Collagen/glycosaminoglycan compositions for use as terminally sterilizable matrices
a glycosaminoglycan and composition technology, applied in the field can solve the problems of collagen/gag matrix materials that collagen/gag matrix materials cannot be labeled as sterile, collagen/gag matrix materials cannot be used sub-dermally, etc., to achieve optimal biological performance and increase the cross-link density of collagen and glycosaminoglycan compositions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Standard Preparation of Collagen / GAG Matrices
[0067] Chondroitin-6-sulfate solution in acetic acid is prepared by mixing 828 grams of deionized water with 2.5 mL of glacial acetic acid and adding to the mixture 2.2 grams of chondroitin-6-sulfate (dry weight). The solution is stirred until the chondroitin-6-sulfate is completely dissolved, preferably for about one hour.
[0068] A collagen dispersion of 0.5% collagen in 0.05 M acetic acid is prepared in a mixing vessel with a cooling system cooled to 4° C. Deionized water (4153 grams) is added to the mixing vessel followed by 12.5 mL of acetic acid and the solution is mixed for ten minutes. Purified collagen (25 grams dry weight) is cut into pieces not larger than 1 cm2 and mixed for 5 minutes at 1 to 10° C. at 25 Hz. The emulsifier and disperser is then increased to approximately 50 Hz and run for 30 minutes while maintaining a temperature of <25° C.
[0069] While continuing to emulsify and disperse, the chondroitin-6-sulfate solution ...
example 2
Application of Silicone Layer
[0072] For collagen / GAG construct to be used for dermal regeneration, a thin layer of silicone is applied to the matrices after dehydrothermal treatment and prior to cross-linking. For this purpose, the Gardener knife is used to apply an even layer of silicone. A Gardener knife is set to an appropriate thickness of about 10 mil and two sheets of polyethylene sheeting is cut to a size approximately 6 inches longer than the matrices. A bead of silicone is placed on one of the polyethylene sheets in between the edges of the small Gardener Knife. The Gardener knife is then drawn slowly to the opposite end of the polyethylene sheet. A collagen / GAG matrix is placed onto the silicone and the other sheet of polyethylene is put on top of the collagen / GAG matrix. A second Gardener knife, set to about 50 mil is drawn over the length of the polyethylene-sponge-polyethylene sandwich. The upper sheet of the polyethylene sheeting is removed and the collagen / GAG-silico...
example 3
Modified Method for Production for Collagen / GAG Matrices of Present Invention
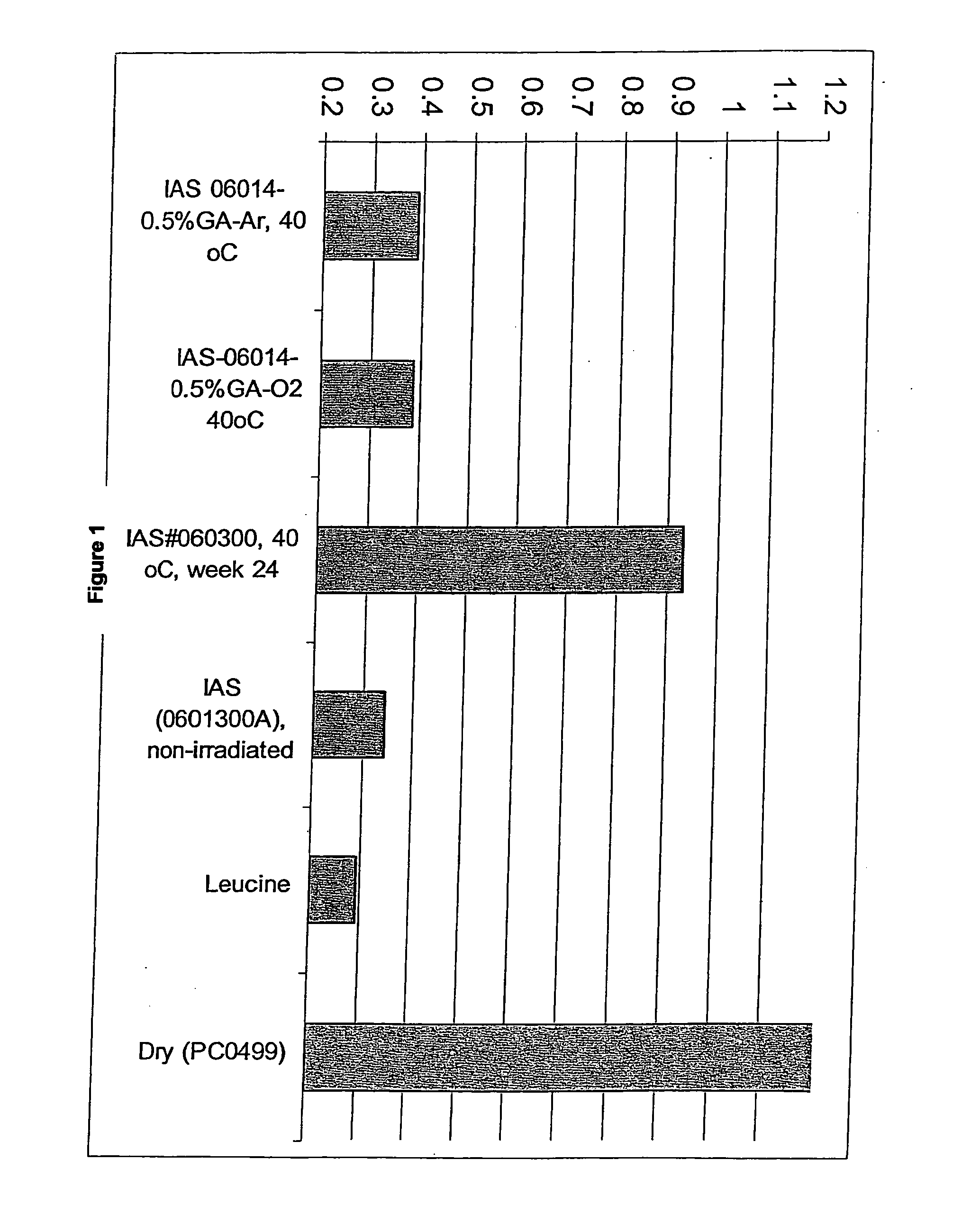
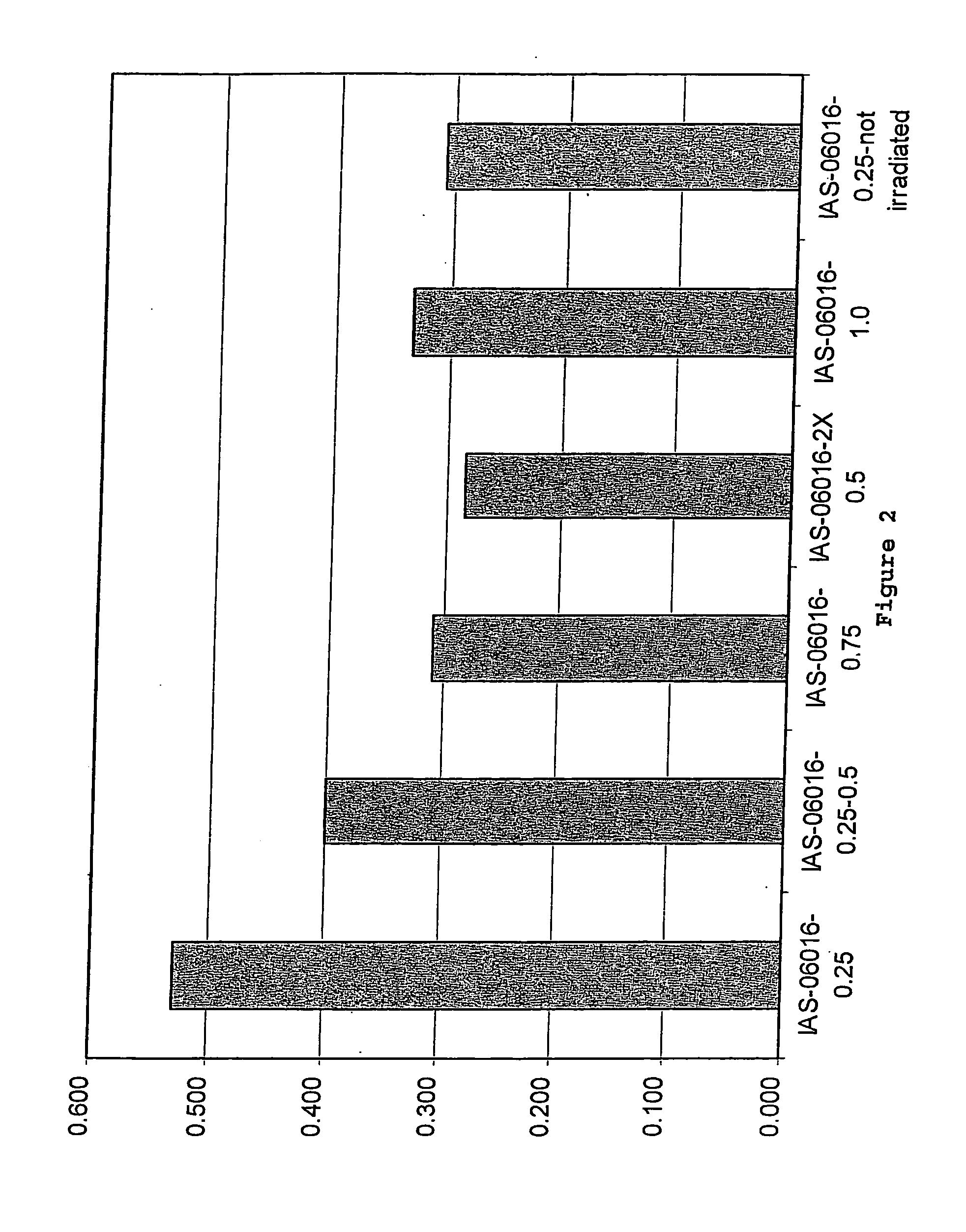
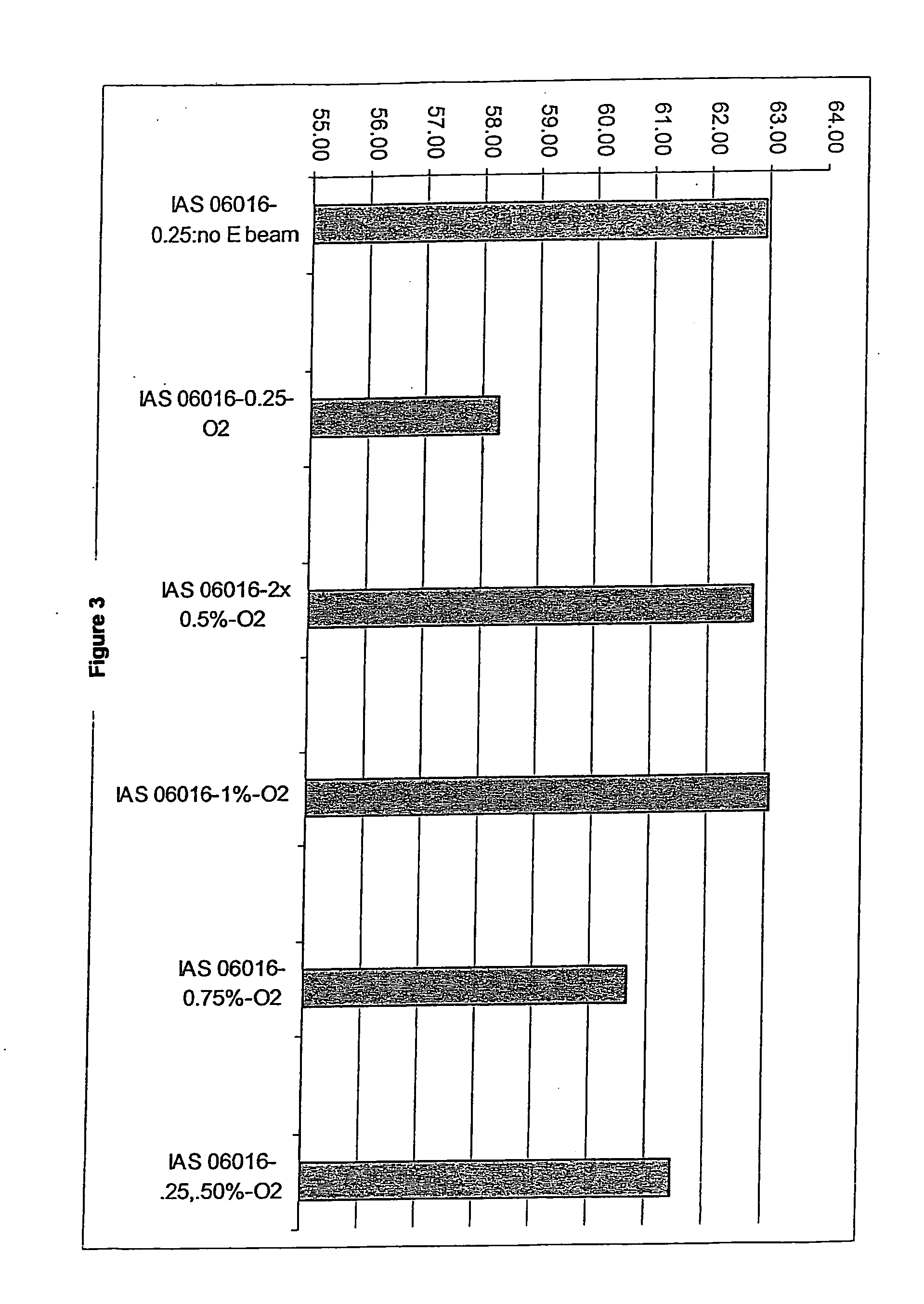
[0073] Collagen / GAG matrices cross-linked sufficiently to compensate for irradiation damage after E-beam sterilization were produced. In this procedure, the collagen / GAG matrices were cross-linked using a 0.5% glutaraldehyde solution in 0.3% acetic acid. The collagen / GAG matrices were first placed between polyethylene sheets and meshes of an appropriate mesh size. The matrices were held in place by a polypropylene frame. For cross-linking, the polyethylene sheeting was placed into the lower part of the cross-link frame. The matrices were then placed onto the polyethylene sheeting and covered with the mesh. The top part of the frame was placed over the mesh and sheet and the frame was closed. The matrices in the frames were then placed into cross-link racks to air dry for 1 to 5 hours.
[0074] A 0.3% acetic acid solution in deionized water was prepared in a tub sufficiently large to hold 2 liters of solution...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperatures | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com