Patents
Literature
293 results about "Percutaneous angioplasty" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
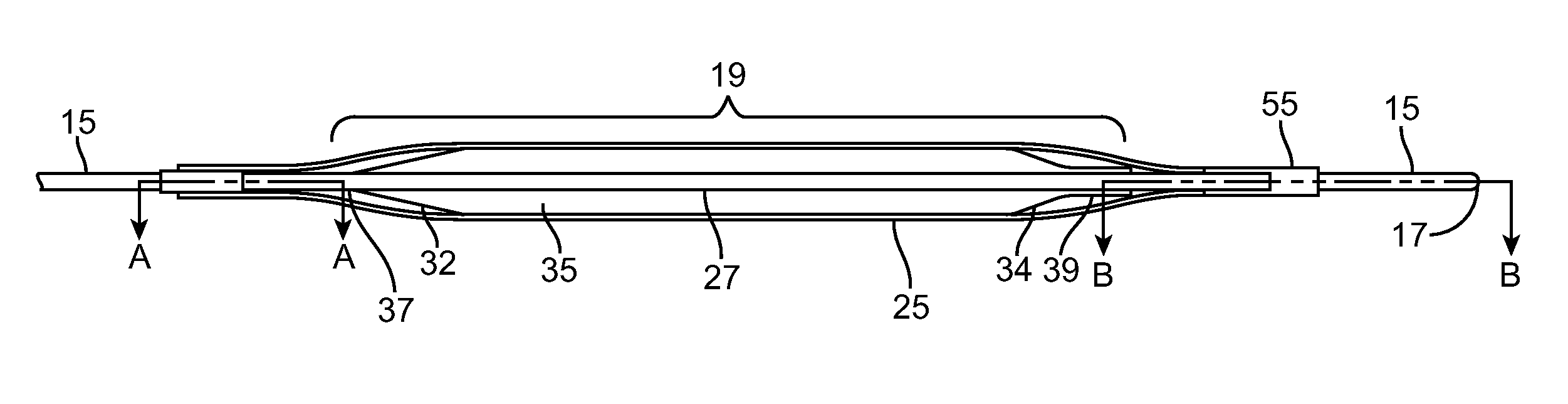
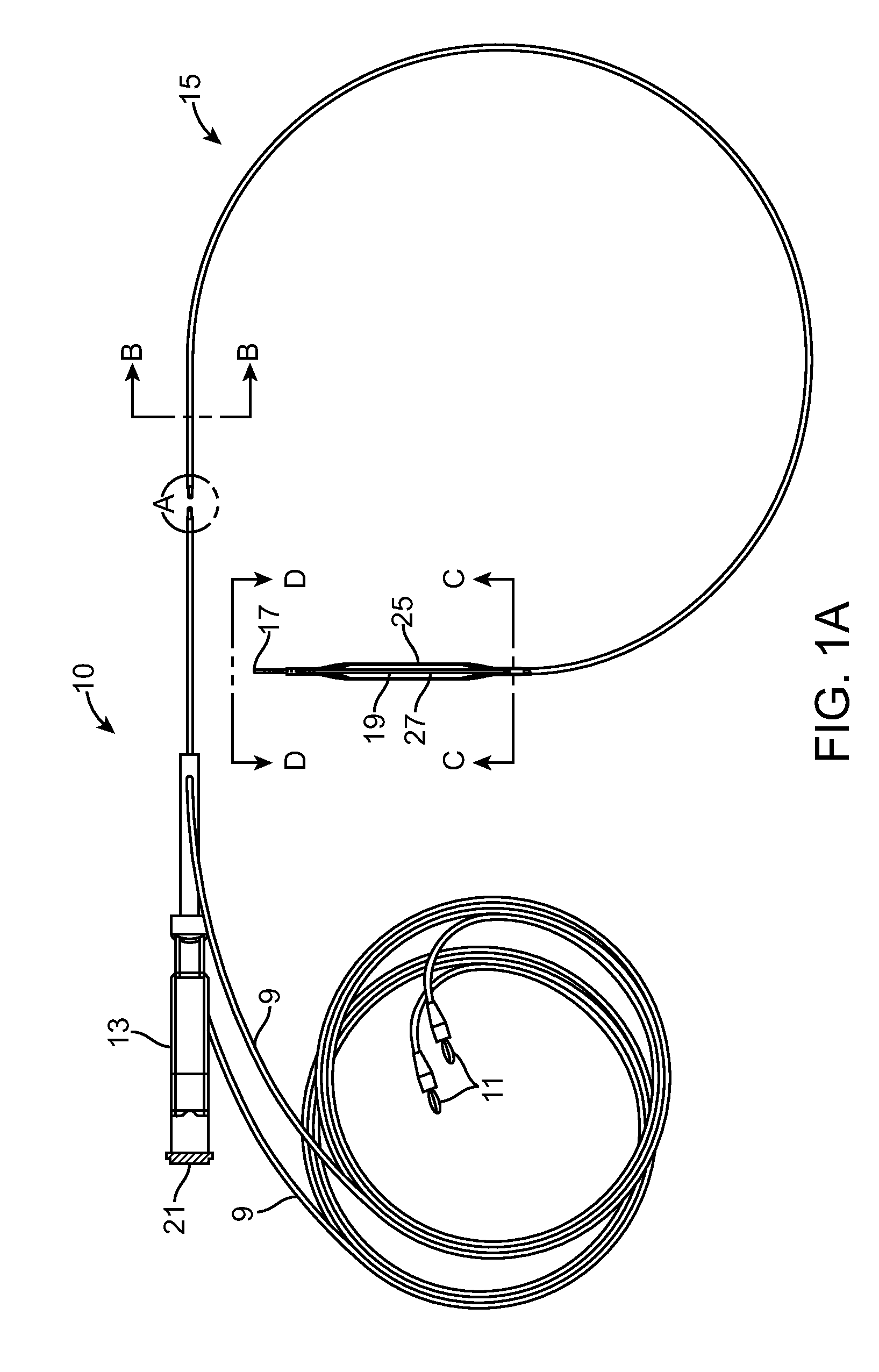
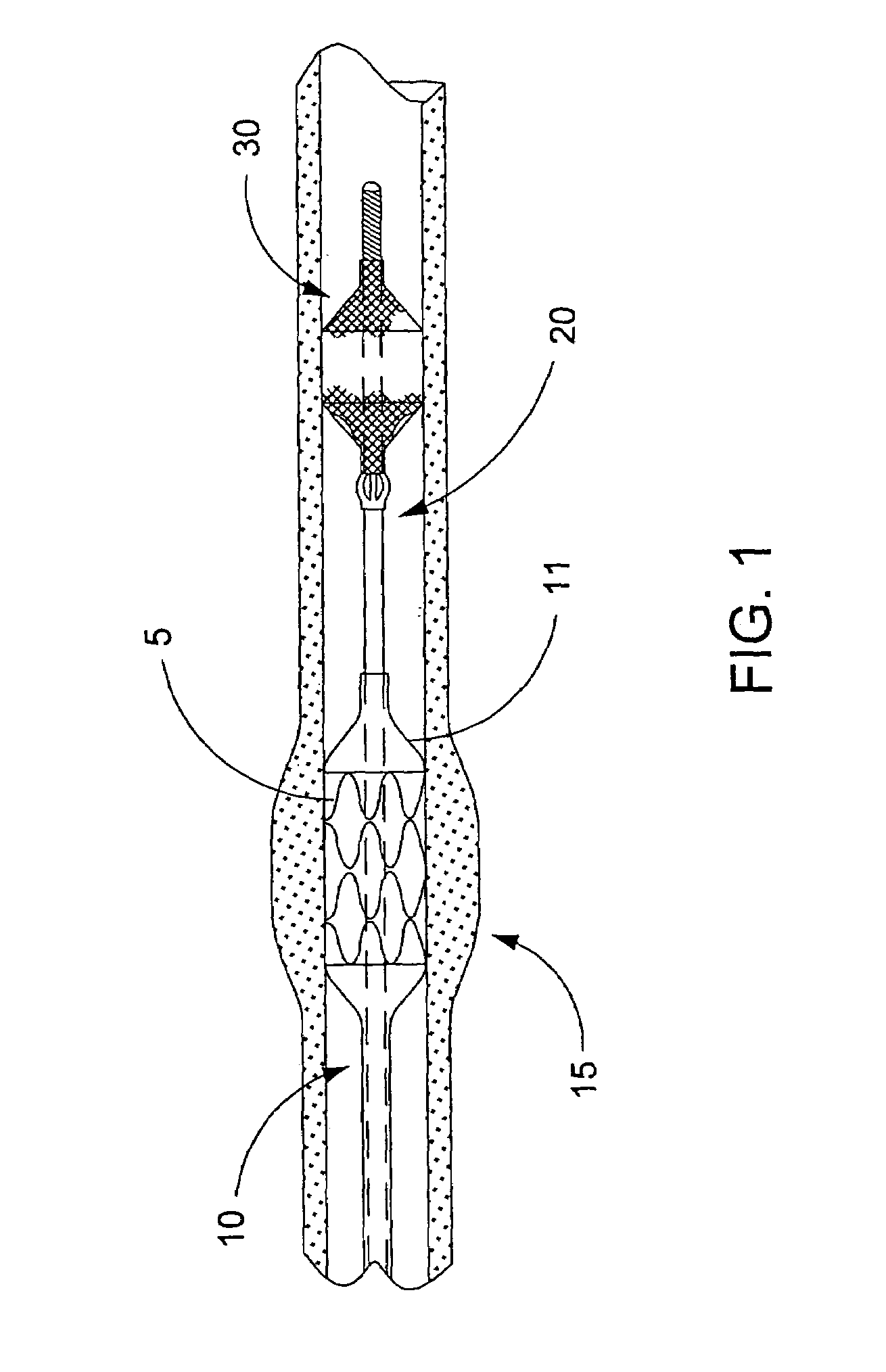
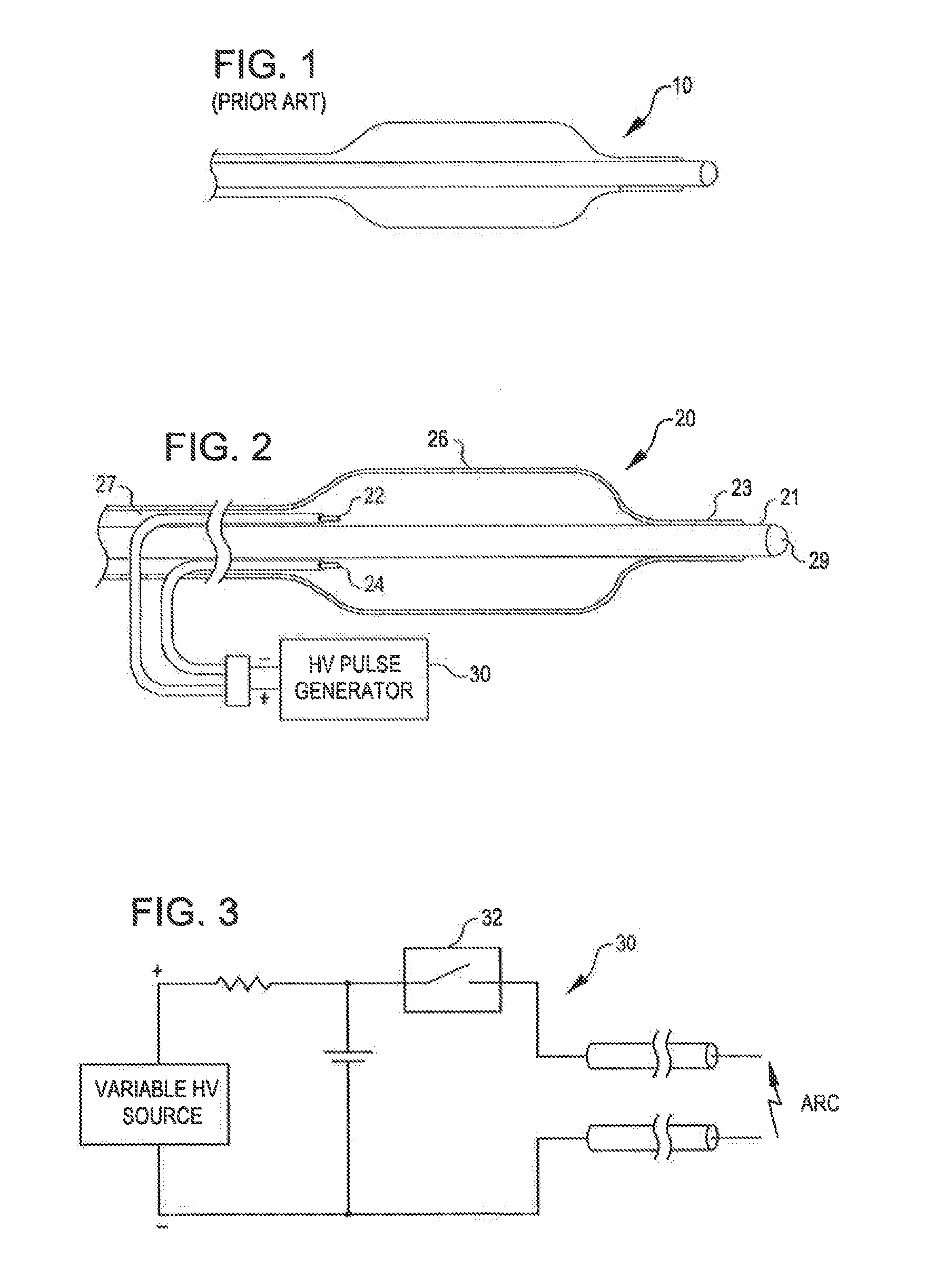
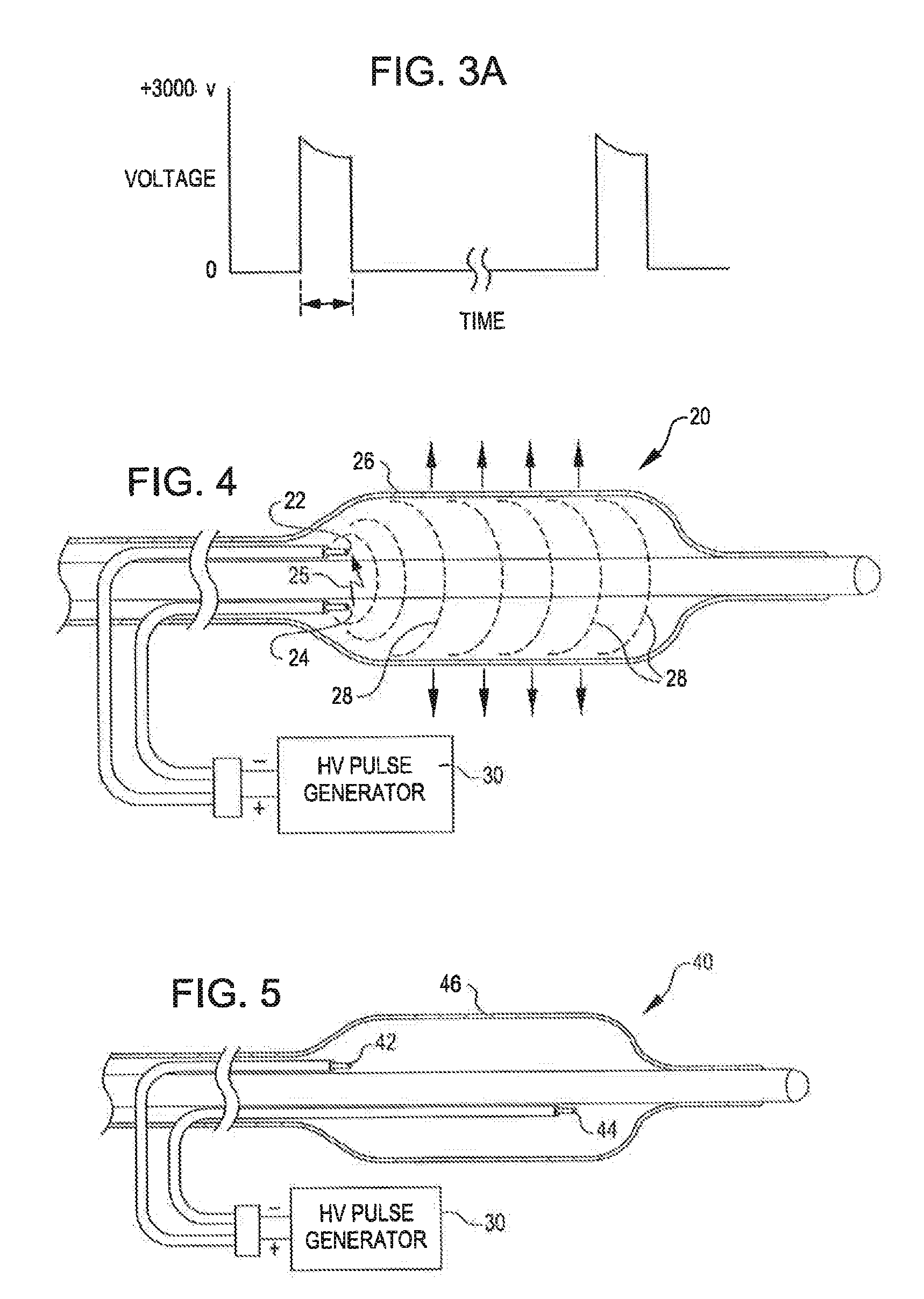
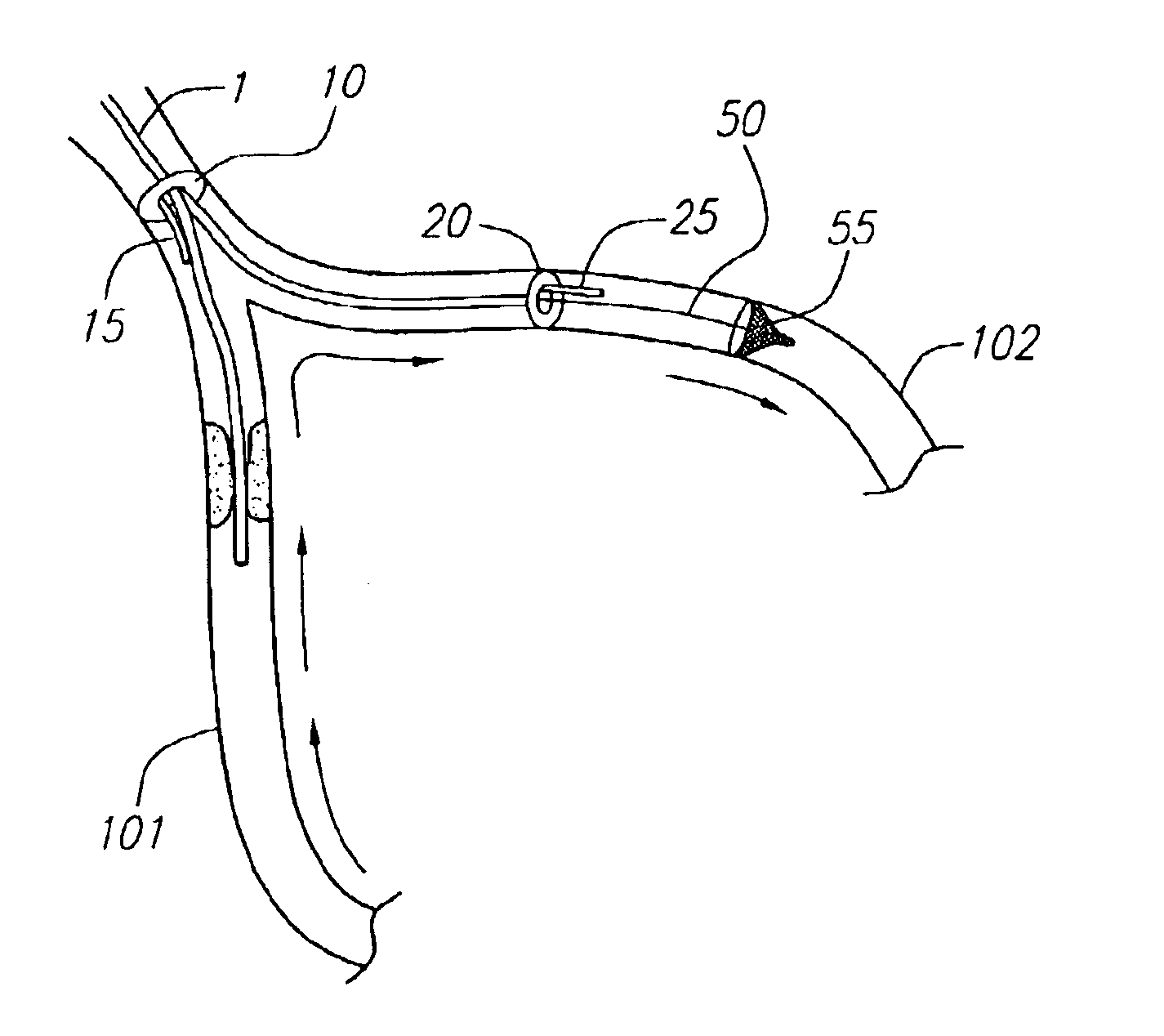
Angioplasty, also known as balloon angioplasty and percutaneous transluminal angioplasty (PTA), is a minimally invasive, endovascular procedure to widen narrowed or obstructed arteries or veins, typically to treat arterial atherosclerosis.
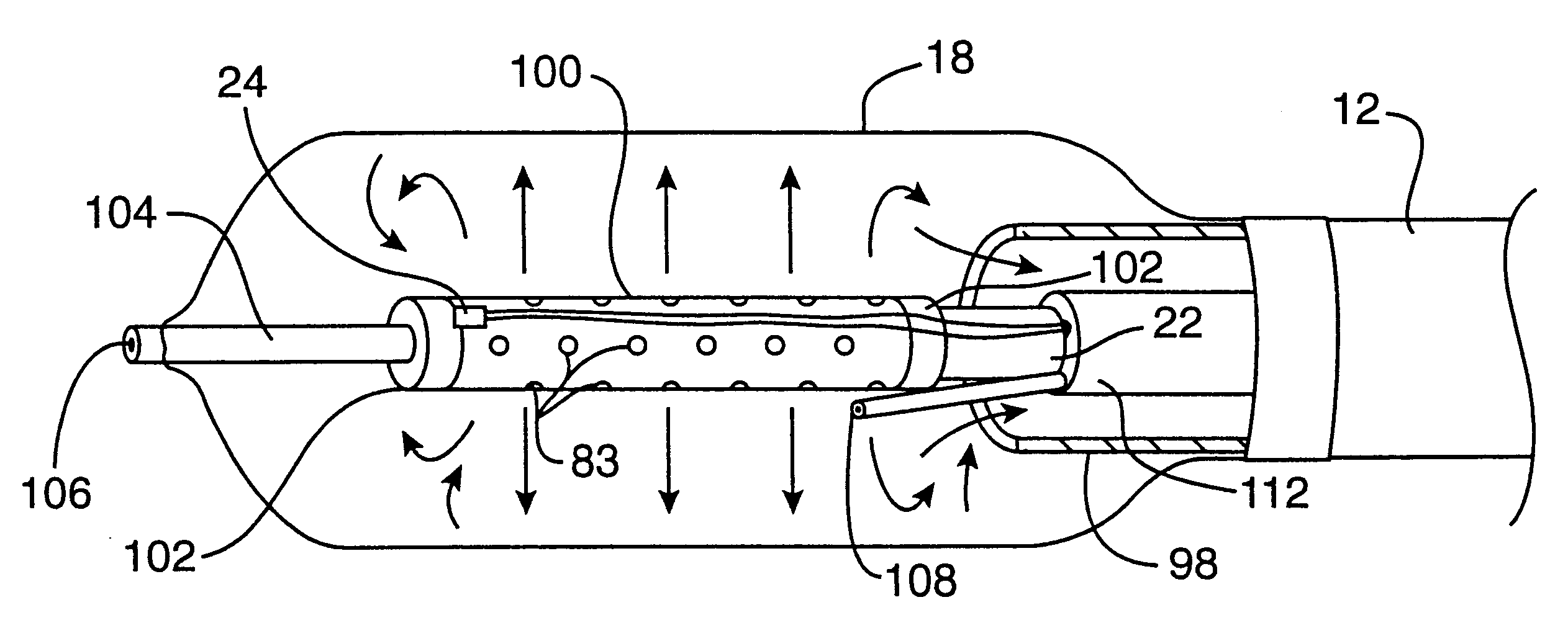
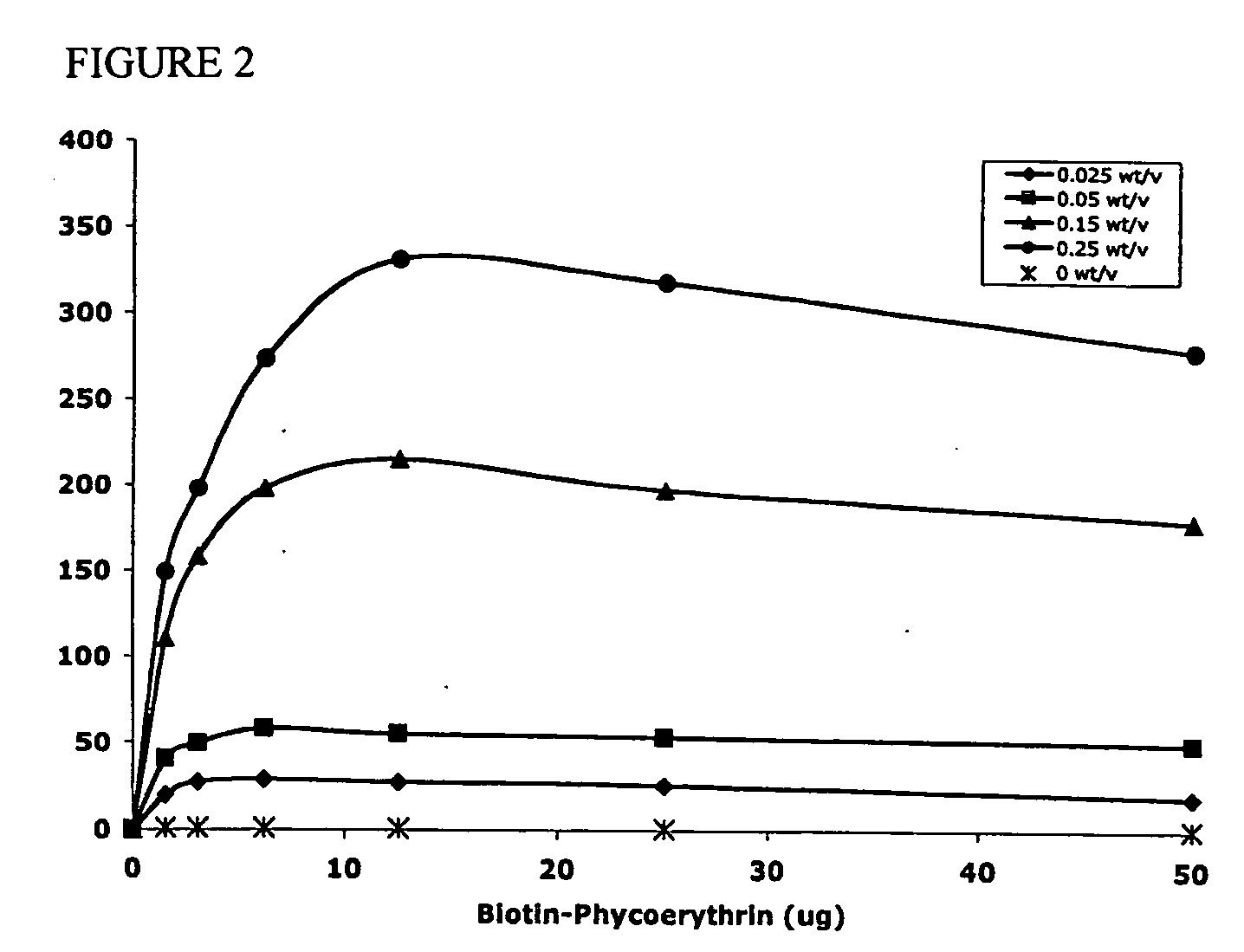
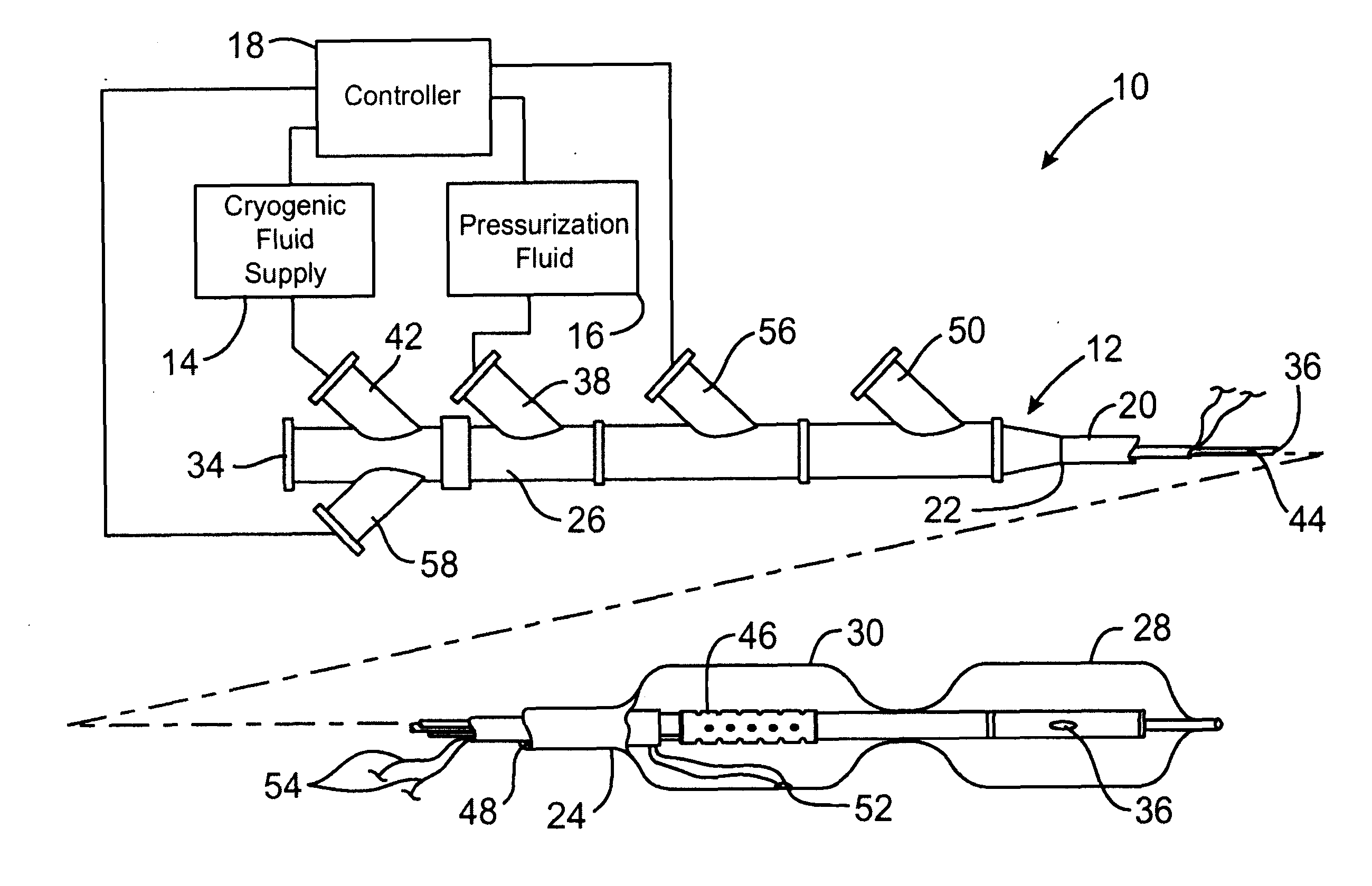
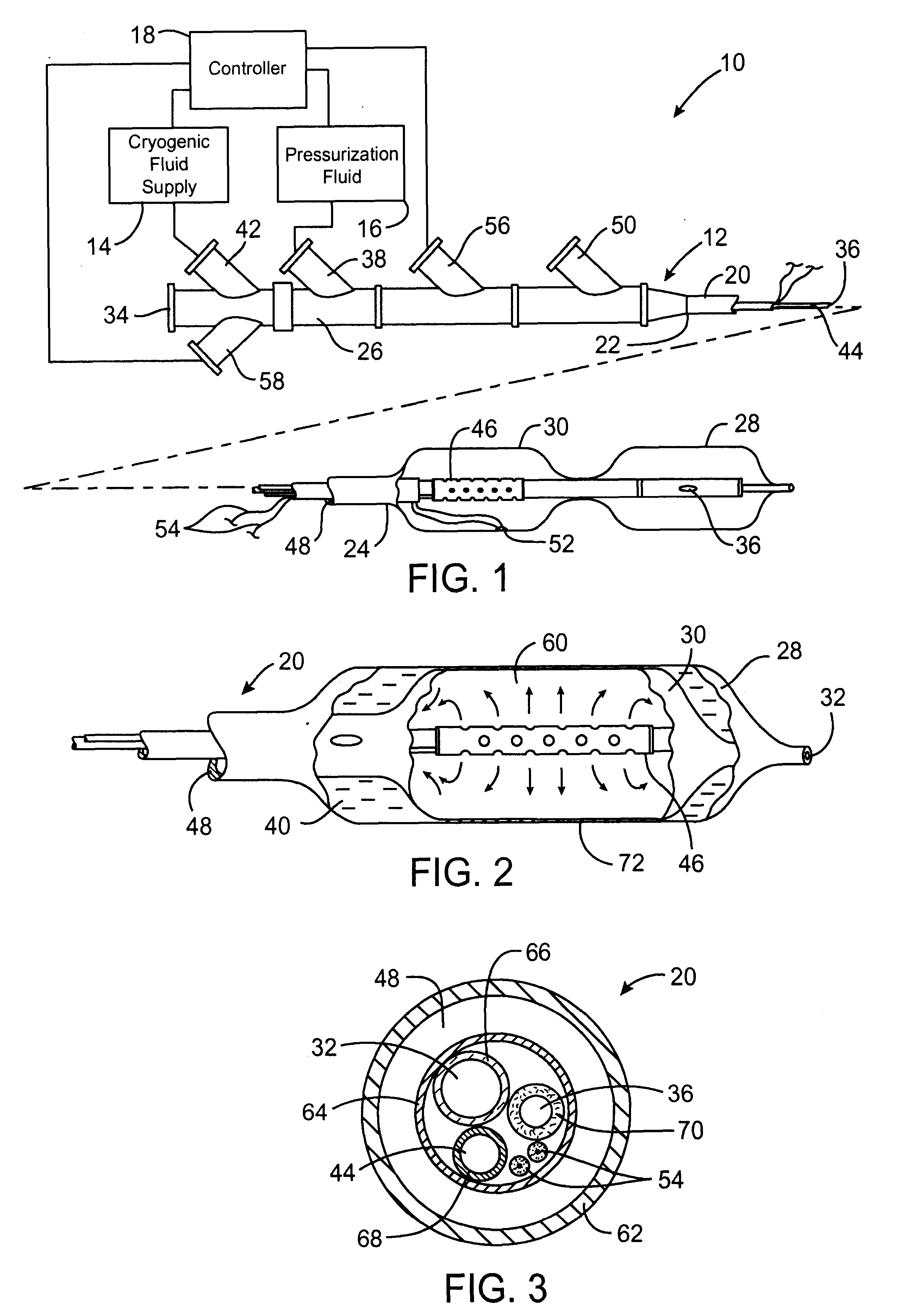
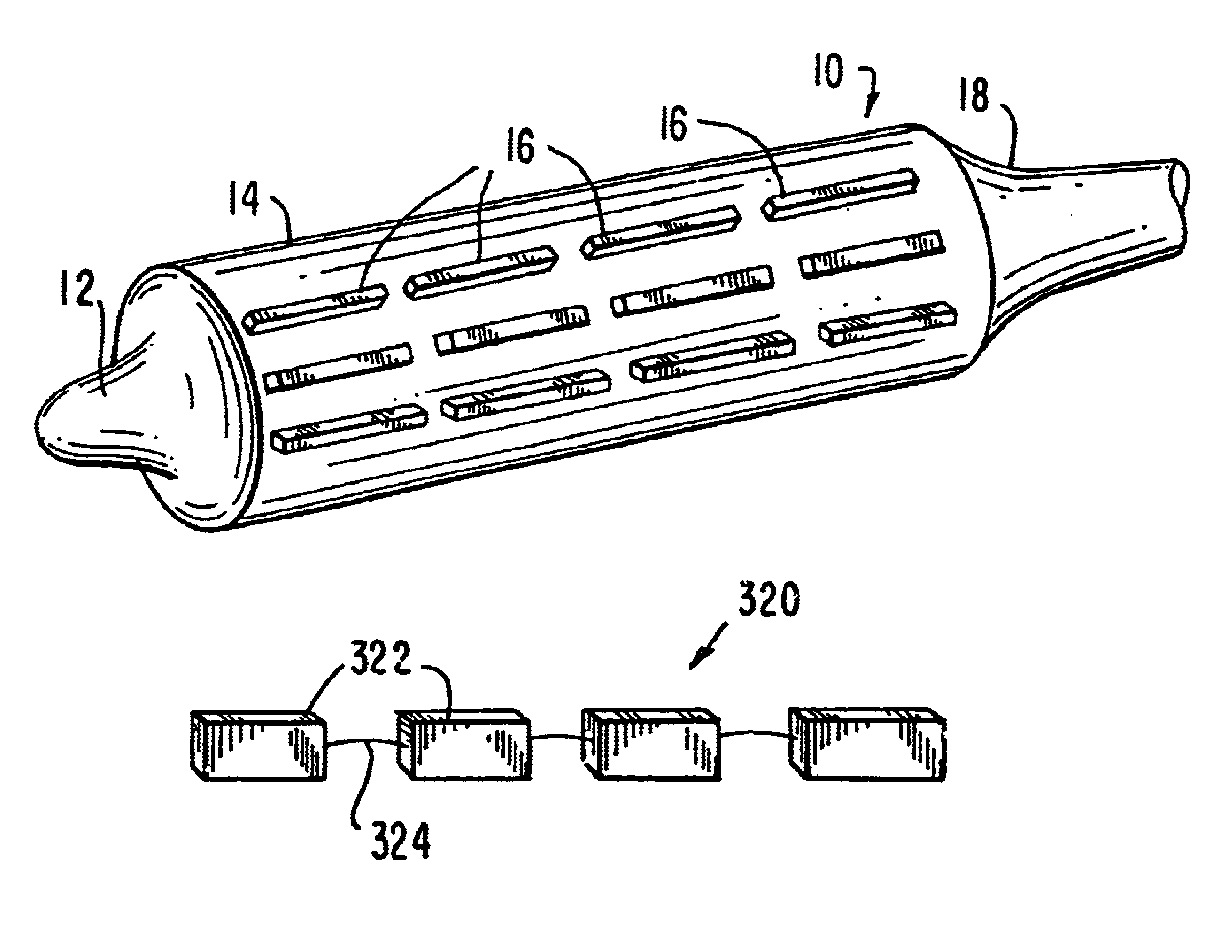
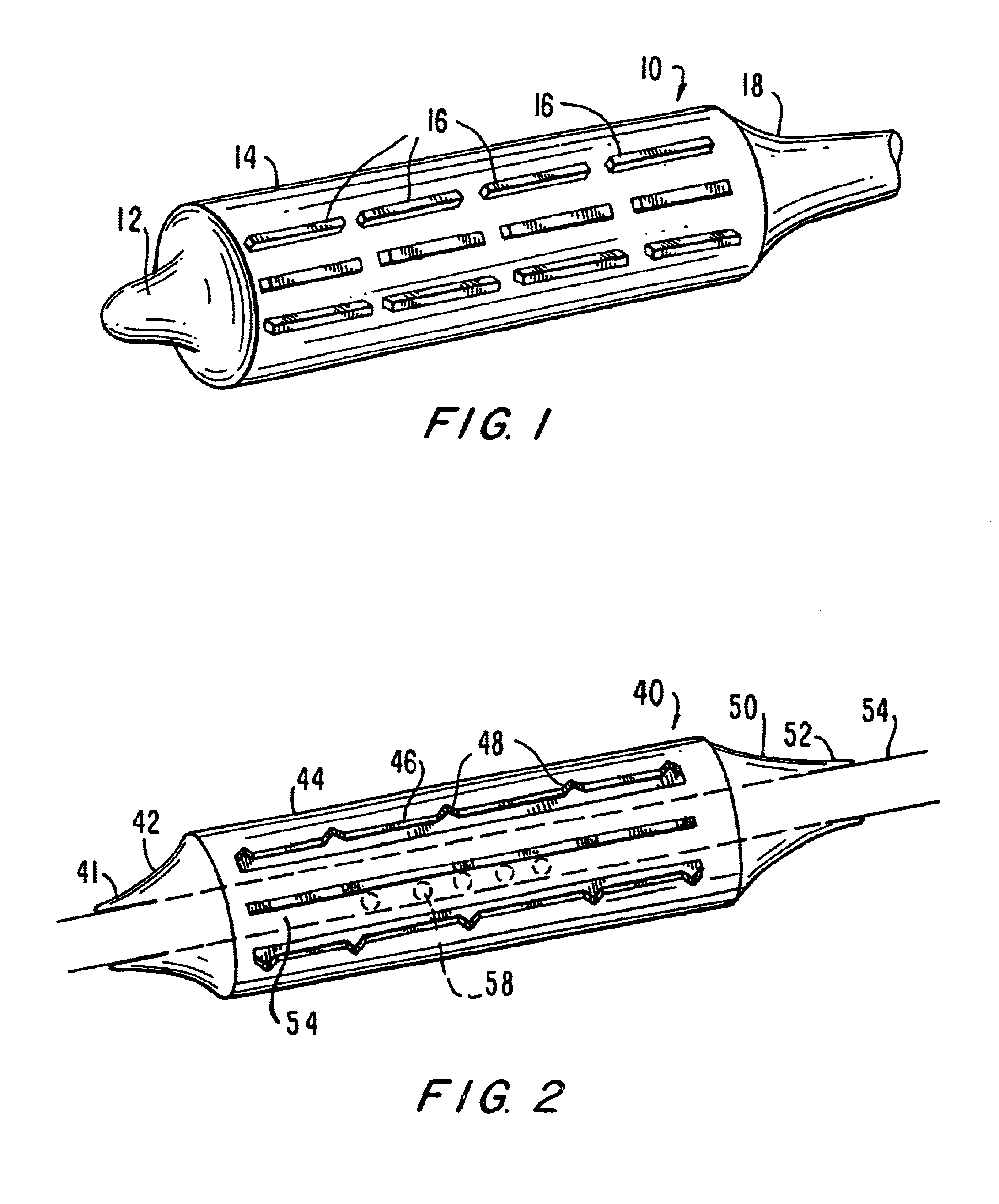
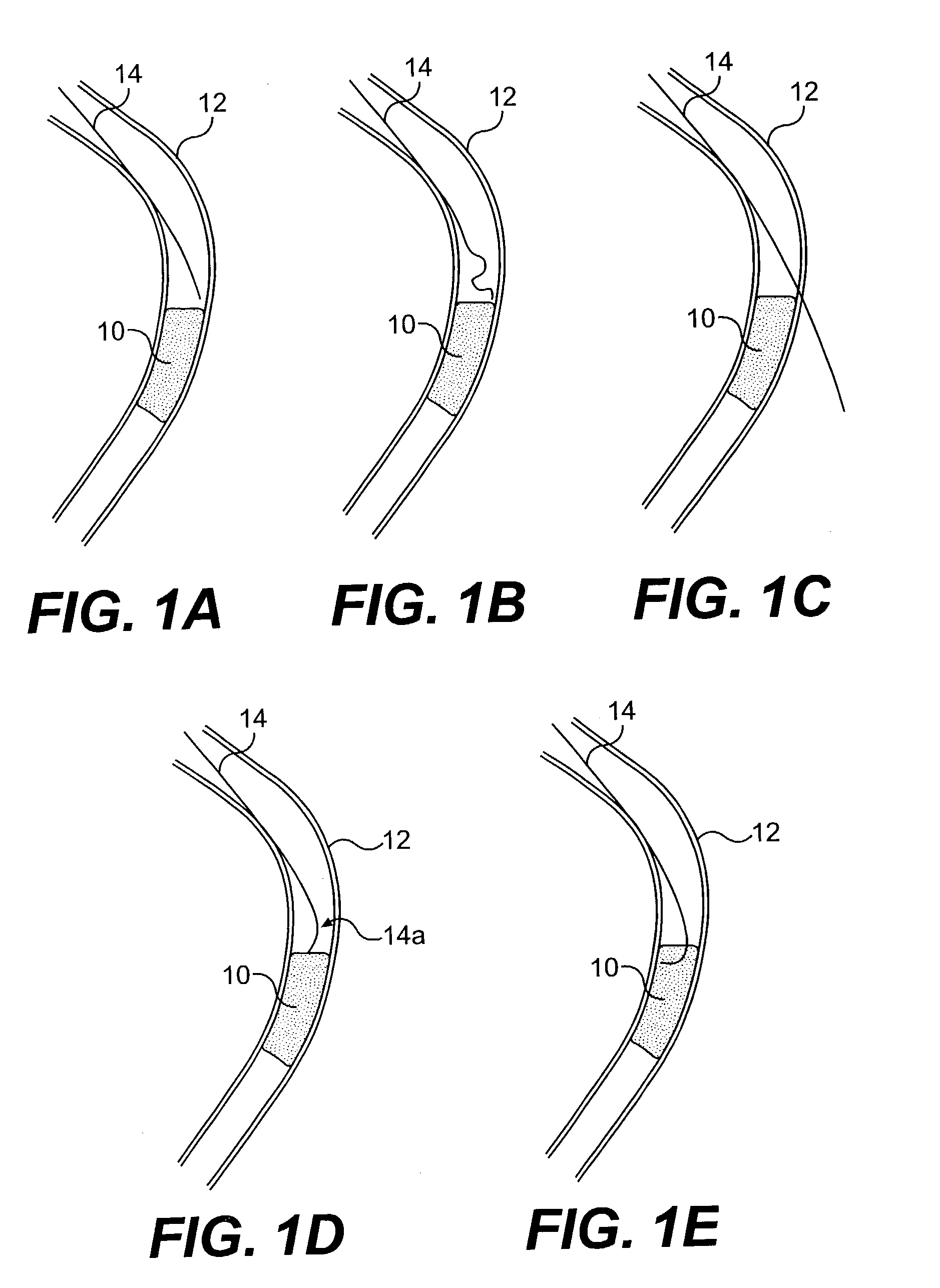
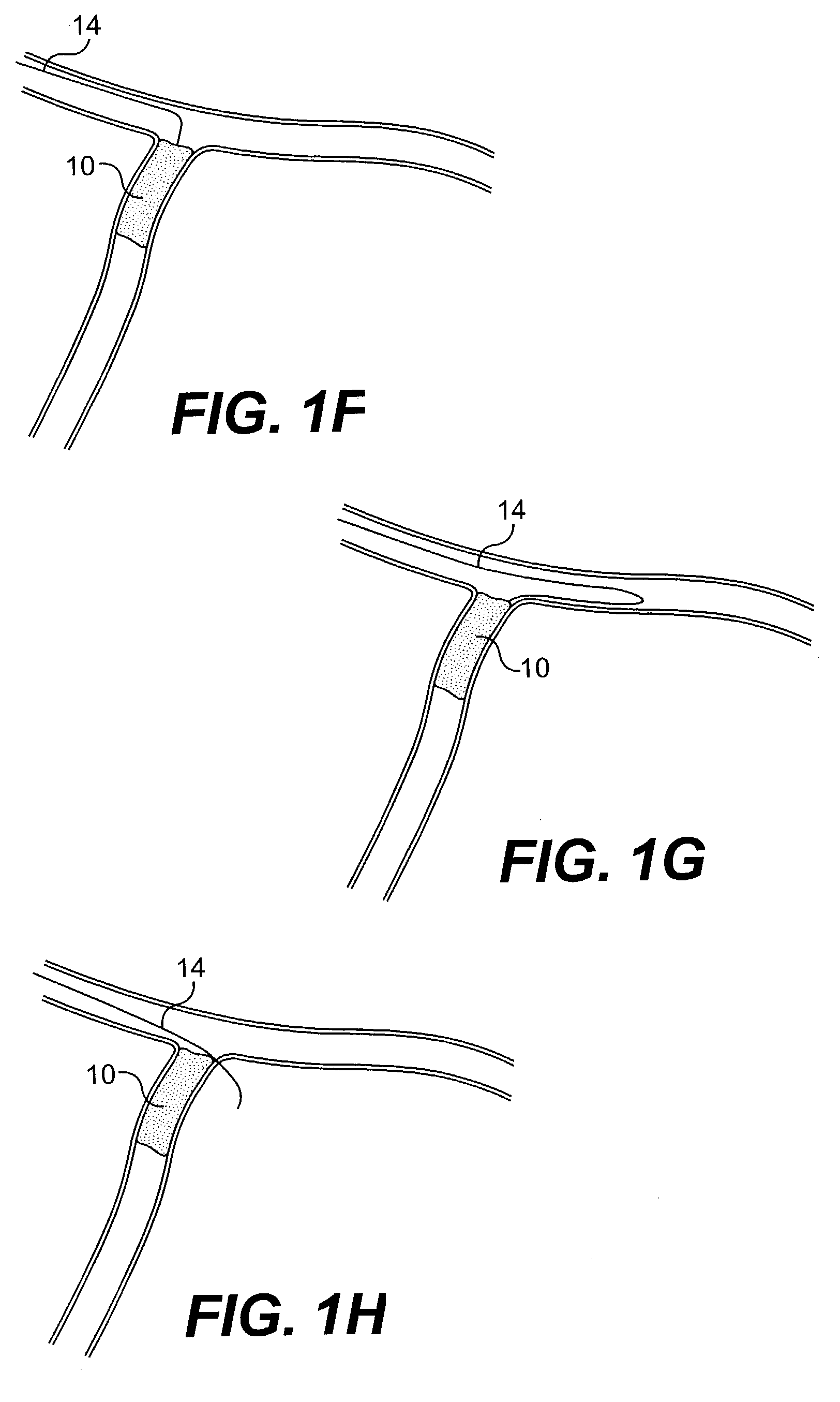
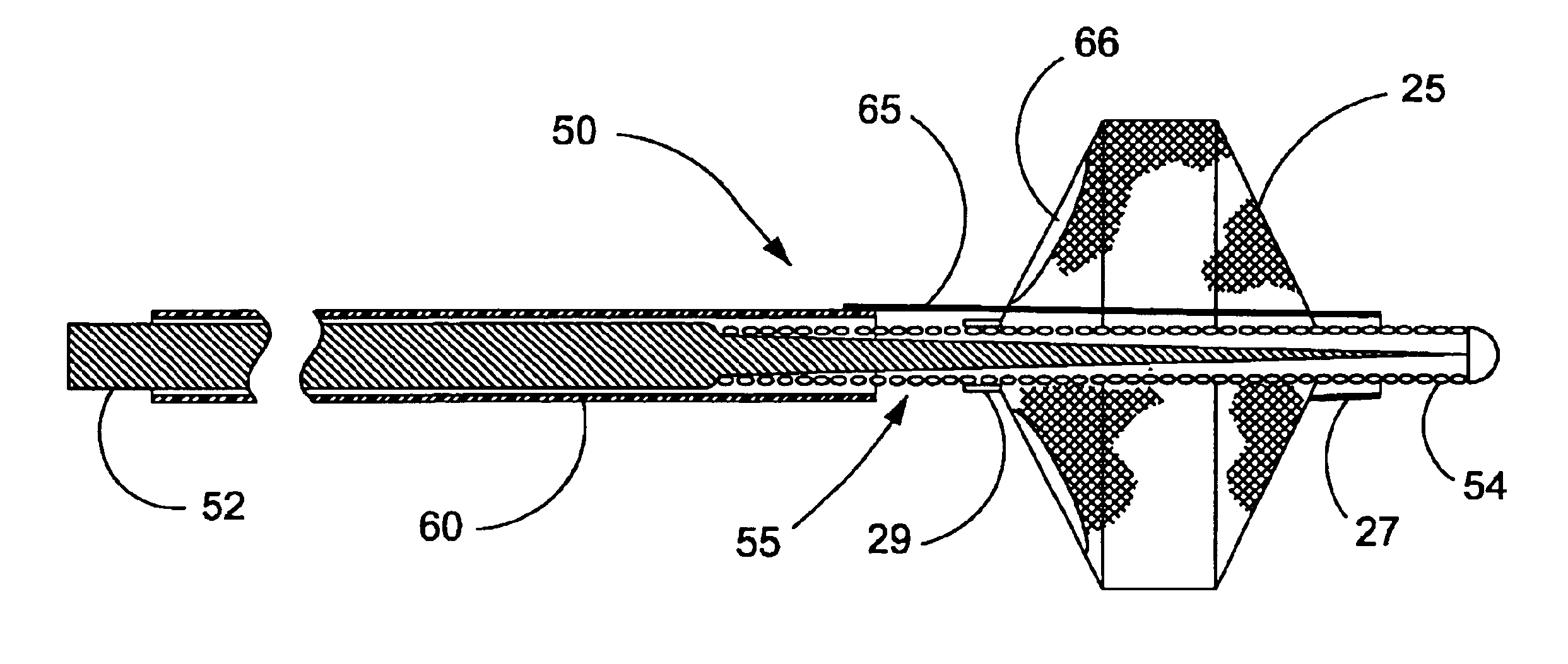
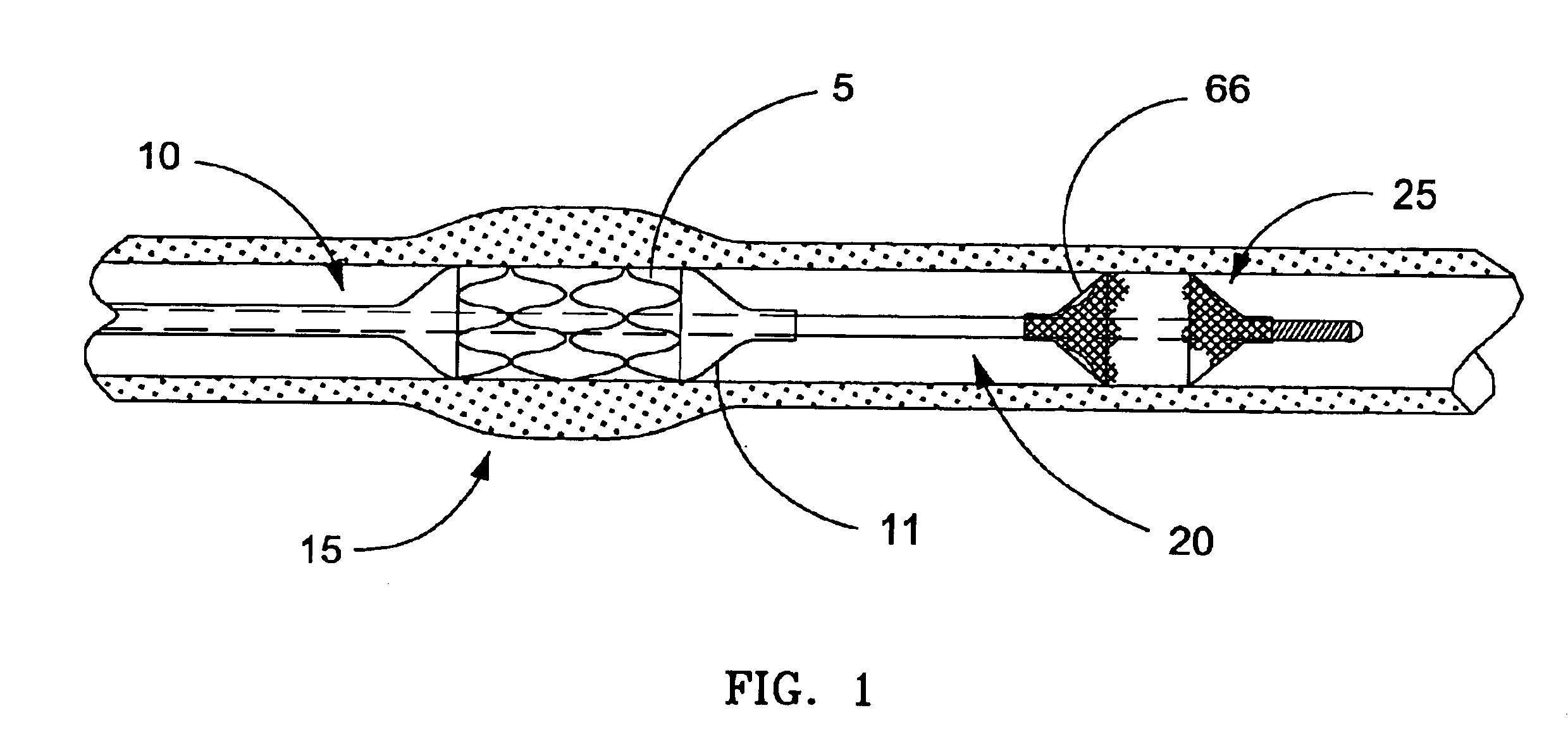
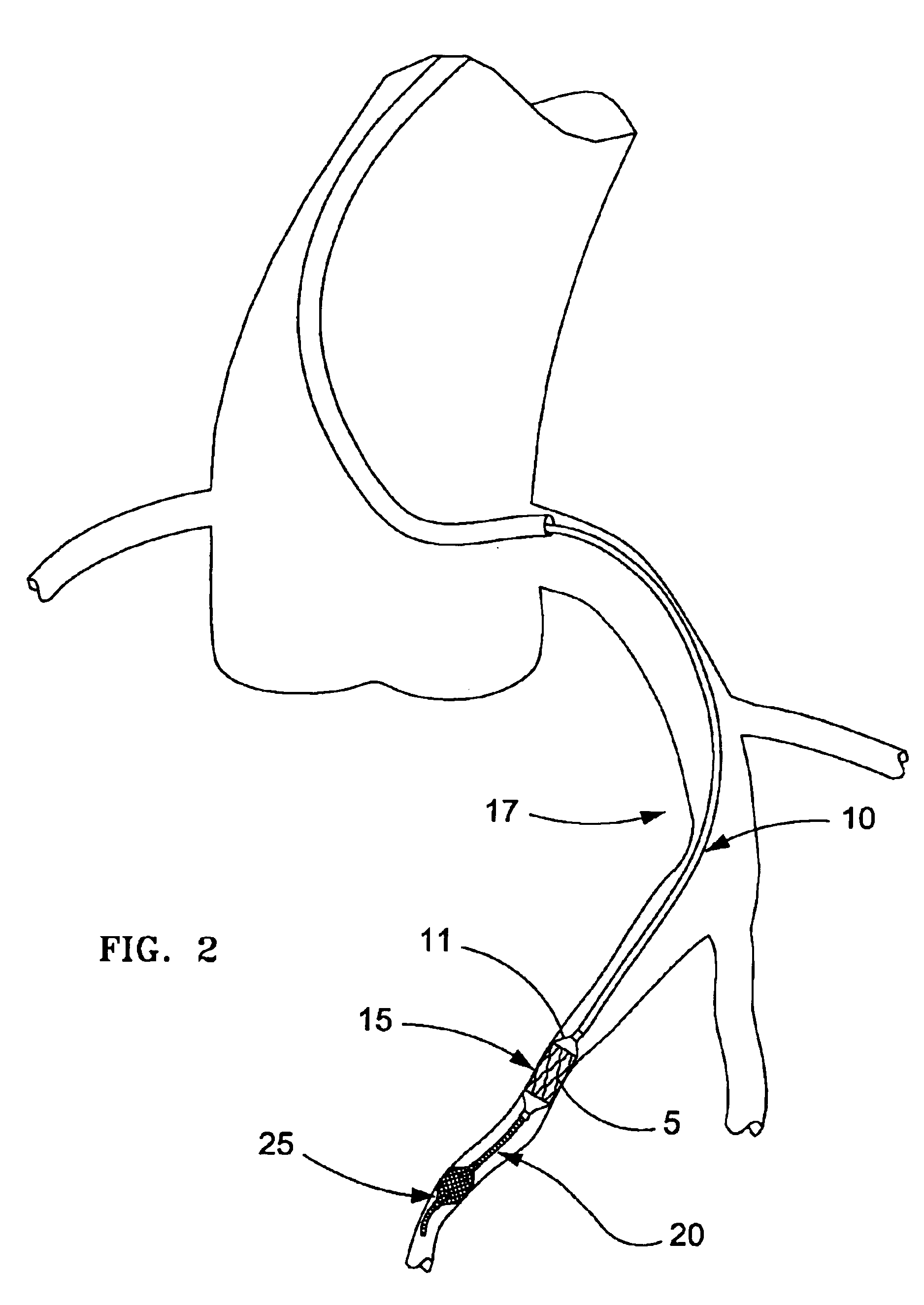
Apparatus and method for cryogenic inhibition of hyperplasia
InactiveUS6355029B1Enhanced inhibitory effectEasy to controlCatheterSurgical instruments for coolingPercutaneous angioplastyBalloon catheter
Post-angioplasty hyperplasia in blood vessels is treated using a cryosurgical balloon catheter. The balloon catheter is positioned at a target region within the blood vessel, and the balloon inflated by expanding a cryogenic fluid, such as liquid nitrogen, across an expansion orifice into a balloon. The balloon will be constructed so that cooling is achieved primarily in the central regions of the balloon, with the proximal and distal regions being less cold and acting to insulate adjacent regions of the blood vessel from excessive cooling.
Owner:BOSTON SCI SCIMED INC
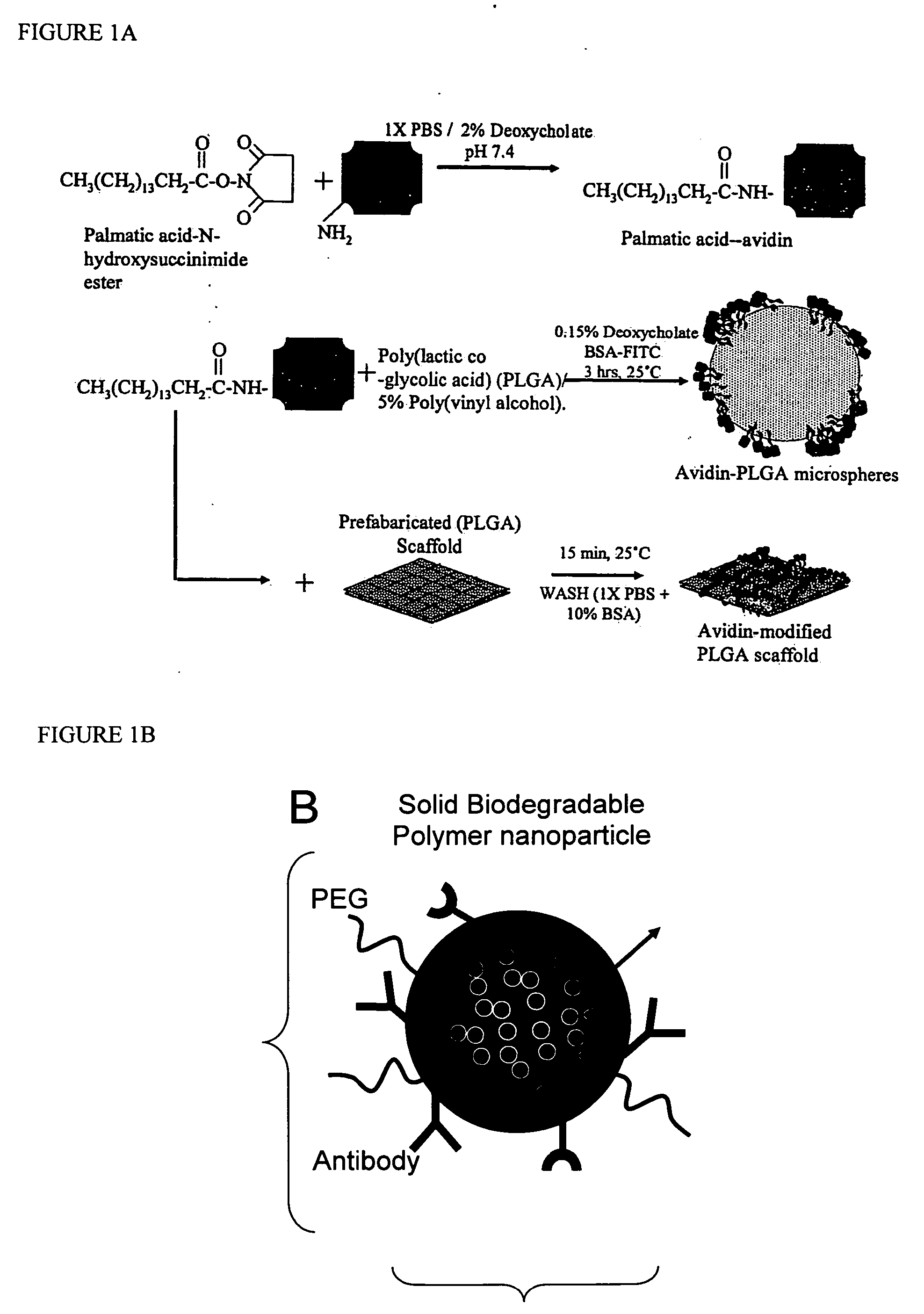
Targeted and high density drug loaded polymeric materials
ActiveUS20060002852A1Increase molecular densityHigh densityPowder deliveryBiocideAntigenWound dressing
Polymeric delivery devices have been developed which combine high loading / high density of molecules to be delivered with the option of targeting. As used herein, “high density” refers to microparticles having a high density of ligands or coupling agents, which is in the range of 1000-10,000,000, more preferably between 10,000 and 1,000,000 ligands per square micron of microparticle surface area. A general method for incorporating molecules into the surface of biocompatible polymers using materials with an HLB of less than 10, more preferably less than 5, such as fatty acids, has been developed. Because of its ease, generality and flexibility, this method has widespread utility in modifying the surface of polymeric materials for applications in drug delivery and tissue engineering, as well other other fields. Targeted polymeric microparticles have also been developed which encapsulate therapeutic compounds such as drugs, cellular materials or components, and antigens, and have targeting ligands directly bound to the microparticle surface. Preferred applications include use in tissue engineering matrices, wound dressings, bone repair or regeneration materials, and other applications where the microparticles are retained at the site of application or implantation. Another preferred application is in the use of microparticles to deliver anti-proliferative agents to the lining of blood vessels following angioplasty, transplantation or bypass surgery to prevent or decrease restenosis, and in cancer therapy. In still another application, the microparticles are used to treat or prevent macular degeneration when administered to the eye, where agents such as complement inhibitors are administered.
Owner:YALE UNIV
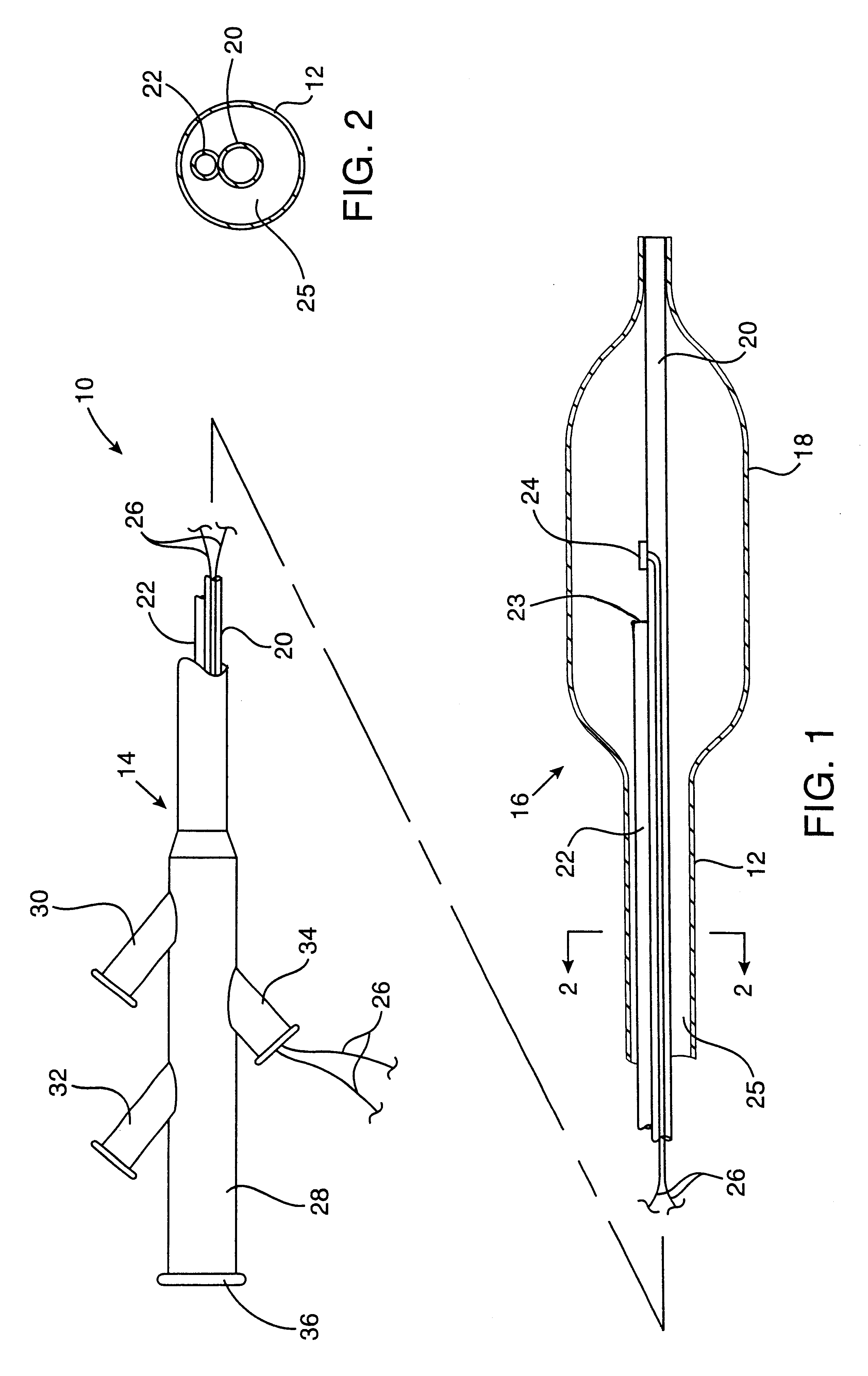
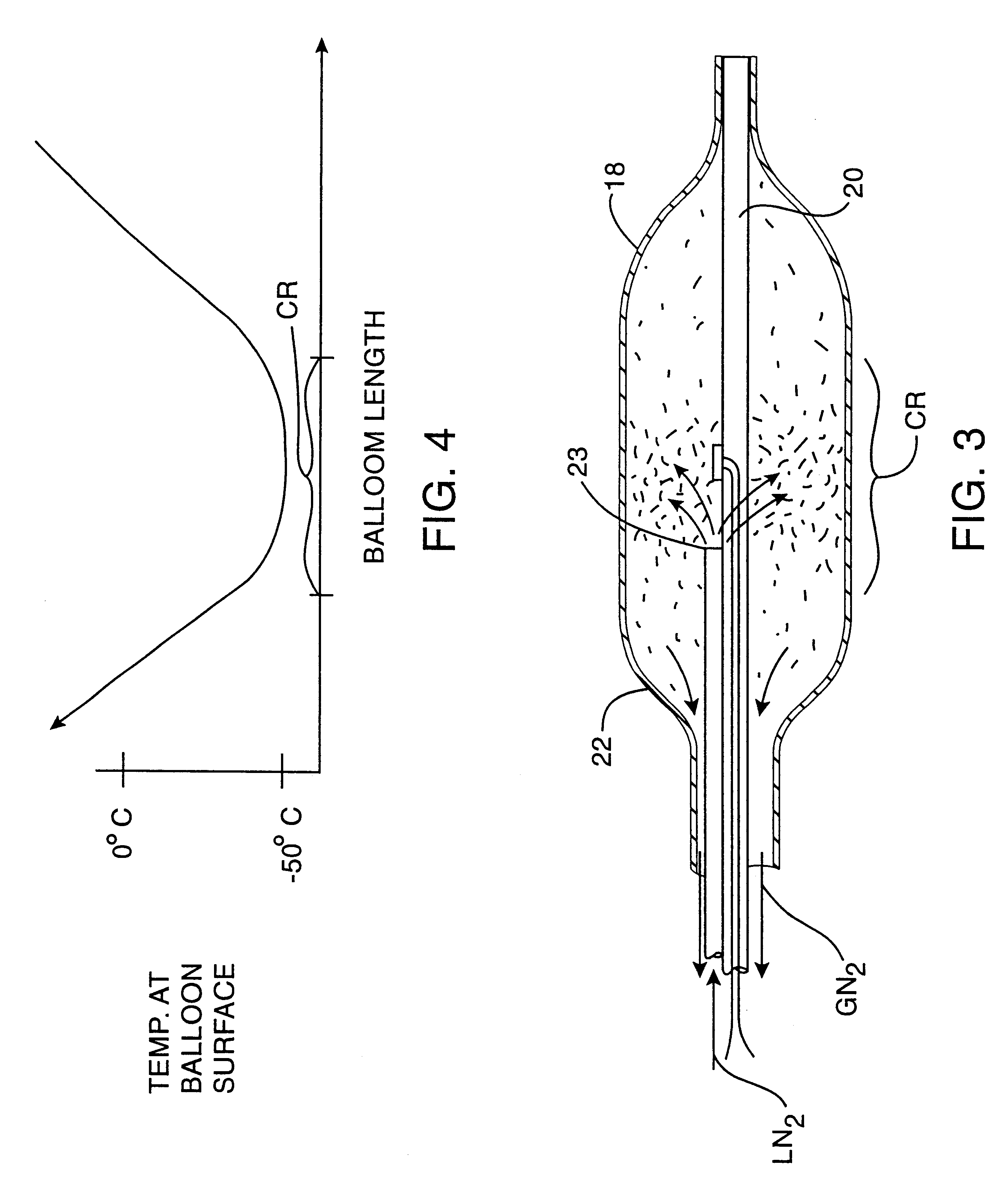
Cryogenically enhanced intravascular interventions
InactiveUS6468297B1Less stenosisLowering indexCatheterSurgical instruments for coolingPercent Diameter StenosisPercutaneous angioplasty
Techniques and devices for treating atherosclerotic disease use controlled cryogenic cooling, often in combination with angioplasty and / or stenting. A combination cryogenic / angioplasty catheter may cool the diseased blood vessel before, during, and / or after dilation. Controlled cooling of the vessel wall reduces actual / observed hyperplasia as compared to conventional uncooled angioplasty. Similar reductions in restenosis may be provided for other primary treatments of the blood vessel, including directional arthrectomy, rotational arthrectomy, laser angioplasty, stenting, and the like. Cooling of vessel wall tissues will often be performed through plaque, and the cooling process will preferably take the thermodynamic effects of the plaque into account.
Owner:BOSTON SCI SCIMED INC
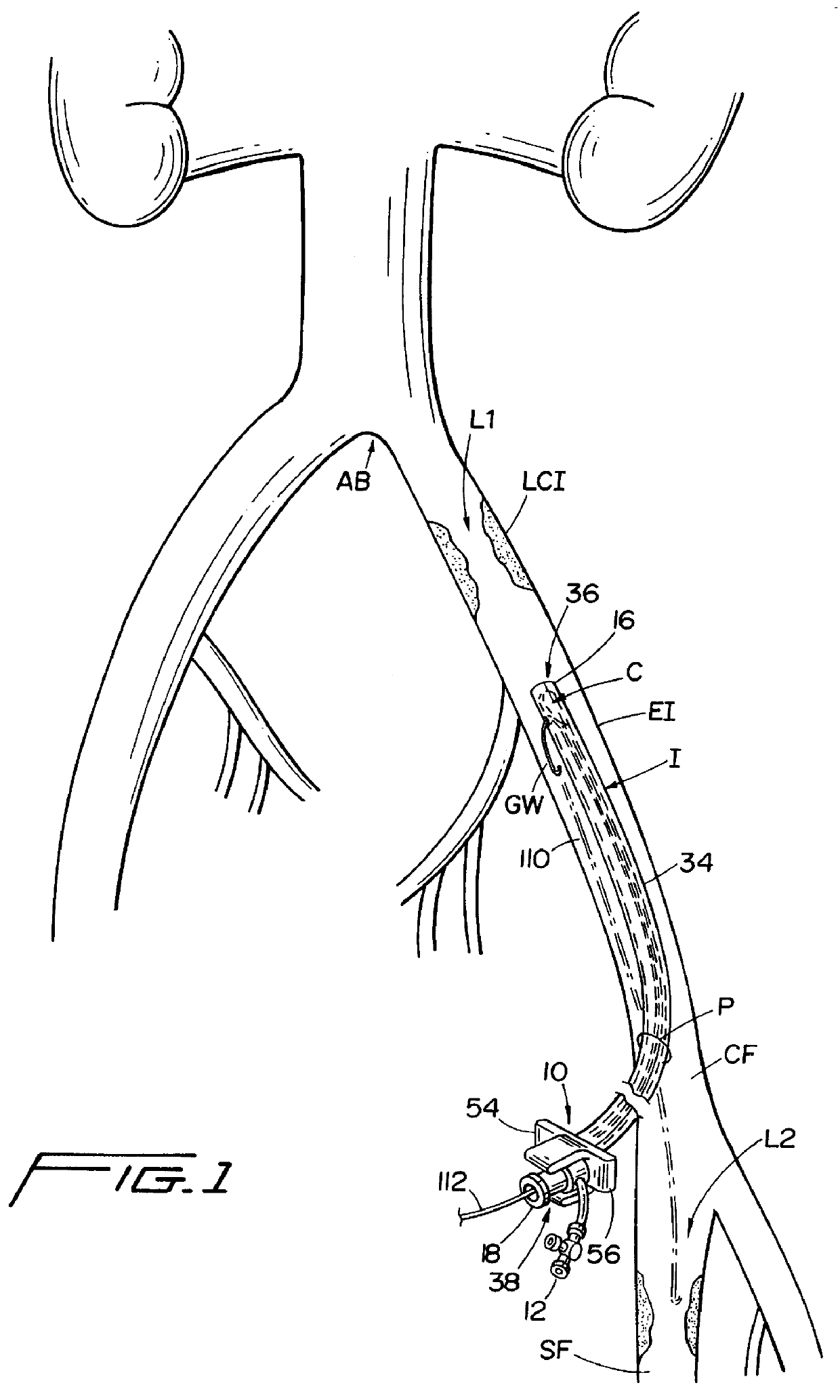
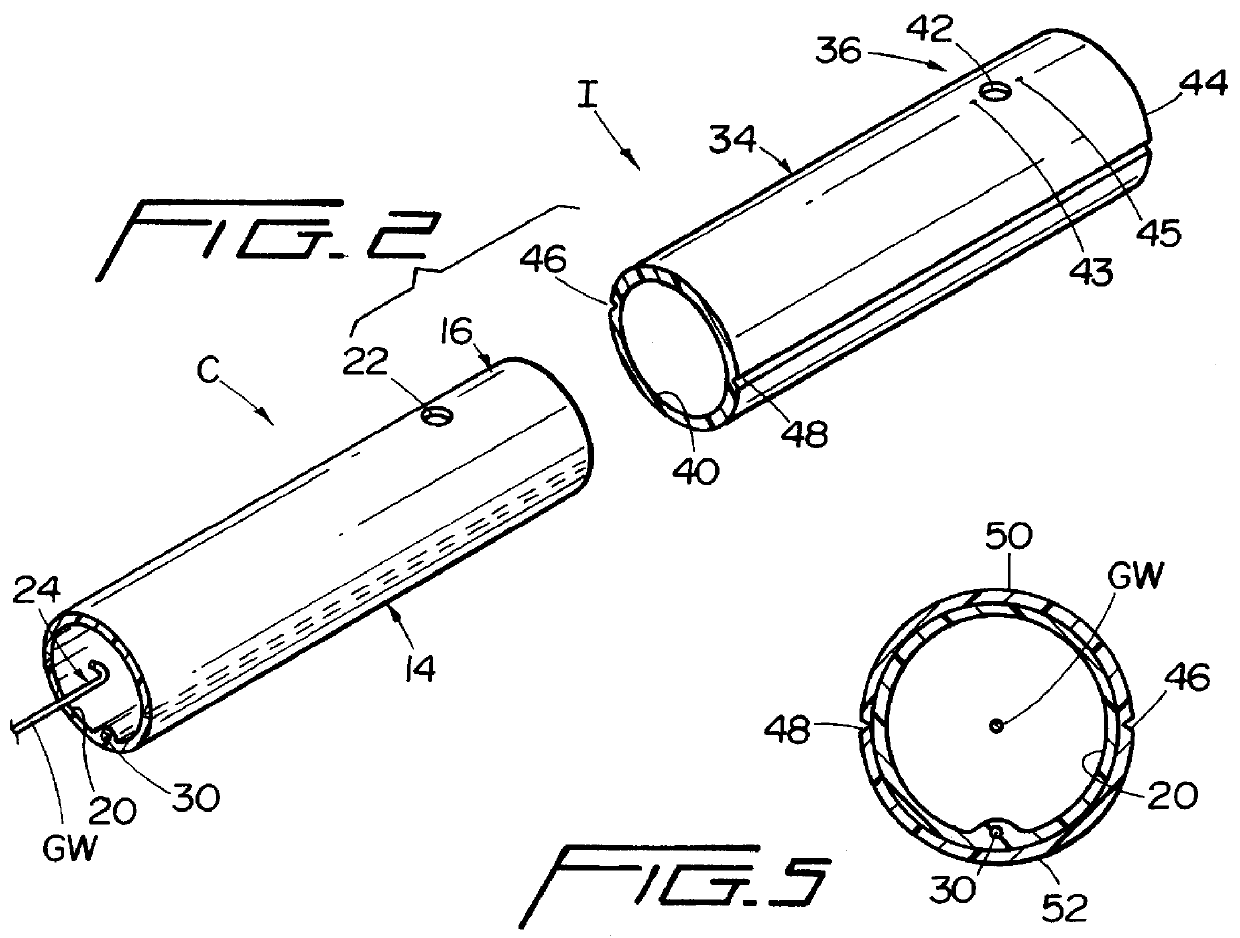
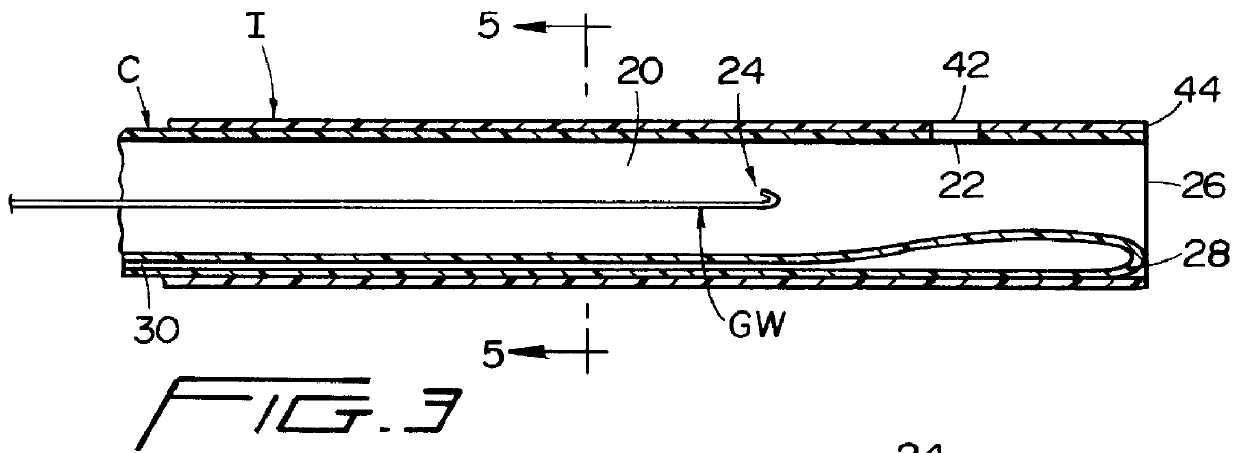
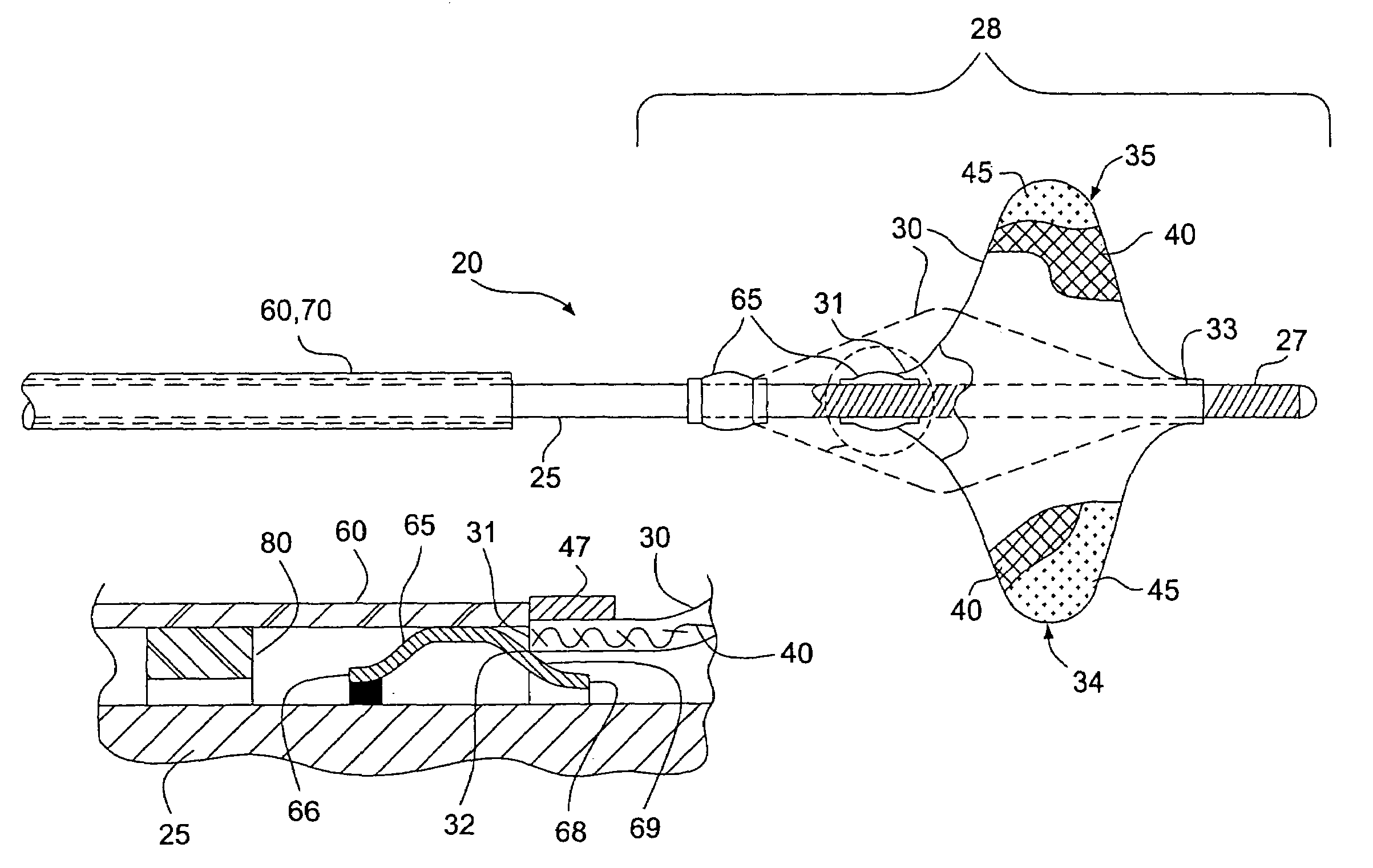
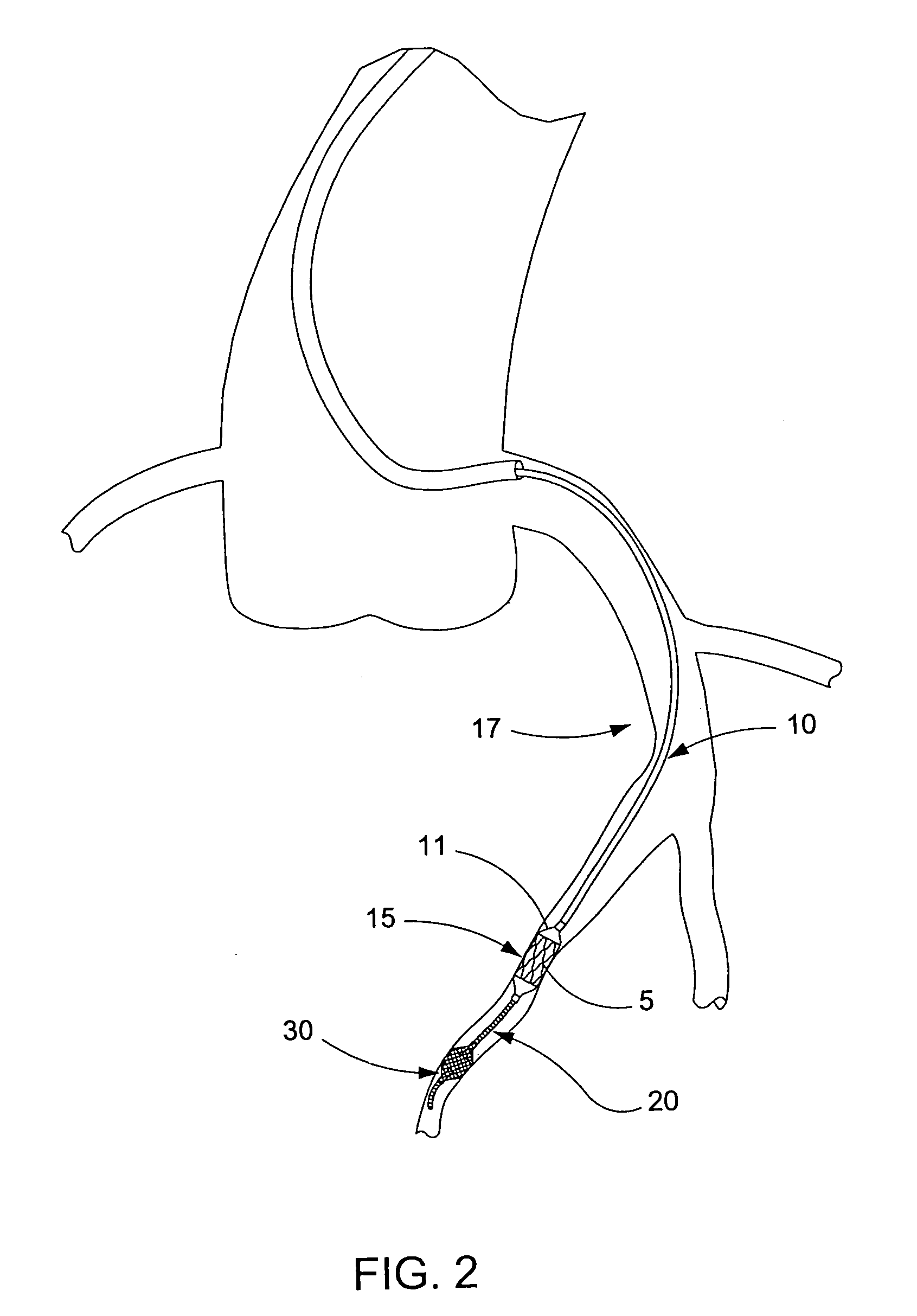
Guidewire apparatus for temporary distal embolic protection
A guidewire apparatus for use during percutaneous catheter interventions, such as angioplasty or stent deployment. A protection element comprising a filter or an occluder is mounted near the distal end of a steerable guidewire, which guides a therapeutic catheter. The guidewire apparatus comprises a hollow shaft movably disposed about a core wire and, optionally, a slippery liner interfitted there between. The shaft and core wire control relative displacement of the ends of the protection element, causing transformation of the protection element between a deployed configuration and a collapsed configuration.
Owner:MEDTRONIC VASCULAR INC
Stiffened balloon catheter for dilatation and stenting
The present invention pertains to a stiffened balloon which can be used in angioplasty, endovascular, and valvuloplasty procedures or as a delivery balloon to deliver a stent or a stent-graft. Longitudinally discontinuous stiffening members connected to the expandable balloon stiffen the balloon but allow it to be navigated through curved passages. Projections on the stiffening members may engage, incise, crush, fracture, or pierce occlusions or retain a stent or stent-graft. Radio-opaque portions may be referenced to determine orientation. Longitudinally continuous stiffening members bearing projections and connected to the balloon perform in a similar manner.
Owner:GRAYZEL JEFFREY +1
Guide wire control catheters for crossing occlusions and related methods of use
InactiveUS20040102719A1Simplify the viewing processStentsBalloon catheterPercutaneous angioplastyThree vessels
A wire control catheter for aligning and guiding a guide wire through a lesion in a vessel is provided. The wire control catheter includes a shaft having a guide wire lumen and a control wire lumen. A control wire passes through the control wire lumen and is used in combination with an articulation structure to deflect or curve a distal tip portion of the catheter. The distal catheter shaft may include a centering device for centering the catheter within the vessel. The distal catheter shaft also may include a pre-dilation balloon for dilating the lesion prior to performing angioplasty or other treatment on the lesion. Additionally, a sliding sheath catheter may be used to provide additional support to the guide wire. The sliding sheath catheter is sized to fit within the guide wire lumen of the control catheter and to allow the guide wire to pass through it. A method of treatment of a blood vessel includes inserting a guide wire into the blood vessel and advancing a control catheter over the guide wire until the distal tip of catheter is near the occlusion in the blood vessel. The tip of the catheter then is deflected via a control wire and an articulation structure. The guide wire is then advanced across the occlusion. The control catheter also may be advanced across the occlusion simultaneously with the guide wire or subsequent to the guide wire crossing. Prior to crossing the occlusion, the wire control catheter may be centered using a centering device. Subsequent to crossing the occlusion, the occlusion may be pre-dilated with a pre-dilation balloon of the wire control catheter.
Owner:ST JUDE MEDICAL CARDILOGY DIV INC
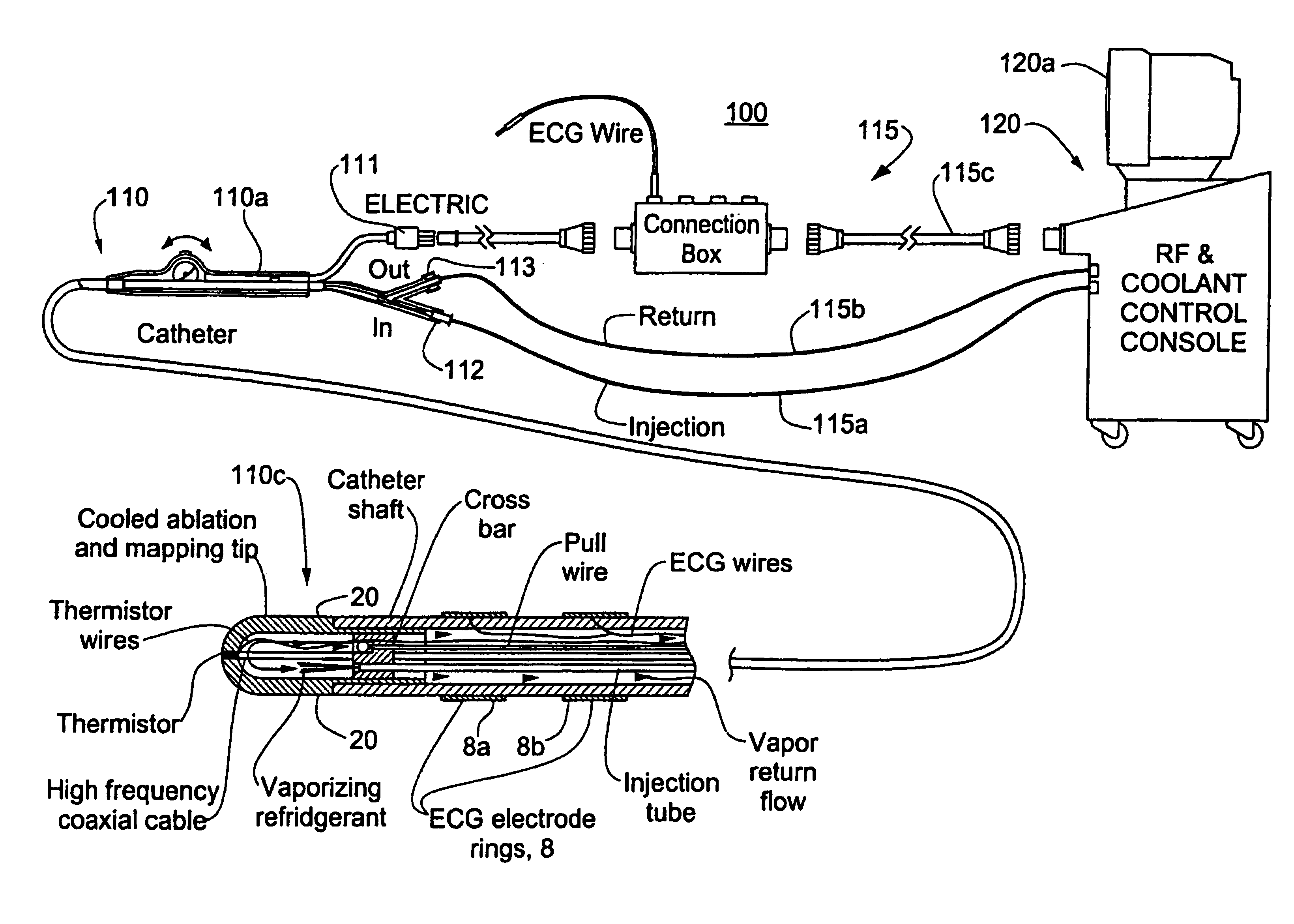
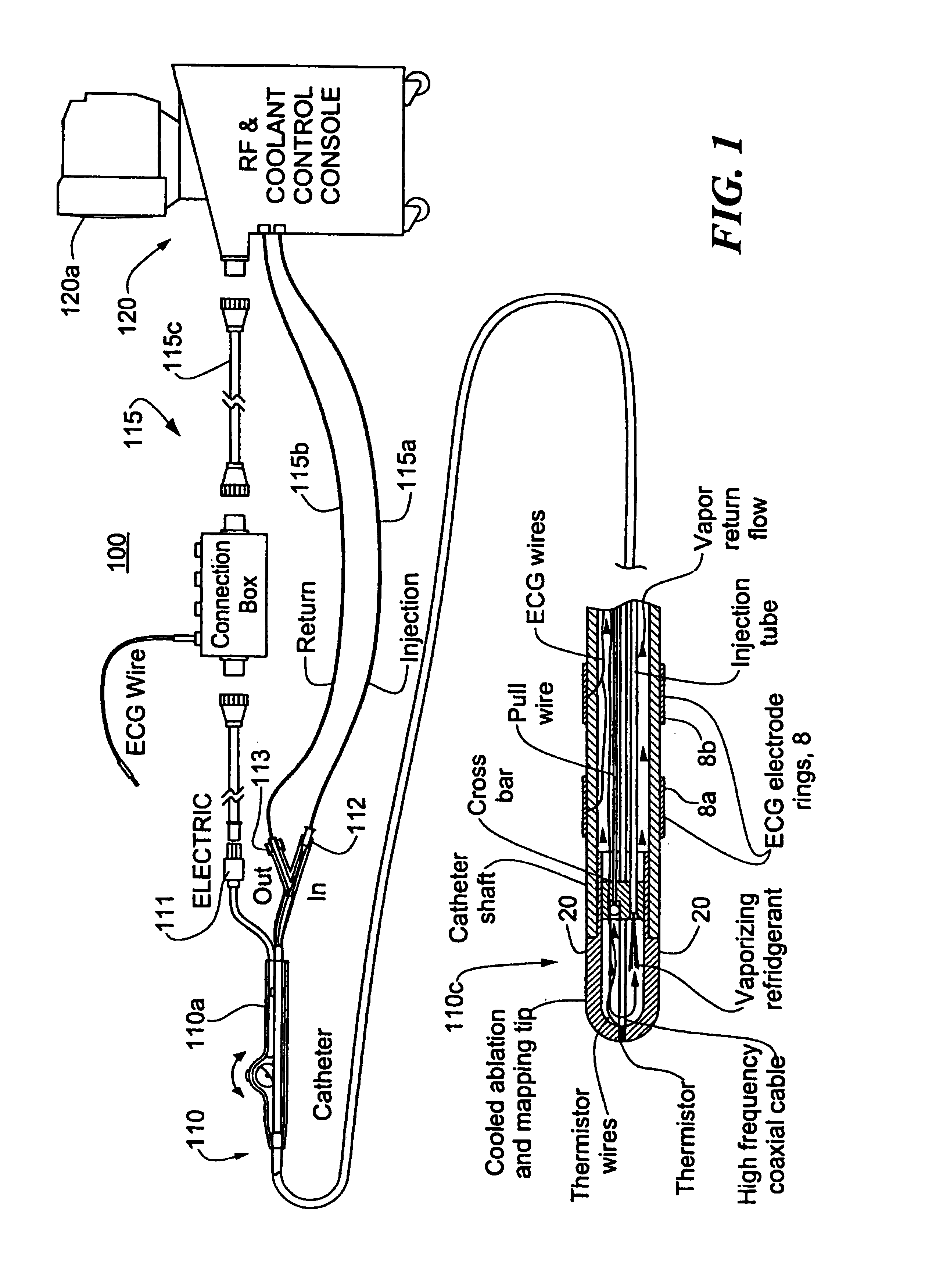
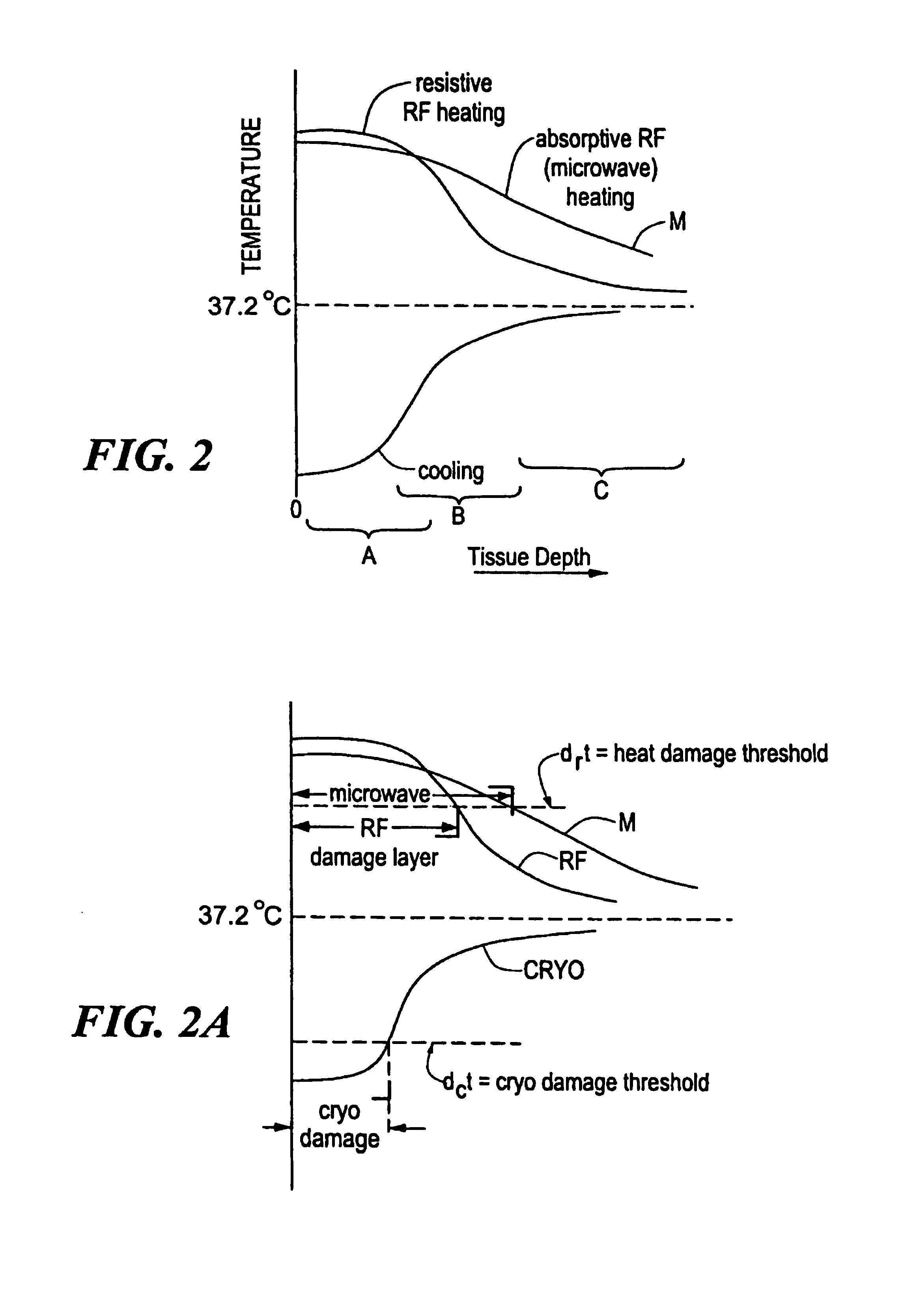
Catheter with cryogenic and heating ablation
InactiveUS7097641B1Improve versatilityEnhancing speed and placement lesionCatheterSurgical instruments for heatingTissue remodelingCelsius Degree
A catheter includes a cryoablation tip with an electrically-driven ablation assembly for heating tissue. The cryoablation tip may be implemented with a cooling chamber through which a controllably injected coolant circulates to lower the tip temperature, and having an RF electrode at its distal end. The RF electrode may be operated to warm cryogenically-cooled tissue, or the coolant may be controlled to conductively cool the tissue in coordination with an RF treatment regimen, allowing greater versatility of operation and enhancing the lesion size, speed or placement of multi-lesion treatment or single lesion re-treatment cycles. In one embodiment a microwave energy source operates at a frequency to extend beyond the thermal conduction depth, or to penetrate the cryogenic ice ball and be absorbed in tissue beyond an ice boundary, thus extending the depth and / or width of a single treatment locus. In another embodiment, the cooling and the application of RF energy are both controlled to position the ablation region away from the surface contacted by the electrode, for example to leave surface tissue unharmed while ablating at depth or to provide an ablation band of greater uniformity with increasing depth. The driver or RF energy source may supply microwave energy at a frequency effective to penetrate the ice ball which develops on a cryocatheter, and different frequencies may be selected for preferential absorption in a layer of defined thickness at depth in the nearby tissue. The catheter may operate between 70 and minus 70 degrees Celsius for different tissue applications, such as angioplasty, cardiac ablation and tissue remodeling, and may preset the temperature of the tip or adjacent tissue, and otherwise overlay or delay the two different profiles to tailor the shape or position where ablation occurs or to speed up a treatment cycle.
Owner:MEDTRONIC CRYOCATH LP
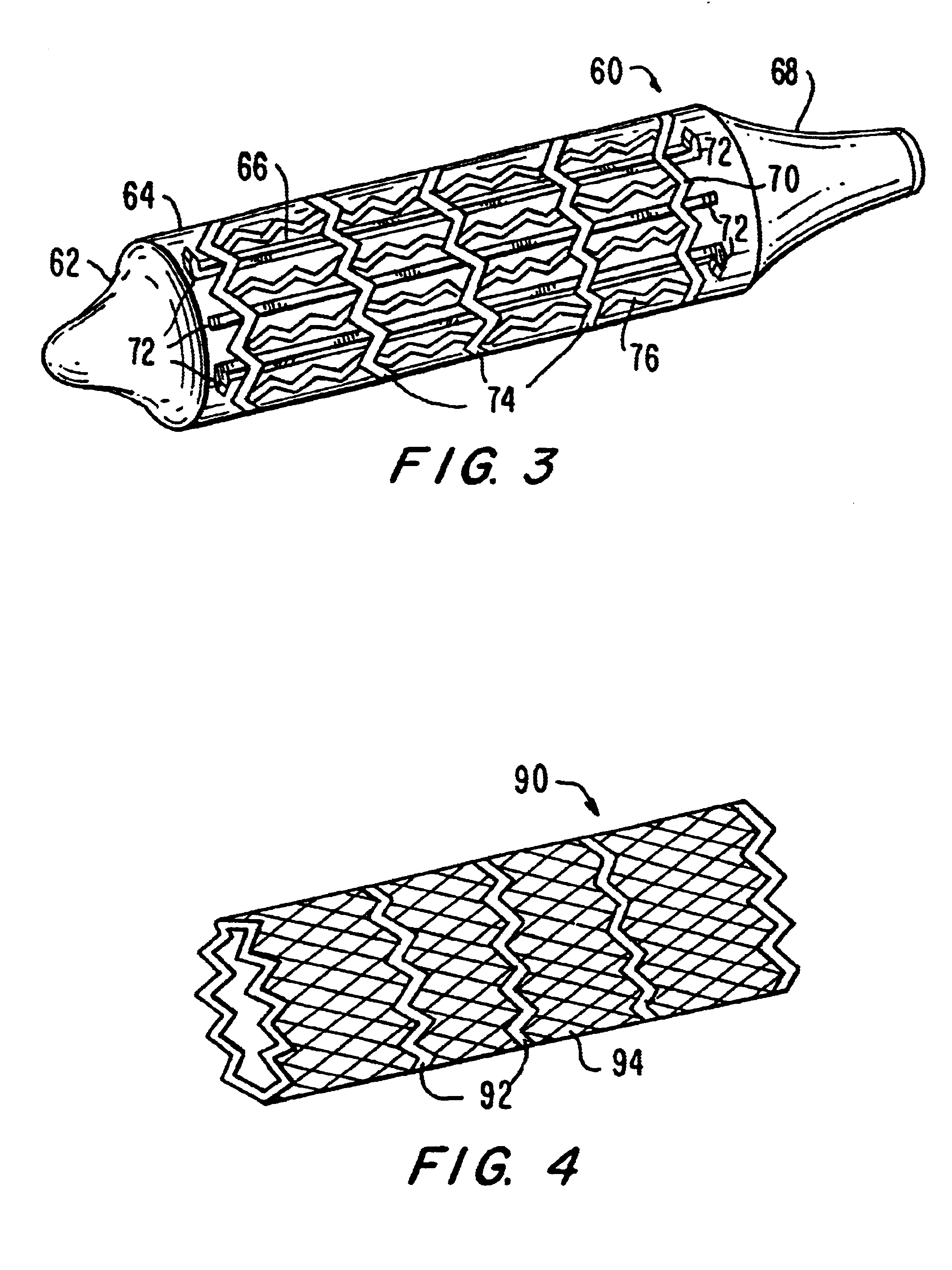
Angioplasty stent
A prosthesis for use in preventing restenosis after angioplasty is formed of plastic or sheet metal, and is expandable and contractable for placement. The prosthesis can be inserted while in a collapsed position, then expanded and locked at the larger diameter. Spring force can be provided by the material itself, or metal springs can be embedded within the walls of the prosthesis. Preferably, the walls have holes therethrough to promote tissue growth; and, in one embodiment, the holes are in the form of slots so that the prosthesis is segmented and can bend longitudinally.
Owner:W H WALL FAMILY HLDG LLLP
Temporary intraluminal filter guidewire and methods of use
InactiveUS6866677B2Restrict movementPrevent movementDilatorsCatheterRelative displacementEngineering
The present invention is a temporary intraluminal filter guidewire for use during interventional procedures, such as angioplasty or stent deployment. A braided filter is mounted near the distal end of a steerable guidewire, which guides a therapeutic catheter. An actuator rod slides over the guidewire and is removably connected to the filter. The rod controls relative displacement of the filter ends, causing transformation of the filter between a deployed configuration and a collapsed configuration. Wire having enhanced radiopacity is included in the filter to provide visualization under fluoroscopy.
Owner:MEDTRONIC AVE
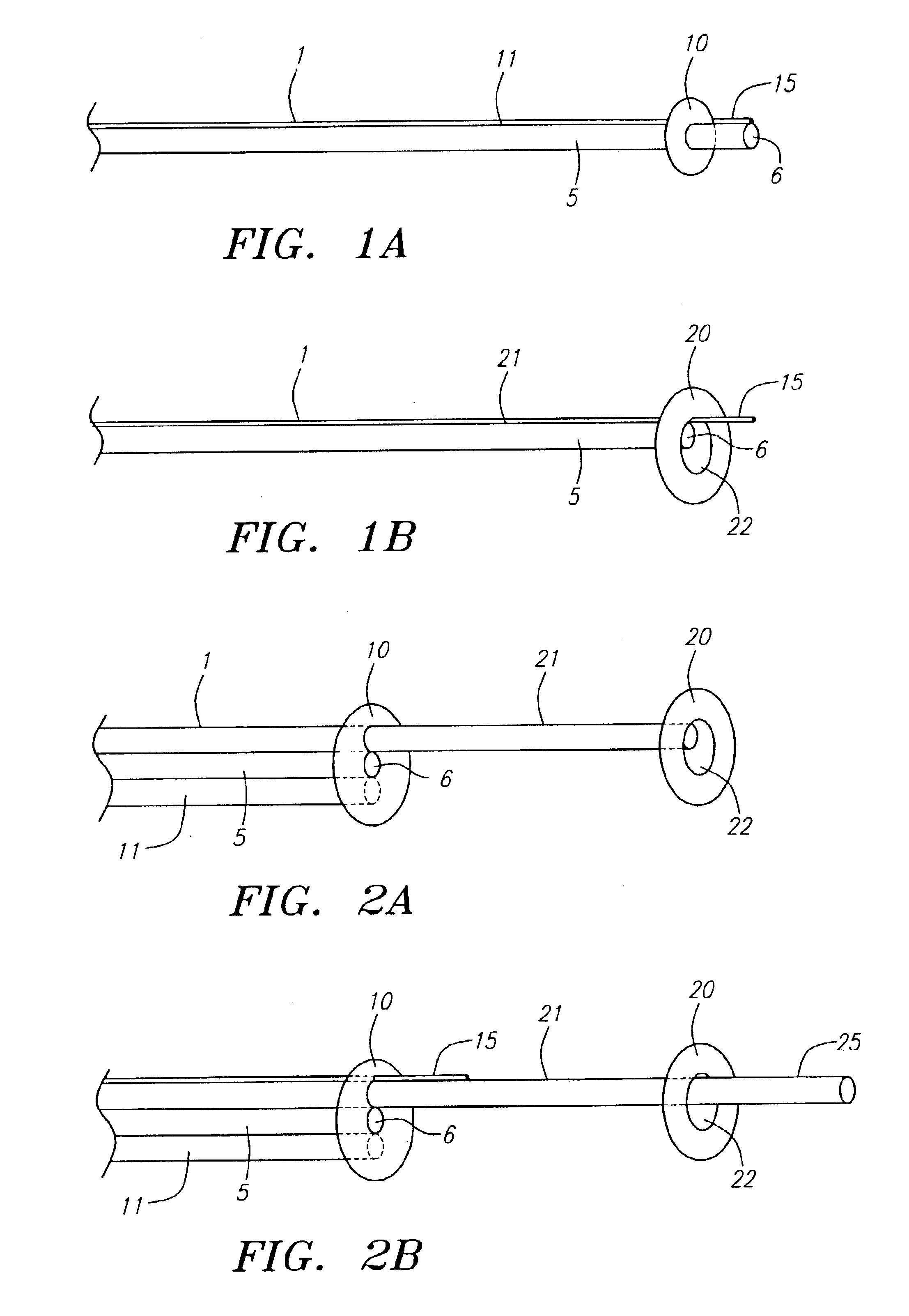
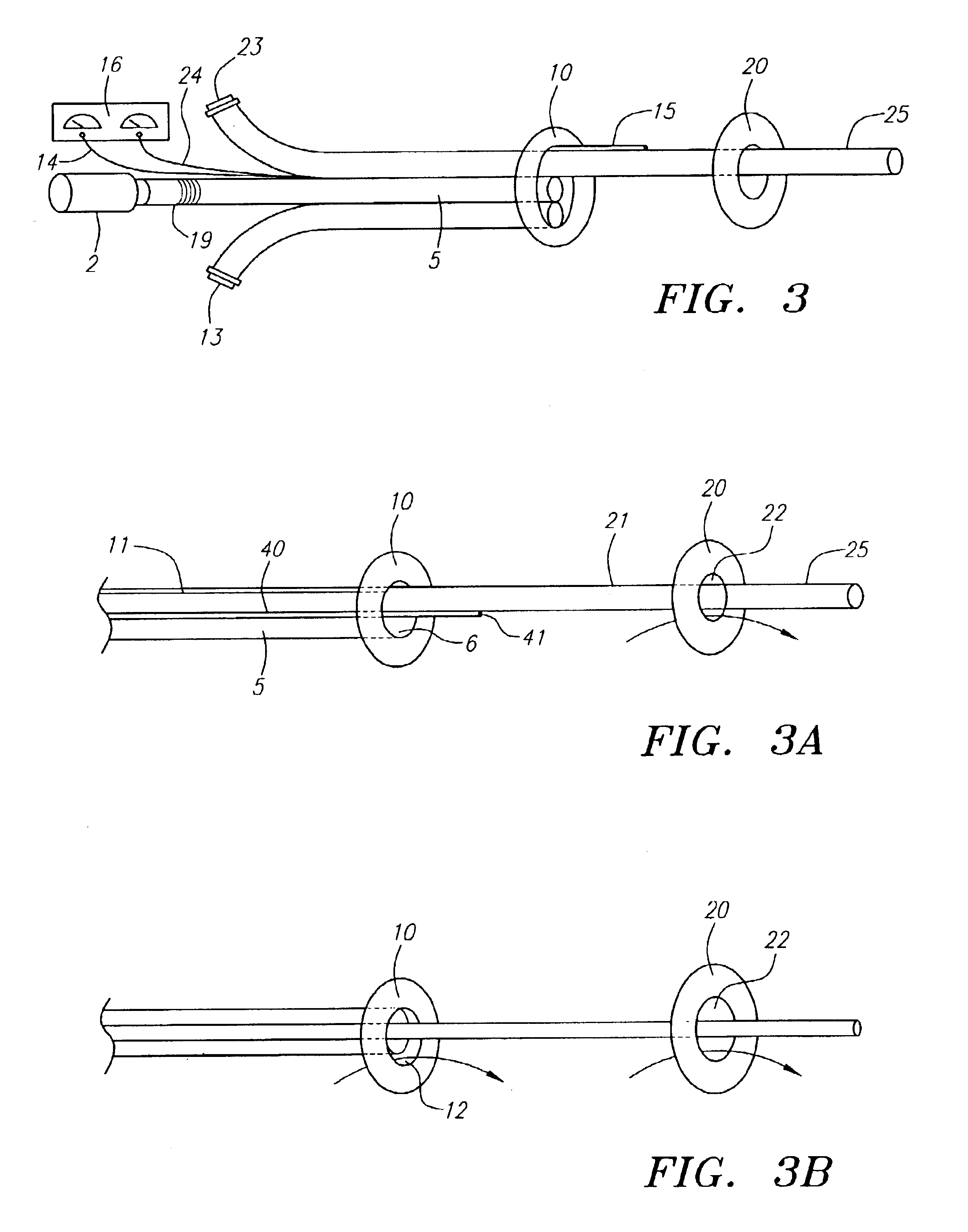
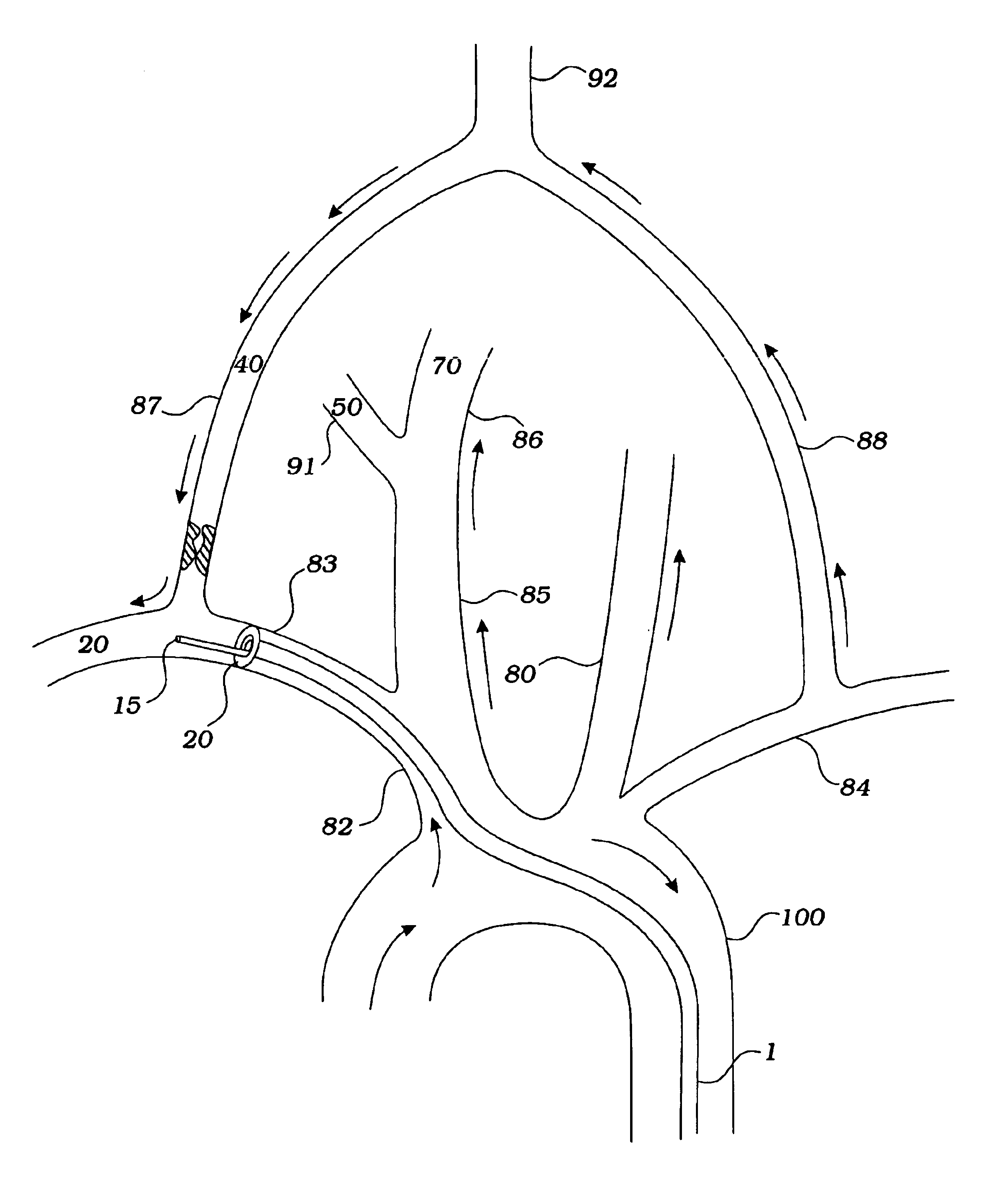
Method and device for performing cooling or cryo-therapies, for, e.g., angioplasty with reduced restenosis or pulmonary vein cell necrosis to inhibit atrial fibrillation employing tissue protection
InactiveUS7001378B2Minimize and inhibit bio-chemical eventRobust designCatheterSurgical instruments for heatingPercent Diameter StenosisPercutaneous angioplasty
An enhanced method and device are provided to treat atrial fibrillation or inhibit or reduce restenosis following angioplasty or stent placement. A balloon-tipped catheter is disposed in the area treated or opened through balloon angioplasty immediately following angioplasty. The balloon, which can have a dual balloon structure, may be delivered through a guiding catheter and over a guidewire already in place. A fluid such as a perfluorocarbon flows into the balloon to freeze the tissue adjacent the balloon, this cooling being associated with reduction of restenosis. A similar catheter may be used to reduce atrial fibrillation by inserting and inflating the balloon such that an exterior surface of the balloon contacts at least a partial circumference of the portion of the pulmonary vein adjacent the left atrium. In another embodiment, blood perfusion is performed simultaneously. In another embodiment, tissue contacted by the cryoablation catheter, undesired to be ablated, is protected against damage by a separate heating step.
Owner:ZOLL CIRCULATION
Apparatus and methods for reducing embolization during treatment of carotid artery disease
InactiveUS6908474B2Reduce riskAvoid developmentGuide needlesBalloon catheterPercutaneous angioplastyPressure difference
Methods and apparatus are provided for removing emboli during an angioplasty, stenting or surgical procedure comprising a catheter having an occlusion element, an aspiration lumen, and a blood outlet port in communication with the lumen, a guide wire having a balloon, a venous return sheath with a blood inlet port, and tubing that couples the blood outlet port to the blood inlet port. Apparatus is also provided for occluding the external carotid artery to prevent reversal of flow into the internal carotid artery. The pressure differential between the artery and the vein provides reverse flow through the artery, thereby flushing emboli. A blood filter may optionally be included in-line with the tubing to filter emboli from blood reperfused into the patient.
Owner:WL GORE & ASSOC INC
Cryotreatment device and method
InactiveUS7220257B1Reduce adverse reactionsReduced responseStentsOther blood circulation devicesCoronary artery angioplastyPercent Diameter Stenosis
Devices and methods for cooling vessel walls to inhibit restenosis in conjunction with medical procedures such as coronary artery angioplasty. Stenosed vessel walls can be cooled prior to angioplasty, after angioplasty, or both. The invention is believed to inhibit restenosis through cooling to a temperature near freezing, preferably without causing substantial vessel wall cell death. One catheter device includes a distal tube region having coolant delivery holes radially and longitudinally distributed along the distal region. In some devices, holes spray coolant directly onto the vessel walls, with the coolant absorbed into the blood stream. In other embodiments, a balloon or envelope is interposed between the coolant and the vessel walls and the coolant returned out of the catheter through a coolant return lumen. Some direct spray devices include an occlusion device to restrict blood flow past the region being cooled. Pressure, temperature, and ultrasonic probes are included in some cooling catheters. Pressure control valves are included in some devices to regulate balloon interior pressure within acceptable limits. In applications using liquid carbon dioxide as coolant, the balloon interior pressure can be maintained above the triple point of carbon dioxide to inhibit dry ice formation. Some cooling catheters are coiled perfusion catheters supporting longer cooling periods by allowing perfusing blood flow simultaneously with vessel wall cooling. One coiled catheter is biased to assume a coiled shape when unconstrained and can be introduced into the body in a relatively straight shape, having a stiffening wire inserted through the coil strands.
Owner:BOSTON SCI SCIMED INC
Apparatus and methods for reducing embolization during treatment of carotid artery disease
InactiveUS6905490B2Reduce riskAvoid developmentGuide needlesBalloon catheterPercutaneous angioplastyPressure difference
Methods and apparatus are provided for removing emboli during an angioplasty, stenting or surgical procedure comprising a catheter having an occlusion element, an aspiration lumen, and a blood outlet port in communication with the lumen, a guide wire having a balloon, a venous return catheter with a blood inlet port, and tubing that couples the blood outlet port to the blood inlet port. Apparatus is also provided for occluding the external carotid artery to prevent reversal of flow into the internal carotid artery. The pressure differential between the artery and the vein provides reverse flow through the artery, thereby flushing emboli. A blood filter may optionally be included in-line with the tubing to filter emboli from blood reperfused into the patient.
Owner:WL GORE & ASSOC INC
Method and device for performing cooling- or cryo-therapies for, e.g., angioplasty with reduced restenosis or pulmonary vein cell necrosis to inhibit atrial fibrillation employing tissue protection
InactiveUS6905494B2Minimize and inhibit biochemical eventRobust designCatheterSurgical instruments for heatingPercent Diameter StenosisPercutaneous angioplasty
An enhanced method and device are provided to treat atrial fibrillation or inhibit or reduce restenosis following angioplasty or stent placement. A balloon-tipped catheter is disposed in the area treated or opened through balloon angioplasty immediately following angioplasty. The balloon, which can have a dual balloon structure, may be delivered through a guiding catheter and over a guidewire already in place. A fluid such as a perfluorocarbon flows into the balloon to freeze the tissue adjacent the balloon, this cooling being associated with reduction of restenosis. A similar catheter may be used to reduce atrial fibrillation by inserting and inflating the balloon such that an exterior surface of the balloon contacts at least a partial circumference of the portion of the pulmonary vein adjacent the left atrium. In another embodiment, blood perfusion is performed simultaneously. In another embodiment, tissue contacted by the cryoablation catheter, undesired to be ablated, is protected against damage by a separate heating step.
Owner:ZOLL CIRCULATION
Inflatable medical devices
InactiveUS20090299327A1Prevent slippingStentsBalloon catheterPercutaneous aortic valve replacementHigh intensity
Inflatable medical devices and methods for making and using the same are disclosed. The inflatable medical devices can be medical balloons. The balloons can be configured to have a through-lumen or no through-lumen and a wide variety of geometries. The device can have a high-strength, non-compliant, fiber-reinforced, multi-layered wall. The inflatable medical device can be used for angioplasty, kyphoplasty, percutaneous aortic valve replacement, or other procedures described herein.
Owner:LOMA VISTA MEDICAL
Cryoplasty device and method
InactiveUS7022120B2Preventing or slowing the post-procedure reclosure of a dilated lesionPrevent and slow recoilDilatorsCatheterPercent Diameter StenosisPercutaneous angioplasty
A cryoplasty catheter and method for preventing or slowing reclosure of a lesion following angioplasty. The cryoplasty catheter includes a shaft having proximal and distal ends and a dilatation balloon disposed at the distal end. An intake lumen and exhaust lumen are defined by the shaft to deliver coolant to the balloon and to exhaust or drain coolant from the balloon. The method in accordance with the present invention includes cooling a lesion to aid in remodeling the lesion through dilatation and / or freezing a portion of the lesion adjacent the dilatation balloon to kill cells within the lesion to prevent or retard restenosis.
Owner:BOSTON SCI SCIMED INC
Irreversible electroporation device and method for attenuating neointimal
InactiveUS20090248012A1Promote resultsPrevent excessive cell lysingElectrotherapyInfusion devicesPercent Diameter StenosisTunica intima
Restenosis or neointimal formation may occur following angioplasty or other trauma to an artery such as by-pass surgery. This presents a major clinical problem which narrows the artery. The invention provides a device and a method whereby vascular cells in the area of the artery subjected to the trauma are subjected to irreversible electroporation which is a non-thermal, non-pharmaceutical method of applying electrical pulses to the cells so that substantially all of the cells in the area are ablated while leaving the structure of the vessel in place and substantially unharmed due to the non-thermal nature of the procedure.
Owner:RGT UNIV OF CALIFORNIA
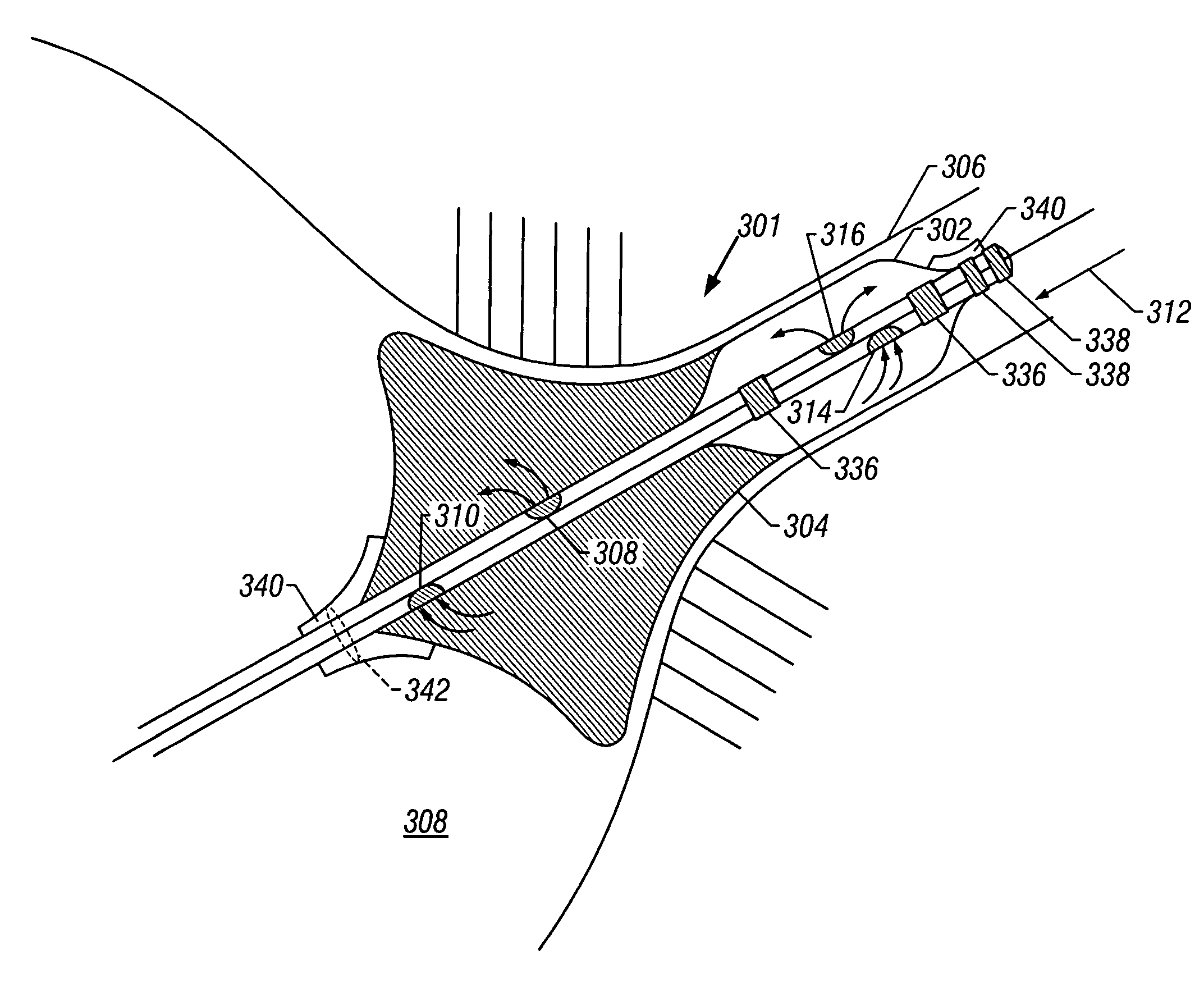
Devices and methods for preventing distal embolization using flow reversal by partial occlusion of the brachiocephalic artery
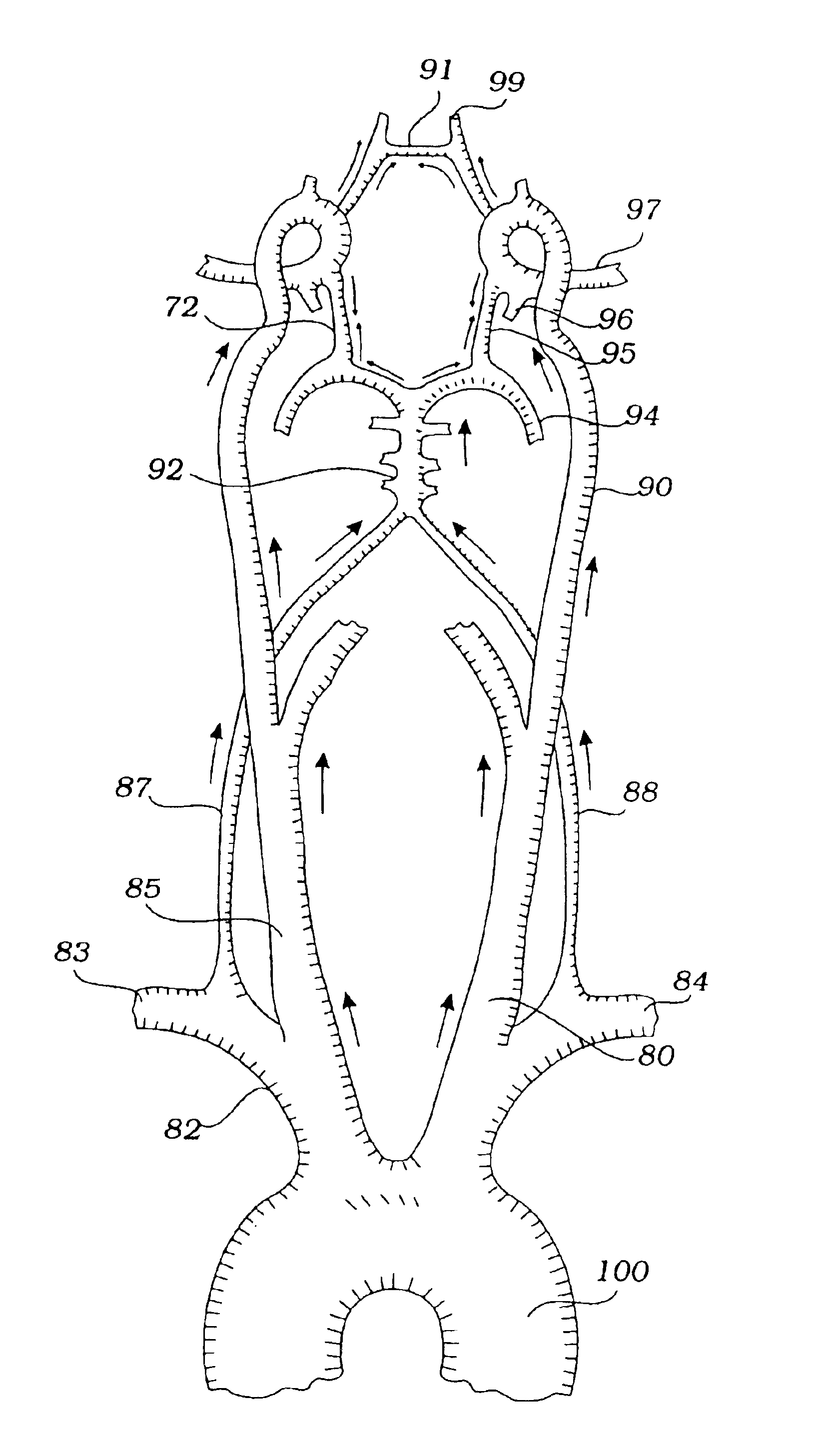
InactiveUS6837881B1Prevention of distal embolizationMinimizing blood lossStentsBalloon catheterAtherectomyPercutaneous angioplasty
The invention provides a medical device having a catheter and one or more expandable constricting / occluding members. The catheter is adapted for use with therapeutic or diagnostic devices, including an angioplasty / stent catheter and an atherectomy catheter. The constrictor / occluder is mounted at the distal end of the catheter. Manometers may be mounted distal to one or more constrictors for measuring pressure distal to the constrictor(s). Methods of using the devices for preventing distal embolization during extracranial or intracranial carotid procedures or vertebral artery procedures by reversing blood flow in an internal carotid artery, an external carotid artery, and / or a common carotid artery toward the subclavian artery are disclosed.
Owner:ZOLL CIRCULATION
Electromagnetic photonic catheter for reducing restenosis
InactiveUS6962584B1Reduce spreadImprove scalabilitySurgical instrument detailsCatheterPhotonicsPercent Diameter Stenosis
The method of vascular treatment for restenosis or vulnerable plaque after an invasive procedure, such as for example angioplasty, stenting with or without drug coating, or drug delivery, comprises: inserting a catheter or hollow guide wire to the treatment location; delivering light through the catheter in the wavelength range of about 700–2500 nm; and moving the light to treat the affected region.
Owner:STONE GREGG W +5
Vascular filter system
A removable vascular filter system for capture and retrieval of emboli while allowing continuous perfusion of blood, comprising a porous filter membrane and a filter membrane support structure. This system is useful for any percutaneous angioplasty, stenting, thrombolysis or tissue ablation procedure. The system may minimize the incidence of stroke, myocardial infarction or other clinical complications that may be associated with these procedures.
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH
Catheter introducer for antegrade and retrograde medical procedures
InactiveUS6022342AAvoid delayEasy to implementGuide wiresSurgeryPercutaneous angioplastyCatheter introducer
A catheter introducer for antegrade and retrograde medical procedures includes a generally tubular introducer member having proximal and distal ends. The introducer member includes a lumen for receiving a guidewire during a medical procedure, which guidewire includes a distal end traveling in a first direction. The catheter introducer further includes an occlusion member adjacent the distal end thereof for altering the traveling direction of the distal end of the guidewire to a second direction. The catheter introducer of the invention can be easily used for performing angioplasty on tandem or segmental lesions in one procedure using antegrade and retrograde approaches. The novel technique of the present invention involves performing angioplasty on one lesion using one of the antegrade and retrograde approaches, and then using the other of the antegrade and retrograde approaches to perform angioplasty on the second lesion. Both lesions are attended to during one medical procedure.
Owner:MUKHERJEE DIPANKAR
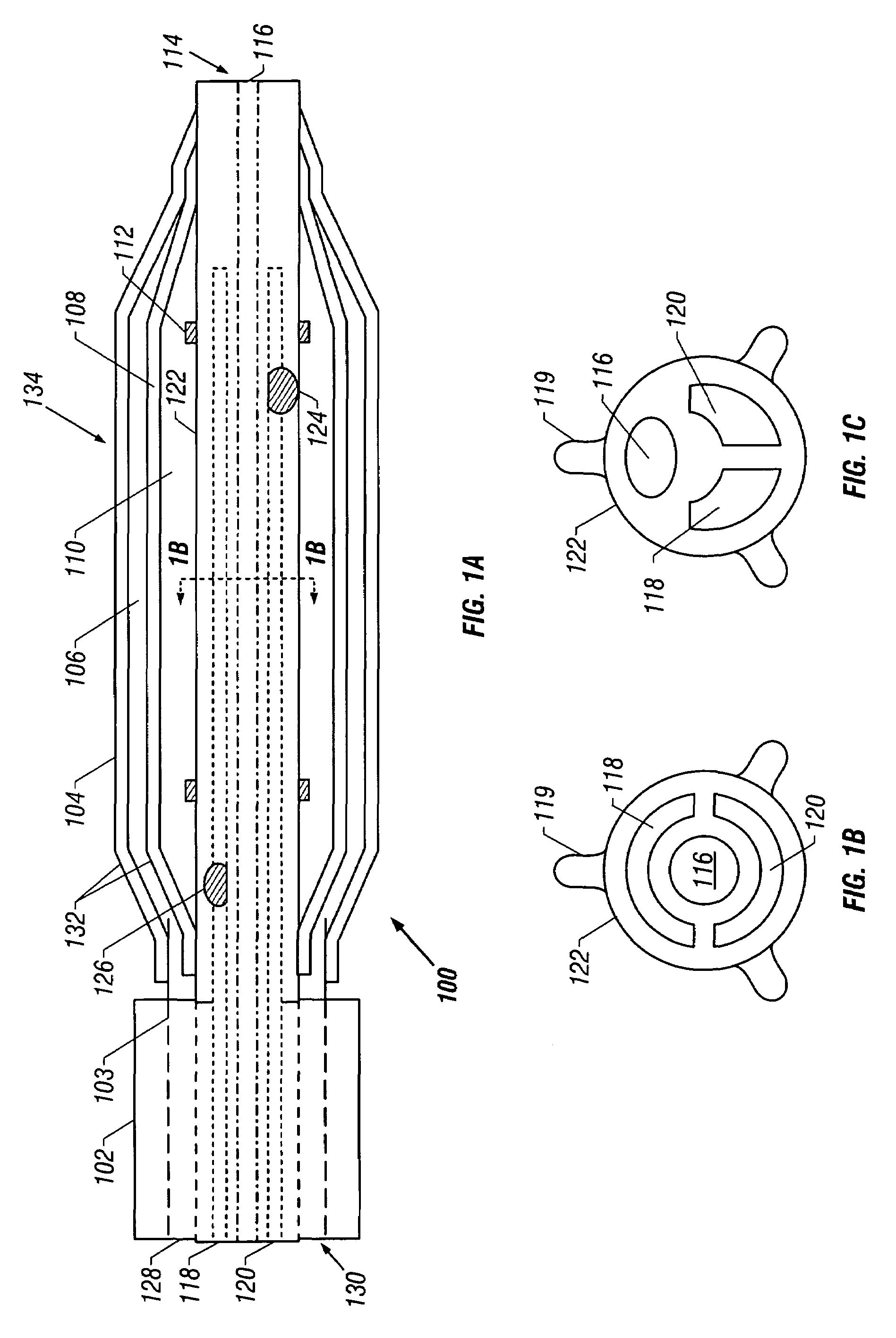
Temporary device for capturing embolic material
InactiveUS7044958B2Improve radiopacityUndiminished physical performanceSurgeryDilatorsRelative displacementPercutaneous angioplasty
The present invention is a temporary device for capturing and removing embolic material during interventional procedures, such as angioplasty or stent deployment. A self-closing, tubular capture element, which may be either a filter or an occluder, is mounted near the distal end of a guidewire. The guidewire is capable of guiding a therapeutic catheter. Relative displacement of the capture element ends causes transformation of the capture element between a closed configuration and a deployed configuration that spans the vessel to be treated. A latch fixed to the guidewire temporarily retains the capture element in the deployed configuration. A first hollow rod slides over the guidewire to deploy the capture element and engage the capture element proximal end with the latch. A second hollow rod slides over the guidewire to release the latch and close the capture element.
Owner:MEDTRONIC VASCULAR INC
Method and device for performing cooling- or cryo-therapies for, e.g., angioplasty with reduced restenosis or pulmonary vein cell necrosis to inhibit atrial fibrillation employing microporous balloon
InactiveUS7449018B2Inhibit and reduce rateMinimize and inhibit biochemical eventCatheterDiagnostic recording/measuringAtrial cavityWorking fluid
An enhanced method and device are provided to inhibit or reduce restenosis following angioplasty or stent placement. A porous balloon-tipped catheter is disposed in the area treated or opened through balloon angioplasty immediately following angioplasty. The balloon, which can have a dual balloon structure, may be delivered through a guiding catheter and over a guidewire already in place. A fluid such as a perfluorocarbon flows into the balloon to freeze the tissue adjacent the balloon, this cooling being associated with reduction of restenosis. A similar catheter may be used to reduce atrial fibrillation by inserting and inflating the porous balloon such that an exterior surface of the balloon, as well as a portion of the cold working fluid, from the microporosity contacts at least a partial circumference of the portion of the pulmonary vein adjacent the left atrium.
Owner:ZOLL CIRCULATION
System and methods for imaging within a body lumen
InactiveUS6947787B2Reduce traumaEfficient procedureUltrasonic/sonic/infrasonic diagnosticsSurgeryPercutaneous angioplastyOptical image
Systems, methods and apparatus for acquiring an image from within a body lumen are provided. Systems embodying features of the invention include an intracorporeal imaging component for acquiring imaging information, an image information recording component, and an image information playback component. Methods for acquiring image information from within a body lumen include steps of acquiring imaging information, storing image information, and playing back imaging information. Imaging information may be played back as acquired, or non-sequentially, or composite images may be formed by combining, filtering, enhancing, or subtracting images and the composite images displayed. Devices embodying features of the invention include optical imaging components such as an optical IGW, an optical imaging recording component, and an optical image playback component. The systems, methods and devices are suitable for use with angiopoplasty, stent delivery, and other intracorporeal clinical procedures.
Owner:ABBOTT CARDIOVASCULAR
Non-cavitation shockwave balloon catheter system
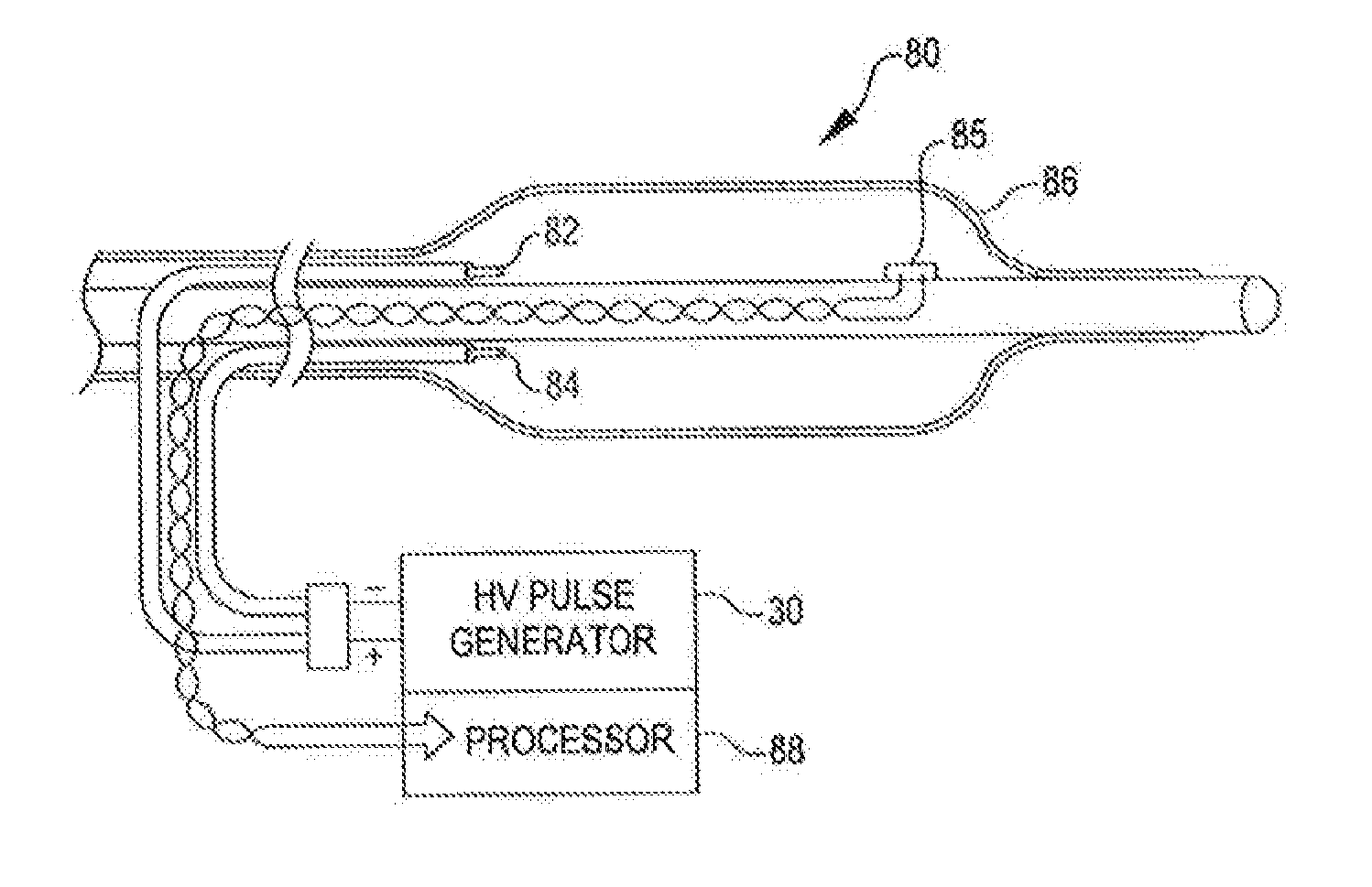
An angioplasty catheter includes an elongated carrier, and an angioplasty balloon about the carrier in sealed relation thereto. The balloon is arranged to receive a fluid therein that inflates the balloon. The catheter further includes a shock wave generator within the balloon that forms a rapidly expanding and collapsing bubble within the balloon to form mechanical shock waves within the balloon. The expanding bubble forms a first shock and the collapsing balloon forms a second shock wave. The shock wave generator is arranged such that the energy of the first shock wave is greater than the energy of the second shock wave.
Owner:SHOCKWAVE MEDICAL
Drug-eluting medical device
The present invention relates to a drug-eluting medical device, in particular a balloon for angioplasty catheters with drug elution to prevent the restenosis of the vessel subjected to angioplasty. More particularly, the present invention relates to a catheter balloon completely or partially coated with paclitaxel in hydrated crystalline form or in hydrated solvated crystalline form, having an immediate release and bioavailability of a therapeutically effective amount of paclitaxel at the site of intervention. The balloon can be made of a polyether-polyamide block copolymer, or a polyester amide, or polyamide-12.
Owner:INVATEC TECH CENT
Devices and methods for preventing distal embolization using flow reversal in arteries having collateral blood flow
InactiveUS6840949B2Prevention of distal embolizationReversed blood flowStentsBalloon catheterAtherectomyPercutaneous angioplasty
The invention provides a medical device having a catheter and one or more expandable constricting / occluding members. The catheter is adapted for use with therapeutic or diagnostic devices, including an angioplasty / stent catheter and an atherectomy catheter. The constrictor / occluder is mounted at the distal end of the catheter. Manometers may be mounted distal to one or more constrictors for measuring pressure distal to the constrictor(s). Methods of using the devices are disclosed for preventing distal embolization during extracranial or intracranial carotid artery, vertebral artery, or coronary artery procedures, or procedures involving any vessel having collateral flow by reversing flow in the diseased vessel.
Owner:ZOLL CIRCULATION
Devices and methods for preventing distal embolization from the vertebrobasilar artery using flow reversal
InactiveUS6887227B1Minimizing distal embolizationPreventing ischemic strokeStentsBalloon catheterAtherectomyPercutaneous angioplasty
The invention provides a medical device having a catheter and one or more expandable constricting / occluding members. The catheter has a lumen communicating with a port at its distal end. The lumen and port are adapted for introduction of therapeutic or diagnostic devices, including an angioplasty / stent catheter and an atherectomy catheter, into a vertebral or basilar artery. The constrictor / occluder is mounted proximal to the port of the catheter. Manometers may be mounted distal to one or more constrictors for measuring pressure distal to the constrictor(s). Methods of using the devices for preventing distal embolization during vertebral and / or basilar procedures by reversing blood flow in the vertebral artery toward the subclavian artery are disclosed.
Owner:ZOLL CIRCULATION
Cryotreatment device and method
InactiveUS20070250050A1Reduce adverse reactionsReduced responseStentsOther blood circulation devicesCoronary artery angioplastyPercent Diameter Stenosis
Devices and methods for cooling vessel walls to inhibit restenosis in conjunction with medical procedures such as coronary artery angioplasty. Stenosed vessel walls can be cooled prior to angioplasty, after angioplasty, or both. The invention is believed to inhibit restenosis through cooling to a temperature near freezing, preferably without causing substantial vessel wall cell death. One catheter device includes a distal tube region having coolant delivery holes radially and longitudinally distributed along the distal region. In some devices, holes spray coolant directly onto the vessel walls, with the coolant absorbed into the blood stream. In other embodiments, a balloon or envelope is interposed between the coolant and the vessel walls and the coolant returned out of the catheter through a coolant return lumen. Some direct spray devices include an occlusion device to restrict blood now past the region being cooled. Pressure, temperature, and ultrasonic probes are included in some cooling catheters. Pressure control valves are included in some devices to regulate balloon interior pressure within acceptable limits. In applications using liquid carbon dioxide as coolant, the balloon interior pressure can be maintained above the triple point of carbon dioxide to inhibit dry ice formation. Some cooling catheters are coiled perfusion catheters supporting longer cooling periods by allowing perfusing blood flow simultaneously with vessel wall cooling. One coiled catheter is biased to assume a coiled shape when unconstrained and can be introduced into the body in a relatively straight shape, having a stiffeninig wire inserted through the coil strands.
Owner:BOSTON SCI SCIMED INC
Method of providing embolic protection and shockwave angioplasty therapy to a vessel
A system comprises a guide wire, an embolic protection basket carried on the guide wire, and a catheter carried on the guide wire adjacent the embolic protection basket. The catheter includes an elongated carrier and a balloon about the carrier in sealed relation thereto. The balloon is arranged to receive a fluid therein that inflates the balloon, and an arc generator including at least one electrode within the balloon forms a mechanical shock wave within the balloon.
Owner:SHOCKWAVE MEDICAL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com