Treatment of bioprosthetic tissues to mitigate post implantation calcification
a bioprosthetic tissue and calcification technology, applied in the field of biomaterials, can solve the problems of undesirable stiffening or degradation of bioprosthetic materials, calcification of connective tissue proteins (i.e., collagen and elastin) within these materials, and achieve the effect of lessening the potential for untoward or undesirable reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0023] The following examples are provided for the purpose of describing and illustrating a few exemplary embodiments of the invention only. One skilled in the art will recognize that other embodiments of the invention are possible, but are not described in detail here. Thus, these examples are not intended to limit the scope of the invention in any way.
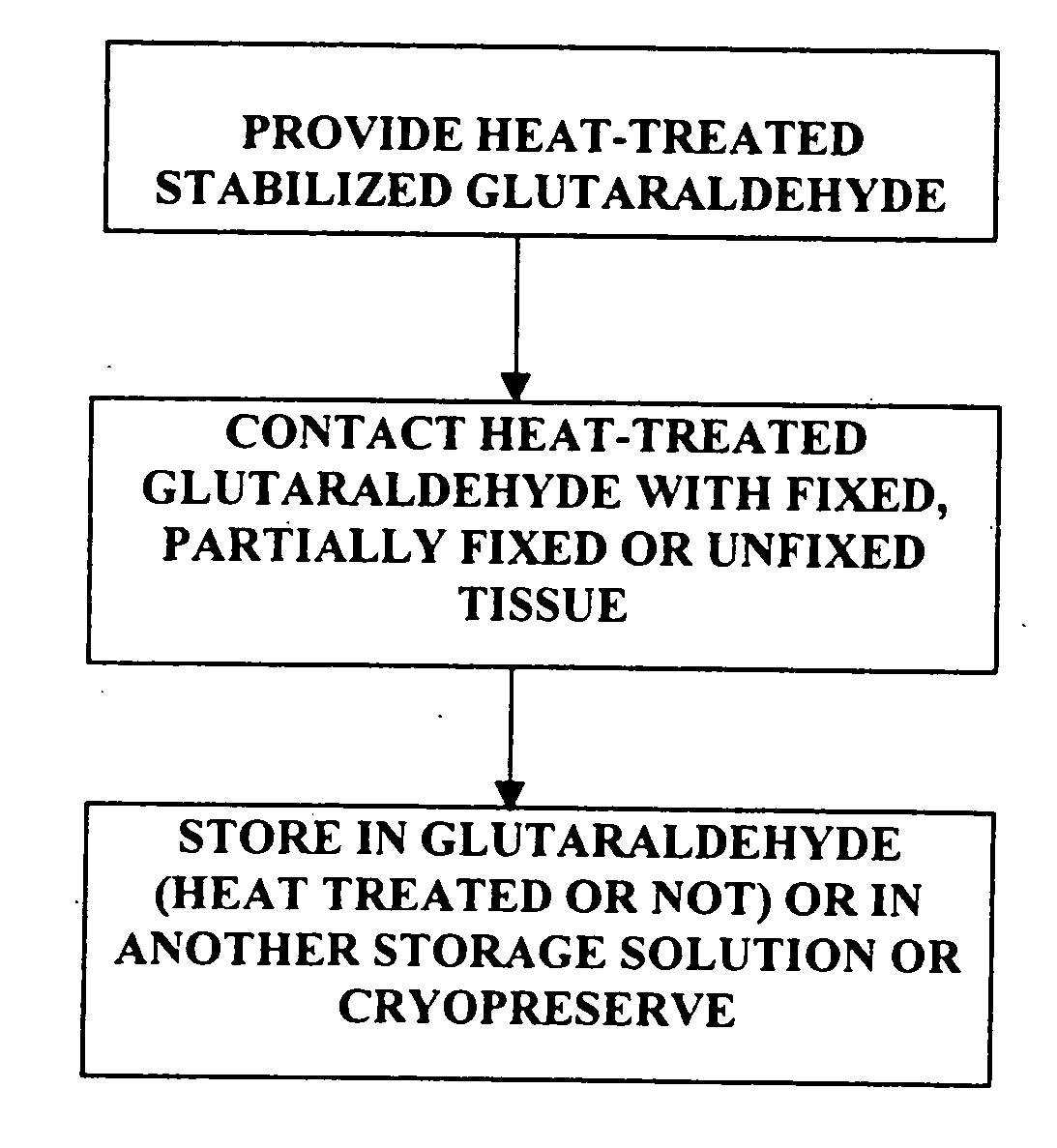
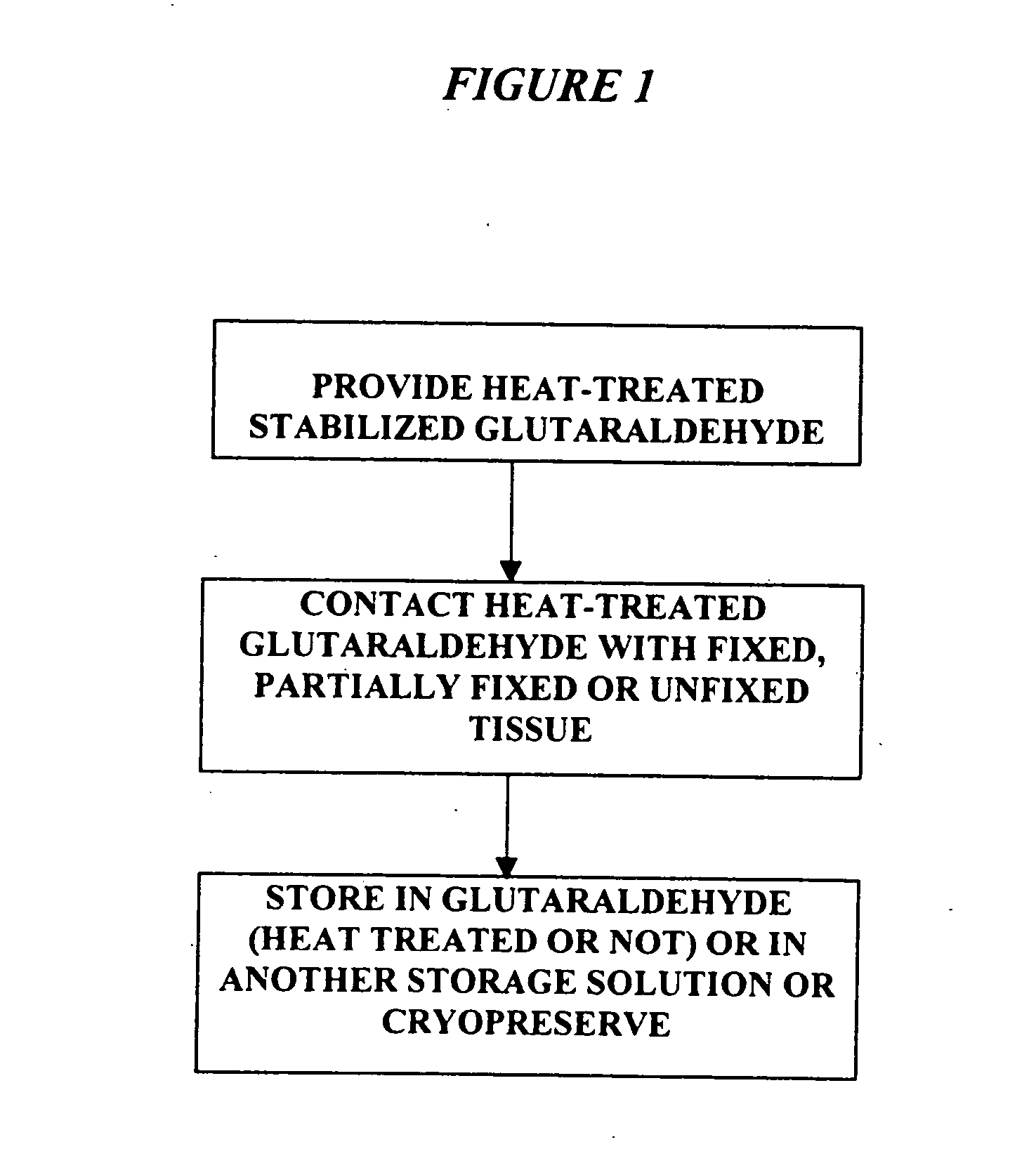
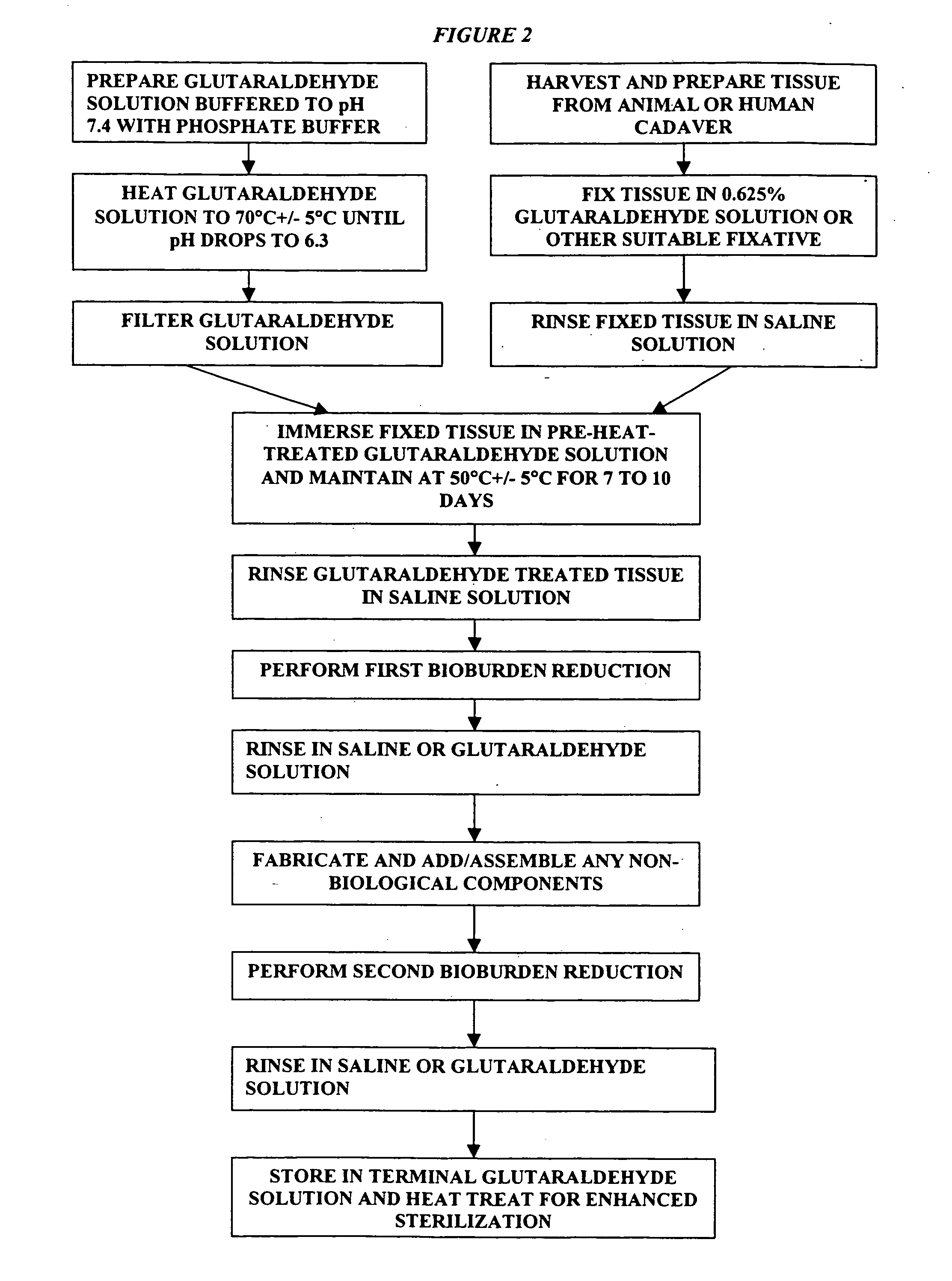
[0024] It has previously been reported that cross-linked bioprosthetic tissue post-treated in 0.625% glutaraldehyde phosphate solution for 2 months at 50° C., with fluid movement (e.g., shaking), exhibited less calcification in the rat subcutaneous and rabbit intramuscular implant models than control cross-linked bioprosthetic tissue fixed in 0.625% glutaraldehyde phosphate solution under typical conditions (i.e., room temperature for 1-14 days). See 66 Ann. Thoracic Surgery 264-6 (1998). Tissues treated under these conditions exhibited a characteristic tan to brown appearance. The heated 0.625% glutaraldehyde phosphate solution als...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com