Transgenically produced non-secreted proteins
a technology of transgenes and proteins, applied in the field of protein production and secretion, can solve the problems of difficult or expensive production of/or in the required quantities using conventional methods, and the difficulty of traditional bacteria or yeast systems to produce many complex proteins in a functional form, etc., to achieve the effect of improving the expression level, increasing the yield of desired proteins, and stabilizing the rna of the expression sequen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
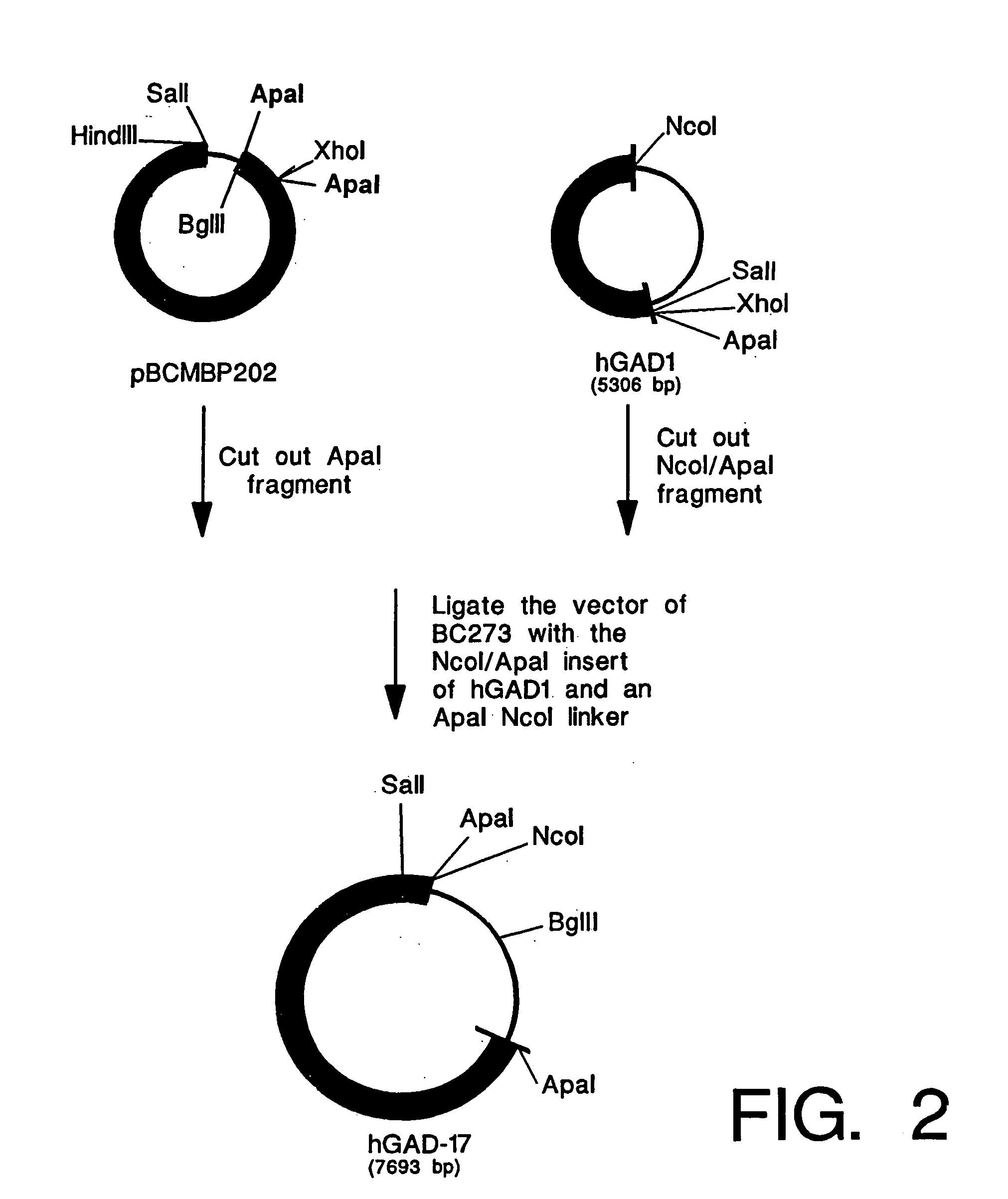
1. Cloning of β-Casein-MBP Fusion Gene
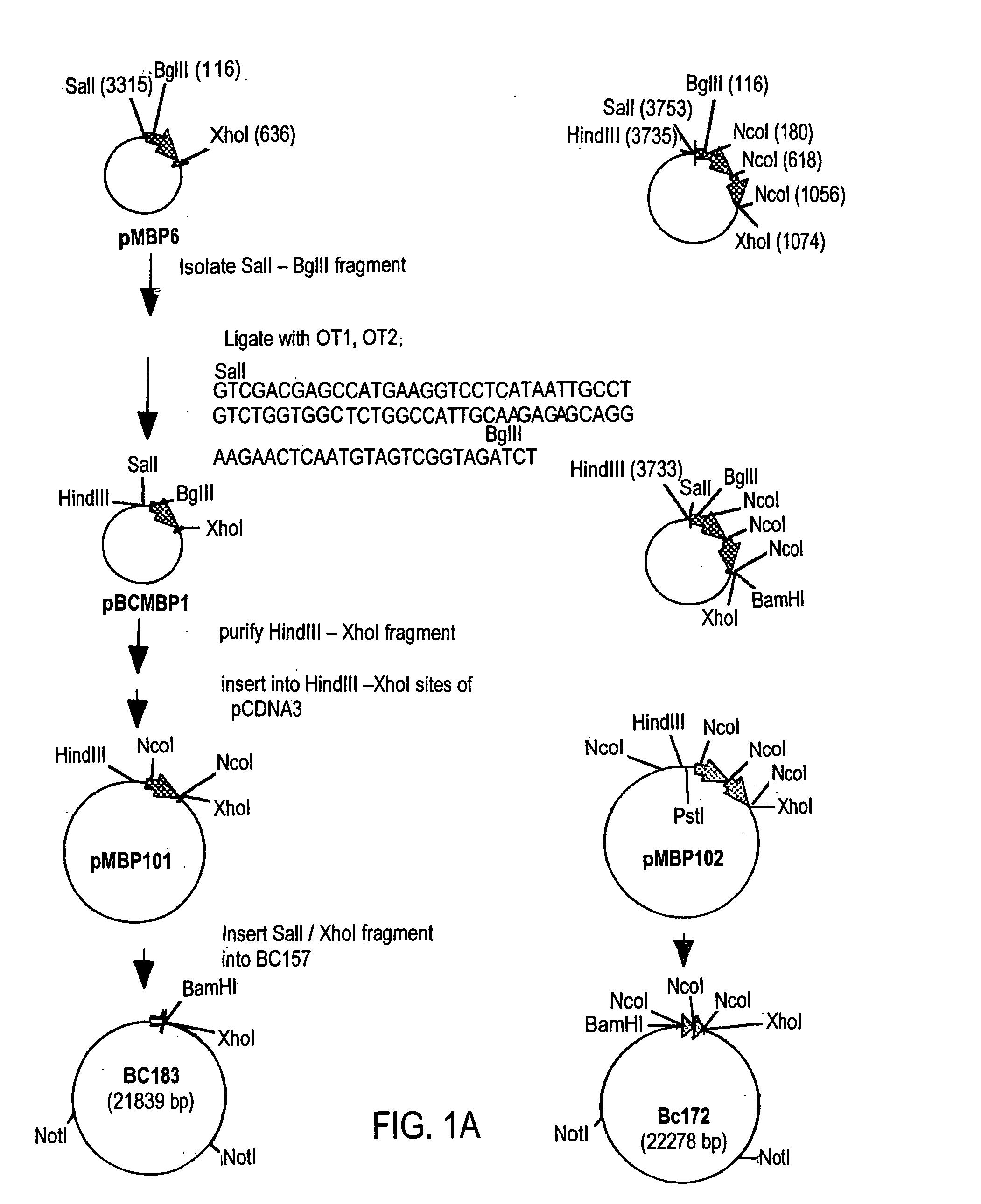
[0137] Five plasmids containing the β-casein / MBP fusing gene were constructed. As depicted in FIG. 1, plasmid pBCMBP1 was generated by ligating goat β-casein signal sequences (oligos OT1 AND OT2) to SalI / BglII sites of pMBP6. Cloning the HindIII-XhoI fragment of pBCMBP1 into pCDNA3 generated pBCMBP101. Plasmid pBCMBP102 was generated by inserting an additional NcoI fragment pMBP6 into the NcoI site of pBCMBP101. SalI / XhoI fragments of pBCMBP101, pBCMBP102 were inserted into BC157 to generated BC183 and BC172, respectively.
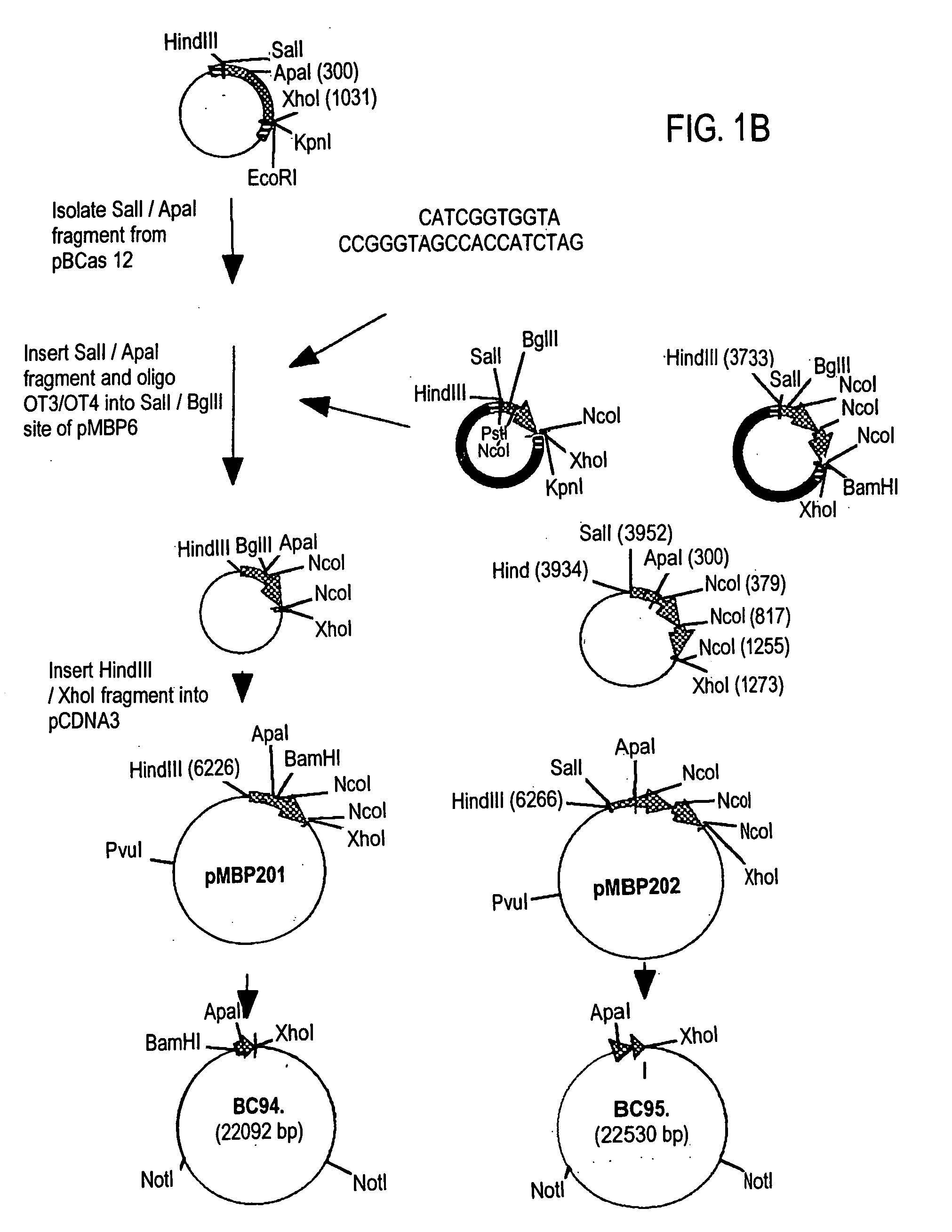
[0138] Plasmid pBCMBP2 was generated by ligating SalI-ApaI fragment of pBC12 (which contains β-casein signal sequence and 30% of the N-terminal coding region of goat β-casein) and a pair of adapter oligos (oligos OT3 and OT4) into the SalI-BglII site of pMBP6. The HindIII / EcoRI fragment of the pBCMBP2, which carries sequences encoding the β-casein signal and N terminal portion followed by the entire MBP coding region was clo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| acid | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com