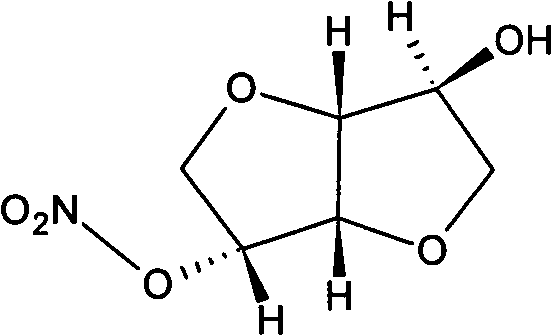
Isosorbide mononitrate liquid preparation and preparing method thereof
A technology of isosorbide dinitrate and liquid preparations, which is applied in the field of isosorbide mononitrate liquid preparations and its preparation, can solve problems such as allergies, pyrogens, tumor-like reactions, contamination particles and impurities, and improve the level of sterility assurance, Effect of increasing solubility and preventing the generation of bacteria
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1: the preparation of isosorbide mononitrate injection (1000ml amount)
[0039] One, the preparation of isosorbide mononitrate injection (1000ml amount)
[0040] In a sterile operating room, dissolve 4 g of isosorbide mononitrate in 400 ml of water for injection at a water temperature of 50°C, then add 9 g of sodium chloride and stir until fully dissolved, add 300 ml of propylene glycol, and stir evenly to obtain solution I;
[0041] Adjust the pH value of solution I to 7.0 with citric acid and sodium hydroxide;
[0042] In the aseptic operation room, the temperature of the above solution I was lowered to 40° C., and then the solution I was filtered with a 0.22 μm pore size filter to obtain the filtrate a.
[0043] The filtrate a is ultrafiltered by ultrafiltration with a molecular weight cut-off of 10,000 Daltons to obtain filtrate b.
[0044] Make up the filtrate b to 1000ml with water for injection, and then filter with a 0.22 μm pore size filter to obta...
Embodiment 2
[0084] Embodiment 2, the preparation of isosorbide mononitrate injection (1000ml amount)
[0085] The preparation process is carried out in the 10,000-class clean area, and the filling process is carried out under the local 100-class clean area of the 10,000-class clean area.
[0086] One, the preparation of isosorbide mononitrate injection
[0087] In a sterile operating room, dissolve 12.5 g of isosorbide mononitrate in water for injection at 65° C., add 26 g of glycerin and stir until fully dissolved, and add 200 ml of propylene glycol to obtain solution I.
[0088] Adjust the pH of solution I to 6.8 with phosphoric acid or disodium hydrogen phosphate.
[0089] In the aseptic operation room, the temperature of the above solution I was lowered to 40° C., and then the solution I was filtered with a 0.22 μm filter to obtain the filtrate a.
[0090] The filtrate a is ultrafiltered with an ultrafilter with a molecular weight cut-off of 10,000 Daltons to obtain a filtrate.
...
Embodiment 3
[0115] Embodiment 3, the preparation of isosorbide mononitrate injection (1000ml amount)
[0116] The preparation process is carried out in the 10,000-class clean area, and the filling process is carried out under the local 100-class clean area of the 10,000-class clean area.
[0117] One, the preparation of isosorbide mononitrate injection
[0118] In a sterile operating room, dissolve 15 g of isosorbide mononitrate in water for injection at 70° C., then add 9 g of sodium chloride and stir until fully dissolved, and add 100 ml of propylene glycol to obtain solution I.
[0119] Adjust the pH of Solution I to 7.2 with glacial acetic acid or disodium hydrogen phosphate.
[0120] In the aseptic operation room, the temperature of the above solution I was lowered to 45° C., and then the solution I was filtered with a 0.22 μm filter to obtain the filtrate a.
[0121] Filtrate a was ultrafiltered with an ultrafilter with a molecular weight cutoff of 10,000 Daltons to obtain filtr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com