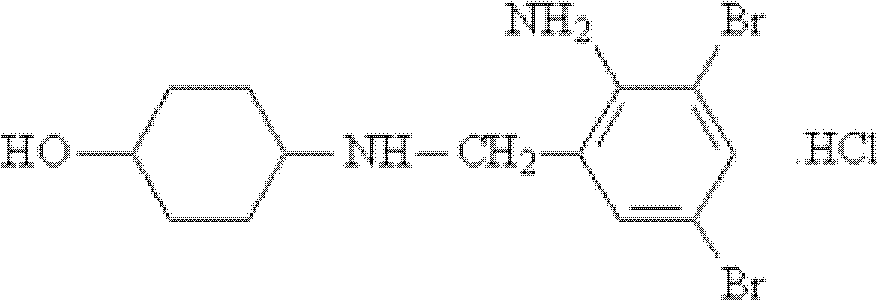Ambroxol hydrochloride glucose injection and preparation method thereof
A technology for glucose injection and ambroxol hydrochloride, which is applied in pharmaceutical formulations, medical preparations with non-active ingredients, and medical preparations containing active ingredients, etc., can solve the problems of decreased content, increased pollution opportunities, poor chemical stability, etc. , to achieve the effect of reducing the level of thermal degradation, improving tolerance and improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0063] Experimental Example 1 Ambroxol Hydrochloride Composition Injection Stability Experiment of the present invention
[0064] 1.1 Stability of Ambroxol Hydrochloride Glucose Injection:
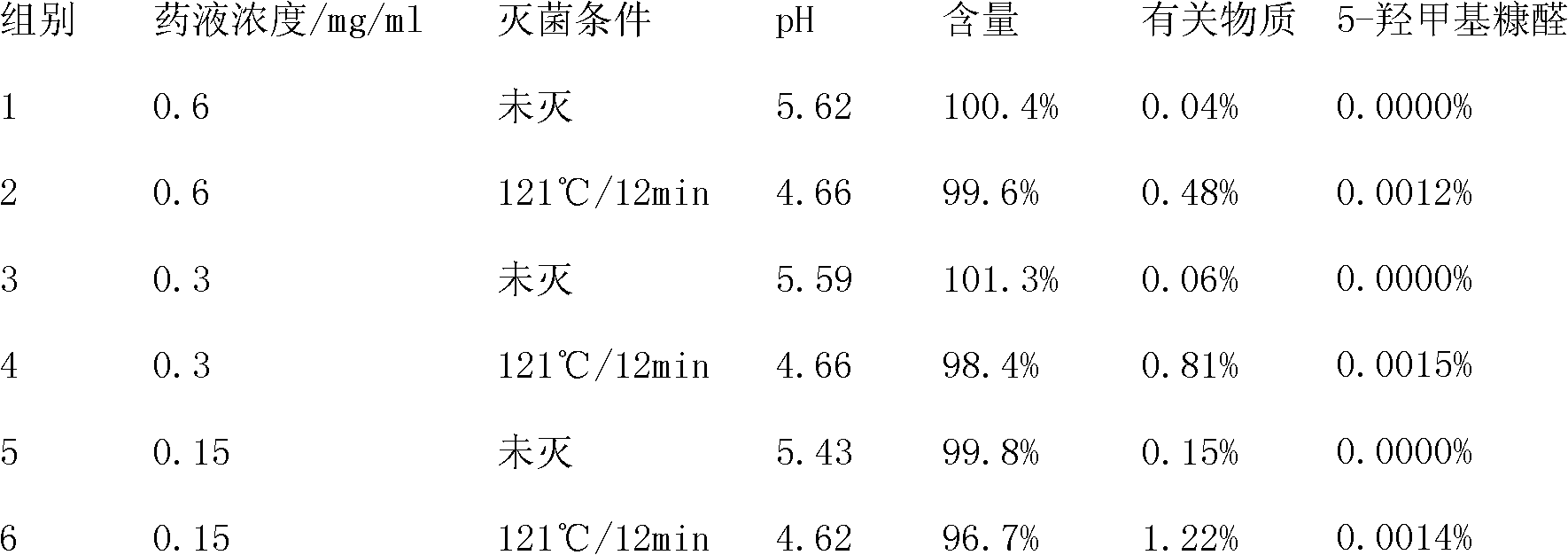
[0065] Directly prepare Ambroxol Hydrochloride Glucose Injection according to conventional methods, promptly take by weighing the prescribed amount of glucose, Ambroxol Hydrochloride and place it in the liquid preparation tank, add water for injection to the full amount, measure pH, fill after filtration, seal at 121 Sterilize at ℃ for 12 minutes, and the test results are shown in Table 1.
[0066] Table 1 Quality Comparison of Ambroxol Hydrochloride Glucose Injection Before and After Sterilization
[0067]
[0068] As can be seen from Table 1, the quality of ambroxol hydrochloride glucose injection prepared with simple prescription and preparation method changes greatly before and after sterilization. The pH drops significantly, and the related substances increase significantly. When...
experiment example 2
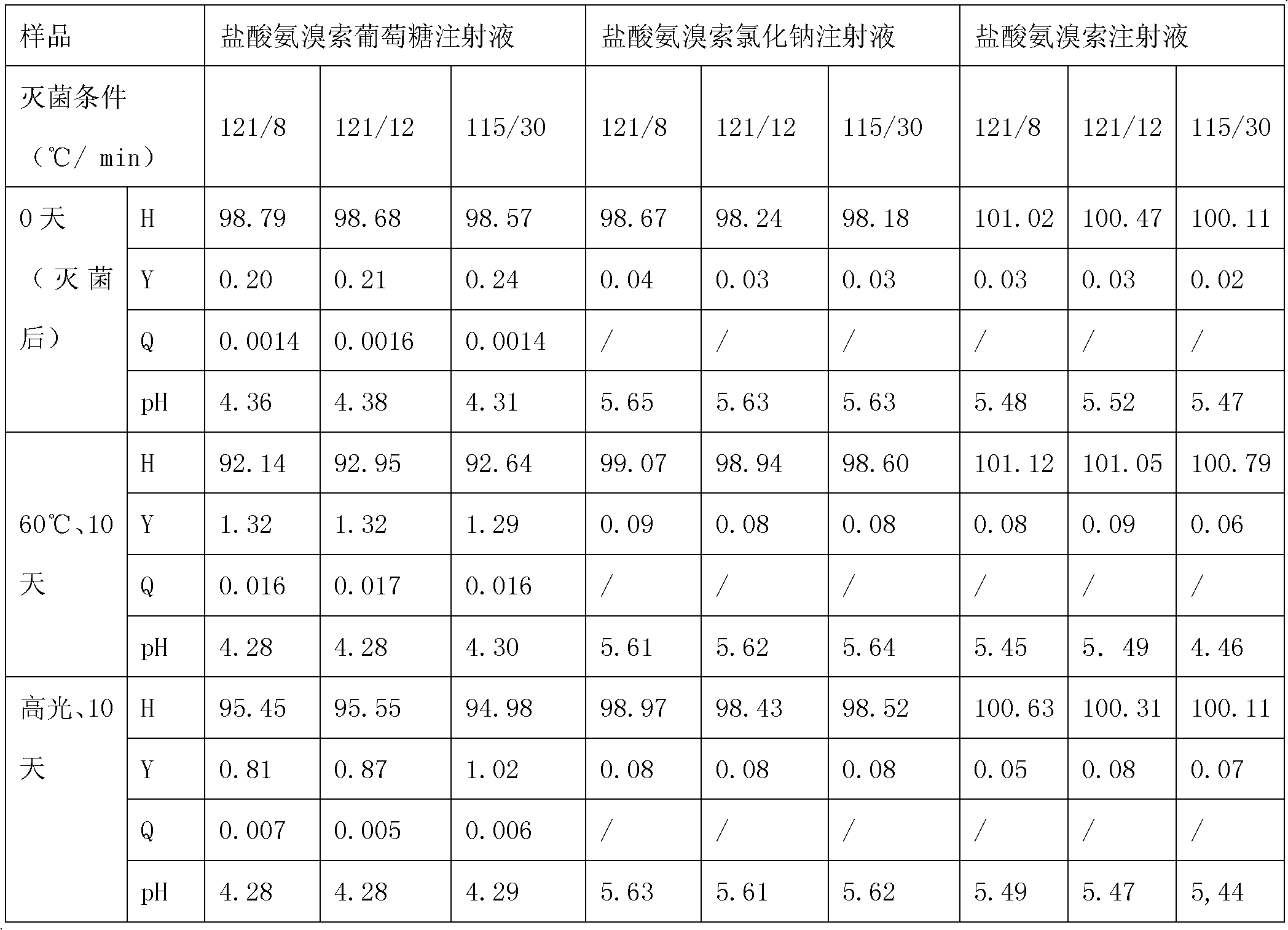
[0119] Experimental example 2 Ambroxol hydrochloride glucose injection detection experiment of the present invention
[0120] The ambroxol hydrochloride glucose injection of the present invention prepared in Examples 1-6 was tested, and the results are shown in Table 12.
[0121] Table 12 Ambroxol hydrochloride glucose injection detection data of the present invention
[0122]
[0123] The following embodiments can all achieve the effects described in the above experimental examples.
Embodiment 1
[0124] The preparation of embodiment 1 injection
[0125] Ambroxol hydrochloride 0.15g glucose 50g
[0126] Weigh glucose and ambroxol hydrochloride and place them in a liquid mixing tank, add water for injection at 40°C to 1000ml, add hydrochloric acid to adjust the pH value to 3.8, measure the pH value and the content of ambroxol hydrochloride to pass, and filter through a 0.22μm micropore Membrane filtration until the medicinal solution is clear, filled, covered, sterilized at 121°C / 12min, ready to serve.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com