Sequestered antagonist formulations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
[0236] In Example 2, a substantially non-releasable form of an opioid antagonist (naltrexone HCL) was prepared by coating naltrexone particles with a coating that renders the antagonist substantially non-releasable.
[0237] Naltrexone HCl 2 mg Capsules (Formulation B)
[0238] Formula:
5 Amt / unit Amt / batch Ingredient (mg) (kg) Naltrexone HCl 2.0 0.04 Eudragit RSPO 96.0 1.92 Stearyl Alcohol 22.0 0.44 Dibasic Calcium 6.0 0.12 Phosphate Butylated Hydroxytoluene 1.0 0.02 (BHT) Size #2 Hard Gelatin N / A N / A Capsules Total 127.0 2.54
[0239] Process:
[0240] 1. Milling Pass stearyl alcohol flakes through a mill.
[0241] 2. Blending Mix Naltrexone HCl, Eudragit, milled Stearyl Alcohol, Dibasic Calcium Phosphate and BHT in a twin shell blender.
[0242] 3. Extrusion Continuously feed the blended material into a twin screw extruder and collect the resultant strands on a conveyor.
[0243] 4. Cooling Allow the strands to cool a Conveyor.
[0244] 5. Pelletizing Cut the cooled strands into pellets using a Pelletize...
example 3
Immediate Release Naltrexone HCl 0.5 mg Tablets
[0257] Formula:
8 Amt / unit Amt / batch Ingredient (mg) (kg) Wet Granulation Naltrexone HCl 0.5 0.1 Plasdone C-30 5.0 1.0 Avicel PH-102 58.0 11.6 Sterile Water for Injection 25.0* 5.0* Dry Mixing Avicel PH-102 58.0 11.6 Cab-O-Sil 0.3 0.06 Ac-Di-Sol 2.5 0.5 Magnesium Stearate 0.7 0.14 Total 125.0 25.0 * Remains as residual moisture only. Not included in total weight.
[0258] Process:
[0259] 1. Solution Preparation Dissolve Naltrexone HCl and Plasdone in Sterile Water for Injection.
[0260] 2. Wet Granulation Granulate Avicel PH-102 with solution as prepared in step 1.
[0261] 3. Drying Dry granulated material from step 2 in a fluid bed dryer.
[0262] 4. Milling Pass dried granulation through a Comil
[0263] 5. Mixing Mix milled granulation with remaining Avicel PH-102, Cab-O-Sil, Ac-Di-Sol, and Magnesium Stearate.
[0264] 6. Compression Compress granulation into tablets using a tablet press.
[0265] Dissolution Method
[0266] 9. Apparatus-USP Type II (Paddle...
example 4
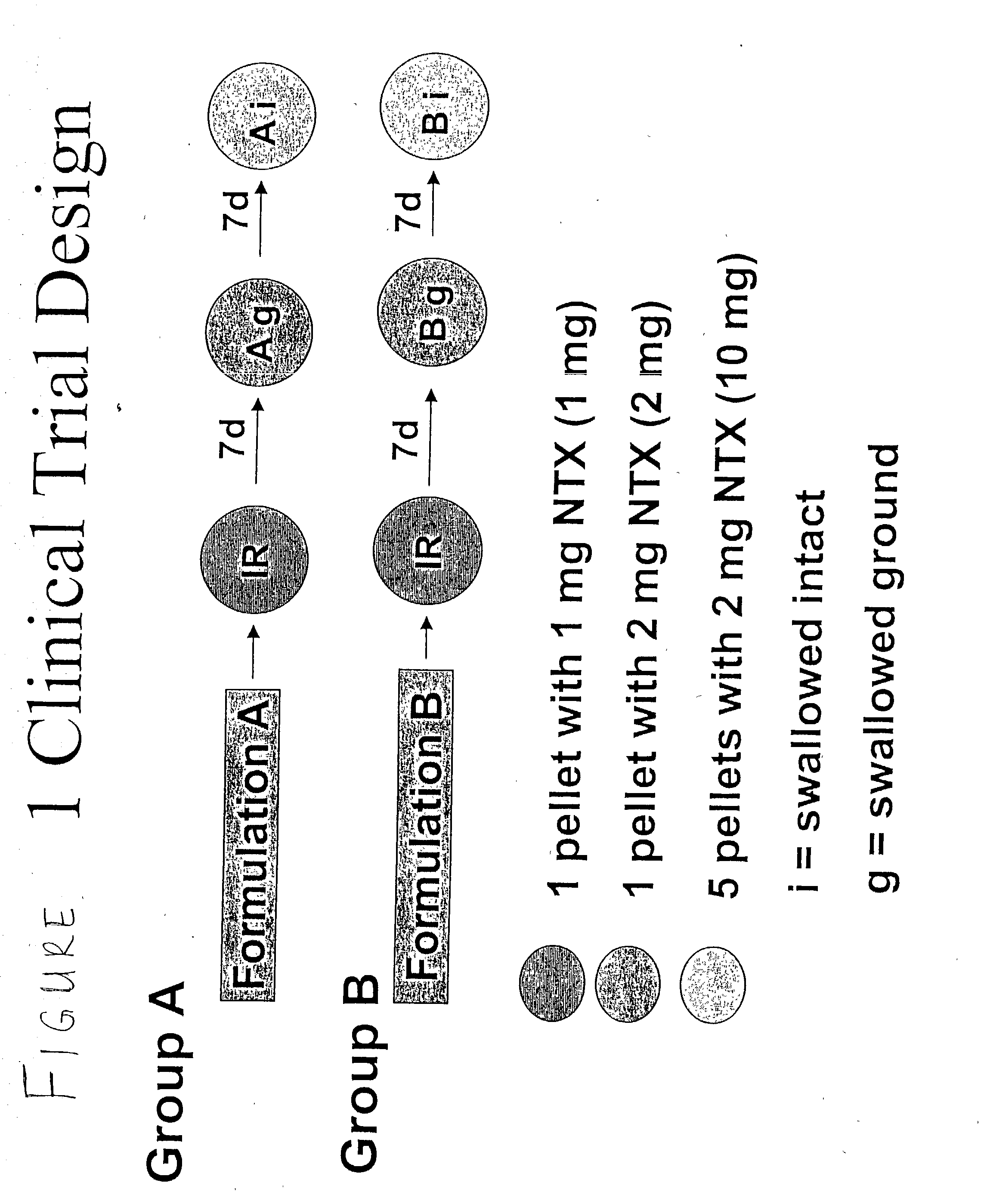
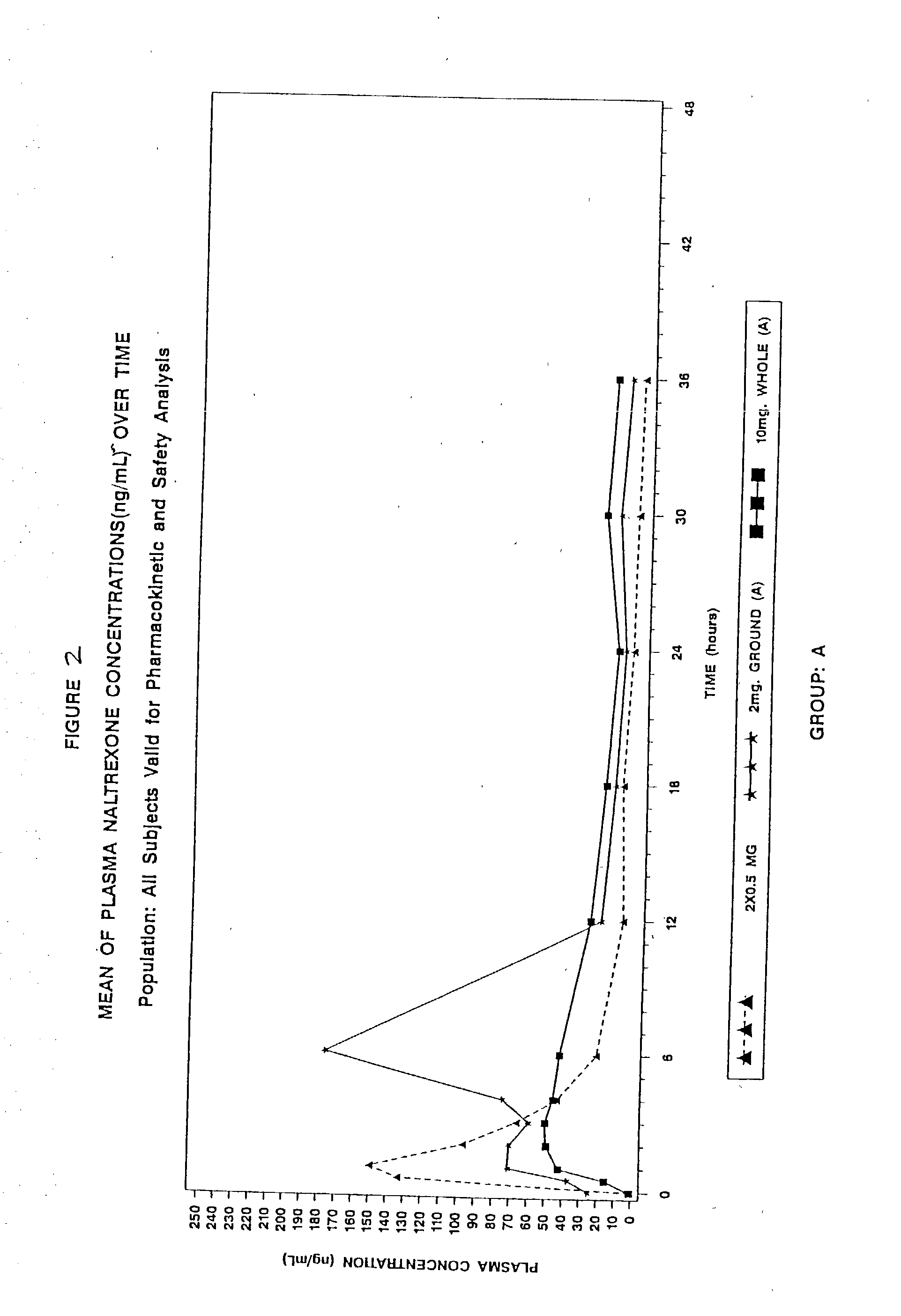
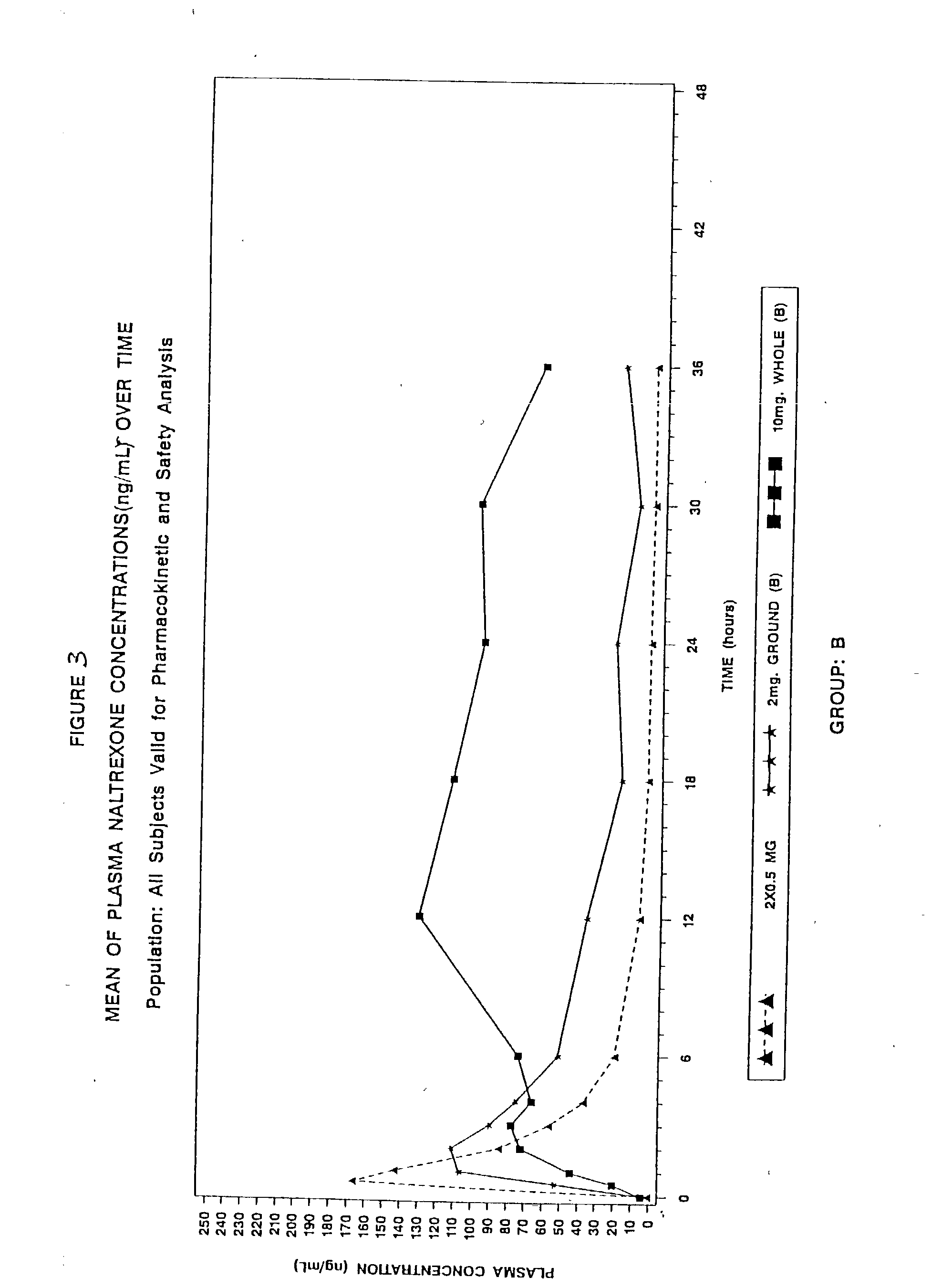
[0271] This was an analytically blind, single dose, five treatments, 3-period crossover, pharmacokinetic study of two naltrexone pellet formulations (Formulation A and Formulation B) swallowed whole or after grinding compared with an immediate-release naltrexone reference swallowed whole in normal healthy subjects. This study assessed the pharmacokinetic (PK) profiles of the two different formulations (Formulation A and Formulation B) of a single dose of one (1) pellet (each containing 2 mg of naltrexone) when ground then swallowed whole and five (5) pellets (each containing 2 mg of naltrexone) when swallowed whole. This study also compared the pharmacokinetics of each formulation administration method to a single dose containing 1 mg of immediate release (IR) naltrexone when swallowed whole.
[0272] FIG. 1 shows the graphical representation of this study, which consisted of two groups (Group A and Group B) with 15 subjects in each group. The treatment order and treatments conditions ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com