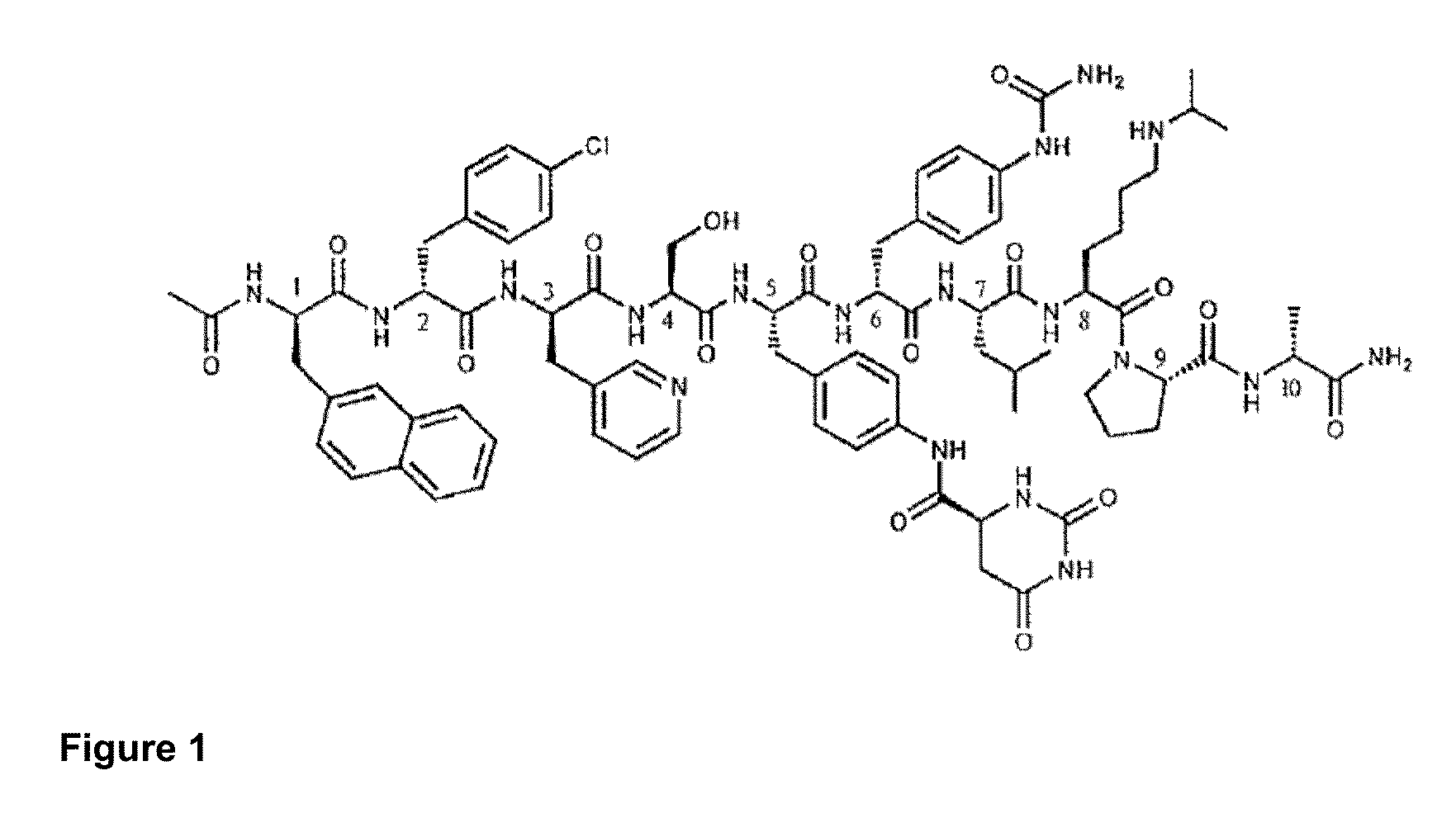
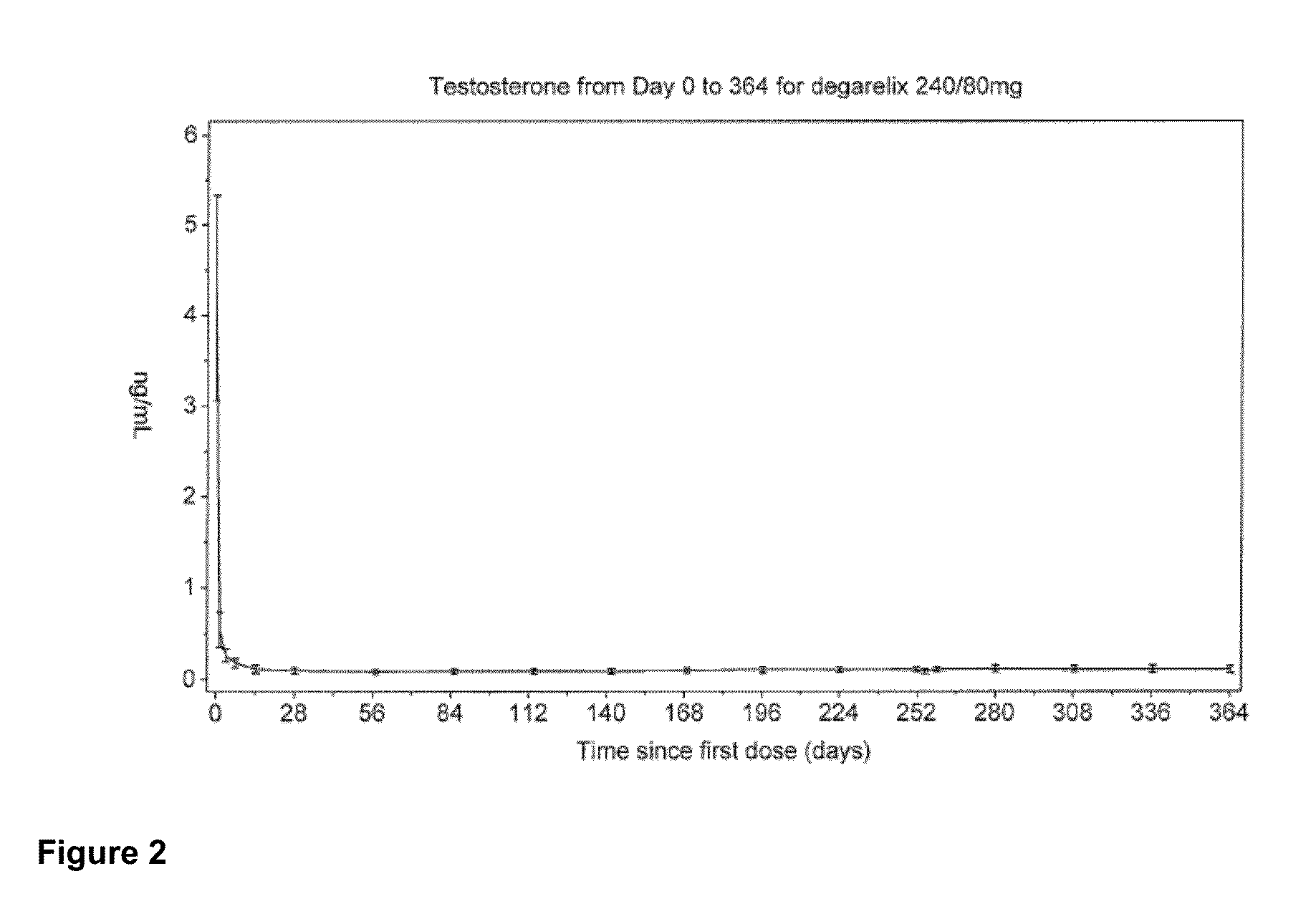
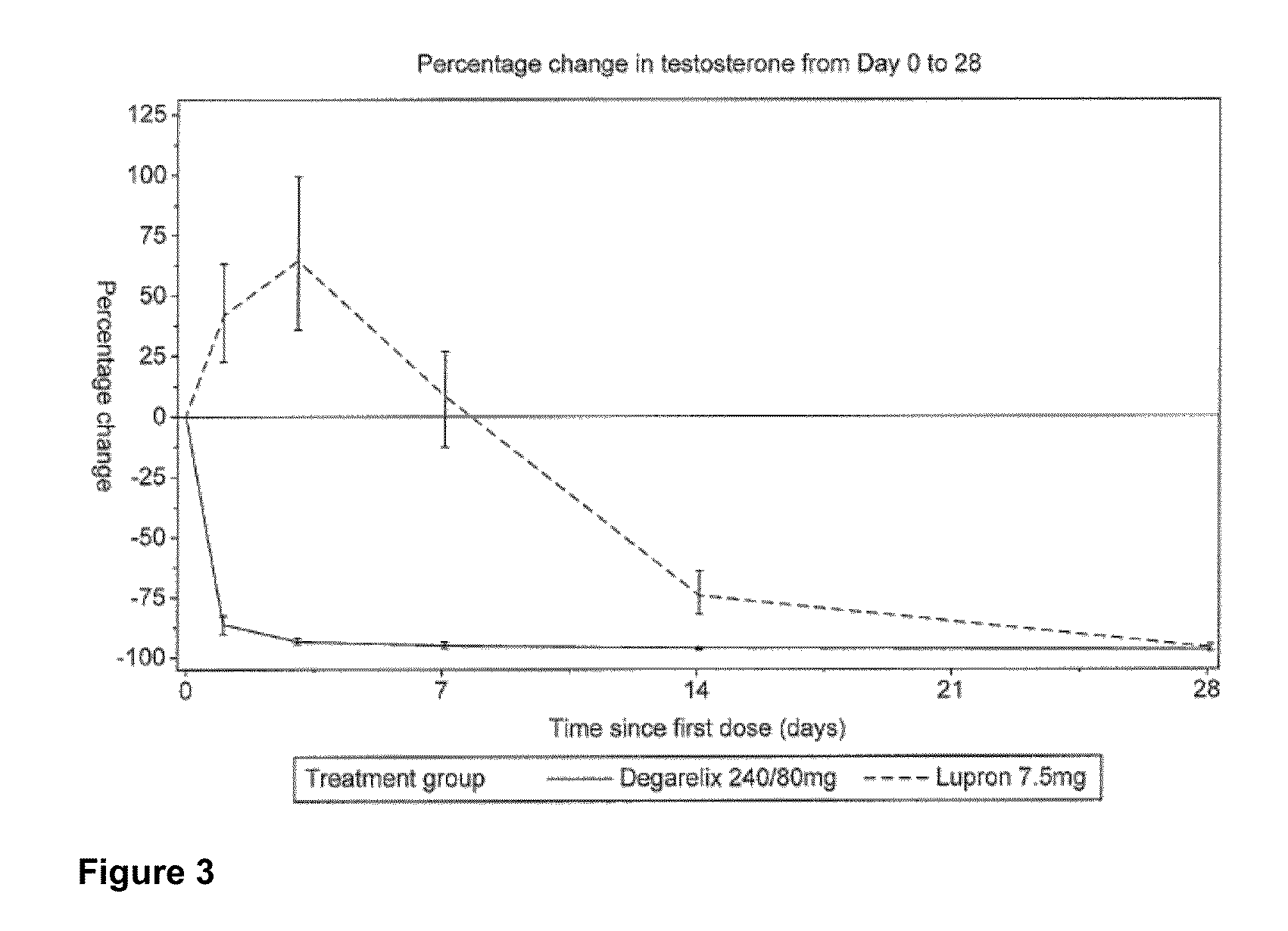
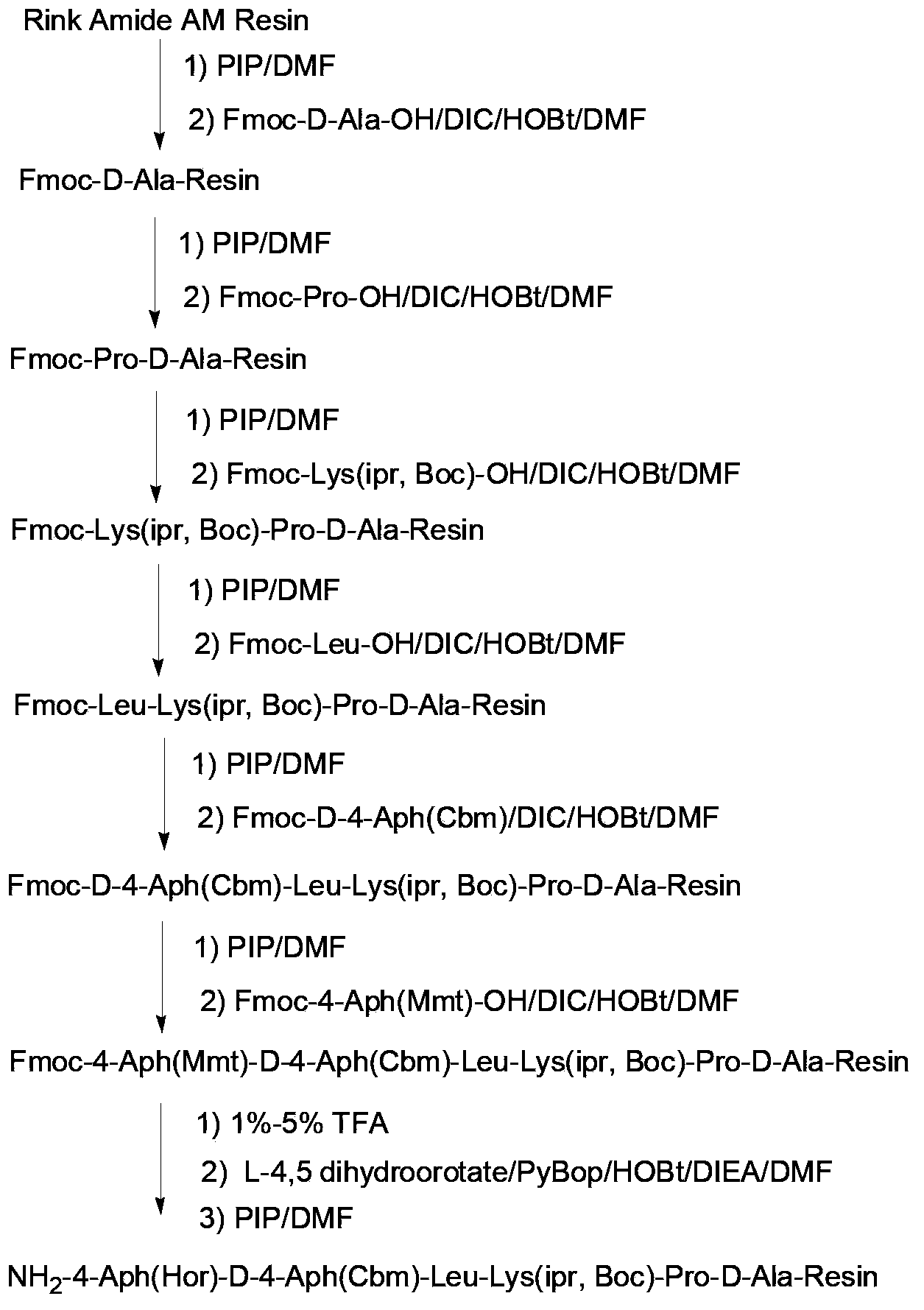
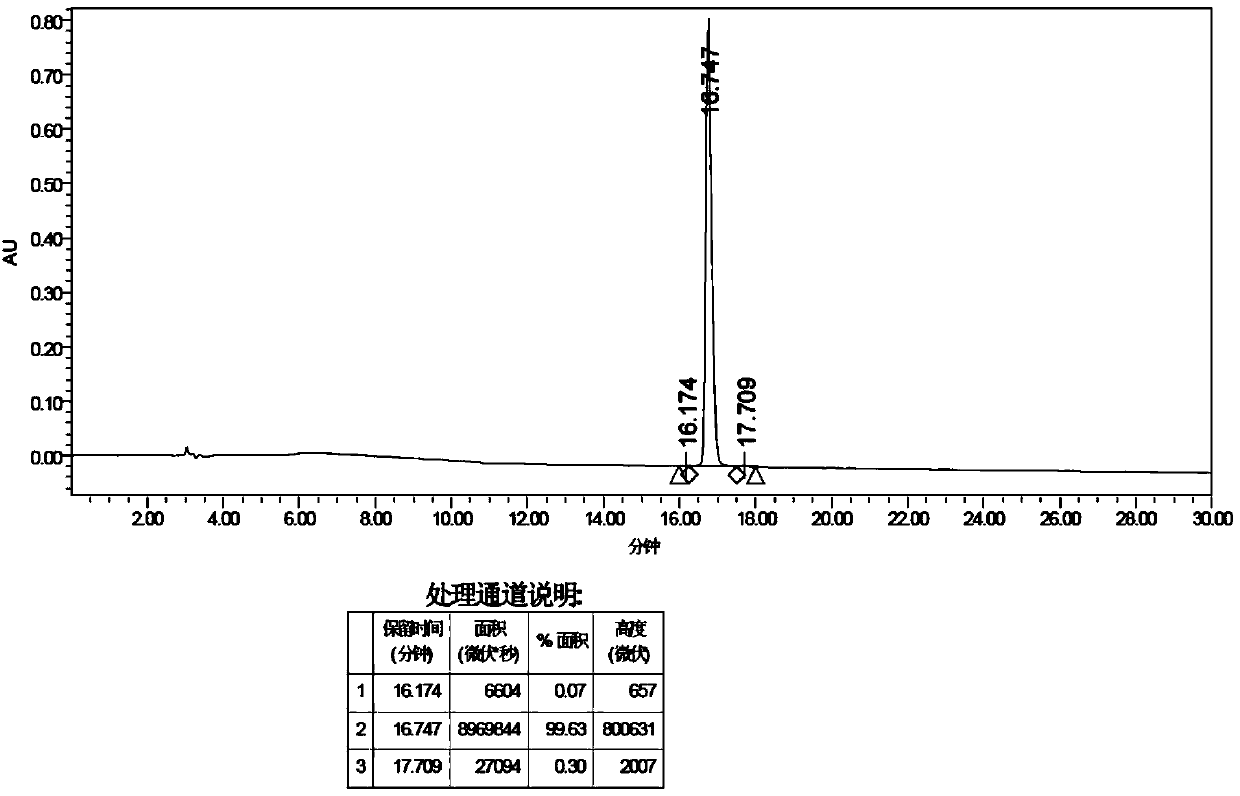
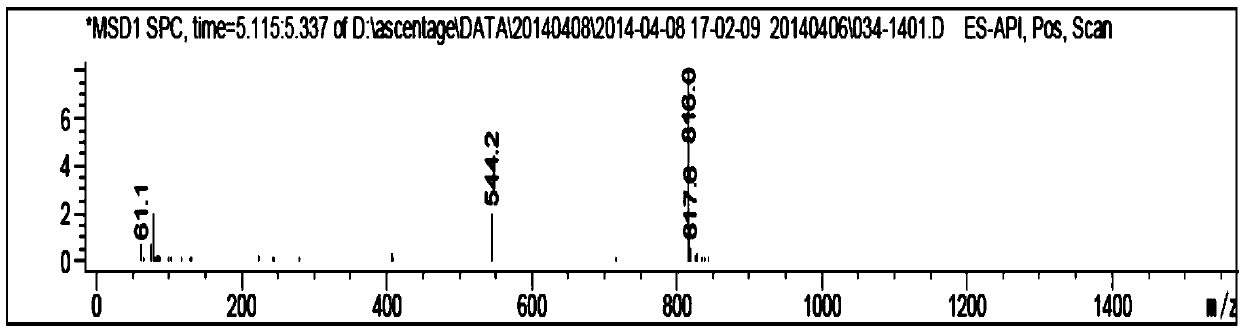
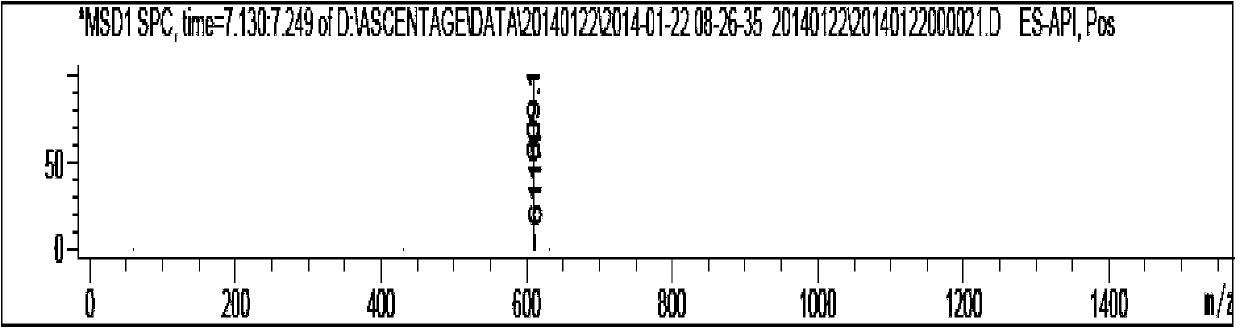
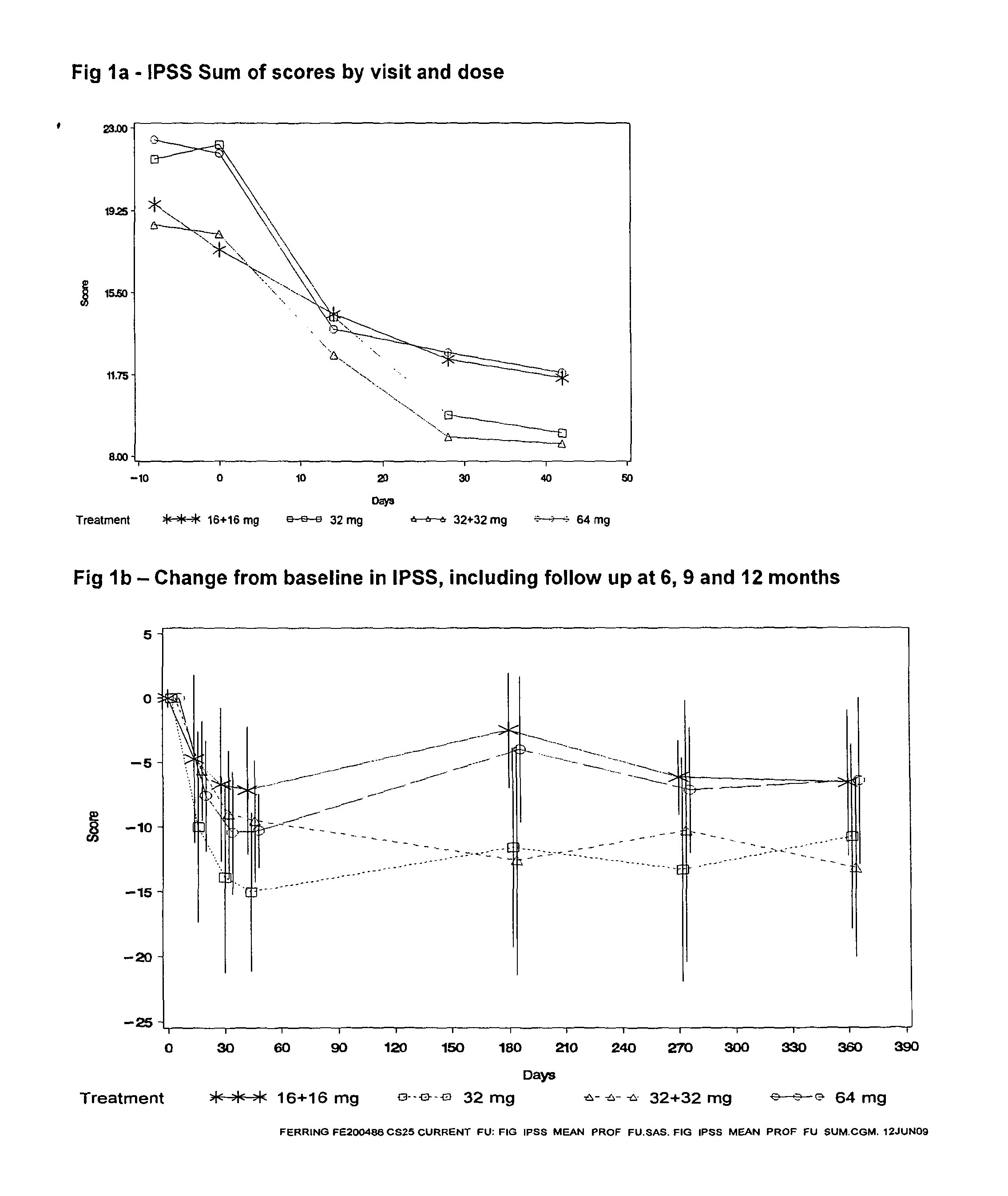
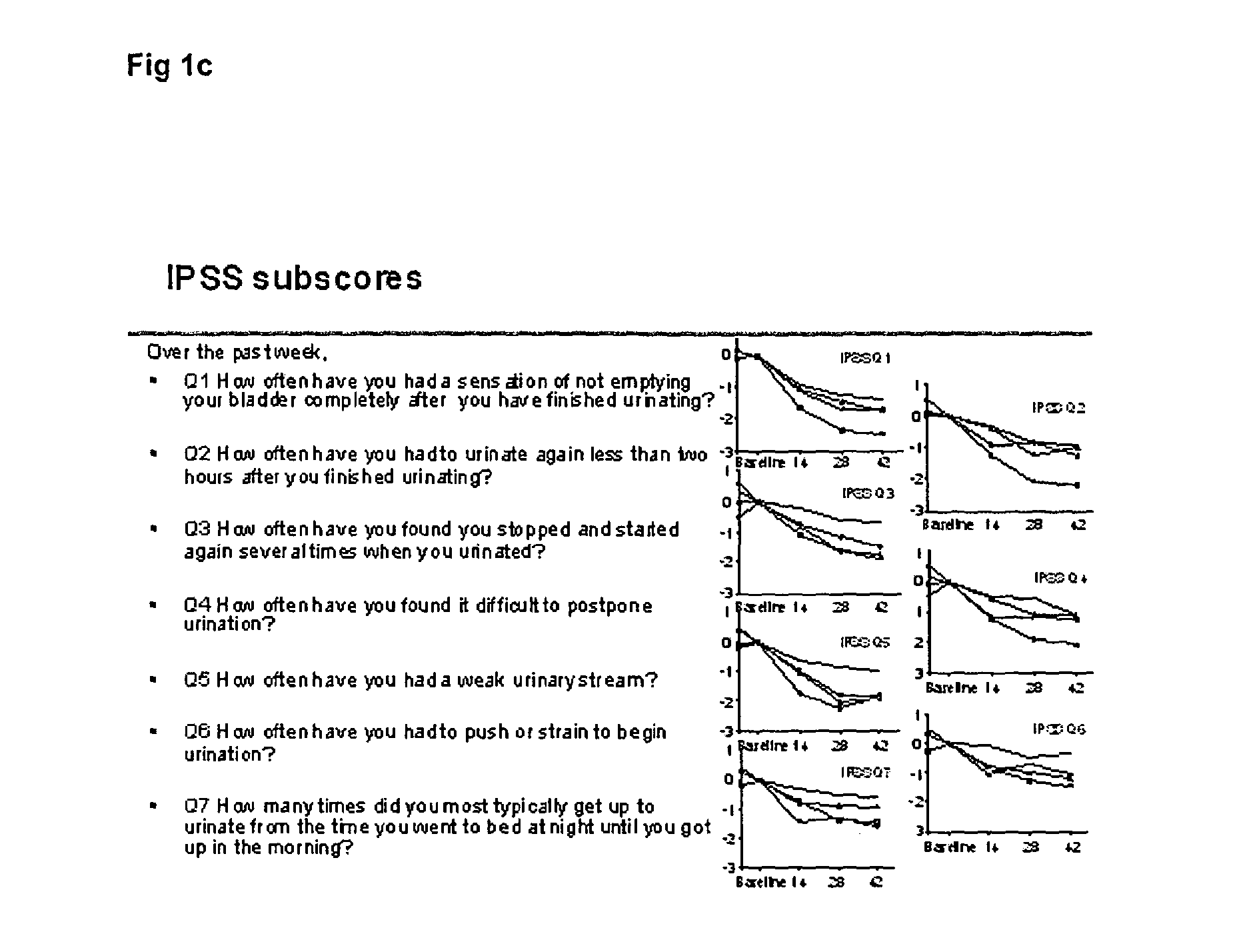
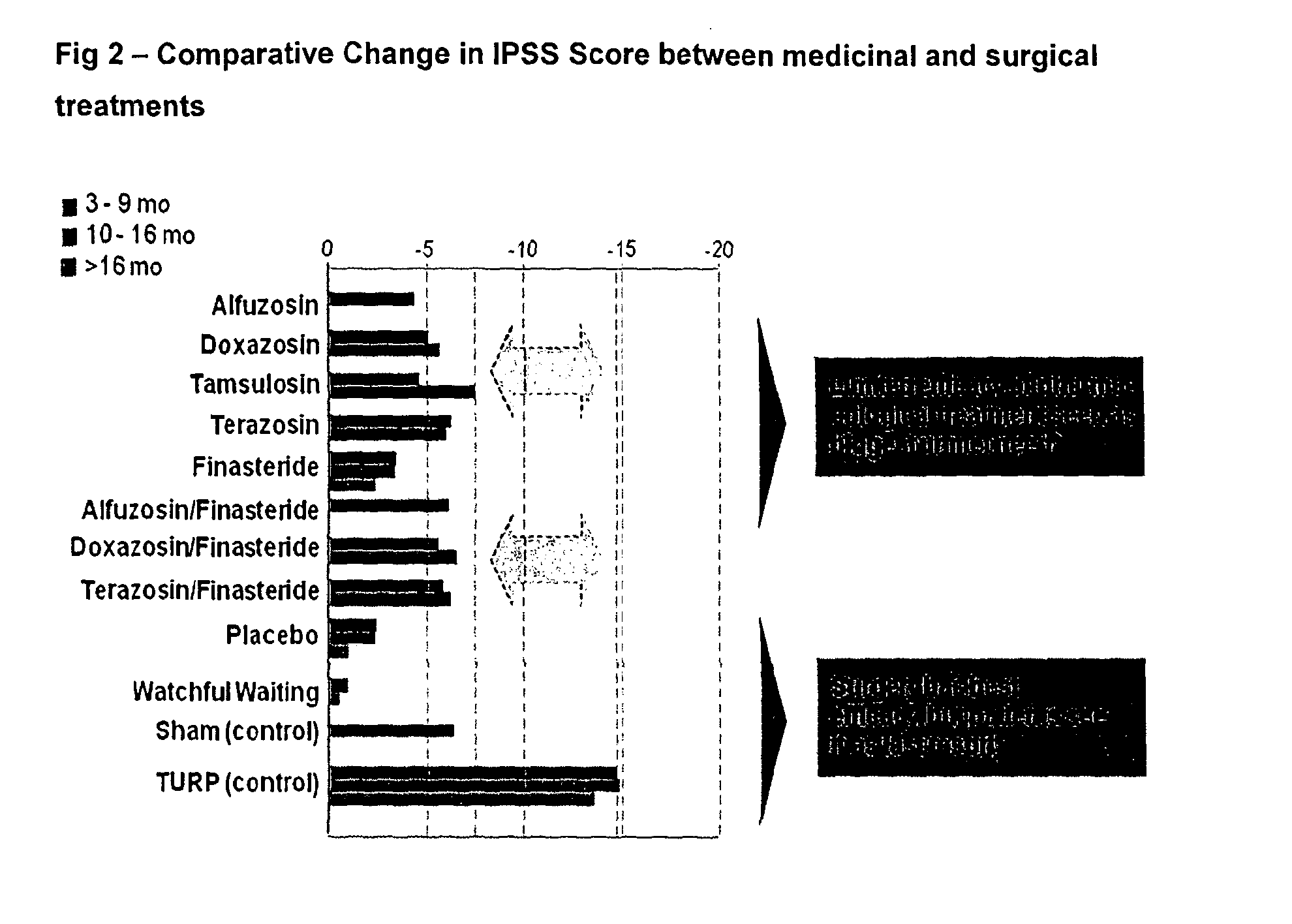
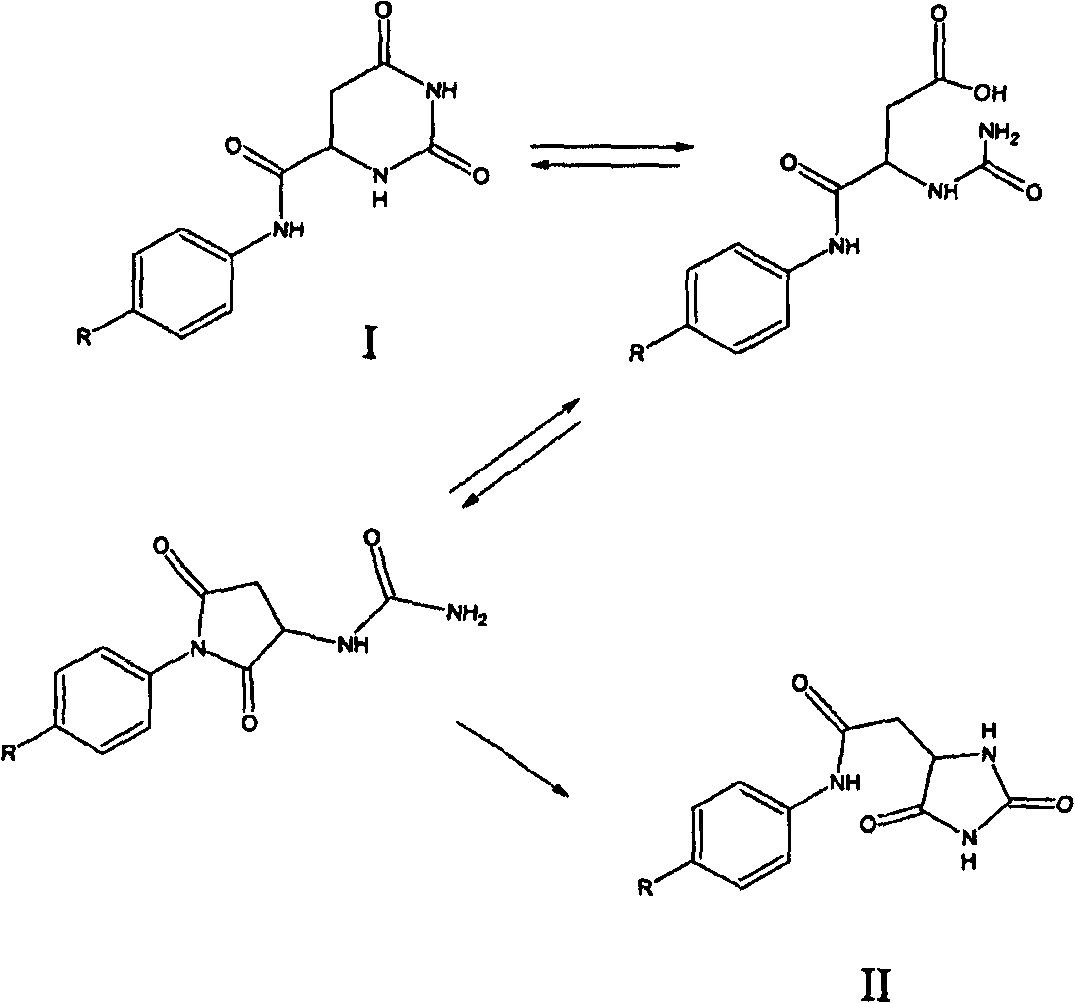
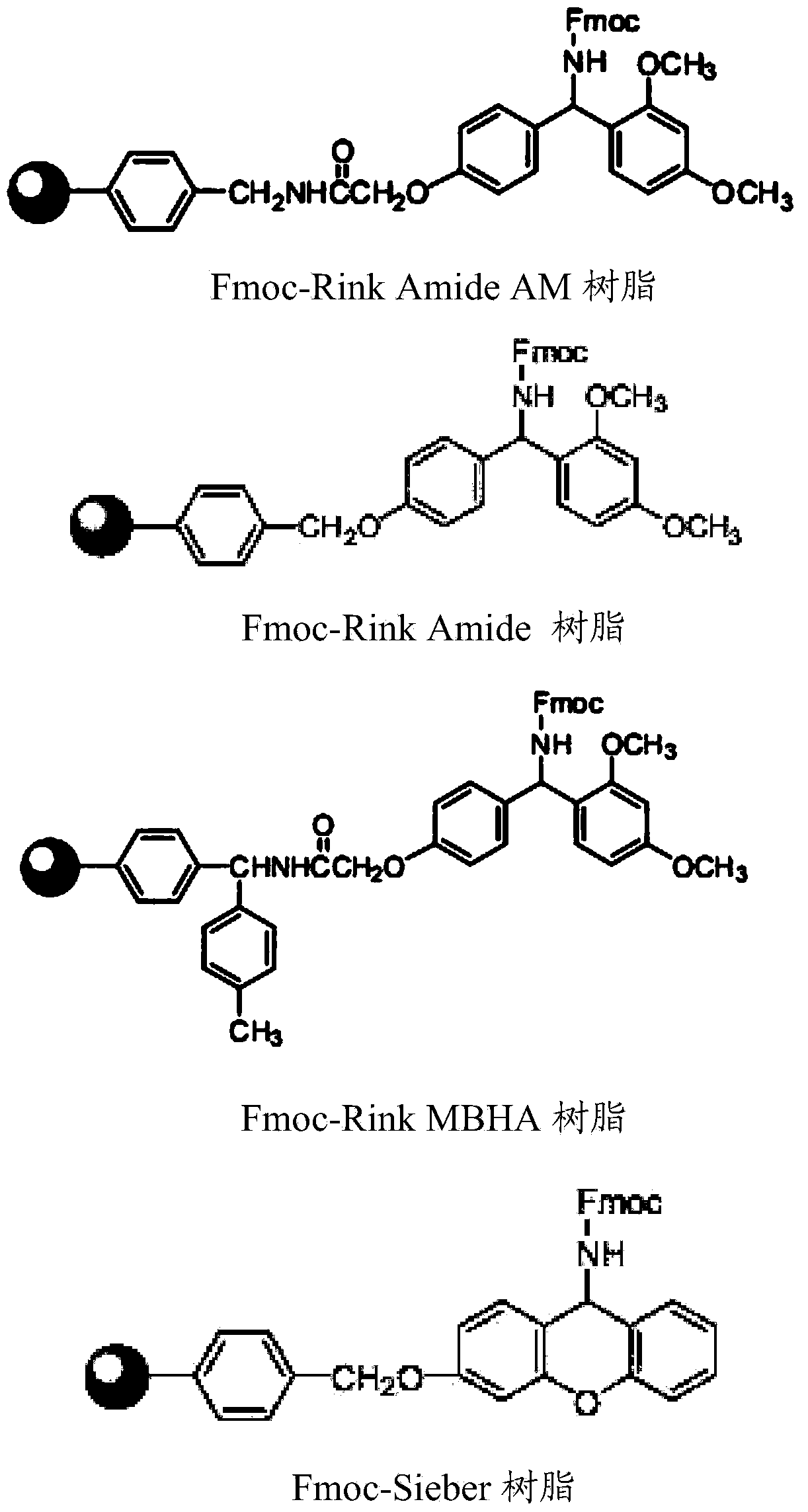
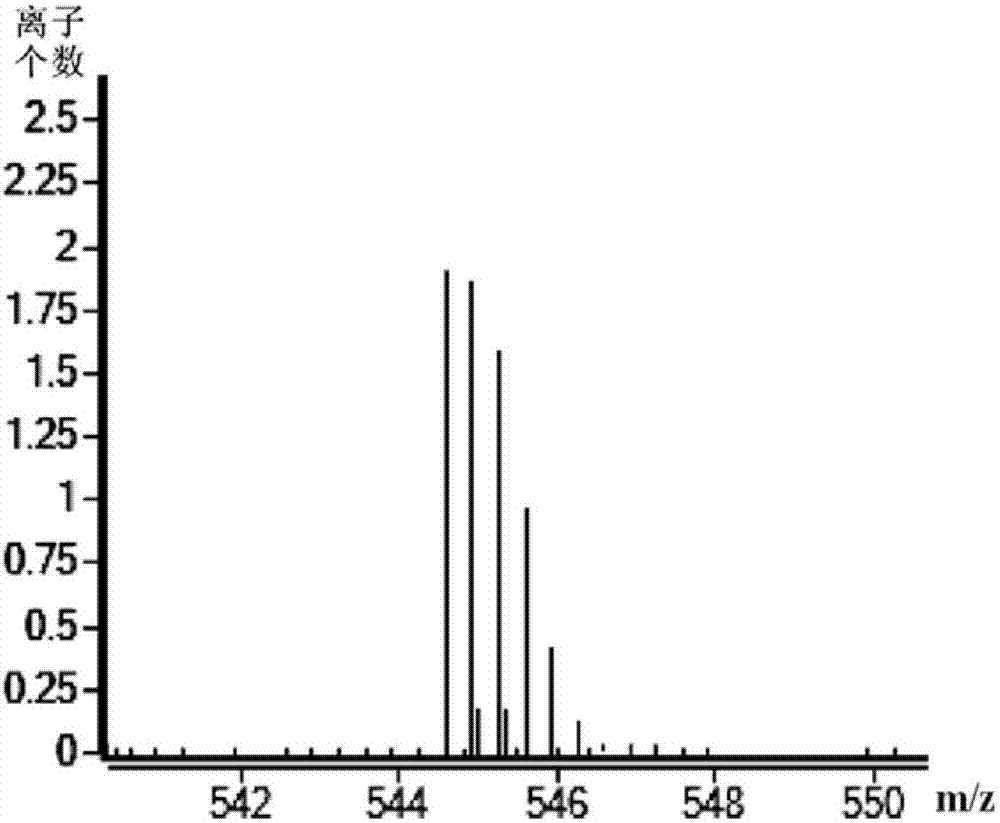
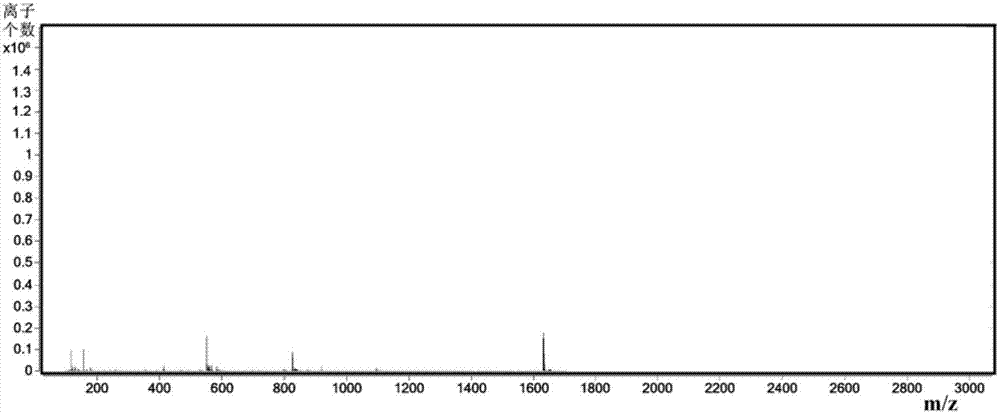
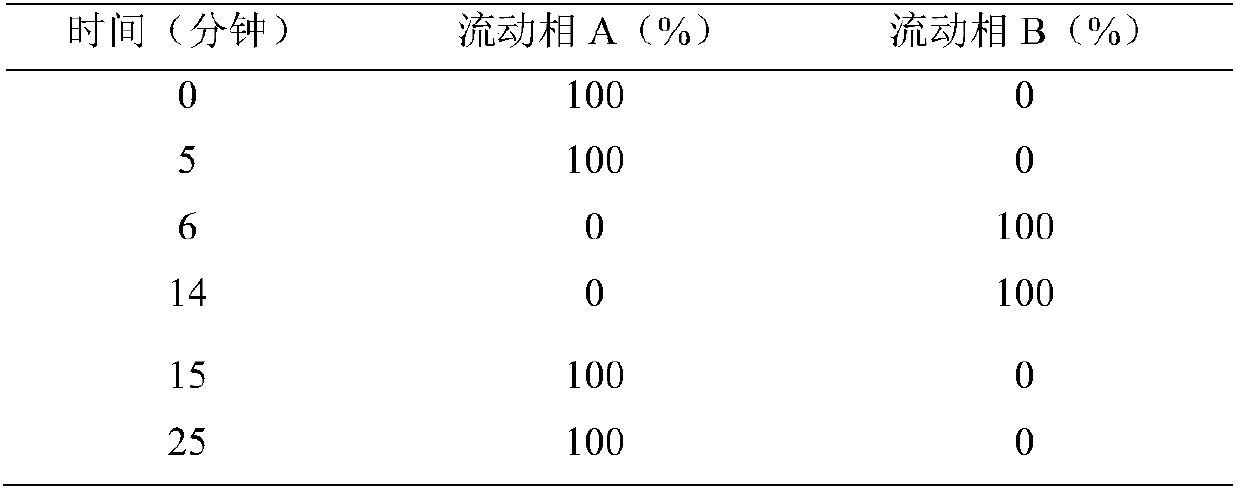
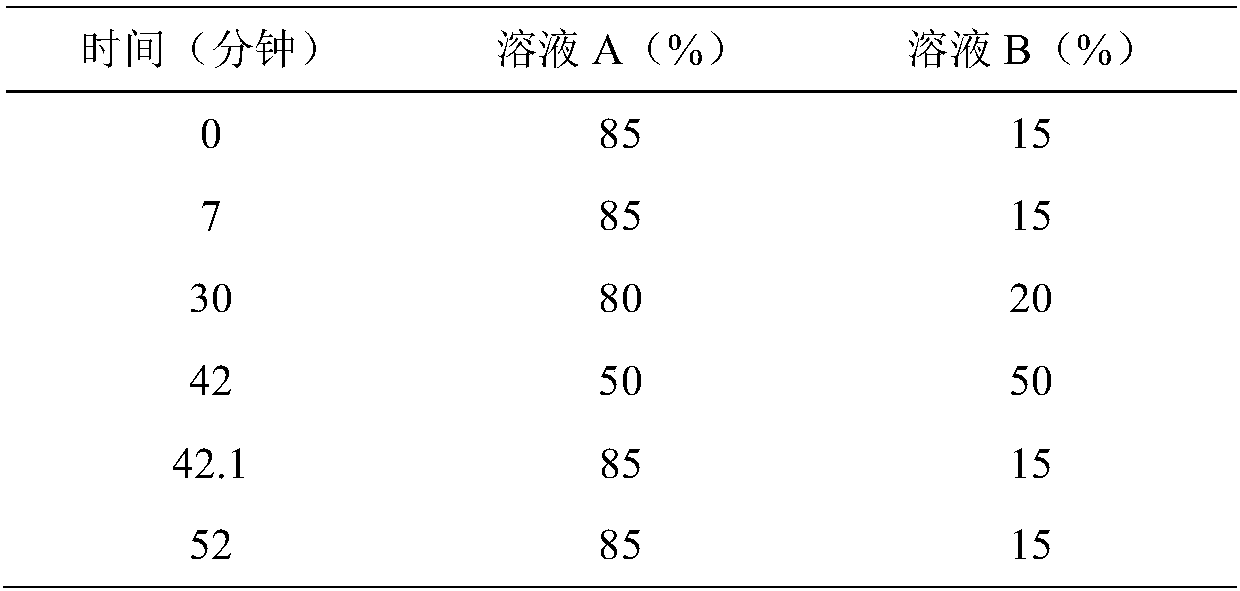
Patents
Literature
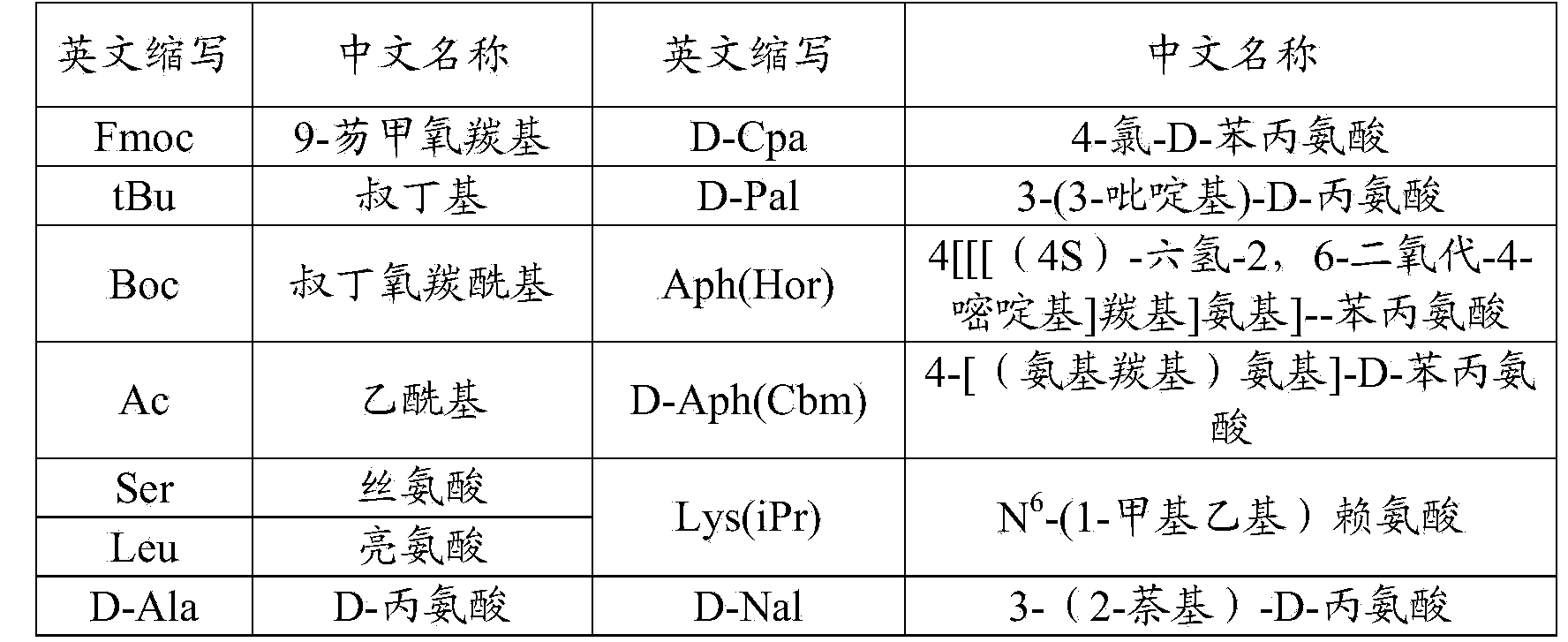
36 results about "Degarelix" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
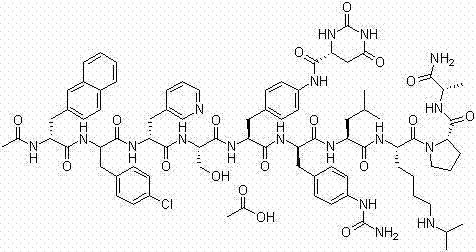
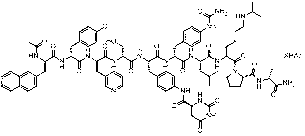
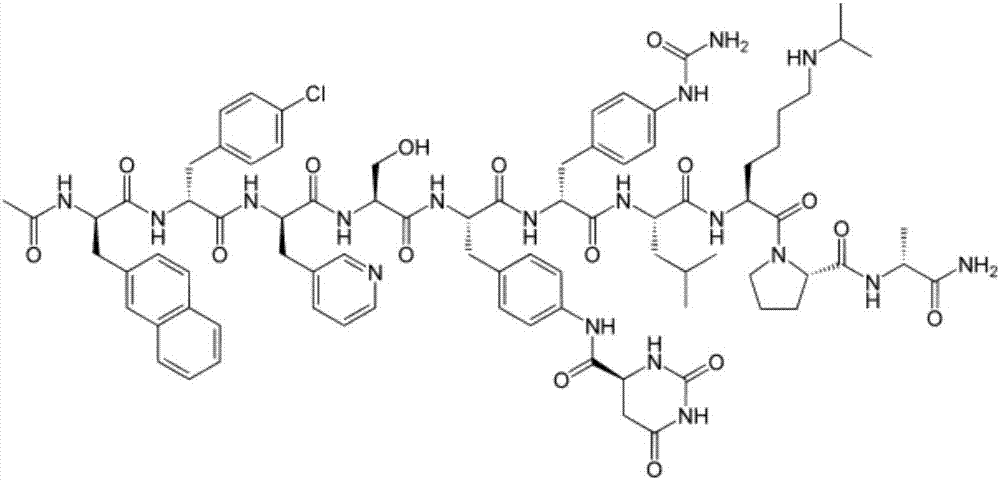
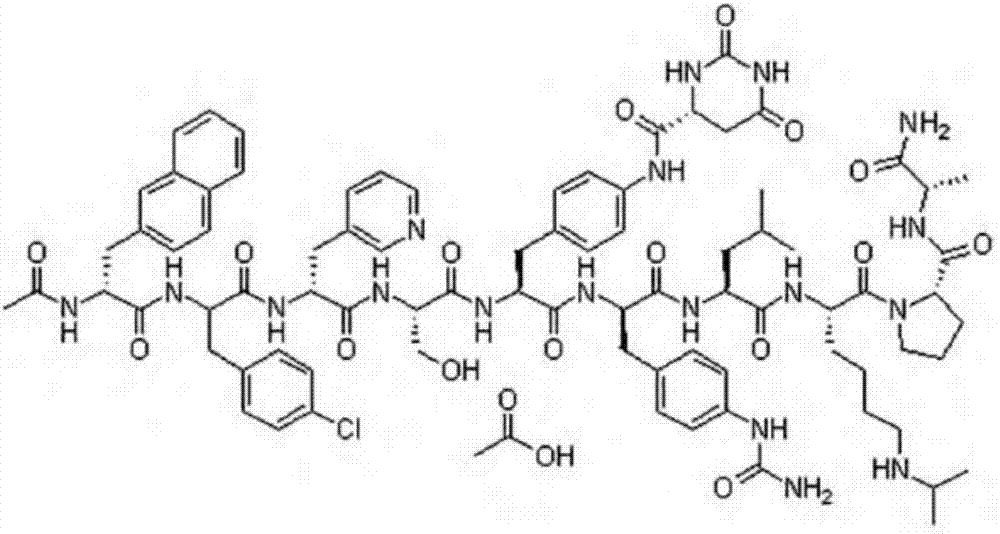
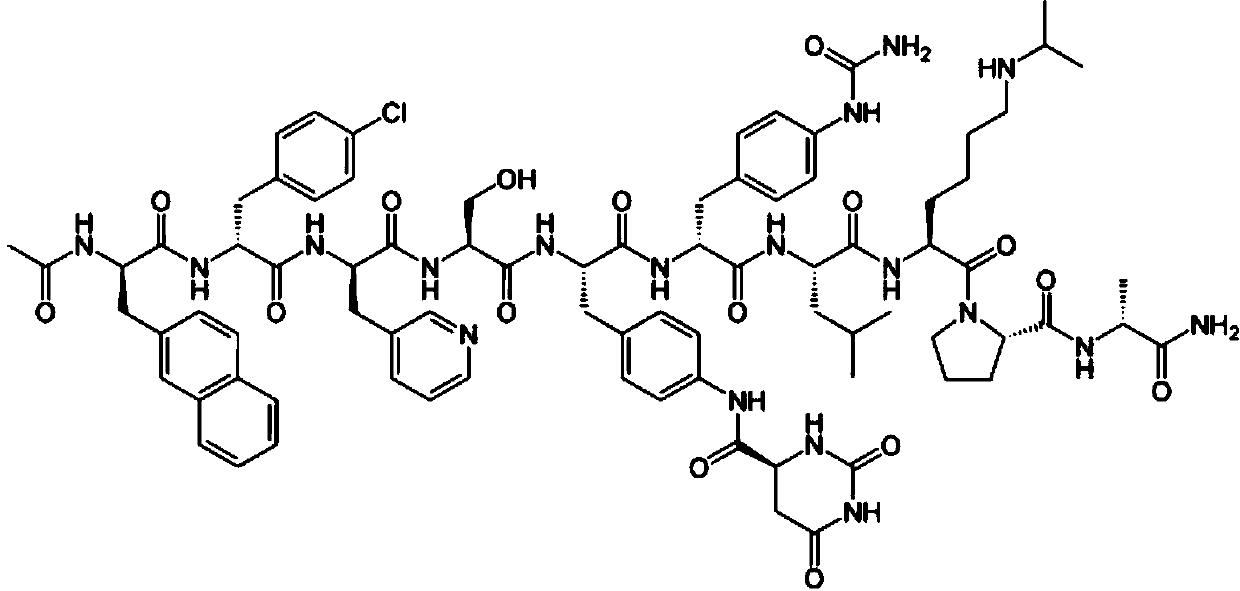
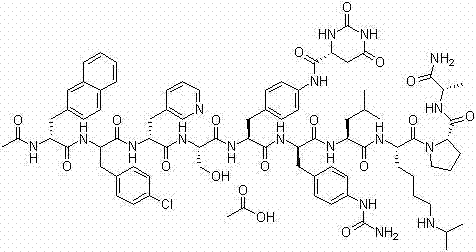
Degarelix is used to treat advanced prostate cancer in men.
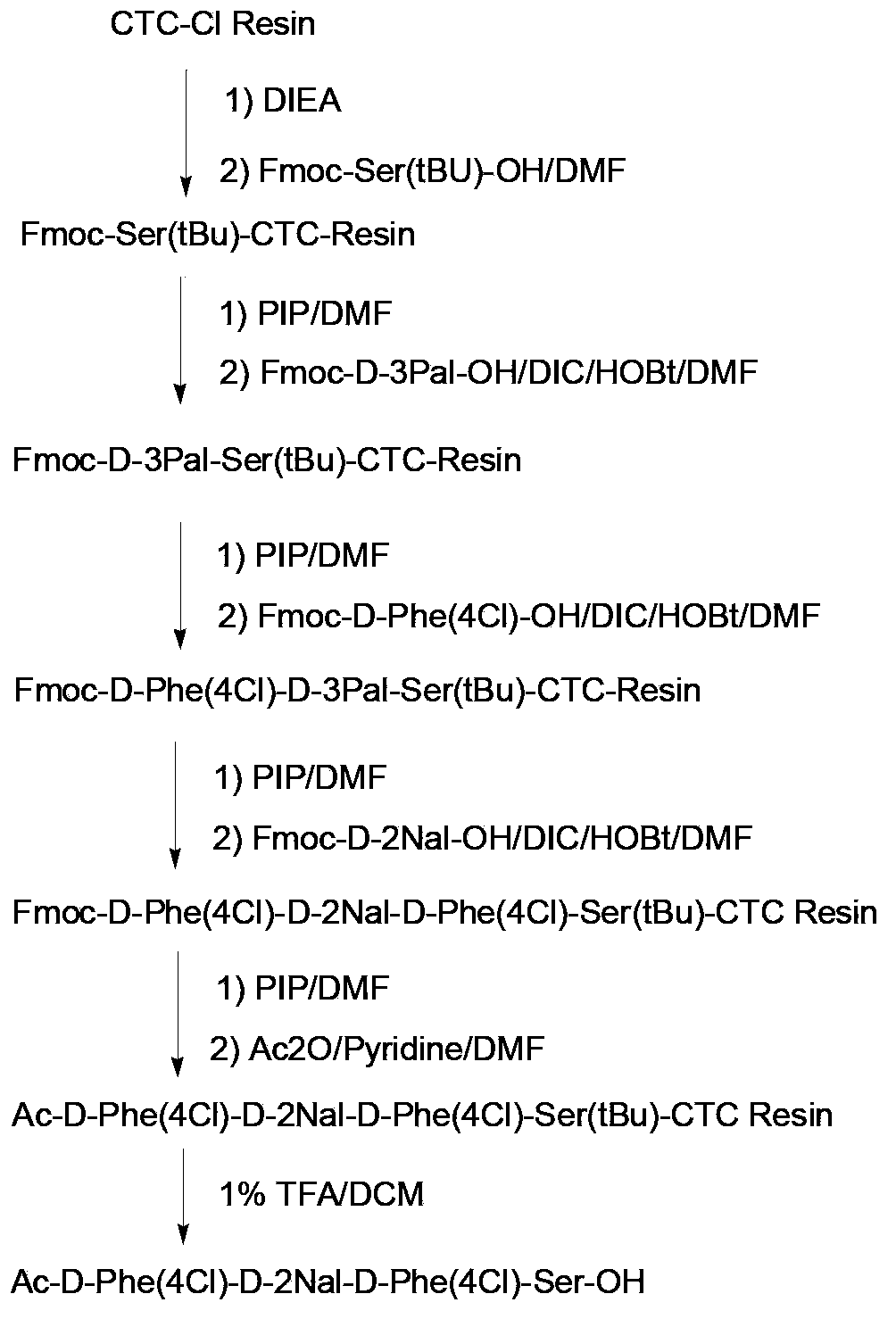
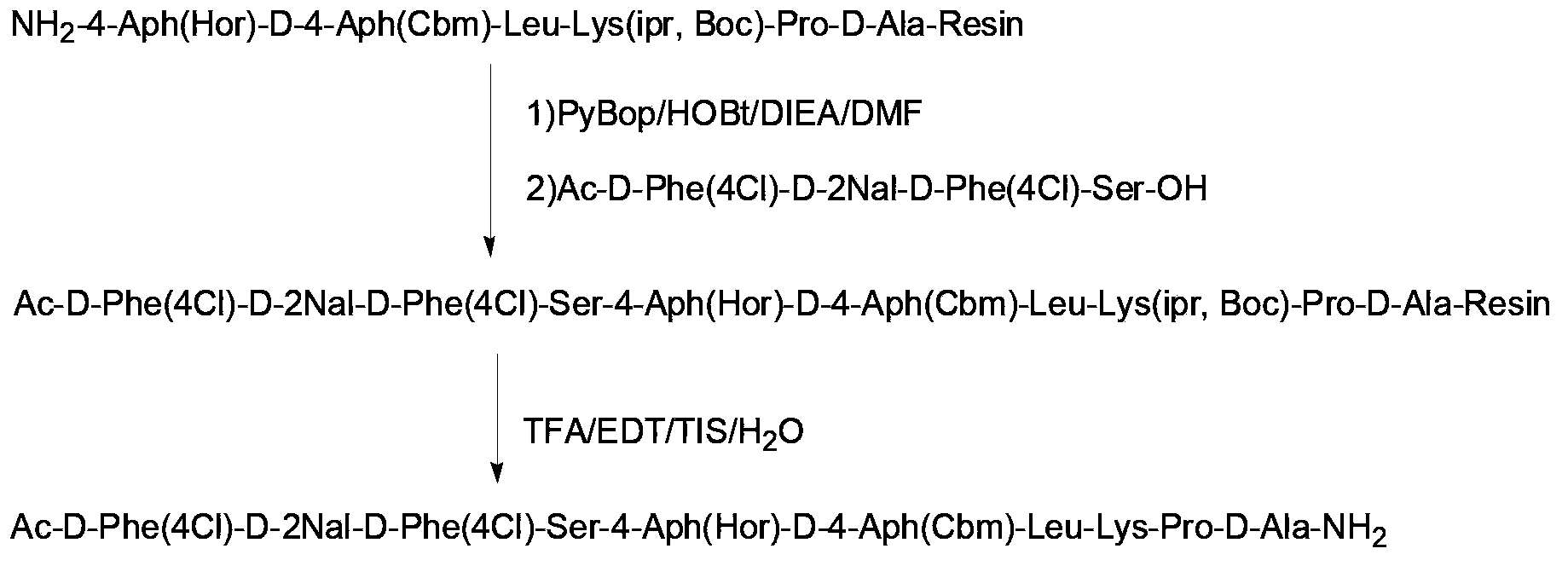
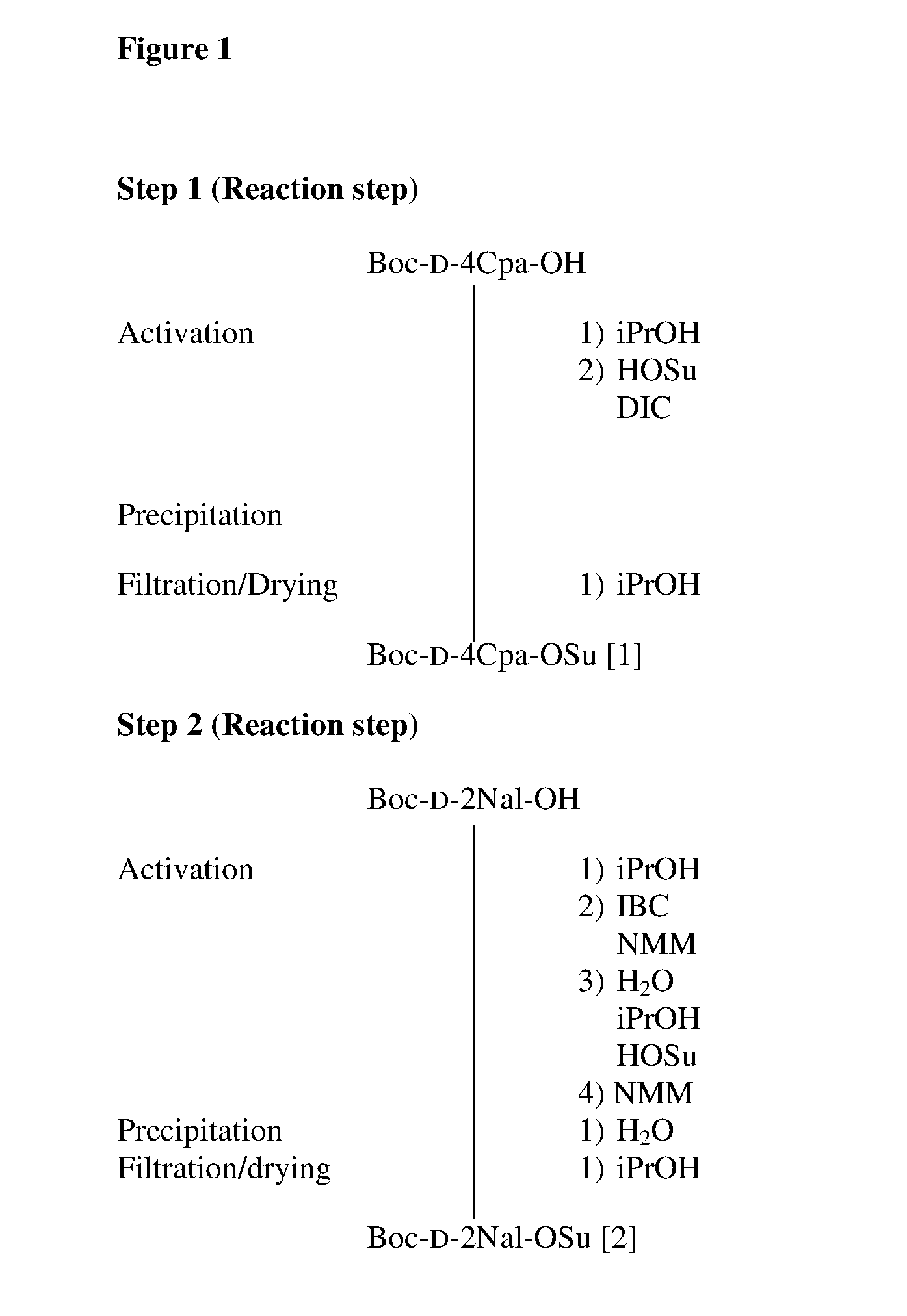
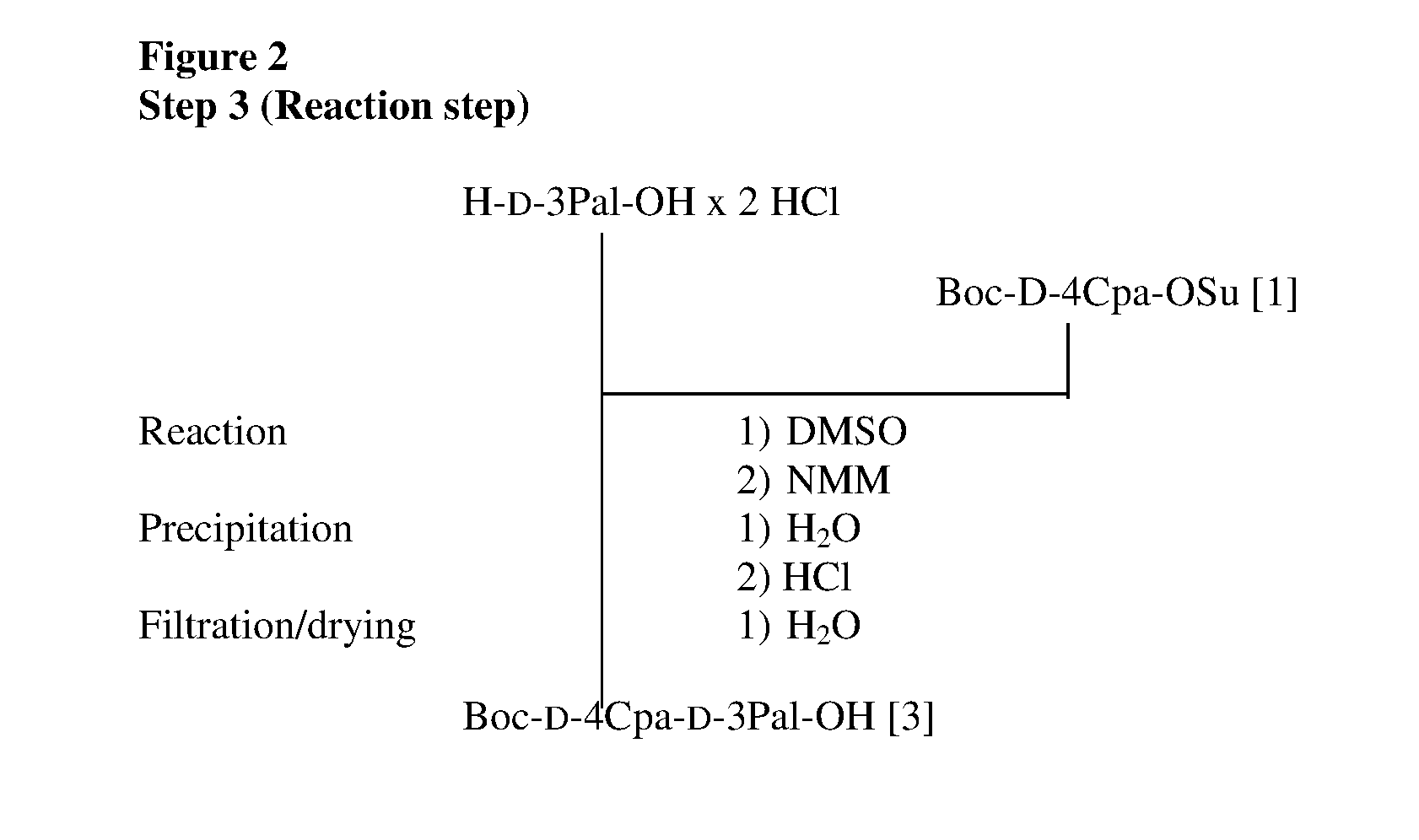
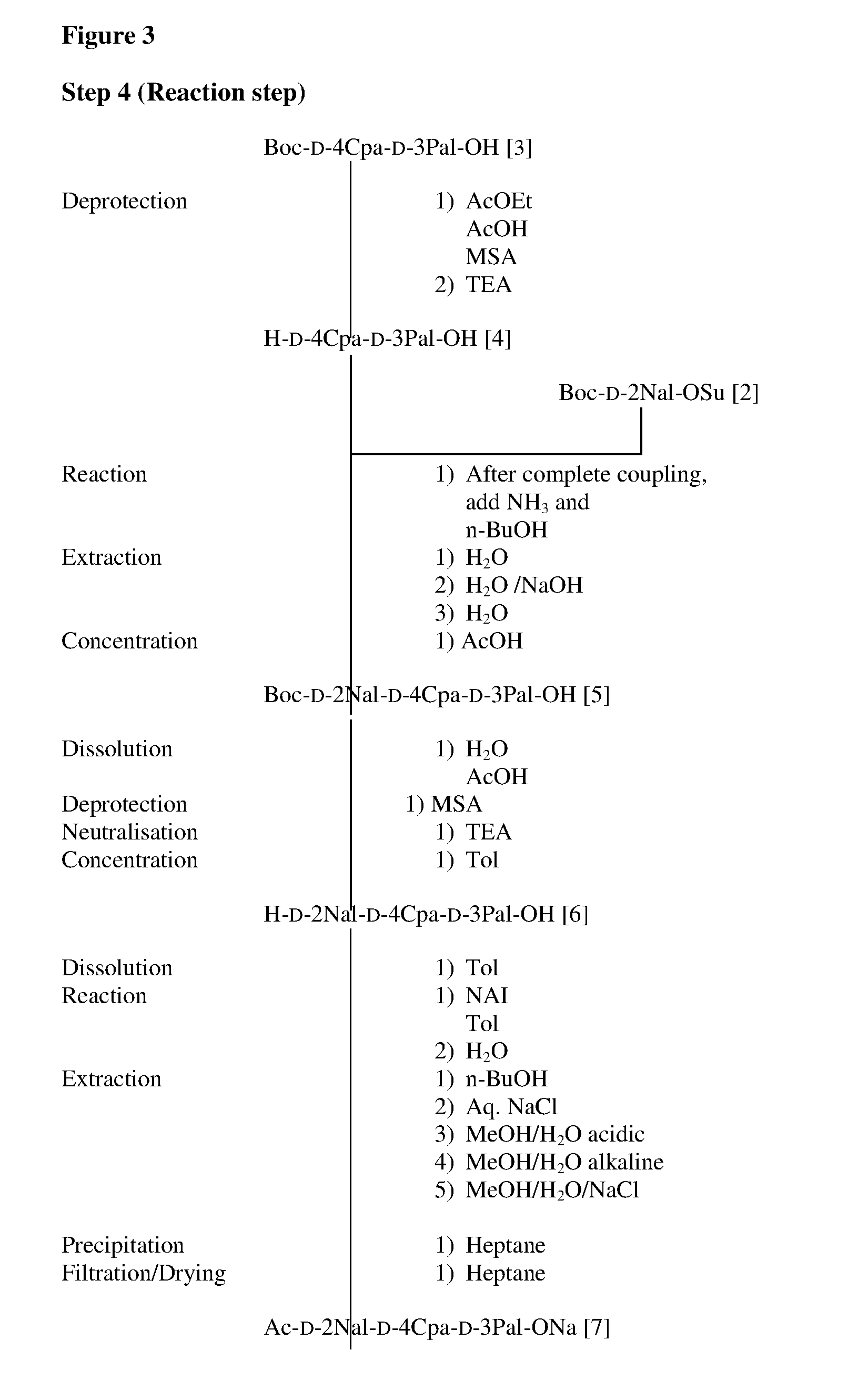
Solid-phase synthetic process for degarelix
ActiveCN102329373AAdvantages of solid phase synthesis processReduce usagePeptide preparation methodsBulk chemical productionAcetic anhydrideSide chain
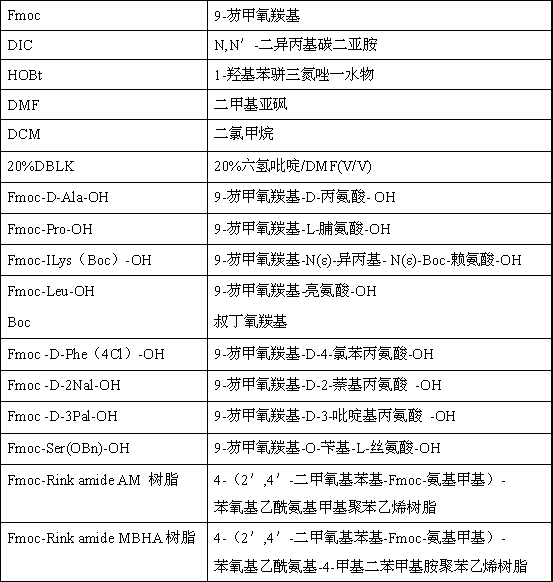
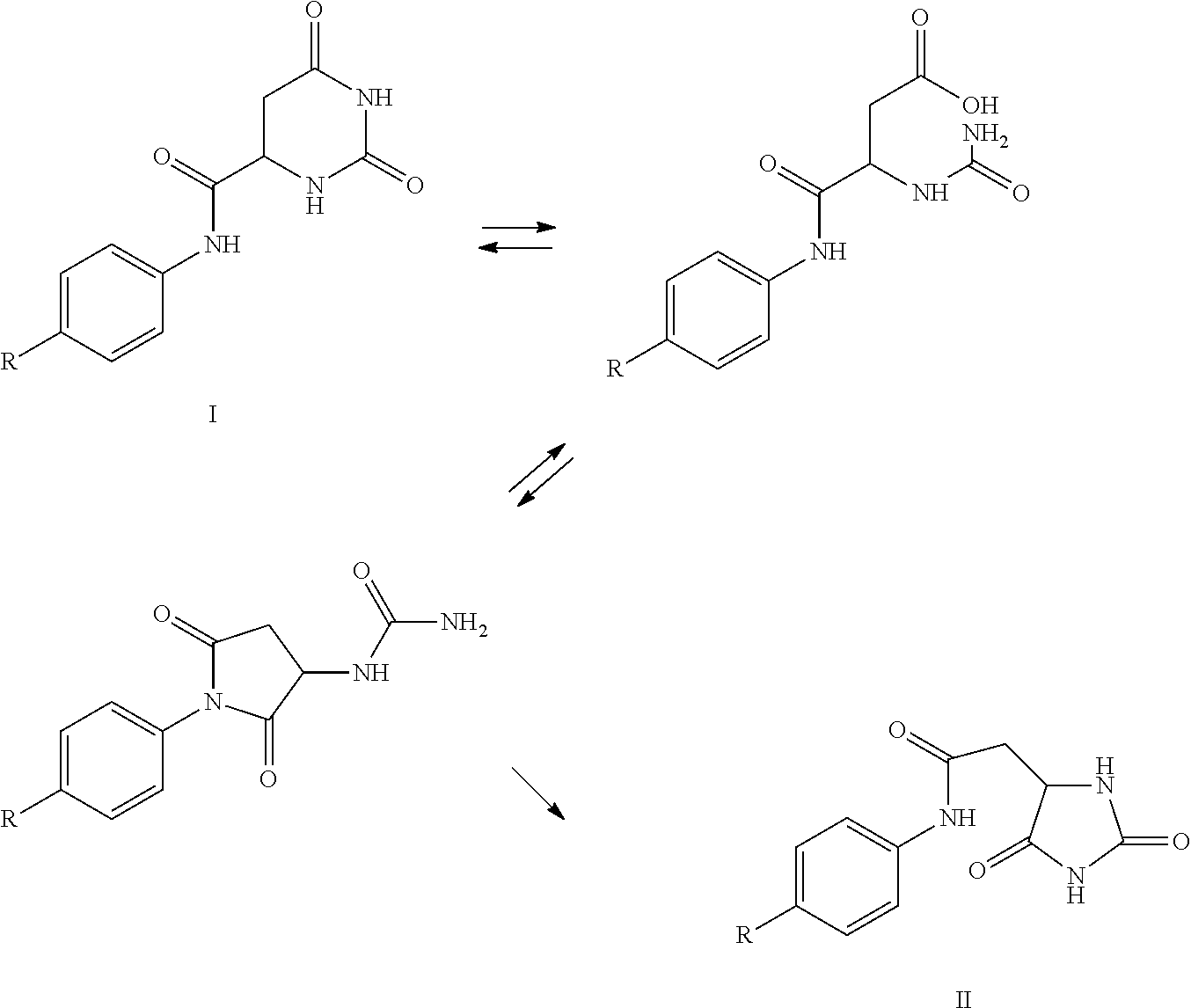
The invention relates to a solid-phase synthetic method for degarelix. The method comprises the following steps of: 1) reacting resin with Fmoc-D-Ala-OH to obtain Fmoc-D-Ala-resin, wherein the resin is amino resin; 2) sequentially connecting according to the amino acid sequence of the degarelix by adopting an Fmoc solid-phase synthetic strategy; 3) removing Fmoc from a N terminal, and acetylating by using acetic anhydride and pyridine; 4) removing a protective group X on the 6th amino acid residue -4Aph(X) from a C terminal; 5) connecting L-4,5-dihydrooroticacid to a side-chain amino group of the 6th amino acid residue -4Aph at the C terminal; 6) cutting peptide resin by using a cracking reagent, precipitating by using anhydrous ether, and centrifuging to obtain crude peptide; and 7) purifying and separating to obtain the degarelix. The method is easy to operate and slightly damages human bodies and environments; and by the process, the content of impurities is effectively reduced, and the large-scale production can be performed.
Owner:HYBIO PHARMA
Method of treating metastatic stage prostate cancer
ActiveUS20090203622A1Deterioration in survival rateImprove life expectancyPeptide/protein ingredientsPharmaceutical delivery mechanismDosing regimenRegimen
The invention provides methods and dosing regimens for treating metastatic stage prostate cancer in a subject using degarelix, as well as related methods of using degarelix in a subject identified as having metastatic stage prostate cancer, and methods of using degarelix to prevent or delay the progression of locally advanced prostate cancer.
Owner:FERRING INT CENT SA
METHOD OF TREATING PROSTATE CANCER WITH GnRH ANTAGONIST
The invention provides methods and dosing regimens for safely and effectively treating androgen-dependent prostate cancer with a gonadotrophin releasing hormone (GnRH) antagonist without causing a testosterone spike and / or other side effect of GnRH agonist therapy such as a urinary tract infection, or an arthralgia-related or cardiovascular side effect. The present disclosure also provides for methods for treating prostate cancer in a patient with a history of at least one cardiovascular event, wherein administration of degarelix to the subject decreases the likelihood of developing or experiencing an additional cardiovascular event compared to treatment with a gonadotrophin releasing hormone (GnRH) agonist.
Owner:FERRING BV
Synthesis of degarelix by solid phase segment method
ActiveCN103351428AReduce usageAvoid repeated contactPeptide preparation methodsBulk chemical productionSide chainTrifluoroacetic acid
The invention discloses synthesis of degarelix by a solid phase segment method, wherein Fmoc-amino acid is connected with resin so that Fmoc-amino acid-resin can be obtained; a solid phase synthesis method is adopted, and the steps of orderly connecting amino acid from the end C to the end N and removing the Fmoc group are carried out, so that Fmoc protected polypeptide resin can be obtained; the method comprises the following steps of: (1) forming polypeptide resin as shown in formula III and polypeptide resin as shown in formula IV, respectively; (2) mixing the polypeptide resin as shown in formula III with 1-5% of trifluoroacetic acid (TFA), connecting L-4,5-dihydroorotate to the side chain amino of the polypeptide resin after the protecting group of the sixth amino acid residue at the end C is removed, and then removing the Fmoc group to obtain polypeptide resin as shown in formula V; removing the Fmoc group of the polypeptide resin as shown in formula IV and acetylating the polypeptide resin, thereby obtaining polypeptide resin as shown in formula VI, and then cutting to obtain segments as shown in formula VII; (3) coupling the polypeptide resin as shown in the formula V with the segments as shown in the formula VII, thereby obtaining the polypeptide resin as shown in formula II; (4) separating polypeptide on the polypeptide resin as shown in the formula II from the resin, thereby obtaining degarelix as shown in formula I.
Owner:HAINAN SHUANGCHENG PHARMA
Method for synthesizing degarelix
InactiveCN102952174AReduce usageAvoid pollutionPeptide preparation methodsBulk chemical productionCombinatorial chemistryPhenylalanine
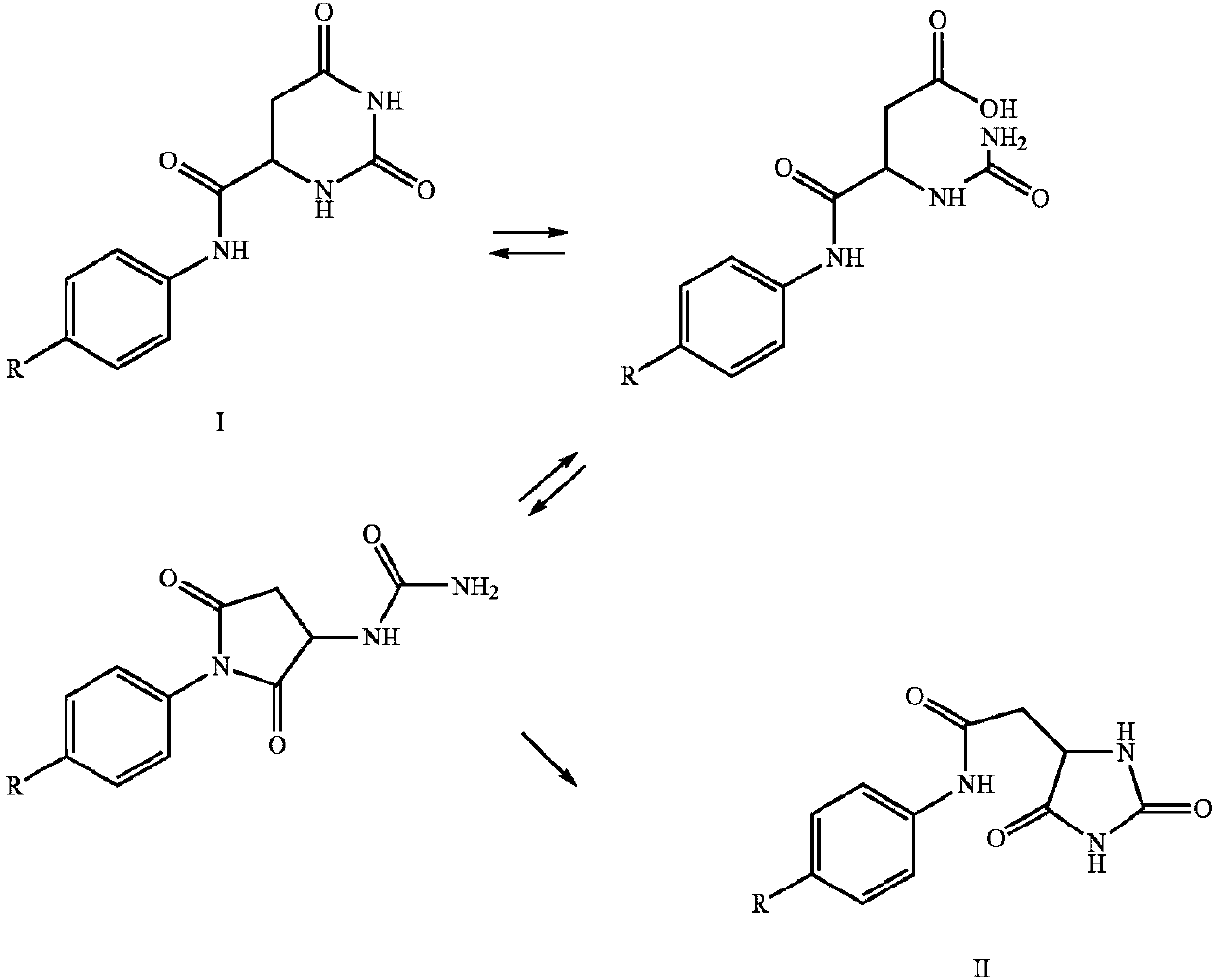
The invention discloses a method for synthesizing degarelix, wherein an amino resin protected with Fmoc is used as a raw material; according to a solid-phase synthesis method, DIC / HOBt (N,N'-diisopropylcarbodiimide / 1-hydroxybenzotrizole) is used as a coupling agent for transpeptidase reaction; 4-serine uses tert-butyl dimethyl to replace tertiary butyl for protection protecting hydroxyl; a hydroorotic acid fragment connected with 4-amino of 5-phenylalanine benzene ring is protected with triphenylmethyl at first, and then introduced, so that the rearrangement side reaction is prevented; and since D-4Aph (Cbm) is used for replacing D-4Aph (Cbm-tBu), a t-Bu removal difficulty is prevented and the occurrence of the side reaction is reduced. A synthesis technology of the method for synthesizing degarelix is simple in steps, easy to control, small in environment pollution and high in yield, thereby being applicable for industrial production.
Owner:济南环肽医药科技有限公司
Solid-phase synthesis method of degarelix
ActiveCN103992392AThe process steps are simpleMild conditionsLuteinising hormone-releasing hormonePeptide preparation methodsAcetic anhydrideDiethyl ether
The invention discloses a solid-phase synthesis method of degarelix. The solid-phase synthesis method of degarelix comprises the following steps: on the basis of taking Fmoc-amino resin as a solid-phase carrier, replacing orotic acid fragments connected with an alanine benzene ring in the fifth site and an amino in the fourth site with ivdde, and performing sequential condensation reaction from the end C to the end N so as to connect 10 protected amino acids, thereby obtaining a full-protective peptide resin; then removing the Fmoc protecting group of end N D-Nal, acetylating by using acetic anhydride and pyridine, replacing ivDde with Hor, cutting the peptide resin by using a splitting agent, and precipitating by using frozen diethyl ether to obtain crude peptide. Besides, the invention also relates to a method for synthesizing a raw material Fmoc-Aph(ivDde)-OH by use of ivDde-OH and Fmoc-Aph-OH. The solid-phase synthesis method of degarelix has the advantages that the ivDde is introduced to synthesize decapeptide firstly and then the Hor is used as a substitute to avoid a rearrangement side reaction; the solid-phase synthesis method is simple in process steps, easy to control, low in influence on a human body and the environment, high in yield, and suitable for large-scale production.
Owner:NANTONG SHIMEIKANG PHARMA CHEM
Composition for the treatment of benign prostate hyperplasia
InactiveUS20120172302A1Low urinary tract symptomReduce riskEnvelopes/bags making machineryPeptide/protein ingredientsProstate hyperplasiaHyperplasia
The present disclosure is directed to compositions and kits comprising degarelix or a pharmaceutically acceptable salt thereof for the treatment of benign prostate hyperplasia (BPH), methods for treating BPH, and methods for preparing compositions of degarelix or a pharmaceutically acceptable salt thereof.
Owner:FERRING BV
Method For The Manufacture Of Degarelix
ActiveCN102428097APeptide/protein ingredientsLuteinising hormone-releasing hormoneOrganic solventOrganic base
In a step-wise synthesis of degarelix comprising 0.3 % by weight or less of 4-([2-(5-hydantoyl)]acetylamino)-phenylalanine analog on (solid support)-NH2 a step comprises providing a solution of an amino acid or peptide of which the a-amino group is protected by Fmoc; contacting the support with the solution in the presence of reagent for forming a peptide bond between a carboxyl group of the amino acid or peptide and (solid support)-NH2; removing Fmoc by contacting the support with an organic base, in particular piperidine, in an organic solvent. Also disclosed is degarelix of high purity prepared by the method of the invention and the use of Fmoc in the synthesis of degarelix.
Owner:POLYPEPTIDE LAB AS
Method for synthesizing degarelix
ActiveCN104177478AHigh purityHigh yieldLuteinising hormone-releasing hormonePeptide preparation methodsD alanineBiological activation
The invention relates to the field of synthesis of medicines and discloses a method for synthesizing degarelix. The method for synthesizing the degarelix comprises the following steps: enabling D-alanine with a protective group coupled with an N end to carry out esterification reaction with amino resin with a protective group coupled with an amino in the presence of a condensation reagent and an activation reagent to obtain peptide resin 1; extending and coupling other protective amino acids one by one by starting from the peptide resin 1 according to a sequence from C end to N end of the amino acid sequence of the degarelix in the presence of the condensation reagent and the activation reagent, to obtain corresponding peptide resins after extending and coupling each time and finally obtain degarelix resin, then carrying out acidolysis to obtain a degarelix crude product, and purifying the degarelix crude product to obtain a degarelix pure product. By virtue of the method for synthesizing the degarelix, a proper synthesizing scheme is selected; the adaptive amino resin and an acidolysis solution are selected; the overall synthesis process is optimized; the purity of the degarelix is obviously improved; the degarelix has a relatively high total yield and is free of pollution to any environment.
Owner:CHENGDU SHENGNUO BIOPHARM
Method for synthesizing Degarelix
ActiveCN105524143AHigh purityThe synthesis process is simpleFollicle-stimulating hormoneLuteinising hormone-releasing hormoneCombinatorial chemistryProtecting group
The invention relates to the field of medicine synthesizing and discloses a method for synthesizing Degarelix. The method is characterized in that the synthesizing of the whole Degarelix is separated into two parts from the fifth amino acid position to the sixth amino acid position, appropriate protecting groups are used for parts of protecting amino acid, and the whole synthesizing process is completed by using specific acidulate agents. The method has the advantages that an appropriate synthesizing scheme is selected, the suitable protecting groups and acidulate agents are selected, the whole synthesizing process is optimized, the purity of the Degarelix is increased evidently, high total yield is achieved, and generation of toxic hydantoin degradation products is avoided.
Owner:CHENGDU SHENGNUO BIOPHARM
Method for preparing degarelix by using solid and liquid combination
InactiveCN107022002AReduce usageAvoid the problem of 4Aph(L-Hor) rearrangement side reactions caused by repeated use of alkaline conditions to remove FmocLuteinising hormone-releasing hormonePeptide preparation methodsCombinatorial chemistrySolid-phase synthesis
The invention relates to the field of polypeptide synthesis, and in particular to a method for preparing degarelix by using a solid and liquid combined chemical method. According to the invention, a fragment peptide Ac-D-2Nal-D-4Cpa-D-3Pal-Ser(tBu)-4Aph(Fmoc)-OH is firstly synthesized for solid phase synthesis of degarelix. The synthesis process is simple, used raw materials are easy to get, and enlargement production is easy to realize; the use of a highly toxic reagent HF is avoided, after spitting by a TFA combination, a refined peptide with purity of 99.5% can be obtained after purification and lyophilization, and the total yield reaches 65%.
Owner:JINAN KANGHE MEDICAL TECH
Method for the manufacture of degarelix
ActiveUS8828938B2Less costlyPeptide/protein ingredientsLuteinising hormone-releasing hormoneOrganic solventOrganic base
Owner:POLYPEPTIDE LAB AS
Degarelix freeze-dried powder injection for injection and preparation method thereof
InactiveCN107693496AImprove stabilityExtended gel timePowder deliveryPeptide/protein ingredientsFreeze-dryingMedical prescription
In the process of researching a degarelix freeze-dried preparation, the inventor finds that viscosity of a medicine-containing solution can be lowered effectively and gelation time can be prolonged byadding a certain amount of tertiary butanol into a water solution before freeze-drying, the inventor further finds that adding of trehalose is conducive to further prolonging the gelation time and improving long-time stability of the preparation. On this basis, a degarelix freeze-dried preparation prescription and process are developed and are simple, easy for quality control and suitable for industrial production.
Owner:天津双硕医药科技有限公司
Preparation method of key intermediate of degarelix
ActiveCN107955061AAvoid racemizationHigh purityLuteinising hormone-releasing hormoneN dimethylformamideTert-Butyloxycarbonyl protecting group
The invention relates to a preparation method of a key intermediate of degarelix. The preparation method comprises that L-lysyl-N6-(1-isopropyl-1-t-butyloxycarboryl)-L-lysyl-L-prolyl-D-alaninamide andFmoc-L-leucine react in the mixed solution of N, N-dimethylformamide and tetrahydrofuran with the presence of organic alkali and a condensing agent to obtain the peptide fragment of the degarelix ofFmoc-L-leucine-N6-(1-isopropyl-1-t-butyloxycarboryl)-L-lysyl-L-prolyl-D-alaninamide. The preparation method of the key intermediate of the degarelix can reduce racemization during peptide condensationand prepare the peptide fragment of the degarelix at high purity and high yield.
Owner:连云港恒运药业有限公司
Preparation method of degarelix
InactiveCN107344960AHigh purityEasy to operateLuteinising hormone-releasing hormonePeptide preparation methodsReaction rateSide chain
The invention provides a preparation method of degarelix. The preparation method comprises the following steps: adopting a solid-phase synthesis method to carry out first condensation reaction on materials including Fmoc-4Nph-OH according to the amino-acid sequence of the degarelix in sequence to obtain a first intermediate product; reducing amino-acid residues 4Nph in the first intermediate product into 4Aph to obtain a second intermediate product; carrying out second condensation reaction on side-chain amino of the 4Aph and L-4, 5-dihydroorotic acid to obtain a third intermediate product; and adopting a cracking agent to crack the third intermediate product so as to obtain the degarelix. The preparation method provided by the invention has the advantages that the material Fmoc-4Nph-O is more stable under an alkali environment, and due to adoption of the material, protective groups do not need to be introduced, and side reaction caused by introduction of the protective groups is effectively avoided, so that the purity of the degarelix product can be increased; the reaction operation is simple, the reaction rate is high, and the benefit for greatly reducing the process cost and the synthesis difficulty is achieved.
Owner:ASYMCHEM LAB TIANJIN
Purification method of degarelix
ActiveCN109748954ASimple and fast operationLow costLuteinising hormone-releasing hormonePeptide preparation methodsPurification methodsAlcohol
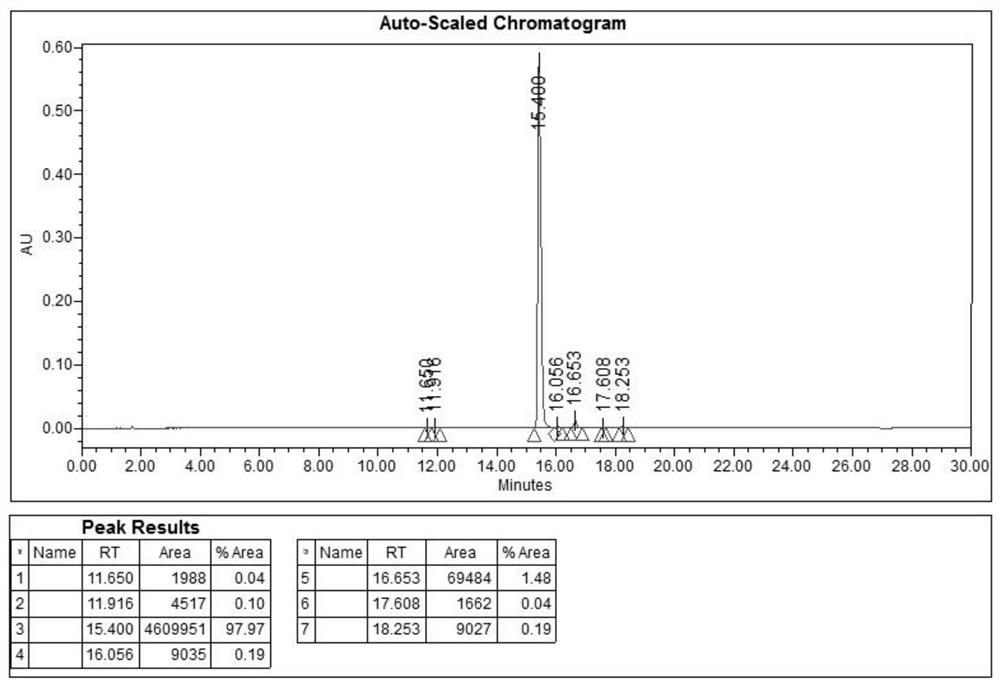
The invention belongs to the technical field of medicine, and relates to a purification method of degarelix, in particular to a method for adding an alkaline acidic salt solution or a weak alkali solution into trifluoroacetic-acid-containing degarelix alcohol for a stirring reaction to remove trifluoroacetic acid from degarelix after filtration, beating, washing and drying. The method is environmentally friendly, cheap, easy to operate, stable in process and suitable for large-scale industrial applications and takes less time.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
Process for the manufacture of Degarelix and its intermediates
ActiveUS9090656B2Peptide/protein ingredientsLuteinising hormone-releasing hormoneHydrogenOrganic solvent
The present invention relates to a liquid (or solution)-phase manufacturing process for preparing the decapeptide Degarelix, its protected precursor, and other useful intermediates. The invention further relates to polypeptides useful in the solution-phase manufacturing process and to the purification of Degarelix itself. Degarelix can be obtained by subjecting a Degarelix precursor according to formula (II): (P1)AA1-AA2-AA3-AA4(P4)-AA5-AA6(P6)-AA7-AA8(P8)-AA9-AA10-NH2 (II) or a salt or solvate thereof, to a treatment with a cleaving agent in an organic solvent, wherein P1 is an amino protecting groups; preferably acetyl; P4 is hydrogen or a hydroxy! protecting group, preferably a hydroxyl protecting group; P6 is hydrogen or an amino protecting groups; preferably an amino protecting groups; and P8 is an amino protecting group.
Owner:FERRING BV
Method for the manufacture of degarelix
ActiveUS20120041172A1Less costlyPeptide/protein ingredientsLuteinising hormone-releasing hormoneOrganic solventOrganic base
In a step-wise synthesis of degarelix comprising 0.3% by weight or less of 4-([2(5-hydantoyl)]acetylamino)-phenylalanine analog on (solid support)-NH2 a step comprises providing a solution of an amino acid or peptide of which the α-amino group is protected by Fmoc; contacting the support with the solution in the presence of reagent for forming a peptide bond between a carboxyl group of the amino acid or peptide and (solid support)-NH2; removing Fmoc by contacting the support with an organic base, in particular piperidine, in an organic solvent. Also disclosed is degarelix of high purity prepared by the method of the invention and the use of Fmoc in the synthesis of degarelix.
Owner:POLYPEPTIDE LAB AS
Process for the preparation of degarelix
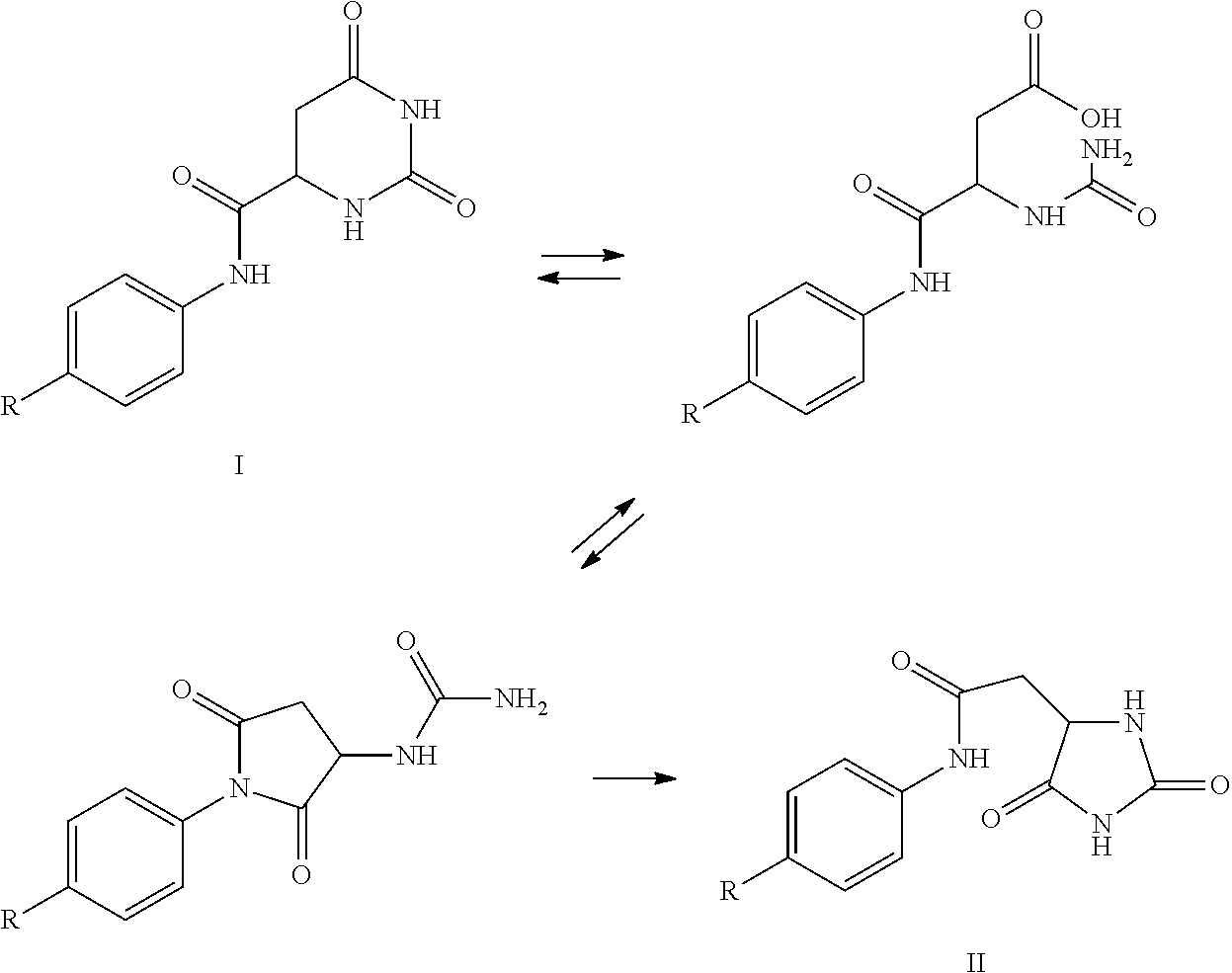
The present invention provides a manufacturing process for the preparation of degarelix by using Fmoc protected amino acids as building blocks, wherein the Fmoc group is cleaved by treatment with tert-butylamine.
Owner:BEIJING FRESENIUS KABI PHARM CO LTD
A kind of solid phase synthesis method of degarelix
ActiveCN103992392BThe process steps are simpleMild conditionsLuteinising hormone-releasing hormonePeptide preparation methodsAcetic anhydrideDiethyl ether
Owner:NANTONG SHIMEIKANG PHARMA CHEM
Degarelix-containing pharmaceutical composition for injection and preparation method and application thereof
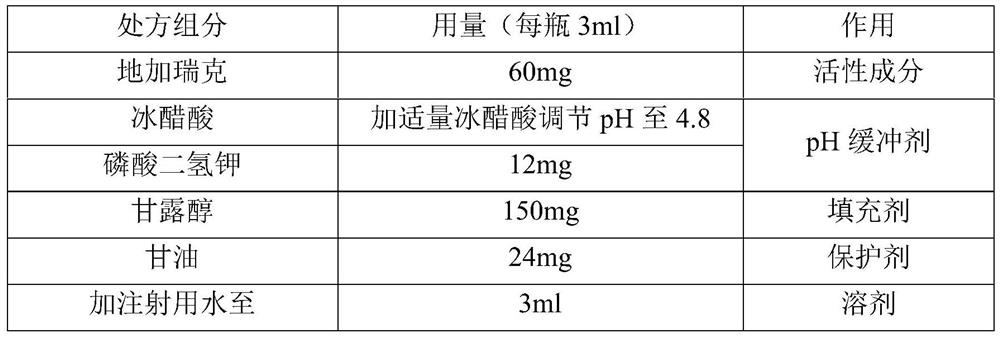
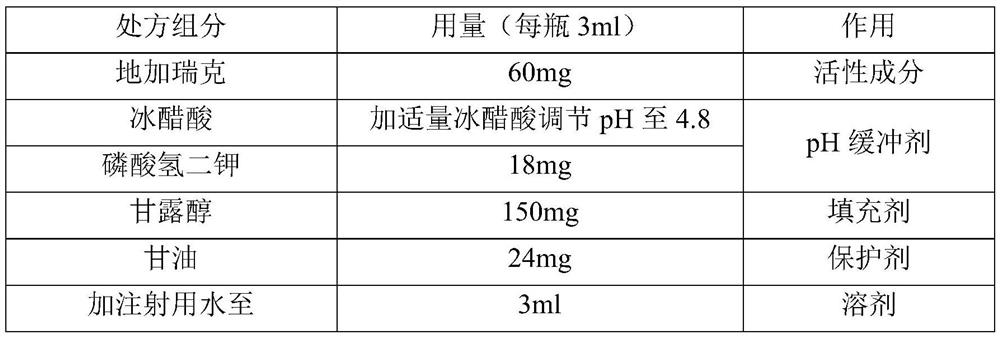
ActiveCN113952297AIncrease fat solubilityImprove permeabilityPowder deliveryPeptide/protein ingredientsDipotassium hydrogen phosphateVentricular tachycardia
The invention discloses a degarelix-containing pharmaceutical composition for injection and a preparation method and application thereof, the pharmaceutical composition comprises degarelix, mannitol and a pH regulator; the pH regulator comprises acetic acid and a pH buffer agent; the pH buffer agent is selected from one of potassium acetate, dipotassium phosphate, monopotassium phosphate and potassium citrate. The degarelix-containing pharmaceutical composition for injection disclosed by the invention is used for treating advanced prostatic cancer, and compared with an original medicine, the degarelix-containing pharmaceutical composition for injection can be used for remarkably improving the symptom of ventricular tachycardia caused by degarelix.
Owner:HYBIO PHARMA
Process for the manufacture of degarelix and its intermediates
ActiveUS20160145301A1Peptide/protein ingredientsLuteinising hormone-releasing hormoneHydrogenOrganic solvent
Owner:FERRING BV
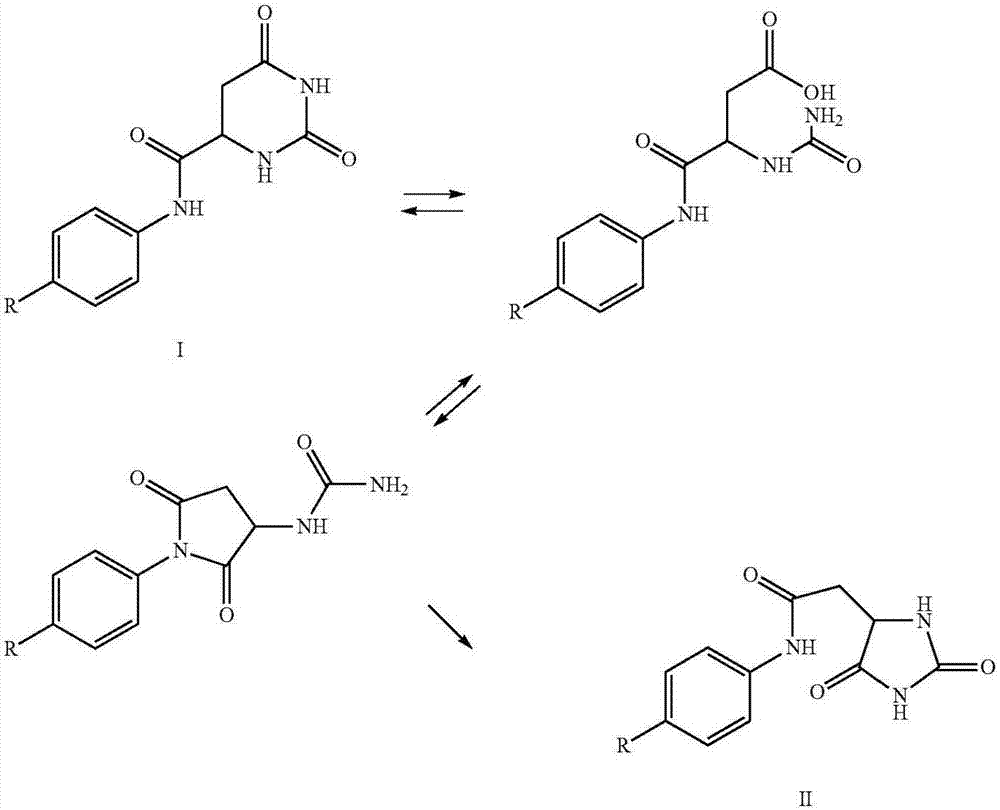
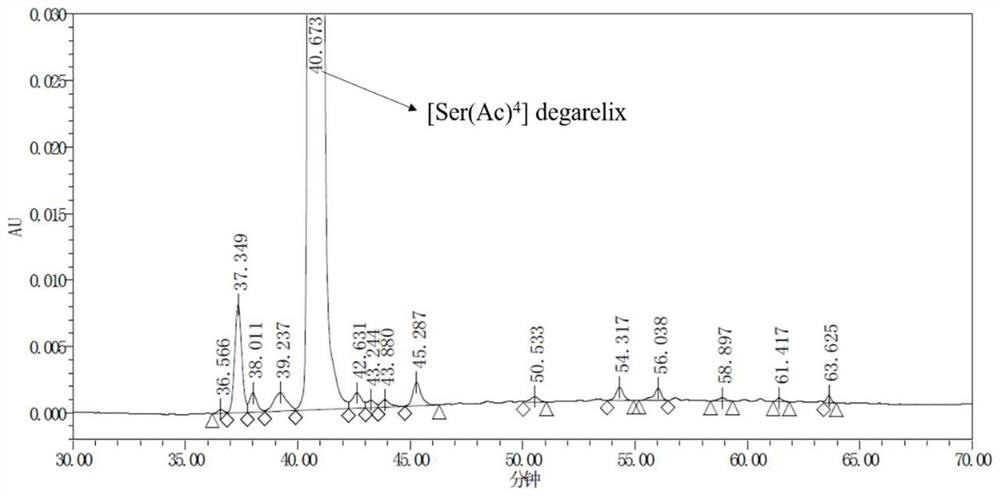
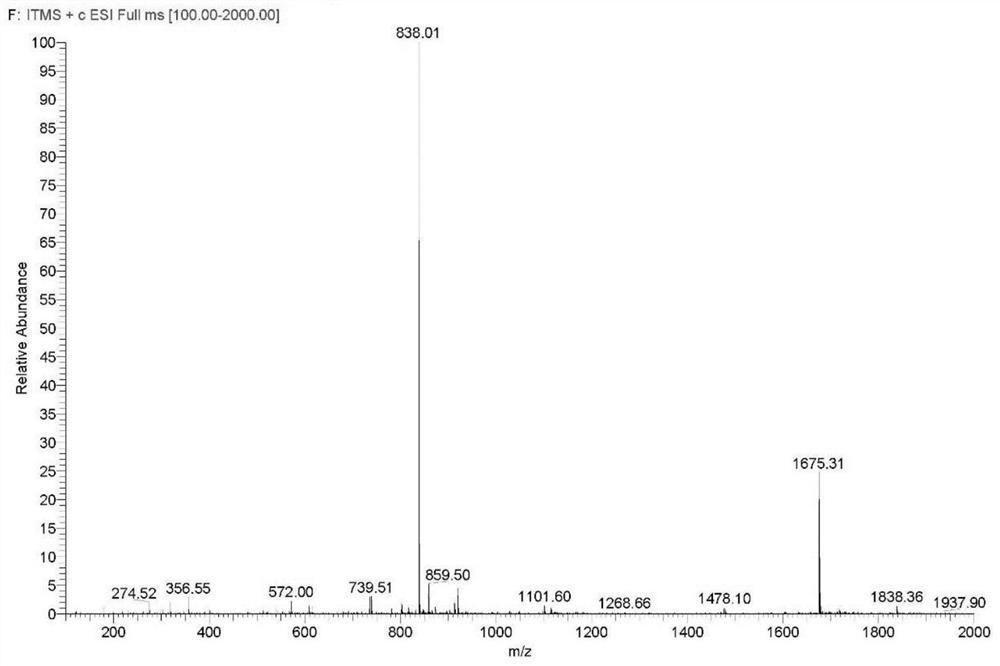
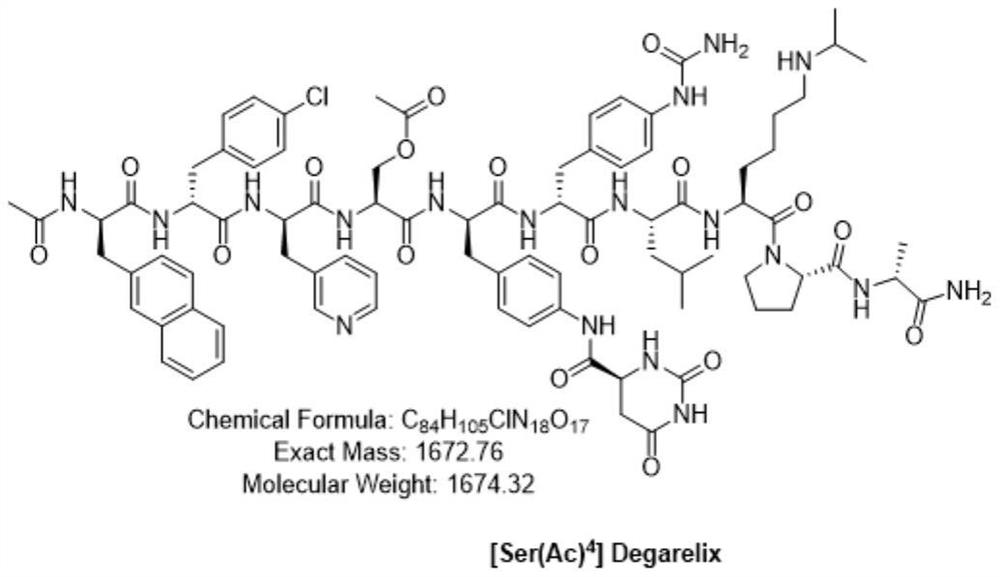
Preparation method of degarelix acetylation impurity
PendingCN114456236AImprove production safetyImprove medication safetyLuteinising hormone-releasing hormonePeptide preparation methodsPeptide sequenceDrugs preparations
The invention provides a preparation method of degarelix acetylation impurities, and belongs to the field of polypeptide drug preparation. The method comprises the following steps that 1, amino resin serves as initial resin, degarelix peptide fragment resin or degarelix peptide resin from the nth site to the tenth site is synthesized according to the degarelix peptide sequence, Fmoc-Ser-OH is adopted for the fourth amino acid, and corresponding protected amino acid or fragments are adopted for the rest; 2) carrying out acetylation by using an acetylation reagent; 3) continuing to couple residual amino acids to obtain degarelix acetylation impurity peptide resin; if the degarelix peptide resin is synthesized in the step 1), the step can be omitted; 4) cracking and purifying to obtain a degarelix acetylation impurity, namely [Ser (Ac) 4] degarelix; wherein n is equal to 1, 2 or 3. The method has the advantages of high purity and high yield, provides a qualified impurity reference substance for impurity analysis and research of degarelix bulk drugs and preparations thereof, and further improves the production and medication safety of degarelix.
Owner:深圳市健翔生物制药有限公司
Use of degarelix in the treatment of endometriosis and related diseases
ActiveUS20150273012A1Rapid and marked hypoestrogenismSuppress symptomsPeptide/protein ingredientsPharmaceutical delivery mechanismDiseaseEndometriotic lesion
The use of degarelix in the treatment of endometriosis, in particular in the treatment of endometriotic ovarian cysts and recurrent endometriotic lesions following surgery and in the treatment of endometriosis and / or endometriotic ovarian cysts in patients who plan to undergo assisted reproduction, is described, wherein this treatment is performed before the patients are subjected to assisted reproduction; degarelix is administered for this purpose as a single dose of 80-120 mg, preferably 80 mg, subcutaneously.
Owner:SCARPELLINI FABIO +1
Synthesis of Degarelix by a Solid Phase Fragmentation Method
ActiveCN103351428BReduce usageAvoid repeated contactPeptide preparation methodsBulk chemical productionSide chainTrifluoroacetic acid
The invention discloses synthesis of degarelix by a solid phase segment method, wherein Fmoc-amino acid is connected with resin so that Fmoc-amino acid-resin can be obtained; a solid phase synthesis method is adopted, and the steps of orderly connecting amino acid from the end C to the end N and removing the Fmoc group are carried out, so that Fmoc protected polypeptide resin can be obtained; the method comprises the following steps of: (1) forming polypeptide resin as shown in formula III and polypeptide resin as shown in formula IV, respectively; (2) mixing the polypeptide resin as shown in formula III with 1-5% of trifluoroacetic acid (TFA), connecting L-4,5-dihydroorotate to the side chain amino of the polypeptide resin after the protecting group of the sixth amino acid residue at the end C is removed, and then removing the Fmoc group to obtain polypeptide resin as shown in formula V; removing the Fmoc group of the polypeptide resin as shown in formula IV and acetylating the polypeptide resin, thereby obtaining polypeptide resin as shown in formula VI, and then cutting to obtain segments as shown in formula VII; (3) coupling the polypeptide resin as shown in the formula V with the segments as shown in the formula VII, thereby obtaining the polypeptide resin as shown in formula II; (4) separating polypeptide on the polypeptide resin as shown in the formula II from the resin, thereby obtaining degarelix as shown in formula I.
Owner:HAINAN SHUANGCHENG PHARMA
A method for synthesizing degarelix
ActiveCN104177478BHigh purityNo harmLuteinising hormone-releasing hormonePeptide preparation methodsD alanineBiological activation
The invention relates to the field of synthesis of medicines and discloses a method for synthesizing degarelix. The method for synthesizing the degarelix comprises the following steps: enabling D-alanine with a protective group coupled with an N end to carry out esterification reaction with amino resin with a protective group coupled with an amino in the presence of a condensation reagent and an activation reagent to obtain peptide resin 1; extending and coupling other protective amino acids one by one by starting from the peptide resin 1 according to a sequence from C end to N end of the amino acid sequence of the degarelix in the presence of the condensation reagent and the activation reagent, to obtain corresponding peptide resins after extending and coupling each time and finally obtain degarelix resin, then carrying out acidolysis to obtain a degarelix crude product, and purifying the degarelix crude product to obtain a degarelix pure product. By virtue of the method for synthesizing the degarelix, a proper synthesizing scheme is selected; the adaptive amino resin and an acidolysis solution are selected; the overall synthesis process is optimized; the purity of the degarelix is obviously improved; the degarelix has a relatively high total yield and is free of pollution to any environment.
Owner:CHENGDU SHENGNUO BIOPHARM
A method for preparing degarelix by solid-liquid combination
InactiveCN107022002BReduce usageThe synthesis process is simpleLuteinising hormone-releasing hormonePeptide preparation methodsCombinatorial chemistryOrganic chemistry
The present invention relates to the field of polypeptide synthesis, in particular to a method for preparing degarelix using a solid-liquid combination chemical method. The present invention synthesizes a fragment peptide Ac-D-2Nal-D-4Cpa-D-3Pal-Ser(tBu) for the first time ‑4Aph(Fmoc)‑OH is used for the solid-phase synthesis of degarelix, the synthesis process is simple, the raw materials used are cheap and easy to obtain, and it is easy to scale up production; the present invention avoids the use of highly toxic reagent HF, and adopts TFA combination cracking, after purification, The refined peptide with a purity of 99.5% was obtained by lyophilization, and the total yield reached 65%.
Owner:JINAN KANGHE MEDICAL TECH
Preparation method of degarelix crude peptide
PendingCN114671927AShort timeReduce consumptionLuteinising hormone-releasing hormonePeptide preparation methodsOrganic solventCentrifugation
The invention relates to the field of polypeptide synthesis, in particular to a preparation method of degarelix crude peptide. The method comprises the following steps: replacing an organic solvent in sedimentation and filtration with water, adding water to dilute a lysate, and stirring and filtering to obtain crude peptide; the method avoids mass use of an organic solvent and repeated operation of a centrifugation step, so that the method is simple, efficient, environment-friendly and low in cost; meanwhile, the precipitation rate of the method is 97.2% + / -1%, which is almost the same as the solid precipitation rate (97.8% in the embodiment 1 of the patent) of the traditional absolute ether precipitation centrifugation.
Owner:HYBIO PHARMA
Solid Phase Synthesis Process of Degarelix
ActiveCN102329373BReduce usageMild reaction conditionsPeptide preparation methodsBulk chemical productionAcetic anhydrideSide chain
The invention relates to a solid-phase synthetic method for degarelix. The method comprises the following steps of: 1) reacting resin with Fmoc-D-Ala-OH to obtain Fmoc-D-Ala-resin, wherein the resin is amino resin; 2) sequentially connecting according to the amino acid sequence of the degarelix by adopting an Fmoc solid-phase synthetic strategy; 3) removing Fmoc from a N terminal, and acetylating by using acetic anhydride and pyridine; 4) removing a protective group X on the 6th amino acid residue -4Aph(X) from a C terminal; 5) connecting L-4,5-dihydrooroticacid to a side-chain amino group of the 6th amino acid residue -4Aph at the C terminal; 6) cutting peptide resin by using a cracking reagent, precipitating by using anhydrous ether, and centrifuging to obtain crude peptide; and 7) purifying and separating to obtain the degarelix. The method is easy to operate and slightly damages human bodies and environments; and by the process, the content of impurities is effectively reduced, and the large-scale production can be performed.
Owner:HYBIO PHARMA
Use of degarelix in the treatment of endometriosis and related diseases
ActiveUS9463213B2Lower Level RequirementsMarked hypoestrogenismPeptide/protein ingredientsPharmaceutical delivery mechanismDiseaseEndometriotic lesion
Owner:SCARPELLINI FABIO +1
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com