Patents
Literature
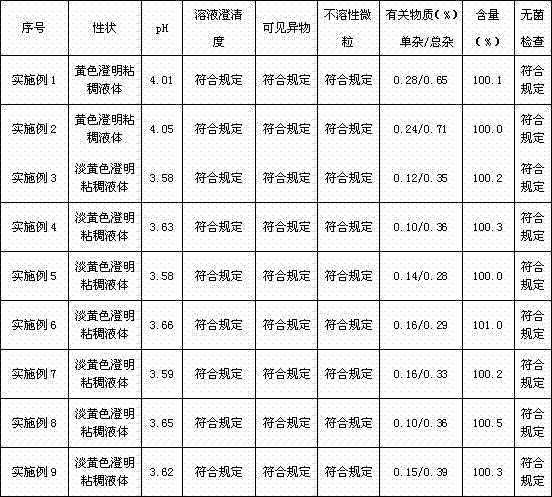
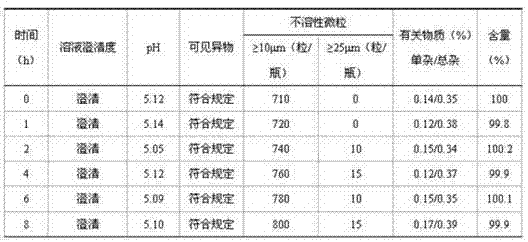
126 results about "Terminal Sterilization" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
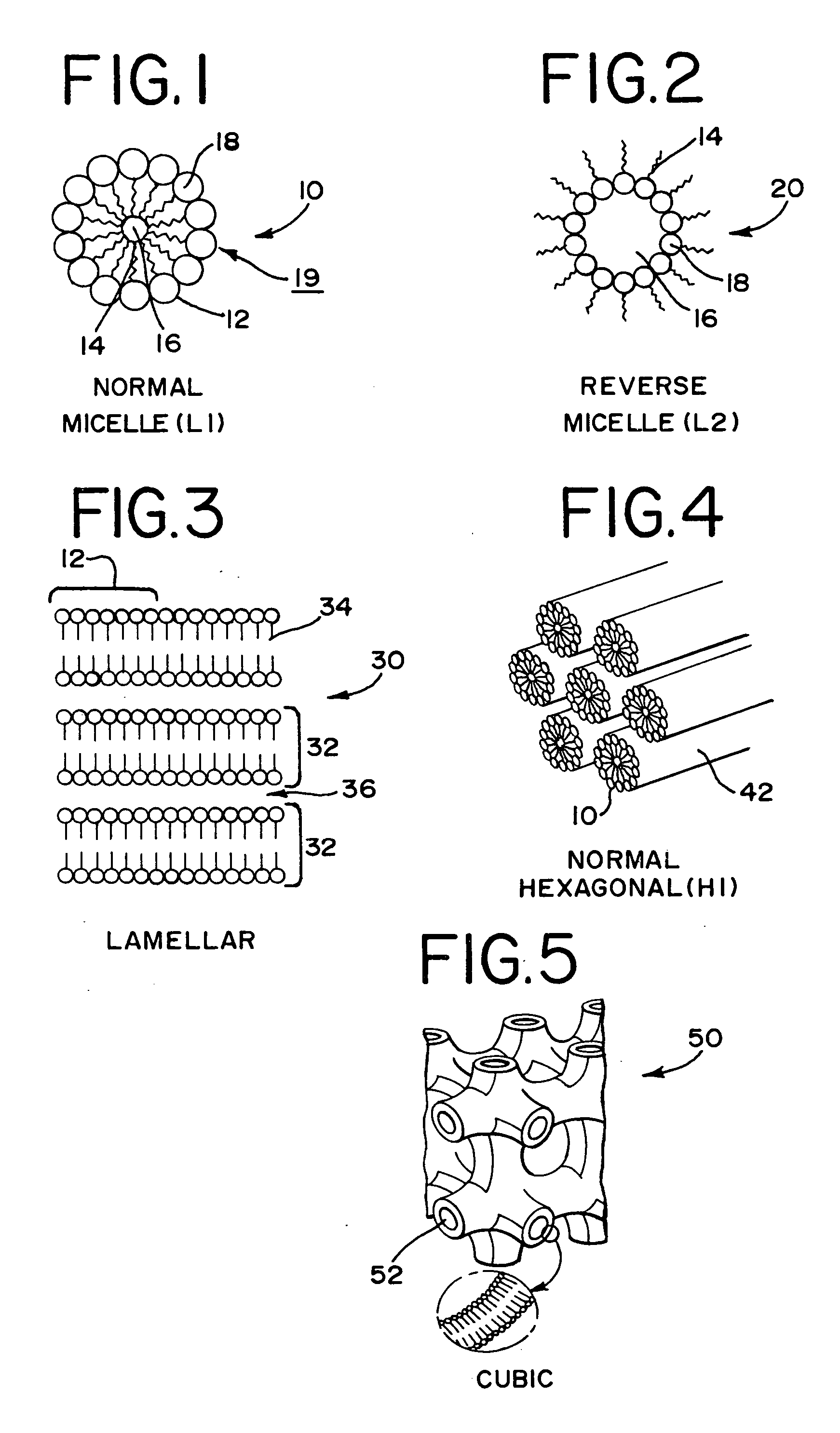
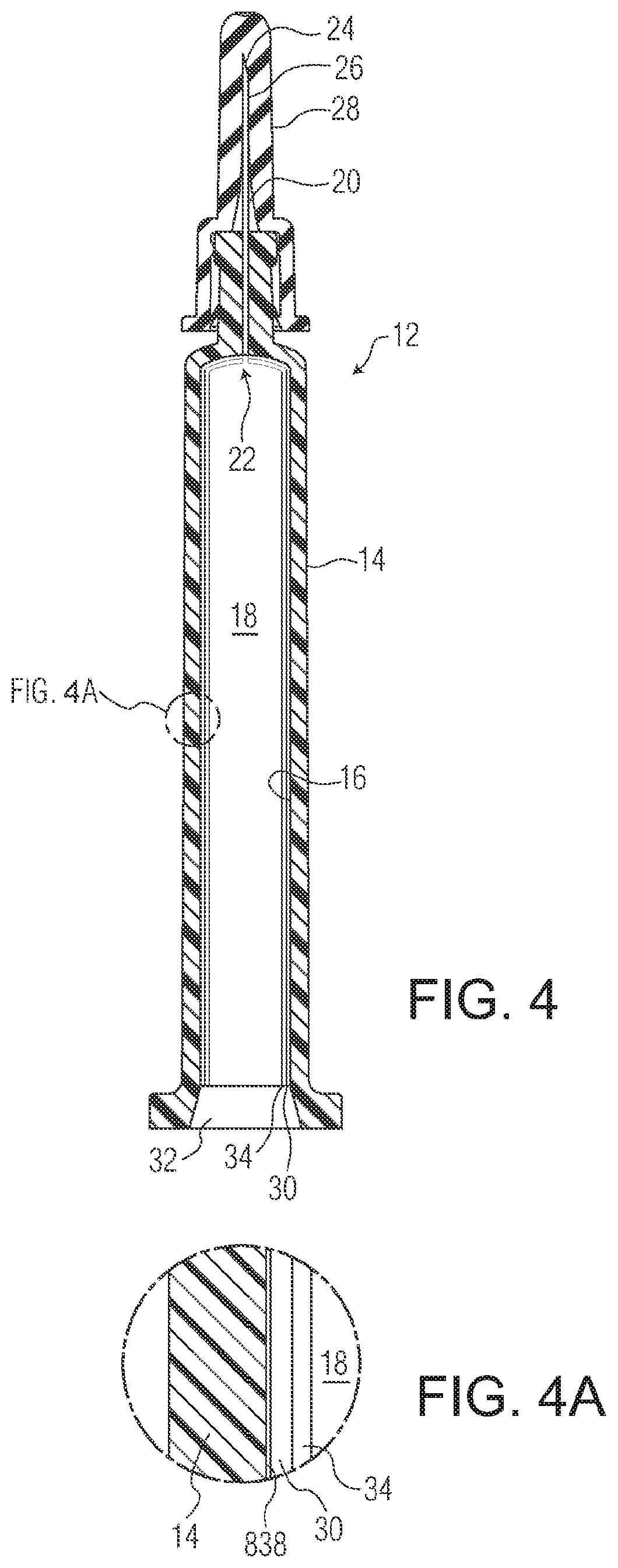
Sterilization of a finished product.
Composition i-ii and products and uses thereof
A curable composition apportioned between at least one Part A and at least one Part B, the Parts sealed within barrier means preventing contamination, the at least one Part A comprising: (i) one or more alkenyl-group containing prepolymers having at least one alkenyl group or moiety per molecule, and the at least one Part B comprising: (ii) one or more SiH-containing prepolymers having at least one Si—H unit per molecule; the composition additionally comprising: (iii) a catalyst for curing by addition of alkenyl-containing prepolymer (i) to SiH-containing prepolymer (ii), wherein prepolymer (ii) is substantially absent from Part A and prepolymer (i) is substantially absent from Part B, methods for preparing the composition, methods for sterilisation thereof, medical and non-medical use thereof, a device incorporating the composition, and a precursor therefor including its sterilisable precursor composition, in particular a terminally sterilisable or terminally sterile composition for medical use, particularly in wound therapy, more particularly as a wound packing material which can be shaped and configured to the shape of a wound, most particularly for application in negative pressure wound therapy (NPWT).
Owner:BLUESTAR SILICONES FRANCE SAS
Treatment of bioprosthetic tissues to mitigate post implantation calcification
InactiveUS6878168B2Lower potentialReduce the temperatureSuture equipmentsPharmaceutical delivery mechanismTreatment resultsMedicine
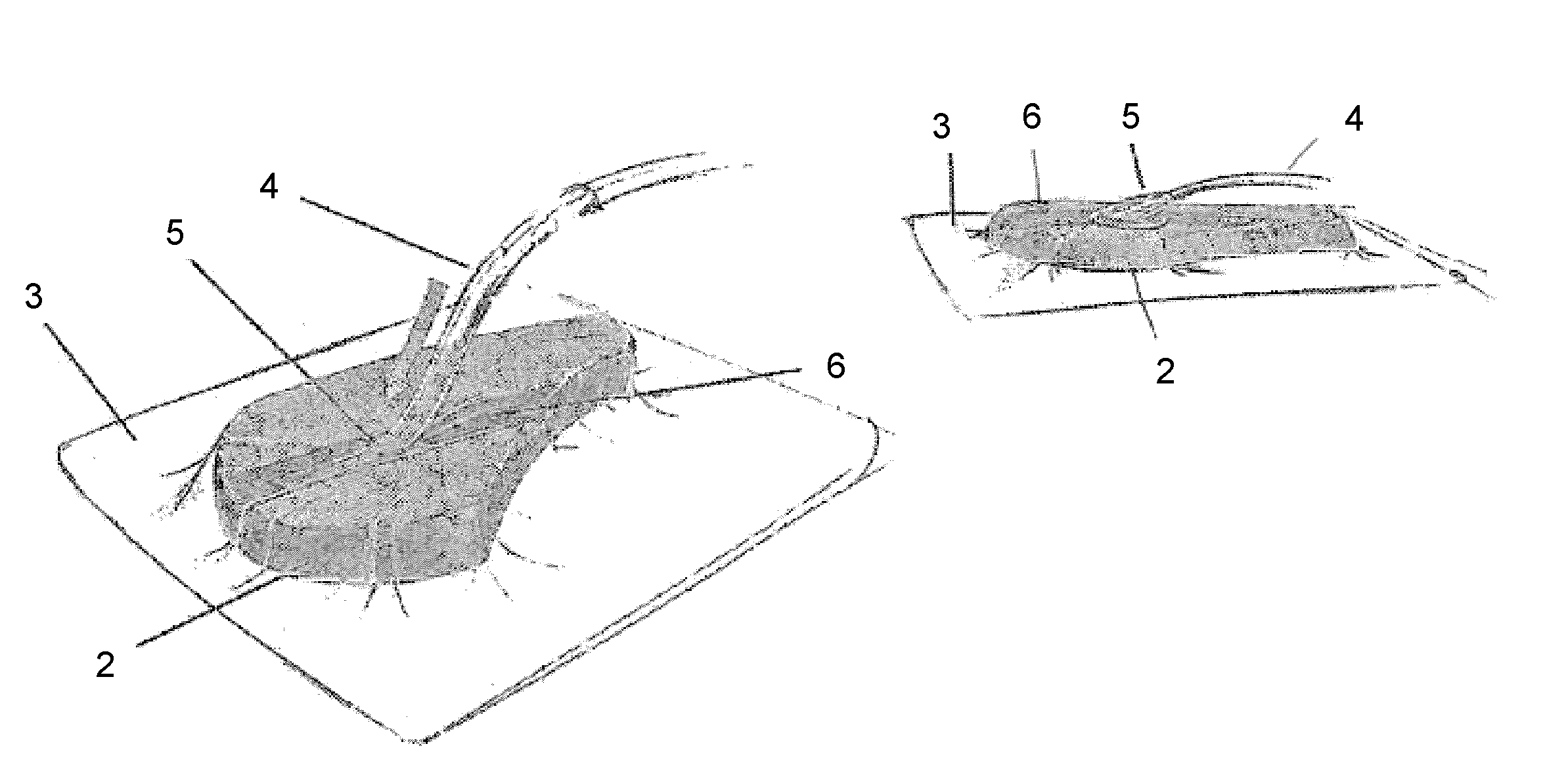
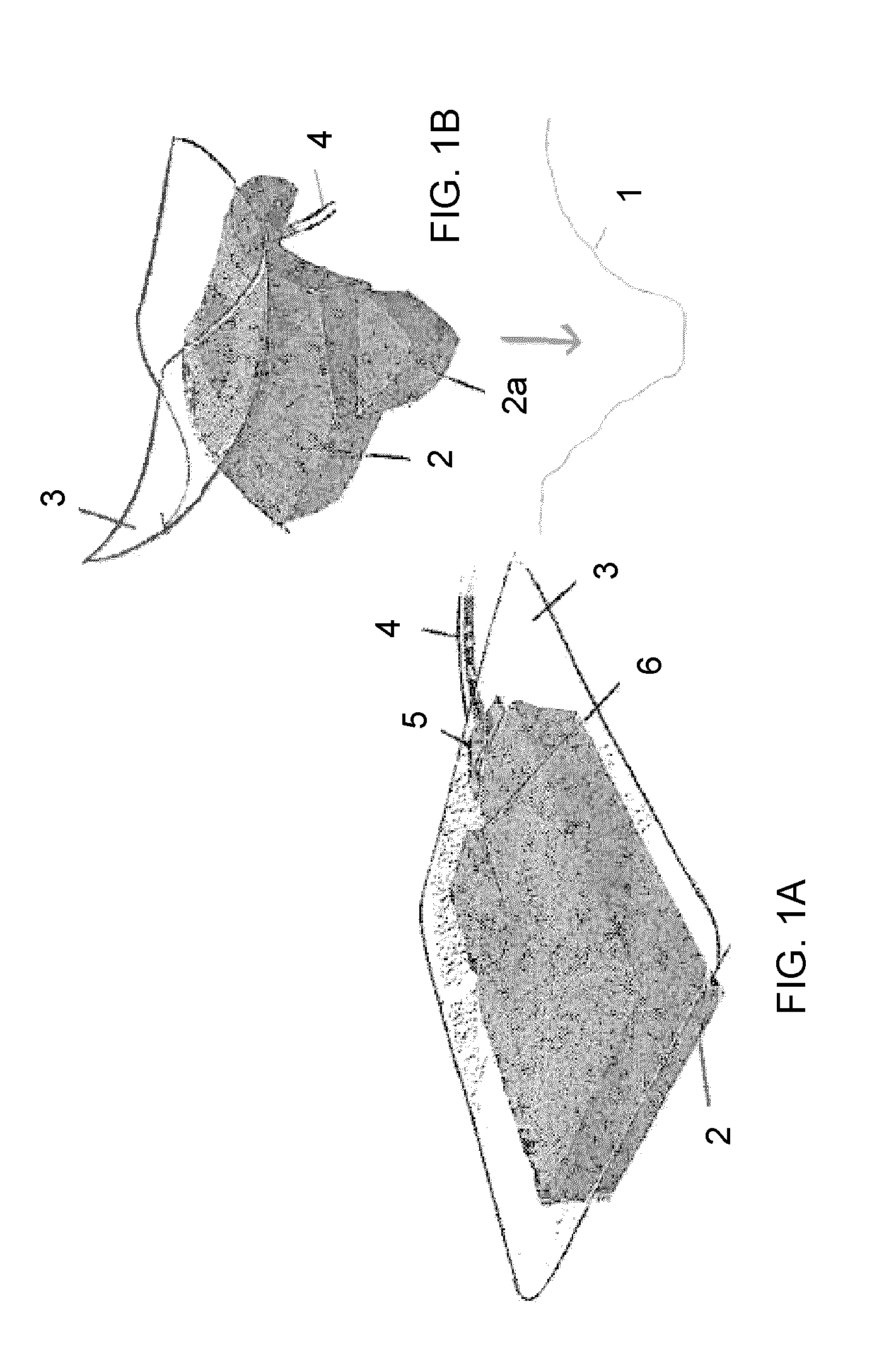
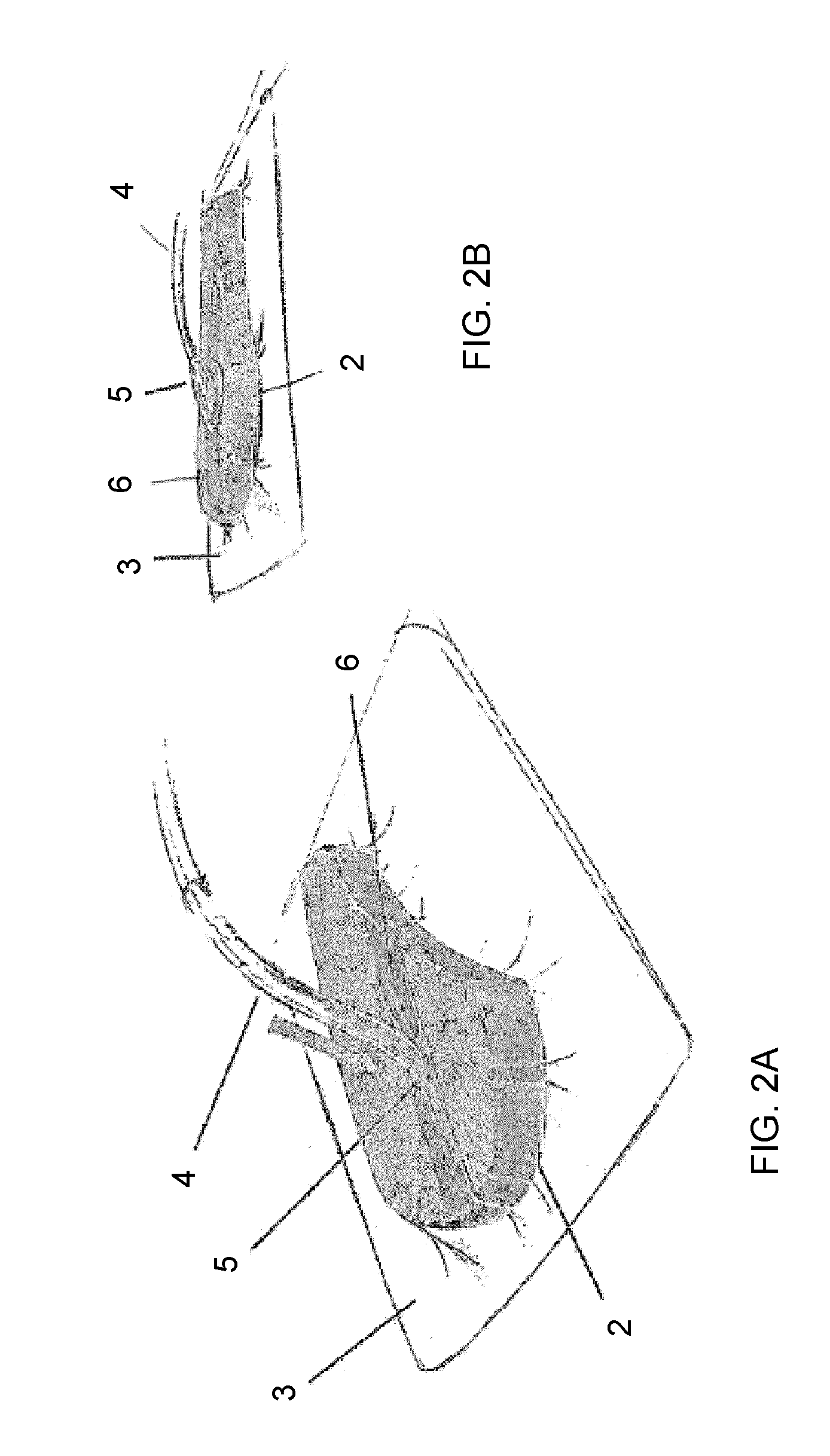
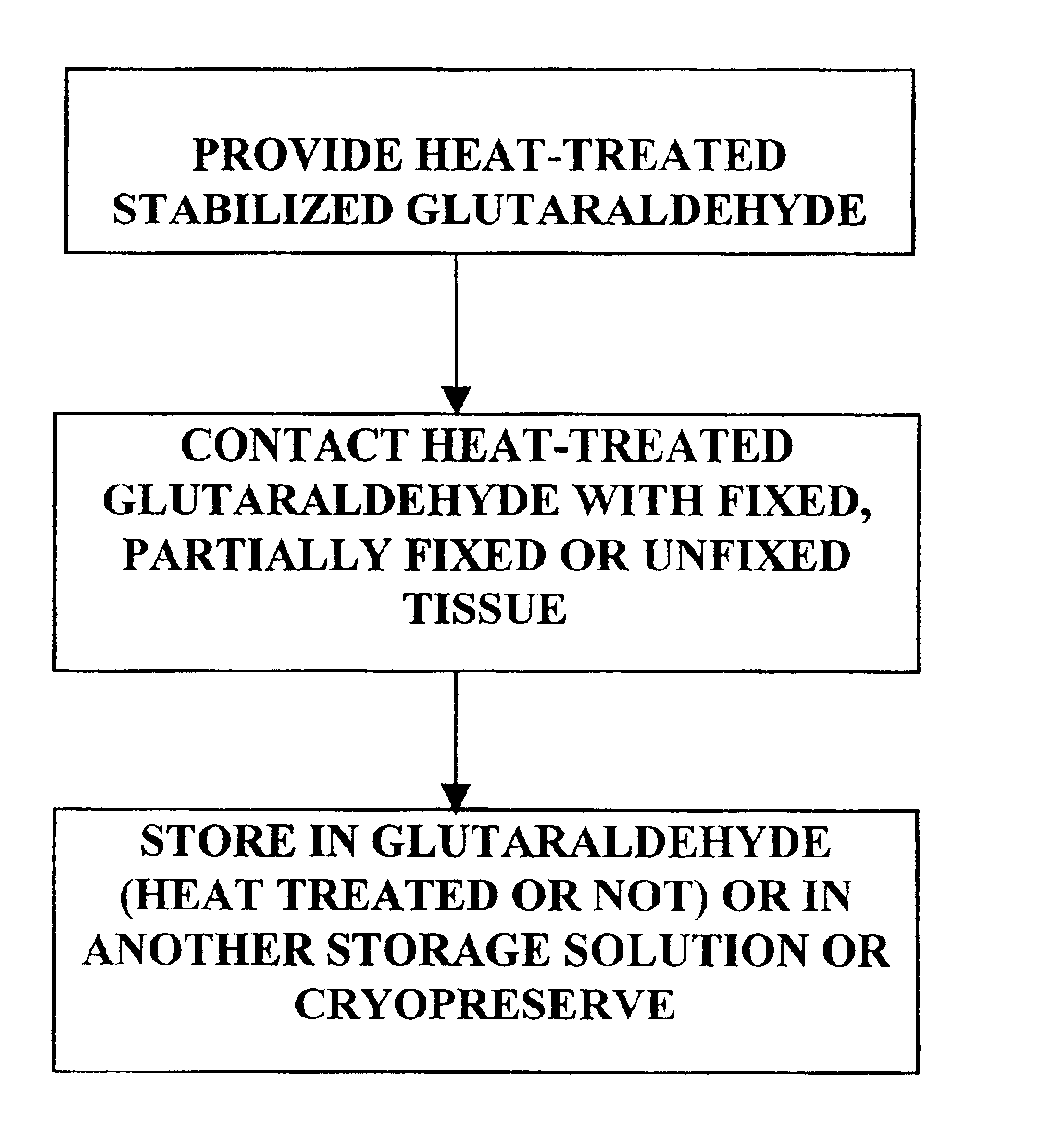
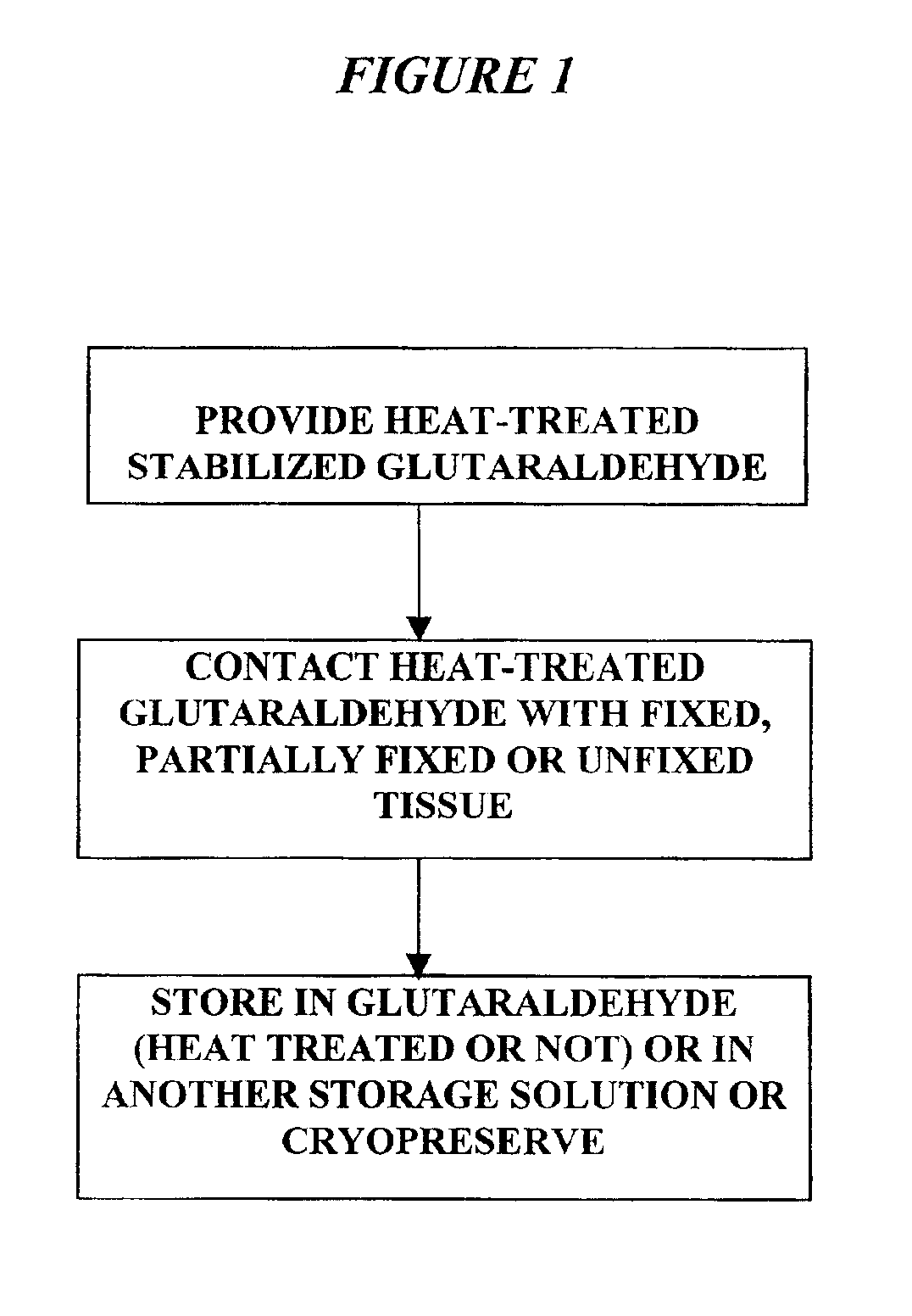
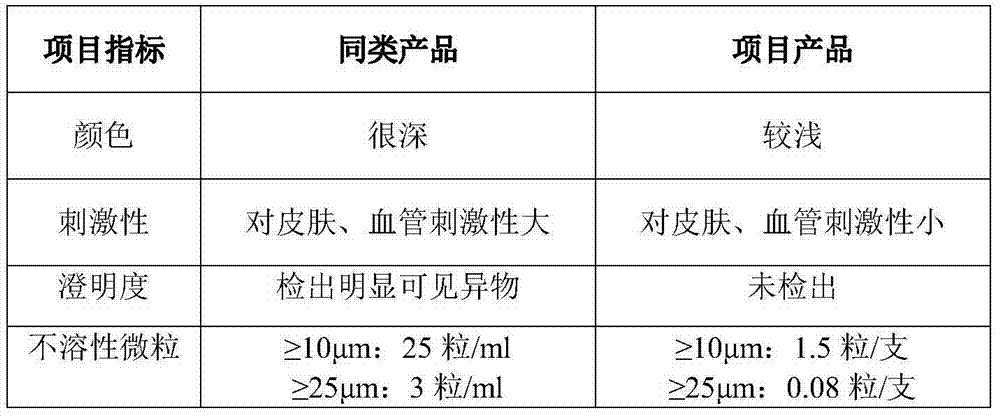
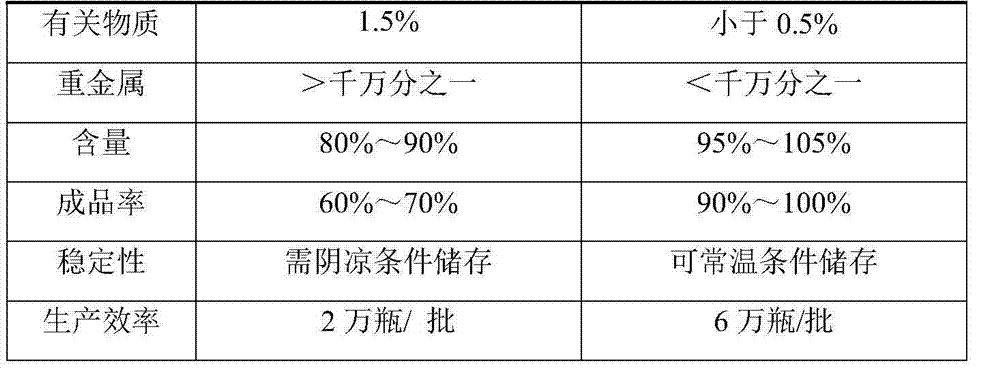
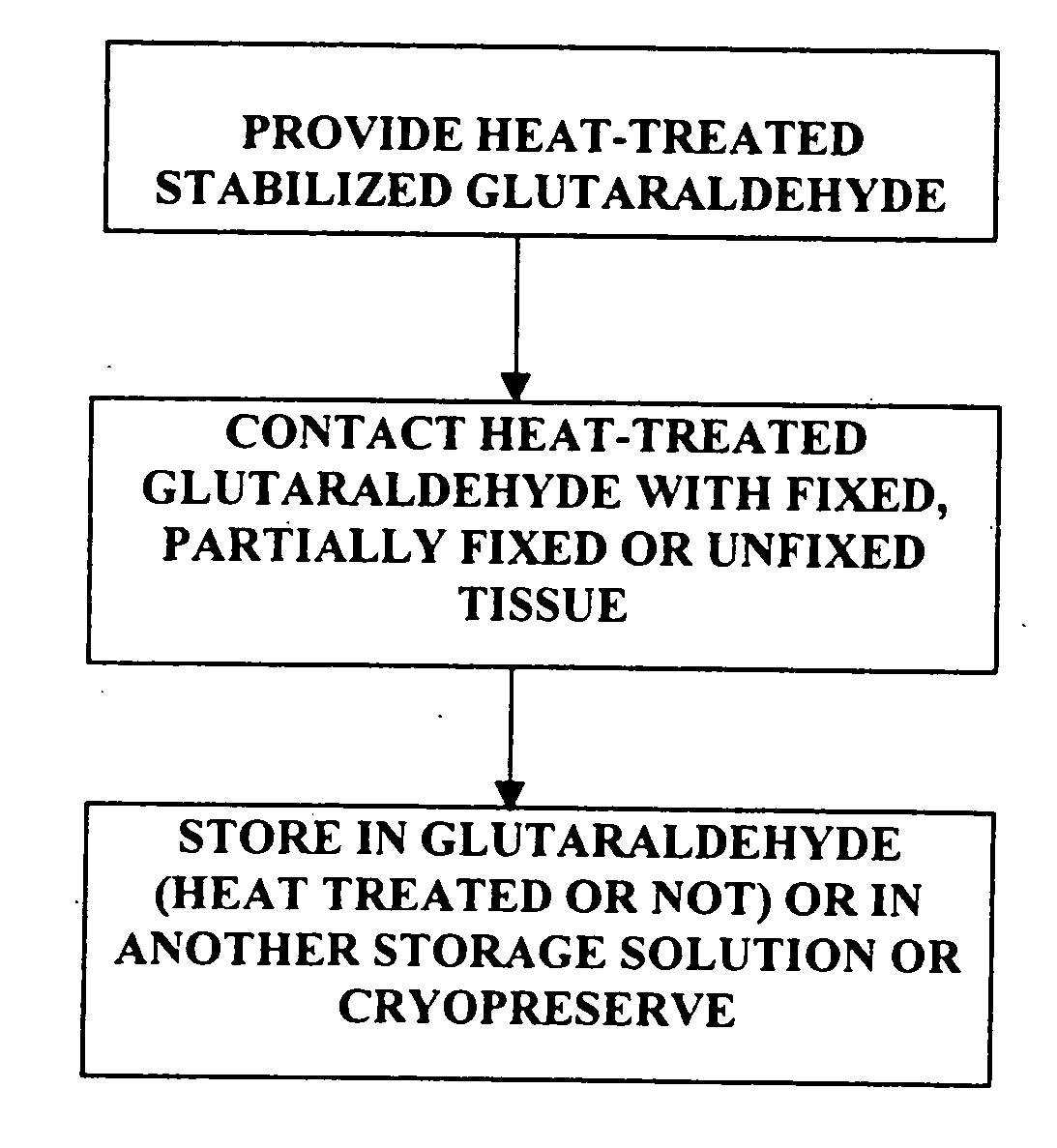
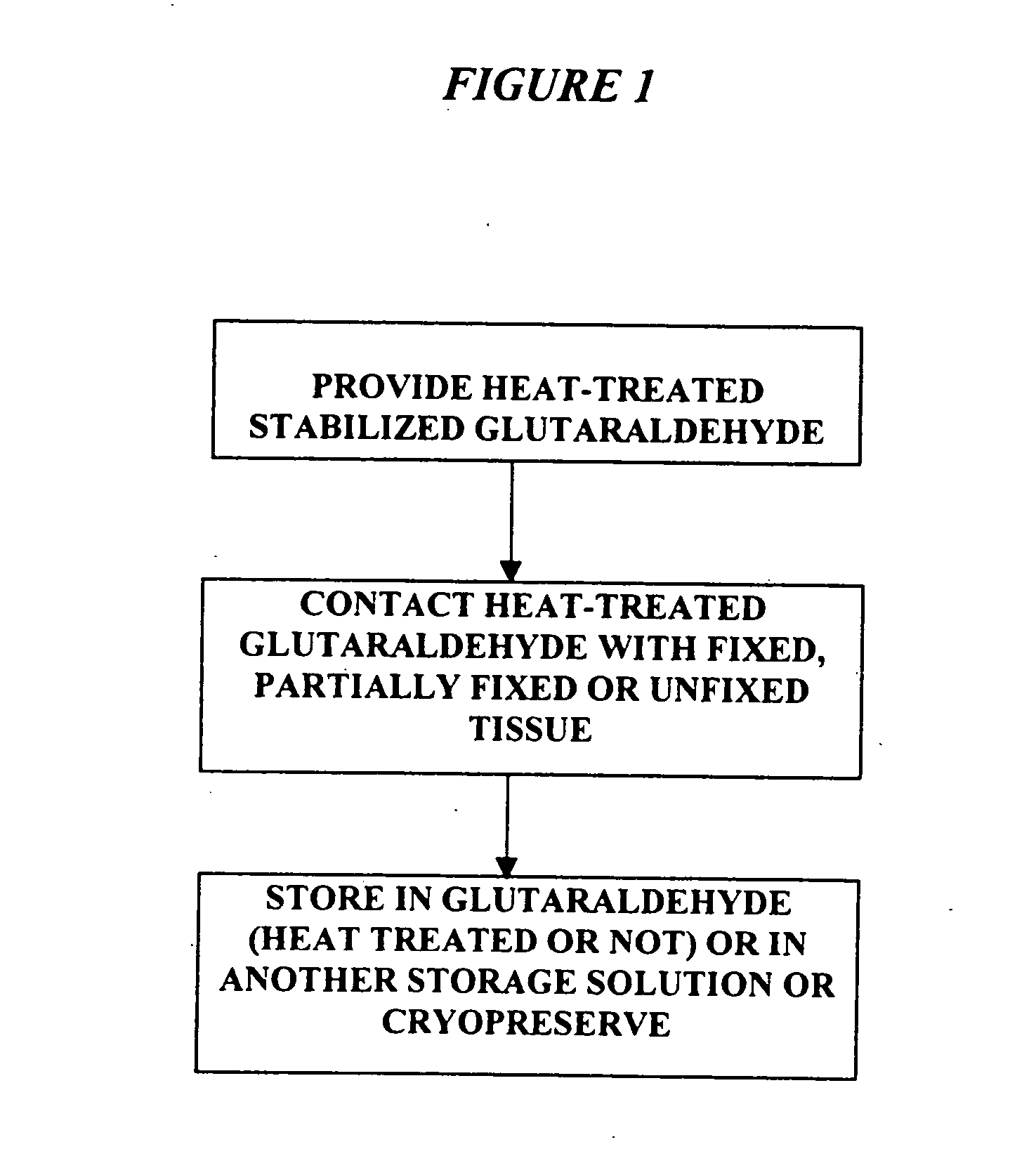
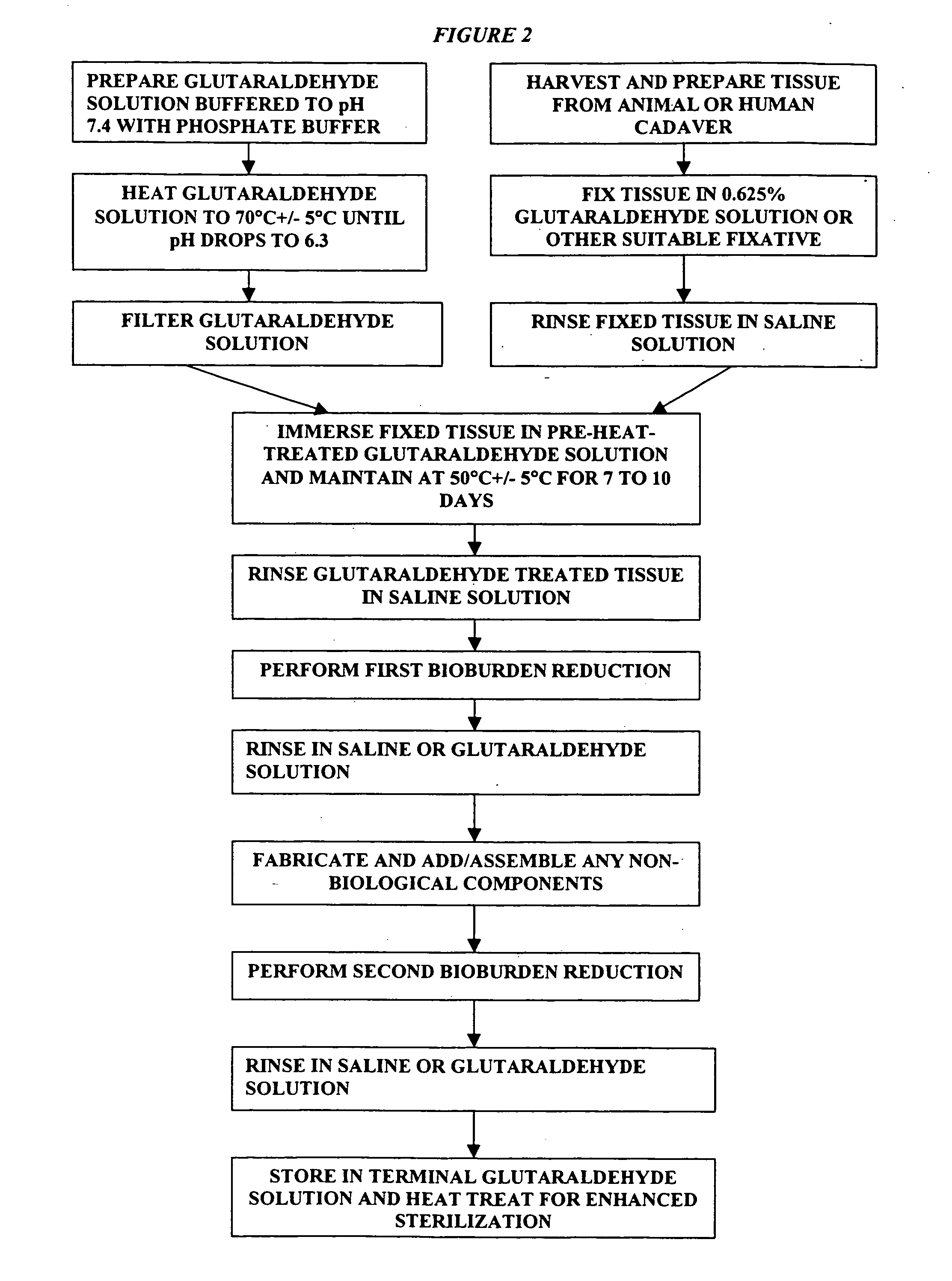
Bioprosthetic tissues are treated by immersing or otherwise contacting fixed, unfixed or partially fixed tissue with a glutaraldehyde solution that has previously been heat-treated or pH adjusted prior to its contact with the tissue. The prior heat treating or pH adjustment of the glutaraldehyde solution causes its free aldehyde concentration to decrease by about 25% or more, preferably by as much as 50%, and allows a “stabilized” glutaraldehyde solution to be obtained at the desired concentration and pH for an optimal fixation of the tissue at high or low temperature. This treatment results in a decrease in the tissue's propensity to calcify after being implanted within the body of a human or animal patient. The heat-treated or pH adjusted glutaraldehyde solution may, in some cases, also be used as a terminal sterilization solution such that the calcification-decreasing treatment with the previously treated glutaraldehyde and a terminal sterilization may be carried out simultaneously and / or in a single container.
Owner:EDWARDS LIFESCIENCES CORP
Method for treatment of biological tissues to mitigate post-implantation calcification and thrombosis
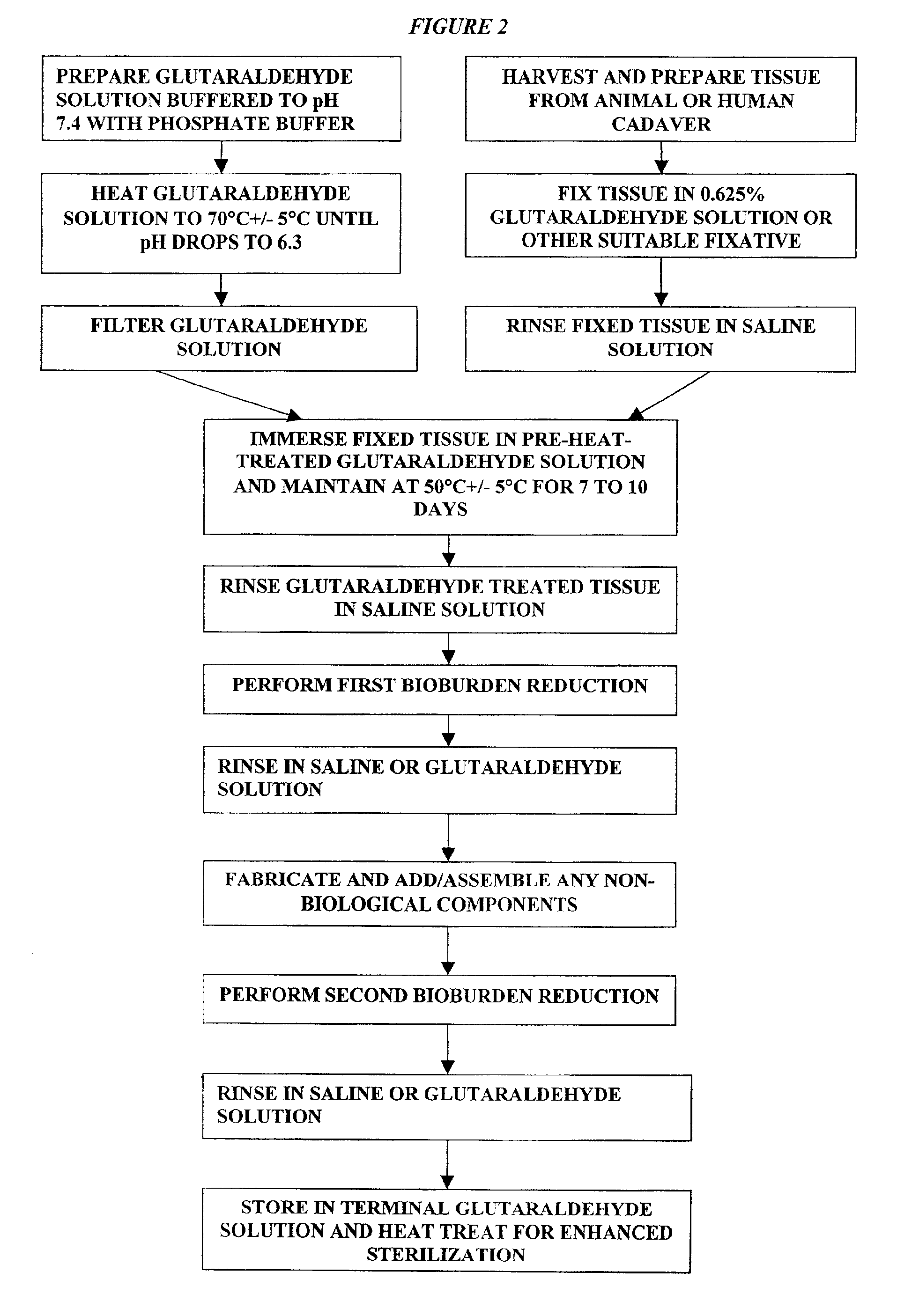
A method of treating a biological tissue including contacting the biological tissue with an aqueous sterilizing solution, and maintaining the aqueous sterilizing solution at a temperature of about 50° C. for a time period of about 1 to 2 days. The method of treating a biological tissue may be utilized as a terminal sterilization step in a method for fixation of biological tissues, and bioprosthetic devices may be prepared by such fixation method. The fixation method may include the steps of A) fixing the tissue, B) treating the tissue with a mixture of i) a denaturant, ii) a surfactant and iii) a crosslinking agent, C) fabricating or forming the bioprosthesis (e.g., forming the tissue and attaching any non-biological components thereto) and D) subjecting the bioprosthesis to the terminal sterilization method. The aqueous sterilizing solution may be glutaraldehyde of about 0.625 weight percent buffered to a pH of about 7.4.
Owner:EDWARDS LIFESCIENCES CORP
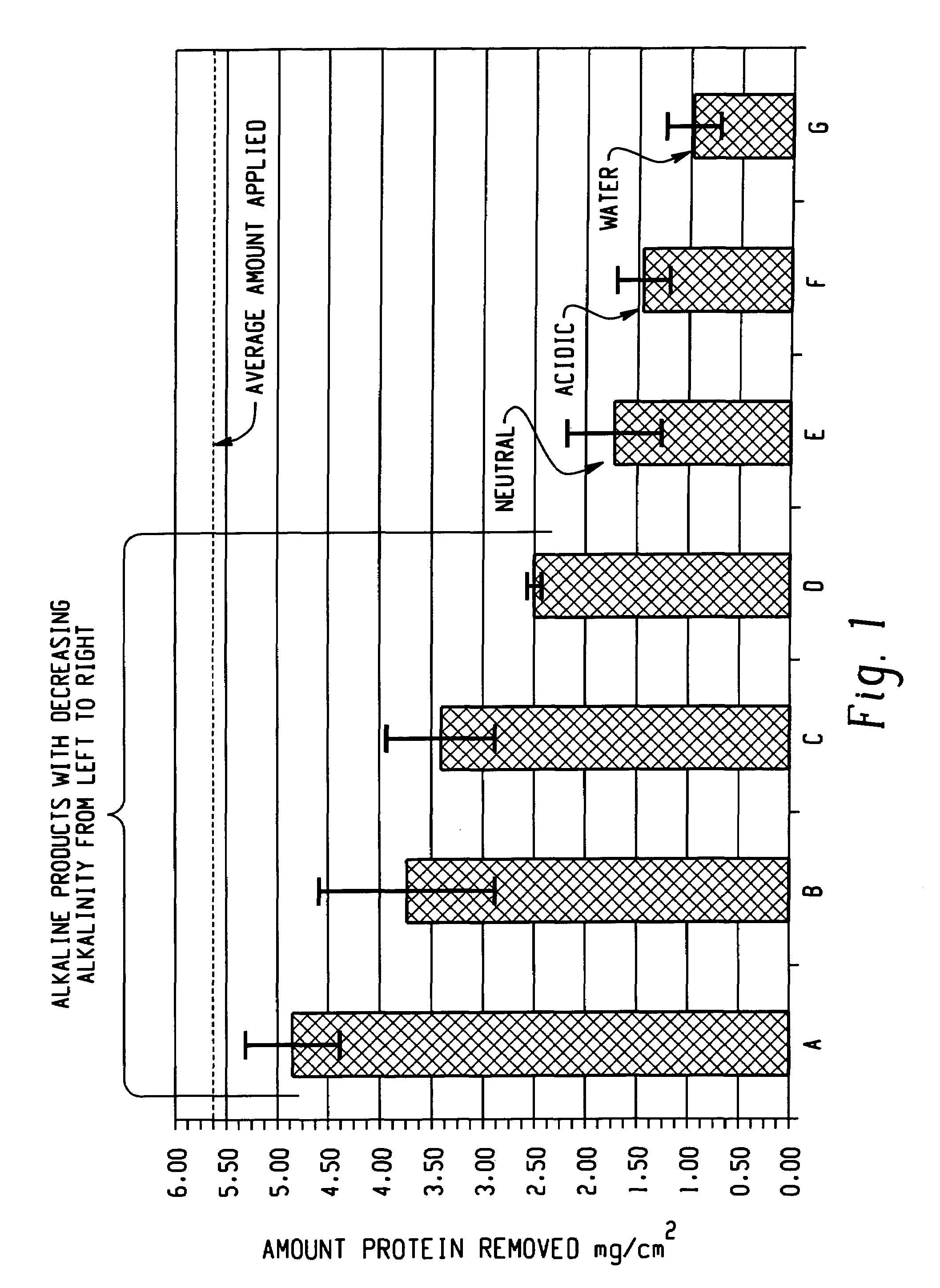
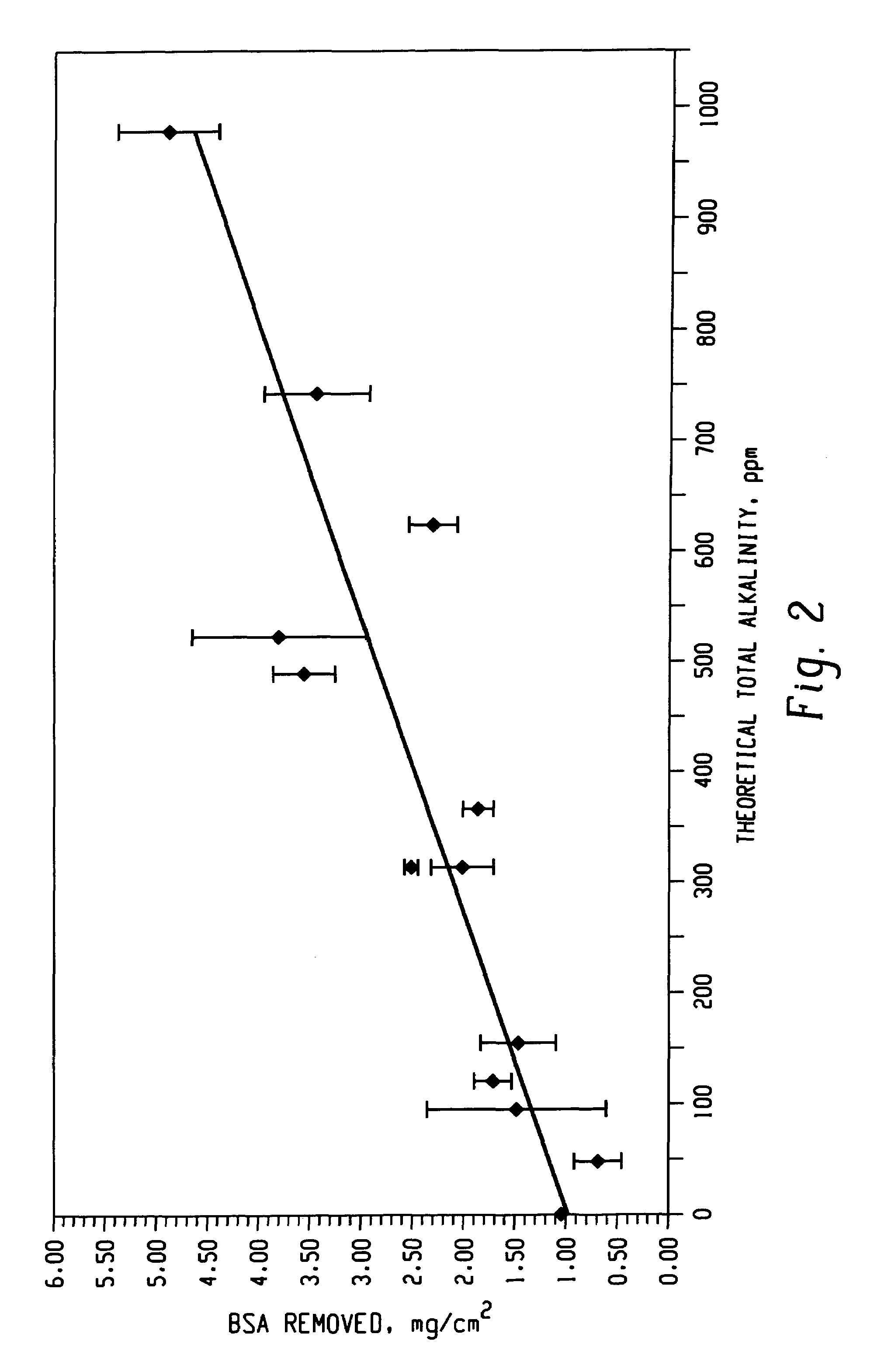
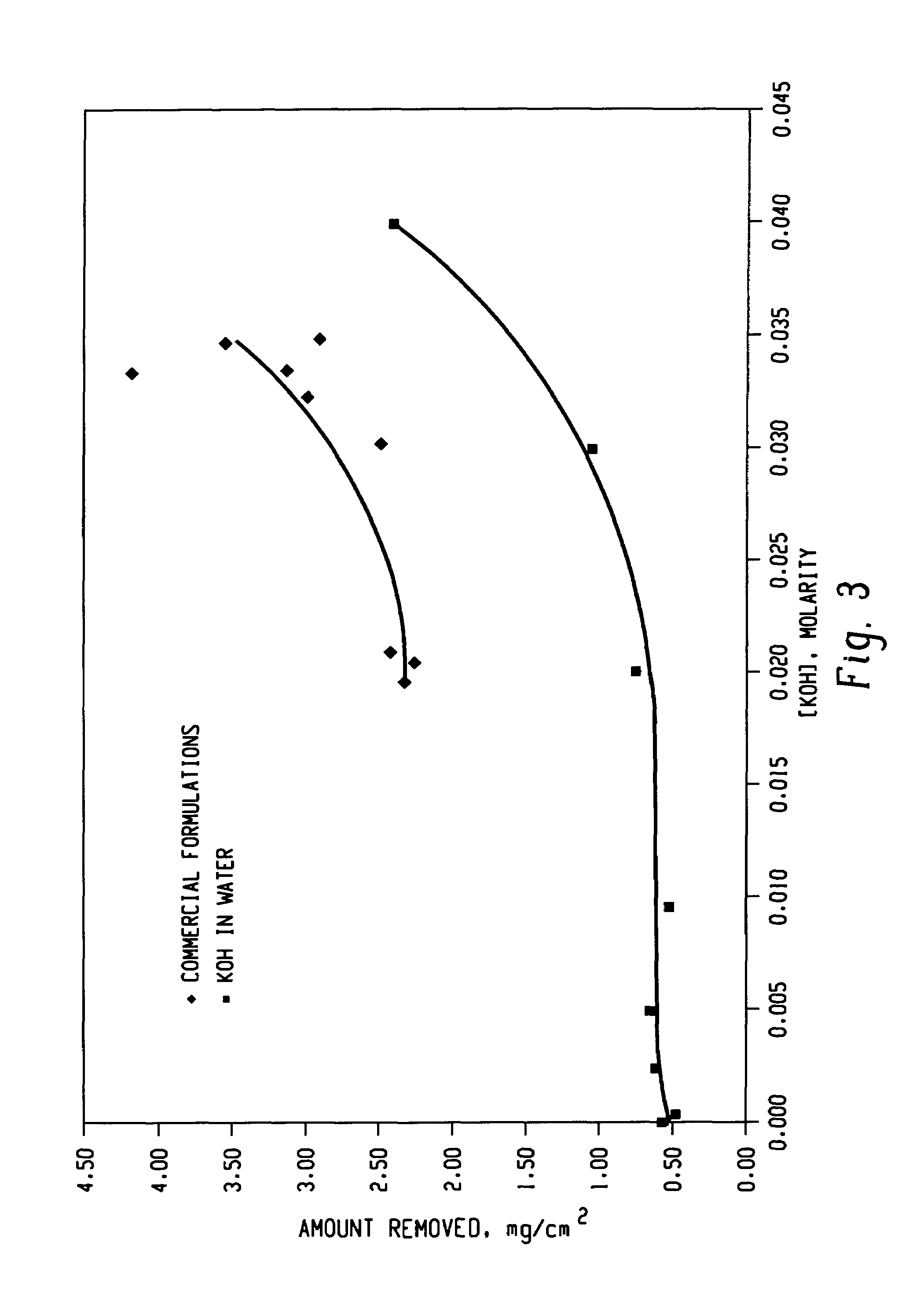
Decontamination of surfaces contaminated with prion-infected material with oxidizing agent-based formulations
InactiveUS7001873B2Gentle processThe process is fast and efficientInorganic/elemental detergent compounding agentsBiocideAcetic acidMicroorganism
A surface which carries a material which is infected with prions is cleaned with an alkaline cleaning solution to remove as much proteinaceous material as possible from the surface. The cleaning agent is an alkaline cleaning agent which attacks prions remaining on the surface and which attacks prions removed from the surface during the cleaning step. After the cleaning solution is drained, a solution of surfactants, buffers, and heavy metal free corrosion inhibitors is circulated over the surface at 50°–60° C. The surfactants disperse and unclump the prion-contaminated material. A strong oxidant, preferably peracetic acid, is added to the solution to bring the peracetic acid concentration to 1,000–2,500 ppm. The peracetic acid or other strong oxidant attacks the prions, particularly the unclumped prion strands, deactivating the prions. After rinsing and drying, the surface may be wrapped in a microbe impermeable barrier and subjected to terminal sterilization, such as steam autoclaving.
Owner:AMERICAN STERILIZER CO
Sterilization of biodegradable hydrogels
ActiveUS20120253071A1Good pharmacokinetic propertiesImproved physicochemicalOrganic chemistryEnergy modified materialsBiodegradable hydrogelsSolvent
The present invention relates to a terminal sterilization process for biodegradable PEG-based insoluble hydrogels using irradiation. The presence of a protective solvent ensures that the hydrogel remains intact with functionally preserved three-dimensional and physicochemical properties.
Owner:ASCENDIS PHARM AS
Terminal sterilization of prefilled containers
InactiveUS20050129569A1Effective sterilizationAvoid adverse reactionsLavatory sanitoryRadiationPolyolefinGamma irradiation
A method for inhibiting adverse reaction of the contents of a prefilled container during a radiation sterilization procedure is disclosed. In the method, a container which is made of a material including a radiation stable polyolefin is prefilled with a medium prior to being subjected to a gamma irradiation sterilization treatment. By using a radiation stable polyolefin material as the container, such as a polyolefin with a radiation stabilizer additive, and by prefilling the container prior to the gamma irradiation treatment, the container can be effectively sterilized without adversely affecting its contents.
Owner:BECTON DICKINSON & CO
Terminal sterilization of injectable collagen products
ActiveUS20060280769A1Lower Level RequirementsAvoid radiationBioreactor/fermenter combinationsBiological substance pretreatmentsMedicineVolumetric Mass Density
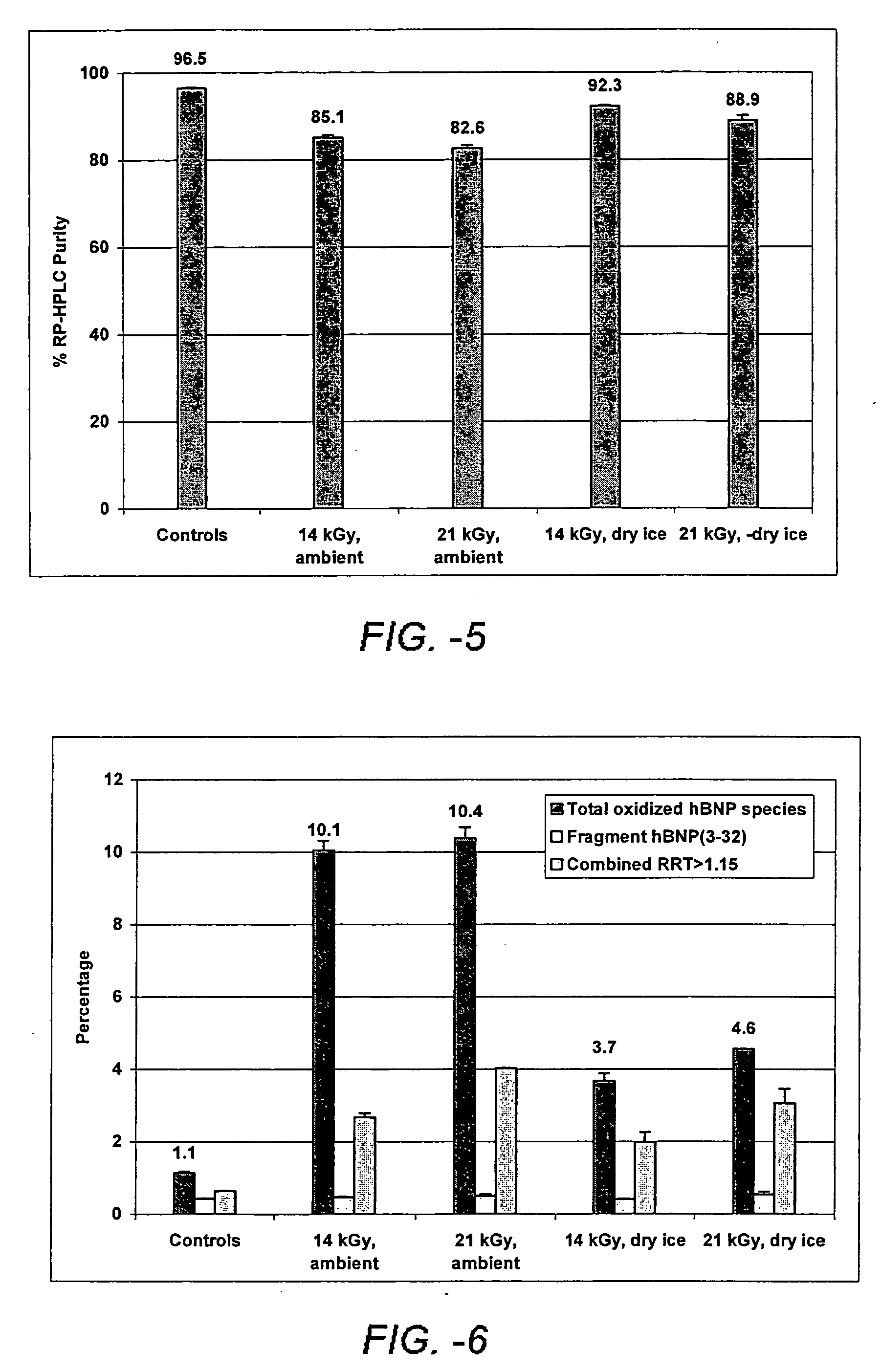
Methods of sterilizing dermal fillers and injectable collagen material have been developed which reduce the level of active biological contaminants or pathogens without adversely affecting the material, i.e., wherein the dermal fillers and injectable collagen material retain their same properties before and after its terminal sterilization. In one embodiment the method for sterilizing the dermal filler or injectable collagen material that is sensitive to radiation contains the steps of protecting the filler or material from radiation, and irradiating the filler or material with a suitable dose of radiation for a time and at a rate effective to sterilize the filler or injectable material. In a preferred embodiment the method for sterilizing the dermal filler or injectable collagen material that is sensitive to radiation includes the steps of a) freezing the filler or material at a temperature below its freezing temperature, which is generally below 0° C. and b) irradiating the filler or material with a suitable dose of radiation at an effective rate for a time effective to sterilize the filler or material. The exposure of the radiation differs depending upon the density of the filler or material, but is preferably between 5kGy and 12kGy and more preferably between 6kGy and 8kGy. These doses result in a sterility assurance level (SAL) of 10−6 SAL for the filler or material.
Owner:MAM HLDG OF WEST FLORIDA L L C
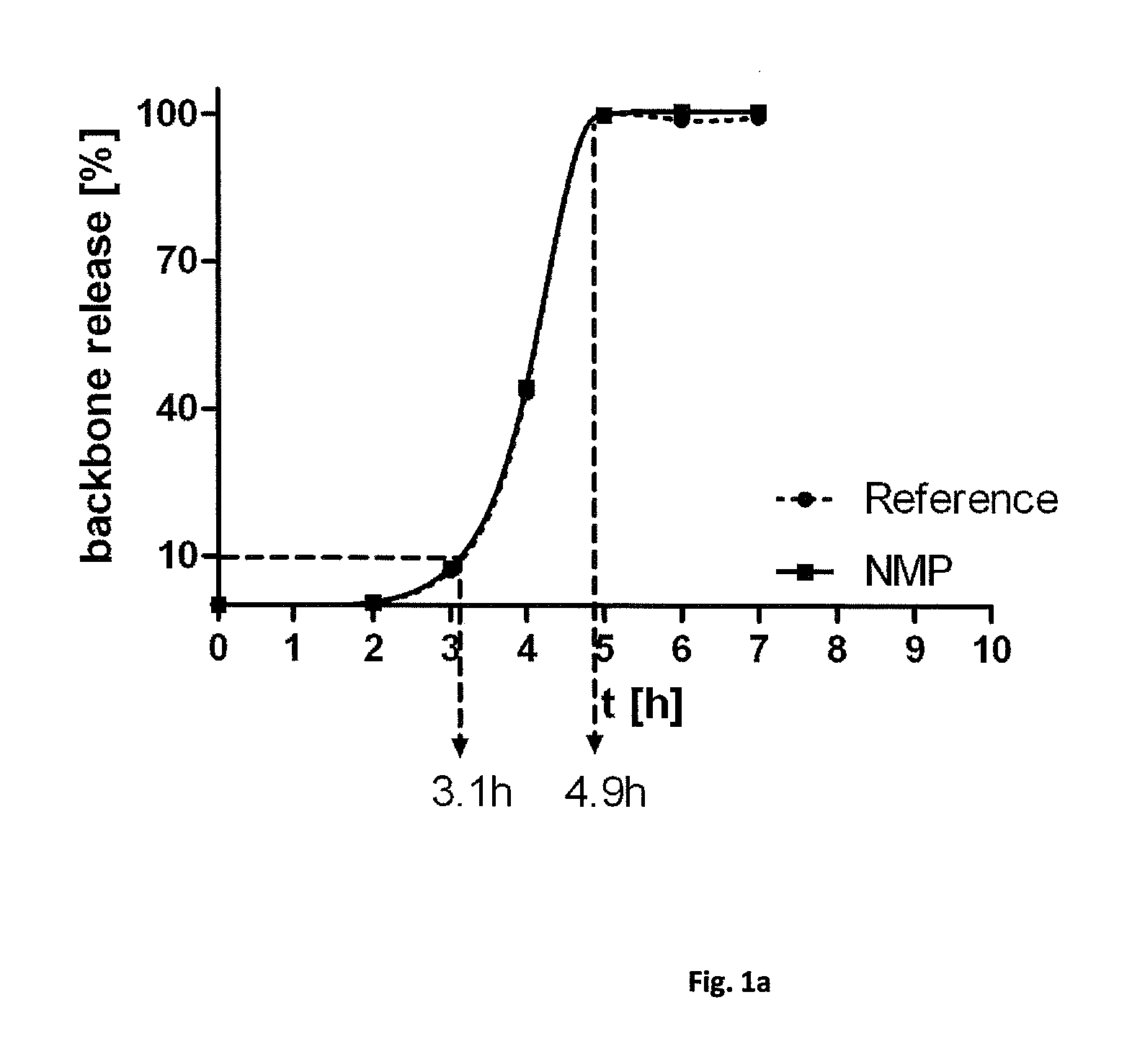
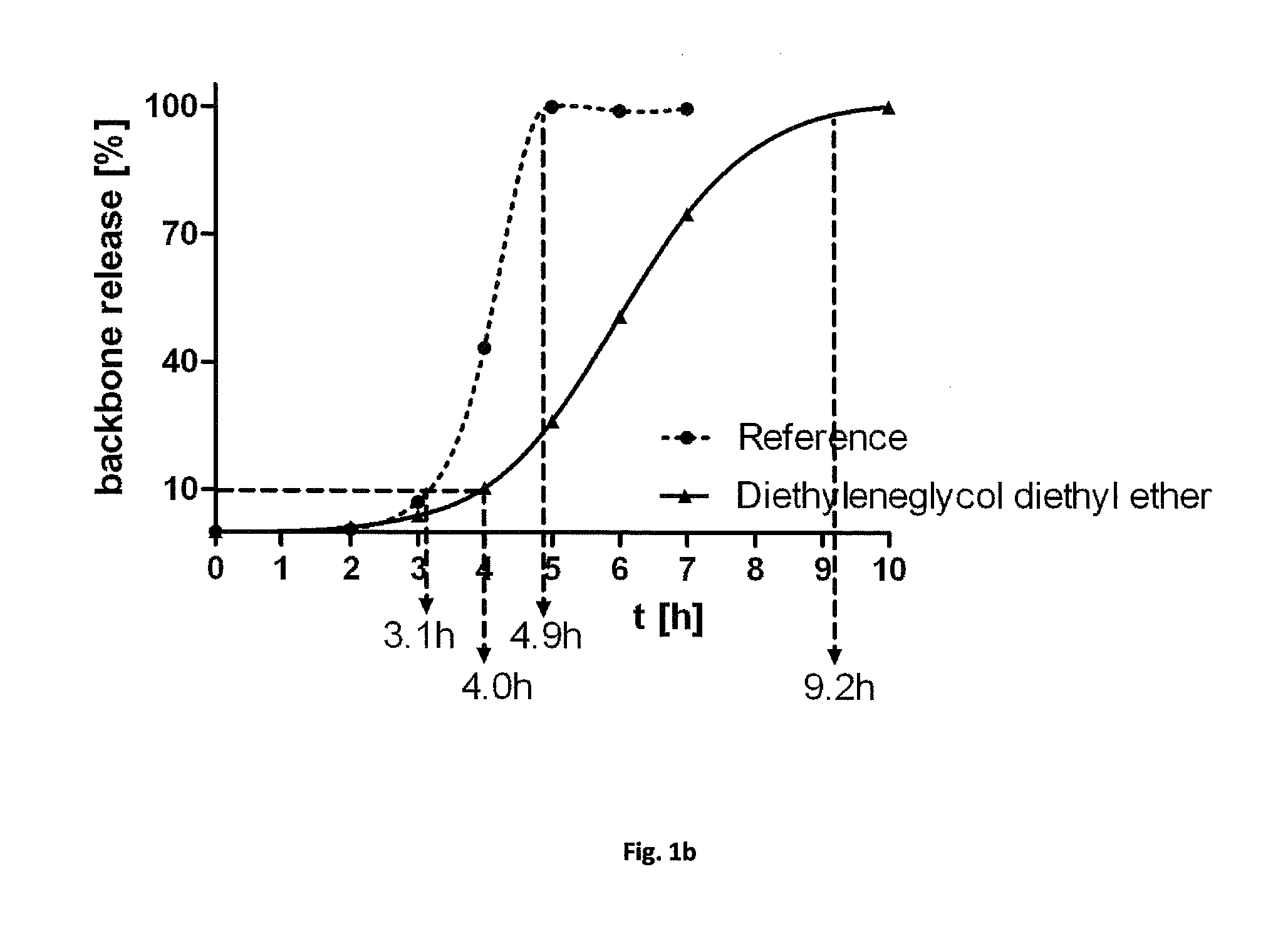
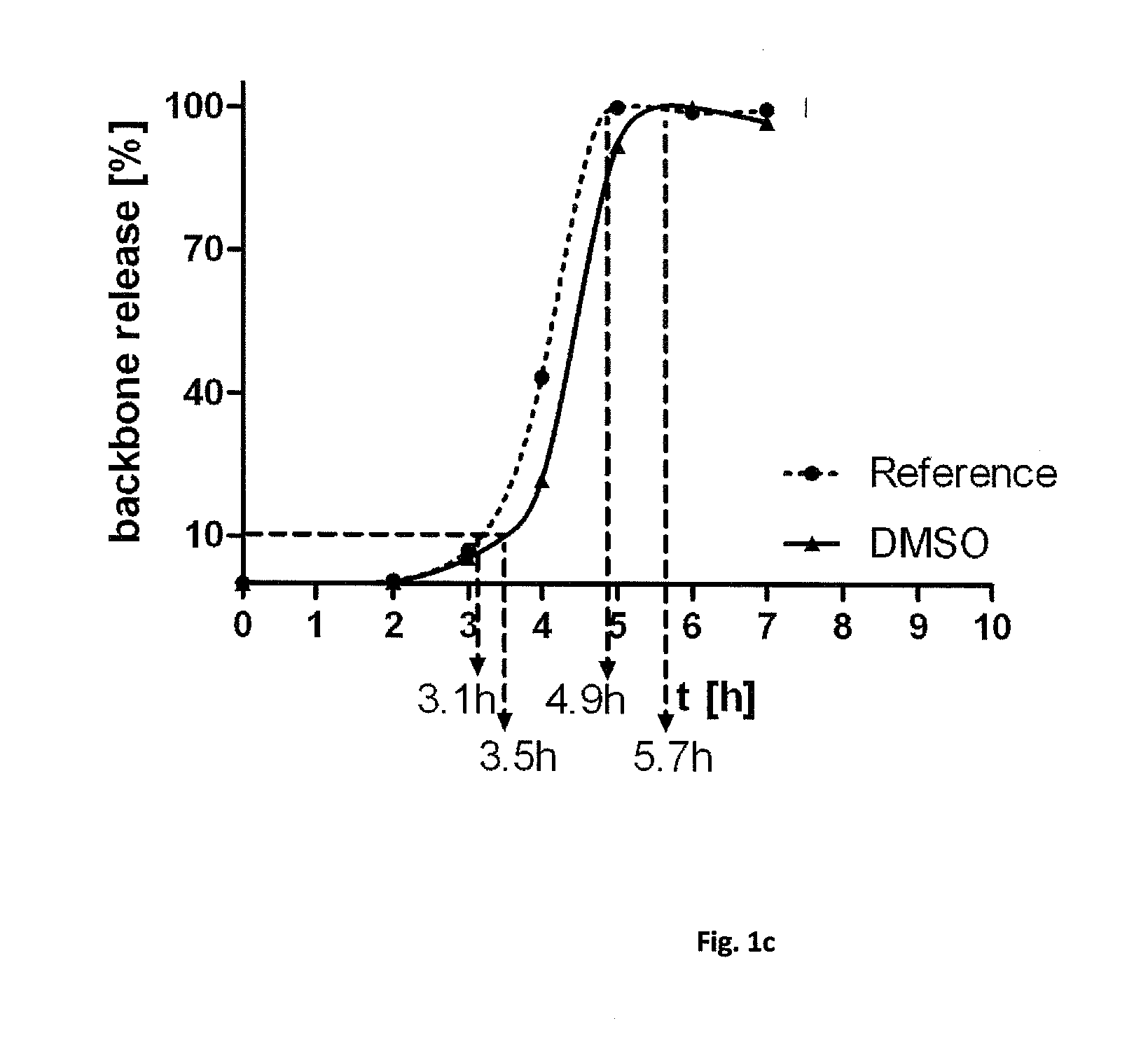
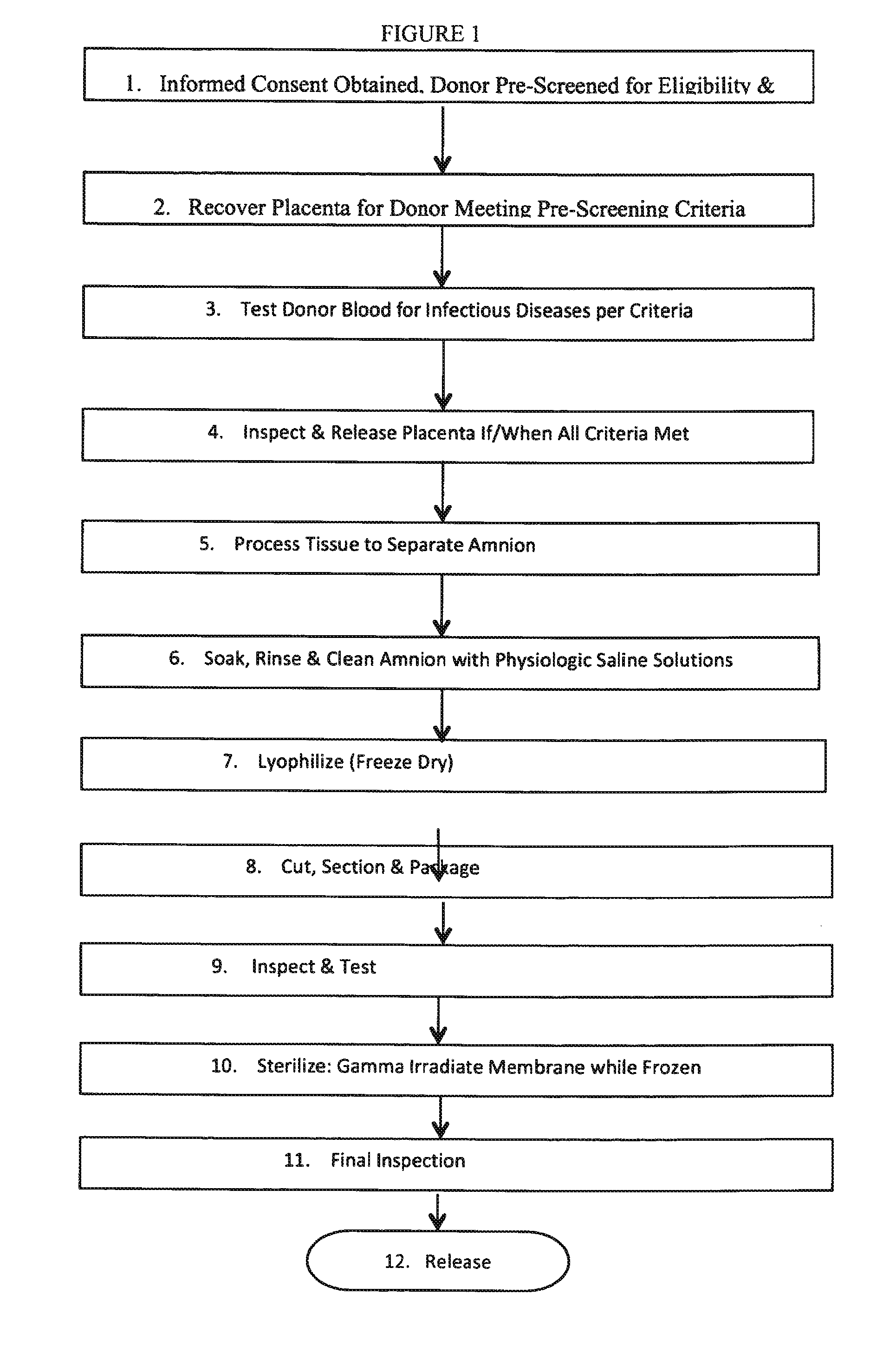
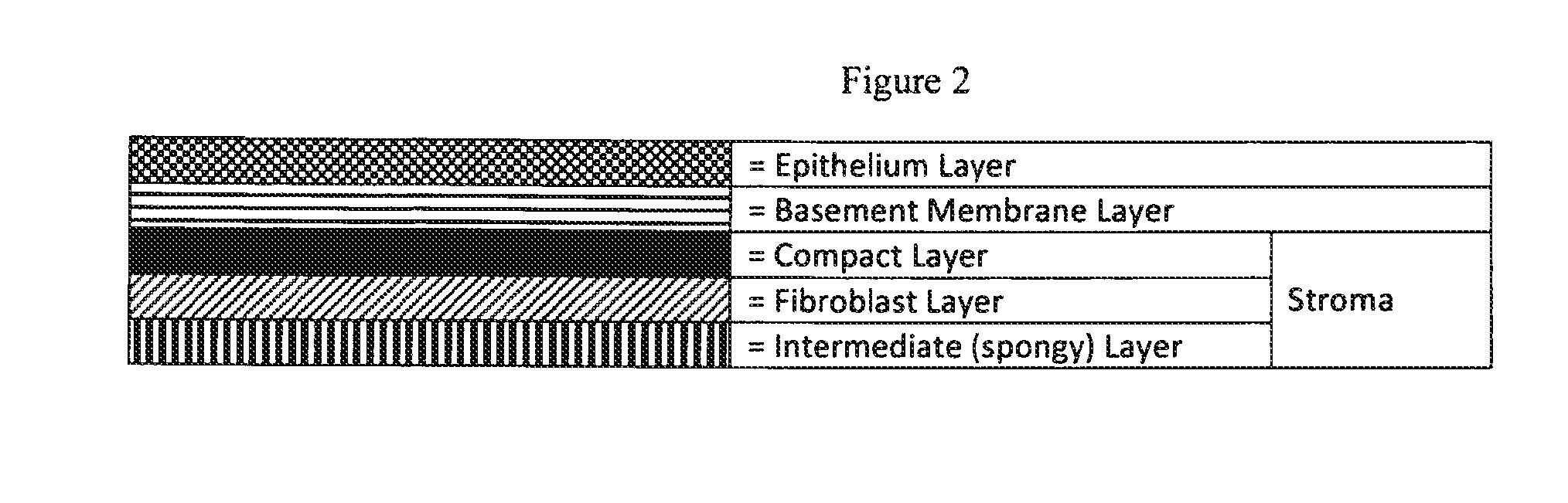
Human Amniotic Membrane Lyophilized Grafts
InactiveUS20140186461A1Retain biological propertiesNeed for refrigerationMammal material medical ingredientsEmbryonic cellsBiological propertyGamma irradiation
Described herein are human amniotic membrane tissue grafts derived from the placenta. The grafts are composed of three layers as seen in the amniotic membrane in utero. These grafts are processed using physiologic solutions, lyophilized and terminal sterilized (via gamma irradiation in a frozen state) that thereby preserves the graft in such a manner as to retain the naturally occurring biological properties of the amniotic membrane and offer a sterile graft for transplantation. By dehydration via lyophilization and terminal sterilization, the graft has the advantage of storage at ambient temperatures for prolonged periods of time prior to transplantation.
Owner:ALPHA TISSUE
Method for terminal sterilization of transdermal delivery devices
InactiveUS20060275170A1Reduce moisture contentLot of radiationEnergy modified materialsMicroneedlesMedicineStratum corneum
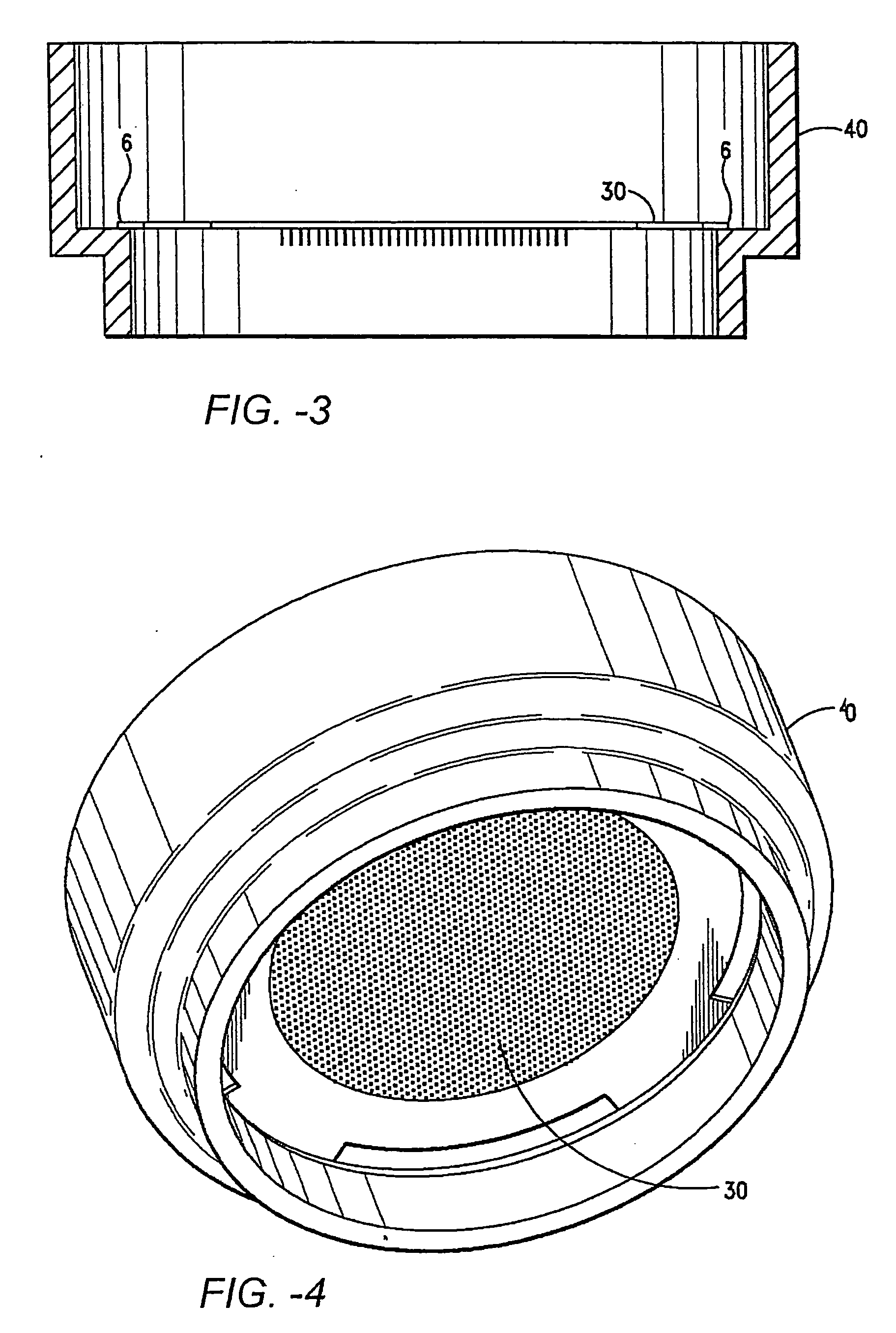
An method and system for providing a terminally sterilized transdermal device adapted to delivery a PTH-based agent. A microprojection member that includes a plurality of stratum comeum-piercing microprojections is coated with PTH-based agent formulation an exposed to sufficient radiation to sterilize the microprojection member while retaining sufficient activity of the PTH-based agent. Preferably, the microprojection member is sealed in packing with an inert atmosphere and reduced moisture. The sterilizing radiation can be gamma radiation or e-beam, preferably delivered in a dose in the range of approximately 5-50 kGy. Also preferably, the irradiation is performed at −78.5-25° C. In preferred embodiments, the radiation is delivered at a rate greater than 3.0 kGy / hr.
Owner:ALZA CORP
Terminal sterilization of injectable collagen products
ActiveUS7902145B2Bioreactor/fermenter combinationsBiological substance pretreatmentsFluenceVolumetric Mass Density
Methods of sterilizing dermal fillers and injectable collagen material have been developed which reduce the level of active biological contaminants or pathogens without adversely affecting the material, i.e., wherein the dermal fillers and injectable collagen material retain their same properties before and after its terminal sterilization. In one embodiment the method for sterilizing the dermal filler or injectable collagen material that is sensitive to radiation contains the steps of protecting the filler or material from radiation, and irradiating the filler or material with a suitable dose of radiation for a time and at a rate effective to sterilize the filler or injectable material. In a preferred embodiment the method for sterilizing the dermal filler or injectable collagen material that is sensitive to radiation includes the steps of a) freezing the filler or material at a temperature below its freezing temperature, which is generally below 0° C. and b) irradiating the filler or material with a suitable dose of radiation at an effective rate for a time effective to sterilize the filler or material. The exposure of the radiation differs depending upon the density of the filler or material, but is preferably between 5 kGy and 12 kGy and more preferably between 6 kGy and 8 kGy. These doses result in a sterility assurance level (SAL) of 10−6 SAL for the filler or material.
Owner:MAM HLDG OF WEST FLORIDA L L C
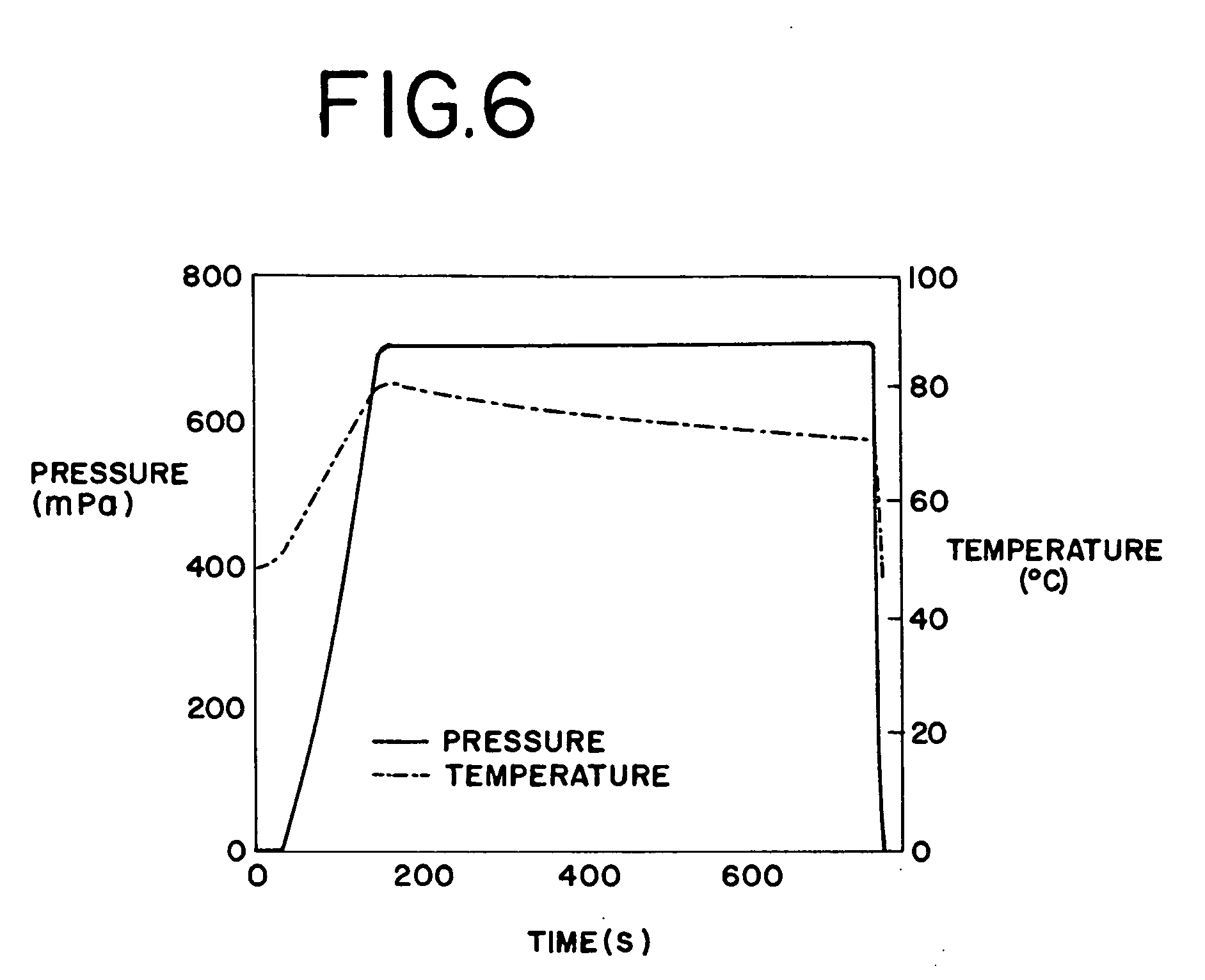
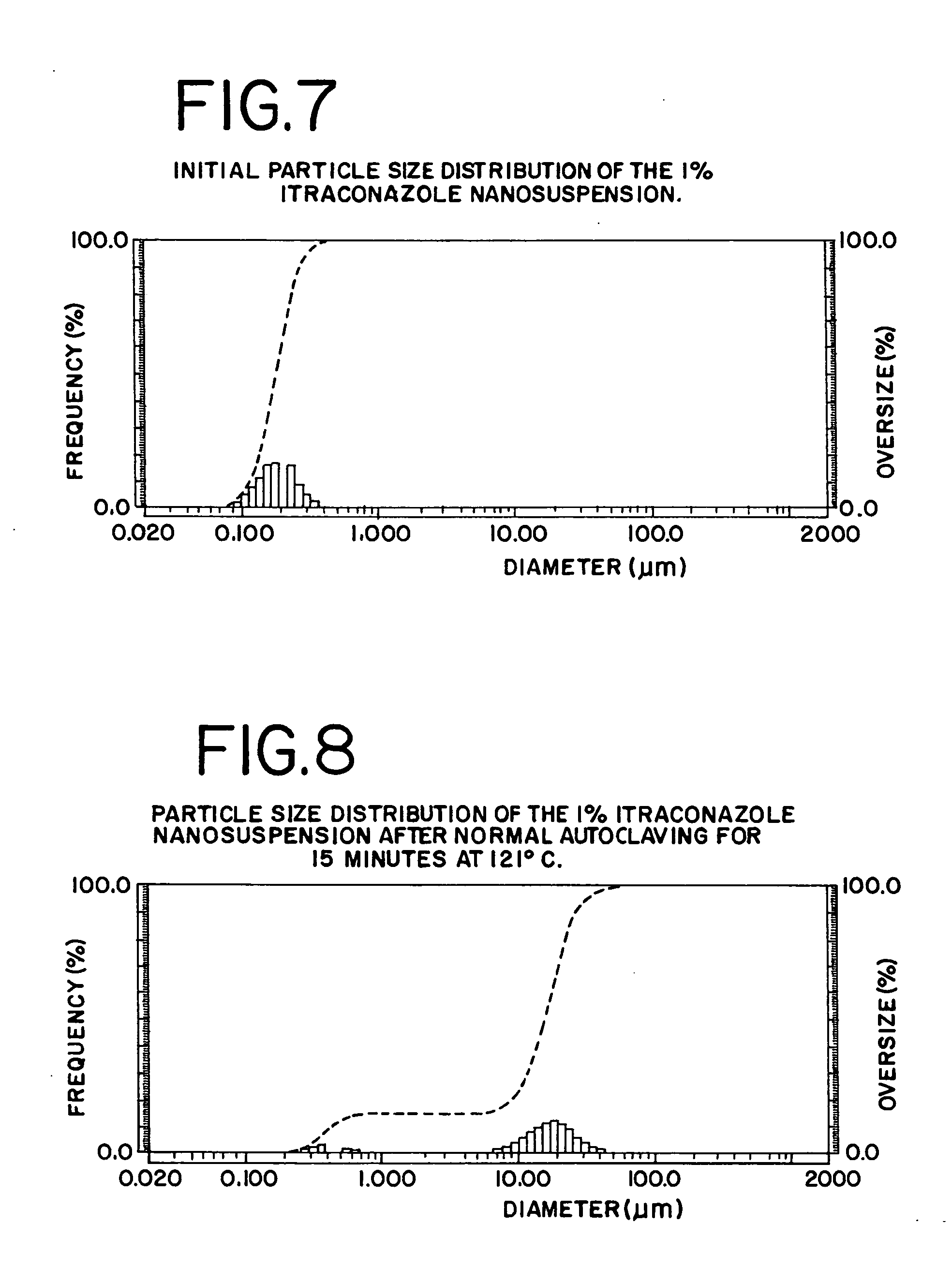
High-pressure sterilization to terminally sterilize pharmaceutical preparations and medical products
ActiveUS20050135963A1Minimize degradationPackage sterilisationMedical devicesMedical productHigh pressure
The present invention provides a process for sterilizing a system, preferably a pharmaceutical preparation such as a dispersion of small particles or droplets of a pharmaceutically active compound using high pressure terminal sterilization techniques and products therefrom.
Owner:BAXTER INT INC +1
Method for terminal sterilization of transdermal delivery devices
InactiveUS20060280645A1Reduce moistureReduce moisture contentPeptide/protein ingredientsMicroneedlesStratum corneumNuclear medicine
A method and system for providing a terminally sterilized transdermal natriuretic peptide delivery device. A microprojection member having a plurality of stratum corneum-piercing microprojections is coated with a natriuretic peptide- formulation an exposed to sufficient radiation to sterilize the microprojection member while retaining sufficient activity of the natriuretic peptide. Preferably, the microprojection member is sealed in packing with an inert atmosphere and reduced moisture. The sterilizing radiation can be gamma radiation or e-beam, preferably delivered in a dose in the range of approximately 14-21 kGy. Also preferably, the irradiation is performed at −78.5-25° C. In preferred embodiments, the radiation is delivered at a rate greater than 3.0 kGy / hr.
Owner:ALZA CORP
Formulation of l-cysteine hydrochloride amenable to terminal sterilization
InactiveUS20190247307A1Amenable to terminal sterilizationOrganic active ingredientsInorganic non-active ingredientsCysteine HydrochloridePharmacology
Stable formulation of L-cysteine hydrochloride amenable to terminal sterilization; a terminally sterilized stable formulation of L-cysteine hydrochloride; a process of manufacturing a stable formulation of L-cysteine hydrochloride amenable to terminal sterilization; and a container comprising the formulations of the invention.
Owner:AL PHARMA INC
Preparation method of carboxymethyl chitin critical hydrogel
ActiveCN106474569AStay stickyStay flexibleSurgeryPharmaceutical delivery mechanismViscous modulusBiocompatibility Testing
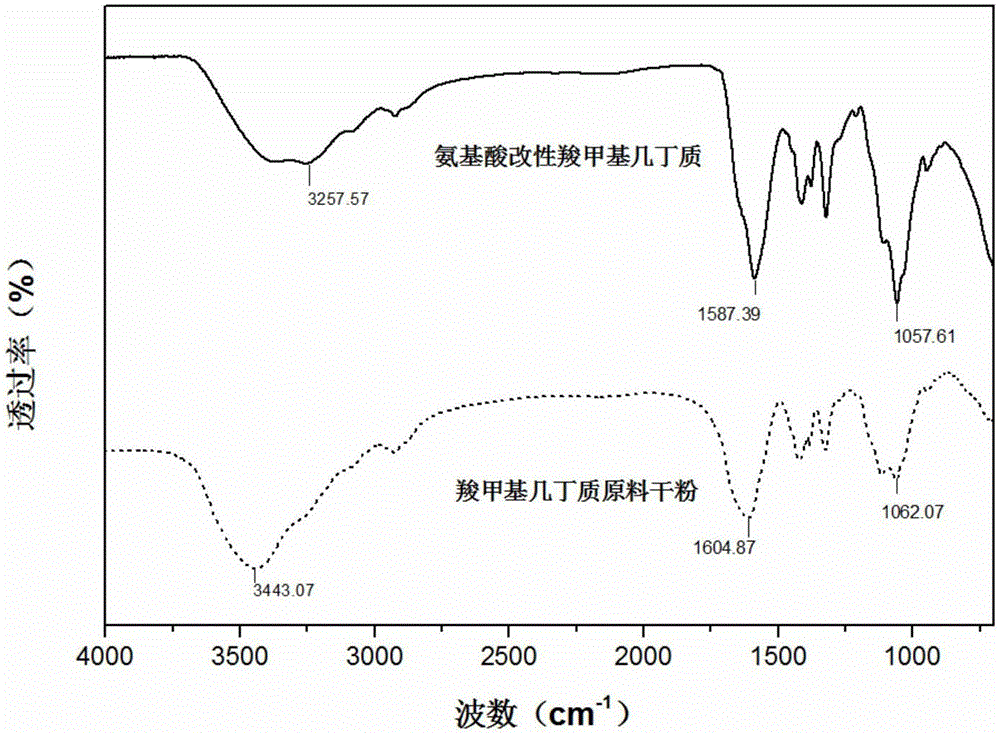
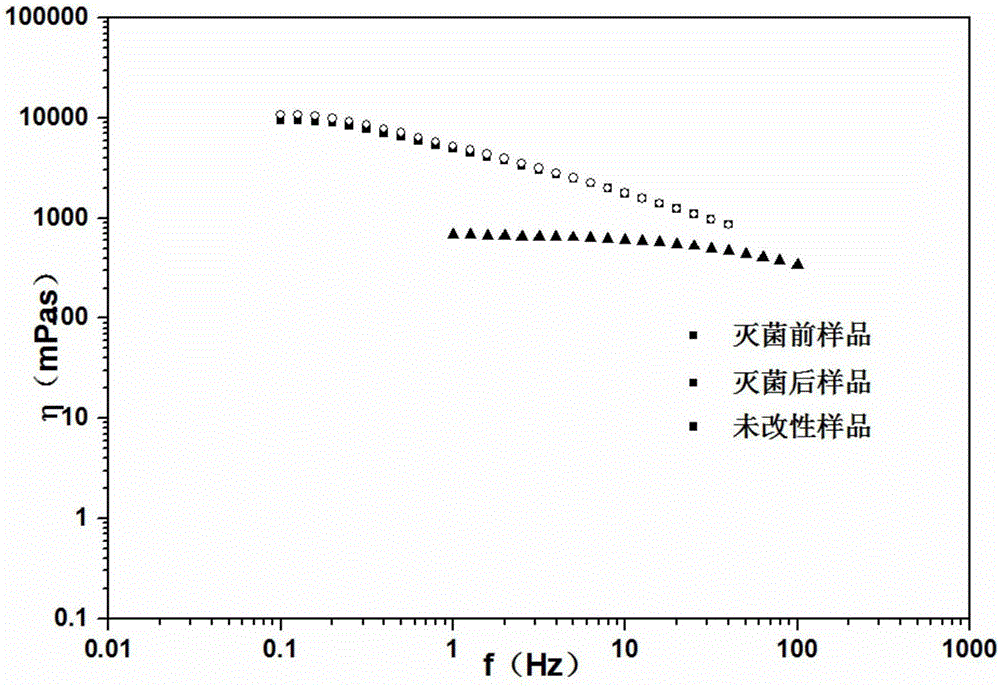
The invention provides a preparation method of a completely new gel form, namely a critical gel, wherein with carboxymethyl chitin as a raw material and natural and non-toxic amino acid, namely lysine, as a modifying substance, the critical gel is prepared under the catalytic action of a coupling reagent, namely 4-(4,6-dimethoxytriazin-2-yl)-4-methyl morpholine hydrochloride (DMTMM). The gel has the main characteristic that the gel can constantly keep viscous modulus and elasticity modulus equal, which is to say the gel possesses two properties, namely high viscosity and high elasticity. The modified gel, which is under a 'critical state', can achieve terminal sterilization, prolong an in vivo residue time and achieve single-dose injection; with the application of the non-toxic amino acid modifying substance, the intrinsic biocompatibility and bio-activity of the carboxymethyl chitin are reserved, and all potential risks are avoided; since the viscous modulus and the elasticity modulus are simultaneously increased and are constantly kept equal, the viscoelasticity of the carboxymethyl chitin is enhanced; and meanwhile, the original liquid state of the carboxymethyl chitin is kept, so that pain in injection can be relieved easily.
Owner:SHANGHAI QISHENG BIOLOGICAL PREPARATION CO LTD
Cyclic adenosine meglumine injection and preparation method thereof
InactiveCN102283804AImprove solubilityImprove stabilityOrganic active ingredientsPharmaceutical delivery mechanismSolubilityFiltration
The invention relates to an adenosine monophosphate meglumine injection and a preparation method thereof. The preparation process is to take an appropriate amount of water for injection, add sodium chloride, cyclic adenosine monophosphate, and meglumine, stir to dissolve completely, add 0.05-0.2% (W / V) activated carbon for needles by volume, and stir for 15-30 minutes. Filter to remove carbon, add water for injection to nearly full amount, use phosphate buffer to adjust the pH value to between 6.0 and 6.5, add water for injection to the full amount, and check that the semi-finished product is qualified, filter, potting (full nitrogen gas in the whole process), and extinguish Bacteria, light inspection, packaging. The method has the advantages of selecting appropriate solvents and additives, improving the solubility and stability of meglumine cyclic adenosine monophosphate, and adopting a terminal sterilization preparation method to effectively ensure the sterility assurance level of the drug. The invention has the characteristics of formula composition, simple process, low production cost, strong drug stability and safety, and the like.
Owner:SHANDONG FANGMING PHARMACEUTICAL CO LTD
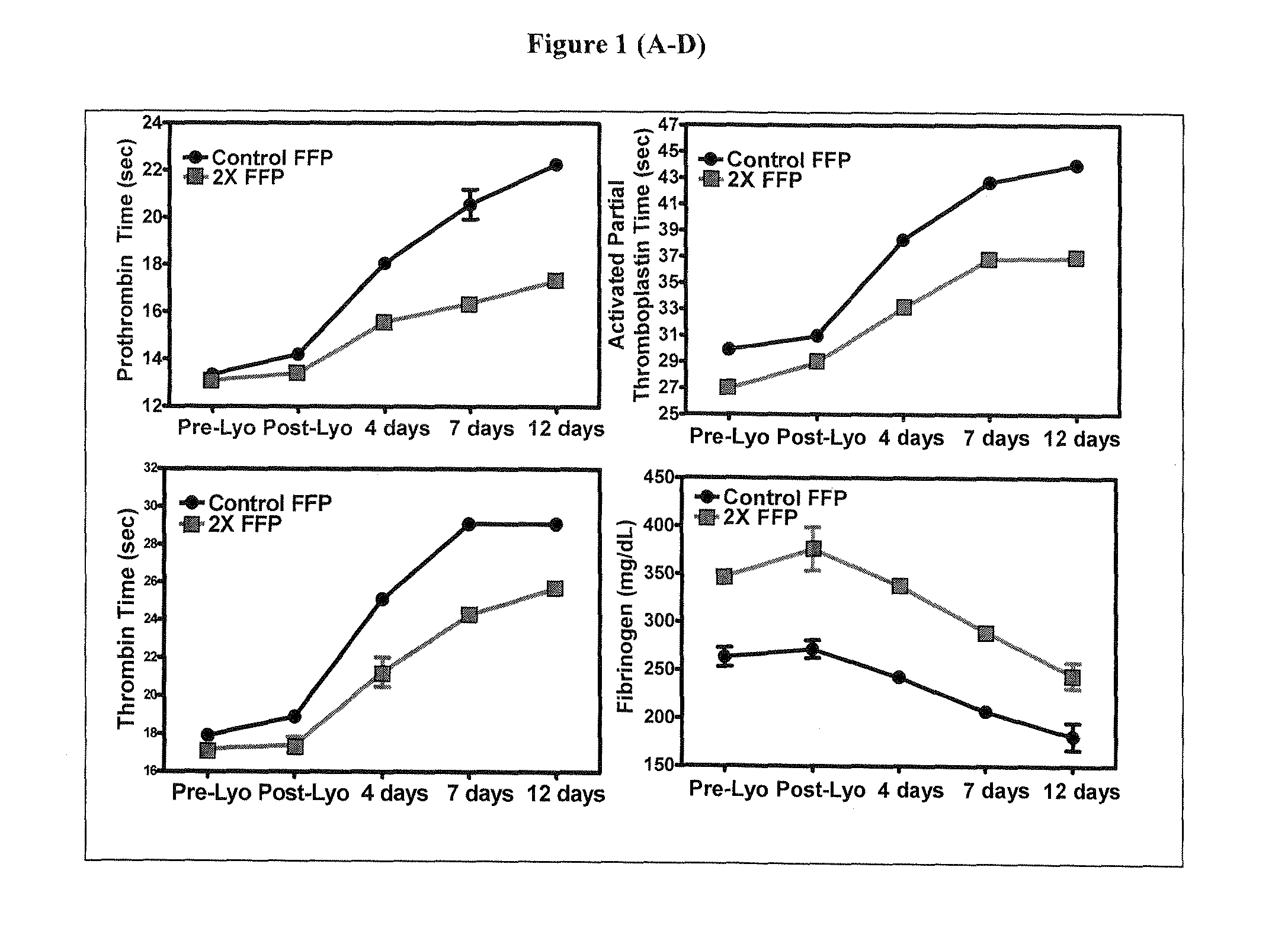
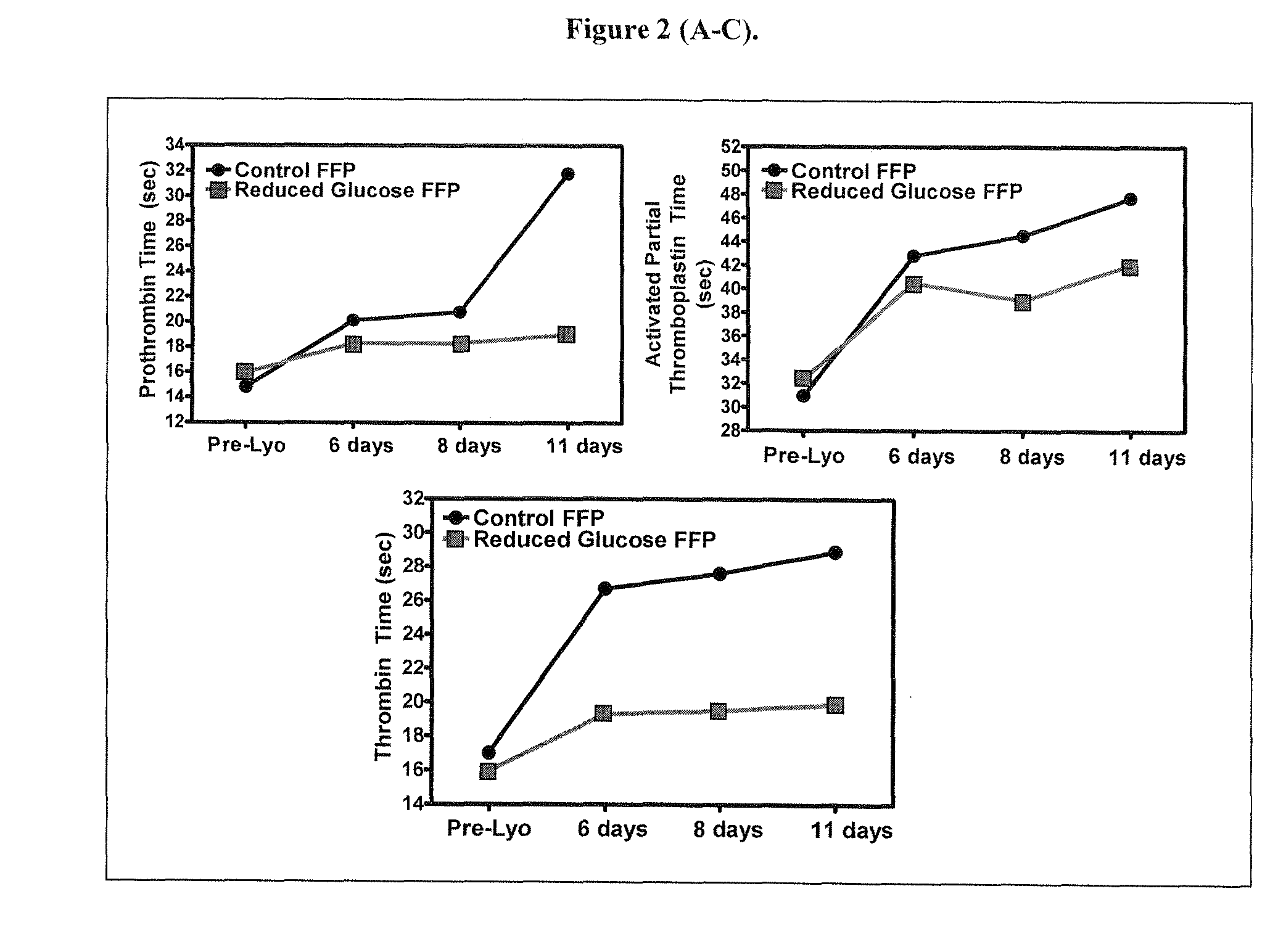
Freeze-dried plasma formats for the trauma care field
InactiveUS20100233671A1Increasing coagulation factor stabilityHigh protein concentrationDipeptide ingredientsMammal material medical ingredientsDipeptideFreeze-drying
Disclosed are freeze-dried plasma formats specifically designed for the trauma care field. Blood plasma is subjected to a glucose removal step, a protein fraction up-concentration step and addition of stabilizers prior to freeze-drying. Preferable stabilizers are glutamine dipeptides, glutamine and glycine. The glutamine based formulation is added direct to plasma and serves three main purposes: 1) Increases stability of plasma proteins and stabilizes pH in freeze-dried state; 2) Increases stability of plasma proteins against Gamma Irradiation and thus allows for the application of a terminal sterilization step; 3) Introduces supplements beneficial to the trauma patient.
Owner:BAKALTCHEVA IRINA B
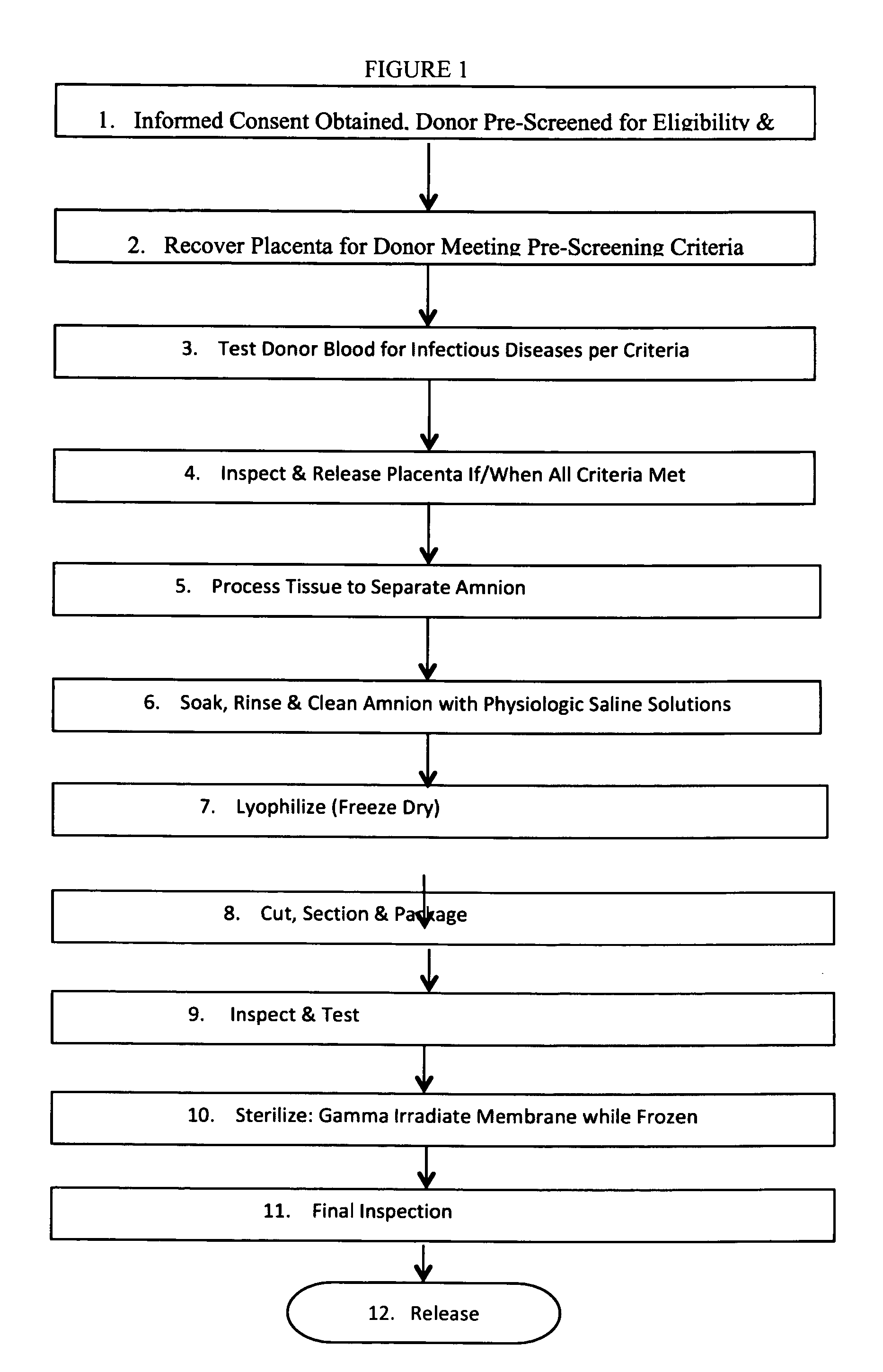
Methods of preparing lyophilized human tissues
Described herein are methods of preparing human amniotic membrane tissue grafts derived from the placenta. The grafts are composed of three layers as seen in the amniotic membrane in utero. These grafts are processed using physiologic solutions, lyophilized and terminal sterilized (via gamma irradiation in a frozen state) that thereby preserves the graft in such a manner as to retain the naturally occurring biological properties of the amniotic membrane and offer a sterile graft for transplantation. By dehydration via lyophilization and terminal sterilization in a frozen state, the graft has the advantage of storage at ambient temperatures for prolonged periods of time prior to transplantation.
Owner:BROUSSARD TERRY W
Sterilizable pharmaceutical package for ophthalmic formulations
PendingUS20200171244A1Reduced stabilityMinimize the numberMedical devicesPharmaceutical delivery mechanismChlorine dioxideOphthalmology
A liquid formulation of an ophthalmic drug in a pre-filled pharmaceutical package, for example a syringe, cartridge, vial or any other vessel made in part or in whole of a thermoplastic polymer, coated on the interior with a tie coating or layer, a barrier coating or layer, a pH protective coating or layer, and optionally a lubricity coating or layer. A blister, a pouch, a bag, a tray or a tub may encompass as a secondary packaging the syringe, vial, cartridge, tube or any other vessel. The package is suitable for sterilization (e.g., surface and / or terminal sterilization) with sterilization gas residuals being minimal and / or lower than required by ISO 10993-7; and / or the stability of the ophthalmic drug is maintained, during a prolonged time period following the sterilization. The sterilization gas may be EO, propylene oxide, chlorine dioxide, nitrogen dioxide, or vaporized hydrogen peroxide (VHP), among others.
Owner:SI02 MEDICAL PRODS
Paclitaxel composition for injection and preparation method thereof
InactiveCN102552123AImprove securityLow content of related substancesOrganic active ingredientsPharmaceutical delivery mechanismPolyoxyethylene castor oilEngineering
The invention provides a paclitaxel composition for injection. The composition comprises the following components in percentage by weight: 0.200-1.087% of paclitaxel, 95.000-99.200% of polyoxyethylenated castor oil and ethanol as well as 0.200-1.500% of organic acid. The paclitaxel composition for injection, provided by the invention, can be directly administrated after being diluted by an infusion solution. By using the paclitaxel composition for injection, provided by the invention, the damage caused by terminal sterilization of the product to the main component is avoided because a sterile production process is adopted in the preparation process; meanwhile, the sterile level of the product is more preferably guaranteed because an polyoxyethylenated castor oil sterilizing process is additionally increased in the process. Compared with the prior art, the paclitaxel composition for injection, provided by the invention, has the advantages of reducing the content of the preparation related substances, improving the product stability, reducing the occurring rate of the adverse reaction and improving the safety in clinical use.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
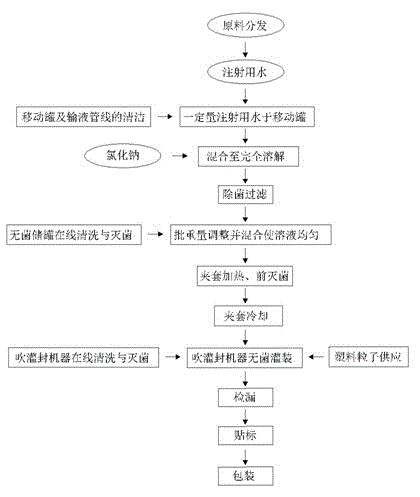
Production technology of sterile oxygen humidification bottles
InactiveCN105129706AGuaranteed to be sterileAvoid infectionRespiratorsBottle-handling machinesProduct inspectionPositive pressure
The invention provides a production technology of sterile oxygen humidification bottles. The production technology comprises the processes: (1), weighing quantitative raw materials and auxiliary materials according to the formula and adding the raw materials and auxiliary materials into a cleaned mobile tank to prepare humidification liquid; (2), after the humidification liquid is filtered, conveying the humidification liquid into a sterile storage tank through a sterile air source arranged on the gas inlet in the top of the mobile tank; (3), adding filtered injection water into the sterile storage tank and diluting the humidification liquid to a metered volume; (4), guiding industrial steam into a jacket of the sterile storage tank, carrying out heating sterilization, conveying the diluted humidification liquid into a blowing, filling and sealing machine after sterile preparation is completed, carrying sterile filling and then carrying out sealing; (5), carrying out leakage detection and finished product inspection on sealed products; (6), carrying out labeling, box packing and encasement. According to the invention, sterilization is carried out before filling of the humidification liquid, blowing, filling and sealing are completed at one station, the whole production process is carried out under protection of sealing, laminar flow and positive pressure, and therefore sterile production of whole products is achieved, and terminal sterilization is not needed.
Owner:ZHONGQI PHARMA IND HENGSHUN ZHONGQI
Method for preparing levofloxacin hydrochloride sodium chloride injection
InactiveCN103479522ASubstance reductionHigh clarityAntibacterial agentsOrganic active ingredientsWater bathsSodium Chloride Injection
The invention provides a method for preparing levofloxacin hydrochloride sodium chloride injection. By researching a liquid medicine preparation method, medicinal charcoal pretreatment and a terminal sterilization process, product quality is ensured by the aid of technologies of a concentrated solution and diluted solution method, charcoal slurry preparation from medicinal charcoal, water bath sterilization and the like, production is facilitated, obtained finished products have higher stability, so that the storage life of the finished products can be prolonged, and clinical use effects are better.
Owner:HAINAN HOTMED TIANYA PHARMA
Terminal Sterilization of Prefilled Containers
InactiveUS20090012466A1Avoid adverse reactionsEffective sterilizationInfusion syringesMedical devicesPolyolefinGamma irradiation
Owner:BECTON DICKINSON & CO
Treatment of bioprosthetic tissues to mitigate post implantation calcification
InactiveUS20050071926A1Lower potentialReduce the temperatureSuture equipmentsPharmaceutical delivery mechanismTreatment resultsMedicine
Bioprosthetic tissues are treated by immersing or otherwise contacting fixed, unfixed or partially fixed tissue with a glutaraldehyde solution that has previously been heat-treated or pH adjusted prior to its contact with the tissue. The prior heat treating or pH adjustment of the glutaraldehyde solution causes its free aldehyde concentration to decrease by about 25% or more, preferably by as much as 50%, and allows a “stabilized” glutaraldehyde solution to be obtained at the desired concentration and pH for an optimal fixation of the tissue at high or low temperature. This treatment results in a decrease in the tissue's propensity to calcify after being implanted within the body of a human or animal patient. The heat-treated or pH adjusted glutaraldehyde solution may, in some cases, also be used as a terminal sterilization solution such that the calcification-decreasing treatment with the previously treated glutaraldehyde and a terminal sterilization may be carried out simultaneously and / or in a single container.
Owner:EDWARDS LIFESCIENCES CORP
Amikacin sulfate injection and preparation method thereof
Owner:SICHUAN KELUN PHARMA RES INST CO LTD
Stable favipiravir injection and preparation method thereof
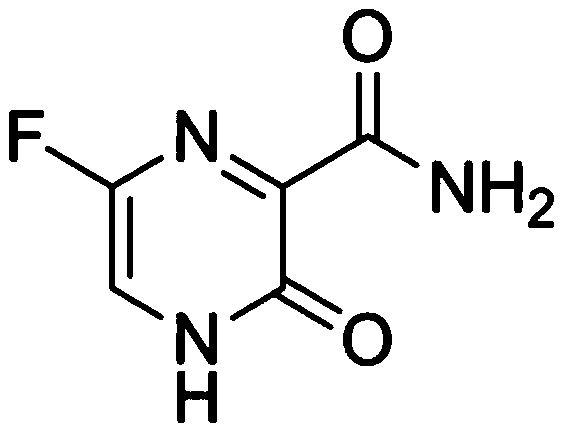
ActiveCN111249229AGood feature propertiesSteady injectionOrganic active ingredientsPharmaceutical delivery mechanismCyclodextrinBiomedical engineering
The invention relates to a stable favipiravir injection and a preparation method thereof. The stable favipiravir injection comprises favipiravir, a solubilizer sulfobutyl ether-beta-cyclodextrin sodium and an acid-base regulator. The stable favipiravir injection can endure terminal sterilization and has good safety and stability.
Owner:FUKANGREN BIO PHARMA
Collagen/glycosaminoglycan compositions for use as terminally sterilizable matrices
InactiveUS20060008905A1Facilitate cross-linkingImprove performanceMechanical working/deformationPaper/cardboard articlesCross-linkMatrix composition
Owner:MATTERN RALPH HEIKO +4
Sterile packaging method for small-volume injection
InactiveCN103057759AGuaranteed chemical propertiesEnsure sterility levelPackage sterilisationBottle-handling machinesBiochemical engineeringFilter system
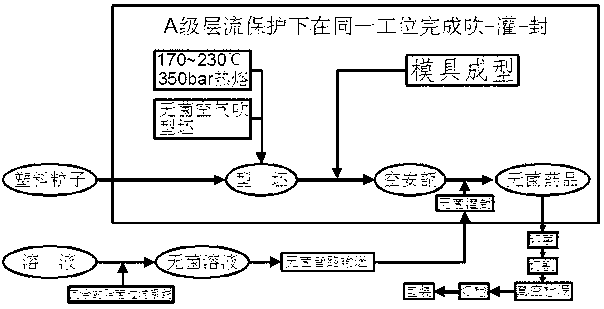
The invention discloses a sterile packaging method for small-volume injection. The sterile packaging method includes steps of (1) weighing injection raw and auxiliary materials under a negative-pressure weighing cover according to the technical proportion, sequentially adding the weighed injection raw and auxiliary materials into a dosing pot which is sterilized in advance, adding injection-used water to dilute and uniformly mix; (2) replenishing injection-used water to the total dosing amount according to the technical proportion by the aid of a weighing system of the dosing pot, adjusting the pH (potential of hydrogen) value of a drug liquor, and filtering and sterilizing the drug liquor through a 0.22-micrometer sterilizing level filter of a redundant filter system; and (3) injecting the filtered sterile drug liquor into an integrated plastic bottle blowing and filling and sealing machine through a sterile pipeline system, and obtaining a finished product after completing bottle blowing, filling and sealing under protection of an A-level laminar flow cover. The sterile packaging method has the advantages that terminal sterilization is omitted, original chemical characteristics of the injection are guaranteed, and further plastic ampoules are used for package, so that no craps are generated during use, embolism of blood vessels is avoided as well, and strong guarantee is provided for safety in clinical use.
Owner:ZHONGQI PHARMA IND HENGSHUN ZHONGQI
Protein A adsorption medium
ActiveCN102698717ASolve the problem that the product cannot be terminally sterilizedOther chemical processesImmune adsorptionOrganic chemistry
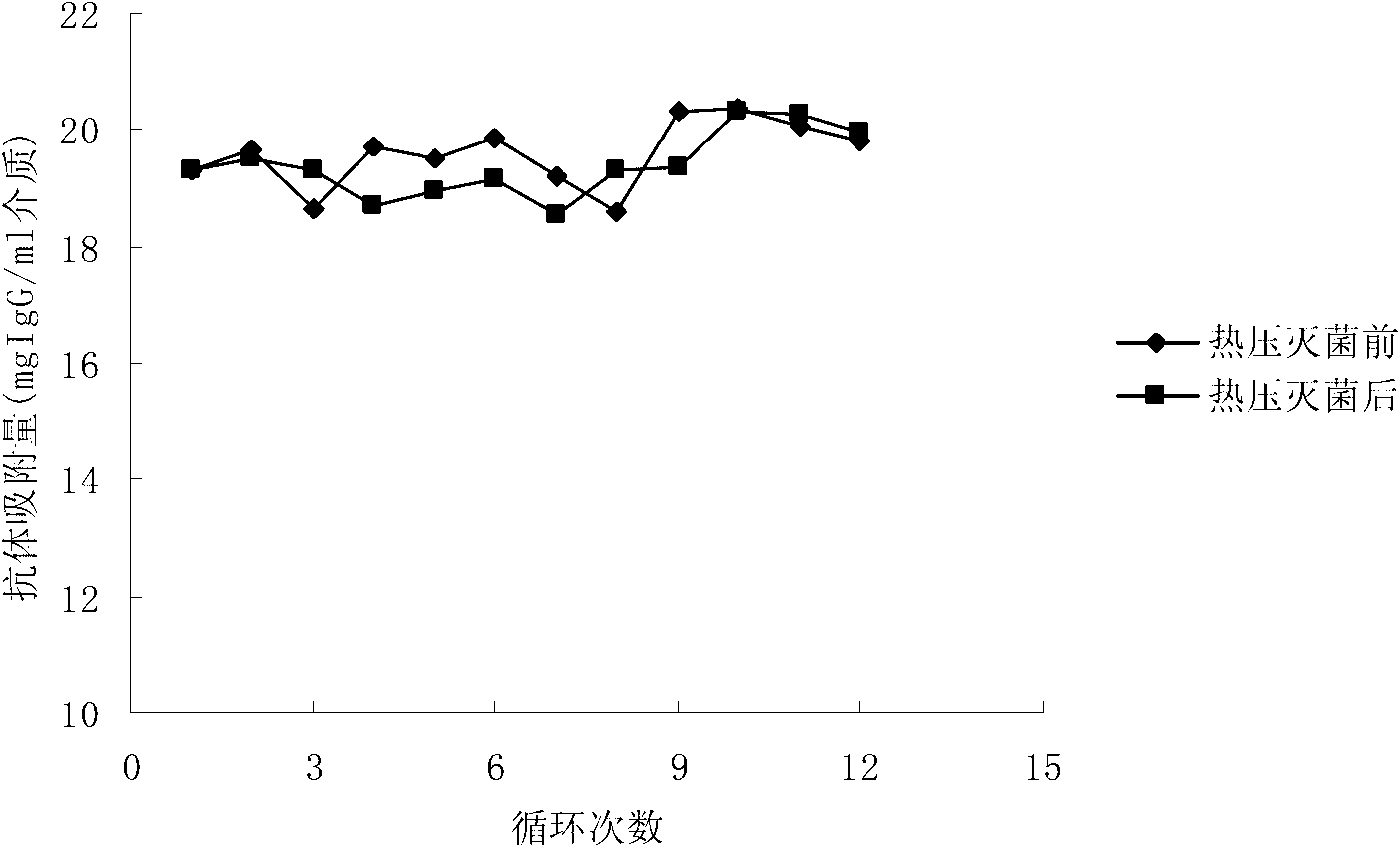
The invention relates to a protein A adsorption medium. The protein A adsorption medium is formed of a solid phase carrier material and a ligand fixed on the carrier by chemical coupling, wherein the ligand is recombinant protein A of an amino acid sequence represented by SEQ ID NO.1 in a sequence table. The protein A adsorption medium is tolerable to 20 minutes of thermal pressure sterilization at the temperature of 121.5 DEG C, so that a problem that a protein A immune adsorption column product is unable to perform final sterilization is resolved.
Owner:GUANGZHOU KONCEN BIOSCI
Ambroxol hydrochloride glucose injection and preparation method thereof
ActiveCN103006548ASimple methodEase of industrial productionOrganic active ingredientsPharmaceutical delivery mechanismMicrofiltration membraneAMBROXOL HYDROCHLORIDE
The invention discloses an ambroxol hydrochloride glucose injection and a preparation method thereof. The ambroxol hydrochloride glucose injection comprises the following components: ambroxol hydrochloride, glucose, water for injection, and a pH regulator. The preparation method for the injection comprises the following steps of: getting the water for injection, adding glucose for dissolving, adding active carbon to adsorb and decarburizing, putting filtrate to a diluted solution tank, adding ambroxol hydrochloride and all water for injection, stirring and dissolving, regulating the pH value of the solution, filtering the solution through a microfiltration membrane until the solution is clear, filling the clear solution, capping the filled solution and carrying out terminal sterilization; or weighing and adding glucose and ambroxol hydrochloride to a preparation tank; adding all water for injection, stirring and dissolving, regulating the pH value of the solution to a determined range, filtering the solution until the medical solution is clear after that the pH value of the solution and the content of the ambroxol hydrochloride and glucose are qualified, filling, capping and carrying out terminal sterilization to the clear solution to obtain the ambroxol hydrochloride glucose injection. The ambroxol hydrochloride glucose injection has good stability, medication safety and effectiveness and a simple process, and is applicable to large-scale industrial production.
Owner:SICHUAN KELUN PHARMA RES INST CO LTD
Bag manufacturing filling and sealing machine
ActiveCN104443539AAchieve productionImprove cleanlinessWrapping material feeding apparatusWrapper twisting/gatheringEngineeringMechanical engineering
The invention discloses a bag manufacturing filling and sealing machine. The bag manufacturing filling and sealing machine comprises a rack and further comprises a film supplying mechanism and an annular conveying assembly. The film supplying mechanism is used for conveying films backwards in the vertical state. The annular conveying assembly is provided with a bag manufacturing mechanism, a filling and sealing mechanism and an output mechanism, all of which are arranged in the conveying direction in sequence. The bag manufacturing mechanism is used for manufacturing the films conveyed to the bag manufacturing mechanism in the vertical state into bags with the upward openings. The filling and sealing mechanism is used for filling the bags conveyed to the filling and sealing mechanism with medicament and sealing the bags. The output mechanism is used for outputting the bags. The film supplying mechanism outputs the films to the bag manufacturing mechanism in the vertical state, the bag manufacturing mechanism can manufacture the bags with the upward openings, the openings of the bags face upwards all the time in the later conveying process, the number of mechanisms above the working faces of the bag openings can be reduced to the maximum degree, interference is avoided, the laminar flow of the cleanliness of a working area is ensured, and large-volume bagging and non-terminal sterilization preparation production can be achieved.
Owner:SHINVA MEDICAL INSTR CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com