Sterilization method of protein A immuno-absorbent column
An immunoadsorption and sterilization method technology, which is applied in the field of protein A immunoadsorption column sterilization, can solve the problems of active ingredient protein A degradation and denaturation, loss of removal of pathogenic substances, etc., and achieve low production cost and high level of sterility assurance , Solve the effect that can not be sterilized
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The sterilization method of the present embodiment is as follows:
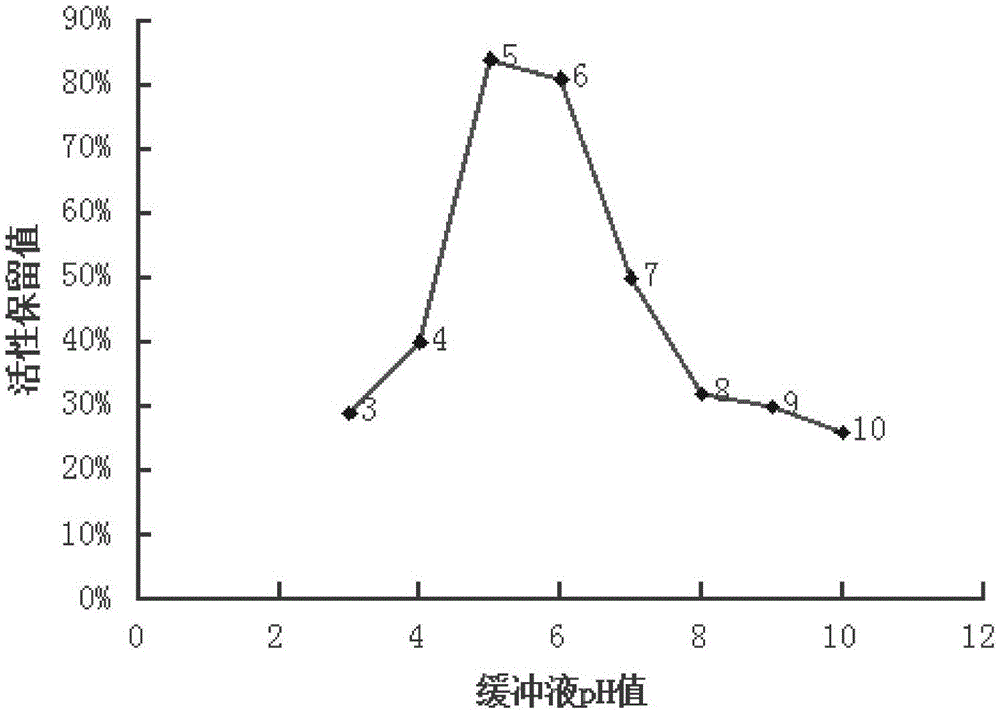
[0032] Take 5g of protein A adsorbent, add 5ml of buffer solution with ion concentration of 0.2M, pH3~10 (choose citric acid buffer solution for pH3~6, phosphate buffer solution for pH7~8, glycine-sodium hydroxide buffer solution for pH9~10 liquid), bottled and steam sterilized at 100°C for 60 minutes.
[0033] Then detect the adsorption performance of the adsorbent before and after sterilization as follows:
[0034] Wash the adsorbent with a large amount of water, take 4.4ml and pack it into the column, first equilibrate the column with 10 times the column volume of the equilibrium solution (1×PBS, pH7.4), and then use 10 times the column volume of the eluent (0.1M, Glycine at pH 2.5) to wash the column, and then equilibrate the column with 10 times column volume of equilibration solution. Take 17ml of plasma to pass through the column at a flow rate of 1.7ml / min. After passing through the plasma, fi...
Embodiment 2
[0037] The sterilization method of the present embodiment is as follows:
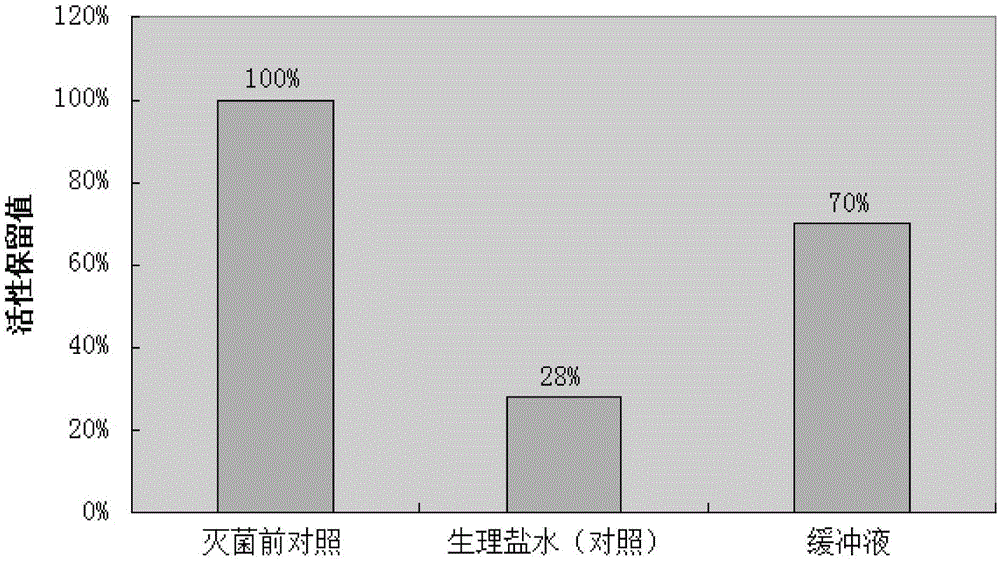
[0038] Take 5g of protein A adsorbent, add 5ml of citric acid buffer (or physiological saline) with an ionic strength of 0.2M and pH6.0, bottle it for steam sterilization, and the sterilization conditions are 121°C and 15min (equivalent to the standard sterilization time F0=15min).
[0039] Then detect the adsorption performance of the adsorbent before and after sterilization with the method of embodiment 1, the results are shown in figure 2 ,from figure 2 It can be seen that under the sterilization conditions of 121°C and 15min, compared with the non-sterilized group, the protein A adsorbent with normal saline as the medium lost a lot of activity after sterilization (the activity retention value was only 28% ), and the protein A adsorbent added with the buffer solution can restore the activity to a greater extent after sterilization (the activity retention value is restored to 70%).
Embodiment 3
[0041] The sterilization method of the present embodiment is as follows:
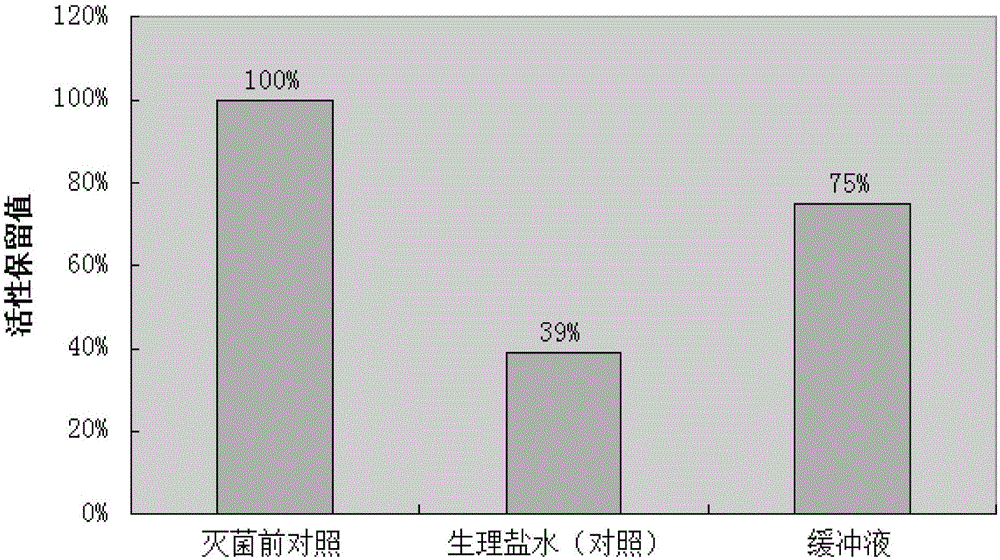
[0042]Take 5g of protein A adsorbent, add 5ml of citric acid buffer solution (or physiological saline) with an ionic strength of 0.2M and pH 6.0, bottle it for steam sterilization, and the sterilization conditions are 115°C and 30min (equivalent to the standard sterilization time F0=8min).
[0043] Then detect the adsorption performance of the adsorbent before and after sterilization with the method of embodiment 1, the results are shown in image 3 ,from image 3 It can be seen from the figure that under the sterilization conditions of 115°C and 30 minutes, compared with the non-sterilized group, the protein A adsorbent with normal saline as the medium had an activity retention value of 39% after sterilization, while adding buffer After sterilization of the protein A adsorbent, the activity retention value is 75%. Compared with Example 2, it can be seen that the reduced steam sterilization condition...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com