Submicron emulsion injection liquid of CoQ10 and preparation process thereof
A sub-microemulsion and injection technology, applied in the fields of health care products and medicines, can solve problems such as application difficulties, unstable injection, and increased side effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Coenzyme Q of the present invention 10 Submicroemulsion injection is composed of the following raw materials in weight ratio:
[0047] coenzyme Q 10 : soybean oil: lecithin: glycerin: water for injection = 0.1:7:0.5:2:89;
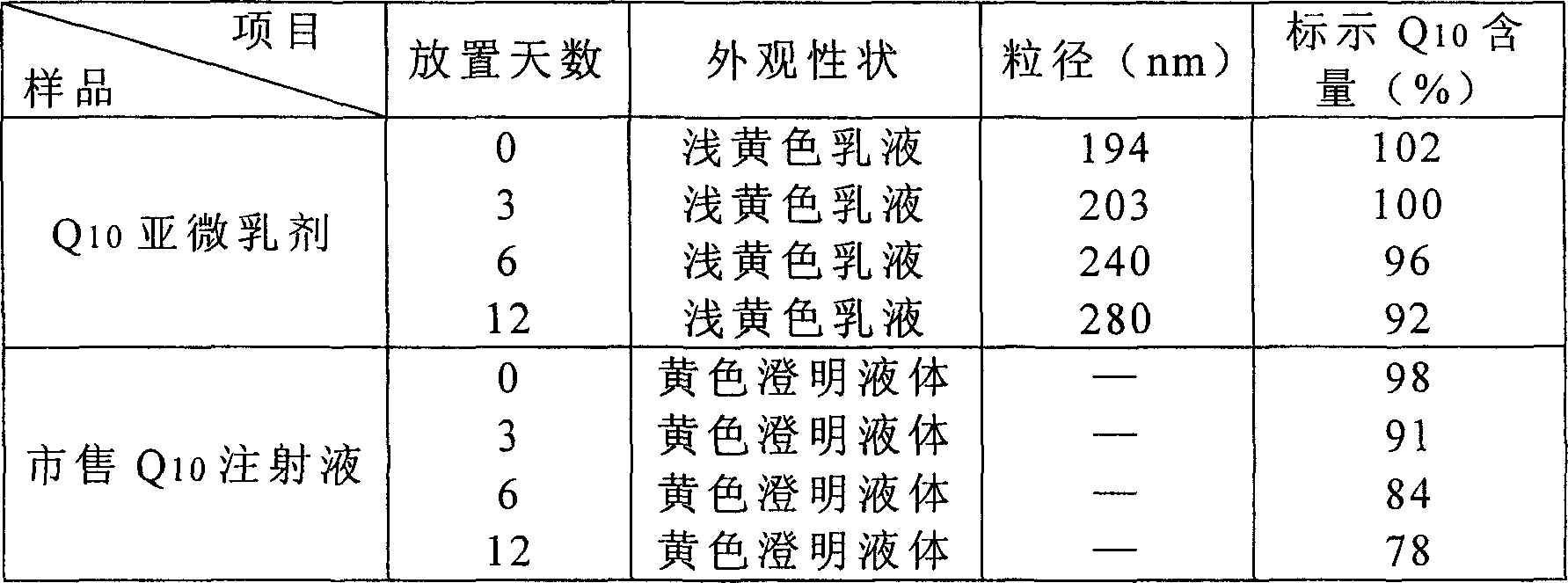
[0048] Weigh coenzyme Q according to the above weight ratio 10 1g, join in the 70g injection grade soybean oil (Tieling City Beiya Medicinal Oil Company), make dissolving; 5g refined egg yolk lecithin (German lipoid company) is dispersed in 100g water; Apparatus factory) with 10000rpm, mix and homogenize for 2-5 minutes, add 790g water for injection, 20g glycerol, with 10000rpm, homogenize for 3-5 minutes, form colostrum, colostrum is joined in high-pressure milk homogenizer (Italian ATS company ), pass N 2 Protection, the initial pressure is 20-30MPa, circulate for 2 minutes, then increase to 80-100Mpa, circulate 6 times, release homogeneous milk. In the 100-level purification workshop, microporous membrane equipment is used to pass through a 0...
Embodiment 2
[0050] Coenzyme Q of the present invention 10 Submicroemulsion injection is composed of the following raw materials in weight ratio:
[0051] coenzyme Q 10 : soybean oil: lecithin: glycerin: oleic acid: water for injection = 2:30:3:2.5:1:65;
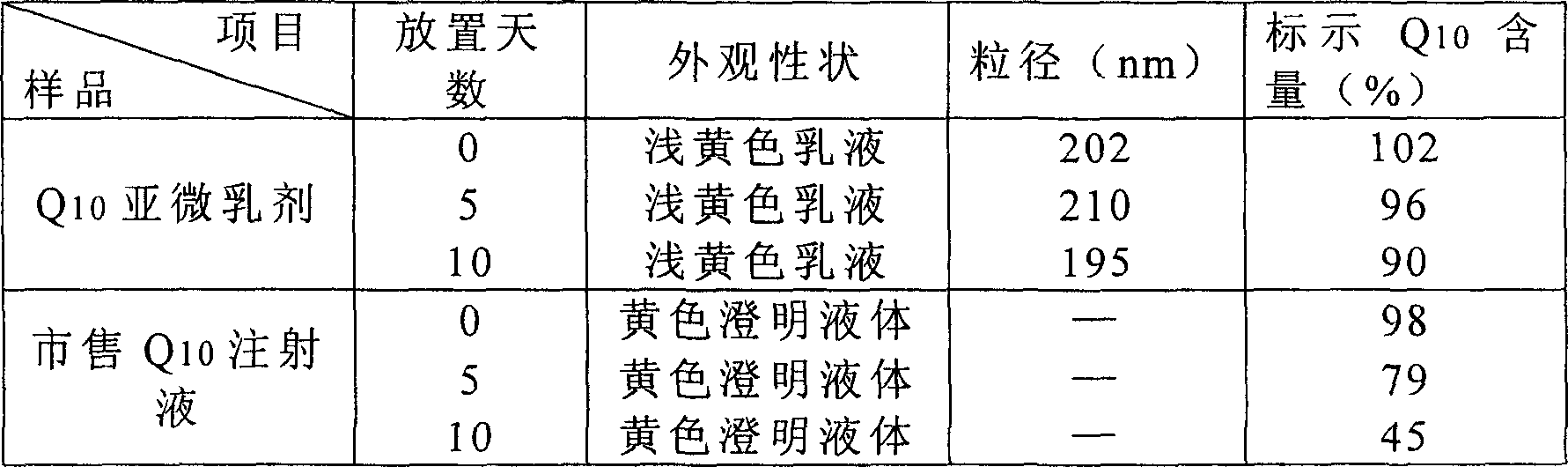
[0052] Take coenzyme Q according to the above weight ratio 10 20g, join in 300g injection grade soybean oil, make dissolving, oleic acid 10g; 30g refined egg yolk lecithin (German lipoid company) is dispersed in 150g water; , mix and homogenize for 2-5 minutes, add 500g water for injection, 25g glycerin, and homogenize for 3-5 minutes at 15000rpm to form colostrum, then, use a high-pressure homogenizer, under a pressure of 20MPa, pass N 2 Protected, after 1 cycle, a uniform emulsion is formed. All the other are with embodiment 1. As determined by liquid chromatography, contains coenzyme Q 10 18mg / ml, the emulsion particle size below 1000nm is greater than 85%.
Embodiment 3
[0054] Coenzyme Q of the present invention 10 Submicroemulsion injection is composed of the following raw materials in weight ratio:
[0055] coenzyme Q 10 : soybean oil: lecithin: glycerin: oleic acid: water for injection = 1:17:2:2.2:0.5:75;
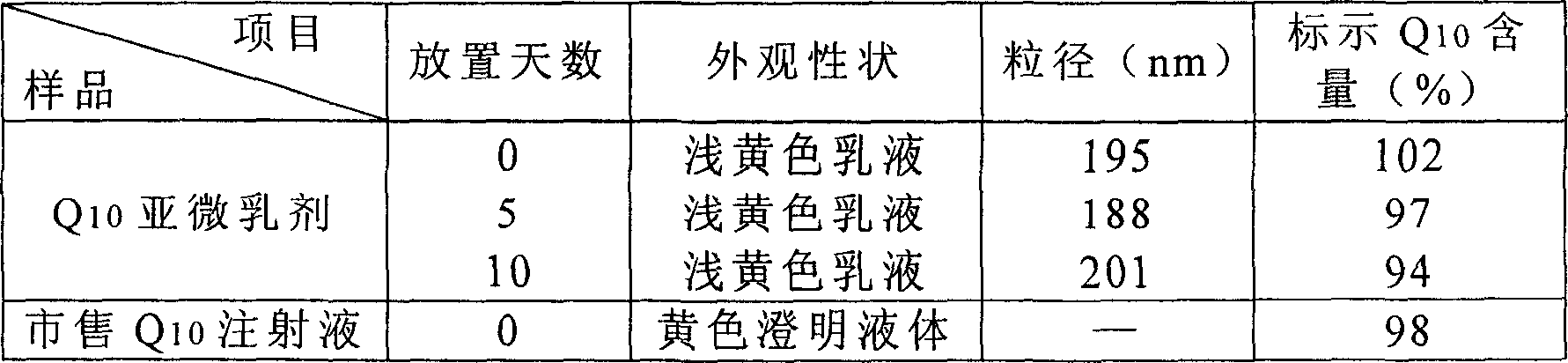
[0056] Weigh coenzyme Q according to the above weight ratio 10 10g, join in 170g injection grade soybean oil, make dissolving, oleic acid 5g; 20g refined egg yolk lecithin (German lipoid company) is dispersed in 200g water; , mix and homogenize for 2-5 minutes, add 550g water for injection, 22g glycerin, and homogenize for 3-5 minutes at 20000rpm to form colostrum, then, use a high-pressure homogenizer, under a pressure of 60MPa, pass N 2 Protected, after 5 cycles, a uniform emulsion was formed. All the other are with embodiment 1. As determined by liquid chromatography, contains coenzyme Q 10 11mg / ml, 1000nm emulsion is greater than 93%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com