Degarelix-containing pharmaceutical composition for injection and preparation method and application thereof
A degarelix and injection technology, applied in the field of pharmaceutical preparations, can solve problems such as sudden cardiac death, and achieve the effects of reducing swelling, increasing clinical safety, and reducing swelling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
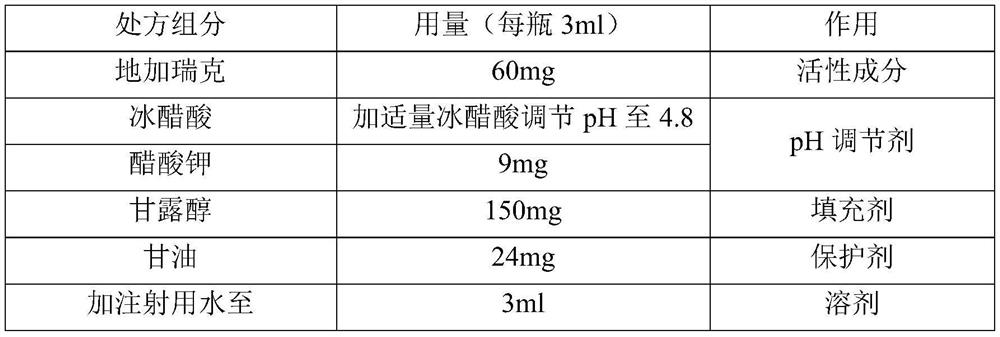
[0037]
[0038] The preparation method is as follows:
[0039] 1) Stir and dissolve 0.90g of potassium acetate in 250ml of water for injection, then add 15g of mannitol and stir to dissolve;
[0040] 2) Dissolving 6g of degarelix in 1);
[0041] 3) Add 2.4g of glycerin to 2), control the temperature to 40°C, stir to dissolve, add water for injection to 90% of the total volume;
[0042] 4) Add an appropriate amount of glacial acetic acid to adjust the pH to 4.8, and add water for injection to make the volume to 300ml;
[0043] 5) Sterilize and filter with a 0.22 μm filter; the sterilized medicinal solution is divided into 100 bottles of vials for injection (3ml each bottle), half-tightened and placed in a lyophilizer to freeze-dry to obtain final product.
Embodiment 2
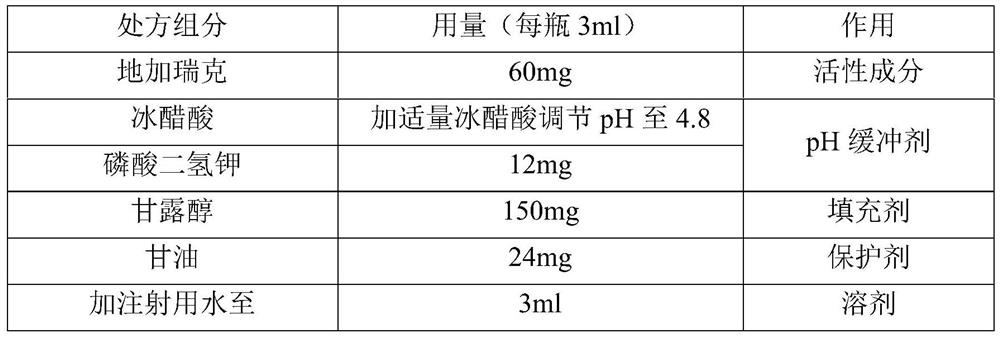
[0045]
[0046] The preparation method is as follows:
[0047] 1) Stir and dissolve 1.20 g of potassium dihydrogen phosphate in 250 ml of water for injection, then add 15 g of mannitol and stir to dissolve;
[0048] 2) Dissolving 6g of degarelix in 1);
[0049] 3) Add 2.4g of glycerin to 2), control the temperature to 40°C, stir to dissolve, add water for injection to 90% of the total volume;
[0050] 4) Add an appropriate amount of glacial acetic acid to adjust the pH to 4.8, and add water for injection to make the volume to 300ml;
[0051] 5) Sterilize and filter with a 0.22 μm filter; the sterilized medicinal solution is divided into 100 bottles of vials for injection (3ml each bottle), half-tightened and placed in a lyophilizer to freeze-dry to obtain final product.
Embodiment 3
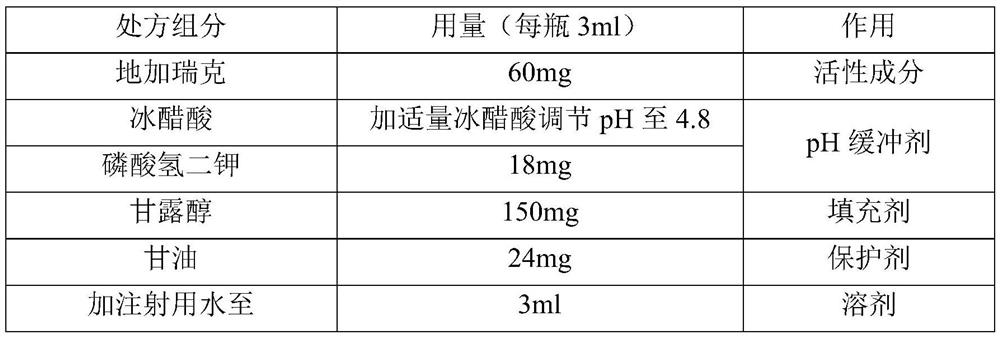
[0053]
[0054] The preparation method is as follows:
[0055] 1) Stir and dissolve 1.80 g of dipotassium hydrogen phosphate in 250 ml of water for injection, then add 15 g of mannitol and stir to dissolve;
[0056] 2) Dissolving 6g of degarelix in 1);
[0057] 3) Add 2.4g of glycerin to 2), control the temperature to 40°C, stir to dissolve, add water for injection to 90% of the total volume;
[0058] 4) Add an appropriate amount of glacial acetic acid to adjust the pH to 4.8, and add water for injection to make the volume to 300ml;
[0059] 5) Sterilize and filter with a 0.22 μm filter; the sterilized medicinal solution is divided into 100 bottles of vials for injection (3ml each bottle), half-tightened and placed in a lyophilizer to freeze-dry to obtain final product.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com