Method For The Manufacture Of Degarelix
A technology of degarelix and a manufacturing method, applied in the field of synthetic peptide manufacturing, can solve the problems of polluting the environment, high disposal cost, dangerous manufacturing personnel, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
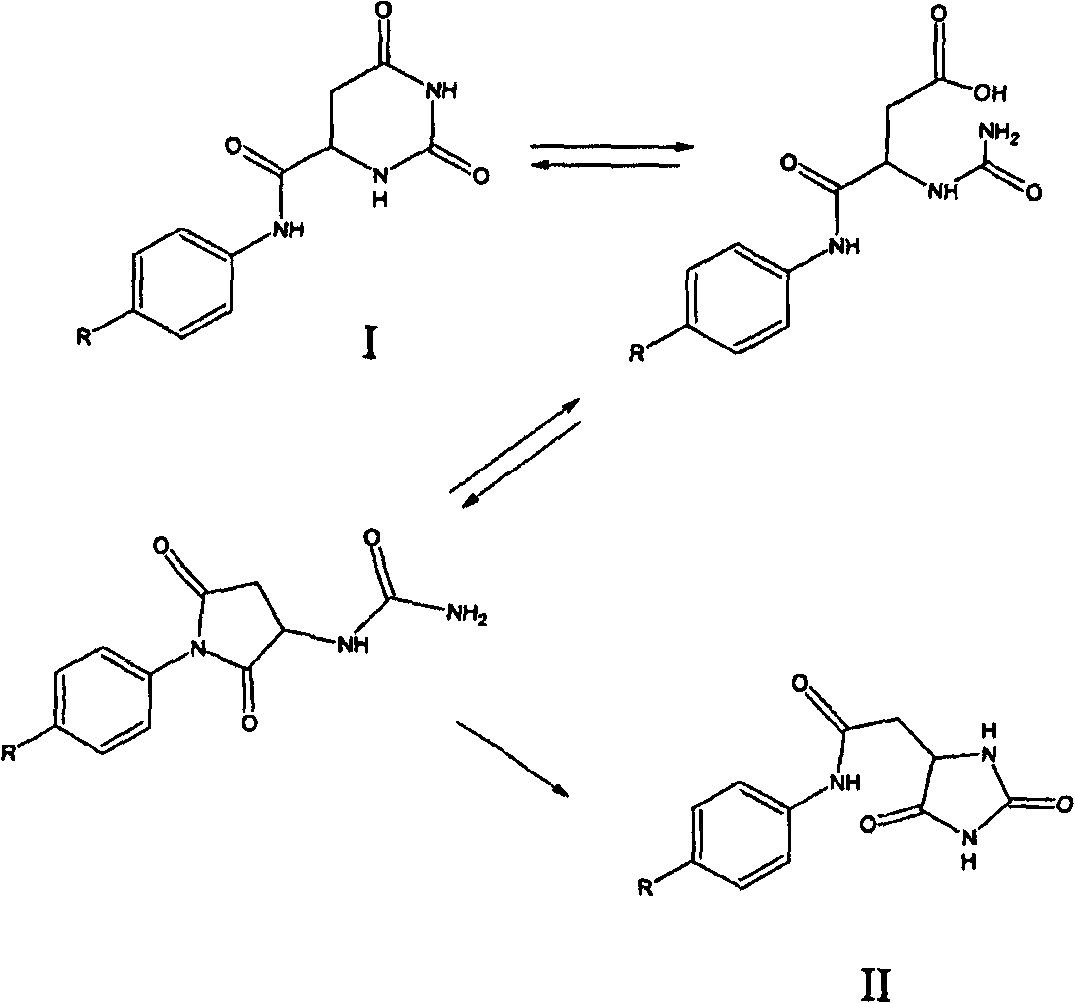
[0062] Hydantoin formation in the synthesis of degarelix. During the production of degarelix, a rearrangement of the hydrogenated orotic acid group to the hydantoin acetyl group was observed in two stages and under two alkaline conditions.
[0063] The first rearrangement occurs in the fragment Z-Ser(tBu)-4Aph(Hor)-D-4Aph(tBu-Cbm)-Leu-ILys(Boc)-Pro-D-Ala-NH 2 during the alkaline extraction process. The pH was adjusted to 9.1 with concentrated NaOH solution in an organic / aqueous biphasic system, whereby 4.5% by weight of the hydantoin analog was formed. The mechanism appears to consist of two steps: (a) hydrolysis of the six-membered hydroorotic acid moiety under basic conditions followed by ring closure under acidic conditions to the five-membered hydantoin analog.
[0064] The second rearrangement occurs during the evaporation of the fragment Z-Ser(tBu)-4Aph(Hor)-D-4Aph(tBu-Cbm)-Leu-OH DCHA. After the above extraction, Z-Ser(tBu)-4Aph(Hor)-D-4Aph(tBu-Cbm)-Leu-OH was dissol...
Embodiment 2
[0067] Stability of degarelix in DBL VDMF and piperidine / DMF. The stability of degarelix was determined under conditions corresponding to the conditions used to hide the Fmoc group during SPPS. It is known that the 5th amino acid residue in the degarelix sequence, ie, the hydroorotic acid group on the side chain of 4Aph (Hor), is sensitive to alkali and rearranges into a hydantoin acetyl group. All SPPS processes known to the inventors are based on Boc-chemistry.
[0068] Degarelix samples were dissolved in 20% piperidine / DMF, 2% DBU in DMF, and 2% DBU+5% water in DMF, respectively. Samples were analyzed by HPLC after 20 hours to determine the amount of hydantoin analogs.
[0069] 2% DBU / DMF resulted in 1.8% hydantoin formation. This amount was increased to 7% if 5% water was also present (simulated aqueous DMF). Surprisingly, the use of 20% piperidine in DMF did not result in the formation of any hydantoin analogs, suggesting that this mixture may be useful for Fmoc-based...
Embodiment 3
[0071] Synthesis and Purification of Degarelix Using Fmo- / Rink Amide AM Resin
[0072] Step 1. Fmoc-Rink amide AM resin (64 g; substitution 0.67 mmol / g) was placed in the reactor and washed with 1.9 L of DMF. A DMF solution of 20% piperidine was added to 250 ml of the swollen resin, and stirred for 20 minutes. The reactor was evacuated so that the reactor was vented through the bottom filter and a second treatment was performed with 250 ml of 20% piperidine in DMF for 20 minutes. The reactor was vented again by evacuating it, and the peptide resin was washed with 2 L of DMF. The reactor was then vented by evacuating it. The peptide resin is ready for step 2.
[0073] Step 2. A solution of 27.0 g of Fmoc-D-AIa-OH (2 equivalents), 14.3 g of HOBt and 13.2 ml of DIC was dissolved in 250 ml of DMF, activated for 15 minutes, and then poured into the resin containing peptide of the reactor. After the reaction time reached 1 hour, 2.2ml of NMM was added to the solution, and the r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com