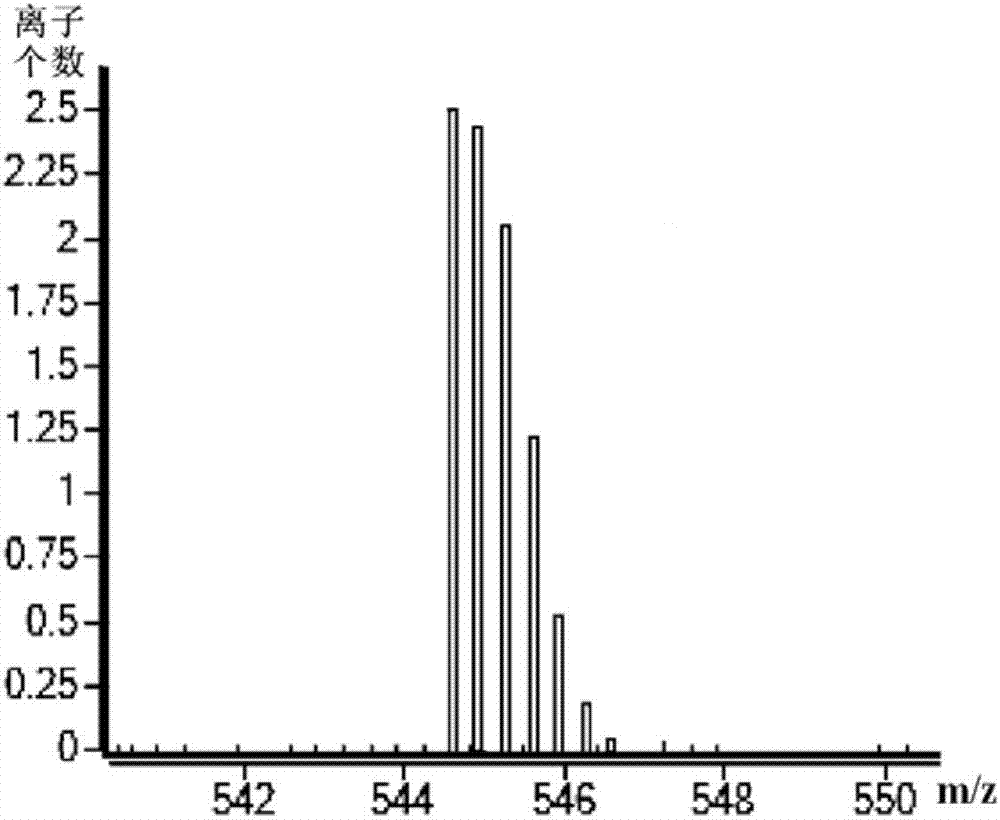
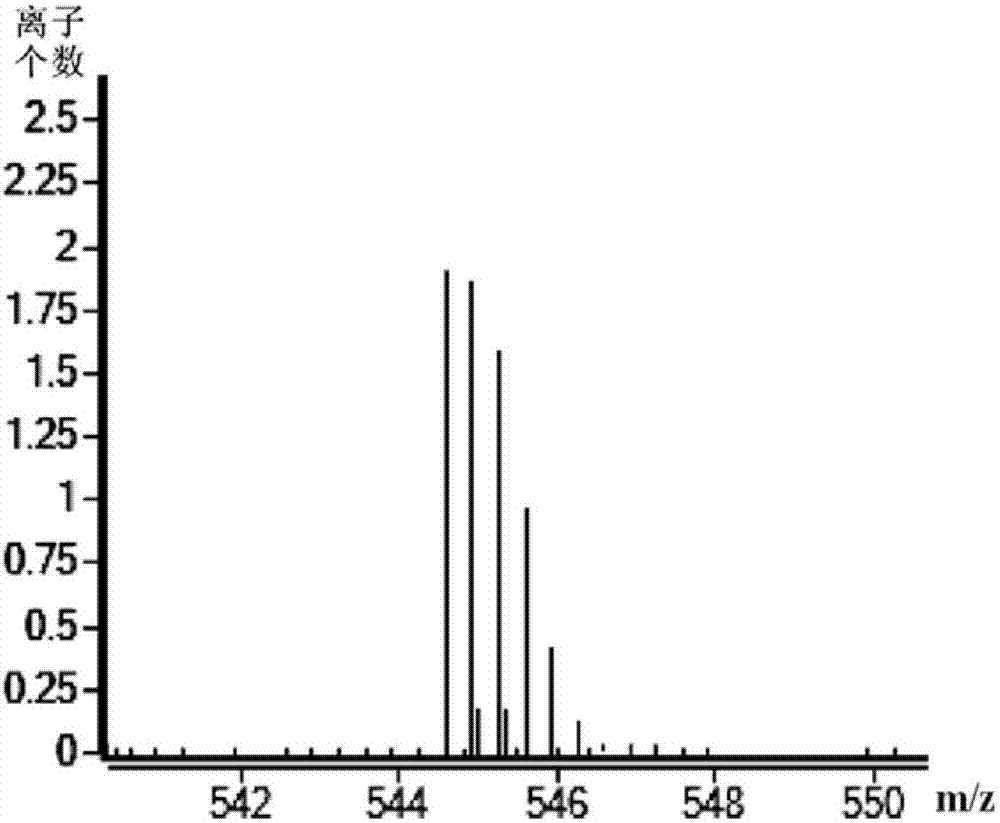
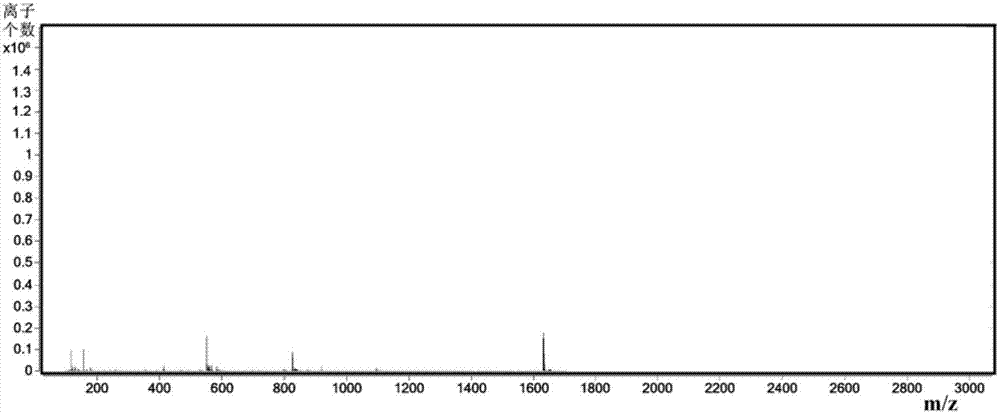
Preparation method of degarelix
A technology of degarelix and resin, which is applied in the field of preparation of degarelix, can solve the problems of high cost of synthesis route process and difficulty in raw material synthesis, and achieve reduction of process cost and synthesis difficulty, reduction of synthesis difficulty and process cost, Simple operation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032]As described in the prior art, in the existing preparation method of degarelix, the raw materials used need to be protected by protecting groups, which will cause the problems of difficult synthesis of raw materials and high cost of synthetic routes. In order to solve the above-mentioned technical problems, the application provides a preparation method of degarelix, which preparation method comprises: Fmoc-D-Ala-resin, Fmoc-Pro-OH, Fmoc-ILys (Boc )-OH, Fmoc-Leu-OH, Fmoc-D-4Aph(Cbm)-OH, Fmoc-4Nph-OH, Fmoc-Ser(tBu)-OH, Fmoc-D-3Pal-OH, Fmoc-D-Phe( 4Cl)-OH and Ac-D-2Nal-OH carry out the first condensation reaction in sequence according to the amino acid sequence of degarelix to obtain the first intermediate product; the amino acid residue 4Nph in the first intermediate product is reduced to 4Aph to obtain the first intermediate product Second intermediate product; make the side chain amino group of above-mentioned 4Aph and L-4,5-dihydroorotic acid carry out the second conden...
Embodiment 1
[0060] 1. Synthesis of Fmoc-D-Ala-Rink resin
[0061] Rink amid AM resin (10mmol, degree of substitution 0.65mmol / g) is loaded into the solid-phase reaction column, washes the above-mentioned solid-phase reaction column twice with DMF, then adds 500mL DMF to the above-mentioned solid-phase reaction column to soak Make the above resin swell, soaking time is 30min. Use 500mL of 20% DBLK to add to the above-mentioned solid-phase reaction column to wash, so as to remove part of Fmoc, and the reaction time is 10min. Then, 500 mL of 20% DBLK was added to the above-mentioned solid-phase reaction column again to remove the remaining Fmoc. The reaction time was 10 min, and then the above-mentioned solid-phase reaction column was washed 6 times with DMF.
[0062] Dissolve 9.34g Fmoc-D-Ala-OH and 8.84g HOBt in 60mL DMF to obtain a mixed solution, and cool the above mixed solution in an ice bath for 10min. Then, 5.05 g of DIC was added to the above mixture to pre-activate Fmoc-D-Ala-OH, a...
Embodiment 2
[0094] The difference from Example 1 is that the amount of reducing agent used is 3.0 times of the resin substitution degree.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com