Human Amniotic Membrane Lyophilized Grafts
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
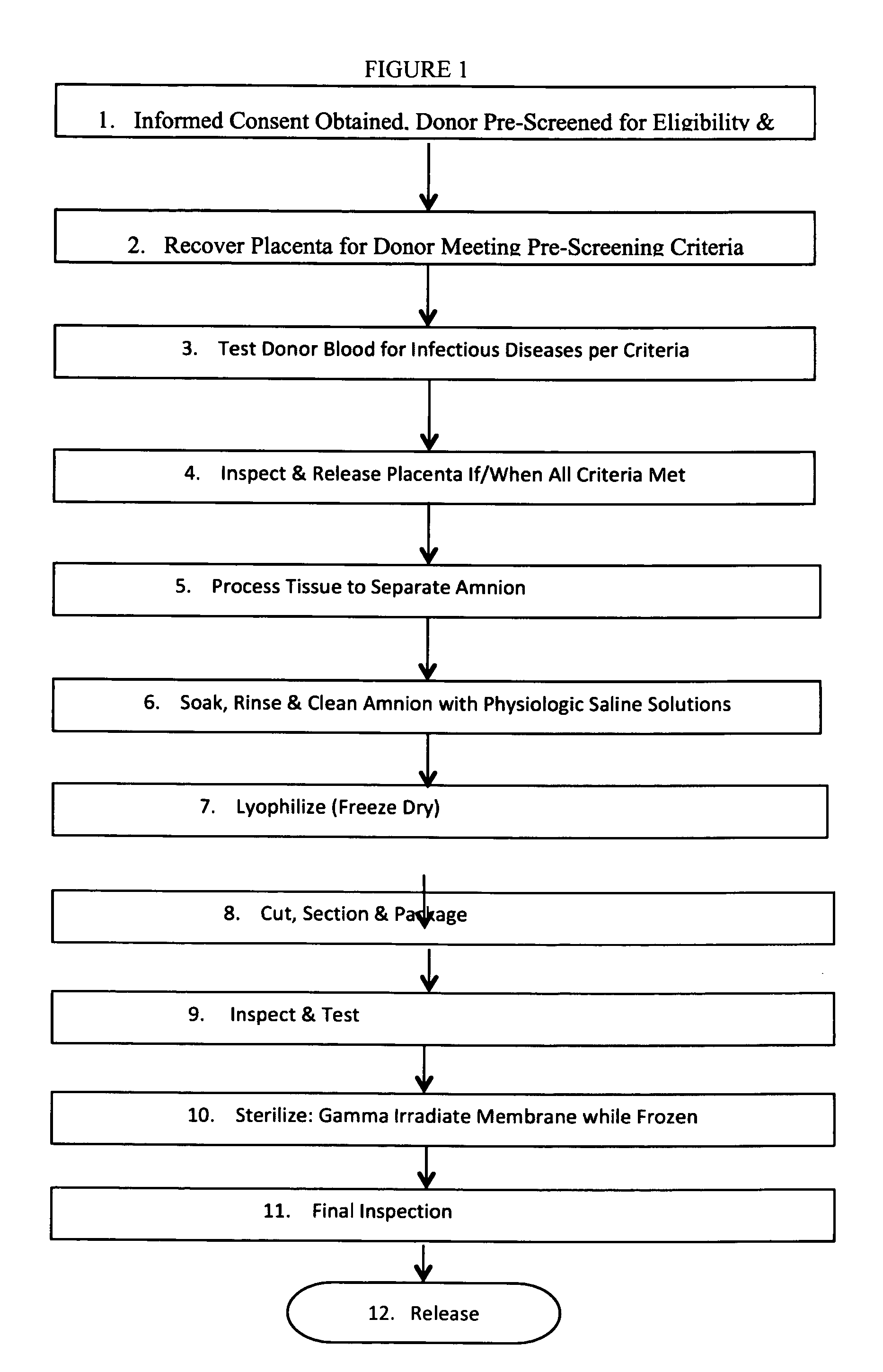
[0013]First it is to be understood that the aspects of the methods to be described are not limited to specific compounds, exact methods described or uses as such may vary. FIG. 1 depicts a process overview flowchart for the recovery and manufacture of the tissue grafts described herein.
[0014]Step 1: Informed Consent Obtained, Donor Pre-Screened for Eligibility and Suitability
[0015]Initially, the potential female donor is approached and written informed consent is obtained following standard industry practices and the guidelines set forth by AATB. The potential donor must fully understand the donation process and give their informed consent to the process and to the testing of their blood for diseases that may affect the suitability of their placenta tissues for use.
[0016]After written consent is obtained, the donor is pre-screened for eligibility and suitability to donate her placenta at delivery. Screening involves assessment for risk factors to communicable diseases as specified i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com