Synthesis method of semaglutide
A synthesis method and peptide resin technology, applied in the field of semaglutide synthesis, can solve the problems of difficult removal of peptide impurities, unfavorable purification, difficult coupling, etc., and achieve the effect of facilitating industrial scale-up and improving the purity of crude peptides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
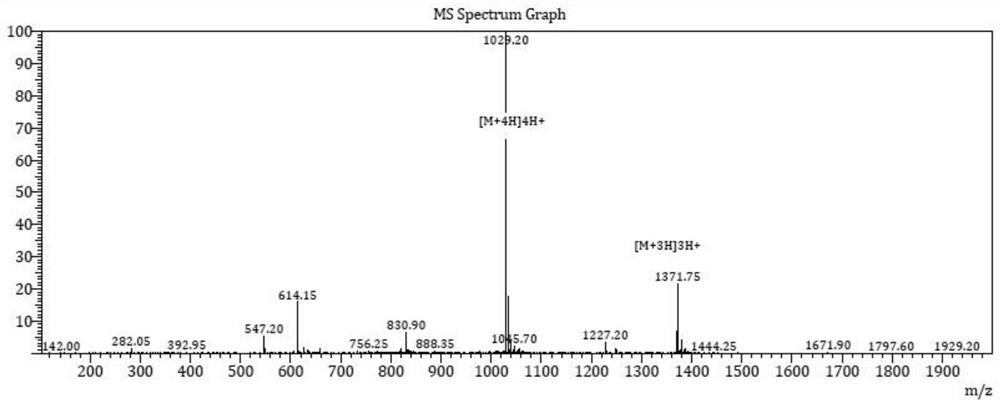
Examples
Embodiment 1
[0059] A synthetic method of semaglutide, the steps are as follows:
[0060] 1. Resin swelling
[0061] Weigh 12.84g of Fmoc-Gly-Wang resin with a substitution degree of 0.39mmol / g and pour it into a solid-phase synthesis reactor, blow it with nitrogen, add 100mL of DMF to swell for 1 hour, and then drain the solvent.
[0062] 2. Remove Fmoc protection
[0063] Add 50 mL of piperidine:DMF (V / V) 1:3 deprotection reagent 1 to the resin, drain it after 5 minutes of reaction, add 50 mL of deprotection reagent 1 again, and drain it after 15 minutes of reaction.
[0064] 3. Washing
[0065] After deprotection, add 50mL DMF to the resin to wash the resin, drain it, add DMF again to wash, and repeat the washing for a total of 6 times. The ninhydrin test of the resin is positive.
[0066] 4. Coupling amino acids
[0067] Weigh Fmoc-Arg(pbf)-OH and 2.04g HOBT that are 3 times the mole number of Fmoc-Gly-Wang resin, add 50mL DMF to dissolve, add DIC 2.4mL under stirring, and stir for...
Embodiment 2
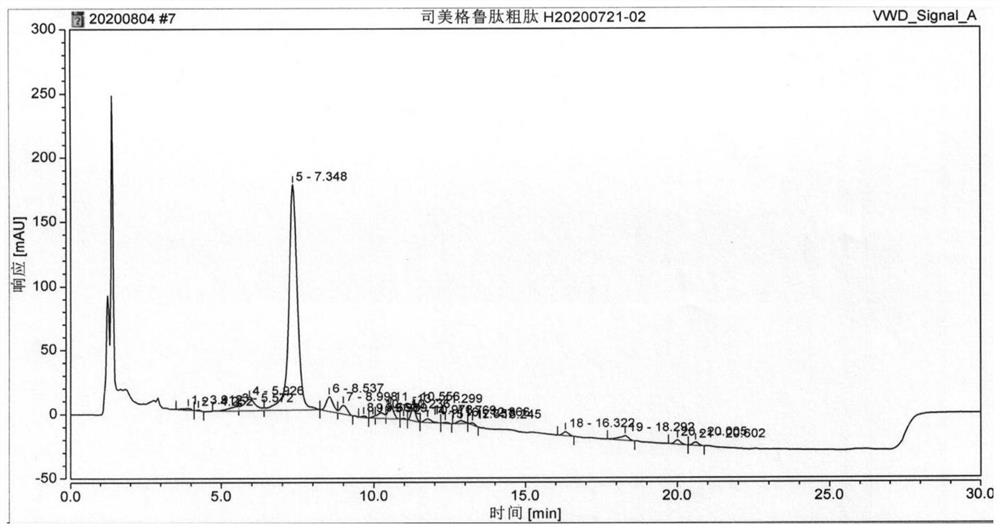
[0105] On the basis of Example 1, the condensing agent used when coupling Boc-His(Trt)-Aib-OH was replaced by TBTU / DIEA, and the obtained semaglutide crude peptide HPLC detection results were as follows Figure 5 As shown, the purity of semaglutide is 72.088%, and the maximum purity is 6.843%.
Embodiment 3
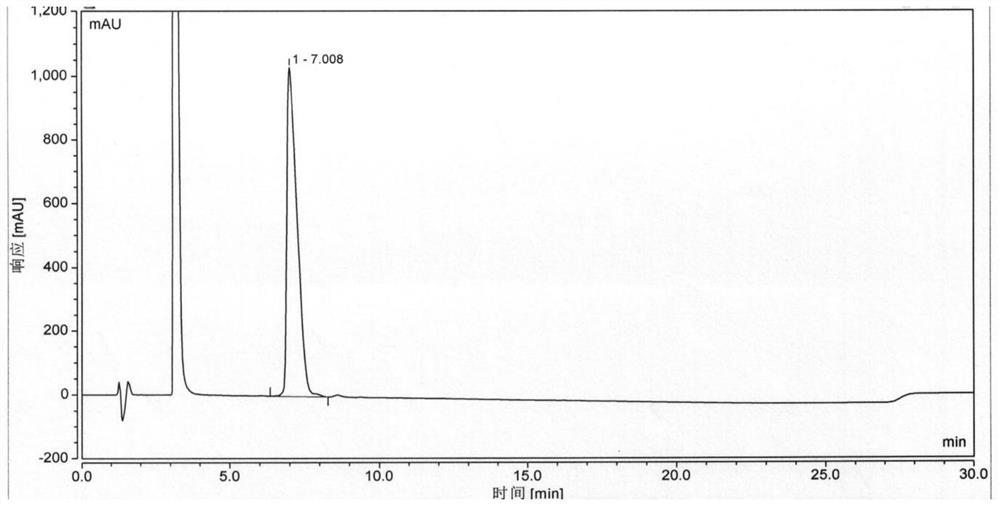
[0107] On the basis of Example 1, the condensing agent used when coupling Boc-His(Trt)-Aib-OH was replaced by HATU / DIEA, and the obtained semaglutide crude peptide HPLC detection results were as follows Image 6 As shown, the purity of semaglutide is 71.369%, and the maximum unmixed value is 4.609%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com