Paclitaxel composition for injection and preparation method thereof
A paclitaxel and composition technology, applied in the directions of drug combination, drug delivery, pharmaceutical formulation, etc., can solve the problems such as the lack of detailed description of the preparation process, the increase in the content of related substances, the increase in the degradation products of the main drug, and the reduction of adverse reactions. Occurrence rate, the effect of reducing the content of related substances and improving the stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
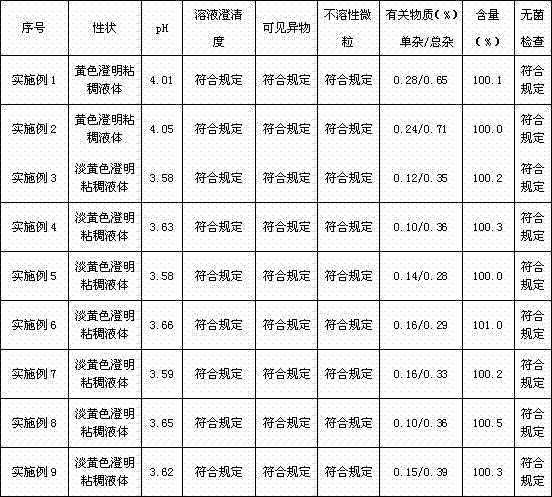
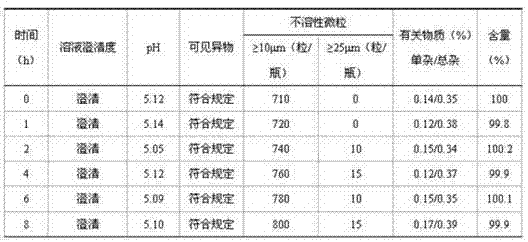
Examples
Embodiment 1
[0033] Sterilization of polyoxyethylene-35-castor oil by conventional methods
[0034] prescription
[0035] Paclitaxel 30g
[0036] Polyoxyl-35-Castor Oil 2650g
[0037] Citric acid 10g
[0038] Ethanol up to 5000ml
[0039]
[0040] A total of 1000 bottles were made
[0041] Polyoxyethylene-35-castor oil, autoclaved at 120°C for 20 minutes, cooled to room temperature for later use.
[0042] Take polyoxyethylene-35-castor oil and put it in a stainless steel bucket, add paclitaxel into it, stir for 30 minutes to mix the raw and auxiliary materials evenly, add 80% ethanol, continue stirring for 15 minutes, then add citric acid, and continue stirring for 5 minutes , add ethanol to the full amount, filter through two-stage 0.22um polytetrafluoroethylene capsule filters, measure the solution content and pH value, check the clarity of the liquid medicine, and fill the liquid medicine in a glass ampoule after passing the test, and seal it , l...
Embodiment 2
[0044] Sterilization of polyoxyethylene-35-castor oil by conventional methods
[0045] prescription
[0046] Paclitaxel 30g
[0047] Polyoxyl-35-Castor Oil 2650g
[0048] Citric acid 10g
[0049] Ethanol up to 5000ml
[0050]
[0051] A total of 1000 bottles were made
[0052] Polyoxyethylene-35-castor oil, autoclave at 115°C for 30 minutes, cool to room temperature for later use.
[0053]Take polyoxyethylene-35-castor oil and put it in a stainless steel bucket, add paclitaxel into it, stir for 30 minutes to mix the raw and auxiliary materials evenly, add 80% ethanol, continue stirring for 15 minutes, then add citric acid, and continue stirring for 5 minutes , add ethanol to the full amount, filter through two-stage 0.22um polytetrafluoroethylene capsule filters, measure the solution content and pH value, check the clarity of the liquid medicine, and fill the liquid medicine in a glass ampoule after passing the test, and seal it , light inspection,...
Embodiment 3
[0055] prescription
[0056] Paclitaxel 30g
[0057] Polyoxyl-35-Castor Oil 2600g
[0058] Citric acid 10g
[0059] Ethanol up to 5000ml
[0060]
[0061] A total of 1000 bottles were made
[0062] Polyoxyethylene-35-castor oil, autoclave at 105°C for 30 minutes, cool to 40°C, repeat the above process twice, cool to room temperature for later use.
[0063] Take polyoxyethylene-35-castor oil and put it in a stainless steel bucket, add paclitaxel into it, stir for 30 minutes to mix the raw and auxiliary materials evenly, add 80% ethanol, continue stirring for 15 minutes, then add citric acid, and continue stirring for 5 minutes , add ethanol to the full amount, filter through two-stage 0.22um polytetrafluoroethylene capsule filters, measure the solution content and pH value, check the clarity of the liquid medicine, and fill the liquid medicine in a glass ampoule after passing the test, and seal it , light inspection, forming paclitaxel composition fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com