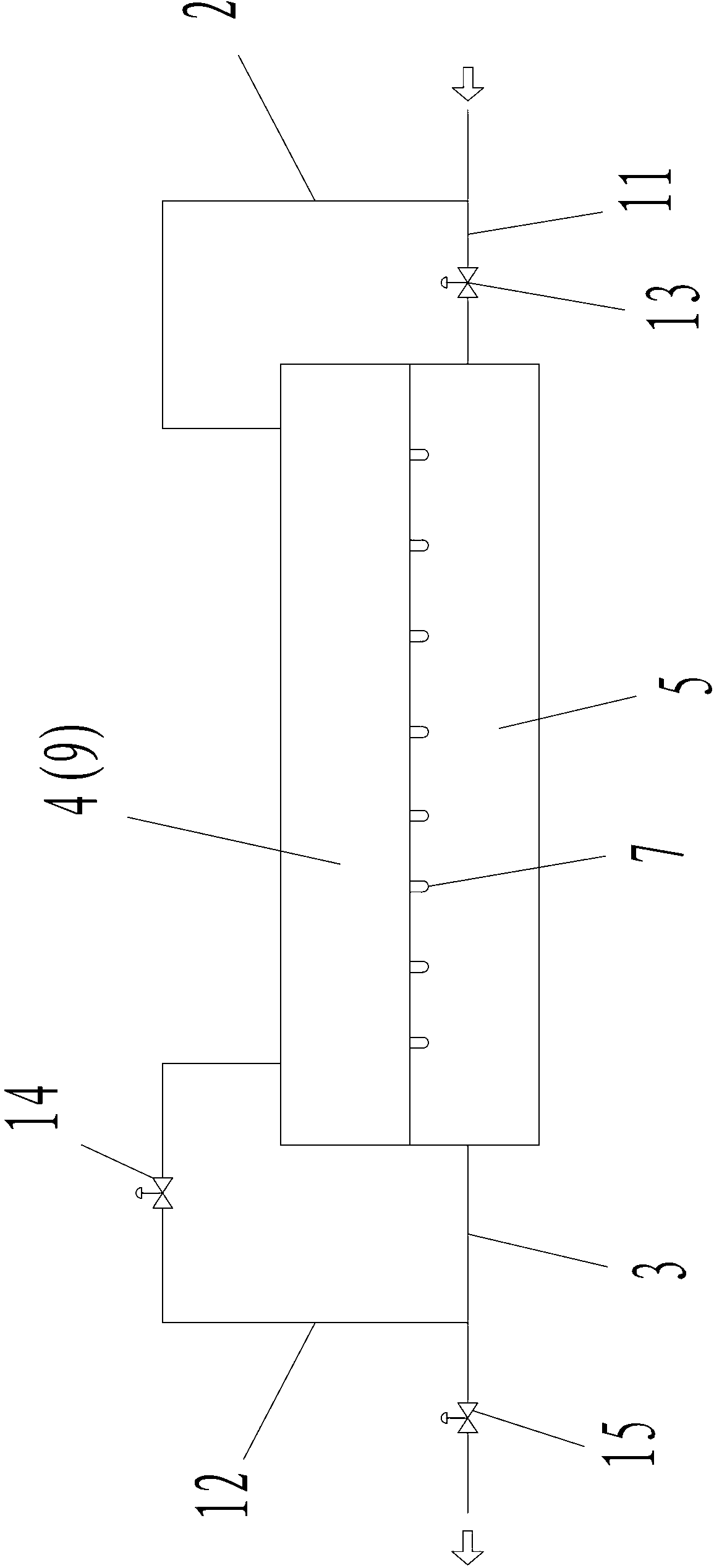
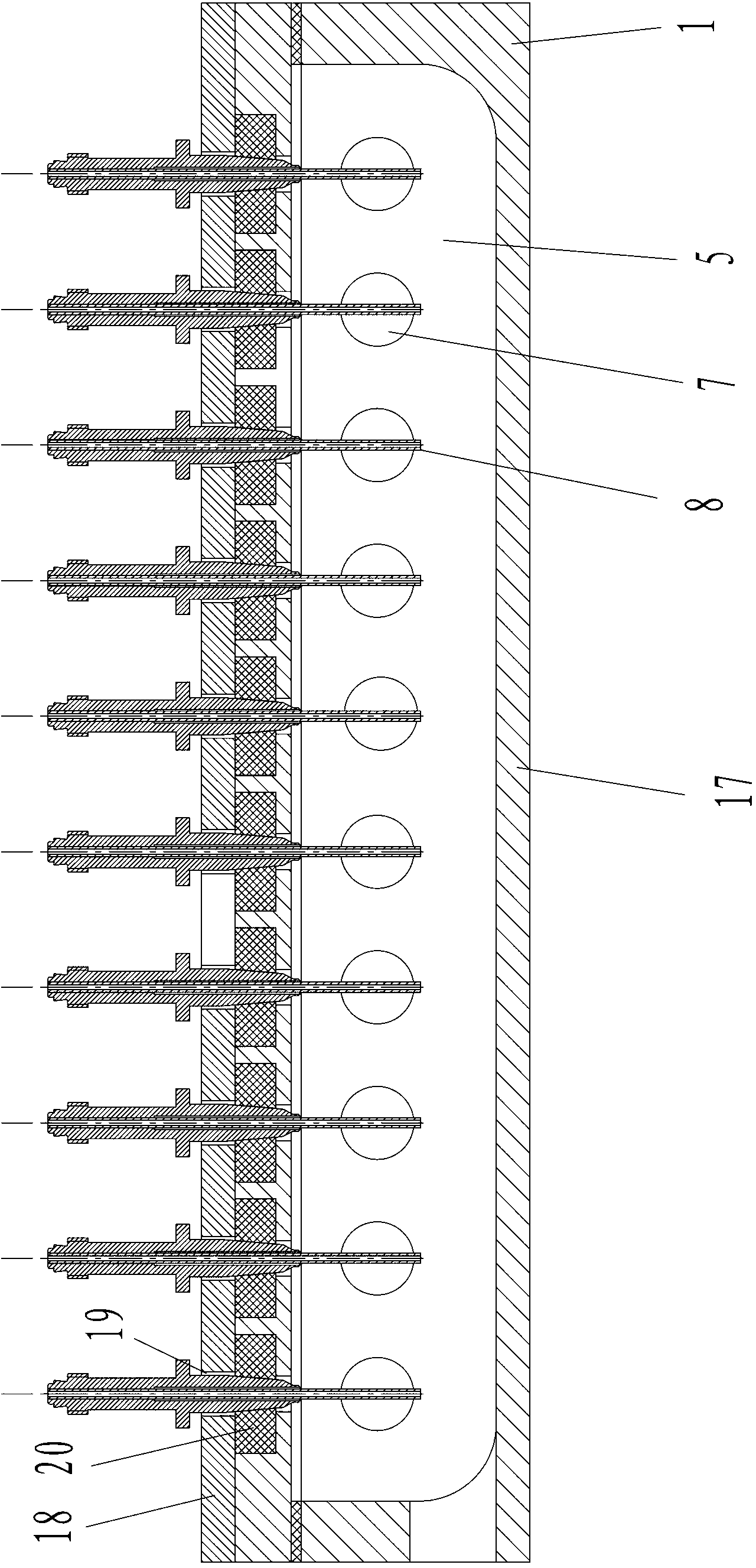
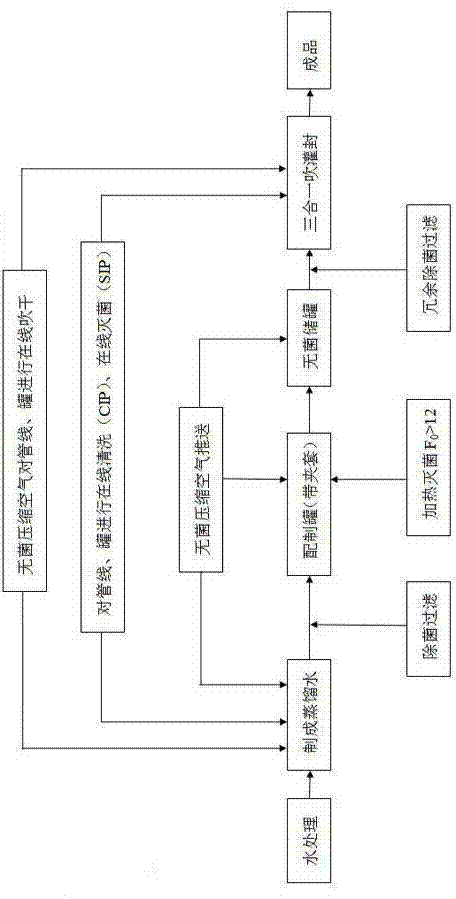
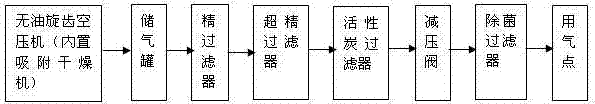
Patents
Literature
41results about How to "Improving Sterility Assurance Levels" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Ambroxol hydrochloride liquid preparation and preparation method thereof
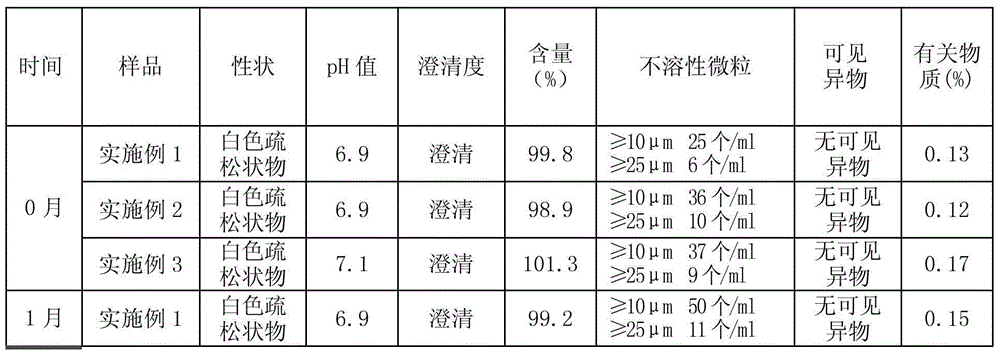
ActiveCN101627967AImprove solubilityOvercome the defect of being slightly soluble in waterOrganic active ingredientsPharmaceutical delivery mechanismWater useBULK ACTIVE INGREDIENT
The invention discloses an ambroxol hydrochloride liquid preparation and a preparation method thereof. The method comprises the steps: dissolving ambroxol hydrochloride, stabilizing agent and osmotic pressure regulator into water used for injection, and evenly mixing together to obtain solution I; then, filtering the solution I, and obtaining the ambroxol hydrochloride liquid preparation. The preparation method does not introduce active carbon, so as to avoid the danger of hurting human body since active carbon particle is introduced into the preparation; meanwhile, the active ingredients in the preparation is ensured to be stable, and the safety (namely, the chemical stability of the ambroxol hydrochloride can be effectively improved, the particle content in the preparation is reduced, and the purity of the preparation is improved) of the finished product can be guaranteed.
Owner:上海华源药业(宁夏)沙赛制药有限公司
Method for preparing sodium-potassium-magnesium-calcium glucose injection
InactiveCN104622896AQuality assuranceImproving Sterility Assurance LevelsOrganic active ingredientsMetabolism disorderSodium acetatePotassium
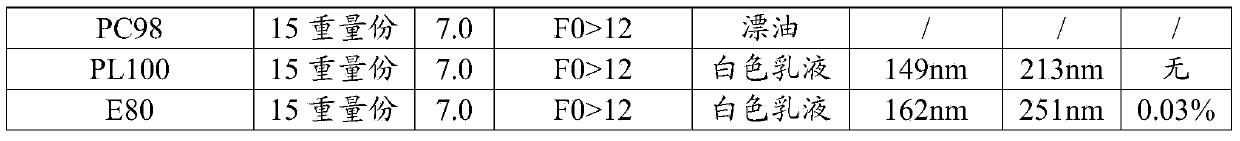
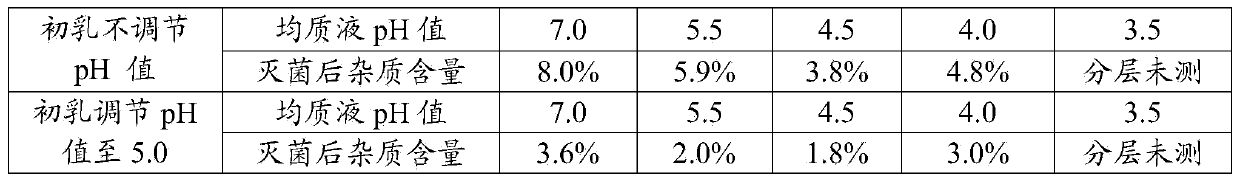
The invention discloses a method for preparing a sodium-potassium-magnesium-calcium glucose injection. The method comprises the following steps: adding water for injection into a preparation tank; sequentially adding calcium gluconate, sodium chloride, potassium chloride, magnesium chloride, glucosum anhydricum, sodium acetate and sodium citrate; dissolving, regulating the pH value to 4.5-5.5 with hydrochloric acid, filtering and filling; sterilizing for 12 minutes at 121 DEG C, inspecting with a lamp, and packaging. According to the method, after the pH value is regulated to 4.5-5.5, a durable overkilling method is carried out at 121 DEG C for 12 minutes (F0 is more than or equal to 12) for sterilizing, the content of glucose is not remarkably reduced, 5-hydroxymethylfurfural and other glucose degrading impurities are in a relatively low level, and the sterility assurance level of the sodium-potassium-magnesium-calcium glucose injection is improved.
Owner:SHANDONG QIDU PHARMA
Muscular amino acids and peptides and nucleosides injection and preparing method thereof
The present invention provides a muscular amino acids and peptides and nucleosides injection, which is extracted from the muscle and the cardiac muscle of healthy rabbit and is a freeze-drying agent which is prepared by the sterilized water solution containing the compositions of polypeptide, amino acids, nucleosides, nucleotides, etc. The muscular amino acids and peptides and nucleosides is used for the adjuvant therapy of brain hypofunction and peripheral nerve disease caused by cerebral apoplexy and cerehral circulation insufficiency. The present invention belongs to the technical field of medicine. The invention introduces a preparing method which comprises the steps of cleaning, cutting into blocks, homogenizing, deep freezing, heating to boiled, separating the supernatant, depositing with acid and the base, depositing with the base, hot pressing, deep freezing, ultra-filtering, detecting, adjusting the proportion between the polypeptide and the hypoxanthine, and ultra-filtering. The muscular amino acids and peptides and nucleosides injection is prepared through the steps of sterilizing filtering, filling into containers, and sterilizing. An appropriate amount of accessory is added and the steps of sterilizing filtering, filling into containers, and freeze drying are executed for preparing the muscular amino acids and peptides and nucleosides for injection. The muscular amino acids and peptides and nucleosides and the preparing method of the invention have the advantages of excellent convenience, excellent wholeness, ensured product which satisfies the national standard, guaranteed virus blanching, injection sterilization F0 larger than 8, and high level of sterilizing guaranteeing.
Owner:石海 +1
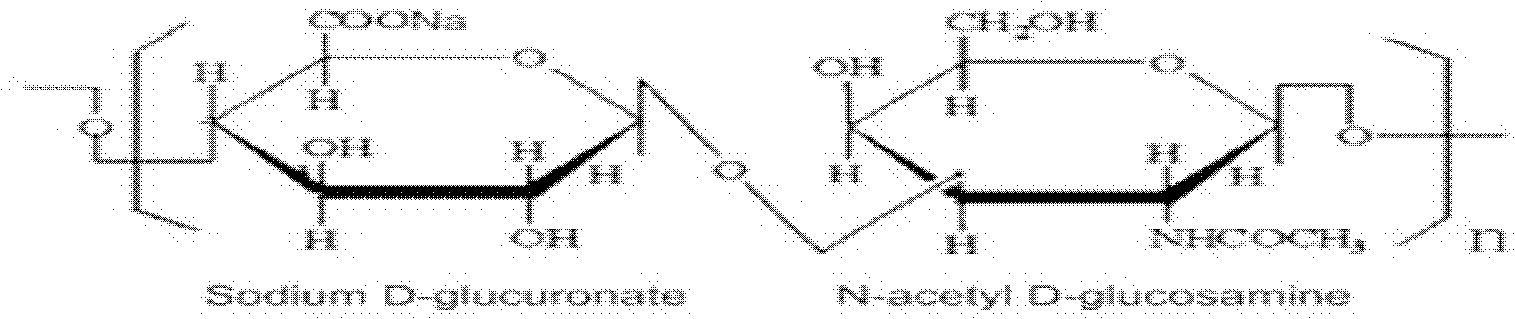
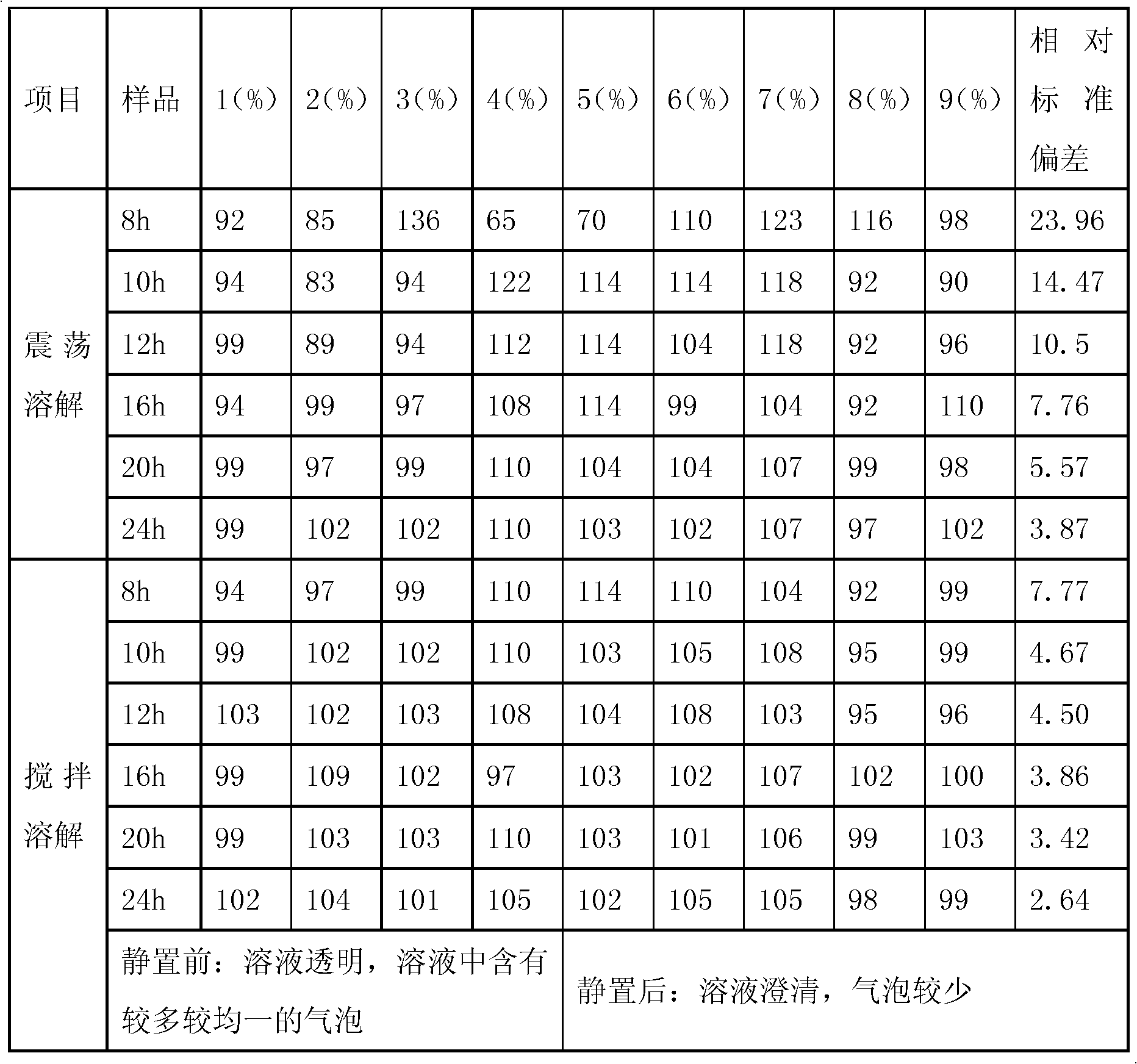
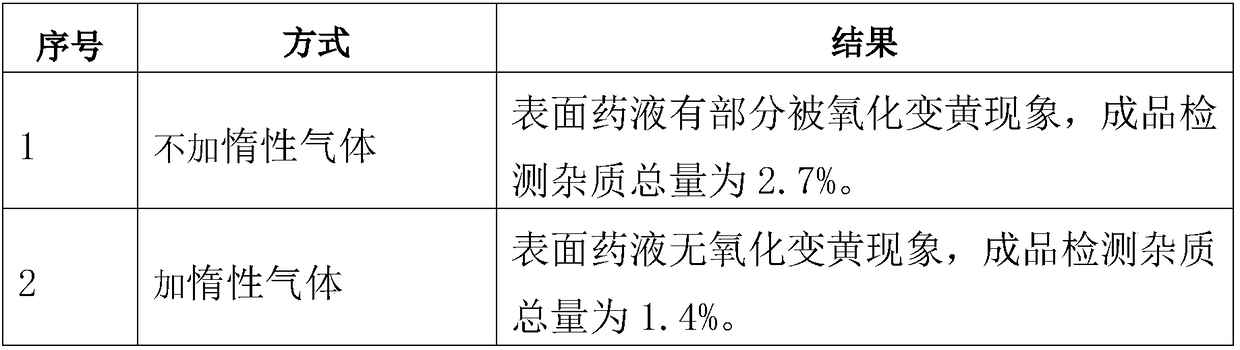
Dissolving method of sodium hyaluronate for solution preparation
ActiveCN102489193AEasy to spreadOvercome the defect of poor uniformityDissolvingHyaluronic acidSodium hyaluronate
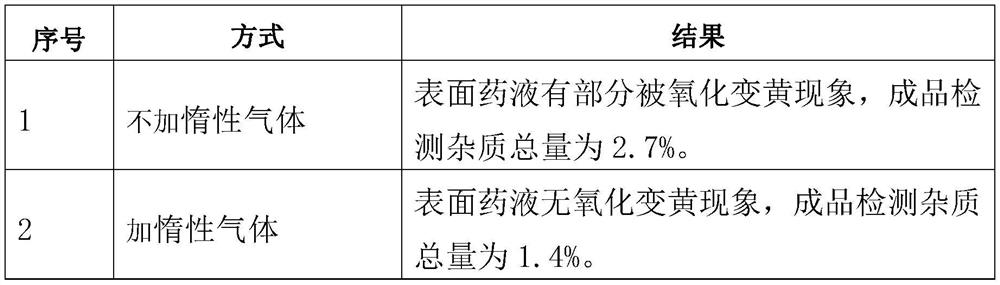
The invention relates to a dissolving method of hyaluronic acid for solution preparation. The method comprises the steps of: first adding a PBS solution into a solution preparation tank and starting stirring; adding sodium hyaluronate with a stirring rotating speed less than 150 round / min; closing a feed inlet after addition, filling inert gas in the solution preparation tank, controlling a pressure within a range of from 0 to 0.1MPa, stirring continuously, predissolving for 40-60 min, stopping stirring, carrying out limit swelling for 1-2 h and carrying out intermittent stirring; finally, finishing stirring, protecting the hyaluronic acid solution in inert gas and carrying out standing. The method for dissolving sodium hyaluronate can substantially increase soup dissolving homogeneity, avoid oxidation of product, enhance aseptic guarantee level during product production process and improve product quality; the standing process employs natural sedimentation to reduce bubble amount in an injection; beside, the method substantially shortens a dissolving time during solution preparation, reduces production cost and especially applies to industrialized production.
Owner:SHANGHAI JINGFENG PHARMA
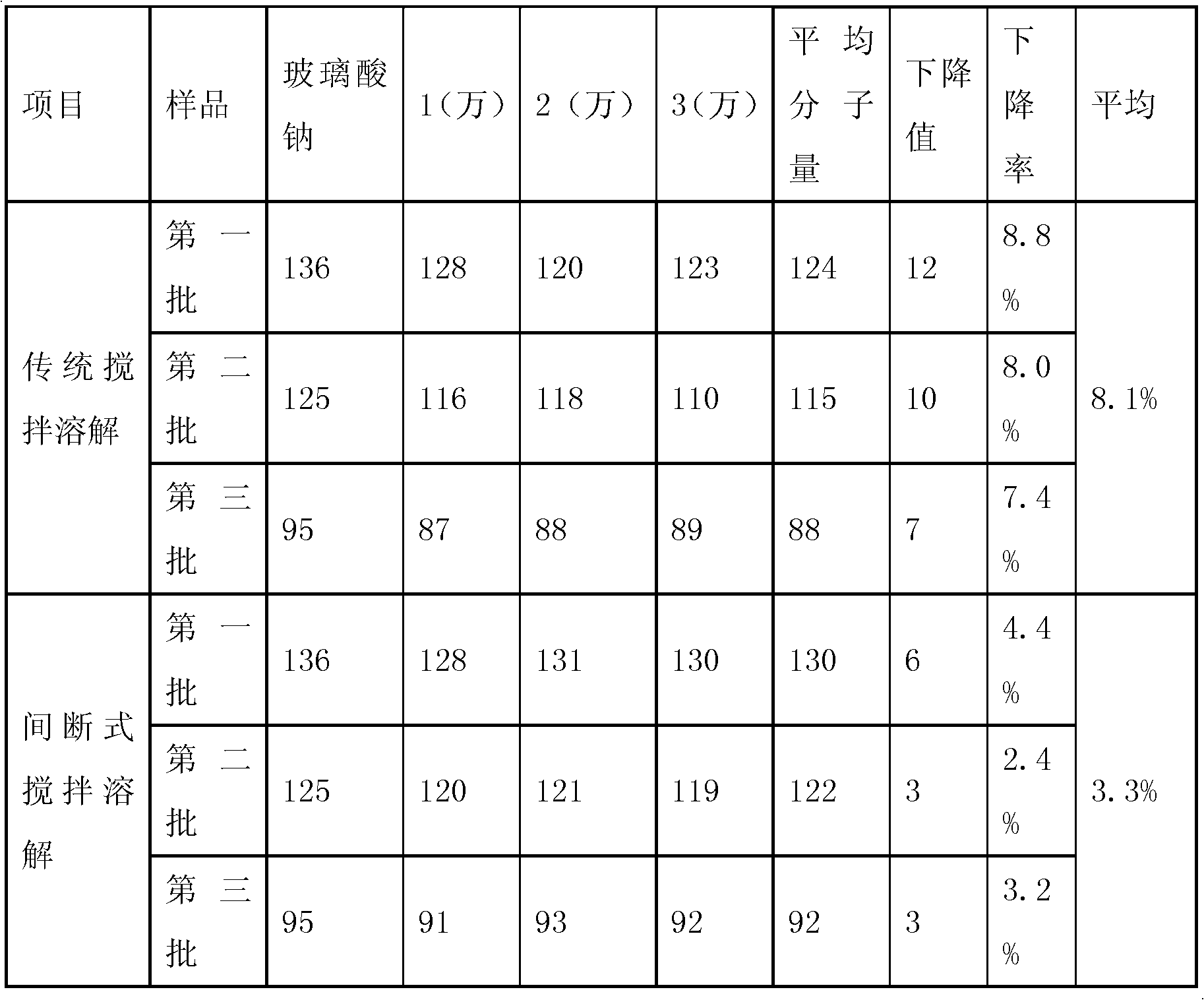
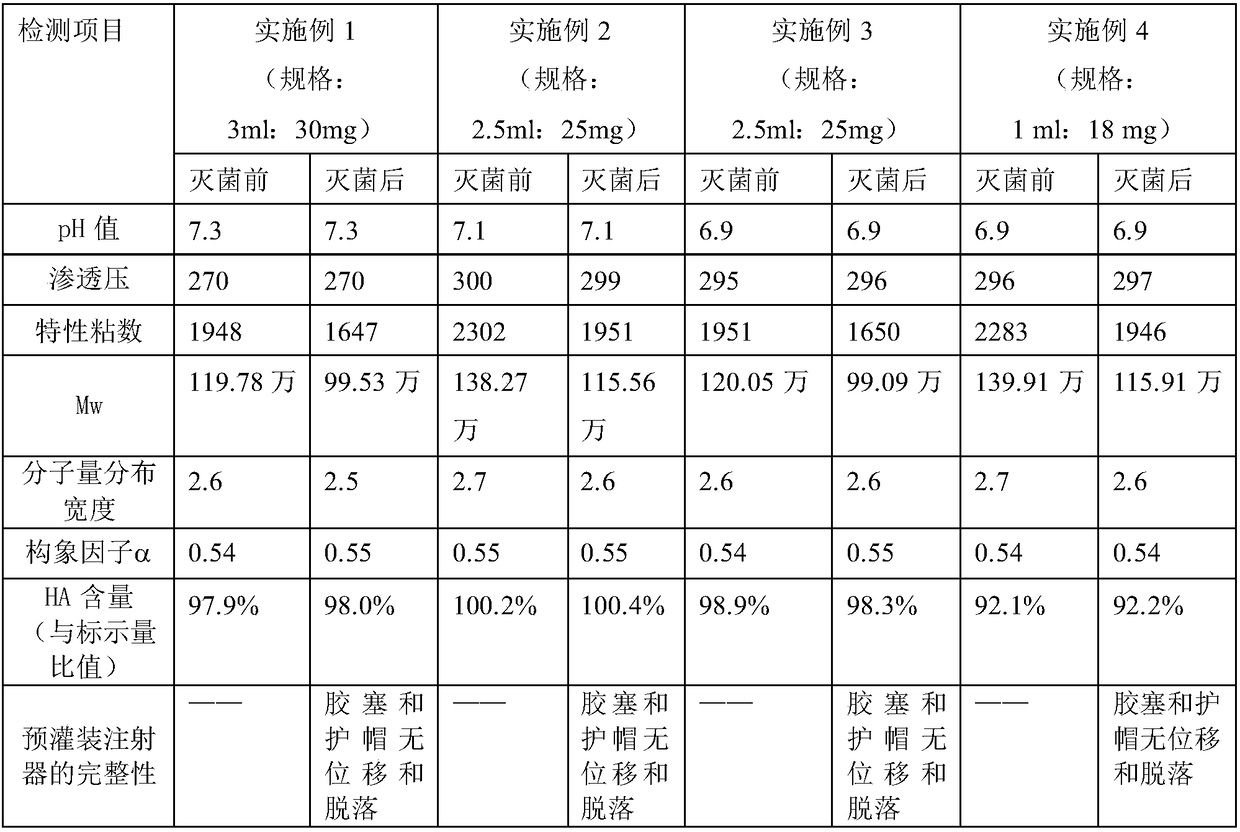
Method for large-scale preparation of mixed steam-air sterilized sodium hyaluronate injections
InactiveCN108261420AReduce degradationImprove product qualityOrganic active ingredientsInorganic non-active ingredientsDrug productSterility assurance level
The invention relates to the technical field of biomedicine, in particular to a method for large-scale preparation of mixed steam-air sterilized sodium hyaluronate injections. According to the method,sterile sodium hyaluronate dry powder is used as a raw material, prepared, intermittently stirred and dissolved, loaded into pre-filled syringes and finally sterilized by means of a mixed steam-air method. The sodium hyaluronate injections obtained by batch preparation are stable and uniform in product quality, and degradation of sodium hyaluronate after sterilization is less. According to the method, the sterility assurance level of the sodium hyaluronate injections is greatly increased, and the clinical requirements for effectiveness and safety of medicine are met.
Owner:SHANGHAI HAOHAI BIOLOGICAL TECH
Composition containing paclitaxel and preparation method thereof
ActiveCN103736096AAvoid hemolytic reactionsReduce drug riskOrganic active ingredientsPowder deliveryPatient complianceMedication risk
The invention relates to the technical field of antitumor drugs, and discloses a composition containing paclitaxel and a preparation method thereof. The composition containing paclitaxel comprises 1 part by weight of paclitaxel and 1.2-15 parts by weight of egg yolk lecithin; the pH value range of the composition is 4.0-6.0. According to the composition containing paclitaxel of the invention, egg yolk lecithin is adopted as an emulsifier; the pH value of the composition is adjusted to be within a proper range; no polyoxyethylated castor oil with large toxicity is adopted; no ethanol is contained; the medication risk of the composition containing paclitaxel is reduced; and the patient compliance is improved.
Owner:李宏 +1
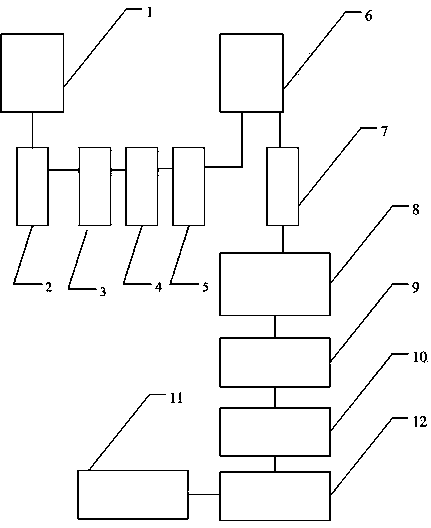
Filtration sterilization device and process for sterile medicines
PendingCN109550297ASolve the problem of clogged filter elementReduce lossSemi-permeable membranesDispersed particle filtrationFiltrationBuffer tank
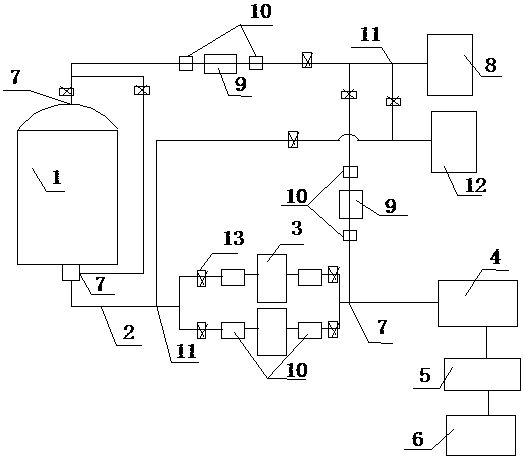
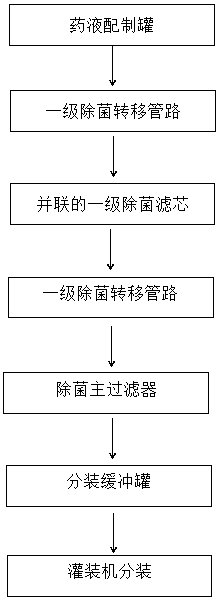
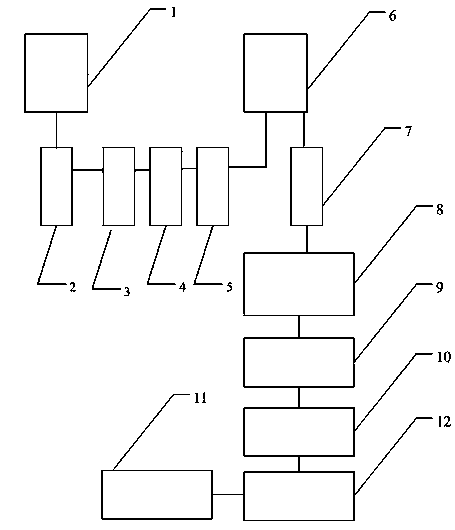
The invention relates to the technical field of sterile medicine production, and particularly relates to a filtration sterilization device and process for sterile medicines. The filtration sterilization device comprises a liquid medicine preparation tank, wherein a discharge port of the liquid medicine preparation tank is sequentially connected with first-stage sterilization filters and a main filter through pipelines; a discharge port of the main filter is sequentially connected with a sub-packaging buffer tank and a filling machine; a plurality of the first-stage sterilization filters are connected in parallel; a sterilizing and transferring pipeline behind the first-stage sterilization filters is provided with a compressed air inlet; and the compressed air inlet is connected with a compressed air source through a pipeline. According to the invention, the yield of product sterilization can be well improved, the liquid medicine transferring pipelines and the filters can be sterilizedwithout dead corners, risks in filtration and sterilization of medicines are reduced to the maximum extent, and the sterility assurance level of the medicines is greatly improved.
Owner:SHANDONG TAIBANG BIOLOGICAL PROD CO LTD
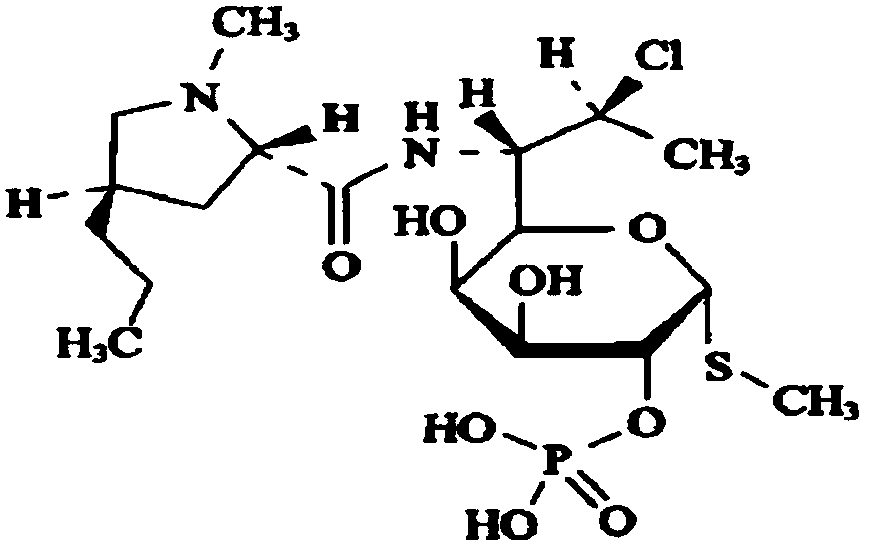
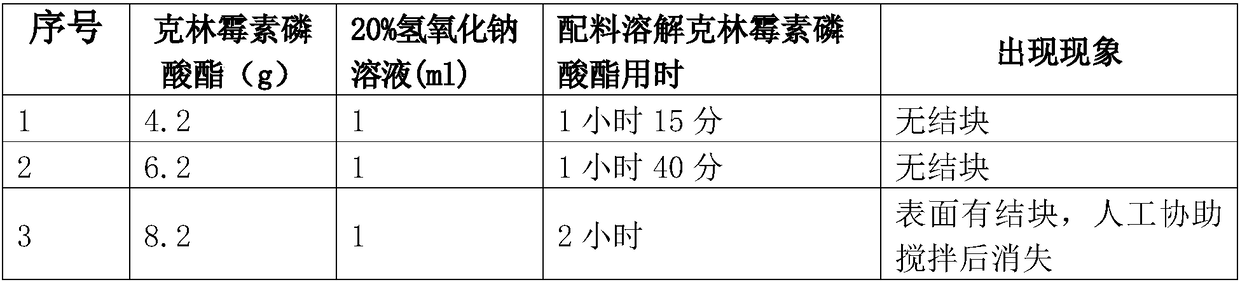
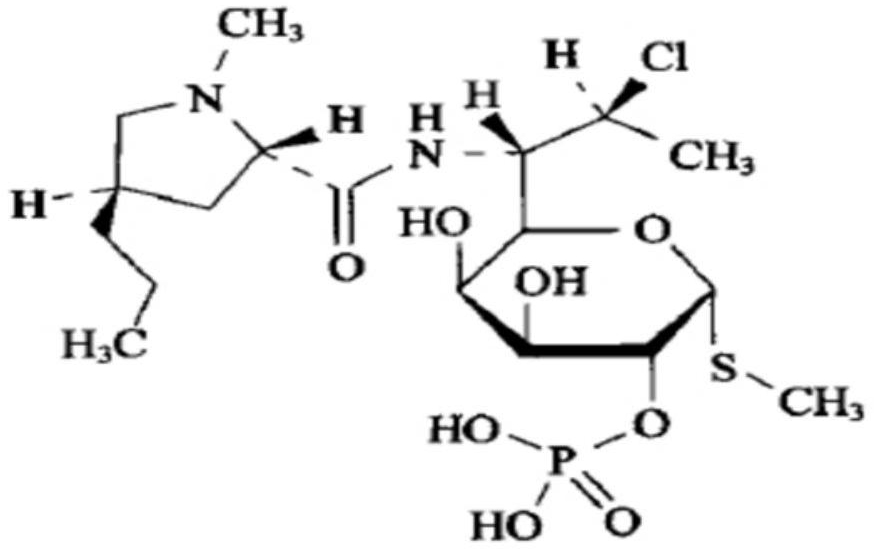
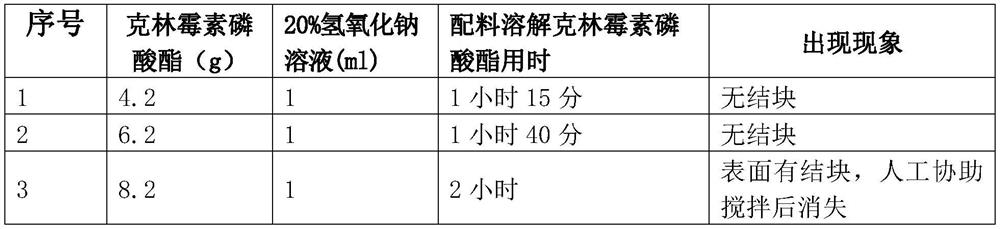
Clindamycin phosphate compound liquid and preparation method thereof
ActiveCN109498564AIncrease temperatureShorten the dissolution timeAntibacterial agentsOrganic active ingredientsActivated carbonFiltration
The invention discloses a clindamycin phosphate compound liquid and a preparation method thereof. The clindamycin phosphate compound liquid is prepared from the following components in proportions: 500-700 parts of clindamycin phosphate, 35-55 parts of sodium hydroxide, 1700-3000 parts of water for injection and 2-10 parts of activated carbon; the clindamycin phosphate dose is prepared by adding raw materials and a PH regulator gradually according to the proportions, dissolving and other processes. By adopting the preparation method, the uniformity of medicinal liquid dissolution is greatly improved, the oxidation process of a product is eliminated, the sterility assurance level in the production process of the product is improved, and the amount of air bubbles in an injection is reduced;in addition, the dissolution time and the filtration time in the liquid preparation process are greatly shortened and the production cost is reduced; therefore, the preparation method is especially suitable for industrial production; the dissolution method is not only applicable to the liquid preparation process of the clindamycin phosphate for injection, but also applicable to the liquid preparation process of other products using the clindamycin phosphate as a raw material and sodium hydroxide as the pH regulator.
Owner:GUIZHOU JINGFENG INJECTION
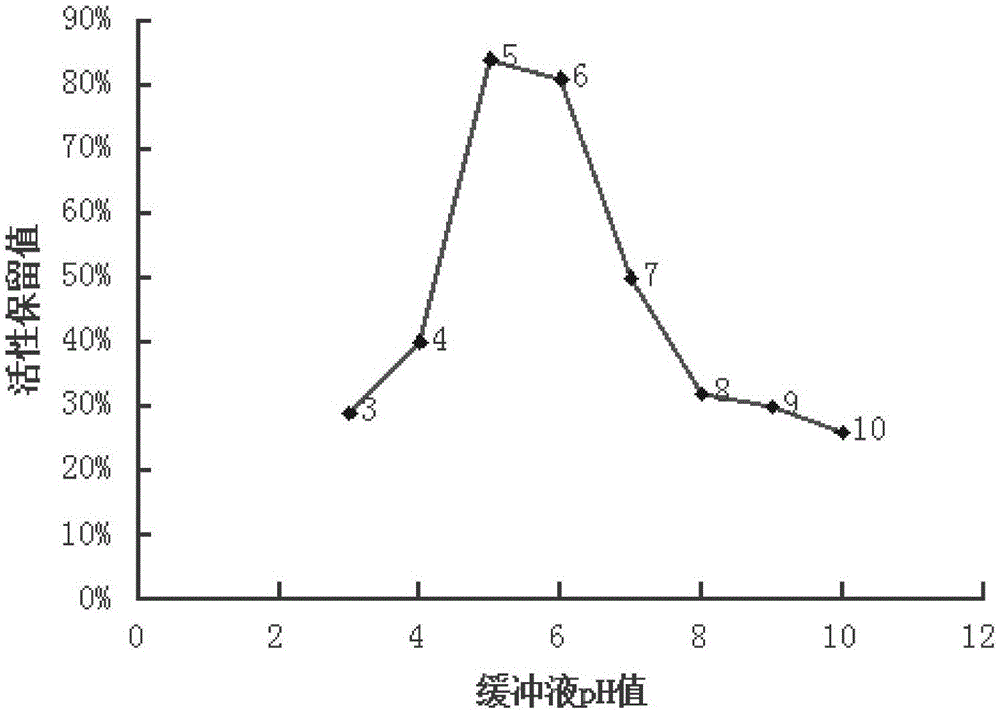
Sterilization method of protein A immuno-absorbent column
ActiveCN105126131AGuaranteed stabilityHigh activitySolid sorbent liquid separationHeatAlcoholImmunosorbents
The invention relates to a sterilization method of a protein A immuno-absorbent column. The sterilization method comprises the following steps: adding a protein A immuno-absorbent to a buffer solution which is 4-7 in pH value, packing a column and sterilizing with steam. The method further comprises a step of adding a stabilizer to the buffer solution, wherein the stabilizer consists of the following components: an antioxidant, polyhydric alcohol, polysaccharide, a chelating agent, a thickening agent, polypeptide or protein. By virtue of the sterilization process, the activity of the absorbent can be protected after sterilization, and the final sterilization of the protein A immuno-absorbent column can be achieved; and the obtained protein A immuno-absorbent column product, compared with products obtained from a sterile process, is higher in sterility assurance level and is lower in production cost.
Owner:GUANGZHOU KONCEN BIOSCI
Nano ganciclovir freeze-drying preparation for injection and preparation method thereof
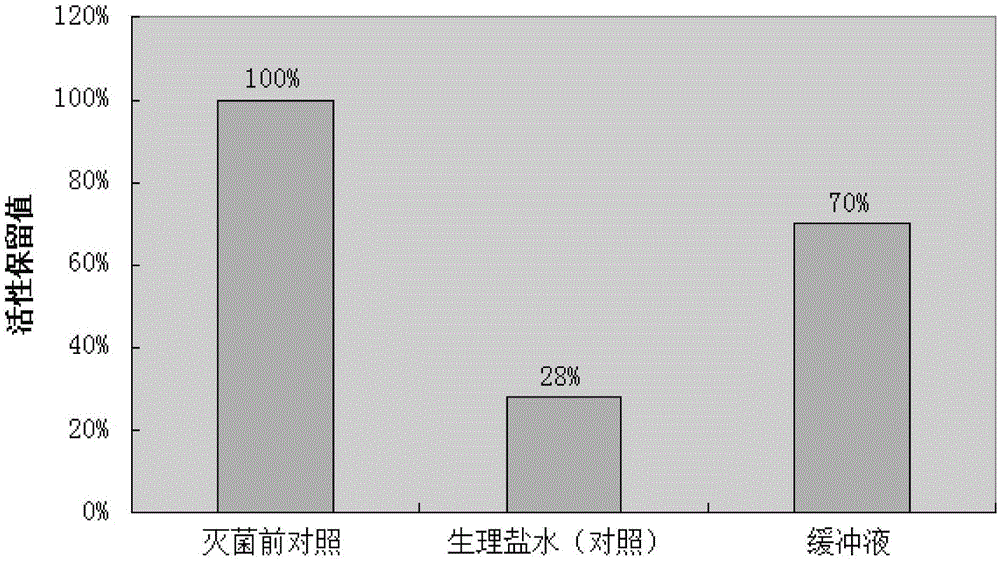
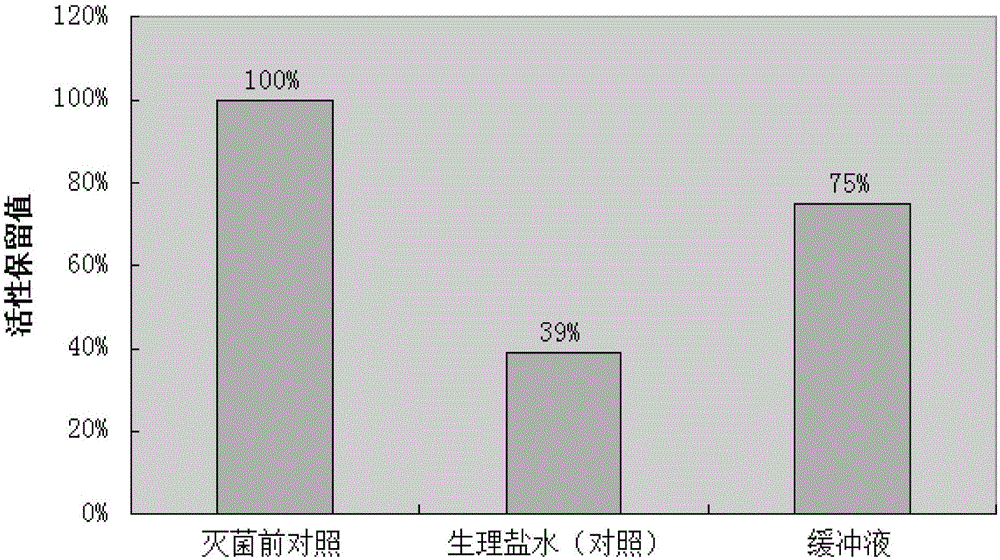
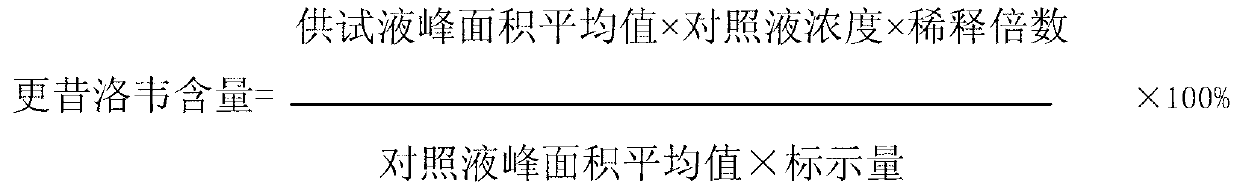
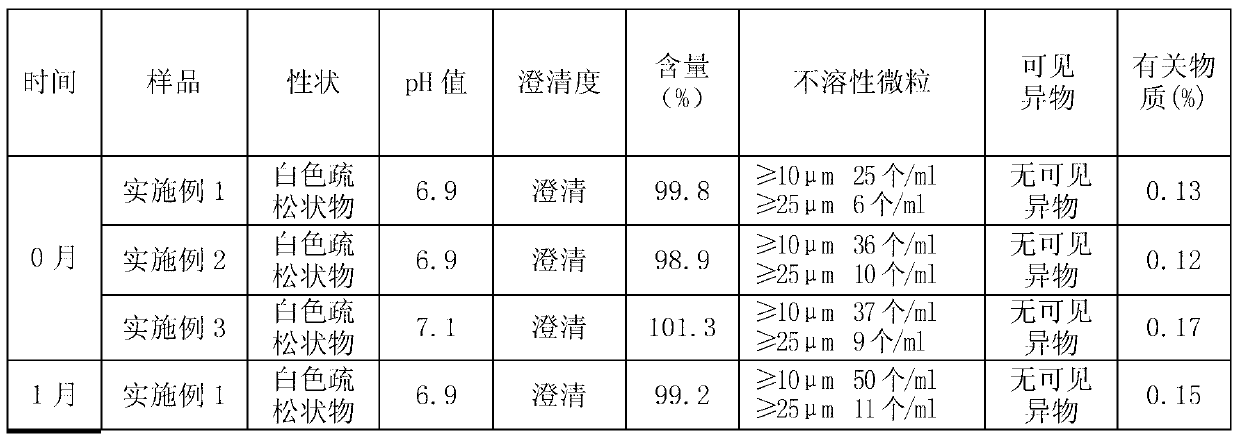
ActiveCN103340830AAvoid local irritation side effectsImprove tolerancePowder deliveryAntiviralsAlkalinityActivated carbon
The invention discloses a nano ganciclovir freeze-drying preparation for injection and a preparation method thereof. The nano ganciclovir freeze-drying preparation for injection comprises the following components in parts by weight: 100-400 parts of ganciclovir, 10-50 parts of dextran 40, 5-50 parts of solubilizer, 10-100 parts of nano carrier material and 10-80 parts of freeze-drying skeleton agent. The preparation method comprises the following steps of: sequentially adding dextran 40, solubilizer, ganciclovir, nano carrier material and freeze-drying skeleton agent into water for injection to be dissolved, filtering a solution step by step, and carrying out freeze-drying, thus a freeze-drying preparation is obtained. According to the preparation method, no activated carbon is introduced, thus a risk that damage is done to a human body when activated carbon particles are introduced into the preparation as the activated carbon is used is avoided; besides, pH of the nano ganciclovir freeze-drying preparation for injection is 6-8 and close to that of plasma, and local irritation produced to the human body owning to overhigh alkalinity is avoided.
Owner:上海华源药业(宁夏)沙赛制药有限公司
Aseptic packaging method of large volume injection
InactiveCN103070823AGood sterility assurance levelImproving Sterility Assurance LevelsPharmaceutical delivery mechanismLavatory sanitoryFilter systemProcess engineering
The invention discloses an aseptic packaging method of a large volume injection. The aseptic packaging method comprises the following steps: (1) weighing raw materials and auxiliary materials of an injection according to a process proportion under a negative-pressure weighing hood, sequentially adding the materials into a pre-sterilized batching pot, adding water for injection to dilute, and mixing uniformly; (2) supplementing the water for injection to achieve the preparation total amount according to the process proportion in combination with a weighing system of a batching pot, adjusting the pH value of the liquor, and filtering and sterilizing the liquor through double 0.22 microns sterilizing grade filters of a redundancy filtering system; and (3) injecting the filtered aseptic liquor to a plastic bottle-blowing, filling and sealing integrated machine through an aseptic pipeline system, finishing the bottle blowing, filling and sealing under the protection of a grade-A laminar flow hood, and finally sterilizing at high temperature to obtain a finished product. According to the aseptic packaging method of the large volume injection, double 0.22 microns sterilizing grade filters are added before filling, and the high-temperature sterilization is performed after filling, so that the aseptic assurance level of the large volume injection is greatly improved in comparison with the traditional terminal sterilization process, the content of a heat source is controlled effectively, and the security of clinical use is strongly guaranteed.
Owner:ZHONGQI PHARMA IND HENGSHUN ZHONGQI
Dry-cleaning sterilizer and method thereof
InactiveCN105148295AAvoid corrosion and other damageShort sterilization timePackage sterilisationCleaning using gasesForeign matterLight energy
The invention discloses a dry-cleaning sterilizer. The dry-cleaning sterilizer comprises an arranging mechanism, a conveying belt, a dry-cleaning device and a sterilization device, wherein a jet nozzle is arranged at the top end of the dry-cleaning device; the conveying belt is used for conveying a material to a material use point through the dry-cleaning device and the sterilization device from the collection opening end of the conveying belt; the sterilization device comprises an inert gas lamp, a pulse laser generator and reflectors on two sides of the sterilization device; the conveying belt positioned in the sterilization device passes through the space between the reflectors on two sides of the sterilization device; and high-intensity pulse laser generated by the pulse laser generator is used for completely illuminating all light energy to a to-be-processed material on the conveying belt by virtue of the reflectors. According to the dry-cleaning sterilizer, pre-treatment and cleaning operation before foreign material cleaning and sterilization of a material surface can be completed by adopting a mode of cleaning, boosting, high-temperature air local dry-cleaning and integral wiping; the material surface is sterilized by adopting a harmless pulse laser irradiation mode; and the material is continuously conveyed by adopting the transmission mechanism, and the effective effect of cleaning and sterilizing can be guaranteed.
Owner:王世庆 +1
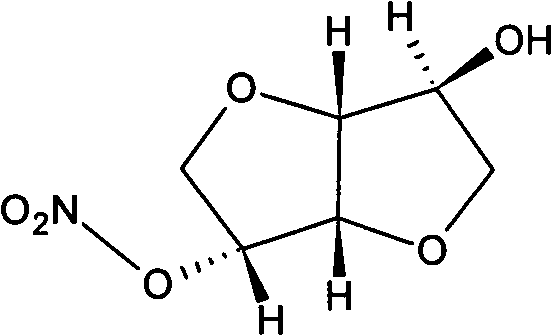
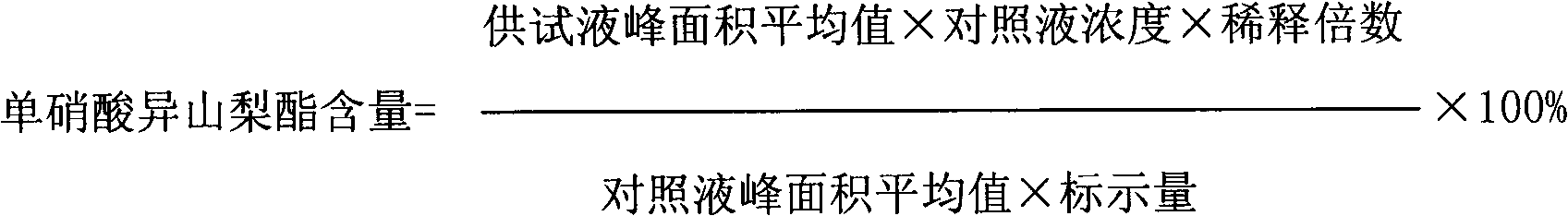
Isosorbide mononitrate liquid preparation and preparing method thereof
ActiveCN101637448AImprove solubilityOvercome the defect of being slightly soluble in waterInorganic non-active ingredientsPharmaceutical delivery mechanismWater useActivated carbon
The invention discloses an isosorbide mononitrate liquid preparation and a preparing method thereof. The method comprises the steps: dissolving isosorbide mononitrate, stabilizing agent and osmotic pressure regulator into water used for injection, and evenly mixing together to obtain solution I; then, filtering the solution I, and obtaining isosorbide mononitrate injection. As active carbon is notintroduced into the preparing method, the active carbon particles are prevented from being introduced into the preparation to be harmful to the human body, and the stability of the active ingredientsin the preparation as well as the safety of the finished product can be ensued (namely, the chemical stability of the isosorbide mononitrate is effectively improved, the particulate content in the preparation is reduced, and the purity of the preparation is increased).
Owner:上海华源药业(宁夏)沙赛制药有限公司
Tafluprost eye drop agent and preparation method thereof
InactiveCN110711175AIncrease contentLess impuritiesOrganic active ingredientsSenses disorderAntioxidantBULK ACTIVE INGREDIENT
The invention belongs to the field of bio-medicine, and discloses a tafluprost eye drop agent and a preparation method thereof. The eye drop agent take tafluprost as an active ingredient, a hydrophilic gel skeleton material is used as a skeleton and a solubilizer, the tafluprost eye drop agent further includes an antioxidant and a stabilizer, and the antioxidant and the stabilizer are macromolecular compounds. A process of the tafluprost eye drop agent improves the asepsis level of a product, the characteristics of simple preparation composition, easy, convenient and quick operation, high compatibility for medicinal packaging materials, low cost, stable properties of liquid medicine and high quality standard are achieved, and the tafluprost eye drop agent is suitable for industrial production.
Owner:南京华盖制药有限公司
Alprostadil lipid microsphere injection and preparation method thereof
ActiveCN103989632AEnhanced Sterile Control ProcessImprove the level ofOrganic active ingredientsPharmaceutical non-active ingredientsEmulsionMicrosphere
The invention provides an alprostadil lipid microsphere injection. The alprostadil lipid microsphere injection is prepared from alprostadil, oil for injection, lecithin for injection, a stabilizing agent, an osmotic pressure regulating agent, a metal complexing agent and injection water. According to the invention, the metal ion complexing agent is employed and exerts main component protection and adjuvant degradation effects; the pH value of the finished injection is controlled by adjusting the pH value of a water phase in advance, so inconvenience and potential quality hazards resulting from adjusting of the pH value of the finished injection in an initial or final emulsion phase after emulsion formation are prevented; unconventional high temperature instant sterilization is employed for sterilization of the finished injection. The preparation method for the alprostadil lipid microsphere injection is simplified, enhances aseptic control process of the injection, improves a sterility assurance level and production efficiency and avoids possibility of a too high pH value of a local part of the injection at an instant; and the prepared alprostadil lipid microsphere injection completely meet clinical needs and has the advantages of a few impurities, long shelf life and small adverse reaction.
Owner:ZHEJIANG SUNDOC PHARMA SCI & TECH CO LTD
Double-layer aseptic package, packaging method and sterility test method for large volume injections
InactiveCN104434517AGuaranteed sterilityExcellent Mechanical ShockMicrobiological testing/measurementPharmaceutical containersMoistureBiomedical engineering
The invention relates to the field of production of large volume injections, and provides a double-layer aseptic package, packaging method and sterility test method for large volume injections. The package comprises an inner packaging bag and an outer packaging bag wrapping the inner packaging bag. The space between the inner packaging bag and the outer packaging bag is vacuumized, so that the outer packaging bag is nearly attached to the inner packaging bag, and the space between the inner packaging bag and the outer packaging bag is aseptic. The mechanical performance and moisture blocking performance of the packaging bags are enhanced, and the problems of product pollution and medical potential safety hazard caused when packages leak or moisture evaporates in the transportation or storage process are solved. Besides, the vacuumized packaging bags are subjected to sterilization, liquid medicine in the inner packaging bag of the package is aseptic, and meanwhile the space between the inner packaging bag and the outer packaging bag is also aseptic. Thus, when the package is applied to the environment like clinical dispensing having high aseptic requirements, it is only required that the outer packaging bag is taken off, and medical staff do not need to disinfect packages one by one.
Owner:XIAN JINGXI SHUANGHE PHARMA
Preparation method of local anesthetic liposome
ActiveCN113041223ASimple preparation processLow requirements for production equipmentAnaestheticsPharmaceutical non-active ingredientsUltrafiltrationLiposome
The invention discloses a preparation method of a local anesthetic liposome, and belongs to the technical field of preparations. The preparation method of the local anesthetic liposome includes the steps of preparing a blank liposome; carrying out ultrafiltration concentration on the blank liposome; co-incubating drug to be encapsulated and the blank liposome, and performing sterilizing and filtering to obtain a drug-loaded liposome; and mixing the drug-loaded liposome with a poloxamer aqueous solution, performing subpackaging, performing freezing after subpackaging, and performing freeze-drying after subpackaging to prepare a liquid preparation, a frozen preparation or a freeze-dried preparation. According to the preparation method of the local anesthetic liposome, a local anesthetic liposome product with lower particle size, higher uniformity and higher entrapment rate can be prepared, the operation is simple, and industrial production is easy to realize.
Owner:NANJING LUYE PHARMA
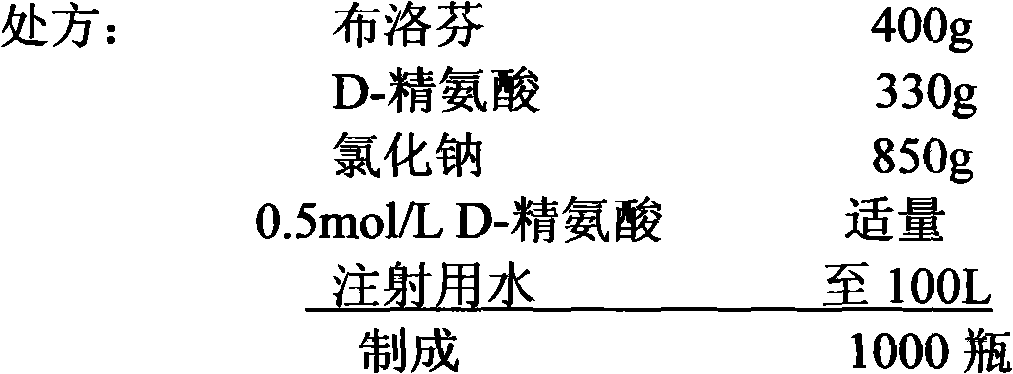
Ibuprofen sodium chloride injection preparation with pH of 6.0-6.5, and preparation method thereof
InactiveCN103565733AReduce dosageSolve the problem of opalescence opacityOrganic active ingredientsAntipyreticActivated carbon filtrationMicrofiltration membrane
The invention provides an ibuprofen sodium chloride injection preparation with pH of 6.0-6.5, and a preparation method thereof. The injection contains 1-10 mg / ml of ibuprofen, and injection water, wherein the pH is adjusted to 6.0-6.5 by an acid-base modifier; the dosage of the required acid-base modifier accounts for about 2-6% by weight / volume of the injection; the sodium chloride content of an isoosmotic adjusting agent is 0.70-0.90% by weight / volume. The preparation technology comprises the following steps: firstly, dissolving the acid-base modifier and the ibuprofen; adding the injection water to 20% of total mass; adding sodium chloride to stir and dissolve; adding activated carbon, filtering and decarburizing; adding water to 90% of total mass, adjusting the pH to 6.0-6.5, and adding water to total mass; filtering by 0.45 microns and 0.22 microns of microfiltration membranes; and filling and sealing and sterilizing at 121 DEG C for 8-20 minutes.
Owner:南京帝易医药科技有限公司
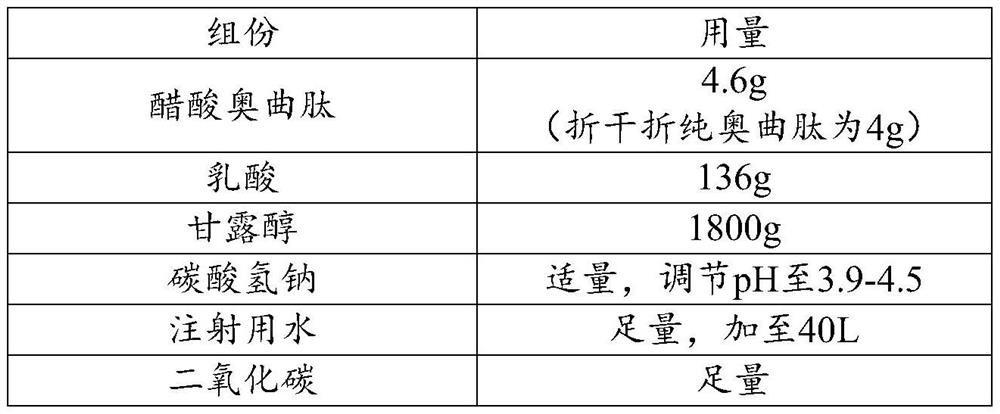
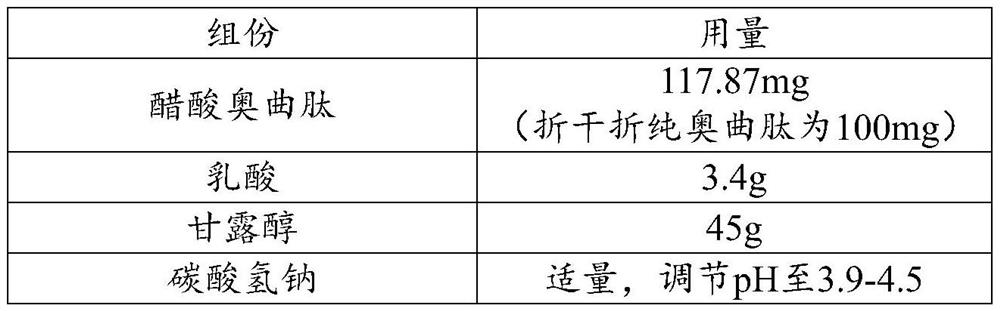
Sterilization and preparation method of octreotide acetate injection
ActiveCN113509564AImproving Sterility Assurance LevelsImprove medication safetyHeatAgainst vector-borne diseasesOctreotide acetateUse medication
The invention relates to the technical field of pharmaceutical preparations, in particular to a sterilization and preparation method of an octreotide acetate liquid preparation. The sterilization and preparation method of the octreotide acetate liquid preparation provided by the invention comprises the following steps: preparing under the protection of inert gas, and then performing 0.22-micron filtration and 115-124 DEG C high-temperature sterilization to obtain the octreotide acetate liquid preparation. The stability of octreotide acetate is maintained through reasonable sterilization conditions, the highest sterility guarantee level is guaranteed, the medication safety is improved, and the product is lower in impurity content, better in stability and better in quality.
Owner:SICHUAN HUIYU PHARMA +1
Water tank assembly for irrigating needle online cleaning and sterilizing
ActiveCN103230908ARealization of online cleaning and sterilizationAvoid diversionHollow article cleaningHeatEnvironmental engineeringMechanical engineering
The invention discloses a water tank assembly for irrigating needle online cleaning and sterilizing. The water tank assembly comprises a cleaning water tank, a cleaning medium input pipe and a cleaning medium discharge pipe. A cleaning medium channel and an irrigating needle cleaning cavity for inserting of an irrigating needle are arranged in the cleaning water tank, the inlet end of the cleaning medium channel is connected with the cleaning medium input pipe, the outlet end of the irrigating needle cleaning cavity is connected with the cleaning medium discharge pipe, a water hole or a nozzle connected into the irrigating needle cleaning cavity is arranged on the cleaning medium channel, and the water hole or the nozzle faces to the irrigating needle in the irrigating needle cleaning cavity and can spray cleaning media to the outer wall of the irrigating needle. The water tank assembly has the advantages of being simple and compact in structure and low in cost, being capable of reducing risks of pollution and cross contamination, improving the asepsis guarantee level and the like.
Owner:TRUKING TECH LTD
Sterile injection water production technique
ActiveCN103693791BImproving Sterility Assurance LevelsControl heat source contentPharmaceutical delivery mechanismAntioxidantDistilled water
The invention discloses a sterile injection water production technique and a sterile compressed air preparation method. The sterile injection water production technique comprises the following steps: firstly, performing online cleaning CIP (Cleaning in Place) and online SIP (Sterilization in Place) and online drying to all pipelines, a preparation tank and a storage tank used in the production of sterile injection water through distilled water or pure steam, and delivering distilled water into the preparation tank; heating and sterilizing the distilled water, and leading into the storage tank and cooling; and finally, performing sterile filtration to the distilled water through a redundant filtration system, and injecting in a blowing-pouring-sealing three-in-one pouring machine through a sterile pipeline system for sterile pouring and sealing, thus obtaining the product. Through the production technique, the injection water is strictly sterilized before pouring and sealing, heat source content can be effectively controlled, an additive and an antioxidant of a plastic bottle can be prevented from diffusing into the injection water and no impurities of scraps and the like do not exist when terminal high-temperature sterilizing is carried out, therefore, the sterile injection water can provide powerful guarantee for the clinical use safety.
Owner:ZHONGQI PHARMA IND HENGSHUN ZHONGQI
Vertical logistics based solvent crystallization method
InactiveCN104117224AAvoid germsImproving Sterility Assurance LevelsCrystallization separationSolventMaterial transfer
The invention relates to the pharmaceutical field, especially to production of bulk drugs by a solvent crystallization method and to a sterile logistics method, particularly a vertical logistics based solvent crystallization method. According to the traditional solvent crystallization method, solid-liquid separation is carried out on a material liquid after crystal growing, and a corresponding material flow system is designed to transfer the separated solid by the action of gravity. With the method provided by the invention, environmental exposure caused pollution problems can be avoided during material transfer, and the prepared bulk drugs have a high sterile level. In addition, the logistics system with gravity as the main driving force has the characteristics of high transport efficiency and low cost.
Owner:CHONGQING LUMMY PHARMA
Anti-cancer drug degarelix acetate injection and preparation method thereof
InactiveCN105749245AImprove preparation qualitySimple processPeptide/protein ingredientsPharmaceutical delivery mechanismHydrogenDegarelix Acetate
The invention belongs to the technical field of medicines, and particularly relates to a degarelix acetate injection and a preparation method thereof. The injection is mainly prepared from active ingredient degarelix acetate, a dispersant, methylparaben, sodium hydrogen sulfite, pH regulator and water for injection. The degarelix acetate injection is stable in quality and simple in process, and is suitable for industrial popularization.
Owner:张光泉
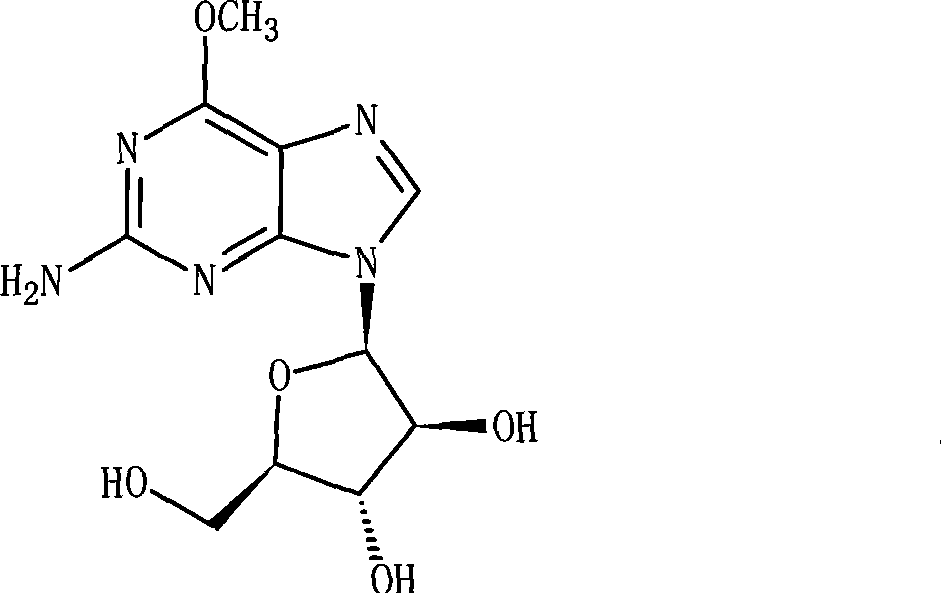
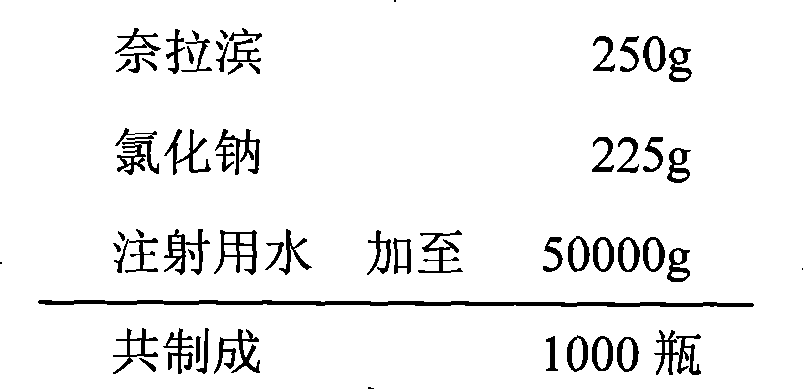
Nelarabine injection
InactiveCN101401786BImprove stabilityLow content of related substancesOrganic active ingredientsInorganic non-active ingredientsActivated carbonAcetic acid
The invention relates to a nelarabine injection, which is characterized by consisting of nelarabine, EDTA or EDTA salt, sodium chloride, and water for injection, wherein the nelarabine accounts for 0.25 to 0.625 percent of the total weight of the injection, the EDTA or the EDTA salt accounts for 0.001 to 0.01 percent of the total weight of the injection, the content of the sodium chloride is 0 orthe dose is used for regulating the osmotic pressure, and the balance is the water for injection. The preparing process comprises the following steps: taking 80 percent of the water for injection, adding raw supplementary materials into the water for injection, stirring the mixture to fully dissolve the raw supplementary materials and mixing the mixture evenly, adjusting the pH value, adding 0.02percent of needle activated carbon into the mixture, keeping the water temperature at 70 DEG C, stirring the mixture for 30 minutes, filtering the mixture to remove the activated carbon when the mixture is hot, cooling the mixture to room temperature, measuring the contents and the pH value of the solution, adding the water for injection with full dose into the solution, mixing the mixture evenly, filtering the mixture through 0.22 mu m of microfiltration membrane until the mixture is clear, packaging the filtrate into infusion bottles after intermediates are measured to be qualified, plugging the bottles, and rolling the mouths of the bottoms to obtain the nelarabine injection after hot pressing sterilization.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
A kind of clindamycin phosphate preparation and preparation method thereof
ActiveCN109498564BIncrease temperatureShorten the dissolution timeAntibacterial agentsOrganic active ingredientsActivated carbonPhosphoric Acid Esters
A clindamycin phosphate compounding solution and a preparation method thereof are prepared from the following components in the following proportions: 500-700 parts of clindamycin phosphate, 35-55 parts of sodium hydroxide, and 1,700 parts of water for injection -3000 parts and 2 parts-10 parts of activated carbon, which are prepared by gradually adding the raw materials and the pH regulator in proportion to the dissolving method and other processes. The sterility guarantee level in the production process reduces the amount of bubbles in the injection; and greatly shortens the dissolution time and filtration time of the liquid dispensing process, reduces the production cost, and is especially suitable for industrial production. This dissolution method is not only suitable for injection. The process of dosing with clindamycin phosphate is more suitable for the dosing process of other products using clindamycin phosphate as raw material and sodium hydroxide as PH regulator.
Owner:GUIZHOU JINGFENG INJECTION
Method for preparation of ceftriaxone sodium in vertical logistics system
InactiveCN104119360AAvoid germsImproving Sterility Assurance LevelsOrganic chemistryDissolutionSolvent
The invention relates to the pharmaceutical field, especially to production of bulk drugs by a solvent crystallization method, and also relates to an aseptic logistics method, and a method for preparation of ceftriaxone sodium in a vertical logistics system. The specific steps include: adding a ceftriaxone sodium crude product into an acetone solution, performing complete dissolution at 20-25DEG C, adding a seed crystal when the temperature drops to 15DEG C, and carrying out stirring until a large number of crystals precipitate; and conducting solid-liquid separation, and transferring the obtained solid from a separation device to a drying device. The separation device is positioned at the upper end, the drying device is located at the lower end, and the separation device and the drying device are in vertical connection through a connecting channel. With the method provided by the invention, the pollution problem caused by environmental exposure in a material transfer process is avoided, and the prepared bulk drug has a high aseptic level. In addition, the logistics system with gravity as the main driving force has the characteristics of high transport efficiency and low cost.
Owner:CHONGQING LUMMY PHARMA
Preparation method of pharmaceutical composition containing deslanoside
InactiveCN112336737AReduce the risk of useImproving Sterility Assurance LevelsOrganic active ingredientsPharmaceutical delivery mechanismMedicineGlycerol
The invention discloses a preparation method of a pharmaceutical composition containing deslanoside. The invention provides a preparation method of a deslanoside injection. The preparation method is characterized by comprising the following step: sterilizing a mixture containing the deslanoside, ethanol, glycerol and water for injection by adopting a terminal sterilization mode of sterilizing at 121 DEG C for 15 minutes. The deslanoside injection prepared by a prescription and process disclosed by the invention is stable in quality and low in impurity content, and can be placed for 5 days and10 days at a high temperature (60 DEG C) and for 1 day and 3 days at a high temperature (80 DEG C), and results show that the deslanoside injection is stable in product quality and relatively small inimpurity increase.
Owner:SHANGHAI XUDONG HAIPU PHARMA
A dry cleaning sterilizer and method thereof
InactiveCN105148295BSave waterAvoid corrosion and other damagePackage sterilisationCleaning using gasesForeign matterLight energy
The invention discloses a dry-cleaning and sterilizing machine, which comprises a sorting mechanism, a conveying belt, a dry-cleaning device and a sterilizing device; the top of the dry-cleaning machine is provided with an air spray head, and the conveying belt passes materials sequentially through the feeding port end of the conveying belt. After passing through the dry cleaning device and the sterilization device to the point of use of the material; the sterilization device includes an inert gas lamp, a pulse laser generator and reflectors on both sides; Through the space; the high-intensity pulse laser generated by the pulse laser generator irradiates all the light energy to the materials to be processed on the conveyor belt through the reflector. In the present invention, cleaning, pressurization, high-temperature air local dry cleaning and overall purging are used to complete the cleaning of foreign matter on the surface of the article and the pretreatment-cleaning work before sterilization. The surface of the object is sterilized by harmless pulsed laser irradiation. The transmission mechanism is used to realize the continuous feeding of items, and to ensure the effective effect of cleaning and sterilization.
Owner:王世庆 +1
Nano ganciclovir freeze-drying preparation for injection and preparation method thereof
ActiveCN103340830BAvoid local irritation side effectsImprove tolerancePowder deliveryAntiviralsActivated carbonAlkalinity
The invention discloses a nano ganciclovir freeze-drying preparation for injection and a preparation method thereof. The nano ganciclovir freeze-drying preparation for injection comprises the following components in parts by weight: 100-400 parts of ganciclovir, 10-50 parts of dextran 40, 5-50 parts of solubilizer, 10-100 parts of nano carrier material and 10-80 parts of freeze-drying skeleton agent. The preparation method comprises the following steps of: sequentially adding dextran 40, solubilizer, ganciclovir, nano carrier material and freeze-drying skeleton agent into water for injection to be dissolved, filtering a solution step by step, and carrying out freeze-drying, thus a freeze-drying preparation is obtained. According to the preparation method, no activated carbon is introduced, thus a risk that damage is done to a human body when activated carbon particles are introduced into the preparation as the activated carbon is used is avoided; besides, pH of the nano ganciclovir freeze-drying preparation for injection is 6-8 and close to that of plasma, and local irritation produced to the human body owning to overhigh alkalinity is avoided.
Owner:上海华源药业(宁夏)沙赛制药有限公司
Vertical logistics based method for sterile preparation of N-(2)-L-alanyl-L-glutamine
InactiveCN104119423AImprove the level of sterility assuranceAvoid germsPeptide preparation methodsSolventChemistry
The invention relates to the pharmaceutical field, especially to production of bulk drugs by a solvent crystallization method and to a sterile logistics method, particularly a vertical logistics based method for sterile preparation of N-(2)-L-alanyl-L-glutamine. According to the traditional solvent crystallization method, a N-(2)-L-alanyl-L-glutamine reaction solution is concentrated and is adjusted to a pH value of 5-6.5, desalination treatment is performed, then solid-liquid separation is conducted, and the separated solid is transferred by action of gravity. Environmental exposure caused pollution problems are avoided during material transfer. The prepared bulk drug has a high sterile level. In addition, the logistics system with gravity as the main driving force has the characteristics of high transport efficiency and low cost.
Owner:CHONGQING LUMMY PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com