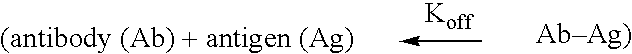Patents
Literature
69results about How to "Suppress symptoms" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
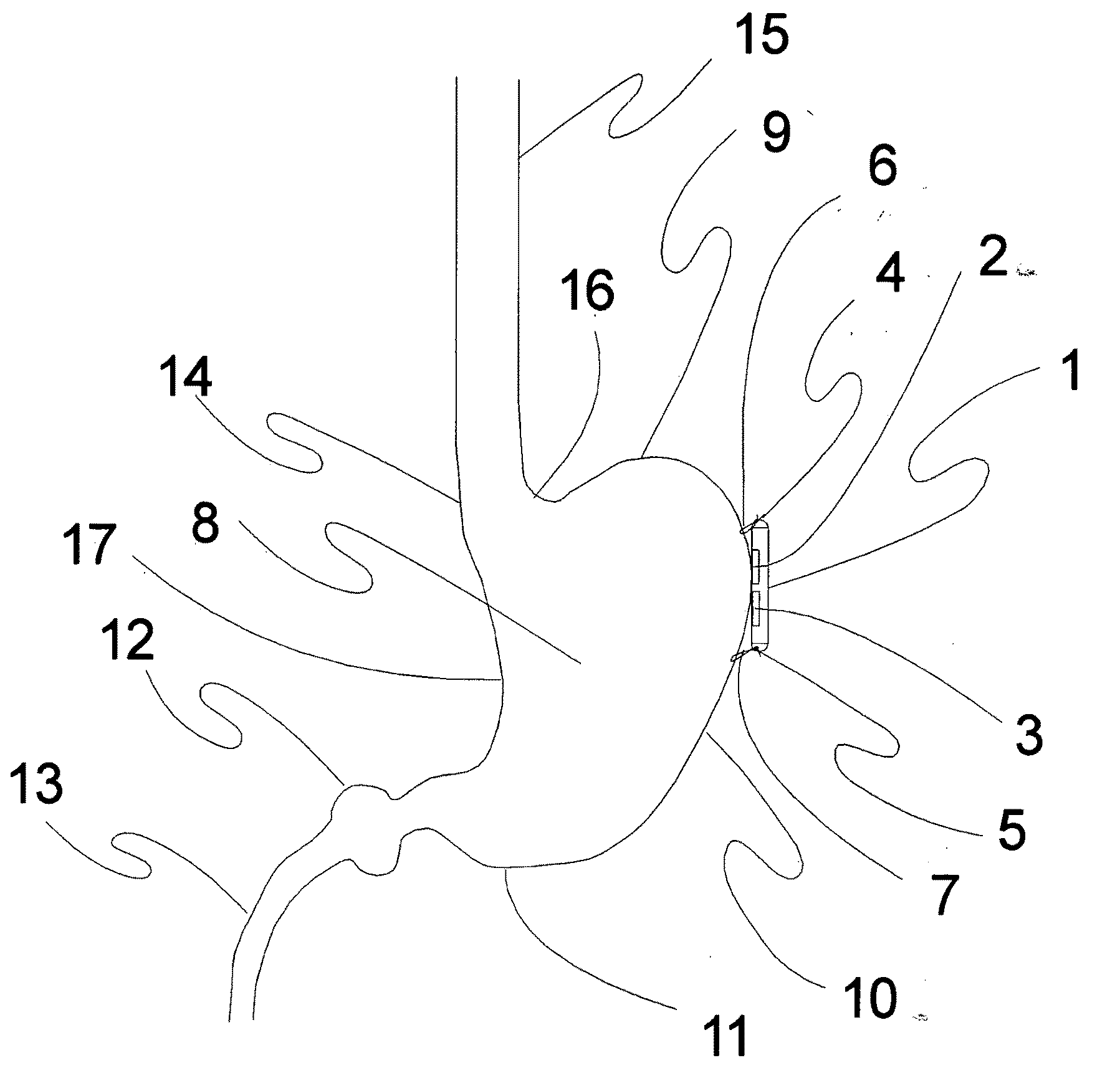
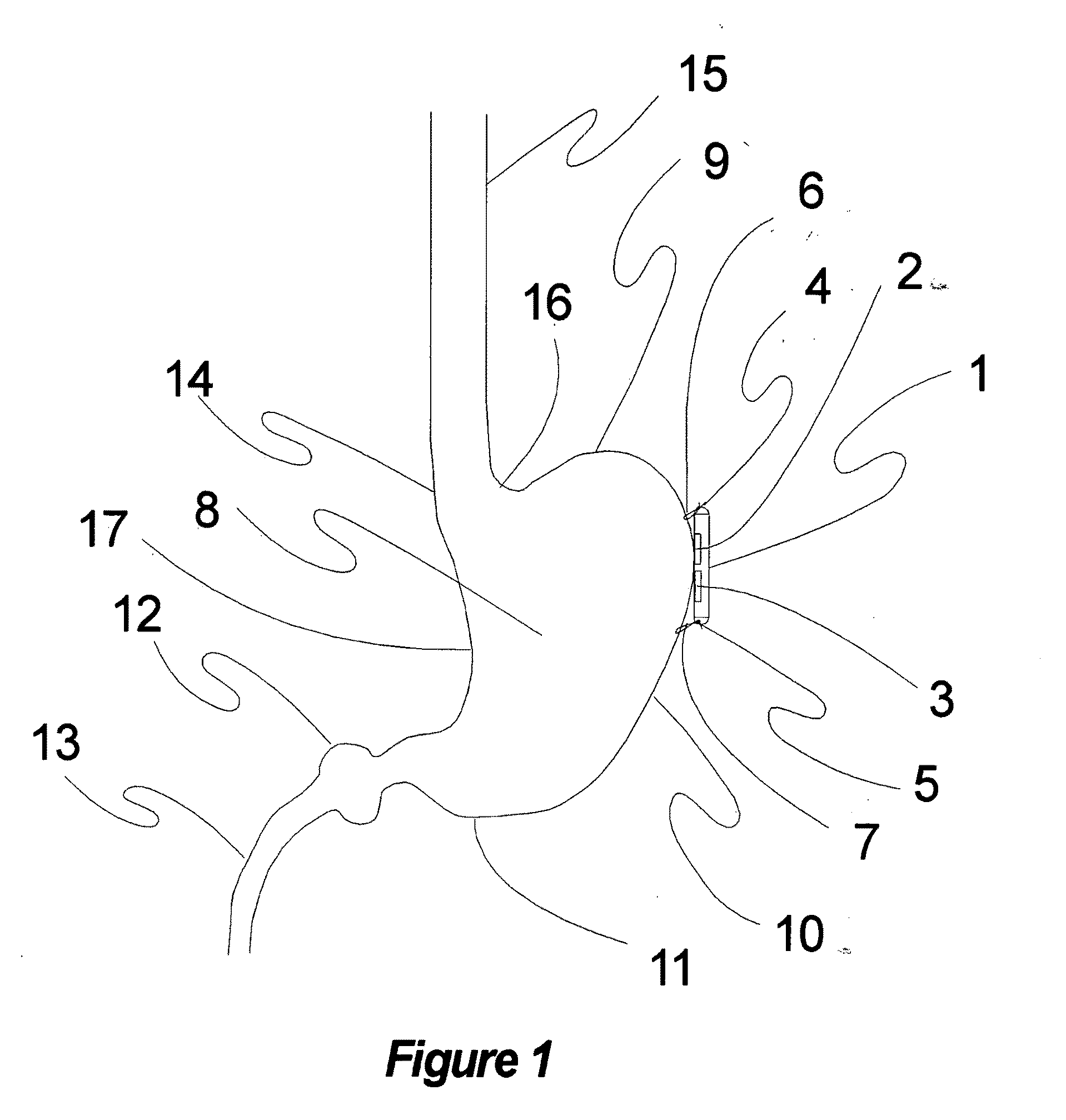
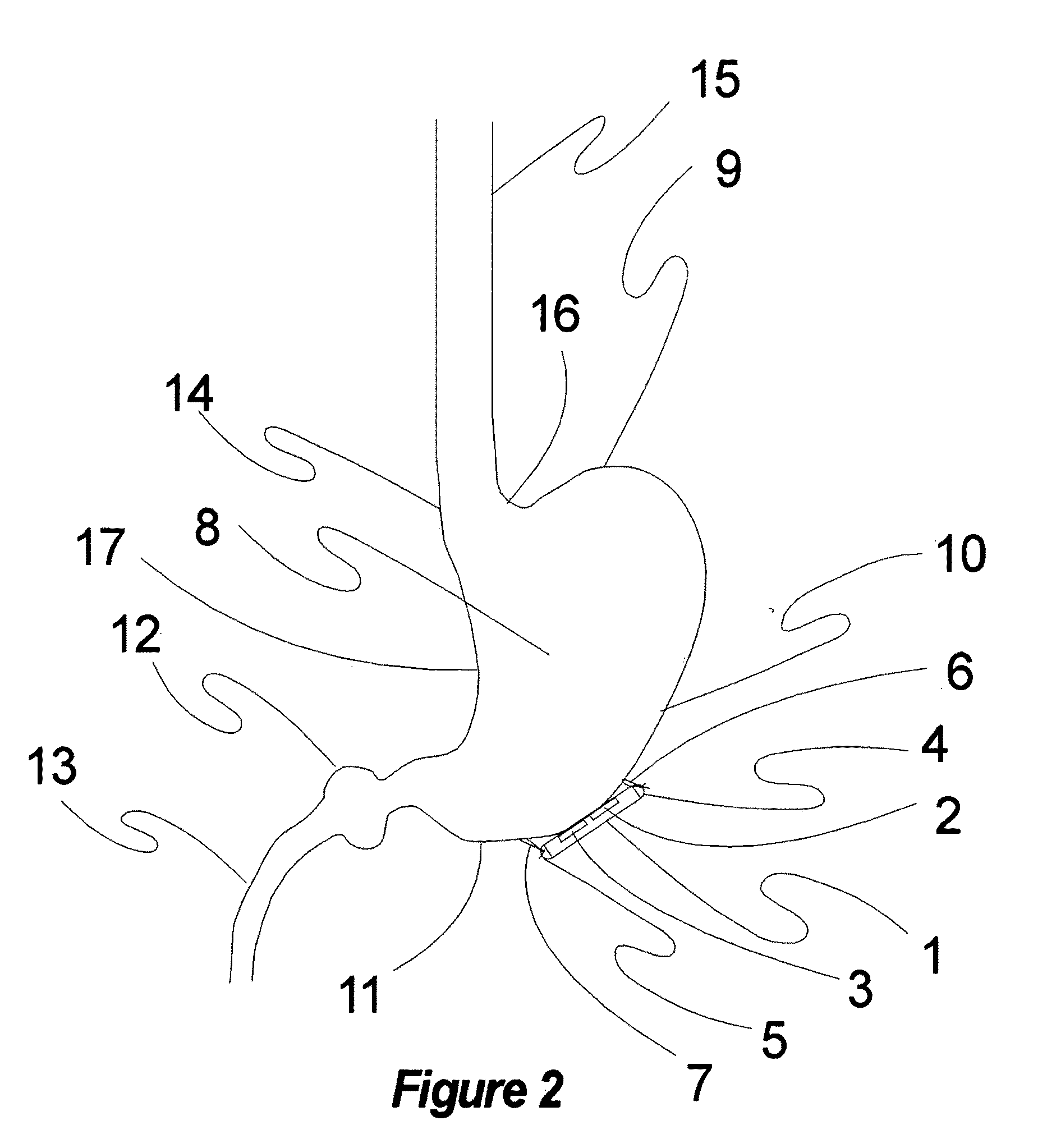
Method and apparatus for conformal electrodes for autonomic neuromodulation for the treatment of obesity and other conditions
InactiveUS20090118780A1Reduce or prevent conditionReducing and preventing symptomSpinal electrodesHead electrodesAfferent NeuronsAutonomic Denervation
The present invention teaches a method and apparatus for user control and operation of physiological modulation, including neural and gastrointestinal modulation, for the purposes of treating several disorders, including obesity, metabolic disease, and other conditions. This includes programming of neuromodulatory signal for the modulation of autonomic neural and neuromuscular modulators, used to modulate tissues, including the afferent neurons of the sympathetic nervous system to induce satiety and efferent neurons to modulate metabolism. The apparatus and methods include a conformal neuromodulator array which facilitates minimally invasive placement of neuromodulators in communication with target tissues.
Owner:DILORENZO DANIEL JOHN
Capsule Stable Against Mastication
InactiveUS20080057115A1Good disintegrationExcellent pharmaceutical preparationAntibacterial agentsNervous disorderSlice thicknessMedicine
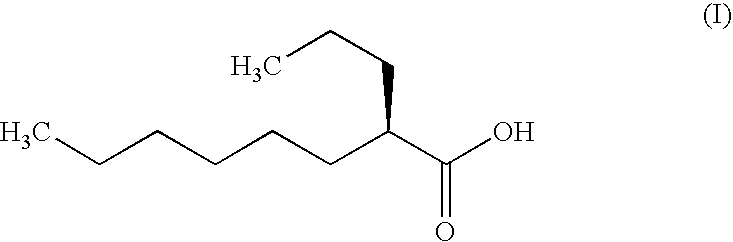
The present invention relates to a soft capsule which is easily disintegrated in the stomach, wherein the contents thereof are not easily leaked at the time of mastication, which is obtained by providing a soft capsule comprising (2R)-2-propyloctanoic acid or a salt thereof with at least one property, preferably all properties, selected from (A) wherein it has a strength of 150 to 400 N by a cracking test; (B) wherein it has a disintegration time of 3 to 10 minutes by the disintegration test stipulated in Japanese Pharmacopoeia; (C) wherein the capsule shell has a shell thickness of 0.05 to 0.50 mm; (D) wherein the capsule shell has a first seam thickness of 0.10 to 0.55 mm; (E) wherein the capsule shell has a second seam thickness of 0.05 to 0.50 mm; (F) wherein the capsule shell has a water content of 5.0 to 9.0%.
Owner:ONO PHARMA CO LTD
Method and apparatus for programming of autonomic neuromodulation for the treatment of obesity
InactiveUS20090187230A1Reduce or prevent conditionReducing and preventing symptomSensorsDigestive electrodesAfferent NeuronsDisease
The present invention teaches methods and apparatus for user control and operation of physiological modulation, including neural and gastrointestinal modulation, for the purposes of treating several disorders, including obesity. This includes programming of neuromodulatory signal for the modulation of autonomic neural and neuromuscular modulators, used to modulate tissues, including the afferent neurons of the sympathetic nervous system to induce satiety and efferent neurons to modulate metabolism.
Owner:DILORENZO DANIEL J
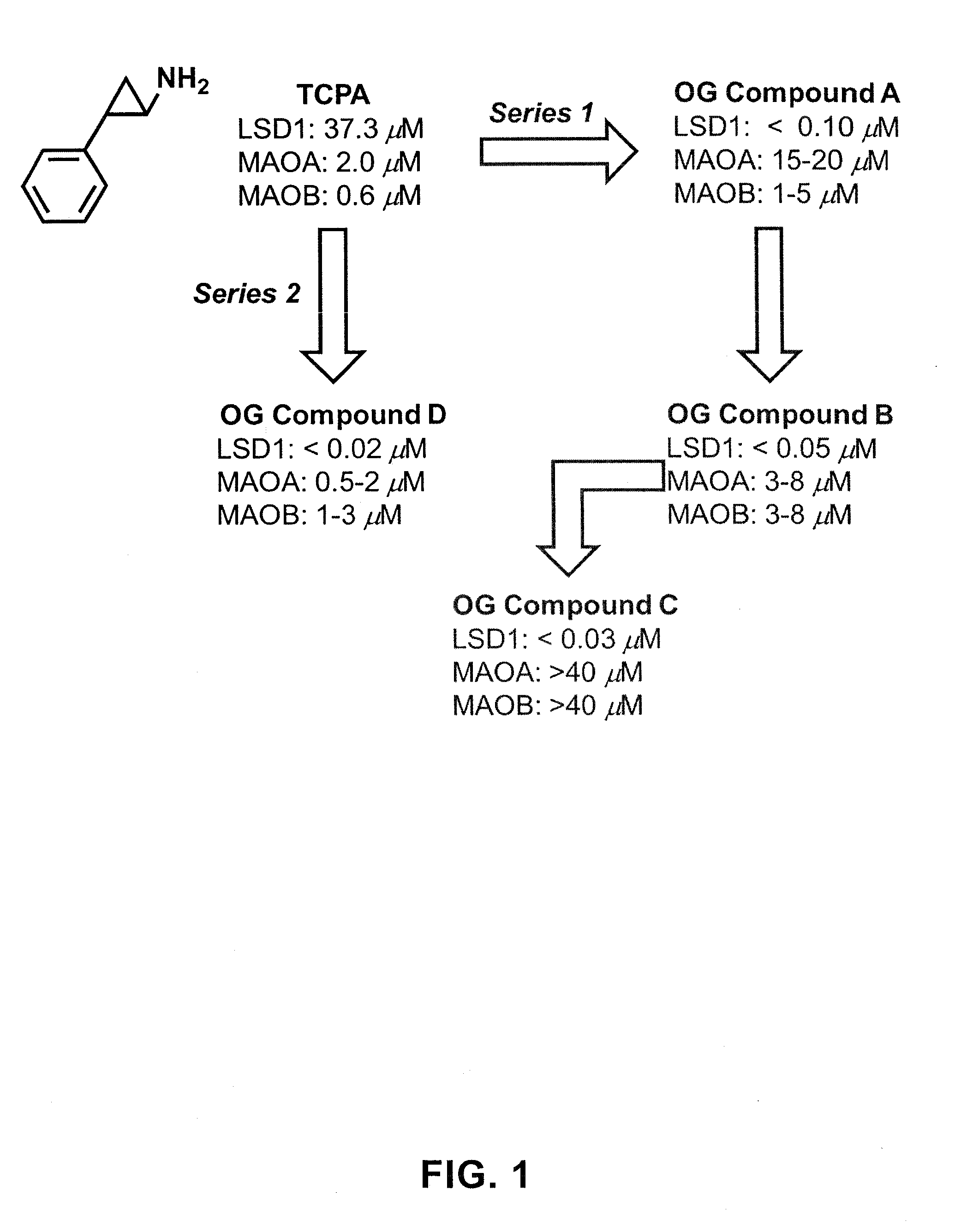
Lysine demethylase inhibitors for diseases and disorders associated with hepadnaviridae
ActiveUS20130095067A1Symptoms improvedReduce probabilityBiocidePeptide/protein ingredientsMammalHepadnavirus
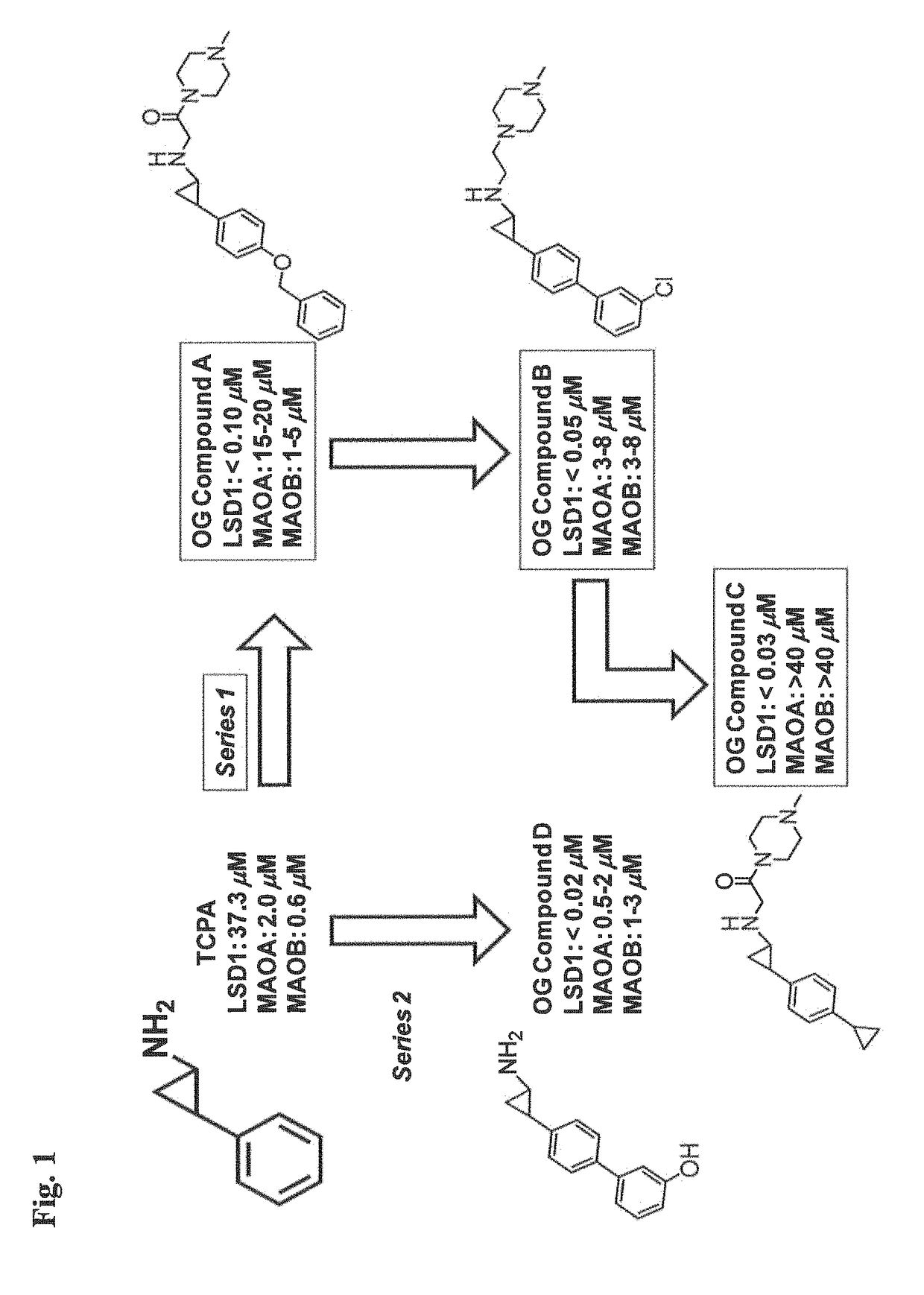
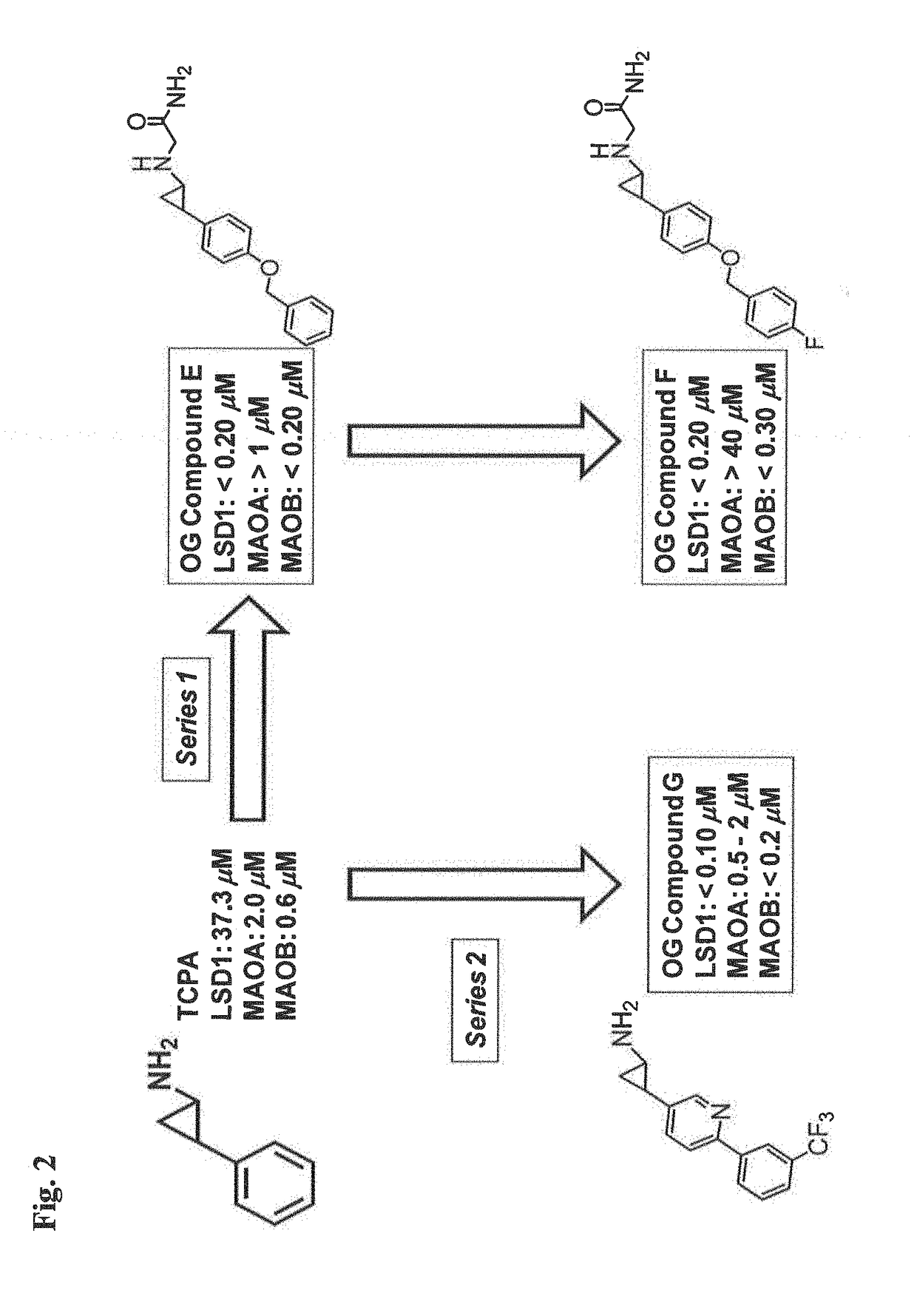
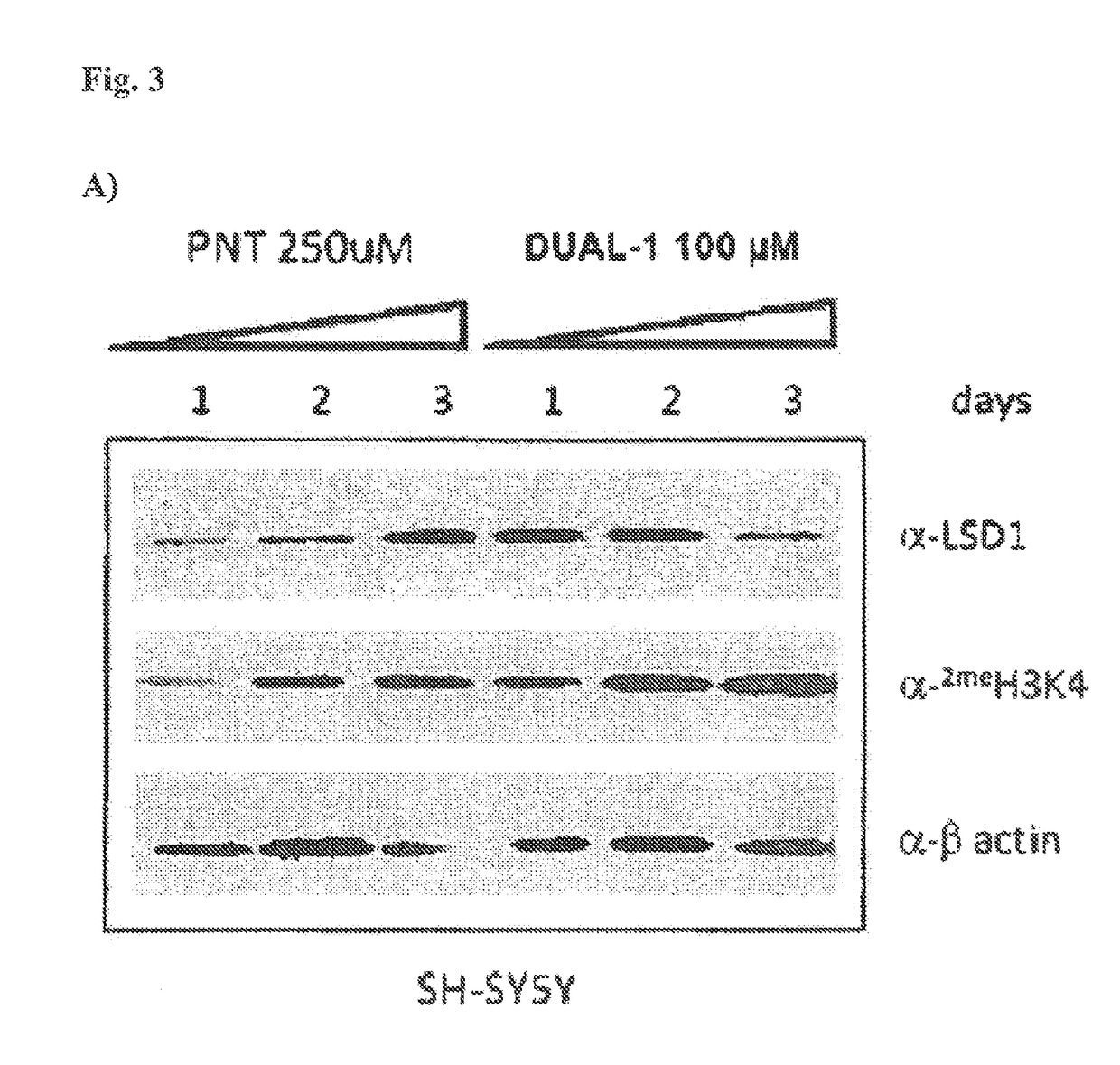
The present invention provides for the treatment or prevention of Hepadnaviridae infection and disease caused by Hepadnaviridae infection. In particular, the invention provides compositions and methods that affect the ability of Hepadnaviridae to utilize the host's cellular machinery as part of the virus' lifecycle. The invention relates to the discovery that interfering with the normal ability of viruses to utilize the host cell machinery with LSD1 inhibitors reduces HBV replication. Thus, the treatment and prevention of Hepadnaviridae infection and disease caused by Hepadnaviridae according to the invention comprises administering to an individual in need of treatment, a therapeutically effective amount of a LSD1 inhibitor. The individual in need of treatment can be a human or, e.g., another mammal.
Owner:ORYZON GENOMICS SA
Lysine demethylase inhibitors for diseases and disorders associated with flaviviridae
ActiveUS20140256742A1Avoids side-effectsReduce duplicationBiocideOrganic active ingredientsLysineLuetic disease
Owner:ORYZON GENOMICS SA
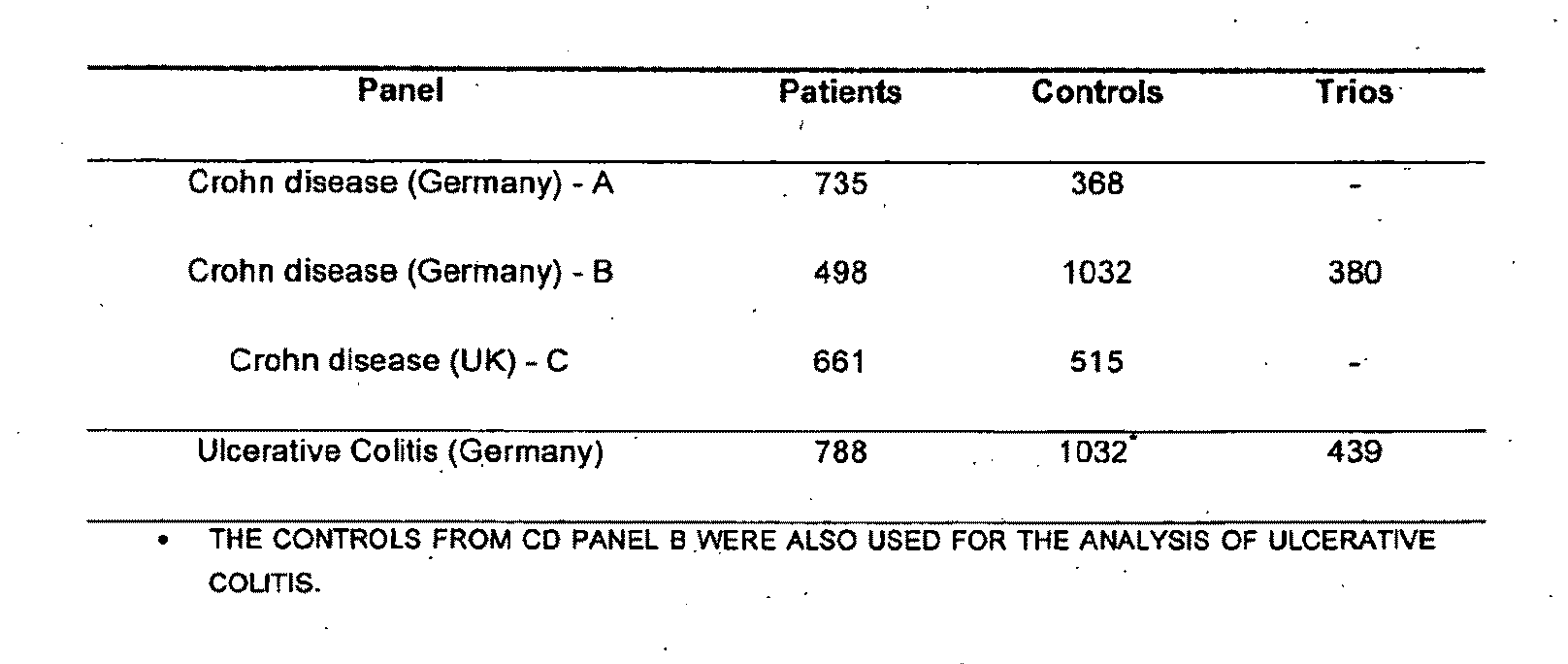
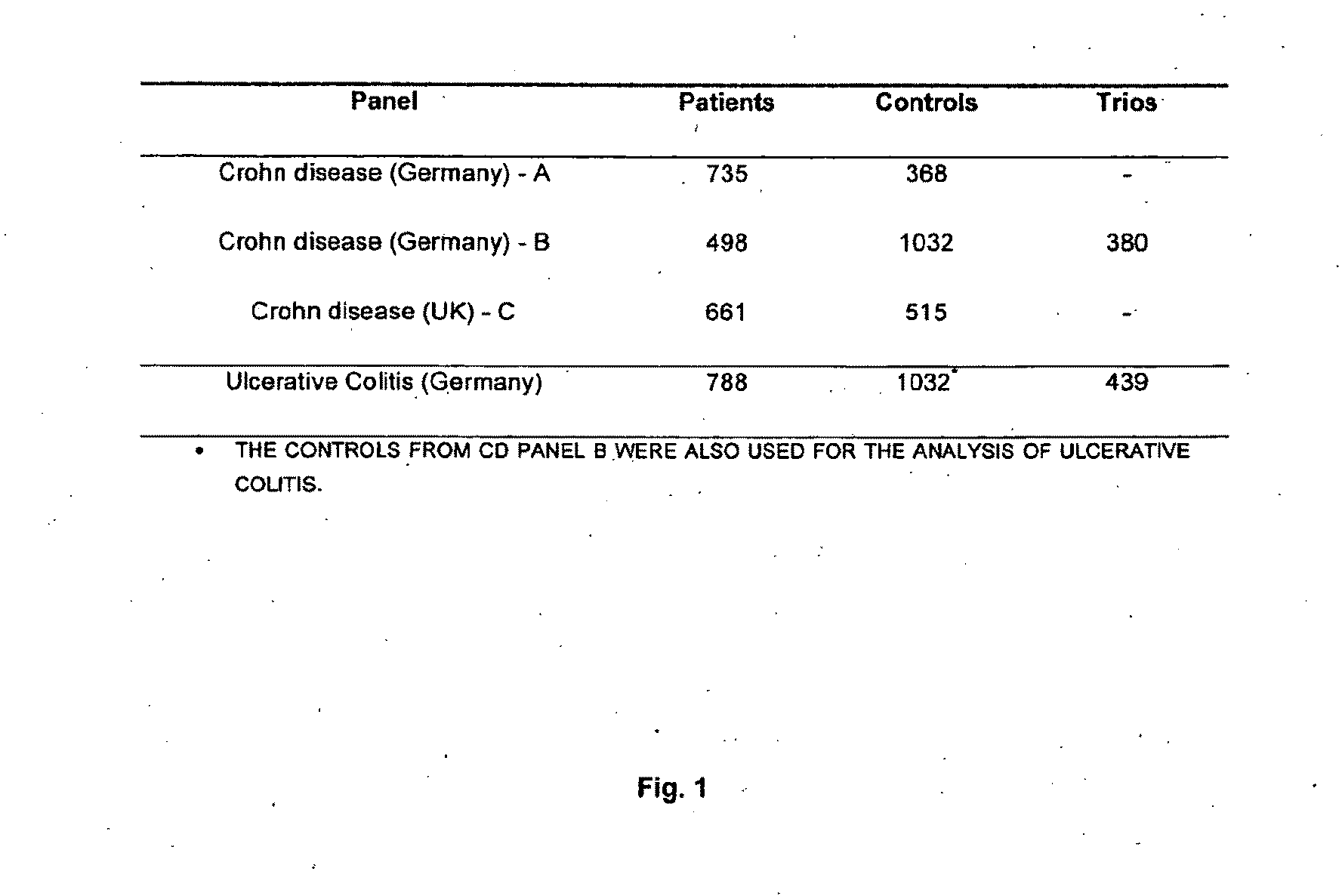
Genemap of the human genes associated with crohn's disease
InactiveUS20090081658A1Reduce frequencyTreatment safetyMicrobiological testing/measurementGenomicsLinkage Disequilibrium Mapping
The present invention relates to the selection of a set of polymorphism markers for use in genome wide association studies based on linkage disequilibrium mapping. In particular, the invention relates to the fields of pharmacogenomics, diagnostics, patient therapy and the use of genetic haplotype information to predict an individual's susceptibility to Crohn's disease and / or their response to a particular drug or drugs.
Owner:GENIZON BIOSCI
Methods and compositions for induction or promotion of immune tolerance
InactiveUS20060193869A1Suppressing autoimmune responseSuppresses autoimmune responsePeptide/protein ingredientsGenetic material ingredientsImmune tolerancePolynucleotide
The invention provides non-immunostimulatory polynucleotide antigen conjugates and methods for treating unwanted immune reactions in individuals using the non-immunostimulatory polynucleotide antigen conjugates.
Owner:DYNAVAX TECH CORP
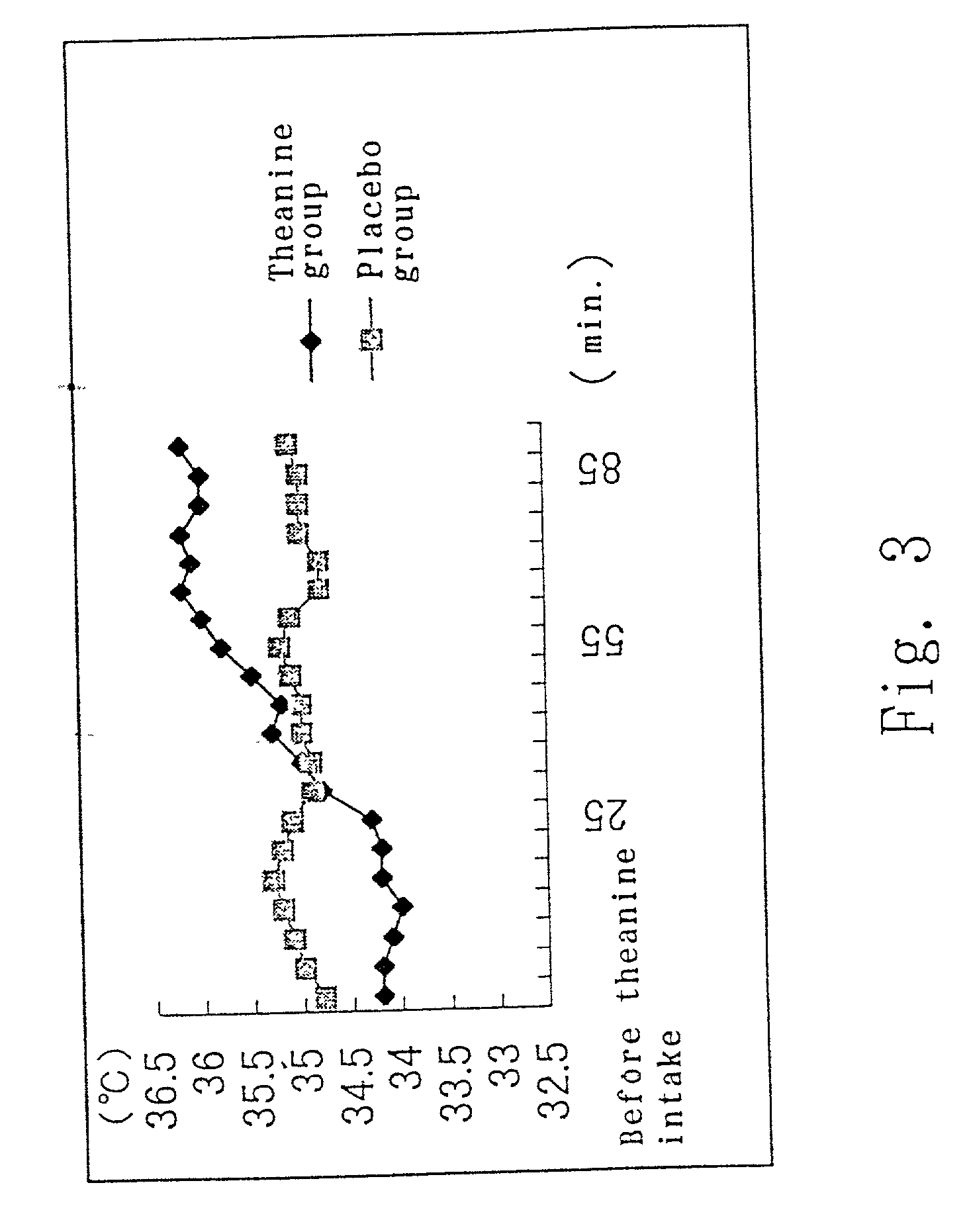
Composition comprising theanine
InactiveUS20010001307A1Low costSuppress symptomsBiocideHeavy metal active ingredientsMedicineTheanine
The present invention relates to a composition for suppressing or ameliorating a symptom accompanying diminished homeostasis, comprising theanine; and a mineral composition comprising theanine and a mineral. According to the present invention, there can be provided a composition for suppressing or ameliorating the above symptoms; and a mineral composition having a reduced peculiar metal taste, of which administration is made easily.
Owner:TAIYO KAGAKU CO LTD
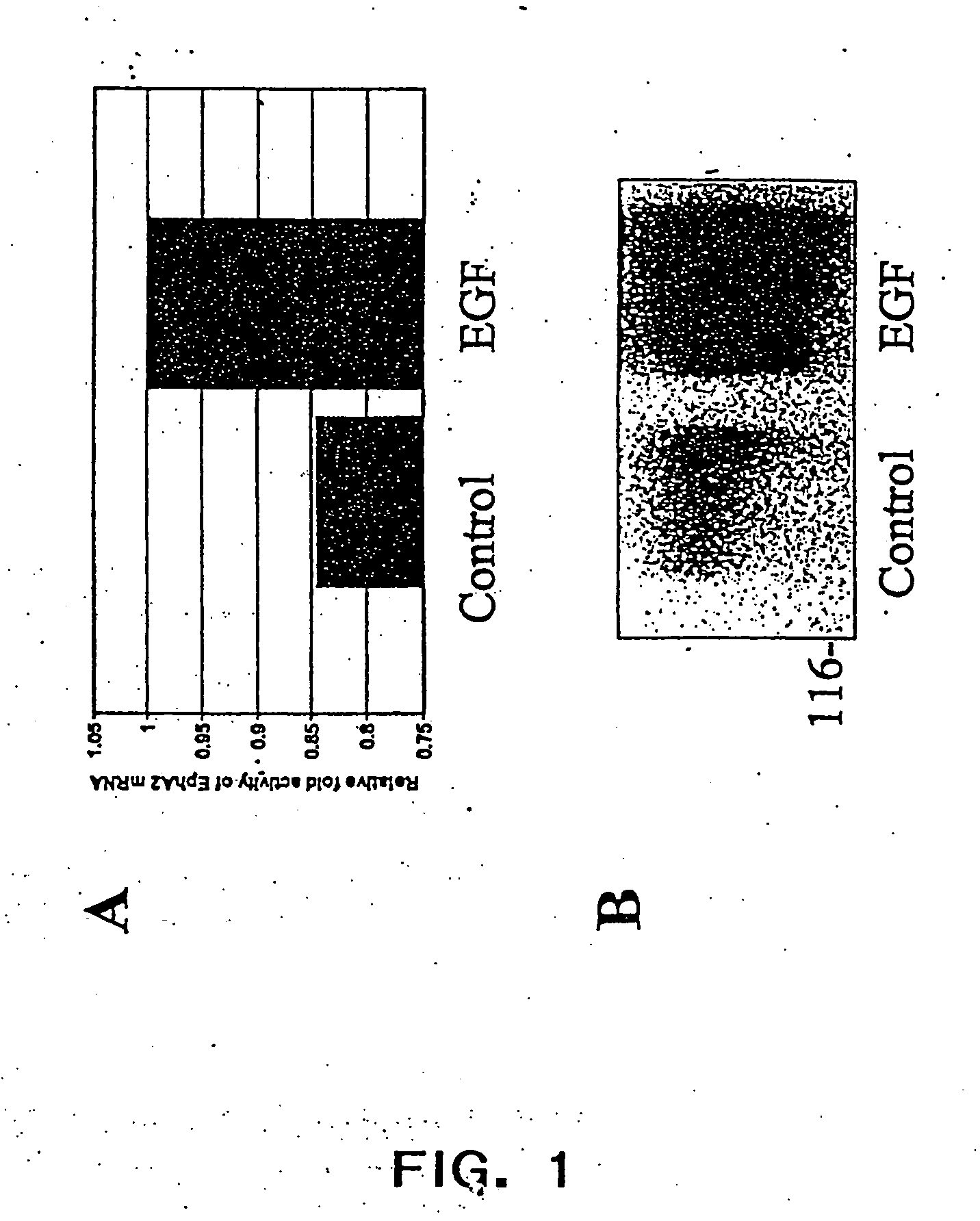
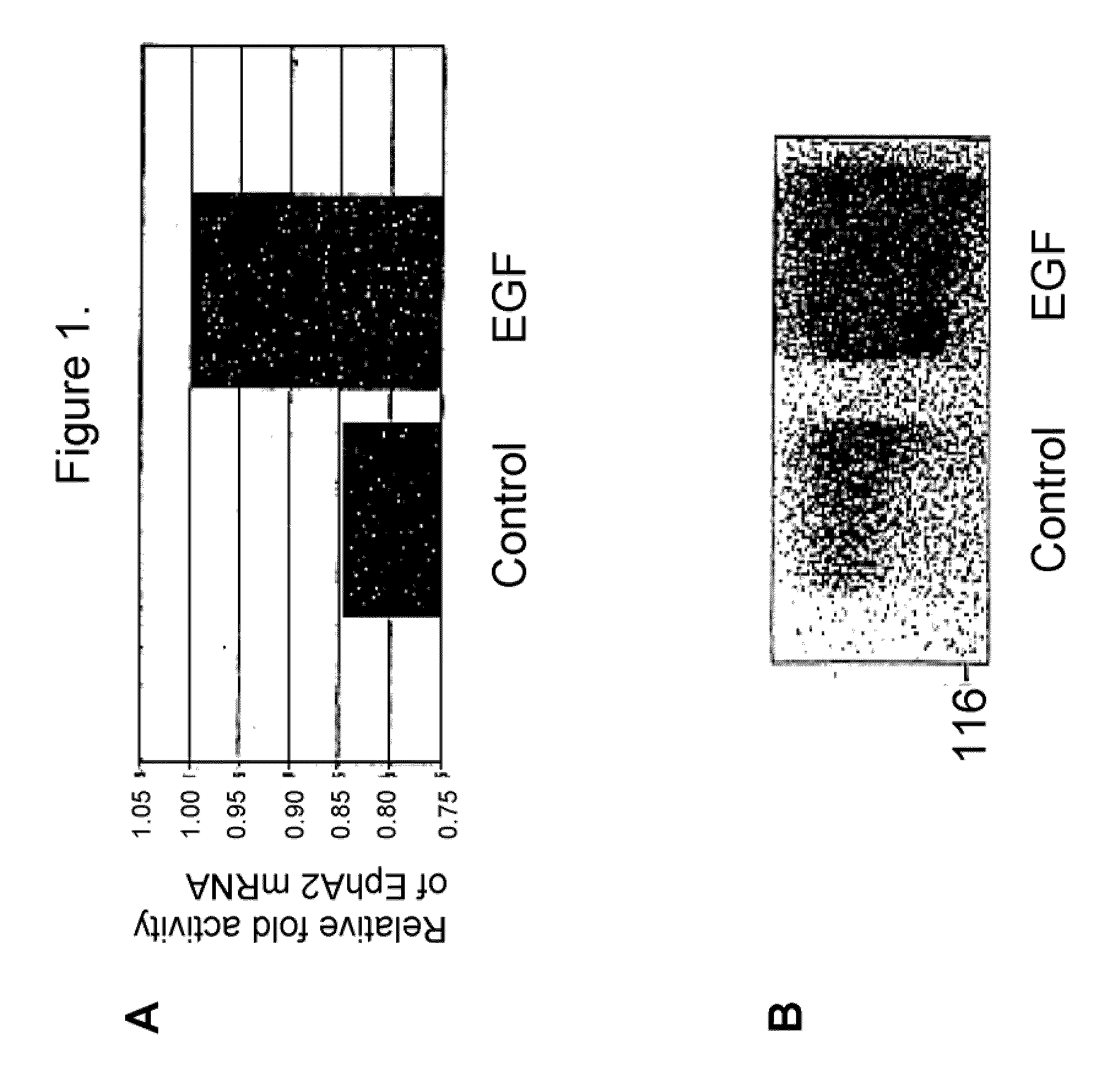
EphA2 and hyperproliferative cell disorders
InactiveUS20050059592A1Increase activityIncreased expressionBiocideSenses disorderDrugTrans-autophosphorylation
The present invention relates to methods and compositions designed for the treatment, management, or prevention of a non-neoplastic hyperproliferative cell or excessive cell accumulation disorders, particularly those involving hyperproliferation of epithelial or endothelial cells. In one embodiment, the methods of the invention comprise the administration of an effective amount of one or more EphA2 agonistic agents that bind to EphA2 and increase EphA2 cytoplasmic tail phosphorylation and / or increase EphA2 autophosphorylation in cells which EphA2 has been agonized. In another embodiment, the methods of the invention comprise the administration of an effective amount of one or more EphA2 agonistic agents that bind to EphA2 and reduce EphA2 activity (other than autophosphorylation). In another embodiment, the methods of the invention comprise administration of an effective amount of one or more EphA2 agonistic agents that bind to EphA2 and decrease a pathology-causing cell phenotype (e.g., a pathology-causing epithelial cell phenotype or a pathology-causing endothelial cell phenotype). In another embodiment, the methods of the invention comprise the administration of an effective amount of one or more EphA2 agonistic agents that are EphA2 antibodies that bind to EphA2 with a very low Koff rate. In preferred embodiments, agents of the invention are monoclonal antibodies. The invention also provides pharmaceutical compositions comprising one or more EphA2 agonistic agents of the invention either alone or in combination with one or more other agents useful in therapy for non-neoplastic hyperproliferative cell or excessive cell accumulation disorders.
Owner:MEDIMMUNE LLC
Composition for preventing and treating type I allergy
InactiveUS7211567B1Suppress symptomsSuppressing typeCompound screeningBiocideType i allergyInternal medicine
Owner:SUNSTAR INC
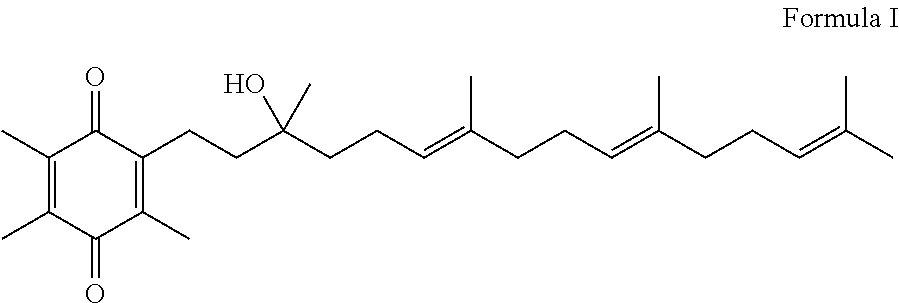
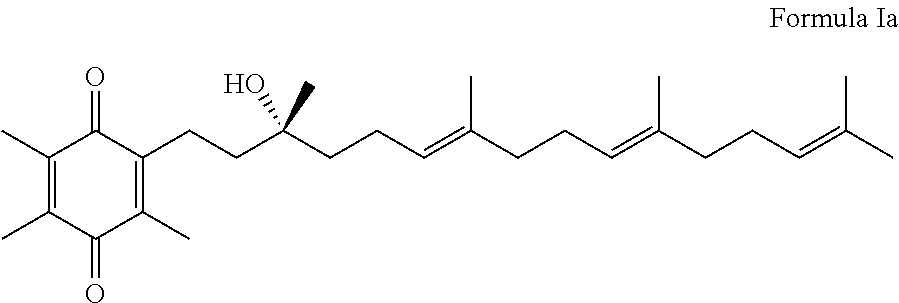
Methods for selective oxidation of alpha tocotrienol in the presence of non-alpha tocotrienols
ActiveUS20140249332A1Shorten the progressReduce or eliminate either a diseaseSenses disorderOrganic compound preparationAlpha-TocotrienolOxidizing agent
A method of producing alpha-tocotrienol quinone or a stereoisomer thereof, the method comprising selective opening of alpha-tocotrienol chroman to alpha-tocotrienol quinone in the presence of non-alpha tocotrienol chromans by oxidizing alpha-tocotrienol with a metal salt oxidizing agent, wherein the stoichiometric ratio of metal salt oxidizing agent / alpha-tocotrienol is at least 4:1 and wherein said metal oxidizing agent is added in sequential additions, in order to reduce oxidation of any amounts of non-alpha tocotrienol chromans that might have been present in the starting alpha-tocotrienol chroman material. This process uses conditions favoring oxidation rates of the alpha tocotrienol chroman vs the non-alpha tocotrienol chromans.
Owner:PTC THERAPEUTICS INC
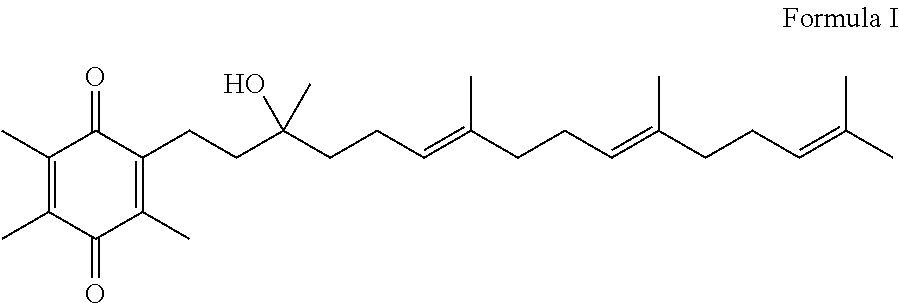
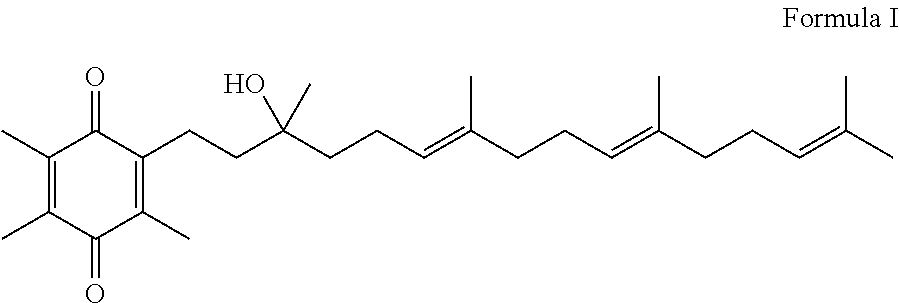
Methods for oxidation of alpha tocotrienol in the presence of non-alpha tocotrienols
ActiveUS9162957B2Shorten the progressReduce or eliminate either a diseaseSenses disorderOrganic compound preparationStereoisomerismAlpha-Tocotrienol
A method of producing alpha-tocotrienol quinone or a stereoisomer thereof, the method comprising selective opening of alpha-tocotrienol chroman to alpha-tocotrienol quinone in the presence of non-alpha tocotrienol chromans by oxidizing alpha-tocotrienol with a metal salt oxidizing agent, wherein the stoichiometric ratio of metal salt oxidizing agent / alpha-tocotrienol is at least 4:1 and wherein said metal oxidizing agent is added in sequential additions, in order to reduce oxidation of any amounts of non-alpha tocotrienol chromans that might have been present in the starting alpha-tocotrienol chroman material. This process uses conditions favoring oxidation rates of the alpha tocotrienol chroman vs. the non-alpha tocotrienol chromans.
Owner:PTC THERAPEUTICS INC
Compound and method for reducing inflammation, pain, allergy, flu and cold symptoms
InactiveUS20180271924A1Effective treatmentRelieve symptomsAntimycoticsMetabolism disorderDiseaseSide effect
The present invention relates to pharmaceutical and nutraceutical compounds and methods for reducing inflammation, blood sugar, gastric acid, symptoms of allergy, common cold and flu, as well as treating infections and pain associated with trauma, medical procedure, and diseases and disorders in subjects in need thereof. The compounds and methods proposed by this invention are related to essentially a mixture of naturally accruing and / or synthetic substances in certain ratios, administration protocols, and delivery systems that amplify their medicinal qualities and reduce side effects, making their clinical application feasible.
Owner:KARIMAN ALEXANDER
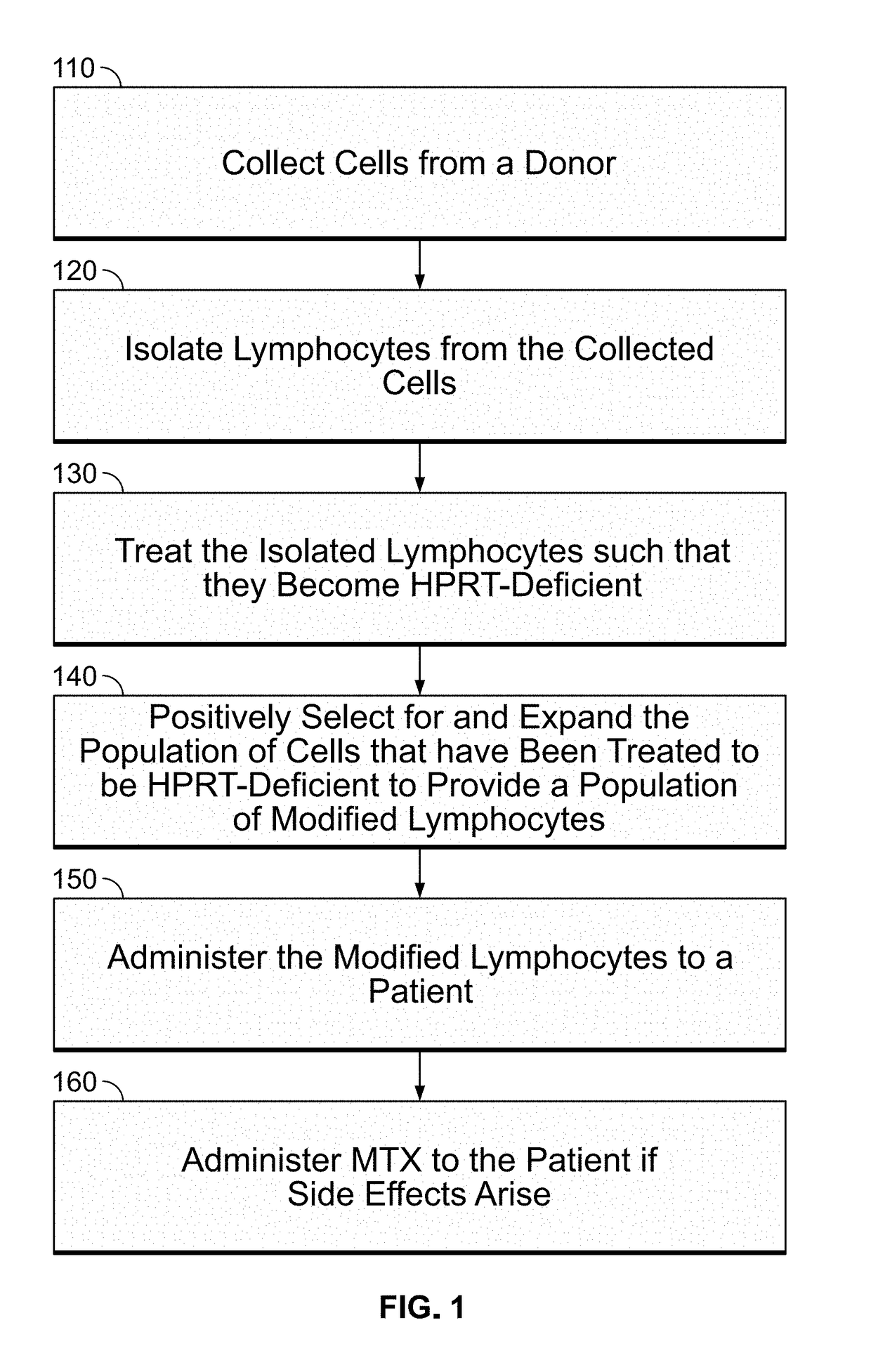
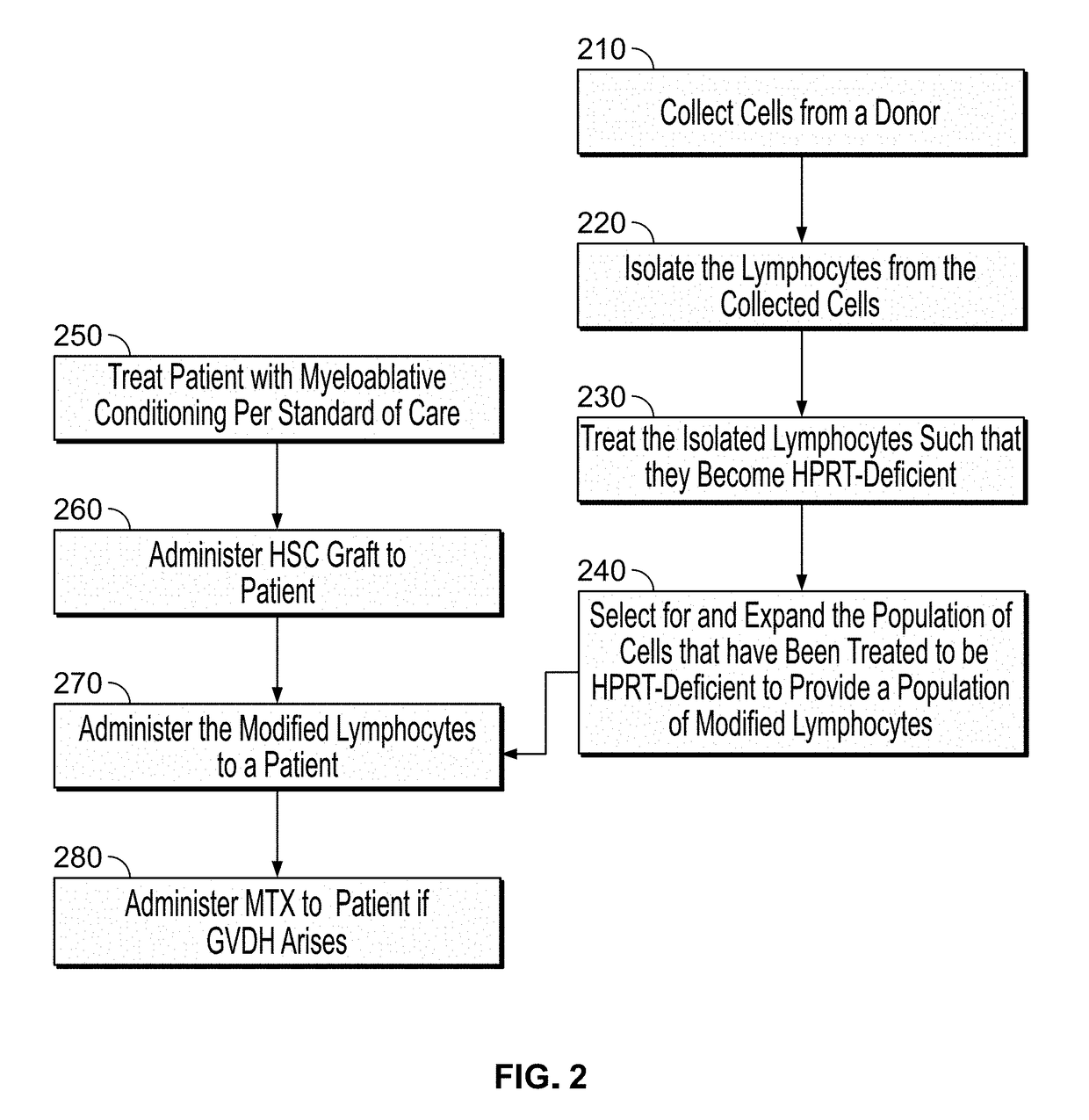
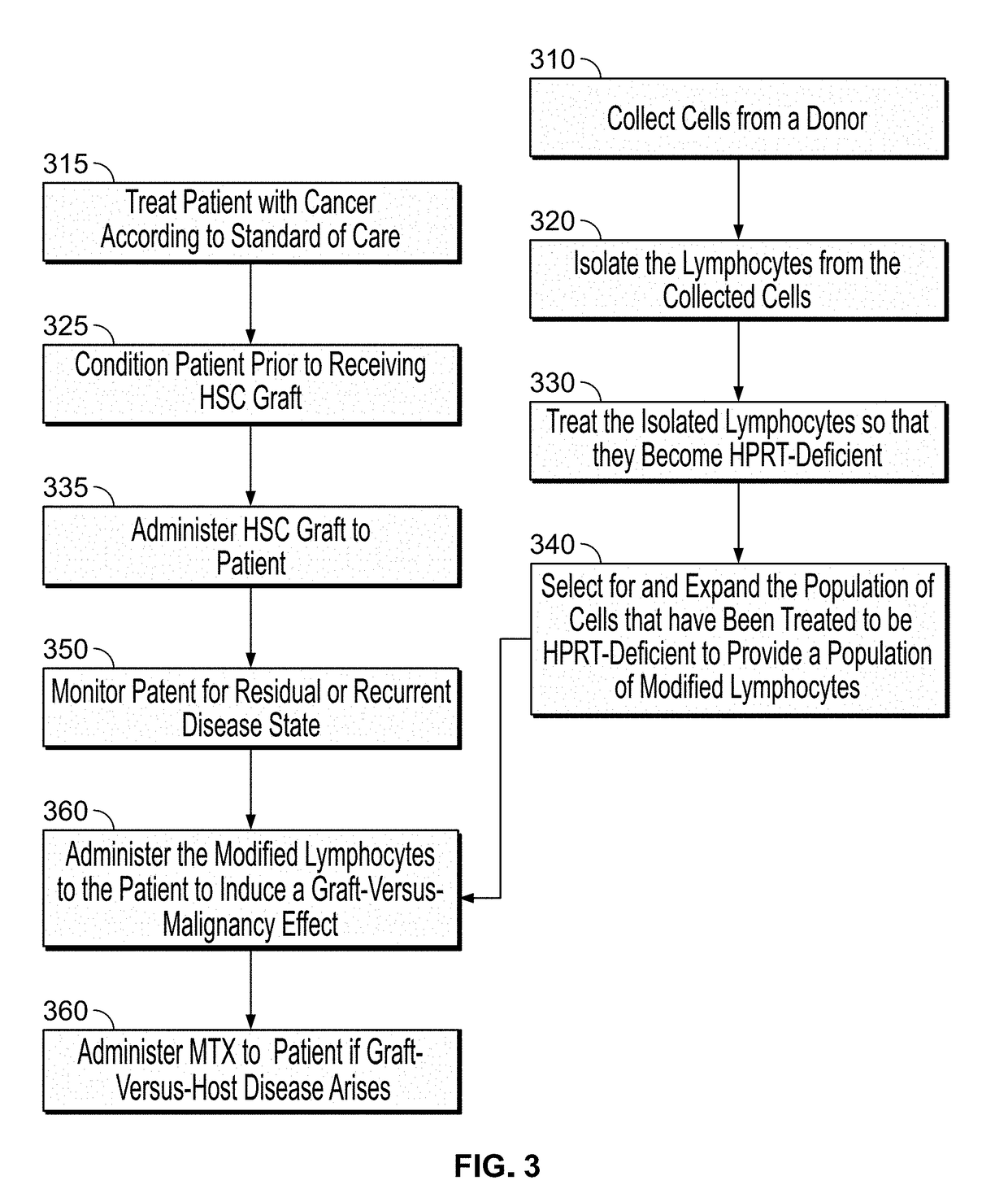
Modulatable switch for selection of donor modified cells
ActiveUS20190054119A1Reducing graft-versus-host-diseaseSuppress and reduce and control side effectMammal material medical ingredientsSkeletal/connective tissue cellsSide effectGenetics
The disclosed methods are generally directed to preventing, treating, suppressing, controlling or otherwise mitigating side effects of T-cell therapy, the T-cell therapy designed to accelerate immune reconstitution, induce a GVM effect, and / or target tumor cells.
Owner:CALIMMUNE INC +1
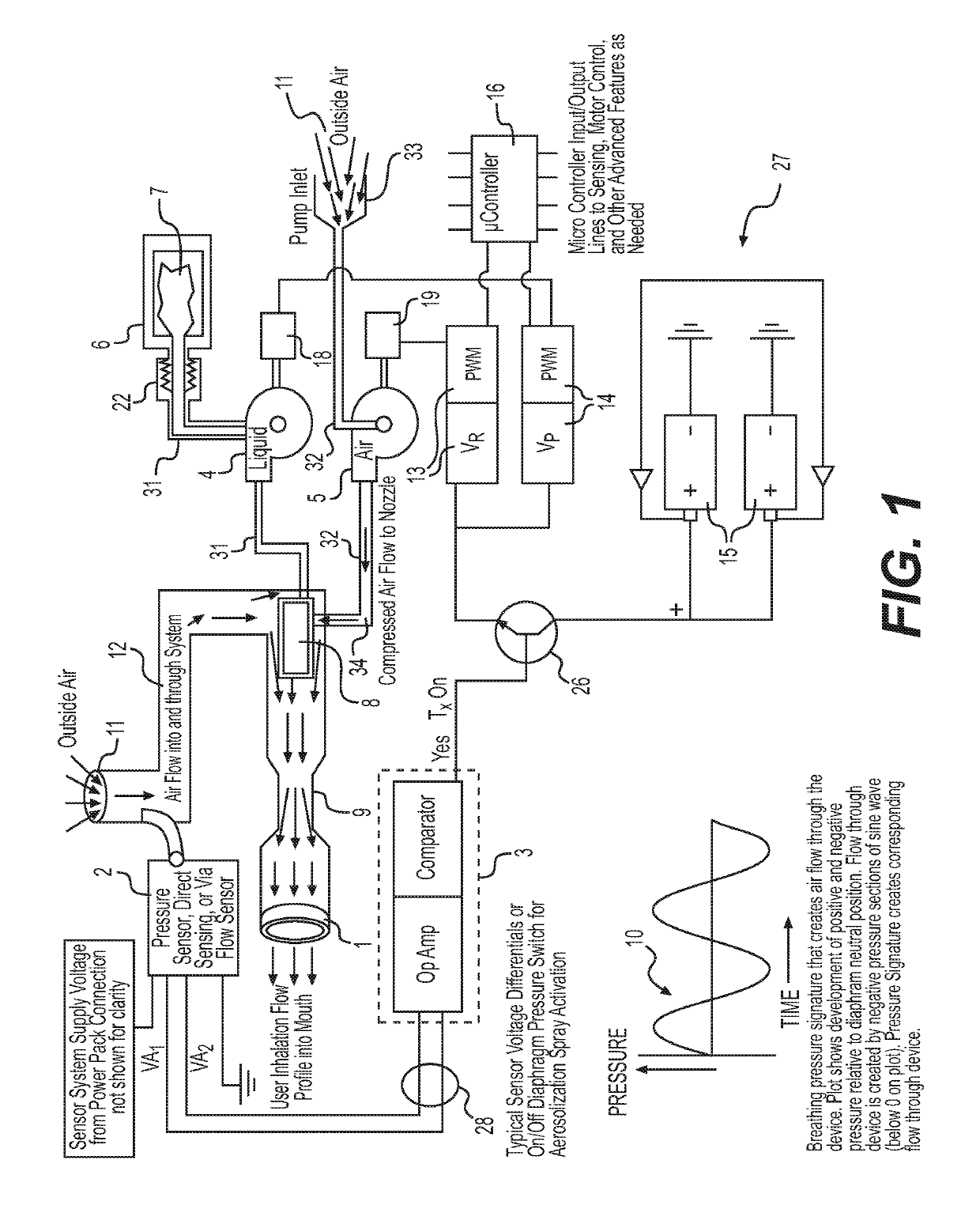
On demand aerosolized delivery inhaler
ActiveUS10286163B1Eliminate needRapid and convenient administrationRespiratorsMedical devicesEngineeringOn demand
A hand-held aerosolized inhaler that delivers an aerosolized solution to the user on demand when he or she inhales is provided. A pressure or flow sensor within the invention detects the change in air flow as the user inhales and triggers spraying the aerosol solution only when the inhaled breath stream will optimally entrain the sprayed aerosol. The pressure or flow sensor sends an electronic signal to turn on or off liquid and air pumps as the user breathes. Liquid, or liquid and air are pumped to the nozzle inside the invention to create an aerosolized mixture.
Owner:PAUSTIAN PHILIP J +1
Preventive or remedy for bedsore
InactiveUS20060035957A1Avoid problemsReduce morbidityBiocideAnimal repellantsHydroxyprolinePharmacology
In accordance with the present invention, there are provided a preventive or of preventing or therapeutic agent for decubitus comprising an N-acylated derivative of hydroxyproline or a salt thereof; the above preventive or therapeutic agent wherein the N-acylated derivative of hydroxyproline or a salt thereof is contained in an amount of 0.1 to 15% by weight to the total weight; and the preventive or therapeutic agent for decubitus wherein the N-acylated group of the N-acylated derivative of hydroxyproline is an N-acylated group having 1 to 24 carbon atoms.
Owner:KYOWA HAKKO KOGYO CO LTD
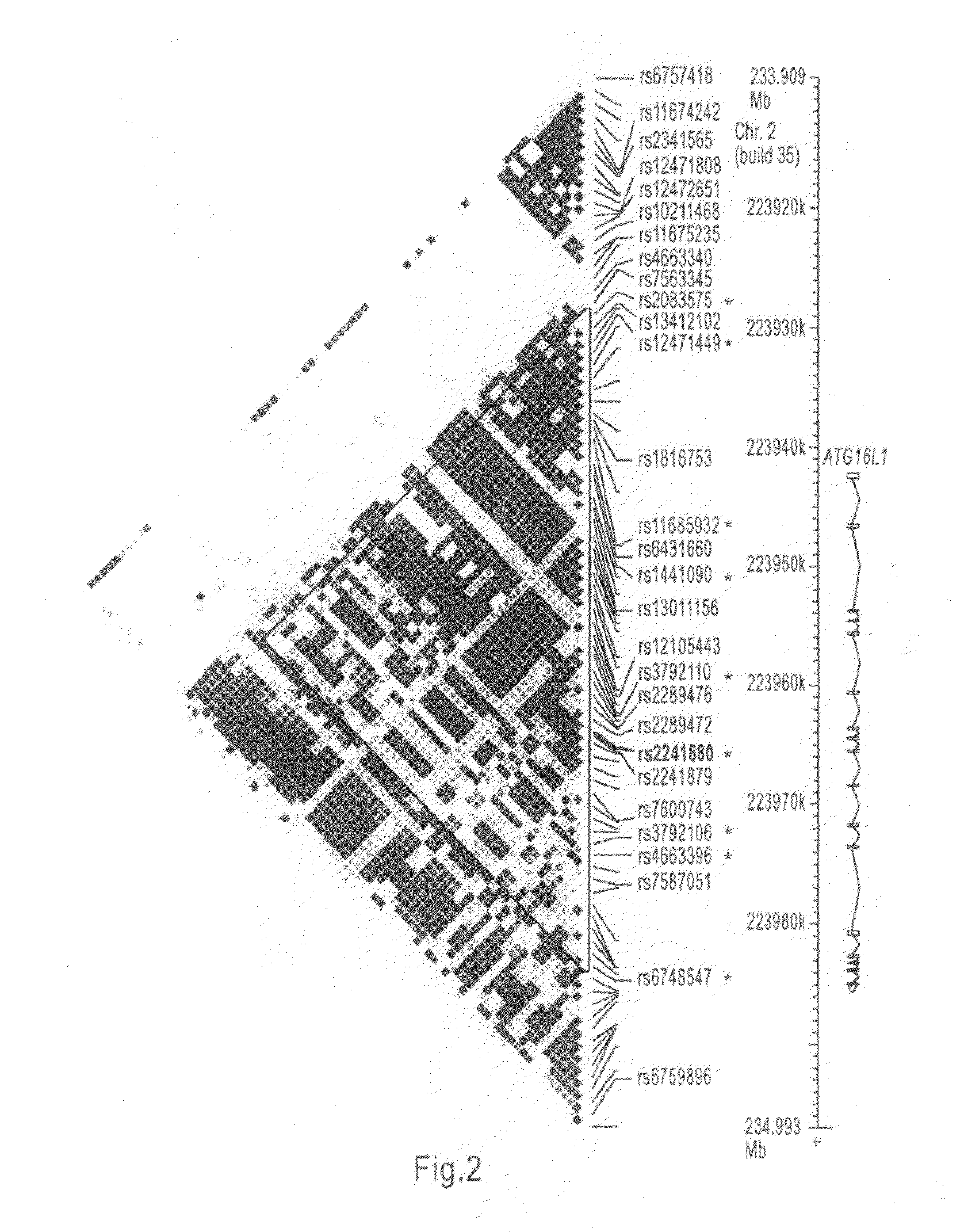
Crohn disease susceptibility gene
InactiveUS20100099083A1Reduce frequencyTreatment safetyMicrobiological testing/measurementLibrary member identificationDiseaseGenomics
The present invention relates to the ATG16l1 gene and genetic variants associated with Crohn's disease. In particular, the invention relates to the fields of pharmacogenomics, diagnostics, patient therapy and the use of genetic haplotype information to predict an individual's susceptibility to Crohn's disease and / or their response to a particular drug or drugs.
Owner:NESTEC SA
Medicine for treating rhinitis and its prepn process
InactiveCN1931247AEasy to useSmall toxicityHydroxy compound active ingredientsAerosol deliverySide effectMedicine
The present invention discloses one kind of medicine for treating rhinitis, and the medicine is prepared with centipeda herb 100-500 weight portions, rhubarb 50-300 weight portions, lily magnolia 50-300 weight portions, cocklebur fruit 50-300 weight portions, mint 50-300 weight portions and borneol 1-10 weight portions. The medicine has obvious systemic and local antiallergic effect and antiphlogistic effect, and is used in treating allergic rhinitis, chronic rhinitis, etc. It has fast acting, determined curative effect, high safety and less toxic side effect. The present invention also discloses the preparation process of the medicine.
Owner:成都南山药业有限公司
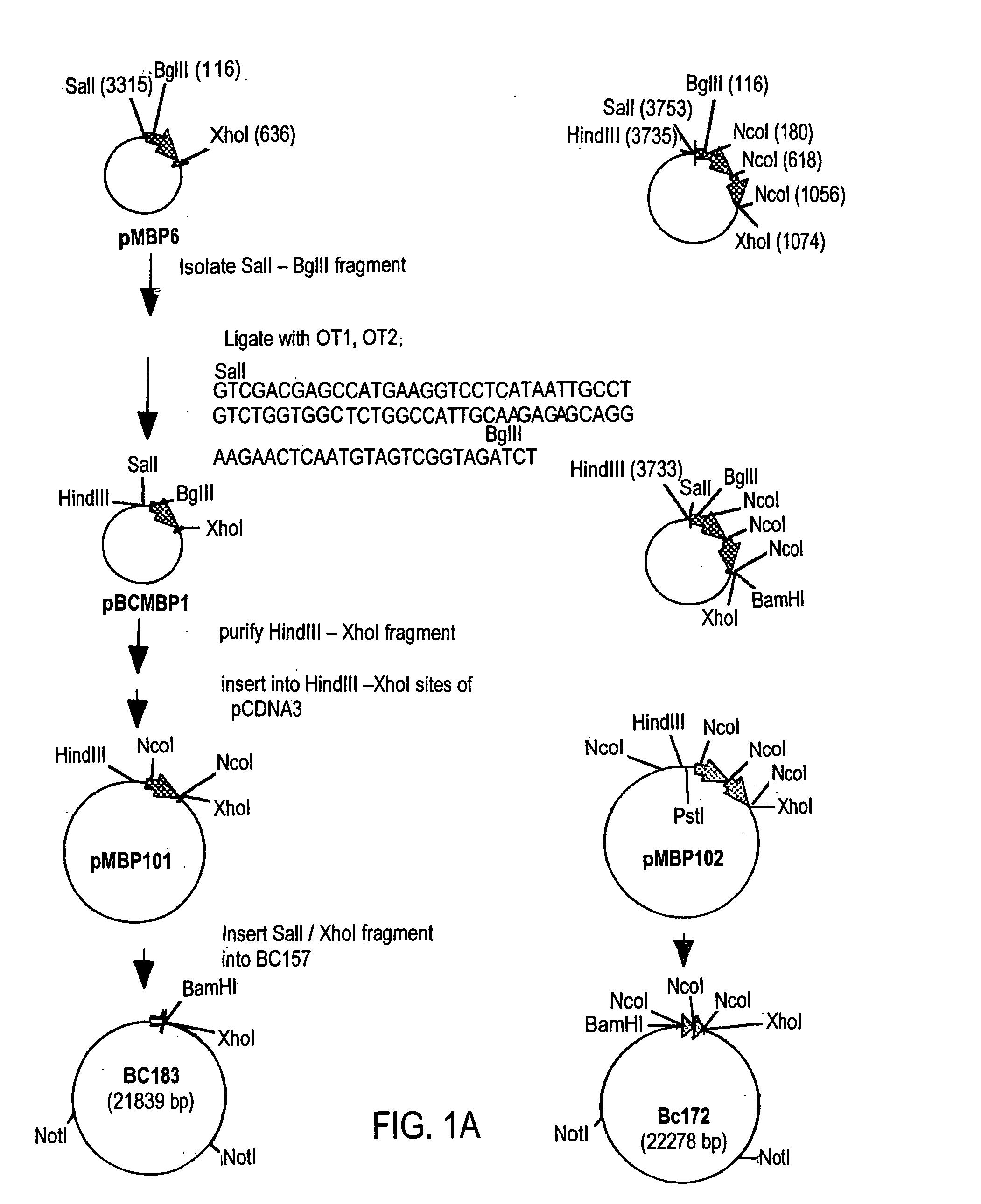
Transgenically produced non-secreted proteins
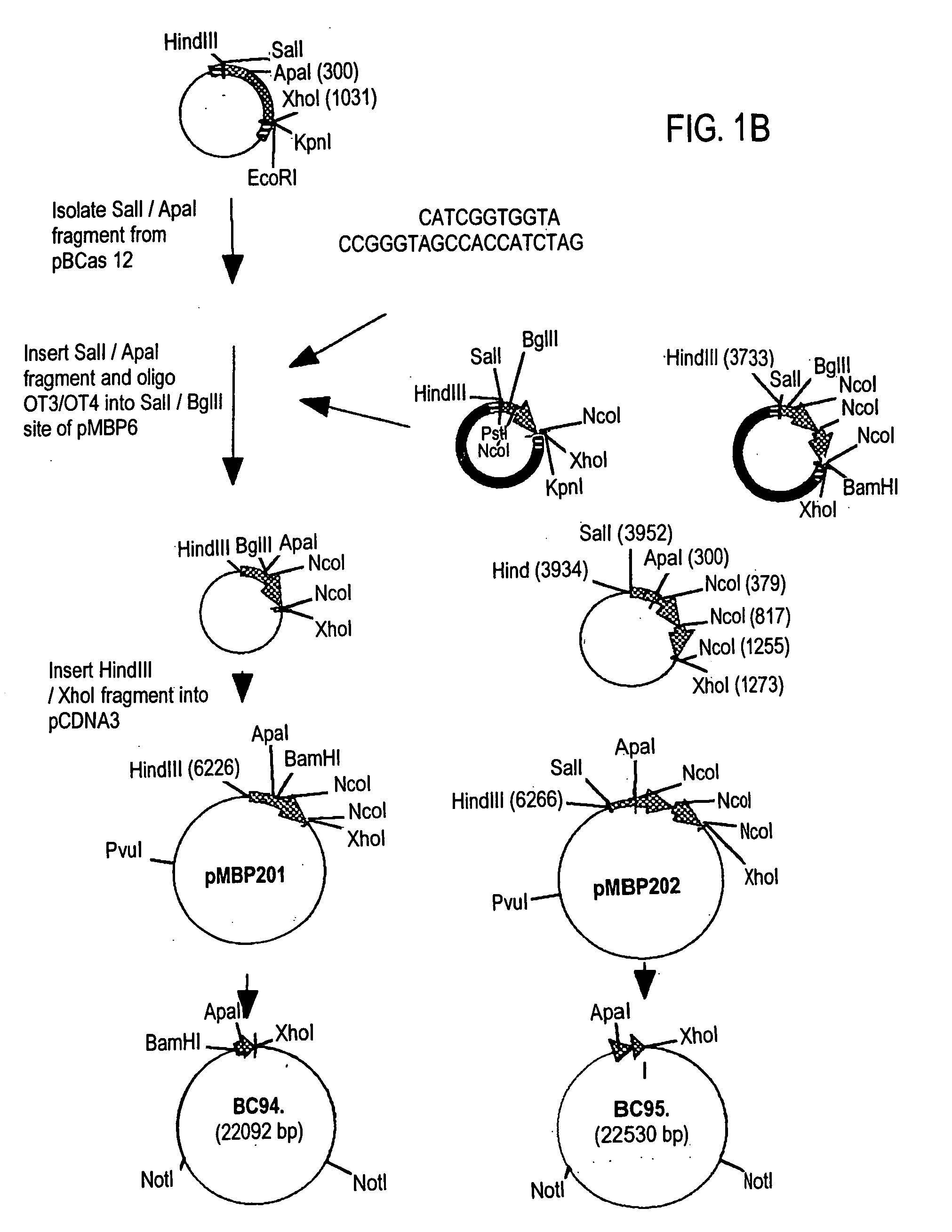
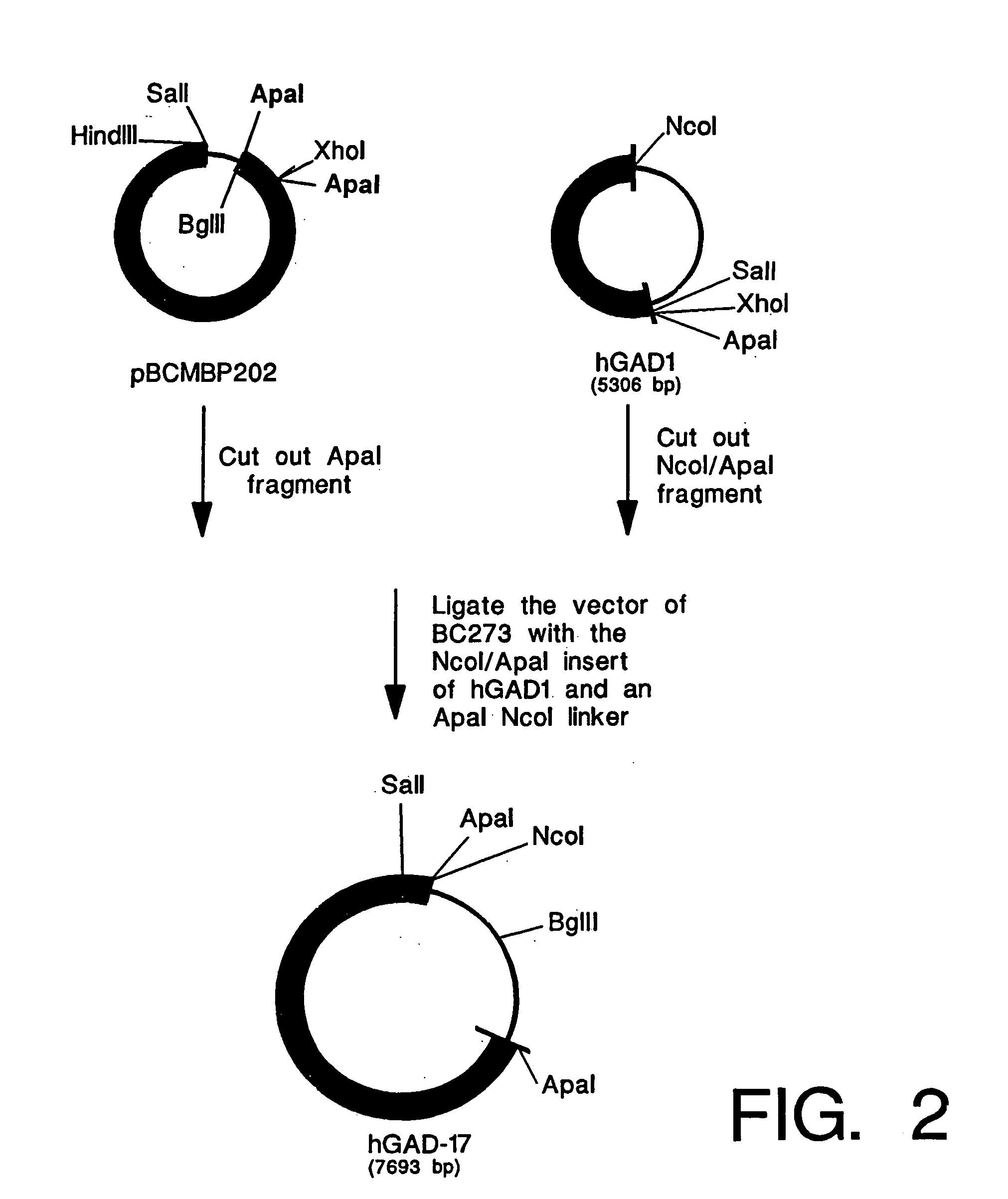
InactiveUS20060179493A1Reduce amountEliminate needPeptide/protein ingredientsAntipyreticMammalGlutamate decarboxylase
The invention provides a method of making and secreting a non-secreted protein. The method includes expressing the protein from a nucleic acid construct which includes: (a) a mammary epithelial specific promoter; (b) a milk protein specific signal sequence which can direct the secretion of a protein; (c) optionally, a sequence which encodes a sufficient portion of the amino terminal coding region of a secreted protein to allow secretion in the milk of a transgenic mammal, of the non-secreted protein; and (d) a sequence which encodes a non-secreted protein, wherein elements (a), (b), optionally (c), and (d) are preferably operatively linked in the order recited. Both glutamic acid decarboxylase (GAD) and myelin basic protein (MBP), which are cytoplasmic proteins, have been produced by the methods of the present invention. The invention also provides methods for treating diabetes and multiple sclerosis using proteins produced by the methods of the present invention.
Owner:GTC BIOTHERAPEUTICS INC
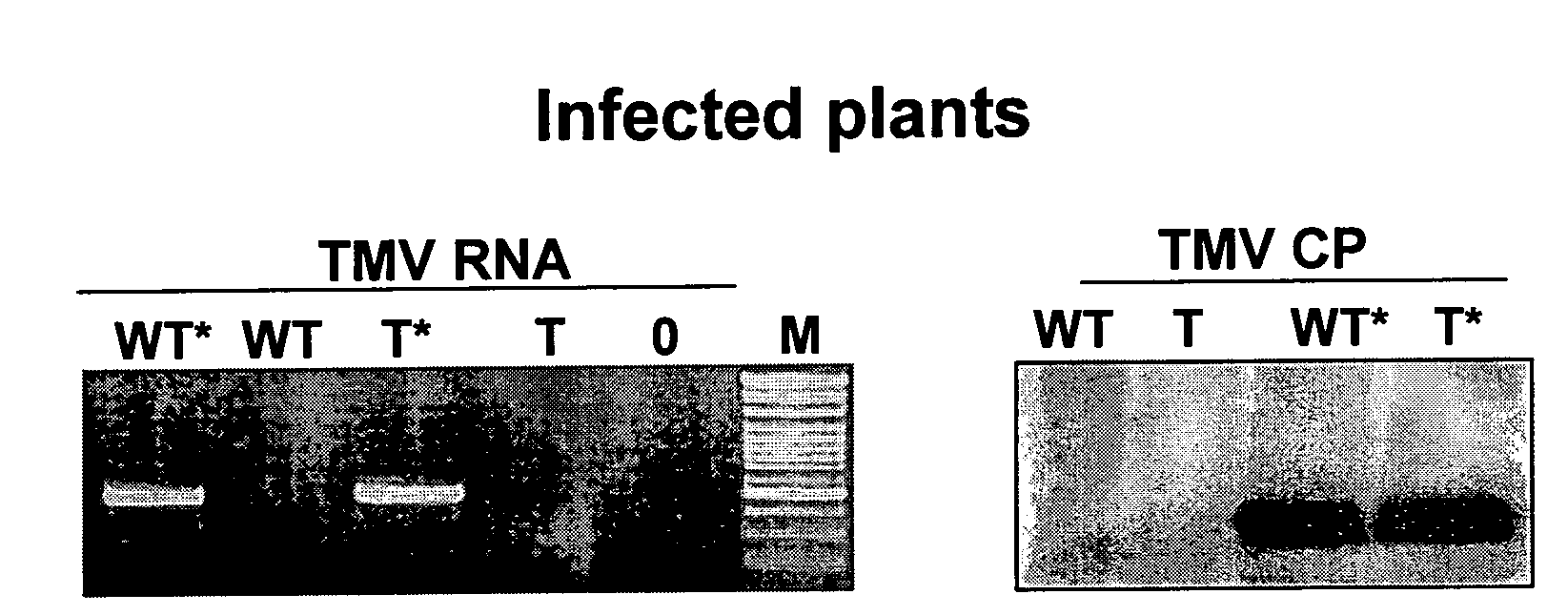
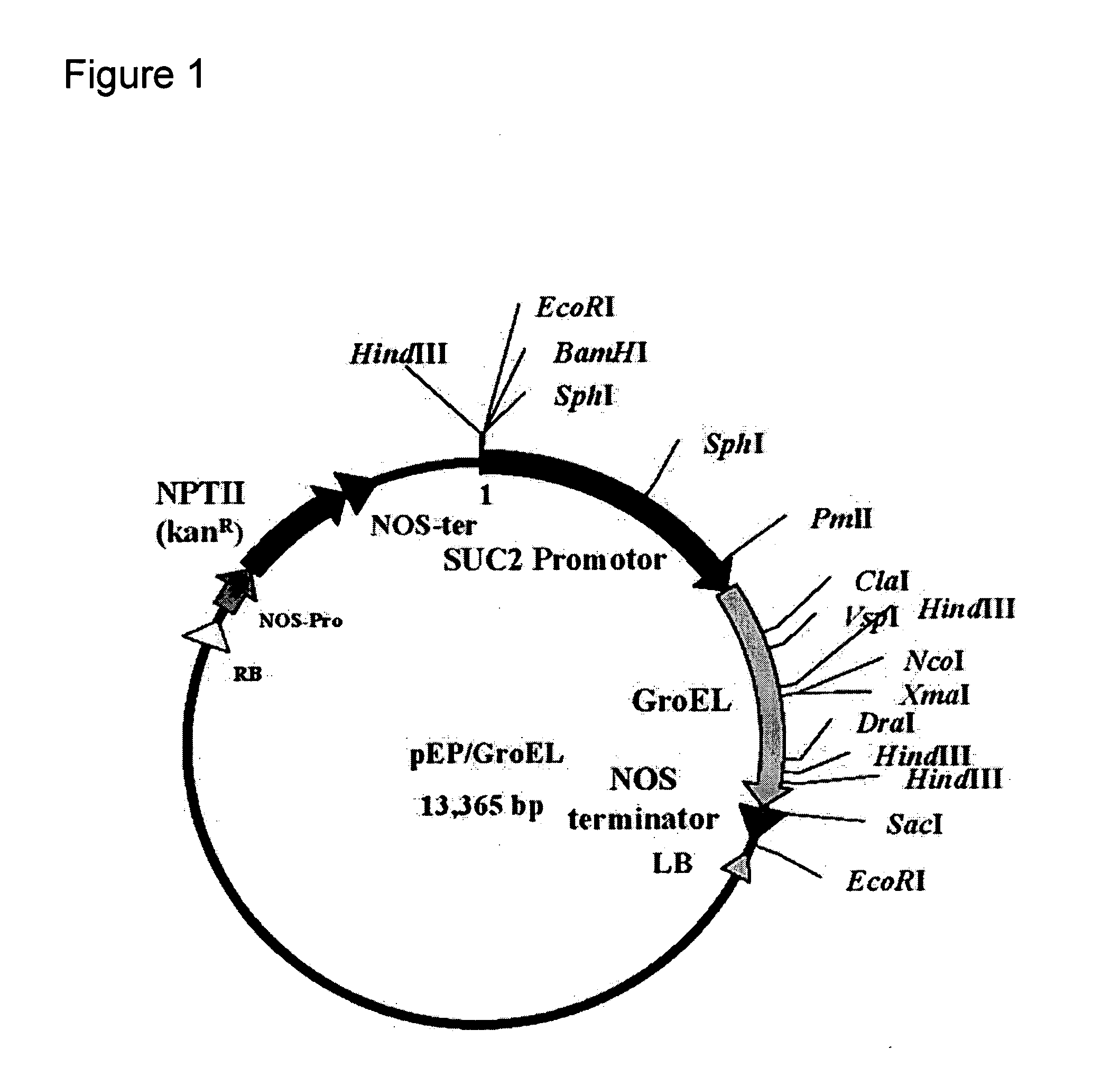
Virus tolerant plants and methods of producing same
InactiveUS20100115665A1Improve toleranceImprove the immunityOther foreign material introduction processesFermentationDiseaseViral disease
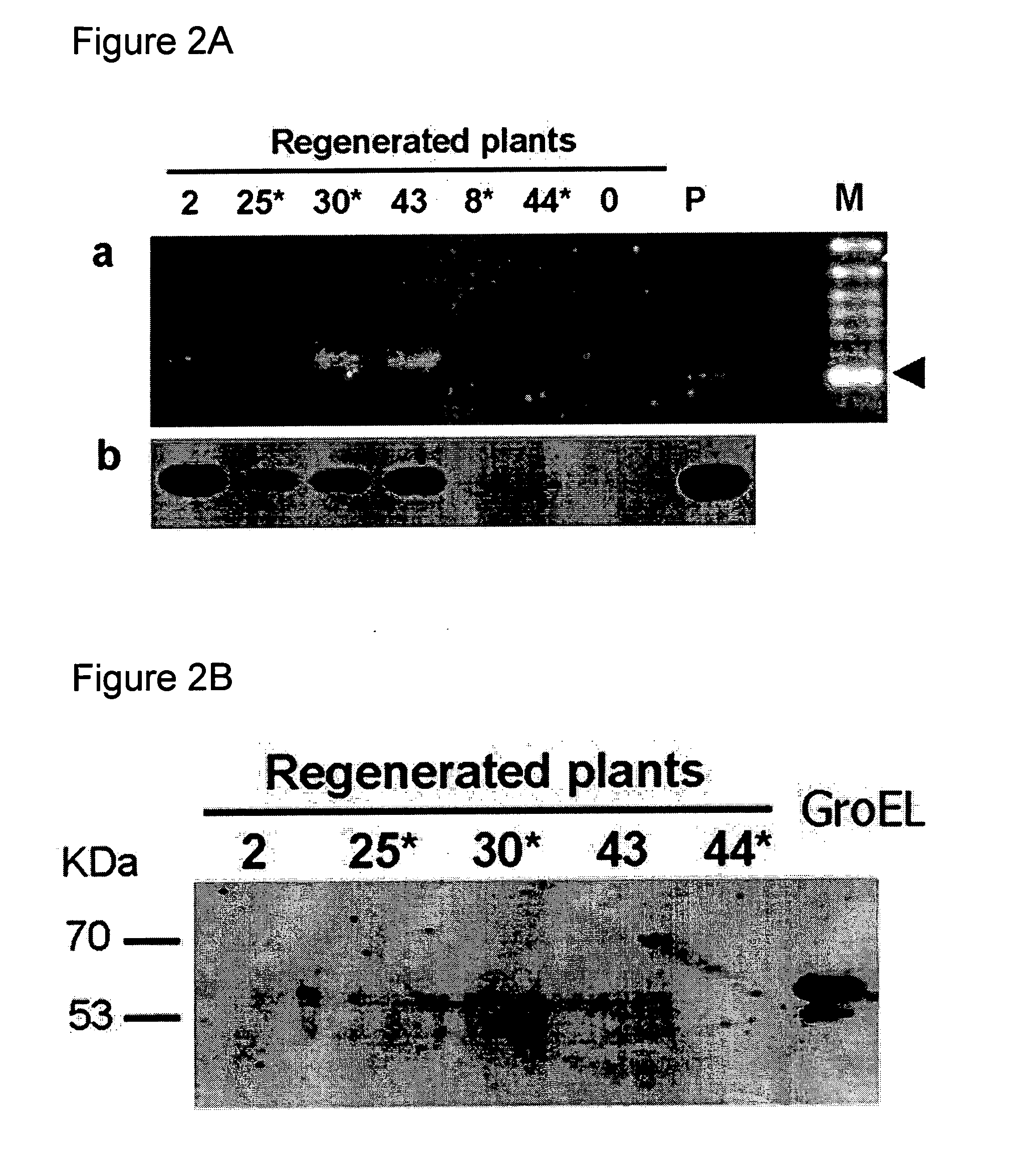
The present invention relates to plants with enhanced tolerance to viral diseases, particularly to diseases caused by insect-transmitted viruses. The invention discloses transgenic plants expressing GroEL protein, and to engrafted plant comprising said transgenic plants or parts thereof, that are tolerant to insect-transmitted viral diseases. The present invention further discloses means and methods of producing same.
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD
Lysine demethylase inhibitors for diseases and disorders associated with Flaviviridae
ActiveUS9790196B2Avoid side effectsReduce duplicationOrganic active ingredientsBiocideLysineLuetic disease
The invention relates to methods and compositions for the treatment or prevention of Flaviviridae infections. In particular, the invention relates to an LSD1 inhibitor for use in treating or preventing Flaviviridae infections, including hepatitis C virus infections.
Owner:ORYZON GENOMICS SA
Dialysis agent a containing acetic acid and acetate salt, and a two-part dialysis agent using thereof
ActiveUS20140097386A1Good storage stabilityReduce acetic acid odorOther chemical processesDialysis systemsAcetic acidAcetate ion
The purpose of the present invention is to provide a dialysis agent A, which is able to set the total acetate ion content in the dialysate to a low value, excellent in storage stability of glucose, able to reduce the acetic acid odor, and able to suppress the corrosion of the dialysate delivery system and the dialysis machine, as well as to provide a two pack type dialysis agent utilizing the dialysis agent A.In the dialysis agent A used in the preparation of a bicarbonate dialysate, which is used as one part of a two pack type dialysis agent, it becomes possible to prepare a bicarbonate dialysate having the total acetate ion concentration of between 2 mEq / L or more and less than 6 mEq / L by allowing to include glucose, acetic acid and acetate salt and to satisfy the molar ratio of 1:0.5 to 2 of acetic acid and acetate salt, and include acetic acid and acetate salt in a total amount of between 2 mEq or more and less than 6 mEq in the dialysis agent A required to prepare 1 L of the bicarbonate dialysate. Moreover, according to the dialysis agent A, in addition to the excellent stability of glucose, it is possible to reduce the acetic acid odor, and furthermore possible to suppress the corrosion of the dialysate delivery system and the dialysis machine.
Owner:TOMITA PHARMA
Preventive or therapeutic agents for decubitus
InactiveUS20080188546A1Avoid problemsReduce morbidityBiocideOrganic chemistryHydroxyprolineCarbon atom
In accordance with the present invention, there are provided a preventive or of preventing or therapeutic agent for decubitus comprising an N-acylated derivative of hydroxyproline or a salt thereof; the above preventive or therapeutic agent wherein the N-acylated derivative of hydroxyproline or a salt thereof is contained in an amount of 0.1 to 15% by weight to the total weight; and the preventive or therapeutic agent for decubitus wherein the N-acylated group of the N-acylated derivative of hydroxyproline is an N-acylated group having 1 to 24 carbon atoms.
Owner:TAKEDA TOSHIAKI +4
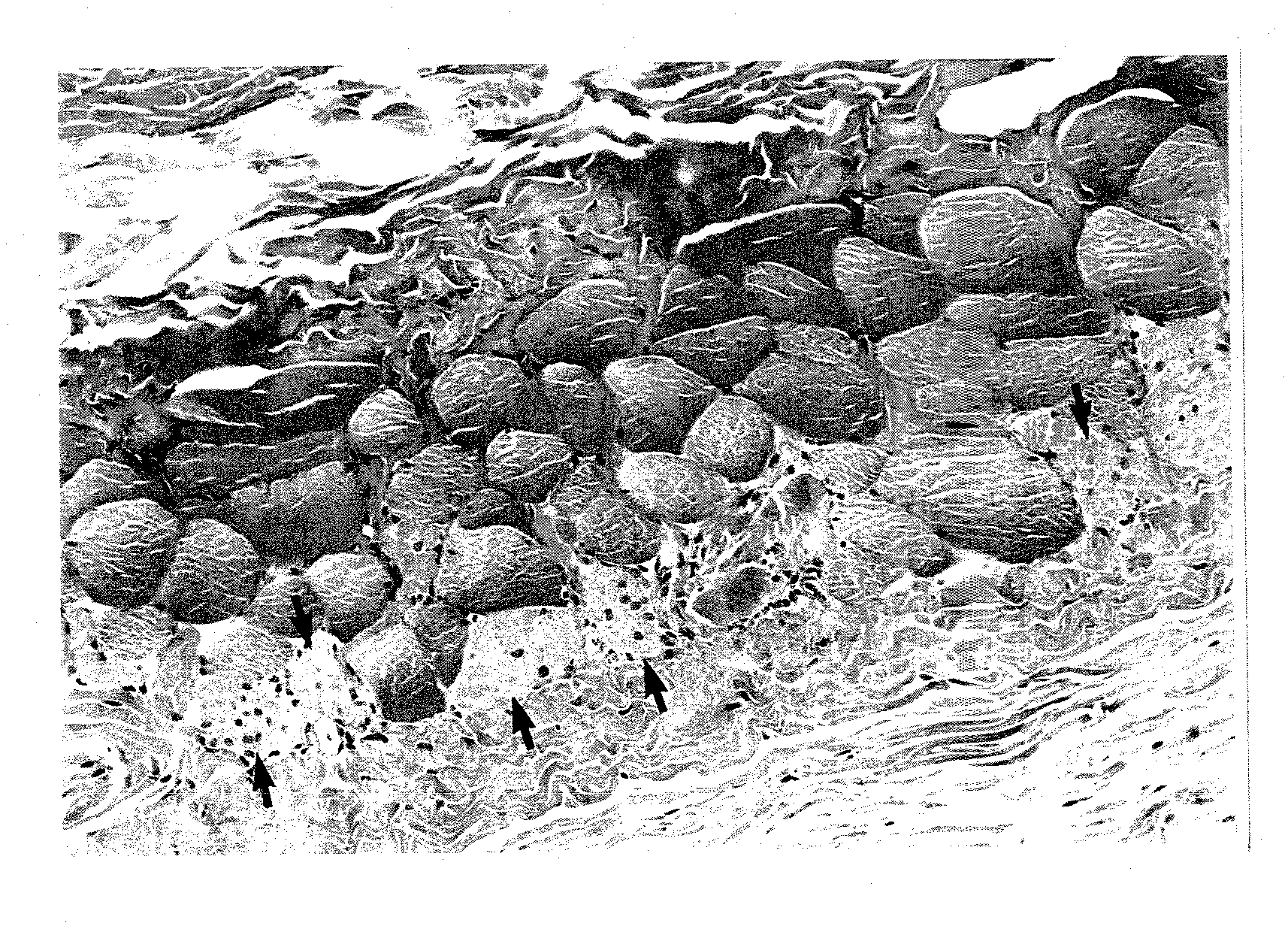
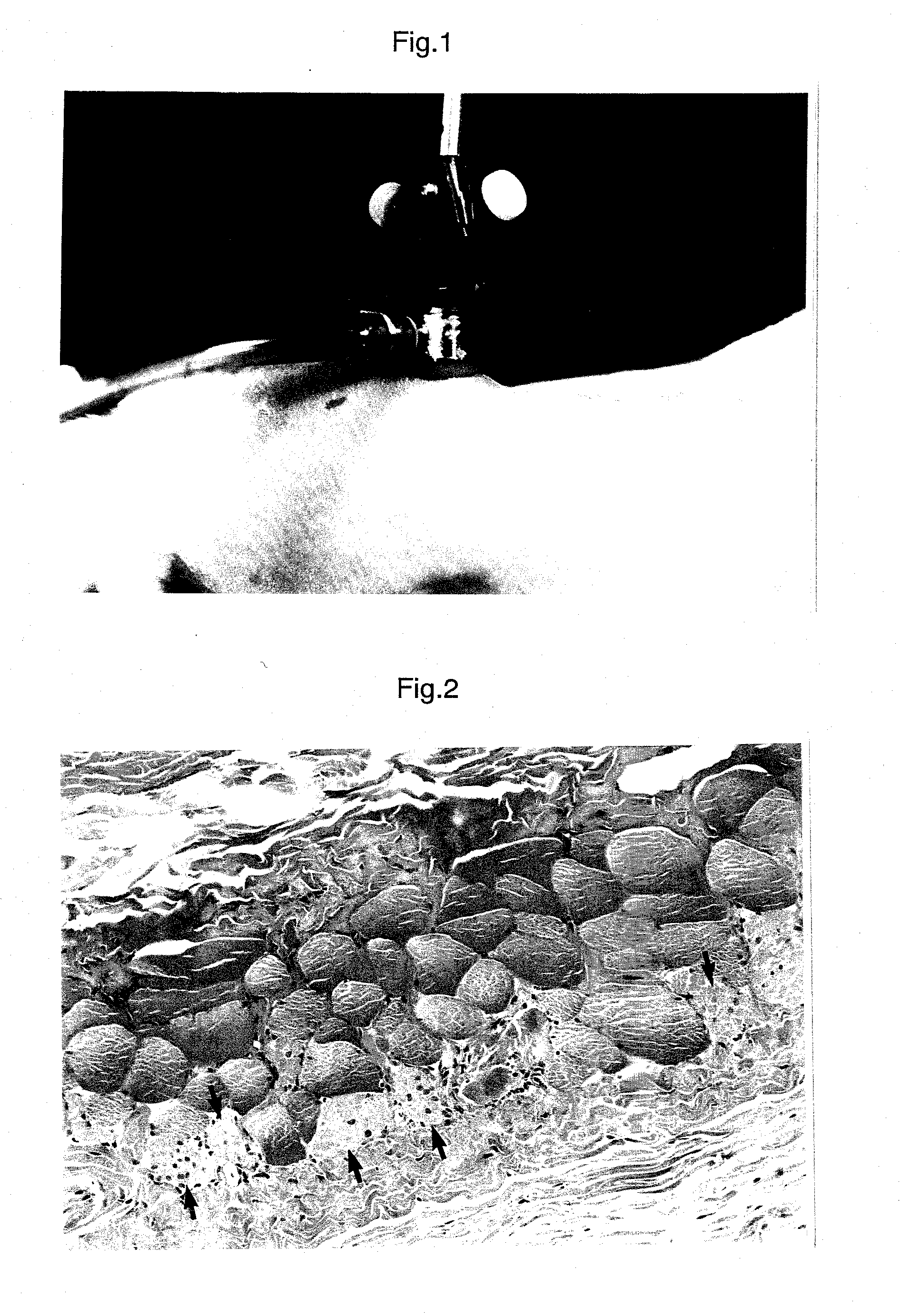
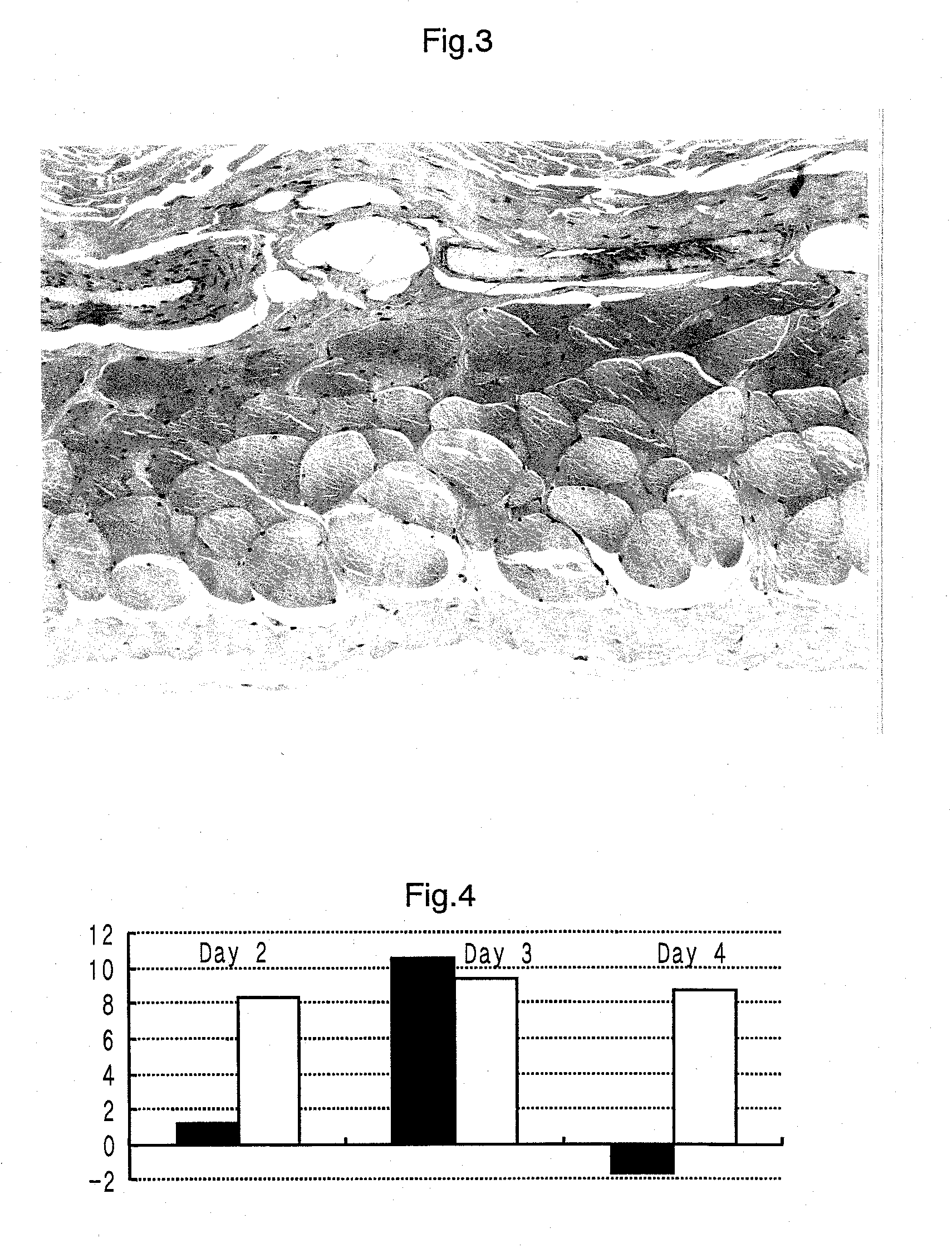
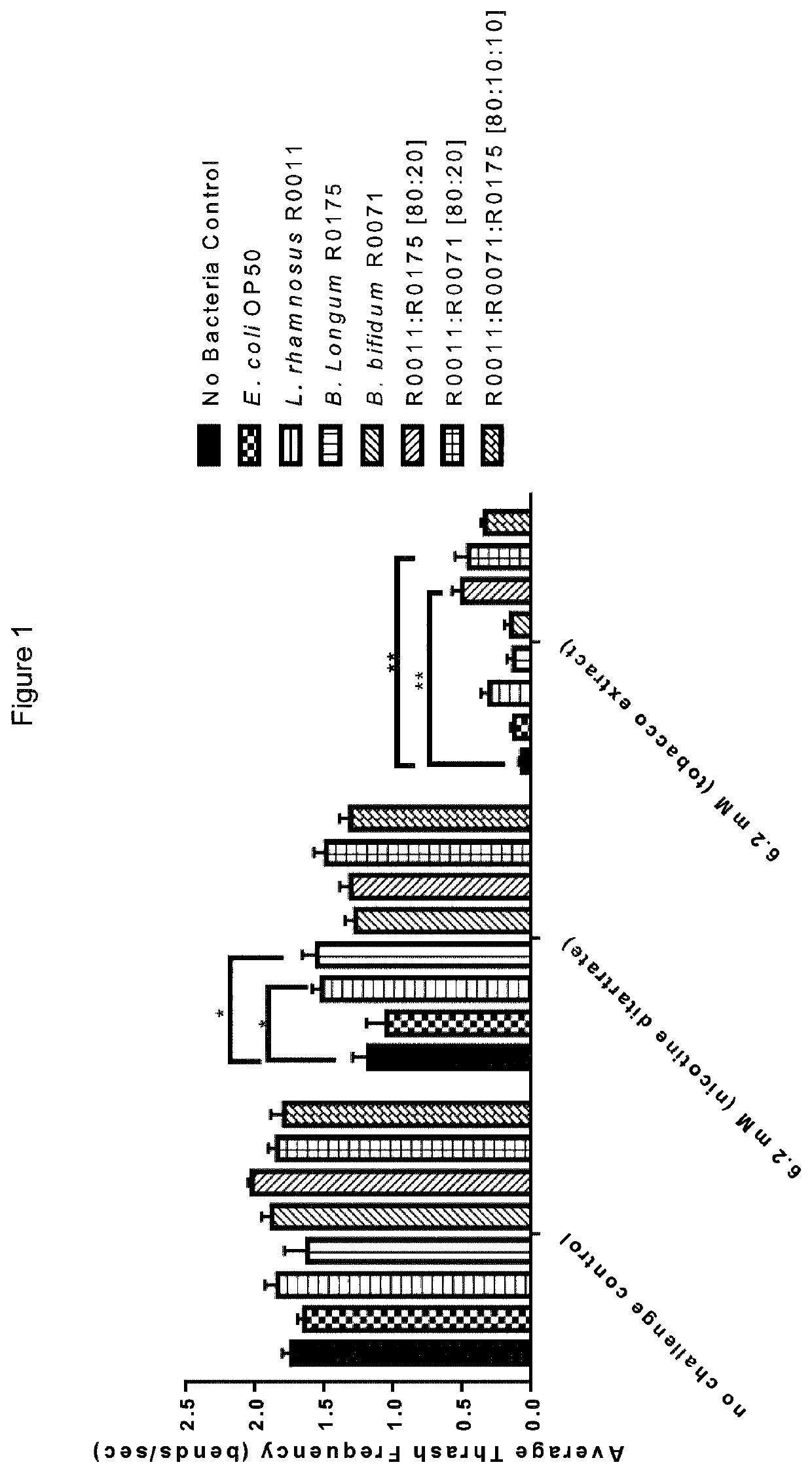
Method for alleviating tobacco or nicotine withdrawal symptoms
PendingUS20210069269A1Alleviating and managing adverse symptomEase smoking cessationNervous disorderLactobacillusMicroorganismTraditional medicine
The present disclosure provides a method for alleviating or suppressing tobacco or nicotine associated withdrawal symptoms and ease smoking cessation in individuals who wish to quit or decrease smoking tobacco or nicotine comprising the use of probiotic microorganisms.
Owner:DANSTAR FERMENT AG
Application of extracellular vesicles in preparation of preparation for treating or preventing metabolic inflammatory syndrome diseases
PendingCN113969304ARelieve or improve symptomsSuppress symptomsMetabolism disorderMicrobiological testing/measurementVesicleDM - Diabetes mellitus
The invention discloses application of extracellular vesicles in preparation of a preparation for treating or preventing metabolic inflammatory syndrome diseases. The metabolic inflammatory syndrome diseases comprise one or more of obesity, atherosclerosis and diabetes mellitus. The invention discloses a plurality of applications of induced extracellular vesicles from the aspects of appearance and mechanism. The invention also discloses application of an apoptosis vesicle detection reagent in preparation of an obesity detection reagent or kit.
Owner:EV CELL BIOTECH GUANGZHOU CO LTD
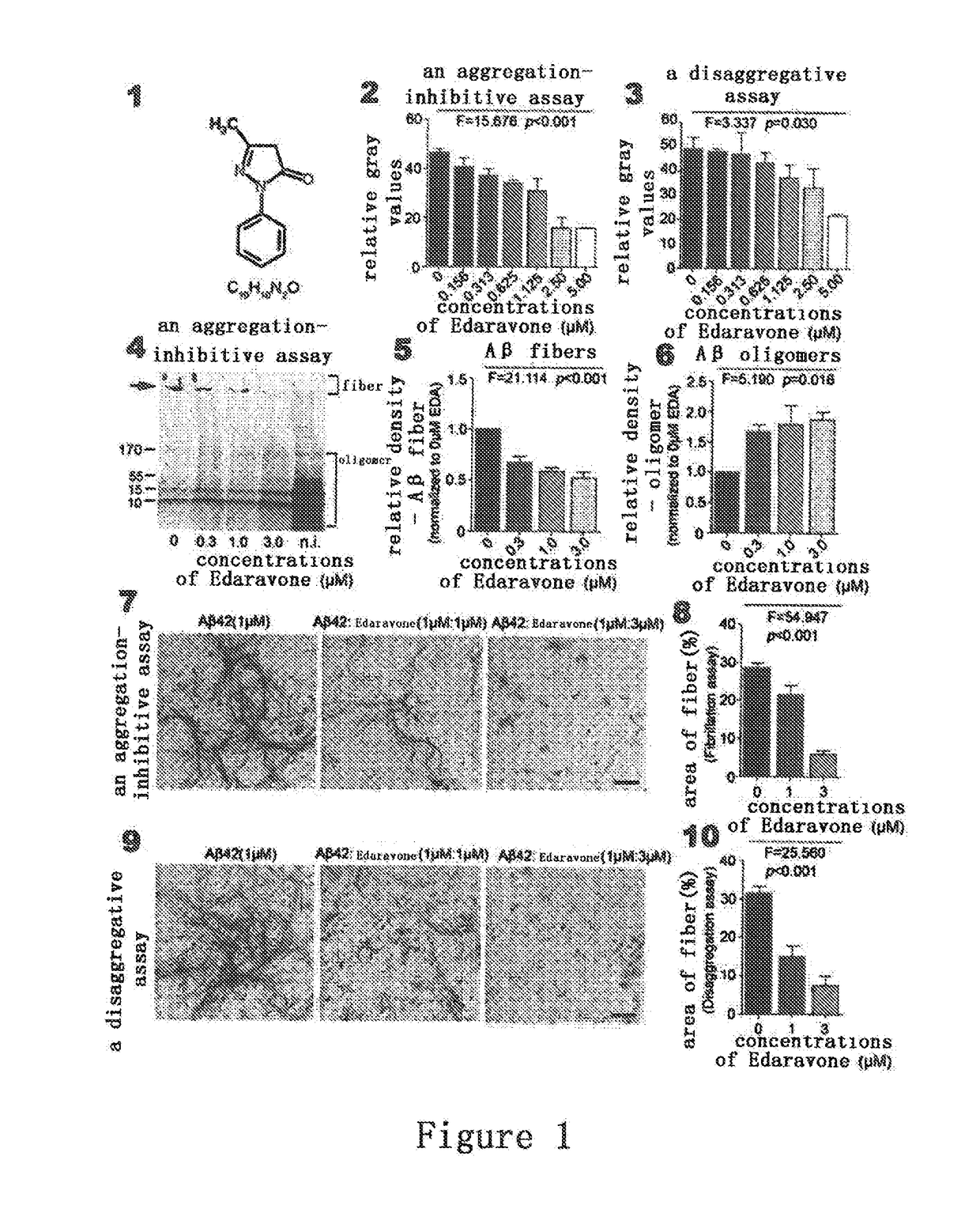
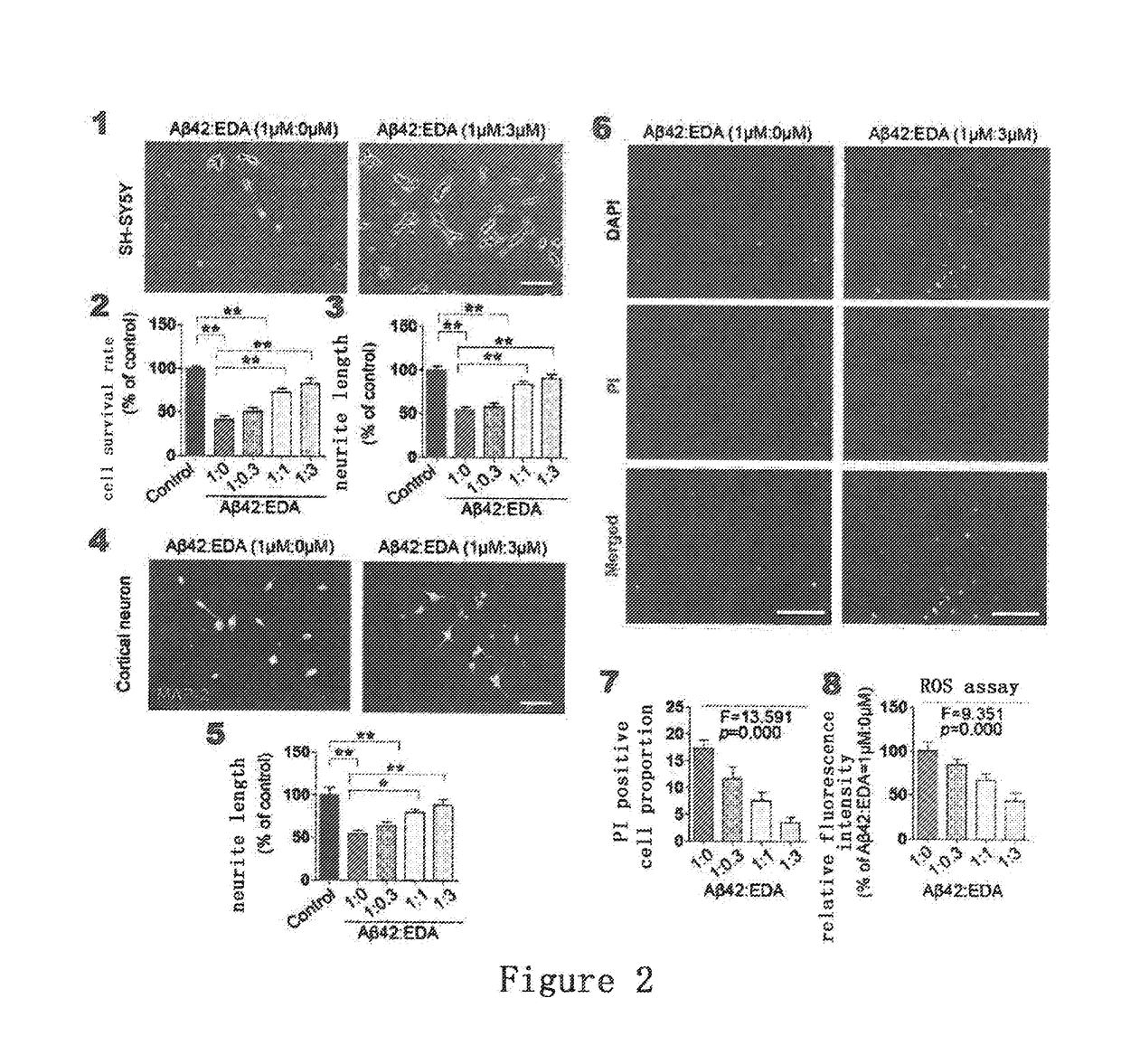
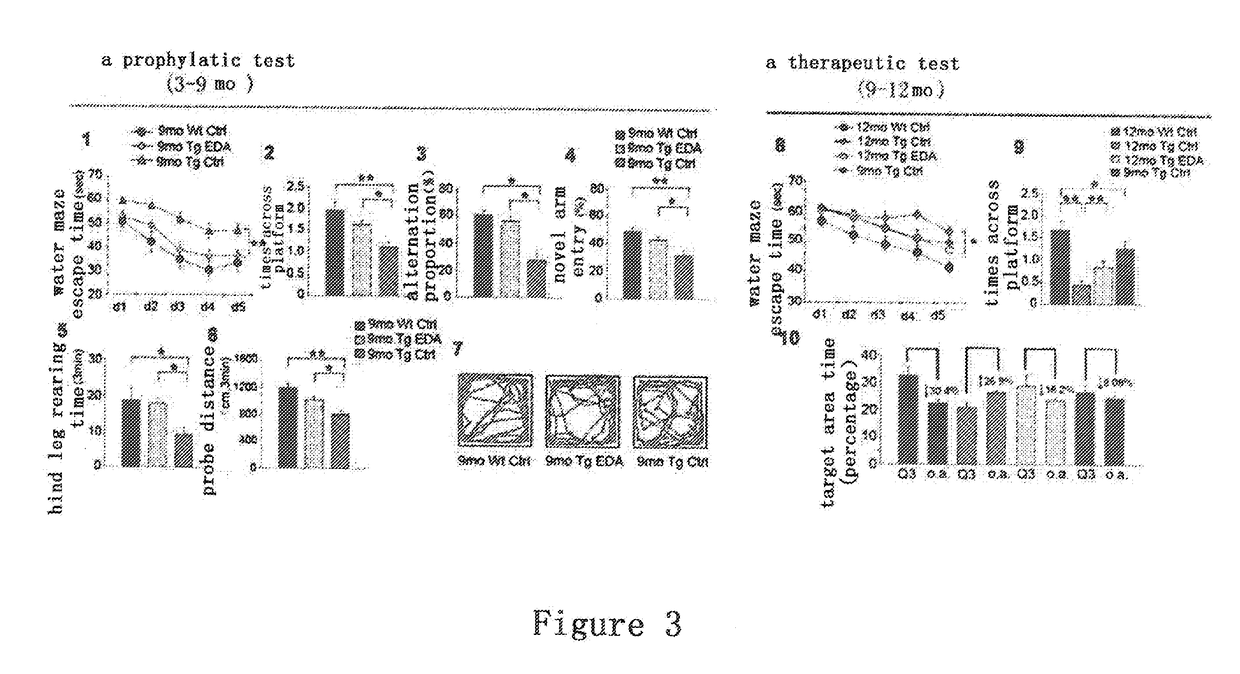
Methods of preventing and treating cerebral amyloid angiopathy (CAA) with an edaravone medicament
InactiveUS20170312253A1Inhibit aggregationPromotes Aβ fiber disaggregationOrganic active ingredientsNervous disorderAmyloid angiopathyEndocrinology
The present invention belongs to pharmaceutical field, specifically, relates to use of Edaravone in the preparation of a medicament for preventing and treating cerebral amyloid angiopathy (CAA). By administering Edaravone, the present invention could scavenge the deposited amyloid (Aβ) in the brain and prevent the intracerebral Aβ deposition.
Owner:THE THIRD AFFILIATED HOSPITAL OF THIRD MILITARY MEDICAL UNIV OF PLA
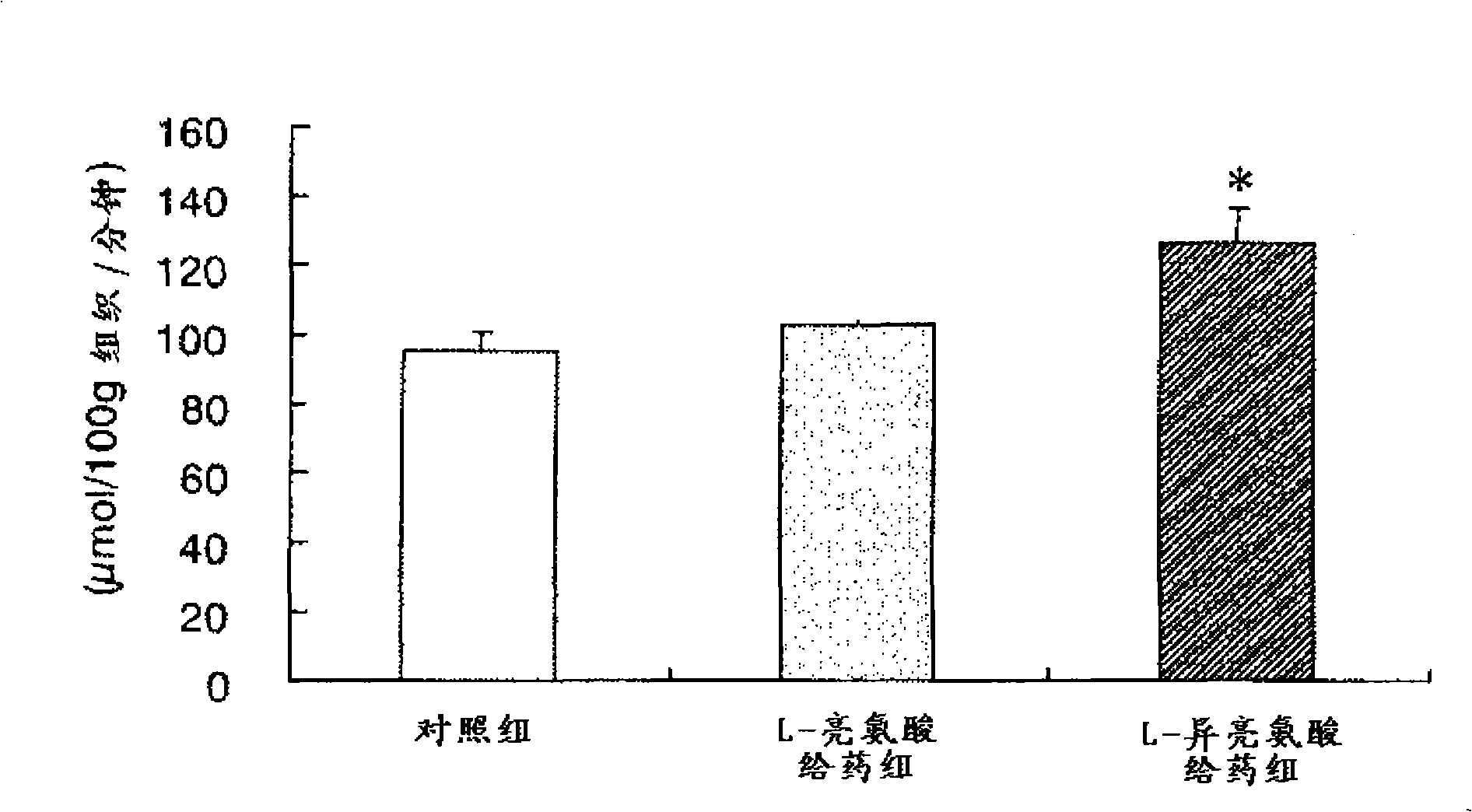
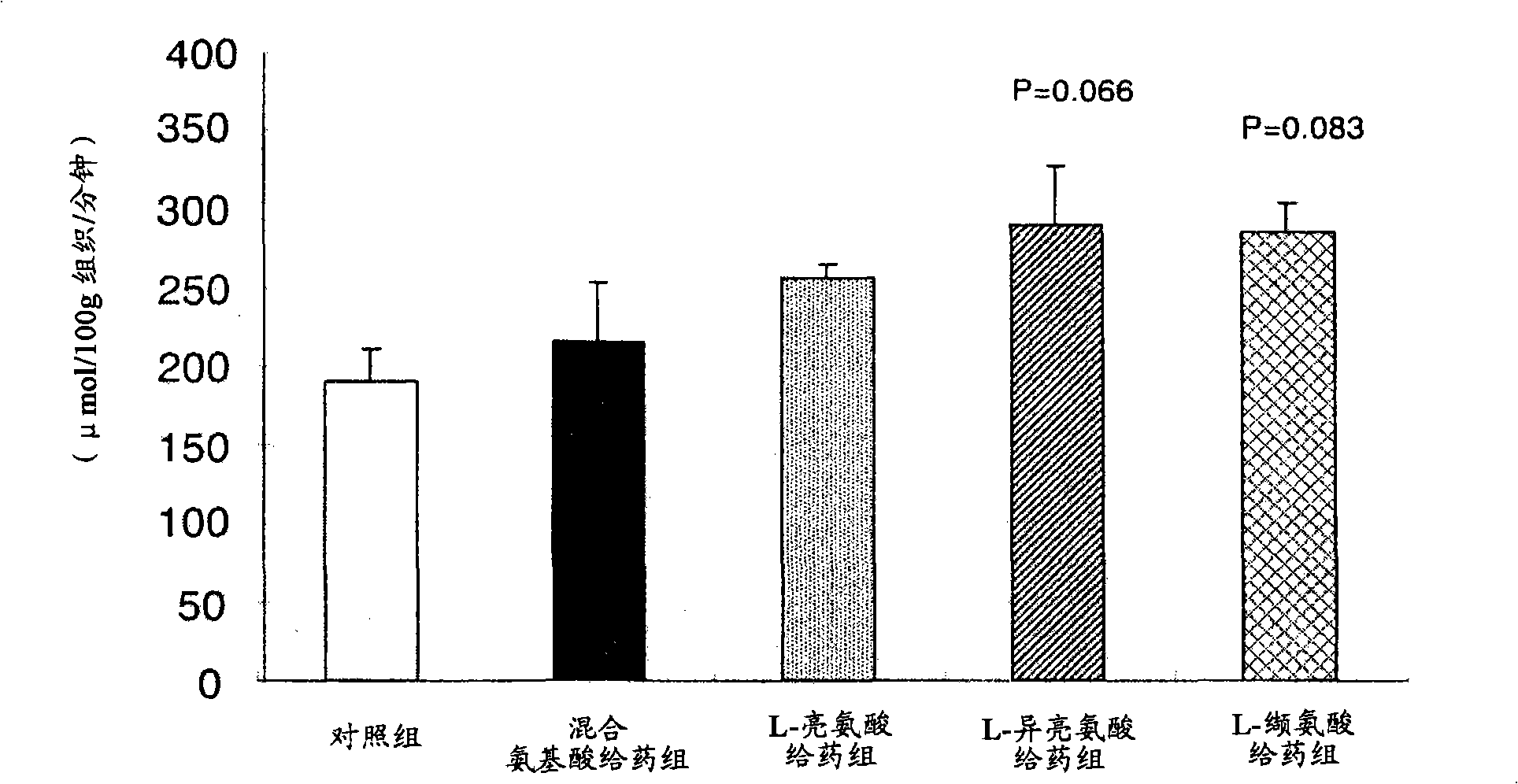
Composition for use in prevention of hypoglycemic condition
InactiveCN101287458AIncrease intakeLower sugar levelsOrganic active ingredientsNervous disorderBULK ACTIVE INGREDIENTActive ingredient
Disclosed is a composition for use in the prevention of a hypoglycemic condition, which comprises at least one compound selected from a branched amino acid, a pharmaceutically acceptable salt thereof and a derivative of the amino acid or salt as an active ingredient. The composition can promote the uptake of a saccharide into a brain cell to thereby prevent a hypoglycemic condition.
Owner:OTSUKA PHARM FAB INC
Traditional Chinese medicine composition for treating ankylosing spondylitis
InactiveCN104138436ASuppress symptomsGood treatment effectAntipyreticAnalgesicsMonkshoodsAnkylosing spondylitis
The invention relates to a traditional Chinese medicine composition for treating ankylosing spondylitis. The traditional Chinese medicine composition comprises the following main components: oldenlandia diffusa, monkshood, radix angelicae tuhuo, cowherb seed, herba epimedii, radix achyranthis bidentatae, rhizoma cibotii, poria cocos, lycopodium clavatum, cistanche, pyrola, ligusticum wallichii and antler. The traditional Chinese medicine composition for treating ankylosing spondylitis, which is provided by the invention, can be used for effectively inhibiting a symptom of ankylosing spondylitis and is remarkable in treatment effect.
Owner:GUILIN HAOXIN TECH SERVICE
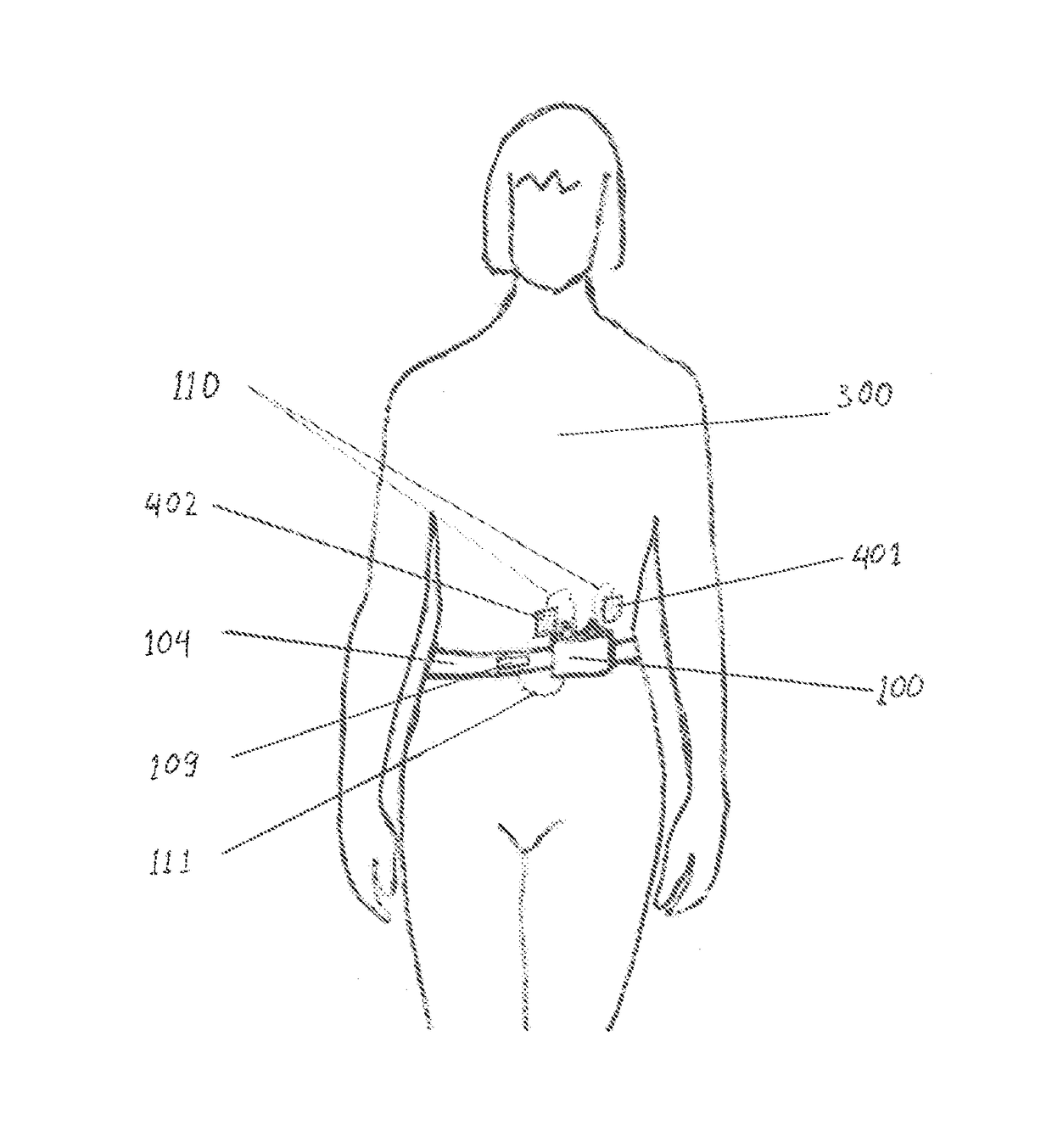
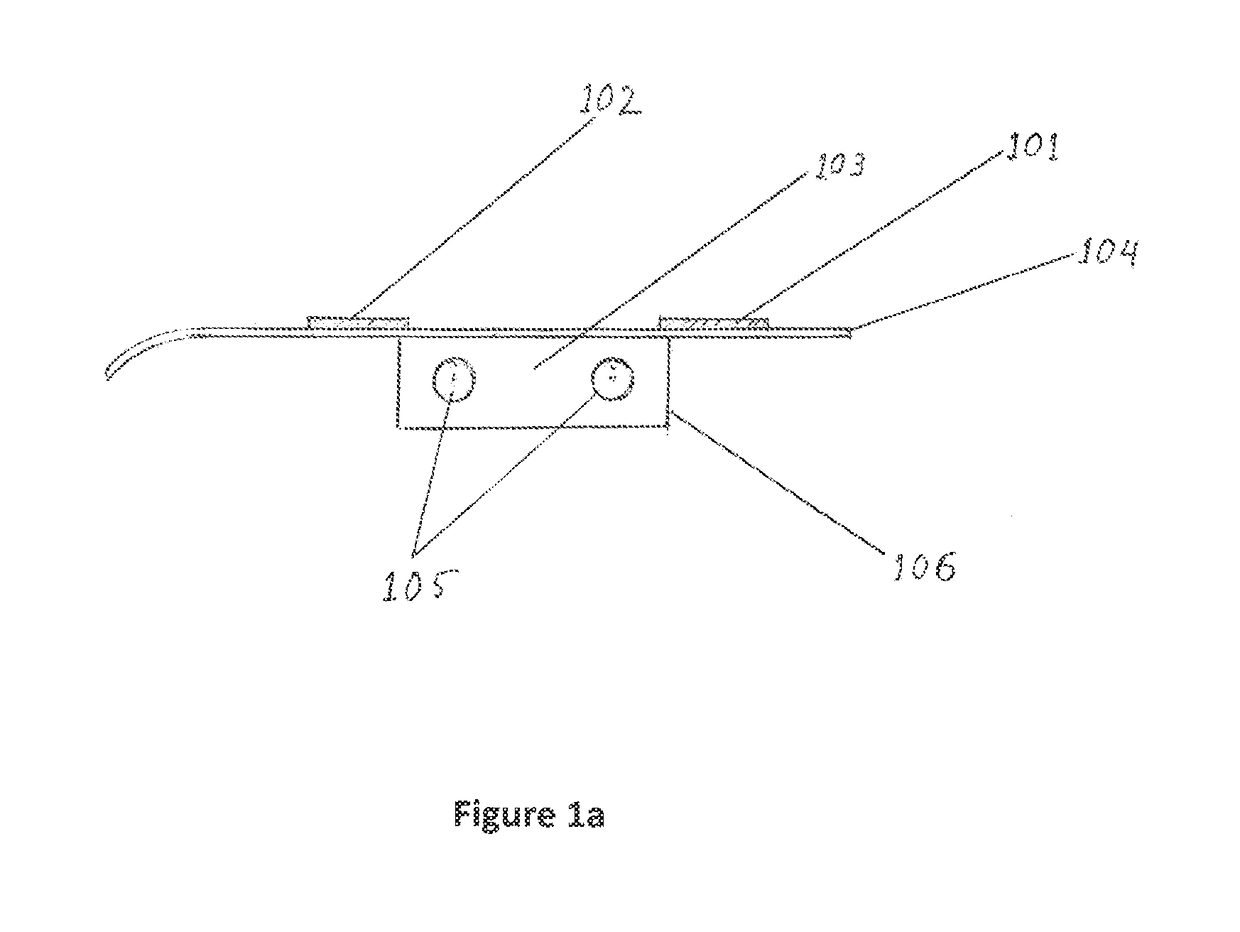
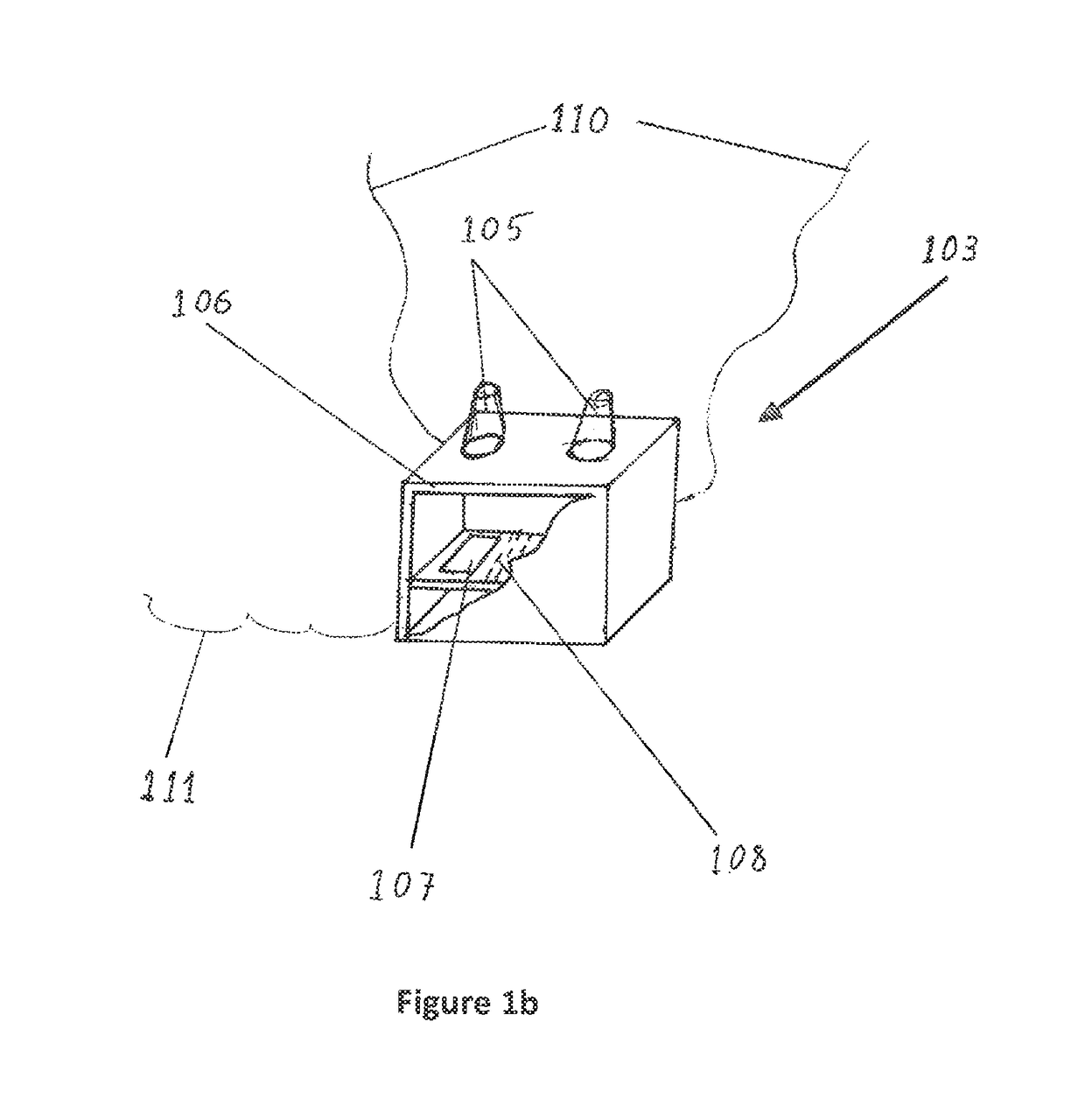
Non-invasive device and method for treating gastro esophageal reflux disease (GERD) and the digestive system
ActiveUS9925375B2Increase of esophagus motilityStrengthens esophagealExternal electrodesArtificial respirationGastro-esophageal reflux diseaseDynamic motion
Owner:GERDCARE MEDICAL LTD
Epha2 and hyperproliferative cell disorders
InactiveUS20090162933A1Promote migrationIncrease secretionSenses disorderDigestive systemCell phenotypeDisease
The present invention relates to methods and compositions designed for the treatment, management, or prevention of a non-neoplastic hyperproliferative cell or excessive cell accumulation disorders, particularly those involving hyperproliferation of epithelial or endothelial cells. In one embodiment, the methods of the invention comprise the administration of an effective amount of one or more EphA2 agonistic agents that bind to EphA2 and increase EphA2 cytoplasmic tail phosphorylation and / or increase EphA2 autophosphorylation, in cells which EphA2 has been agonized. In another embodiment, the methods of the invention comprise the administration of an effective amount of one or more EphA2 agonistic agents that bind to EphA2 and reduce EphA2 activity (other than autophosphorylation). In another embodiment, the methods of the invention comprise administration of an effective amount of one or more EphA2 agonistic agents that bind to EphA2 and decrease a pathology-causing cell phenotype (e.g., a pathology-causing epithelial cell phenotype or a pathology-causing endothelial cell phenotype). In another embodiment, the methods of the invention comprise the administration of an effective amount of one or more EphA2 agonistic agents that are EphA2 antibodies that bind to EphA2 with a very low Koff rate. In preferred embodiments, agents of the invention are monoclonal antibodies. The invention also provides pharmaceutical compositions comprising one or more EphA2 agonistic agents of the invention either alone or in combination with one or more other agents useful in therapy for non-neoplastic hyperproliferative cell or excessive cell accumulation disorders.
Owner:KIENER PETER A +3
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com