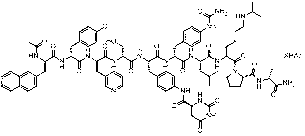
Method for synthesizing degarelix
A technology of degarelix and solid-phase synthesis, which is applied in peptide preparation methods, chemical instruments and methods, organic chemistry, etc., can solve problems such as long reaction time by-products, missing sequence peptides, and large space space, and achieve Avoid the difficulty of removal, reduce the generation of impurities, and reduce the effect of environmental protection pressure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Embodiment 1 The solid-phase synthesis of degarelix
[0056] 1) Swelling and deprotection of Fmoc-protected Rink amide AM resin
[0057] Put Fmoc-protected Rink amide AM (substitution degree 0.49mmol / g, 0.82g, 0.4mmol) into a solid-phase reaction column, wash with DMF for 1-4 times, then swell with DCM for 20-40 minutes, and remove DCM by suction filtration , adding 20% DBLK solution for deprotection for 5 minutes, once every 10 minutes, and then washing with DMF for 4 to 6 times.
[0058] 2) Preparation of Fmoc-D-Ala-Rink amide AM resin
[0059] F-D-Ala-OH (249mg, 0.8mmol), HOBt-Cl (164mg, 0.88mmol)
[0060] Dissolve in 2ml DMF, then add 124μL DIC to activate for 5~10 minutes, add the activated solution repeatedly to the resin obtained in step 1) to react until the ninhydrin detection method detects that the resin is negative, stop the reaction, and wash the resin with DMF for 4 ~6 times, get Fmoc- D-Ala- Rink amide AM resin;
[0061] 3) Preparation of Fmoc-D-Ala...
Embodiment 2
[0084] Example 2 The solid-phase synthesis of degarelix
[0085]The Fmoc-protected Rink amide AM resin in Example 1 was replaced with Fmoc-protected Rink amide MBHA (substitution degree=0.73mmol / g, 0.55g, 0.4mmol). The rest of the operations were the same as in Example 1. After shrinkage, 1.17g of peptide resin was obtained, and after cleavage, 0.52g of crude peptide and 0.21g of refined peptide were obtained, with a yield of 40%.
Embodiment 3
[0086] Example 3 The solid-phase synthesis of degarelix
[0087] The Fmoc-Ser(TBDMS)-OH in step 3) in Example 1 was replaced by Fmoc-Ser(OBn)-OH, and the rest of the operations were the same as in Example 1, and the reaction conditions for removing the Obn protecting group were the same as in Example 1 to obtain refined Peptide, the yield is 47%, the purity is 99.6%
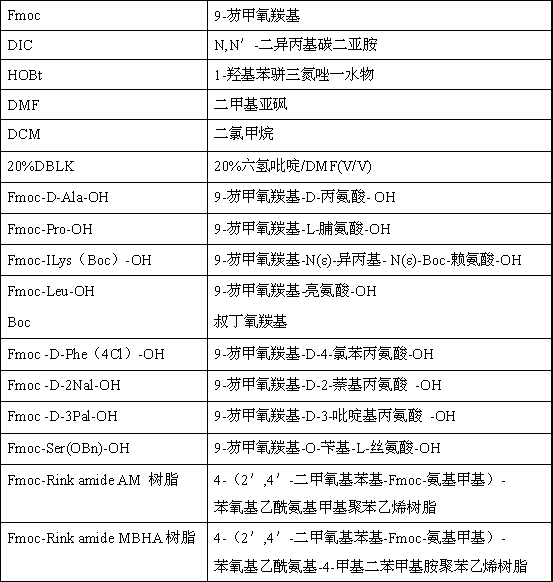
[0088] The abbreviations that embodiment and aforementioned process adopt and the material of representative thereof are as follows:
[0089]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com