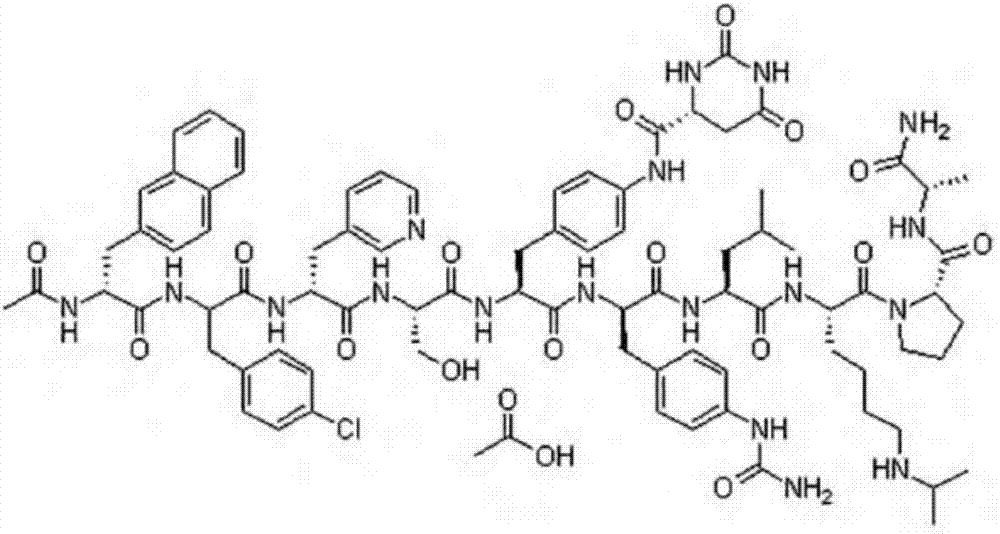
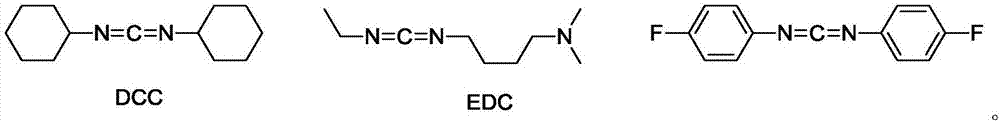
Preparation method of key intermediate of degarelix
A synthetic method and technology of Rick polypeptide, applied in chemical instruments and methods, luteinizing hormone releasing hormone, peptides, etc., can solve the problem of no degarelix polypeptide fragments, etc., and achieve the effect of avoiding racemization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] H-Lys(iPr, Boc)-Pro-D-Ala-NH 2 +Fmoc-Leu-OH→Fmoc-Leu-Lys(iPr, Boc)-Pro-D-Ala-NH 2
[0027] L-lysyl-N6-(1-isopropyl-1-tert-butoxycarbonyl)-L-lysyl-L-prolyl-D-alaninamide (45.5g, 1eq), Fmoc- Add L-leucine (38.8g, 1.1eq), HOBT (14.9g, 1.1eq), N,N-dimethylformamide 200ml, tetrahydrofuran 600ml into the reaction flask, stir and control the temperature at 10-20°C and add N- After methylmorpholine (10 g, 1.0 eq), triethylamine (10 g, 1.0 eq), EDC·HCl (22.9 g, 1.2 eq) was added and stirring continued for 4 hours.
[0028] After the reaction was completed, 1.5 L of water and 3.0 L of ethyl acetate were added to the reaction solution for extraction. The organic phase was washed once with 1.5 L of water, dried by adding anhydrous sodium sulfate, filtered, and concentrated to dryness under reduced pressure to obtain Fmoc-L-leucyl- N6-(1-isopropyl-1-tert-butoxycarbonyl)-L-lysyl-L-prolyl-D-alaninamide crude product 67.1g, yield 85.1%. HPLC detection showed that the content of rac...
Embodiment 2
[0031]L-lysyl-N6-(1-isopropyl-1-tert-butoxycarbonyl)-L-lysyl-L-prolyl-D-alaninamide (45.5g, 1eq), Fmoc- Add L-leucine (38.8g, 1.1eq), HOBT (14.9g, 1.1eq), N,N-dimethylformamide 200ml, tetrahydrofuran 600ml into the reaction flask, stir and control the temperature at 10-20°C and add N- After methylmorpholine (10g, 1.0eq), triethylamine (10g, 1.0eq), bis-p-fluorophenylcarbodiimide (27.6g, 1.2eq) was added and stirring was continued for 4 hours.
[0032] After the reaction was completed, 1.5 L of water and 3.0 L of ethyl acetate were added to the reaction solution for extraction. The organic phase was washed once with 1.5 L of water, dried by adding anhydrous sodium sulfate, filtered, and concentrated to dryness under reduced pressure to obtain Fmoc-L-leucyl- N6-(1-isopropyl-1-tert-butoxycarbonyl)-L-lysyl-L-prolyl-D-alaninamide crude product 67.1g, yield 85.1%. HPLC detection showed that the content of racemic peptide was 0.05%, the total impurity was 0.87%, and the maximum sing...
Embodiment 3
[0035] L-lysyl-N6-(1-isopropyl-1-tert-butoxycarbonyl)-L-lysyl-L-prolyl-D-alaninamide (45.5g, 1eq), Fmoc- Add L-leucine (38.8g, 1.1eq), HOBT (14.9g, 1.1eq), N,N-dimethylformamide 200ml, tetrahydrofuran 600ml into the reaction flask, stir and control the temperature at 10-20°C and add N- After methylmorpholine (10 g, 1.0 eq), triethylamine (10 g, 1.0 eq), DCC (24.7 g, 1.2 eq) was added and stirring was continued for 4 hours.
[0036] After the reaction was completed, 1.5 L of water and 3.0 L of ethyl acetate were added to the reaction solution for extraction. The organic phase was washed once with 1.5 L of water, dried by adding anhydrous sodium sulfate, filtered, and concentrated to dryness under reduced pressure to obtain Fmoc-L-leucyl- N6-(1-isopropyl-1-tert-butoxycarbonyl)-L-lysyl-L-prolyl-D-alaninamide crude product 65.6g, yield 83.1%. HPLC detection showed that the content of racemic peptide was 0.24%, the total impurity was 1.47%, and the largest single impurity was 0.33...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com