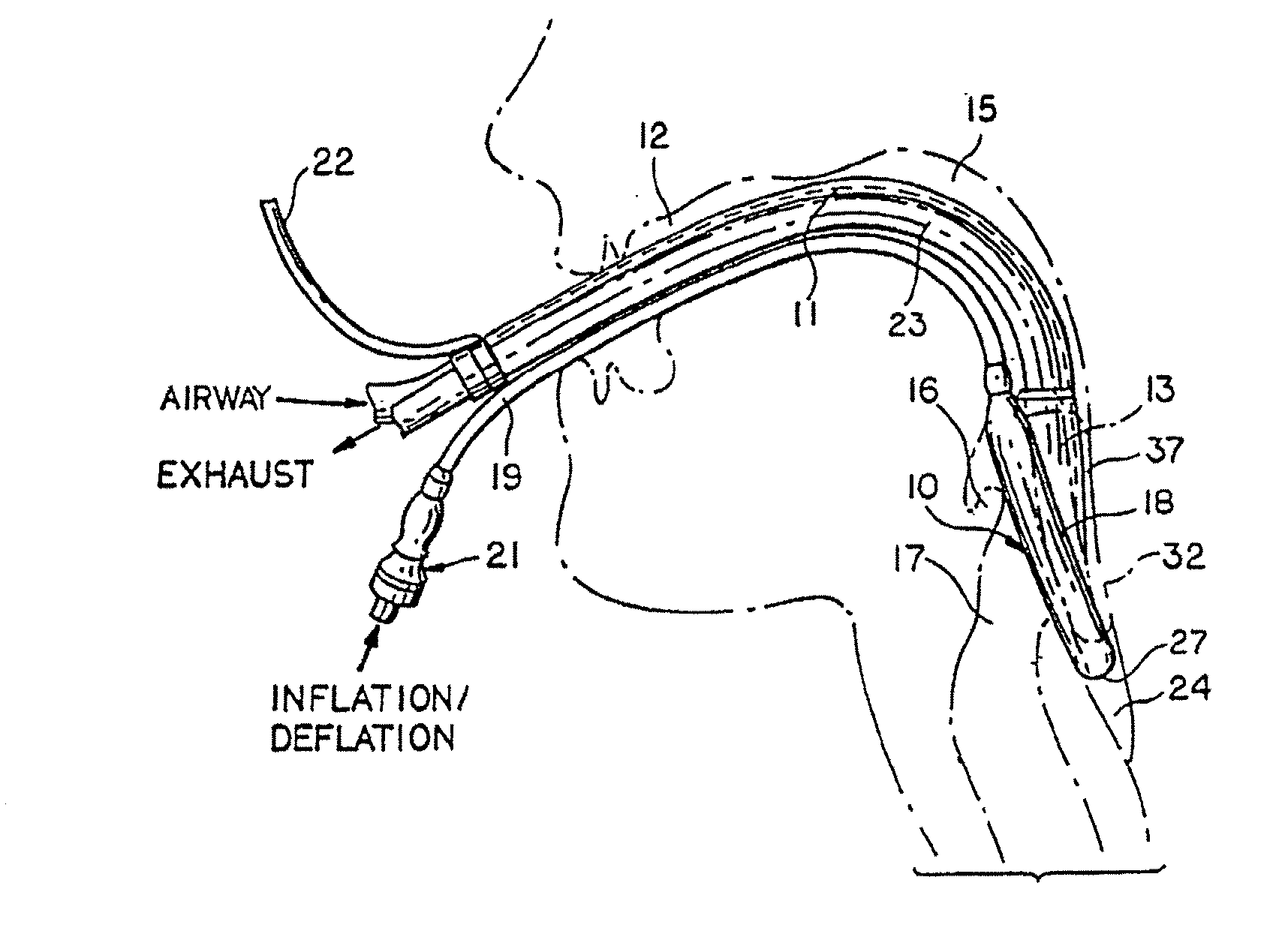
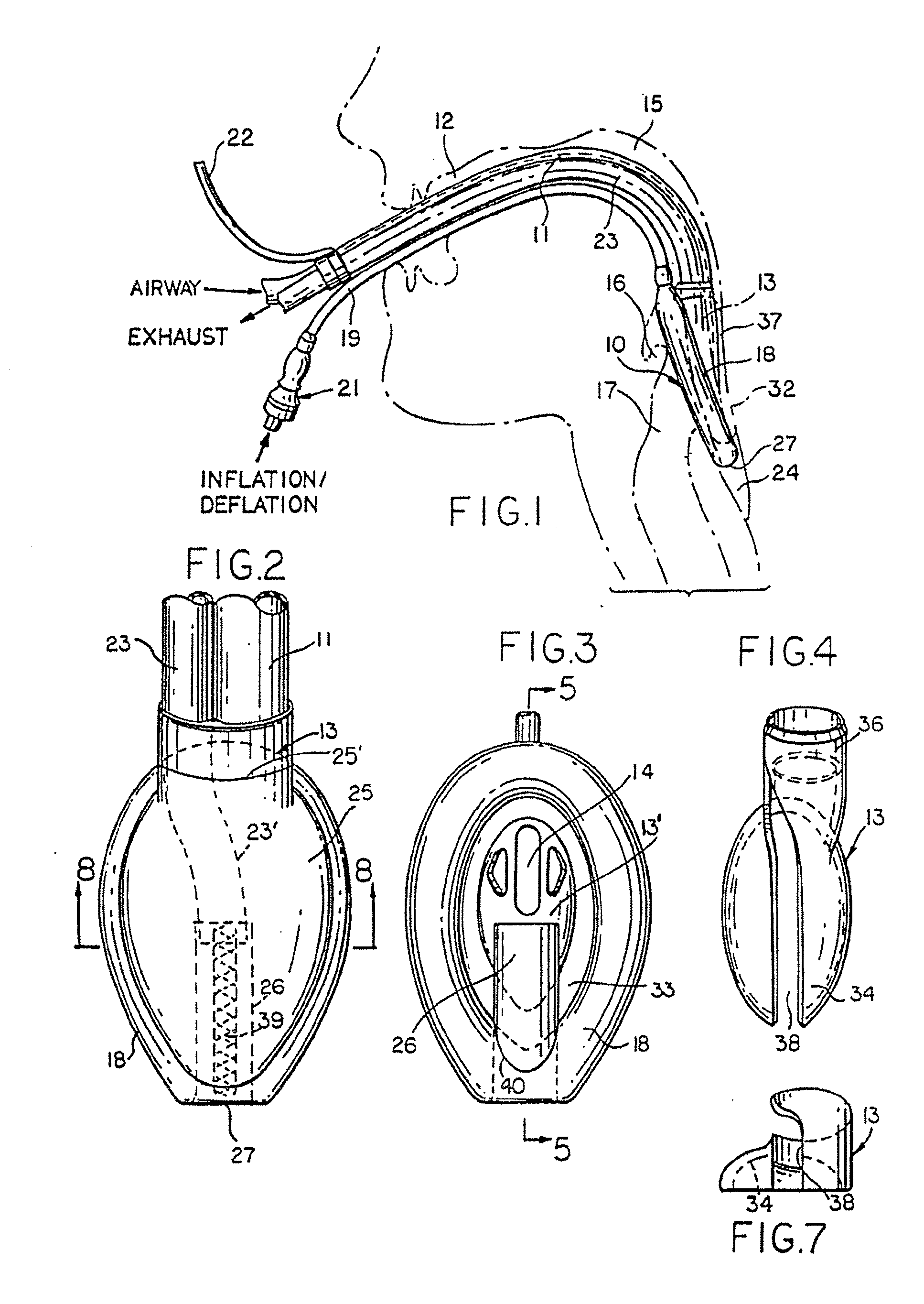
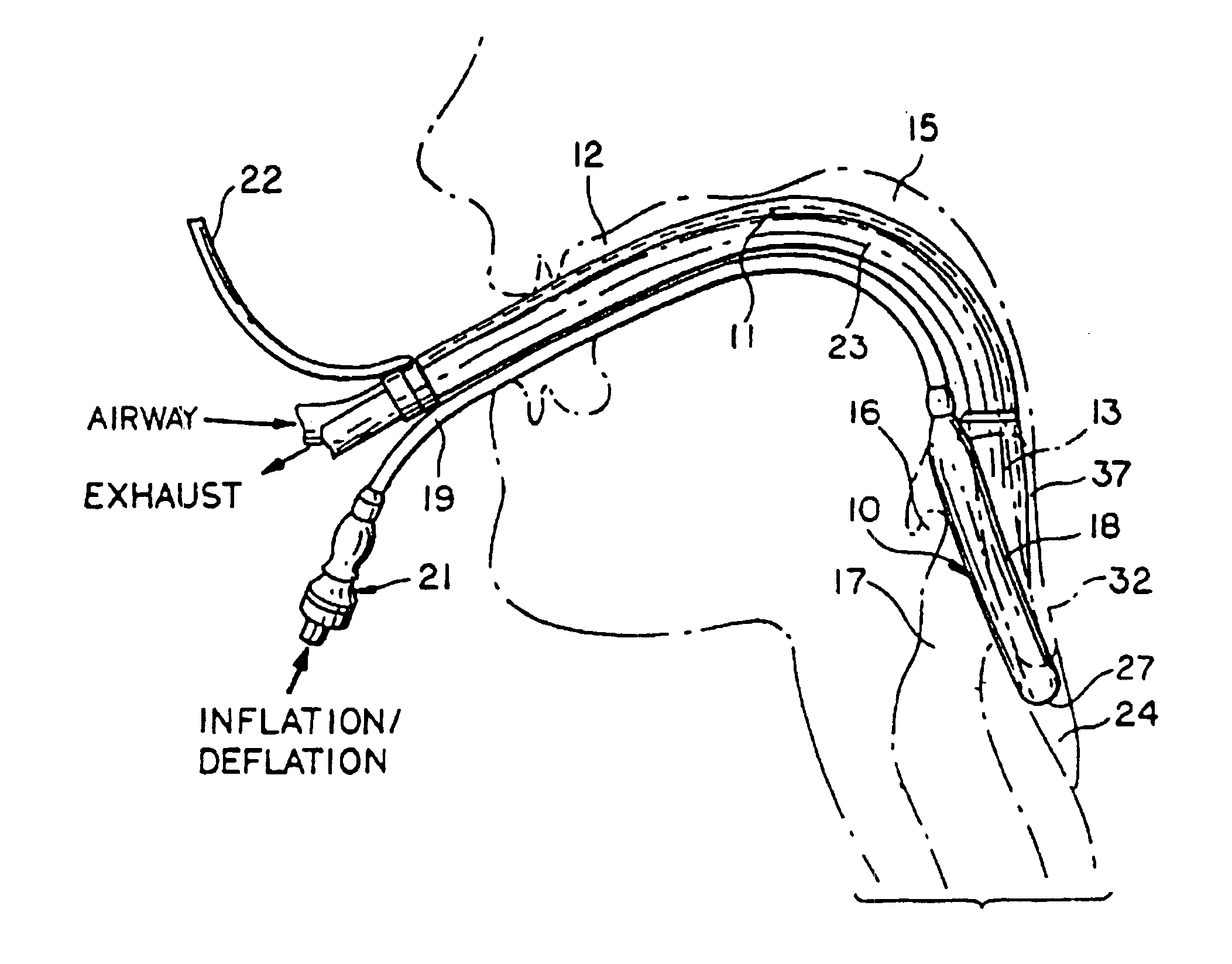
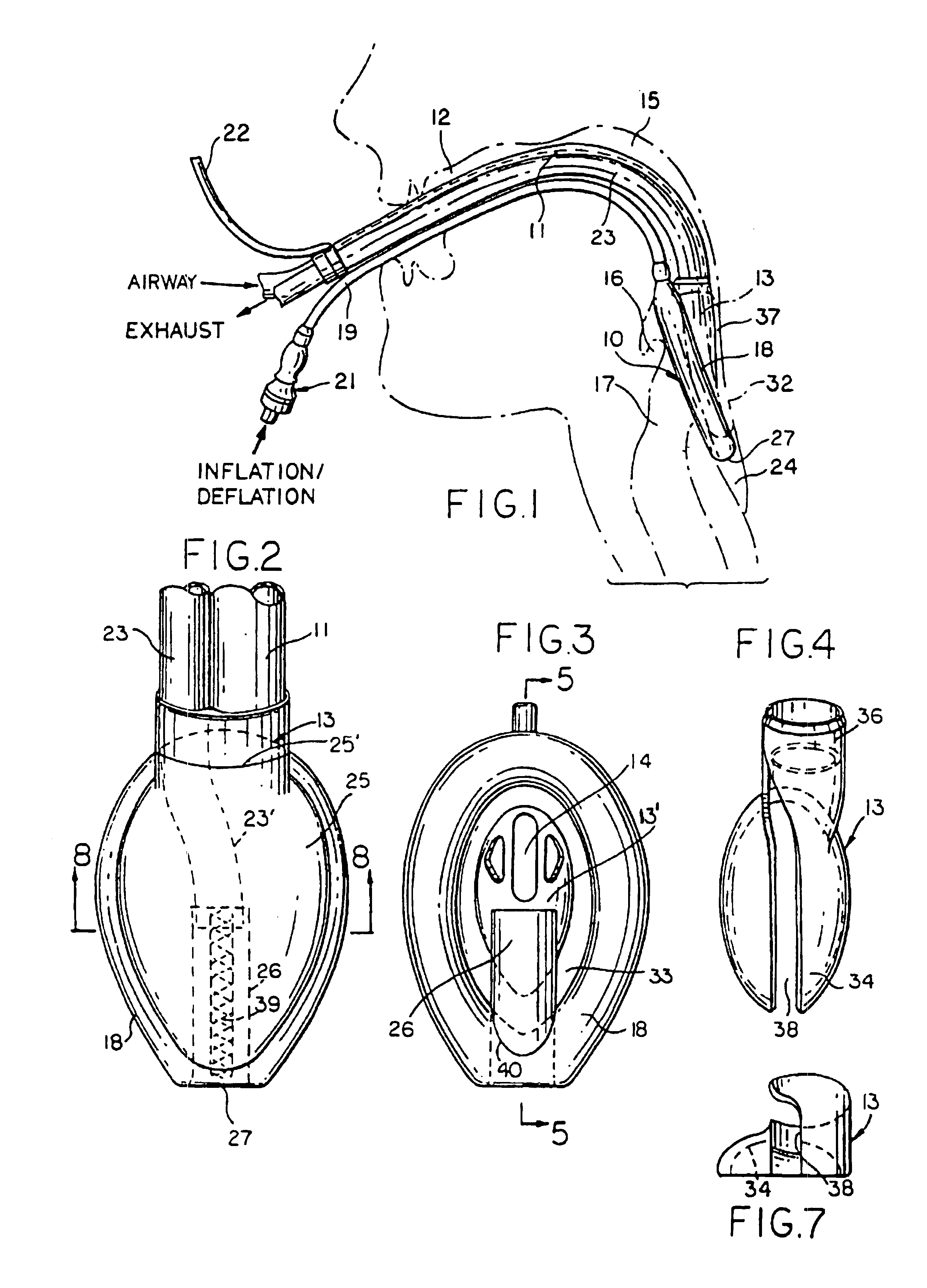
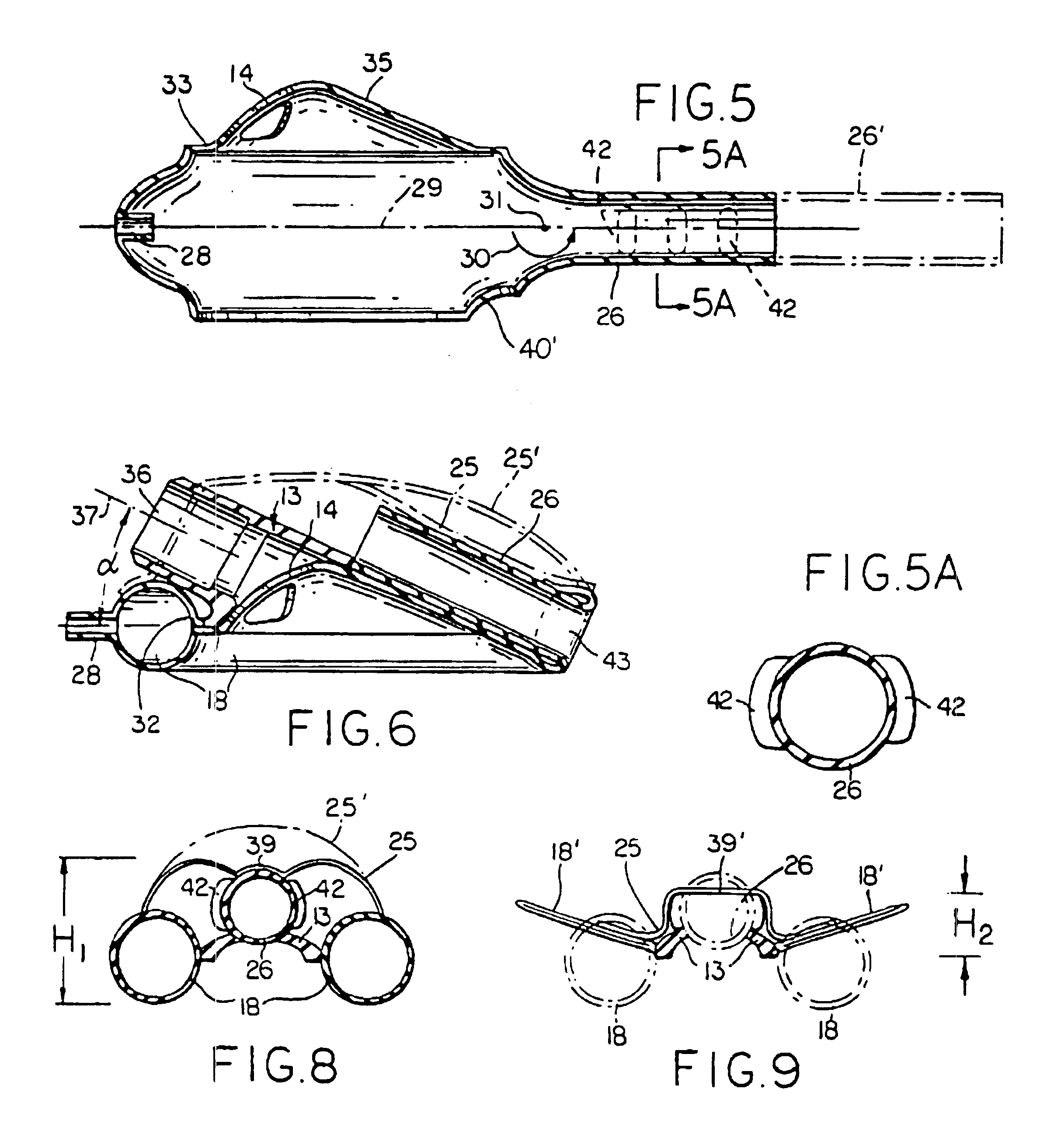
Gastro-Laryngeal Mask
A gastro-laryngeal mask features softly compliant construction of the distal half of the mask, wherein the mask is of generally elliptical configuration, with an inflatable peripheral cuff to seal and support the mask around the laryngeal inlet. A back cushion is inflatable to engage the back wall of the pharynx and thus to forwardly load the peripheral-cuff seal to the laryngeal inlet. An evacuation tube for external removal of a possible gastric discharge completes an evacuation or discharge passage contained within the mask and opening through the distal end of the peripheral cuff. Special provision is made for assuring integrity of the discharge passage within the flexible distal half of the mask, i.e., assuring against collapse of the distal-end half of the softly compliant evacuation tube in the distal region of the mask, such that inflation of the mask does not compromise viability of the evacuation tube by compressing softly compliant material of the evacuation tube during periods of mask inflation. The special provision also favors such collapse of the mask when deflated as to provide a leading flexible edge for piloting a safe and correct advancing insertional advance of the deflated mask in the patient's throat, in avoidance of epiglottis interference and to the point of locating engagement in the upper sphincter of the oesophagus.
Owner:THE LARYNGEAL MASK +1
Methods of delivering an implant to an intervertebral space
ActiveUS20120123548A1Reduce the likelihood of migrationBone implantJoint implantsFilling materialsIntervertebral space
According to some embodiments, a method for promoting spinal fusion using a spinal implant comprises providing a spinal implant, wherein the spinal implant comprises an anterior wall, a posterior wall and two lateral walls configured to extend between the anterior wall and the posterior wall. In some embodiments, the spinal implant further comprises at least one internal chamber generally positioned between the anterior wall, the posterior wall and the two lateral walls, wherein the internal chamber being is adapted to receive at least one graft and / or other fill material.
Owner:SPINE HLDG LLC
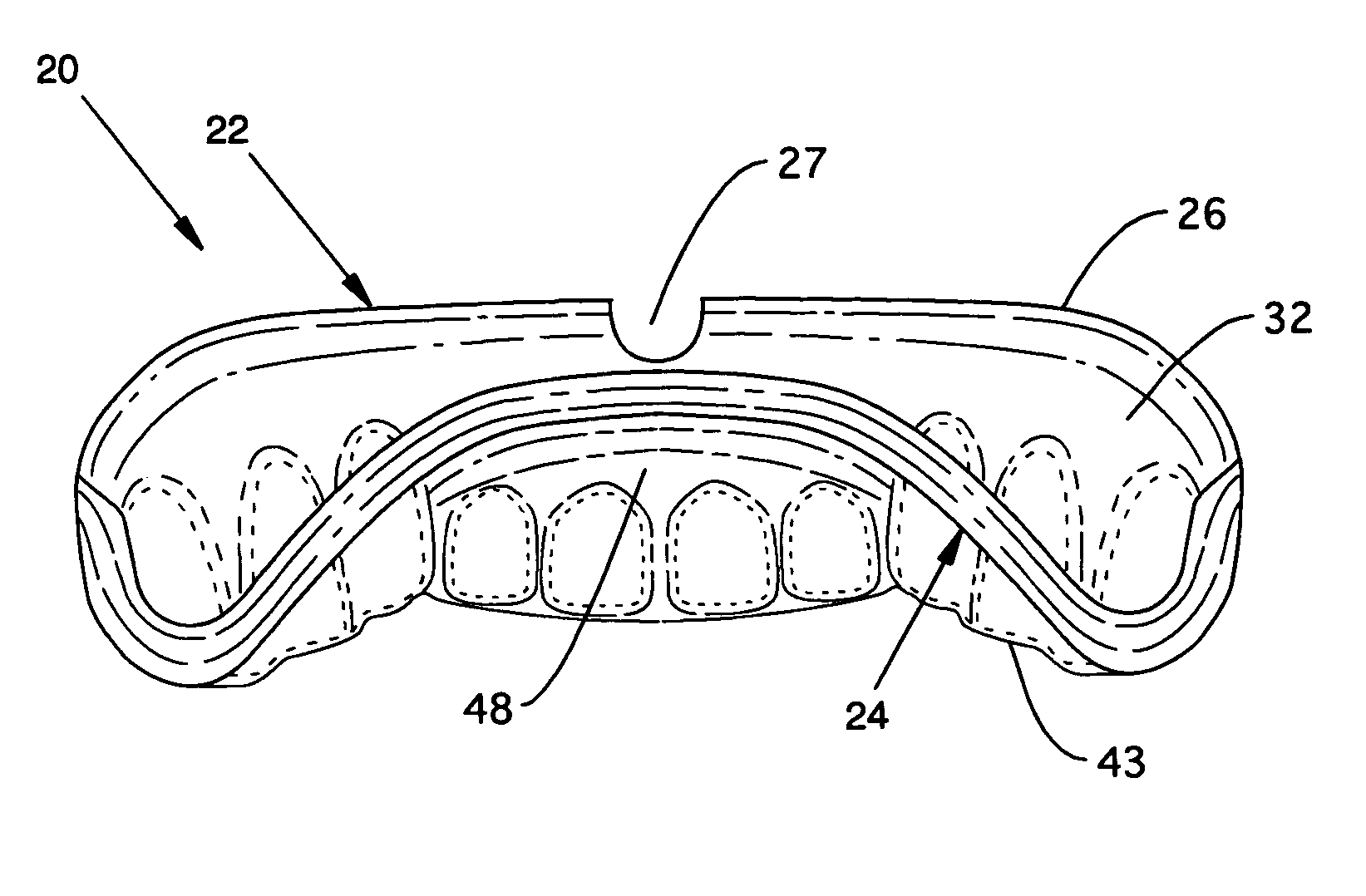
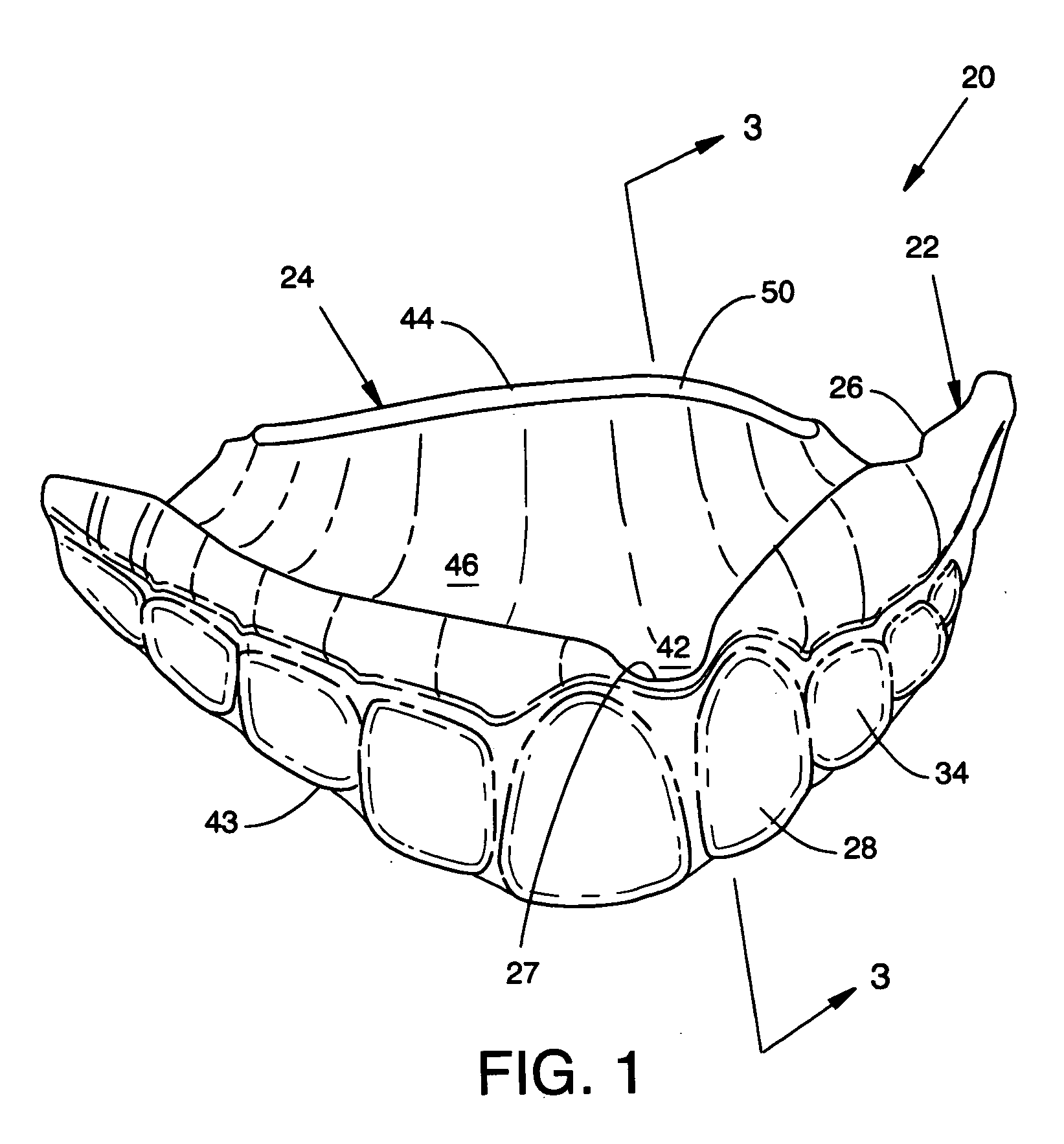
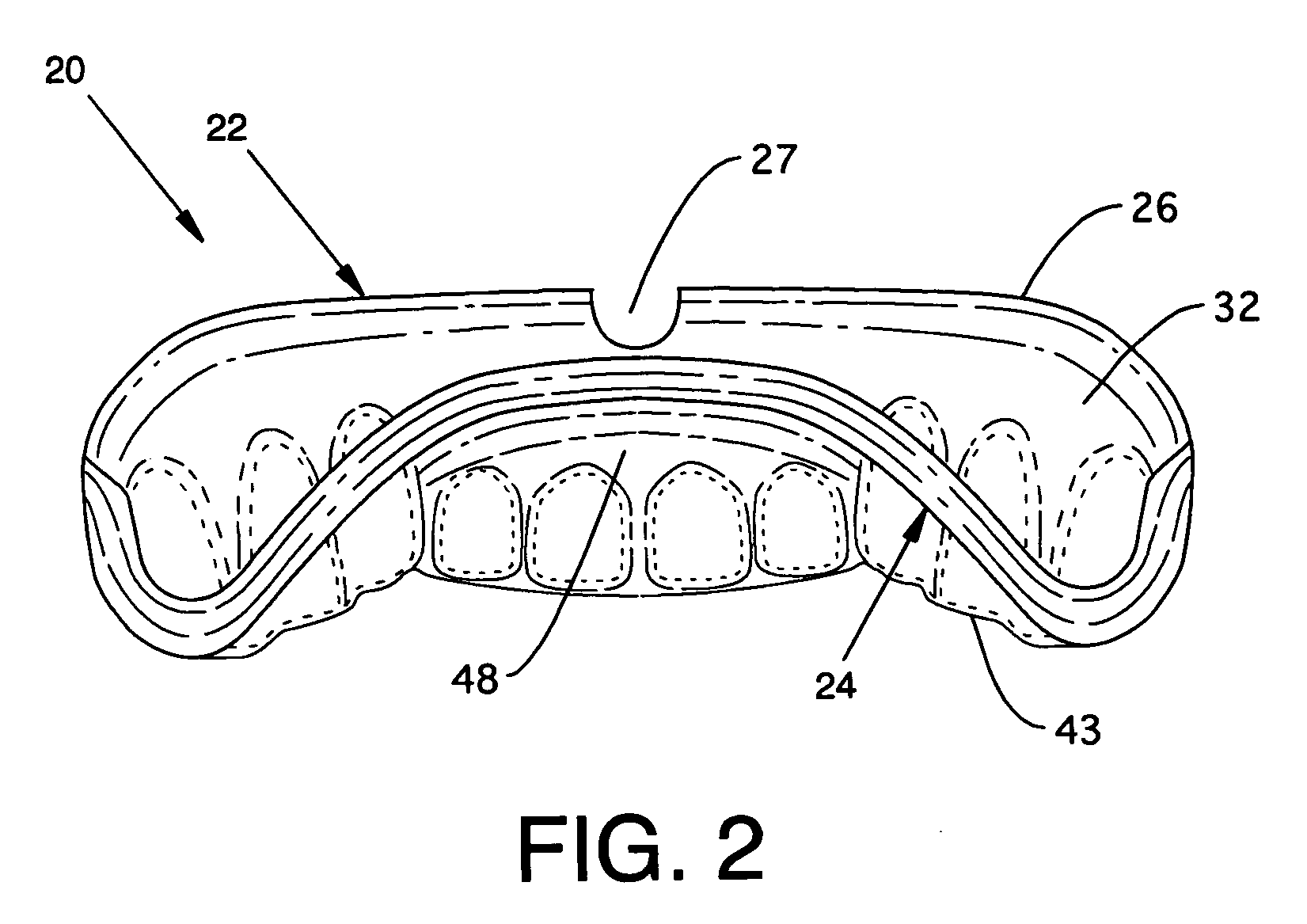
Custom mouthguard
A custom mouthguard has a resilient U-shaped body with an anterior wall and a posterior wall. A post dam on the posterior wall forms a seal with palatal tissue to increase retention of the mouthguard in a wearer's mouth. The increased retention allows a wearer to speak and open mouth breath while wearing the mouthguarrd. The mouth guard also has an indexed region that serves to mutually stabilize maxillary teeth, mandibular teeth, mandible and TMJ components. Mouthguard methods and processes are also disclosed.
Owner:AMBIS JR EDWARD J
Modular prosthetic component with improved body shape
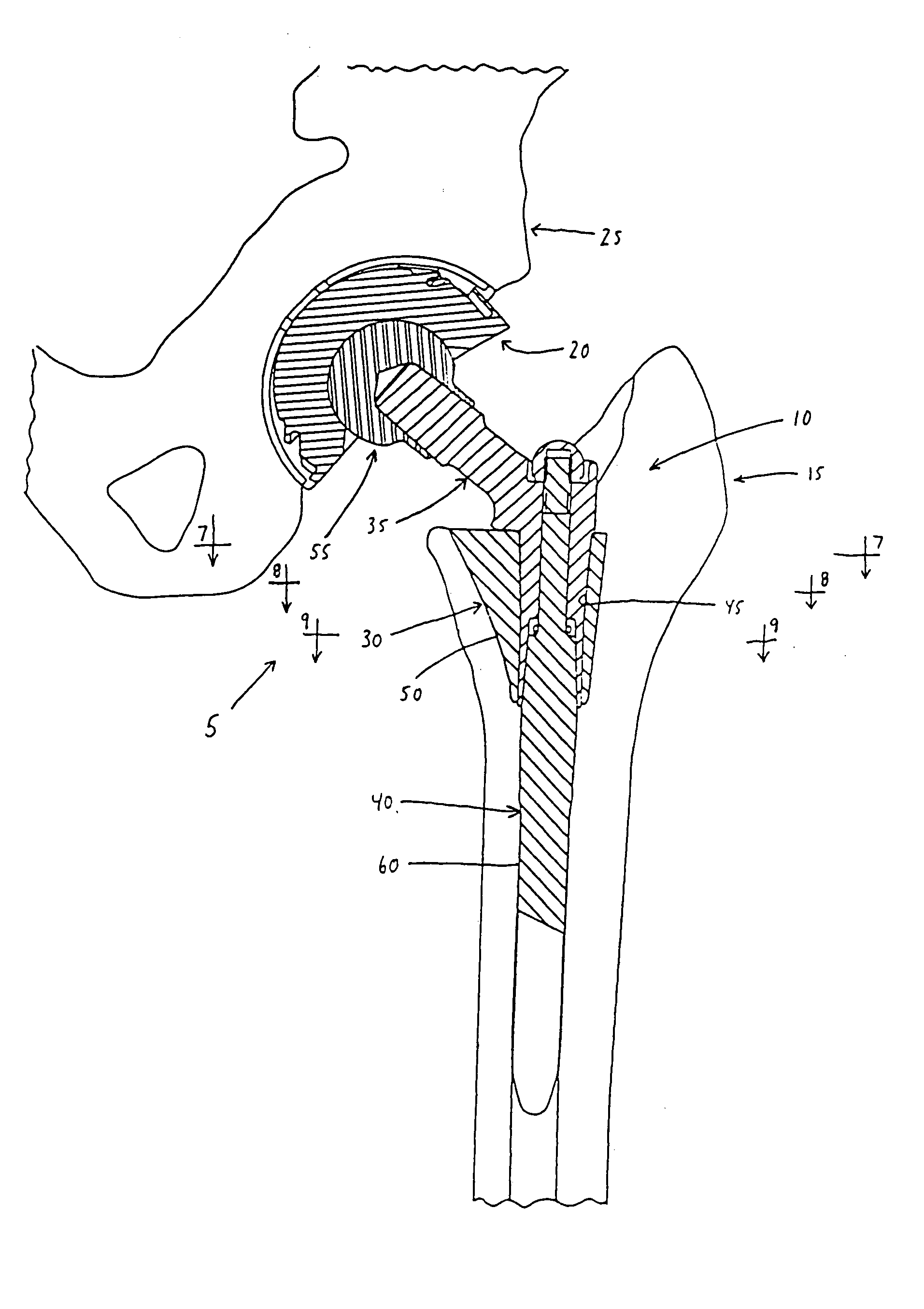
InactiveUS20050283254A1OptimizationRestoring a hip jointJoint implantsFemoral headsBody shapeMedicine
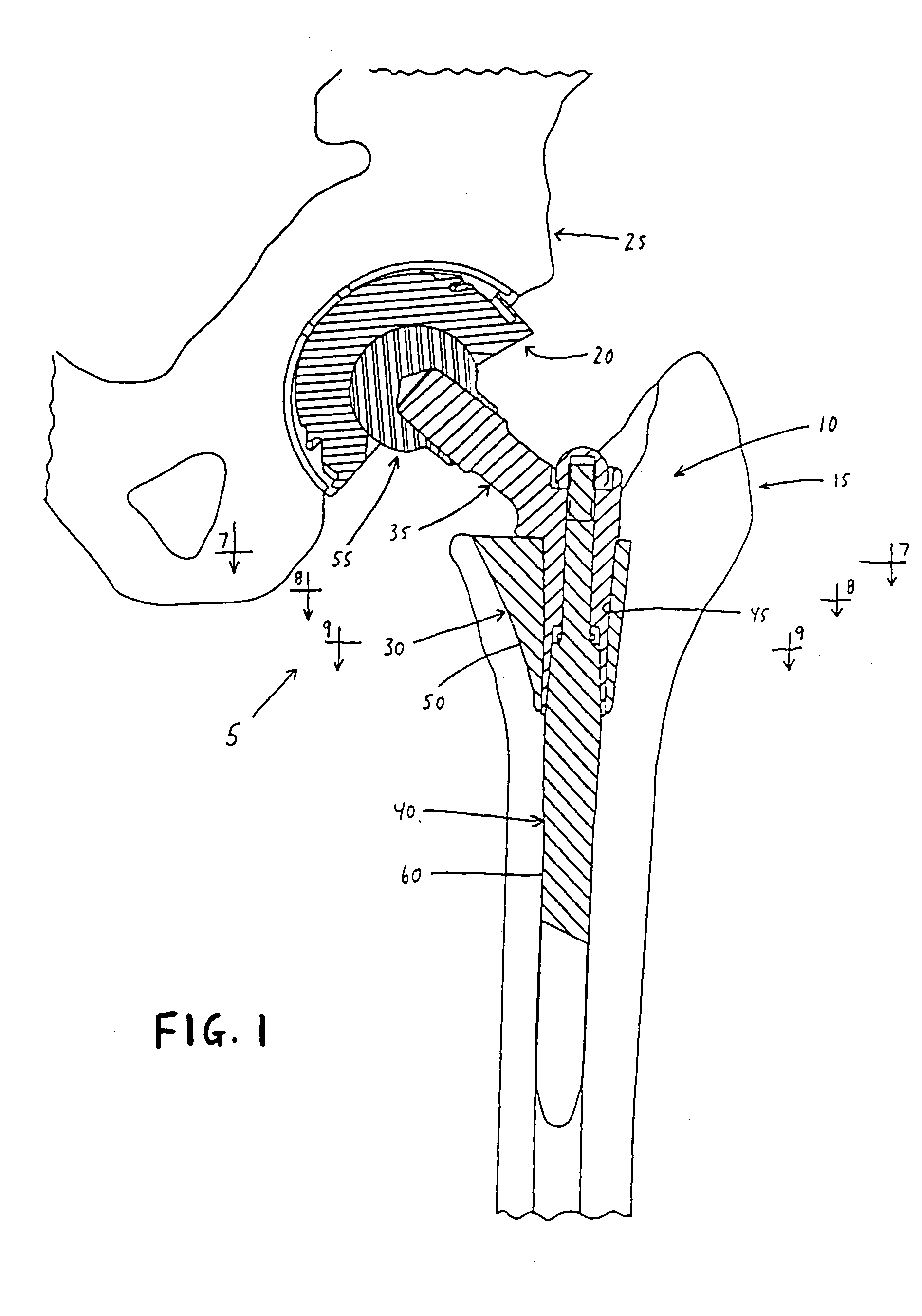
An improved body element for use in a modular prosthetic stem component of the sort comprising a body element and at least one other element, wherein the body element and the at least one other element are joined together by at least one modular connection, wherein the improved body element comprises an anterior wall and a posterior wall, at least one of the anterior wall and the posterior wall converging toward the other on the medial side of the body element and diverging away from the other on the lateral side of the body element, whereby the body element approximates a general wedge shape.
Owner:SHALBY ADVANCED TECH INC
Magnetic devices, systems, and methods placed in or on a tongue
Magnetic structures develop a magnetic force between a tongue and a posterior pharyngeal wall to stabilize an orientation of the tongue. The magnetic structures include magnetic materials that are sized, configured, and arranged on at least one of the first and second magnetic structures, to maintain a substantially mutually repelling orientation between the first and second magnetic structures during a native range of movement of the tongue relative to the pharyngeal wall, i.e., during swallowing and / or drinking / and or speech.
Owner:KONINKLIJKE PHILIPS ELECTRONICS NV
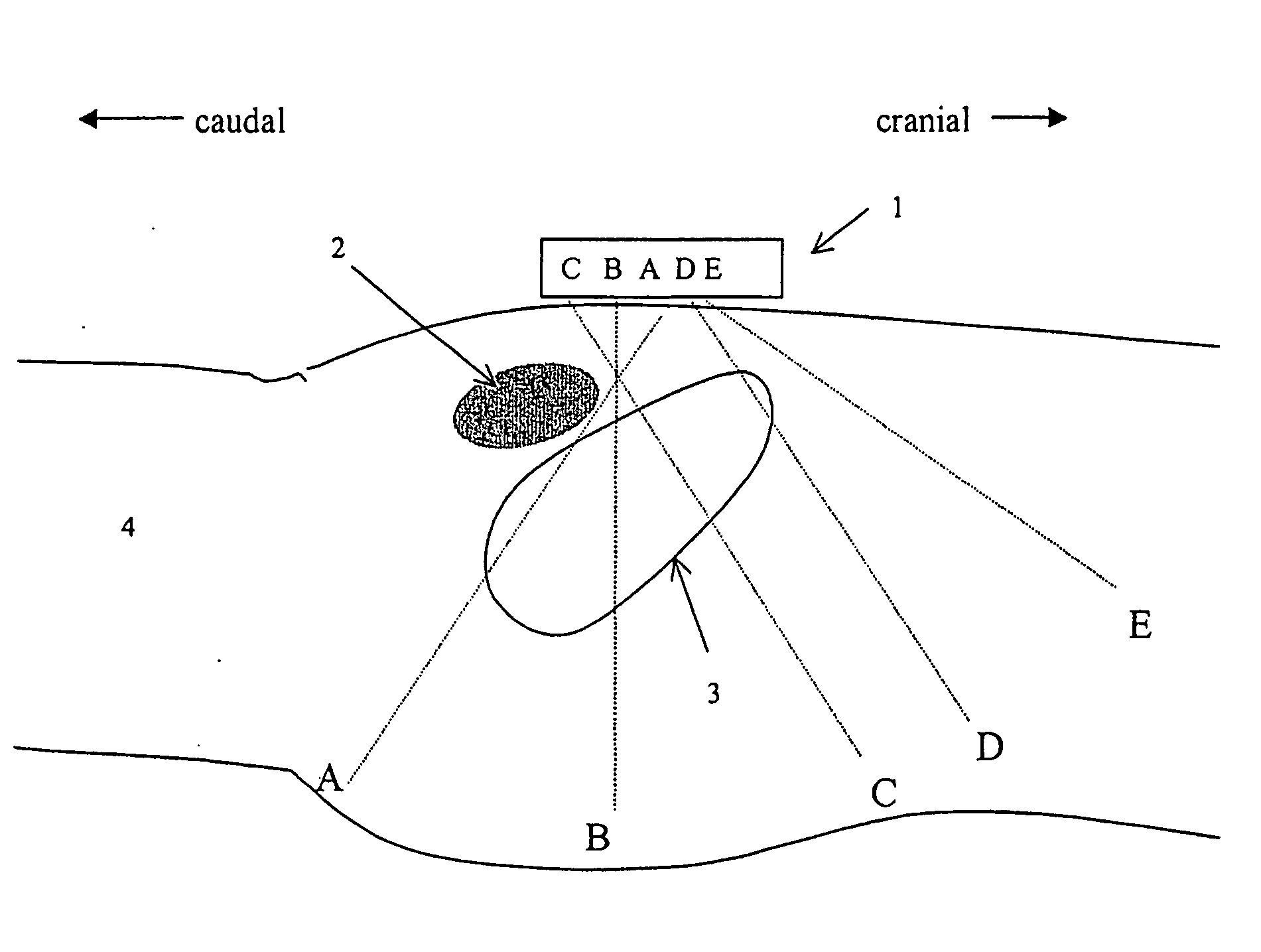
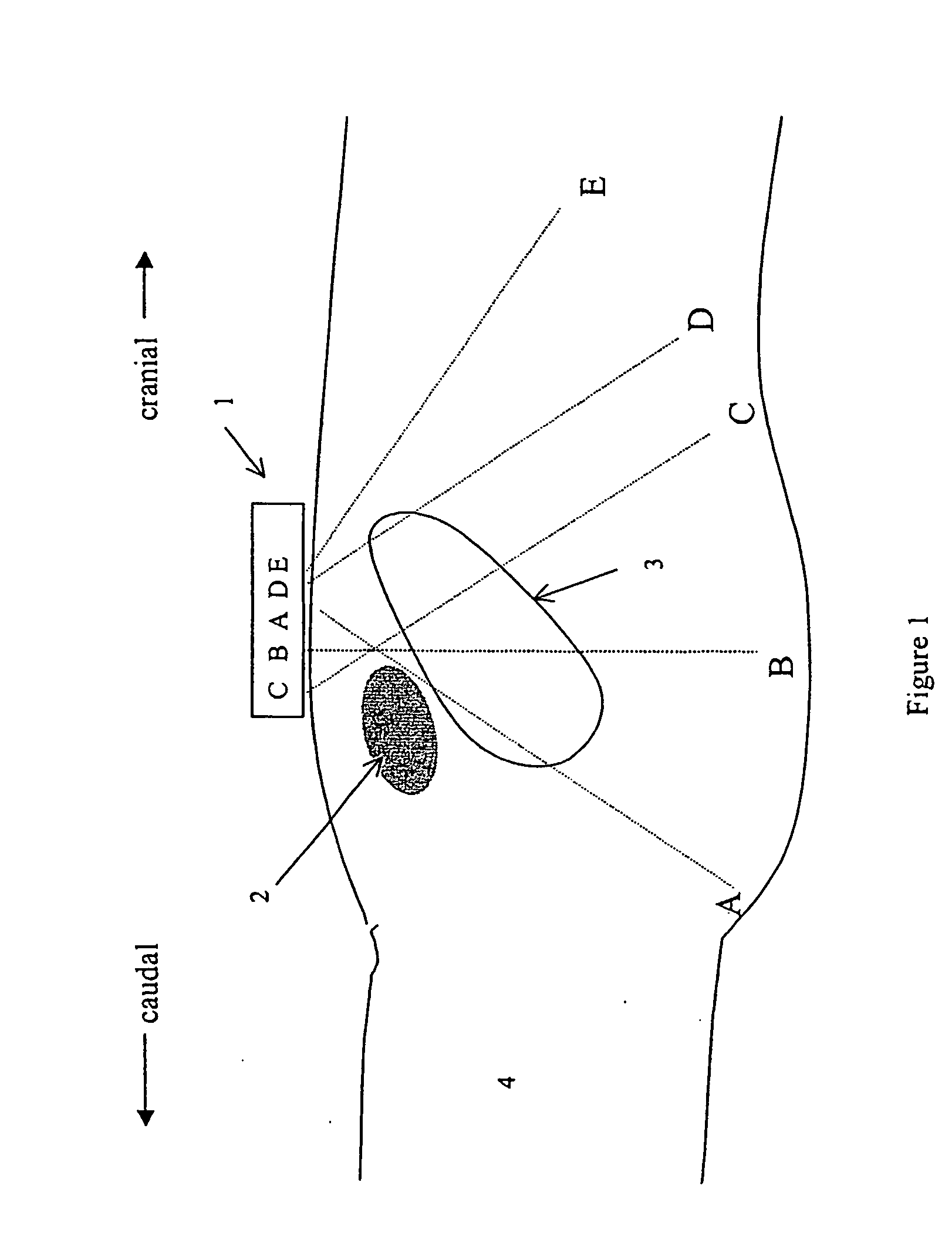
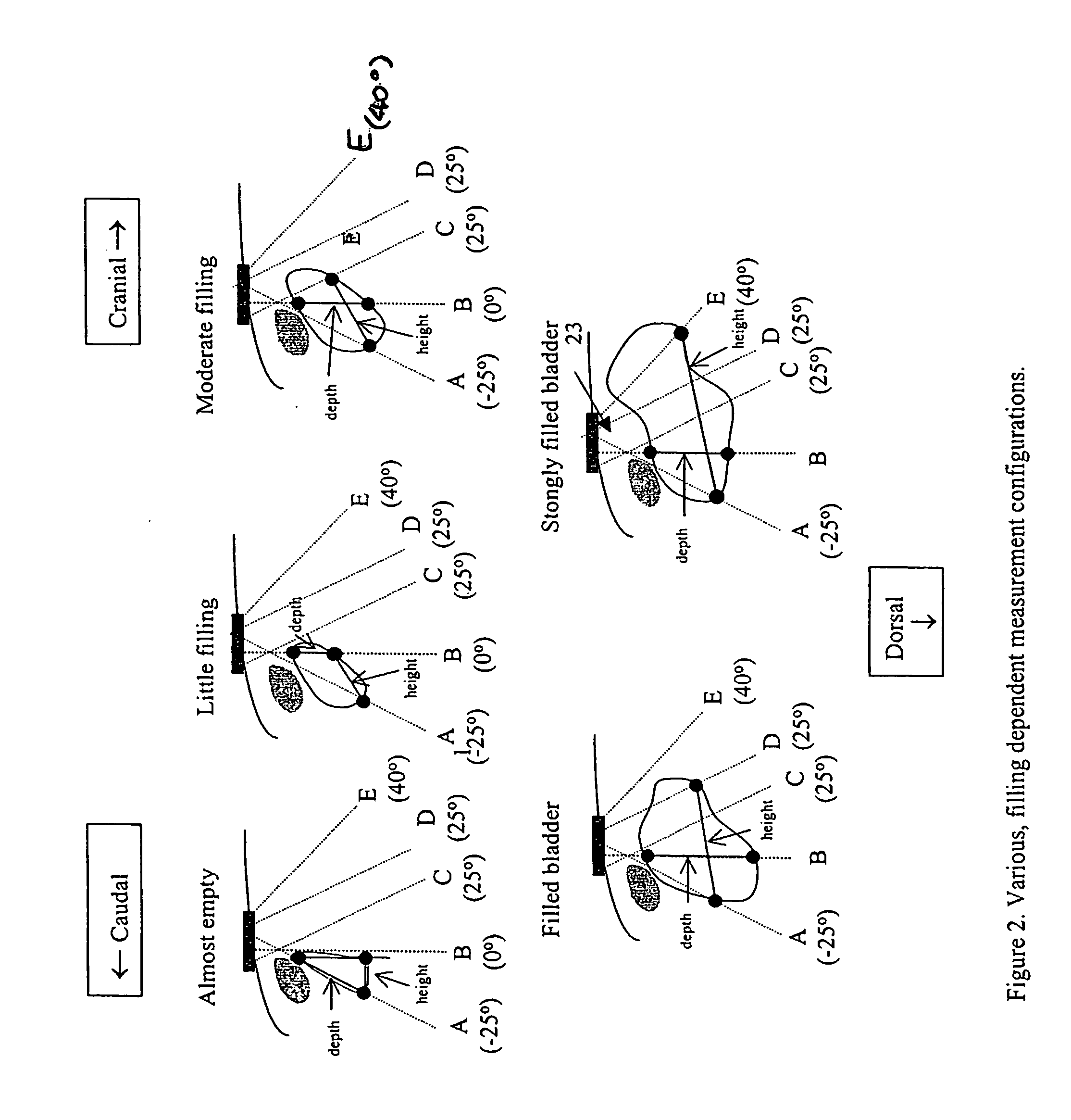
Instantaneous ultrasonic measurement of bladder volume
An apparatus and methods to quantify the volume of urine in a human bladder with a limited number of acoustic beams is disclosed. In a first version the apparatus is composed of a transducers assembly that transmits a plurality of narrow ultrasound beams in different directions towards the bladder and receives the returning ultrasound signals; a receiver detector for processing the returned signals; an analog-to-digital converter; a memory to store the digitized data and a volume display allowing to define the optimal position of the transducer assembly. The apparatus also includes a signal processing software that automatically determines the bladder Depth D and Height H and computes the volume of urine using an empirical formula corrected by specific, empirically measured, filling dependant correction factors. In a second version a single wide angle ultrasound beam transducer transmitting ultrasound signals at fundamental frequency is used to quantify the urine volume. Return signals originating from a depth beyond the usual position of the posterior wall depth of a filled bladder are analyzed for presence of higher harmonic signals which in turn are related to presence or absence of urine. Both methods or a combination thereof can be used us a simple warning device for presence of residual urine after voiding or indicate the presence of a critical bladder urine filling level.
Owner:VERATHON
Gastro-laryngeal mask
A gastro-laryngeal mask features softly compliant construction of the distal half of the mask, wherein the mask is of generally elliptical configuration, with an inflatable peripheral cuff to seal and support the mask around the laryngeal inlet. A back cushion is inflatable to engage the back wall of the pharynx and thus to forwardly load the peripheral-cuff seal to the laryngeal inlet. An evacuation tube for external removal of a possible gastric discharge completes an evacuation or discharge passage contained within the mask and opening through the distal end of the peripheral cuff. Special provision is made for assuring integrity of the discharge passage within the flexible distal half of the mask, i.e., assuring against collapse of the distal-end half of the softly compliant evacuation tube in the distal region of the mask, such that inflation of the mask does not compromise viability of the evacuation tube by compressing softly compliant material of the evacuation tube during periods of mask inflation. The special provision also favors such collapse of the mask when deflated as to provide a leading flexible edge for piloting a safe and correct advancing insertional advance of the deflated mask in the patient's throat, in avoidance of epiglottis interference and to the point of locating engagement in the upper sphincter of the oesophagus.
Owner:THE LARYNGEAL MASK +1
Left ventricular conduits and methods for delivery
Conduits are provided to direct blood flow from the left ventricle to a coronary artery at a location distal to a blockage in the coronary artery. Threaded and nonthreaded conduits are delivered using a guidewire delivered through the posterior and anterior walls of a coronary artery and into the heart wall. A dilator may be provided over the guidewire into the heart wall, and the conduit delivered over the dilator. An introducer sleeve may be provided over the dilator into the heart wall, the dilator removed, and the conduit delivered through the introducer sleeve. A hollow needle also may be inserted into the posterior and anterior walls of the coronary artery prior to inserting the guidewire. A depth measuring tool may determine the appropriate length of the conduit prior to delivery. The depth measuring tool can include the hollow needle with an access port on a proximal end of the needle and an opening on the distal end of the needle in flow communication with the access port so that when the needle is inserted through the heart wall and into the heart chamber, blood flow through the opening.
Owner:HORIZON TECH FUNDING CO LLC
Magnetic device for guiding catheter and method of use therefor
InactiveUS20100145147A1Prevent insertionSufficient magnetic forceSurgeryEndoscopesMagnetic tension forceNose
One or more external magnets that are suitable for placement on the back or front of a patient's neck and methods for using the external magnet(s) to guide a feeding tube containing one or more internal magnets through the esophagus are described herein. The external magnet is applied to the back or the front of the patient's neck and the feeding tube is inserted into the patient's nose and advanced within the patient's body. The magnetic field of the external magnet combines with the magnetic field of the internal magnet to produce a sufficient magnetic force to pull or push the feeding tube apparatus against the posterior wall of the esophagus to prevent placement of the stylet in the patient's trachea or windpipe to prevent insertion into the patient's lungs.
Owner:SYNCRO MEDICAL INNOVATIONS +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com