Sterile packaging method for small-volume injection
A packaging method and injection technology, applied in the field of aseptic packaging of small-volume injections, can solve problems such as hidden safety hazards, easy embolism, and content reduction, and achieve the effect of safety assurance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
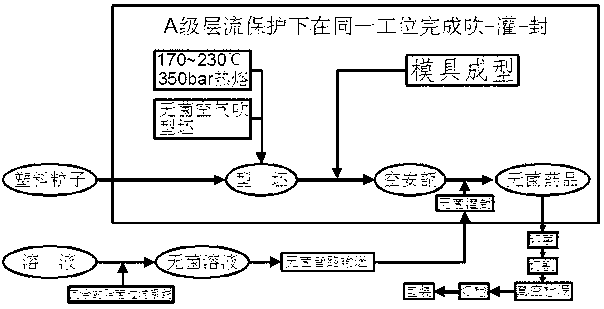
[0018] Take the production of small volume trimethoprim injection as an example, such as figure 1 shown, including the following steps:
[0019] (1) Weigh the raw and auxiliary materials of trimethoprim injection according to the process ratio under the negative pressure weighing hood, then add them to the pre-sterilized batching pot in sequence, add water for injection to dilute, and mix well;
[0020] (2) With the cooperation of the weighing system of the batching pot, supplement the water for injection according to the process ratio to the total amount of the preparation, adjust the pH value of the trimethoprim injection to 3.85-3.95, and then the liquid is removed through the 0.22um redundant filter system Bacteria-grade filter for filtration sterilization;
[0021] (3) The filtered sterile medicinal liquid is injected into the plastic bottle blow-fill-seal integrated machine through the sterile pipeline system, and plastic particles are added to the other end of the plas...
Embodiment 2
[0023] Take the production of small volume racemic anisodamine hydrochloride injection as an example, such as figure 1 shown, including the following steps:
[0024] (1) Under a negative pressure weighing hood, weigh the raw and auxiliary materials of racemic anisodamine hydrochloride injection according to the process ratio, then add them to the pre-sterilized batching pot in sequence, add water for injection to dilute, and mix evenly;
[0025] (2) With the cooperation of the weighing system of the batching pot, supplement the water for injection according to the process ratio to the total amount of preparation, adjust the pH value of racemic anisodamine hydrochloride injection to 4.6-5.3, and then the liquid passes through the redundant filter system at 0.22 um sterilization grade filter for filter sterilization;
[0026] (3) The filtered sterile medicinal liquid is injected into the plastic bottle blow-fill-seal integrated machine through the sterile pipeline system, and p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com