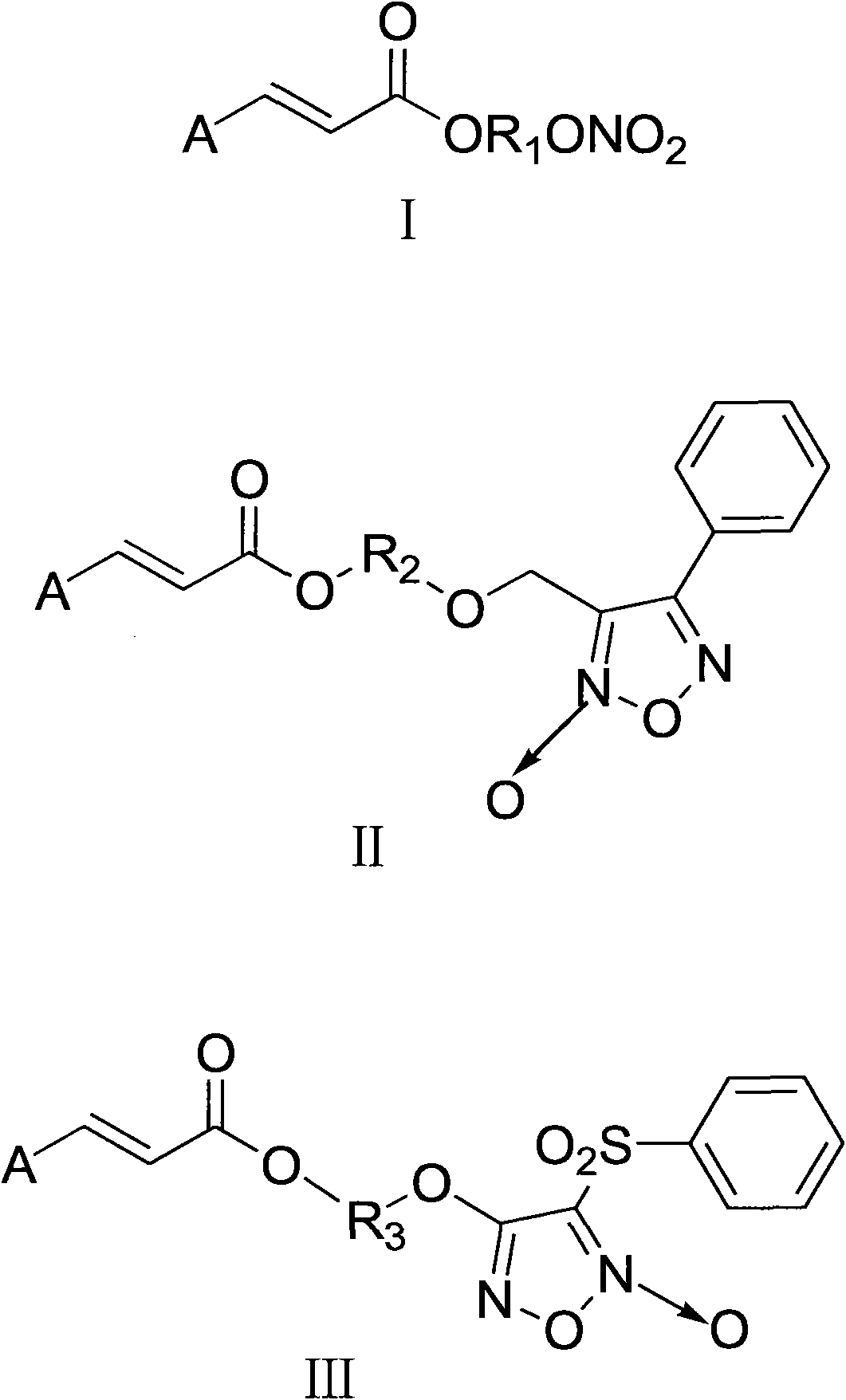
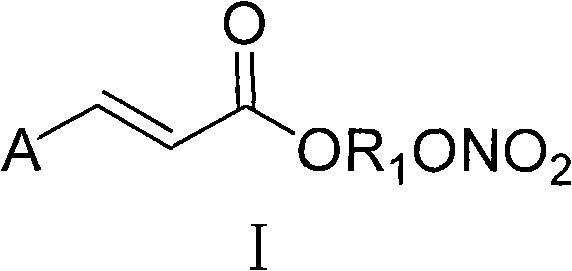
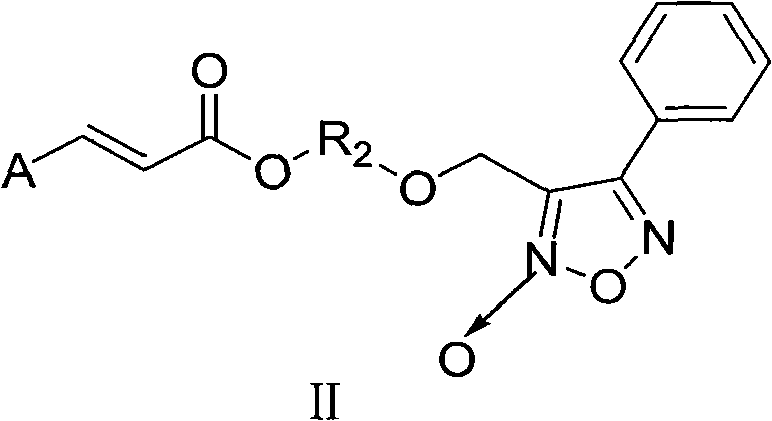
Aromatic acid pro-drug with nitrogen monoxide donor, and preparation method and application thereof
A technology of nitric oxide and prodrugs, applied in the field of medicinal chemistry research, can solve problems affecting drug efficacy, strong hydrophilicity of molecules, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] 1. Preparation of (E)-3-(4-hydroxyl-3-methoxyphenyl)acrylic acid-3-bromopropyl ester (I1a)
[0057] Dissolve 5.0g (25.8mmol) of ferulic acid and 1,3-dibromopropane (100mmol) in 150mL of acetone, add 10mL of triethylamine, heat and stir at 60°C for 4h, take the reaction solution, filter, concentrate, and coat the concentrate with silica gel Column chromatography, eluting with ethyl acetate:petroleum ether=1:4, gave the monobrominated product I1a as a dark red oil with a yield of 55.8%.
[0058] 1 H NMR (CDCl 3 )δ: 2.26 (m, 2H, CH 2 ), 3.52(t, J=3.7Hz, 2H, CH 2 Br), 3.94(s, 3H, OCH 3 ), 4.34(t, J=3.7Hz, 2H, COOCH 2 ), 5.83(s, 1H, OH), 6.30(d, J=9.5Hz, 1H, C=CH), 6.92(d, 1H, Ar-H), 7.06-7.15(m, 2H, Ar-H) , 7.65 (d, J=9.5Hz, 1H, CH=C).ESI-MS: 353 [M+K] + .
[0059] 2. Preparation of (E)-3-(4-hydroxyl-3-methoxyphenyl)acrylic acid-3-nitroxylpropyl ester (Ⅰ1)
[0060] Dissolve I1a (0.7mmol) prepared in 1 in 20mL acetonitrile, add AgNO 3 0.6g (2.5mmol), stirred at 50°...
Embodiment 2
[0063] 1. Preparation of (E)-3-(4-hydroxyl-3-methoxyphenyl)acrylic acid-4-bromobutyl ester (I2a)
[0064] Referring to the preparation method of I1a in Example 1, a white solid was prepared by reacting ferulic acid with 1,4-dibromobutane, the yield was 43.0%, and Mp: 81.9-82.3°C. 1 H NMR (CDCl 3 )δ: 1.87-2.07 (m, 4H, CH 2 ), 3.47(t, J=6.4Hz, 2H, CH 2 Br), 3.93(s, 3H, OCH 3 ), 4.24(t, J=6.4Hz, 2H, COOCH 2), 5.85 (s, 1H, OH), 6.28 (d, J=15.9Hz, 1H, C=CH), 6.92 (d, 1H, Ar-H), 7.62 (d, J=15.9Hz, 1H, CH =C).ESI-MS: 351 [M+Na] + .
[0065] 2. Preparation of (E)-3-(4-hydroxyl-3-methoxyphenyl)acrylic acid-4-nitroxylbutyl ester (I2)
[0066] With reference to the preparation method of I1 in Example 1, by I2a and AgNO 3 The white powder was obtained by reaction with a yield of 91.7%, Mp: 58.1-62.7°C. 1 HNMR (CDCl 3 )δ: 1.81-1.90 (m, 4H, CH 2 ), 3.93 (s, 3H, OCH 3 ), 4.24(t, J=5.9Hz, 2H, COOCH 2 ), 4.52(t, J=6.2Hz, 2H, CH 2 ONO 2 ), 5.90 (s, 1H, OH), 6.28 (d, J=15.9Hz, 1H,...
Embodiment 3
[0068] 1. Preparation of (E)-3-(4-hydroxyl-3-methoxyphenyl)acrylate-5-bromopentyl ester (I3a)
[0069] Referring to the preparation method of I1a in Example 1, it is prepared by reacting ferulic acid with 1,5-dibromopentane. White solid, yield 65.8%, Mp: 66.3-66.6°C. 1 H NMR (CDCl 3 )δ: 1.58 (m, 2H, CH 2 ), 1.70 (m, 2H, CH 2 ), 1.90 (m, 2H, CH 2 ), 3.44 (m, 2H, CH 2 Br), 3.92(s, 3H, OCH 3 ), 4.20 (m, 2H, COOCH 2 ), 5.91 (s, 1H, OH), 6.28 (d, J=15.9Hz, 1H, C=CH), 6.91 (d, 1H, Ar-H), 7.61 (d, J=15.9Hz, 1H, CH =C).ESI-MS: 365[M+Na] + .
[0070] 2. Preparation of (E)-3-(4-hydroxyl-3-methoxyphenyl)acrylate-5-nitroxylpentyl ester (I3)
[0071] Referring to the preparation method of I1 in Example 1, by I3a and AgNO 3 The reaction is made. Blue-yellow crystals, yield 83.0%, Mp: 51.3-53.7°C. 1 H NMR (CDCl 3 )δ: 1.49-1.63 (m, 2H, CH 2 ), 1.71-1.84 (m, 4H, CH 2 ), 3.93 (s, 3H, OCH 3 ), 4.21(t, J=6.4Hz, 2H, COOCH 2 ), 4.47(t, J=6.5Hz, 2H, CH 2 ONO 2 ), 5.91 (s, 1H, OH)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com