Preparation method of pharmaceutical composition containing deslanoside
A technology for deacetylsaurin and mixtures, which is applied in the field of preparation of deacetylsaurin compositions, and can solve the problems of low sterility assurance level, high injection risk, and large impurity level in deacetylsaulicidin injections. , to achieve the effect of improving the level of sterility assurance, ensuring sterility, and eliminating the risk of introducing impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073]The composition of the prescription is as follows:
[0074] prescription Prescription 1 a
Prescription 2 b
Deacetyloside 12.9g43g Ethanol (95%) c
5.29kg 17.63kg glycerin 9.59kg 31.98kg Add water for injection to 65.89kg 219.9kg
[0075]Remarks:
[0076]a: The batch is 30,000, and the filling quantity is 2.15ml / piece. The glycerin manufacturer is Hunan Erkang Pharmaceutical Co., Ltd. The total weight of the liquid medicine is calculated based on the solution density of 1.0216g / ml.
[0077]b: The batch is 100,000 bottles, and the volume is 2.15ml / bottle. The glycerin manufacturer is Aug.Hedinger GmbH&Co.KG. The total weight of the liquid medicine is calculated based on the solution density of 1.0228g / ml.
[0078]c: The density of 95% ethanol is 0.81g / cm3It is calculated that the mass of ethanol in 5.29 kg of 95% ethanol is 4.9 kg, and the mass of ethanol in 17.63 kg of 95% ethanol is 16.33 kg.
[0079]Prescription 1 preparation process: add about 60% (about 39.55kg) of water for injection ...
Embodiment 2
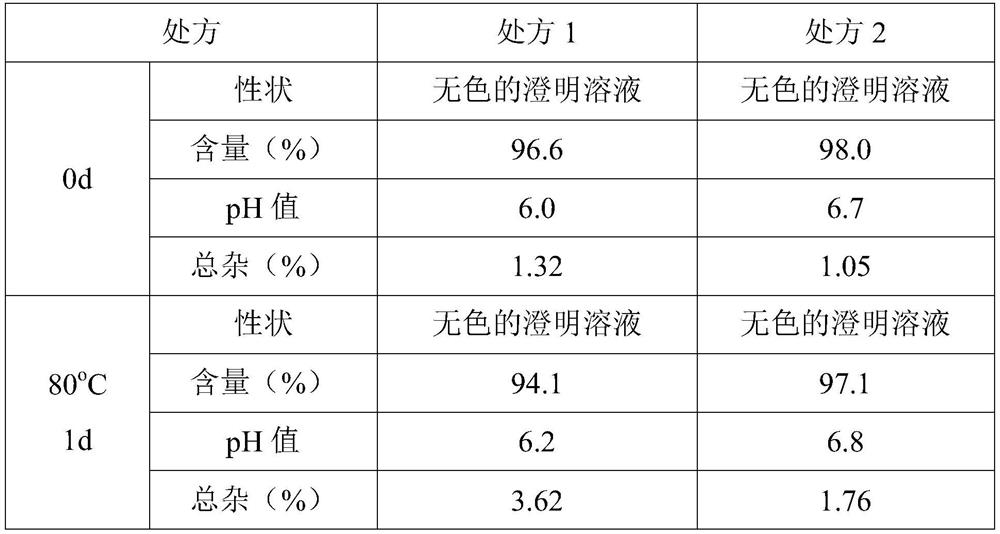
[0086]Terminal sterilization was performed on the samples of prescription 2, and the sterilization conditions were 116°C for 30 minutes and 121°C for 15 minutes. Compare the quality of the products before sterilization and different sterilization conditions, and take samples after sterilization at a high temperature of 60°C, 5 days, 10 days, and a high temperature of 80°C, in 1 day and 3 days to test the product quality (The test method is the same as above). The test results are as follows:
[0087]
[0088]
[0089]The experimental results show that deacetyl lanolin injection can withstand the terminal sterilization process, and the increase of impurities is small during the stability process.
Embodiment 3
[0091]The composition of the prescription is as follows:
[0092] ingredient Dosage (200,000 pieces / batch, 2.15ml / piece) Deacetyloside 86.0g Ethanol (95%) 35.26kg glycerin 63.96kg Add water for injection to a
439.80kg
[0093]a: The total weight of the drug solution is calculated based on the solution density of 1.0228g / ml.
[0094]Add about 60% of the prepared amount (about 264.00kg) of water for injection into the dispensing tank. After the water temperature is reduced to ≤40°C, add the prescription amount of glycerin and start stirring. Add the prescription amount of ethanol to the stainless steel barrel, and then add an appropriate amount of water for injection (about 12.60 kg) to dilute the prescription amount of ethanol into an ethanol solution with a concentration of about 70%. Add the prescribed amount of deacetyl lanolin to the above about 70% ethanol solution, and stir for at least 20 minutes until it is uniformly dissolved. Pour the above-mentioned liquid medicine into the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com