Solid-phase synthesis method of degarelix
A solid-phase synthesis, degarelix technology, applied in the preparation methods of peptides, chemical instruments and methods, organic chemistry, etc., can solve the problems of easy introduction of heavy metal substances, easy shedding, breakage at the joint between peptides and resins, etc. Reduce damage to human body, avoid rearrangement reactions, and have little effect on human body and environment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
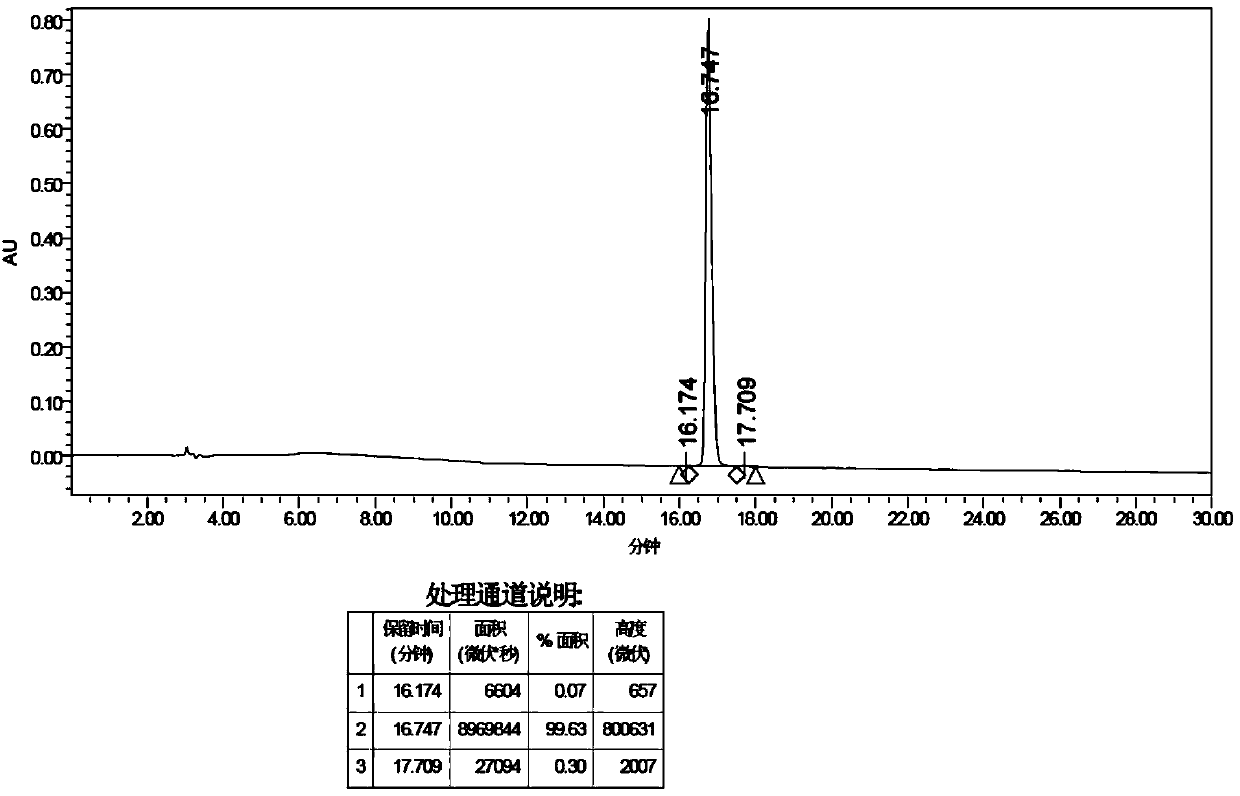
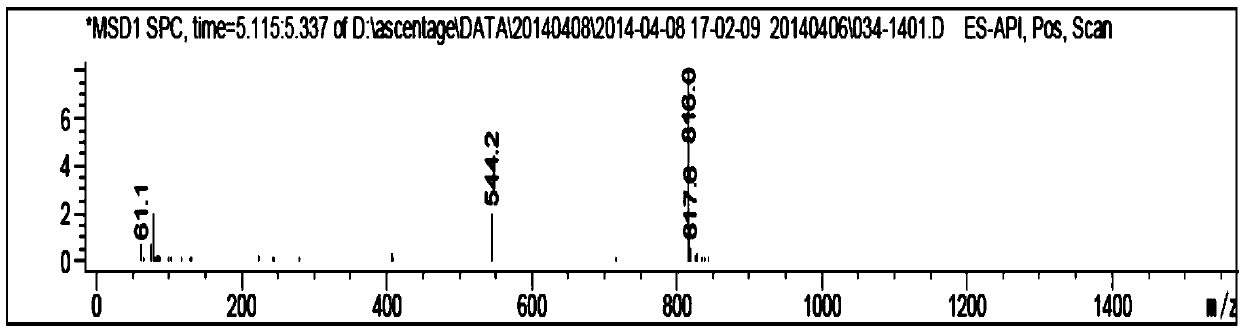
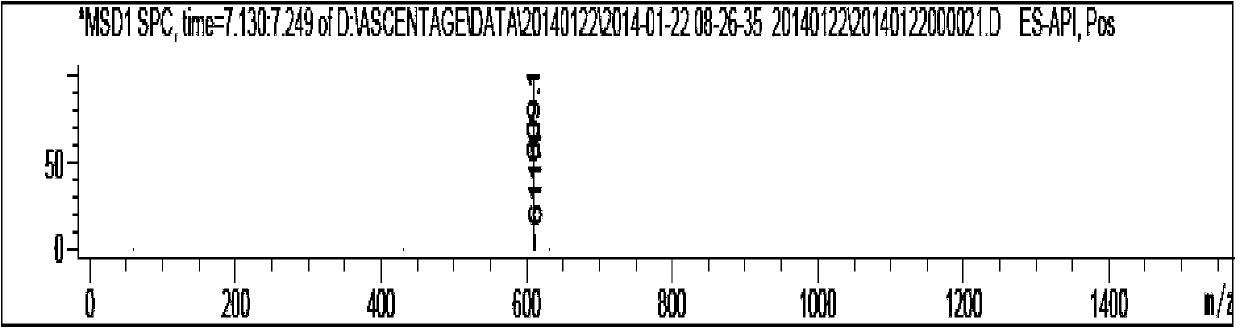
Image
Examples
Embodiment 1
[0035] Example 1: Preparation of Fmoc-D-Ala-Rink-Amide-MBHA.
[0036] Weigh 0.88g (0.3mmol) Fmoc-Rink-Amide-MBHA resin (degree of substitution 0.34mmol / g) into a 20ml BD syringe with a frit (ordinary glass peptide reactor can also), use 3 times the resin volume of DCM Swell twice, 1 hour each time, after the resin is completely swollen, use 20% (v / v) piperidine / DMF solution to remove the Fmoc protecting group twice for 5 min and 20 min respectively, and wash with DMF 3 times. Dissolve 373.56mg (1.2mmol) Fmoc-D-Ala-OH and 162.12mg (1.2mmol) HOBt with 10ml DMF, ice bath for ten minutes, add 0.4ml (2.4mmol) DIEA, activate for 3min, then add 455.04mg (1.2mmol) ) HBTU, the mixed solution was added to the solid-phase reactor, and the reaction was stirred for 2 h. The ninhydrin test was negative, washed three times with DMF, and drained to obtain Fmoc-D-Ala-Rink-Amide-MBHA.
Embodiment 2
[0037] Example 2: Fmoc-D-2Nal-D-Phe(4Cl)-D-3Pal-Ser(tBu)-4Aph(ivDde)-D-4Aph(tBuCbm)-Leu-ILys(Boc)-Pro-D-Ala - Synthesis of Rink-Amide-MBHA.
[0038] Fmoc-D-Ala-Rink-Amide-MBHA (0.3 mmol) was charged into the solid phase reactor, washed twice with DMF, and the Fmoc protecting group was removed twice with 20% (v / v) piperidine / DMF solution, time 5min and 20min respectively, washed 3 times with DMF, and tested by ninhydrin method. Dissolve 404.88mg (1.2mmol) Fmoc-Pro-OH and 162.12mg (1.2mmol) HOBt with 10ml DMF, ice bath for ten minutes, add 0.4ml (2.4mmol) DIEA, activate for 3min, then add 455.04mg (1.2mmol) HBTU , the mixed solution was added to the solid-phase reactor, and the reaction was stirred for 1.5h. The ninhydrin test was negative, and the DMF was washed three times.
[0039] Repeat the above operation, according to the amino acid sequence of degarelix, connect Fmoc-Pro-OH, Fmoc-ILys(Boc)-OH, Fmoc-Leu-OH, Fmoc-D-4Aph(tBuCbm)-OH, Fmoc-4Aph( ivDde)-OH, Fmoc-Ser(tBu)-OH...
Embodiment 3
[0043] Example 3: AC-D-2Nal-D-Phe(4Cl)-D-3Pal-Ser(tBu)-4Aph(ivDde)-D-4Aph(tBuCbm)-Leu-ILys(Boc)-Pro-D-Ala - Synthesis of Rink-Amide-MBHA.
[0044] Fmoc-D-2Nal-D-Phe(4Cl)-D-3Pal-Ser(tBu)-4Aph(ivDde)-D-4Aph(tBuCbm)-Leu-ILys(Boc)-Pro-D-Ala-Rink- Amide-MBHA was loaded into the solid phase reactor, washed twice with DMF, deprotected Fmoc twice with 20% (v / v) piperidine / DMF solution for 5 min and 20 min respectively, washed 3 times with DMF, ninhydrin law test. Then, a mixed solution of acetic anhydride (3 ml) and DCM (9 ml) was added, and the reaction was stirred for 30 min, washed three times with DMF, and detected by ninhydrin detection method.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com