Stable favipiravir injection and preparation method thereof
A technology for favipiravir and injection, which is applied to the field of favipiravir injection and its preparation, can solve the problems of difficulty in redissolving sodium salts, poor reconstitution of freeze-dried preparations, and affecting the convenience of clinical use, and achieves good Effects of Feature Properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] 100 prescriptions
[0030]
[0031] Preparation
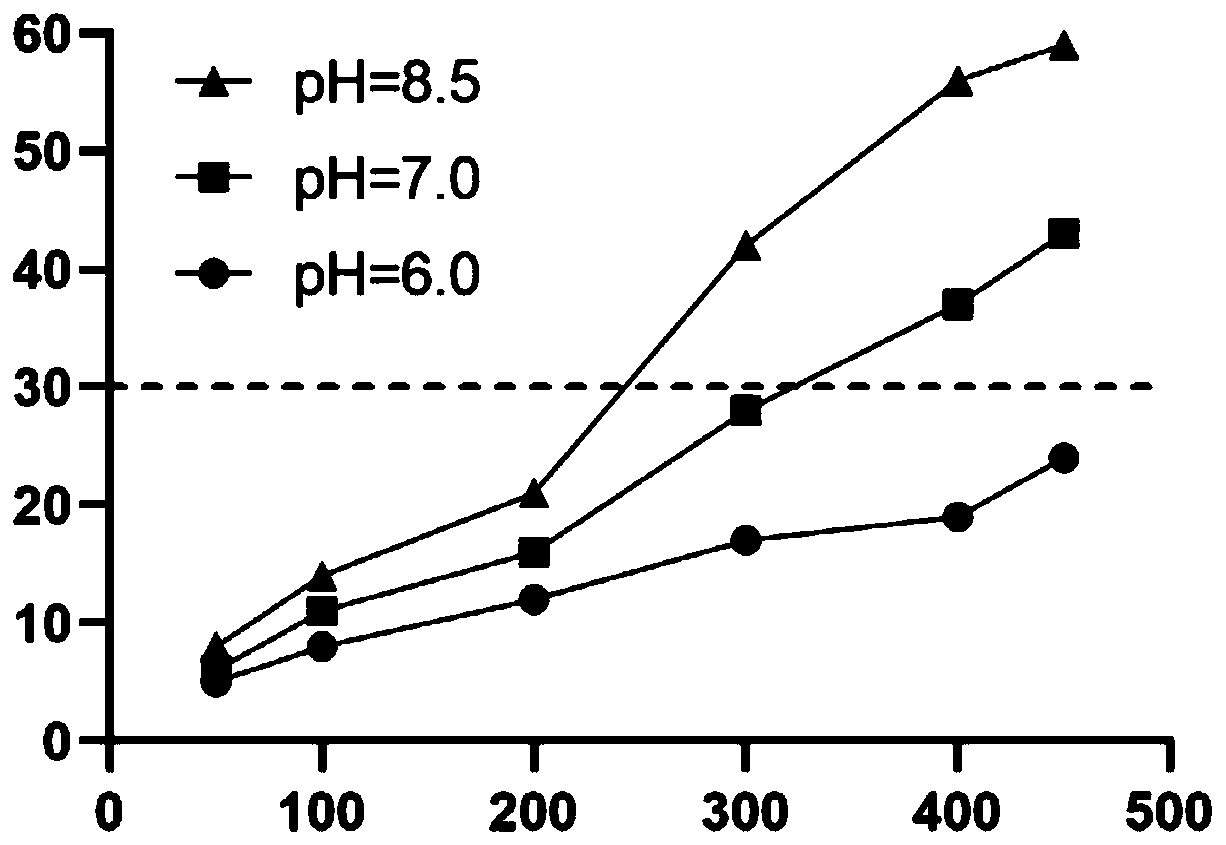
[0032] (1) Take 60% of the prescription amount of water for injection, heat it until the temperature is between 60-70°C, add sulfobutyl ether-β-cyclodextrin sodium, stir to dissolve. Add 1M sodium hydroxide solution to alkalinize the sodium sulfobutyl ether-β-cyclodextrin solution, and measure the pH between 8.5 and 9.5;
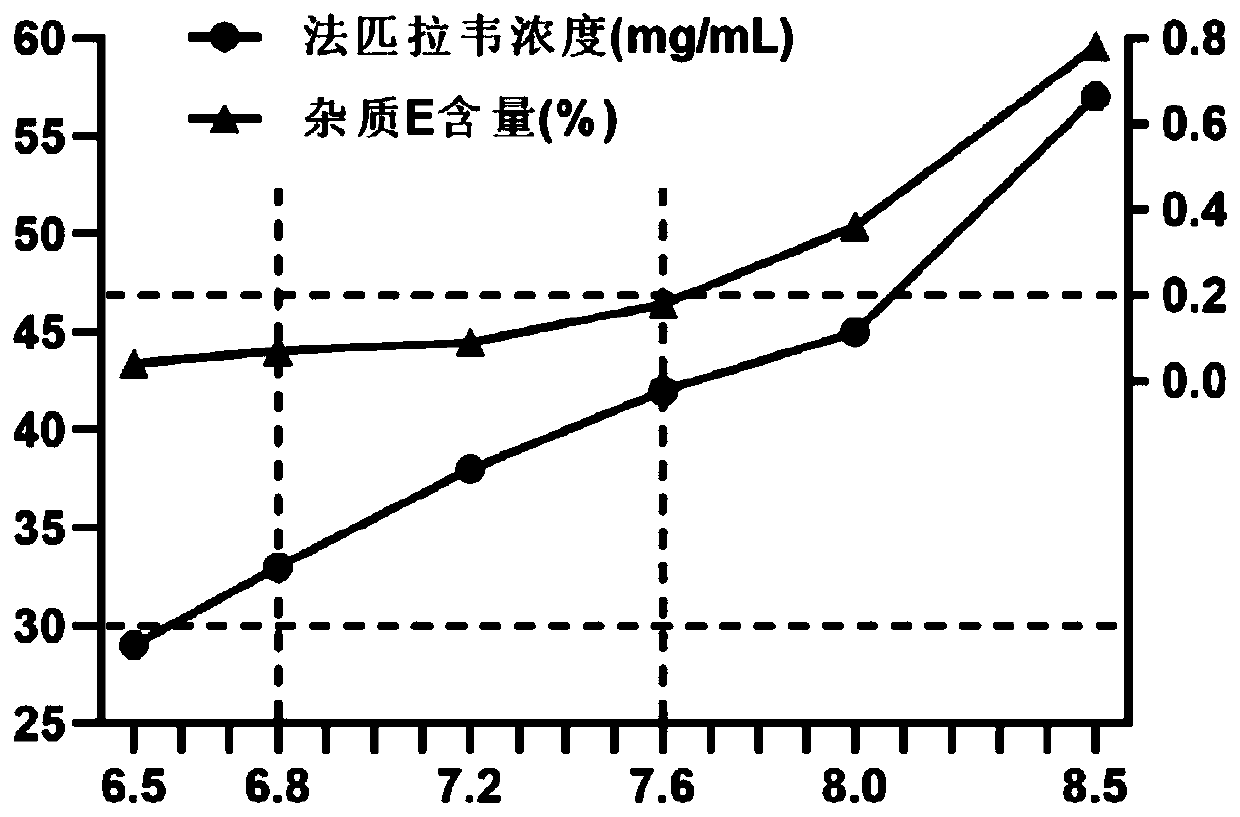
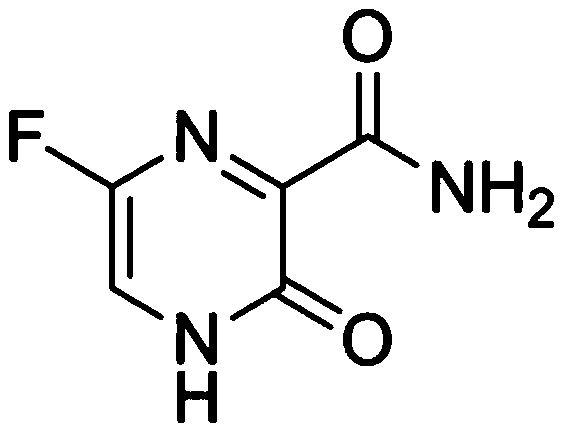
[0033] (2) Add Favipiravir, stir for 0.25 to 0.5 hours to obtain a clear solution, adjust the pH of the solution to between 6.8 and 7.6 with 1M hydrochloric acid or 1M sodium hydroxide solution, preferably pH 7.2, replenish water to full amount, and filter through 0.22 μm membrane filtration;
[0034] (3) The filtrate is filled in ampoules, each with a volume of 10 mL, sealed, terminally sterilized by moist heat (121°C, sterilized for 12 minutes), packaged, and stored.
Embodiment 2
[0036] 100 prescriptions
[0037]
[0038] Preparation
[0039] (1) Take water for injection with 70% of the prescribed amount, heat it until the temperature is between 60-70°C, add sulfobutyl ether-β-cyclodextrin sodium, stir to dissolve. Add 10% ammonia solution to alkalinize the sodium sulfobutyl ether-β-cyclodextrin solution, and measure the pH between 8.5 and 9.5;
[0040] (2) Add Favipiravir, stir for 0.25-0.5h to obtain a clear solution, adjust the pH of the solution to between 6.8 and 7.6 with 1M hydrochloric acid or 10% ammonia solution, preferably pH 7.2, replenish water to full amount, and use a 0.22 μm filter membrane filter;
[0041] (3) Fill the filtrate into 15 or 20mL vials, each with a volume of 10mL, seal it, perform terminal moist heat sterilization (121°C, sterilize for 12min), pack and store.
Embodiment 3
[0043] 100 prescriptions
[0044]
[0045] Preparation
[0046] (1) Take 70% of the prescription amount of water for injection, heat it until the temperature is between 60-70°C, add sulfobutyl ether-β-cyclodextrin sodium, stir to dissolve. Add 1M potassium hydroxide solution to alkalinize the sodium sulfobutyl ether-β-cyclodextrin solution, and measure the pH between 8.5 and 9.5;
[0047] (2) Add Favipiravir, stir for 0.25-0.5h to obtain a clear solution, adjust the pH of the solution to between 6.8 and 7.6 with 1M potassium hydroxide solution or 6M phosphoric acid solution, preferably pH 7.2, replenish water to full amount, 0.22μm Membrane filtration;
[0048](3) Fill the filtrate into 15 or 20mL vials, each with a volume of 10mL, seal it, perform terminal moist heat sterilization (121°C, sterilize for 12min), pack and store.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com