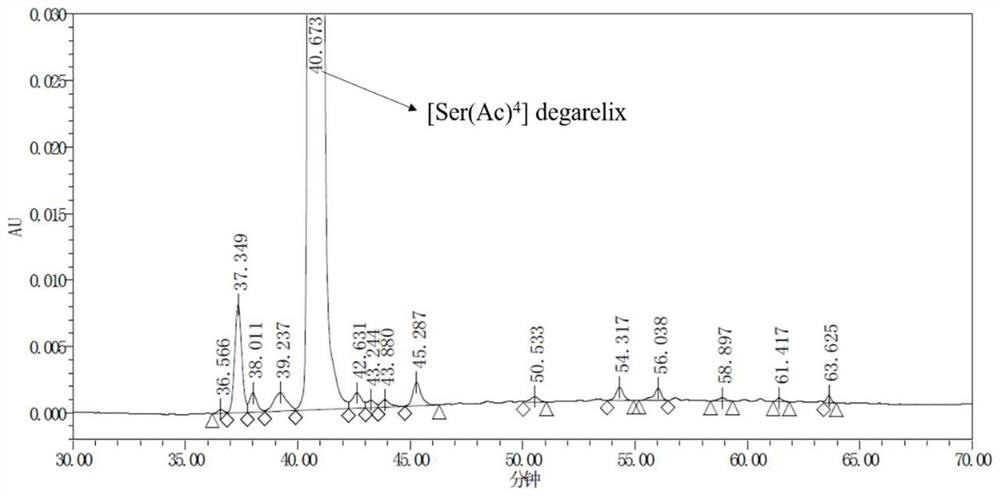
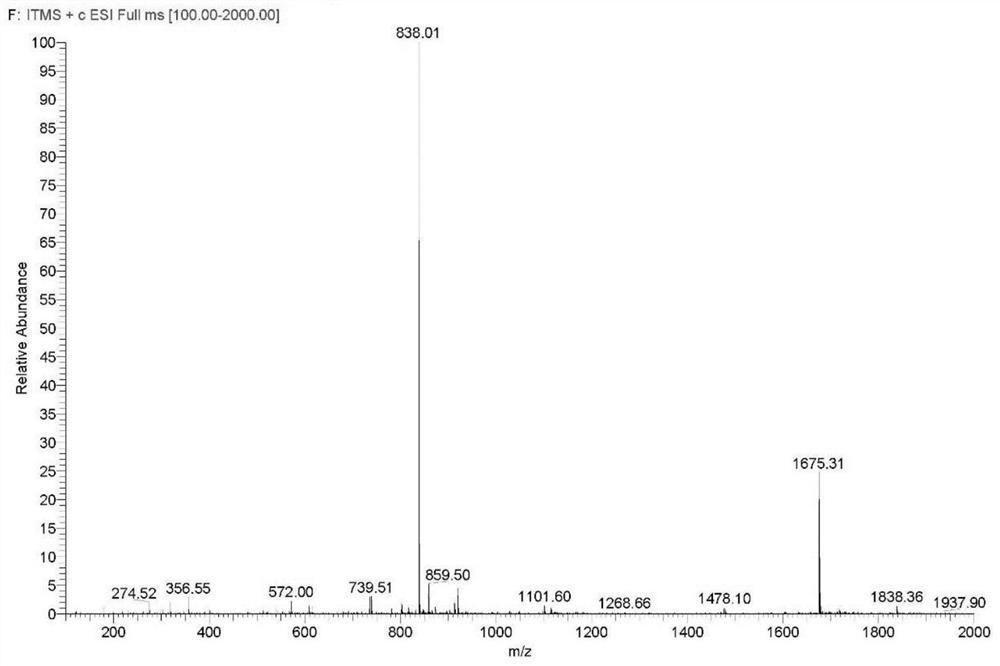
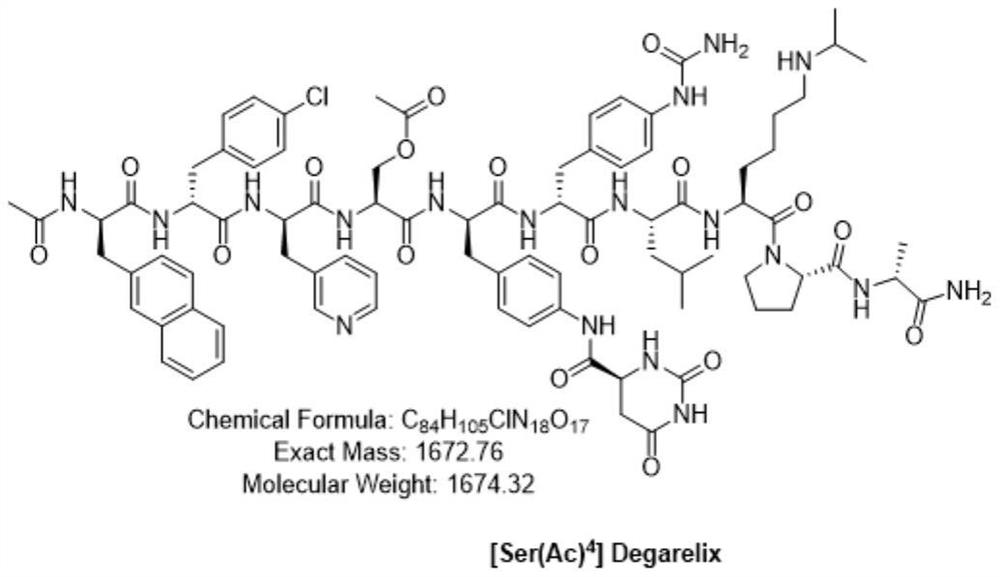
Preparation method of degarelix acetylation impurity
A technology of degarelix and rick acetylation, which is applied in the field of polypeptide drug preparation and can solve problems such as side chain acetylation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] The synthesis of embodiment 1 Fmoc-D-Ala-Rink MBHA resin
[0045] Weigh Rink Amide MBHA resin (75g, 30mmol, degree of substitution: 0.40mmol / g) into a solid-phase reaction synthesis column. Add 500mL DMF to swell for 30min, and remove the DMF. The resin was washed with 3*400mL DMF, and the DMF was aspirated. Add 400 mL of DBLK solution (20% piperidine / DMF solution, V / V), deprotect twice, the first time is 5 min, the second time is 15 min. After deprotection, the resin was washed with 400mL DMF each time for 6 times. After the fourth wash, a little resin was picked with a glass rod, and the ninhydrin test was positive, indicating that Fmoc was completely removed.
[0046] Weigh 18.68g Fmoc-D-Ala-OH and 9.73g HOBt, add 120mL DMF to dissolve, after complete dissolution, cool the solution below 5°C, then add 11.36g DIC (pre-cooled to <0°C), in the solution Activated in medium for about 3-5 minutes, control the activated solution into the reaction column, react at 20-30°C f...
Embodiment 2
[0047] Synthesis of Example 2 Degarelix 1-10 Peptide Resin
[0048] Weigh Fmoc-D-Ala-Rink MBHA resin (30mmol) in the solid phase reaction synthesis column. The resin was washed with 3*400mL DMF, and the DMF was aspirated. Add 400 mL of DBLK solution (20% piperidine / DMF solution, V / V), deprotect twice, the first time is 5 min, the second time is 15 min. After deprotection, the resin was washed with 400mL DMF each time for 6 times. After the fourth wash, a little resin was picked with a glass rod, and the ninhydrin test was positive, indicating that Fmoc was completely removed.
[0049] Weigh 20.24g Fmoc-Pro-OH and 9.73g HOBt, add 120mL DMF to dissolve, after complete dissolution, cool the solution below 5°C, then add 11.36g DIC (pre-cooled to <0°C) to activate in the solution For about 3-5 minutes, add the activated solution to the reaction column under control, and react at 20-30°C for 2-3 hours. When the ninhydrin test is negative, remove the reaction solution, add 400mL of...
Embodiment 3
[0057] Example 3 Synthesis of degarelix acetylated impurity peptide resin
[0058] Continue the reaction of the product of Example 2, weigh 18.02g of acetic acid, 11.00g of DMAP and 48.64g of HOBt, add 120mL of DMF to dissolve, after complete dissolution, cool the solution to below 5°C, add to the reaction column, and then add 56.79 gDIC (pre-cooled to <0°C), react at 20-30°C for 3-5 hours, and end the reaction, add 400mL of DMF each time to wash the resin, and wash 6 times. After washing, the washing liquid was extracted, and then the resin was washed with DCM, 400 mL each time, and washed 4 times to obtain the degarelix acetylated impurity peptide resin: Ac-D-2Nal-D-4Cpa-D-3Pal-Ser( Ac)-4Aph(Hor)-D-4Aph(Cbm)-Leu-ILys(Boc)-Pro-D-Ala-Rink MBHA resin, the resin is vacuum-dried at 20-30°C until it becomes quicksand. The peptide resin was 110.22 g after drying, and the weight gain rate of the resin was 119%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com