Nano ganciclovir freeze-drying preparation for injection and preparation method thereof
A technology of ganciclovir and freeze-dried preparations, applied in the field of ganciclovir nanocapsule freeze-dried preparations for injection and their preparation, can solve the problems of vascular tissue irritation and other problems, achieve elimination of vascular irritation, increase absorption, The effect of reducing toxic side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
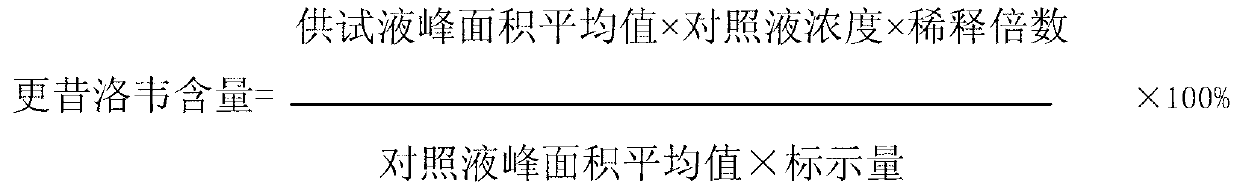
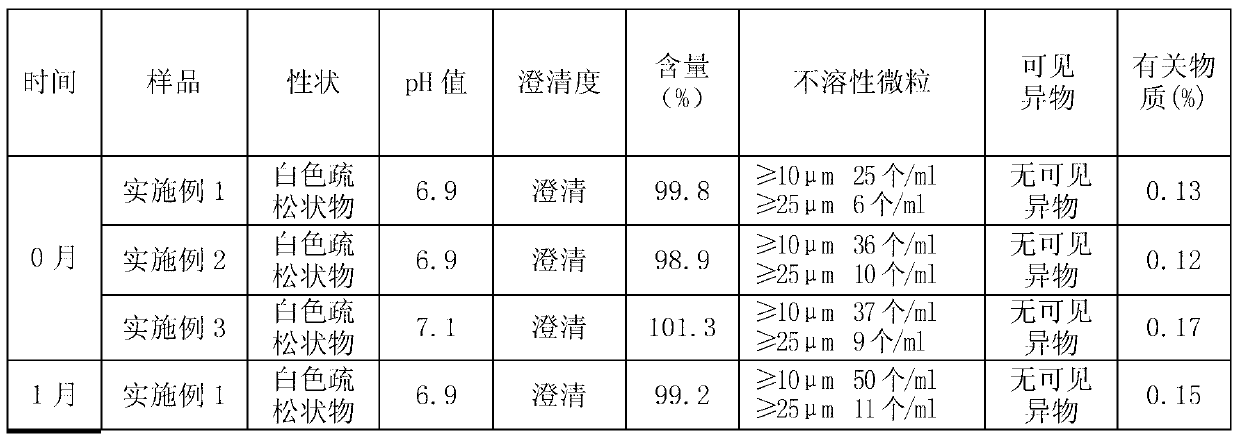
[0032] 1. Preparation of ganciclovir nanocapsule lyophilized preparation for injection (3000ml volume)
[0033] Prescription: ganciclovir 250g, dextran 4010g, hydroxypropyl-β-cyclodextrin 30g, polyethylene glycol-polylactic acid block copolymer (number average molecular weight 1000) 60g, mannitol 30g, sodium sulfite 1g, water for injection Set the volume to 3000ml.
[0034] Preparation:
[0035] ⑴Preparation: After treating the containers and pipes used for dosing, wash them with water for injection; wash vials and rubber stoppers according to conventional methods, sterilize them, and dry them for later use.
[0036] (2) Dosing: Add 80% of the prescription amount of water for injection (2400ml) into the liquid mixing tank (temperature 50-60°C), first weigh the prescribed amount of dextran 40, solubilizer, and sodium sulfite into the liquid mixing tank, and stir until dissolved Weigh the ganciclovir of prescription quantity, add in the above-mentioned liquid medicine, stir unti...
Embodiment 2
[0076] 1. Preparation of ganciclovir nanocapsule lyophilized preparation for injection (3000ml volume)
[0077] Prescription: ganciclovir 250g, dextran 4015g, hydroxypropyl-β-cyclodextrin 40g, polyethylene glycol-polylactic acid block copolymer (number average molecular weight 1000) 70g, mannitol 60g, sodium sulfite 3g, water for injection Set the volume to 3000ml.
[0078] Preparation:
[0079] ⑴Preparation: After treating the containers and pipes used for dosing, wash them with water for injection; wash vials and rubber stoppers according to conventional methods, sterilize them, and dry them for later use.
[0080] (2) Dosing: Add 80% of the prescription amount of water for injection (2400ml) into the liquid mixing tank (temperature 50-60°C), first weigh the prescribed amount of dextran 40, solubilizer, and sodium sulfite into the liquid mixing tank, and stir until dissolved ; Weigh the ganciclovir of prescription quantity, add in the above-mentioned medicinal liquid, stir...
Embodiment 3
[0105] 1. Preparation of ganciclovir nanocapsule lyophilized preparation for injection (3000ml volume)
[0106] Prescription: Ganciclovir 125g, dextran 4020g, hydroxypropyl-β-cyclodextrin 20g, polyethylene glycol-polylactic acid block copolymer (number average molecular weight 2000) 40g, mannitol 40g, sodium sulfite 2g, water for injection Set the volume to 3000ml.
[0107] Preparation:
[0108] ⑴Preparation: After treating the containers and pipes used for dosing, wash them with water for injection; wash vials and rubber stoppers according to conventional methods, sterilize them, and dry them for later use.
[0109] (2) Dosing: Add 80% of the prescription amount of water for injection (2400ml) into the liquid mixing tank (temperature 50-60°C), first weigh the prescribed amount of dextran 40, solubilizer, and sodium sulfite into the liquid mixing tank, and stir until dissolved ; Weigh the ganciclovir of prescription quantity, add in the above-mentioned medicinal liquid, stir...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com