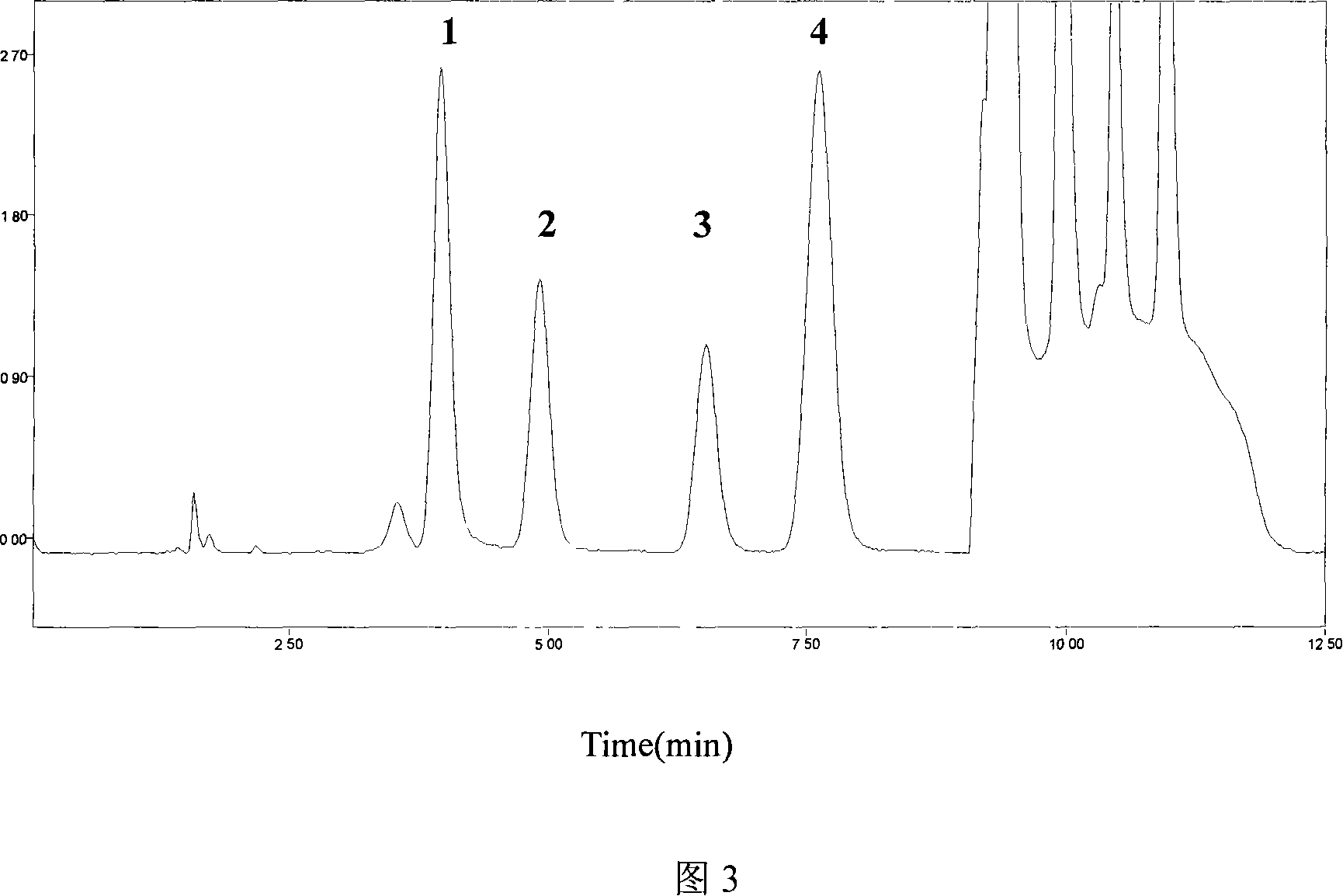
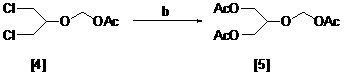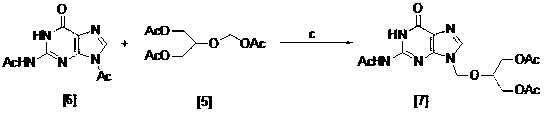Patents
Literature
117 results about "Ganciclovir" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
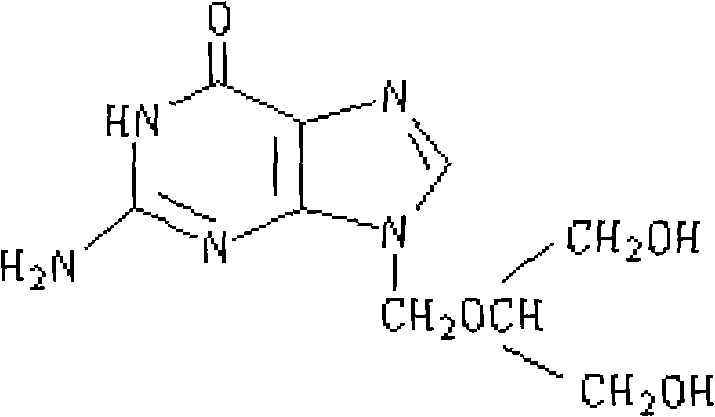
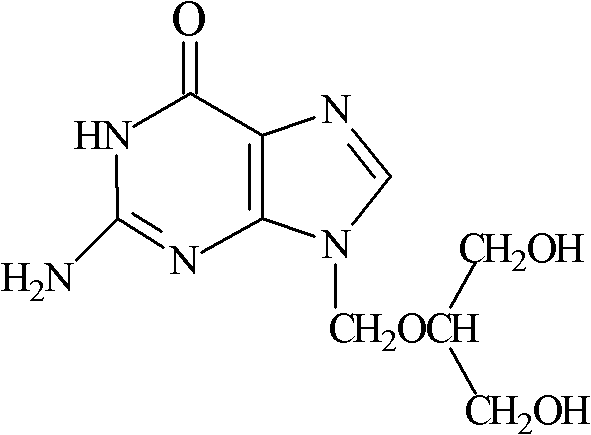
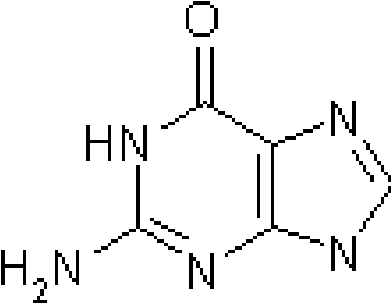
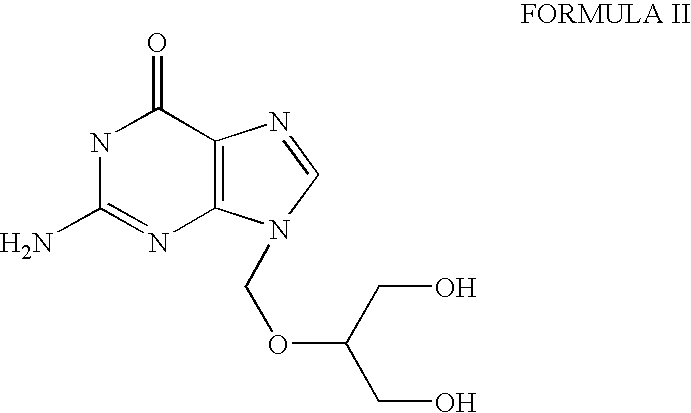
Ganciclovir is an anti-viral drug. It is used to prevent disease caused by a virus called cytomegalovirus (CMV) in people who have received organ or bone marrow transplants. CMV disease can lead to serious infections in the body, including an infection in the eye, called CMV retinitis, that can cause blindness.
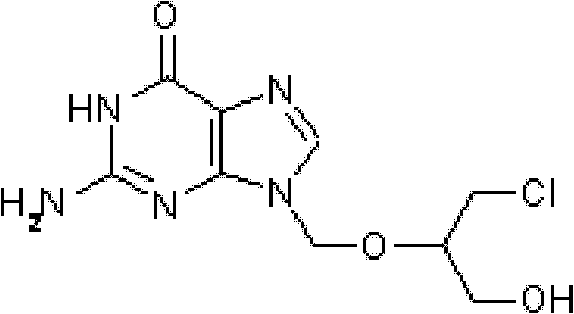
Prodrug composition
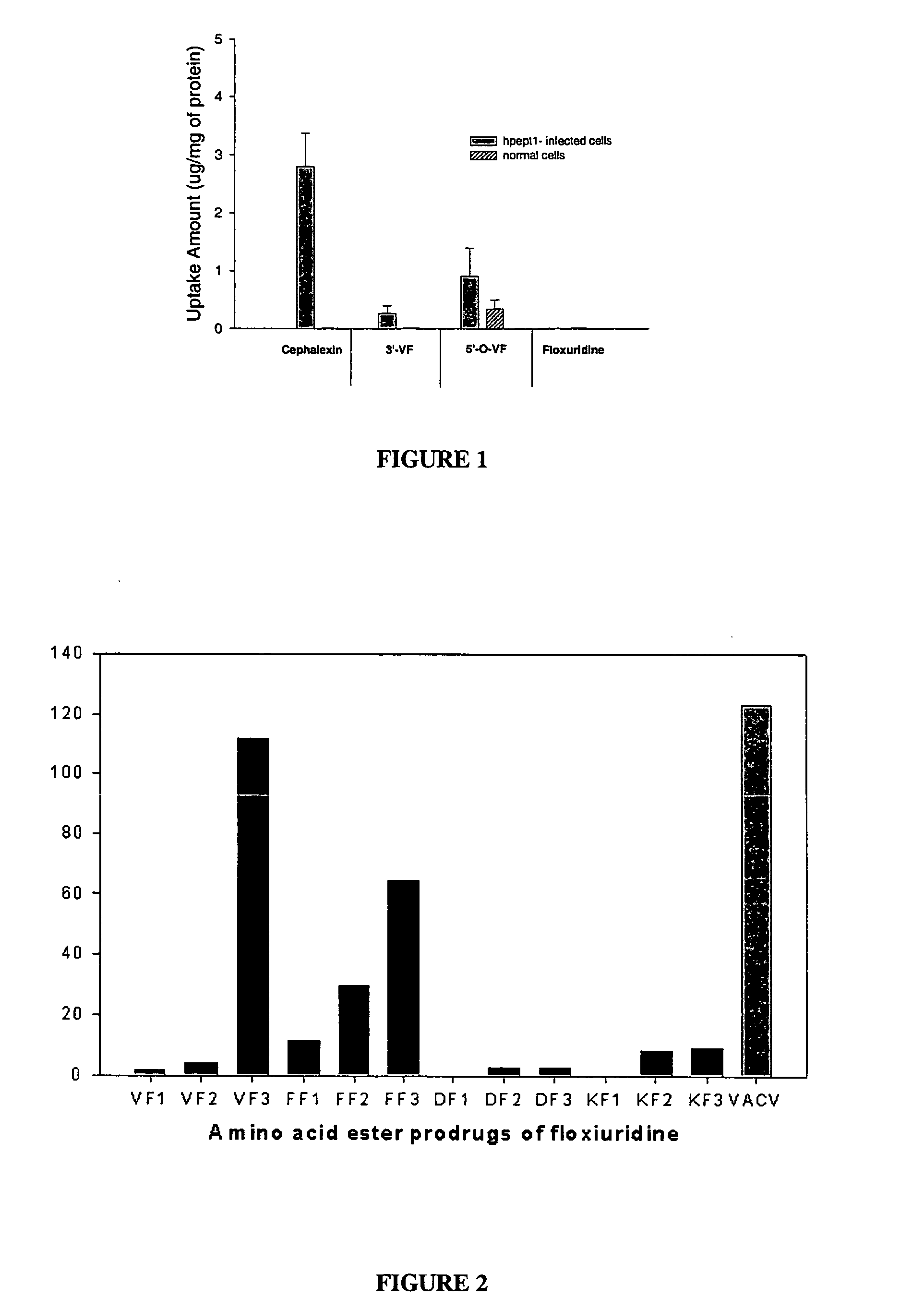
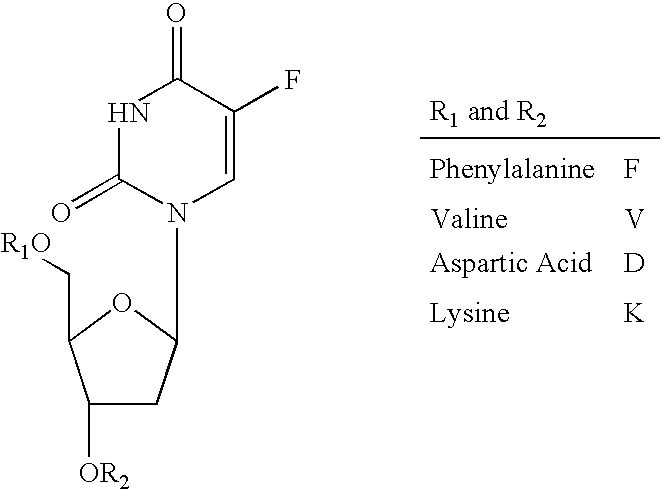
A prodrug composition is provided which includes a pharmaceutical species and an amino acid having a covalent bond to the pharmaceutical species. The pharmaceutical species is characterized by bioavailability of 30% or less and a molecular weight in the range of 100-1000 Daltons. The composition is characterized further in that the pharmaceutical species is not acyclovir, ganciclovir, BRL44385, or penciclovir. Also described is an inventive method of delivering a pharmaceutical species to an individual including the step of orally administering an inventive prodrug to an individual. In one embodiment the prodrug includes a pharmaceutical species characterized by bioavailability of 30% or less, wherein the pharmaceutical species has a molecular weight in the range of 100-1000 Daltons. The inventive prodrug is transported from the gastrointestinal lumen by a specific transporter and is enzymatically cleaved to yield the pharmaceutical species, such that the pharmaceutical species is delivered to the individual.
Owner:HILFINGER JOHN
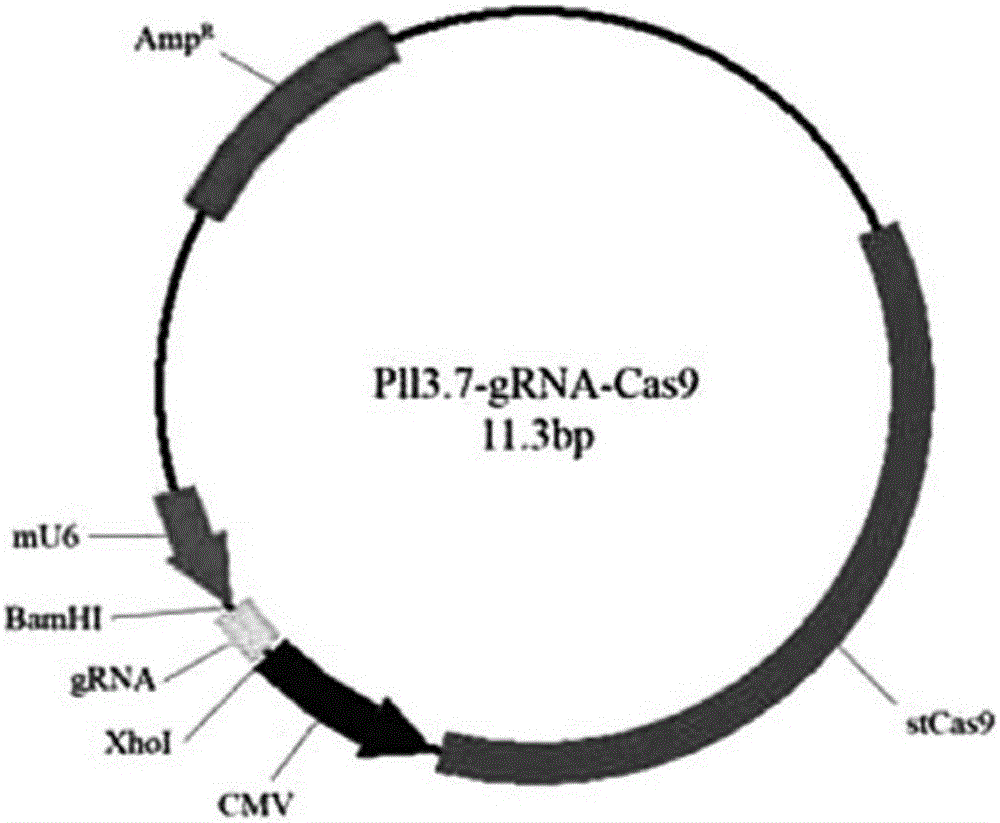
SSA (single-strand annealing) repair-based gene seamless editing method utilizing CRISPR/Cas9 technology
InactiveCN106011171ASeamless editingNucleic acid vectorVector-based foreign material introductionGenome editingPlasmid Vector
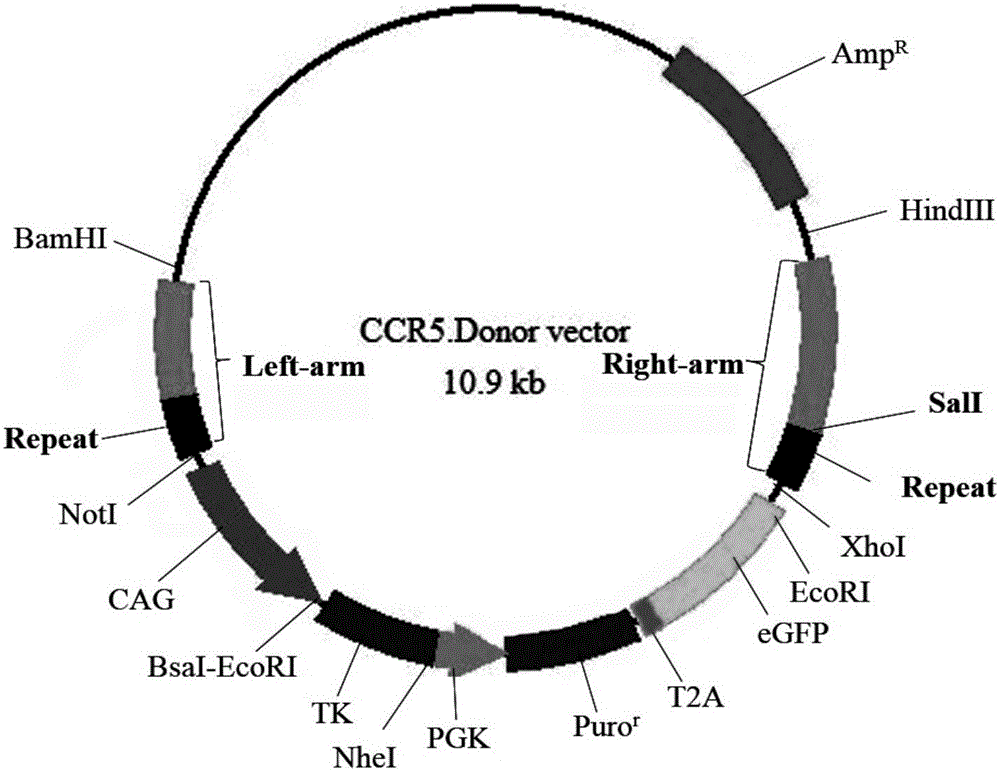
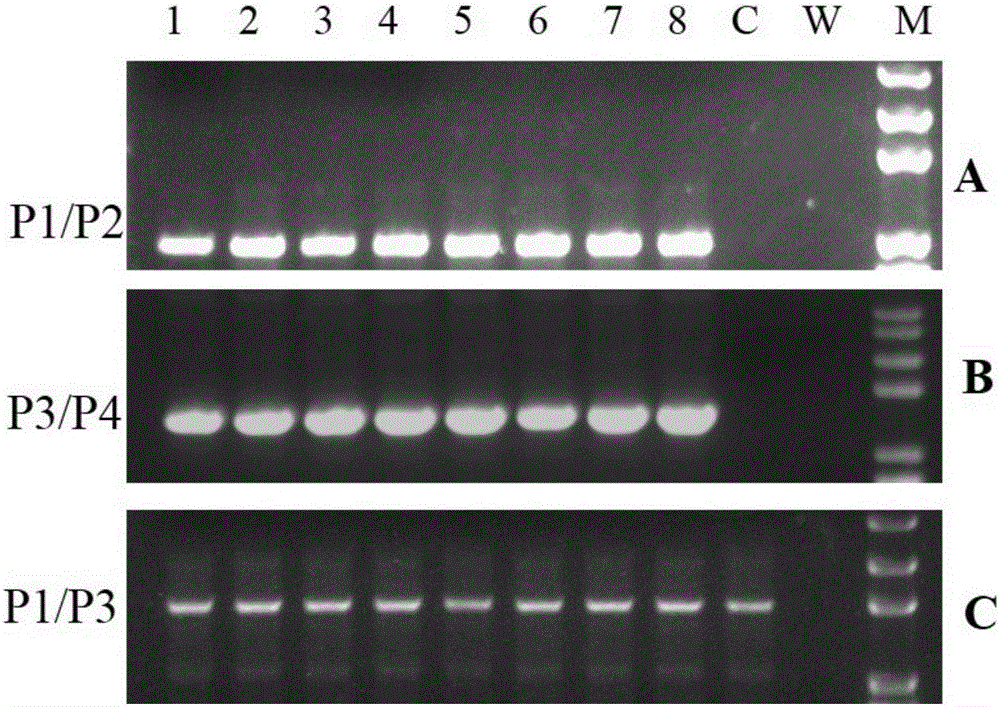
The invention mainly belongs to the technical field of gene editing and particularly relates to an SSA (single-strand annealing) repair-based gene seamless editing method utilizing a CRISPR / Cas9 technology. According to the gene seamless editing method, CRISPR / Cas9 expression vectors CCR5.CRISPR / Cas9 and eGFP.CRISPR / Cas9 targeting CCR5 and eGFP are constructed firstly, a donor CCR5-Donor vector is constructed, a CCR5-Donor plasmid vector adopts the pXL-BACII-L-arm-CAG-TK-PGK-Puro<R>-T2A-eGFP-bGH pA-R-arm structure, L-arm and R-arm are left and right homologous arms, and CAG-TK-PGK-Puro<R>-T2A-eGFP-bGH pA between L-arm and R-arm is a screening marking component; through two times of transfection, seamless editing is completed by using HR and SSA repair mechanisms in the CRISPR-Cas9 system and cells through positive and negative screening of puromycin and ganciclovir of the cells.
Owner:NORTHWEST A & F UNIV
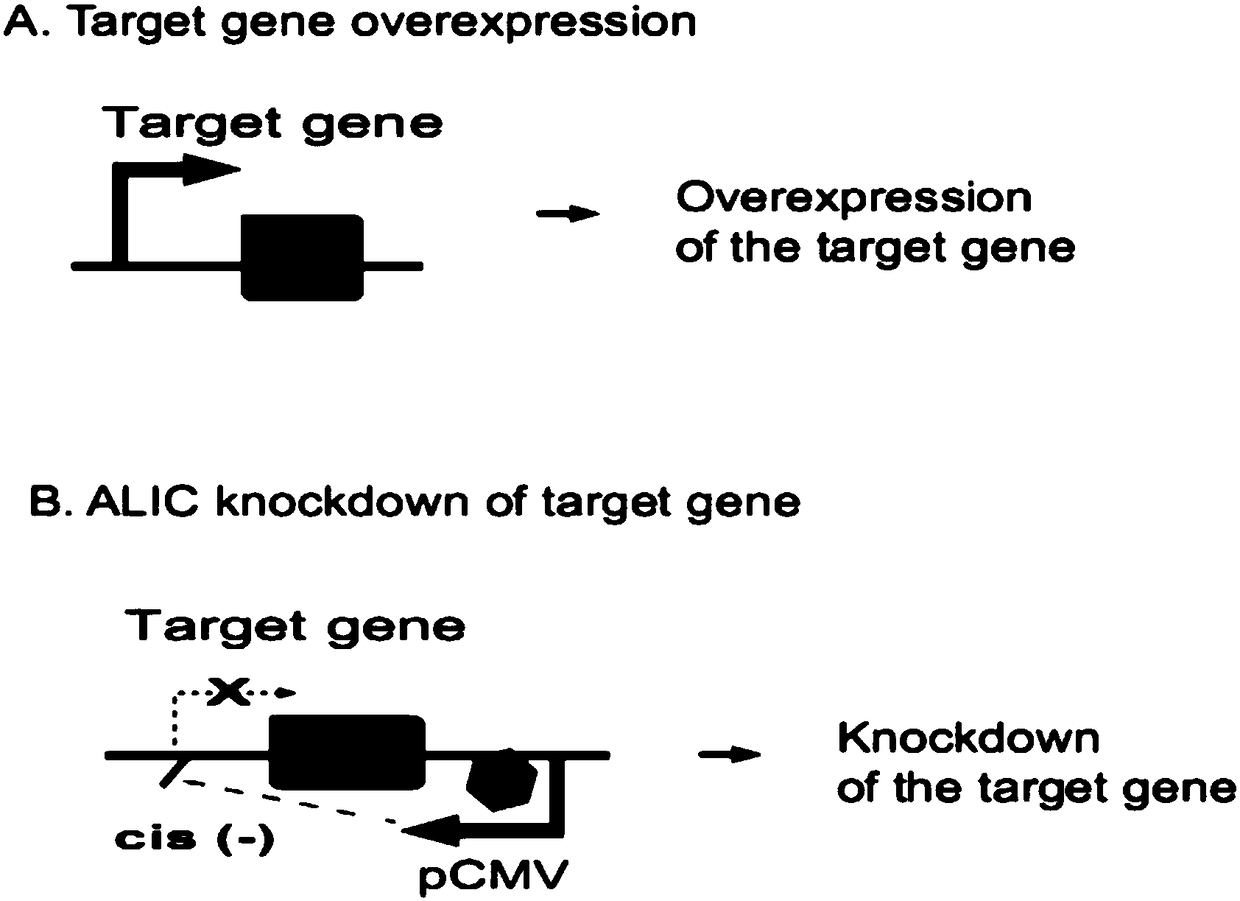
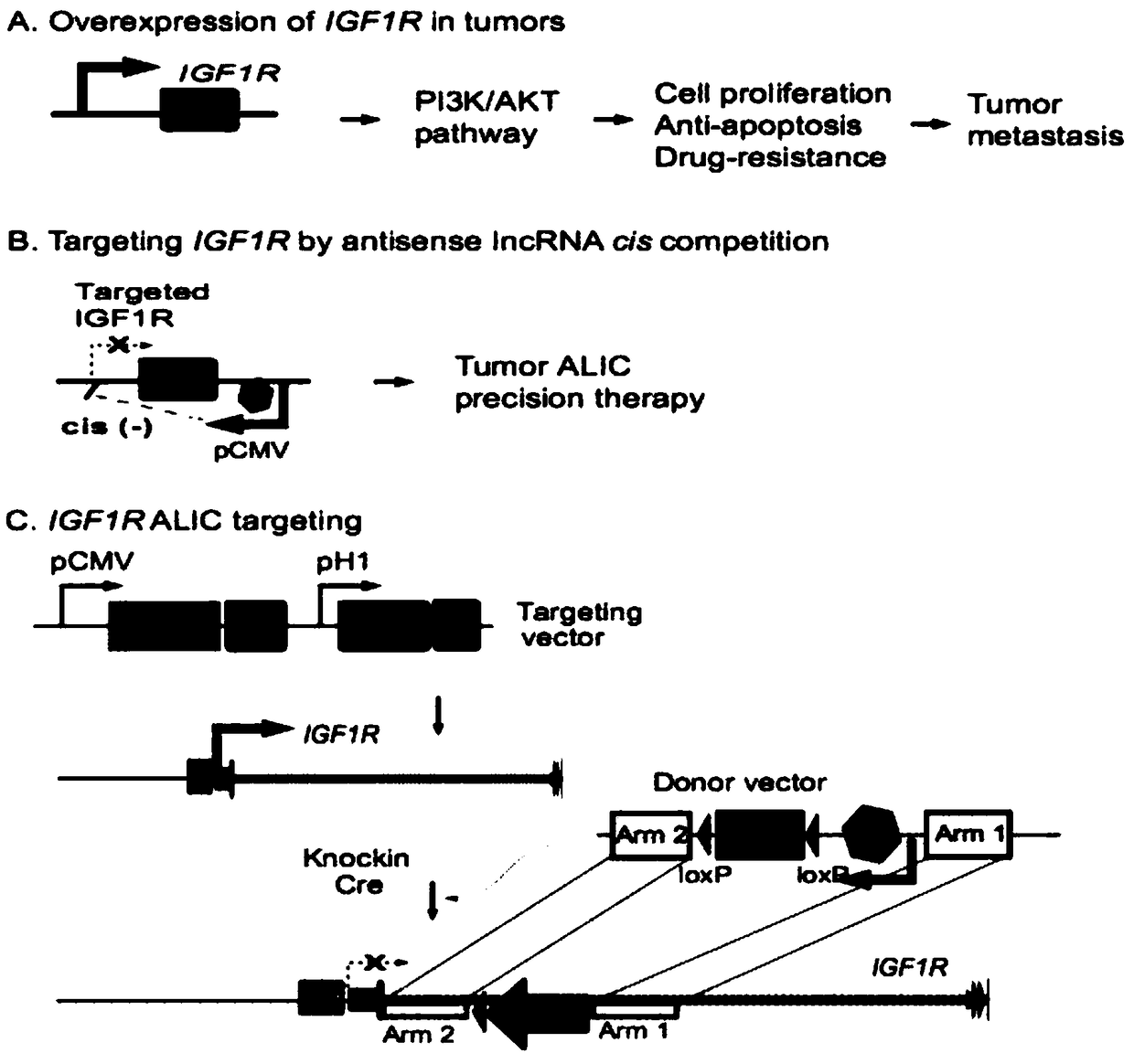
Method for inhibiting target gene expression by antisense lncRNA-mediated cis-regulation
The invention discloses a method for inhibiting target gene expression by antisense lncRNA-mediated cis-regulation. The method for inhibiting gene expression by antisense lncRNA-mediated cis-regulation is characterized in that by utilizing the characteristic that the lncRNA has the effect of competing endogenous target RNA, a strong promoter is inserted on a target gene by taking RNA as a genome positioning tool through a CRISPR Cas9 gene editing method, and a lot of antisense lncRNA complementary with the target gene is synthesized to realize in situ cis-competitive inhibition of expression of the endogenous target gene. The method comprises the following steps: firstly, constructing a targeting vector and a homologous template strand donor vector, simultaneously transfecting the constructed vectors into host cells, performing target cloning by puromycin and ganciclovir co-screening, and finally, performing detection and functional research on expression of the target gene.
Owner:JILIN UNIV
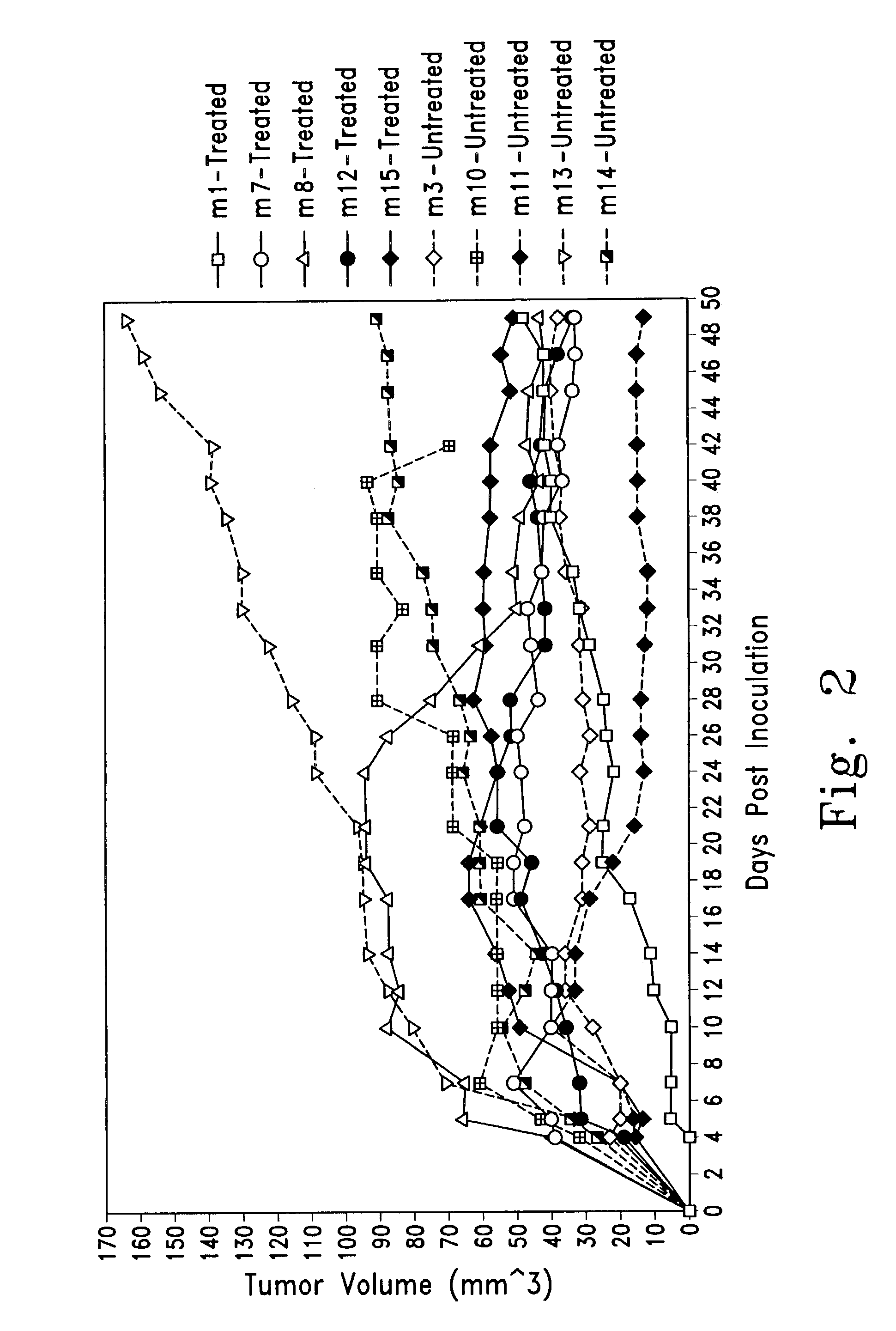
Combined Gene Therapy for the Treatment of Macroscopic Gliomas
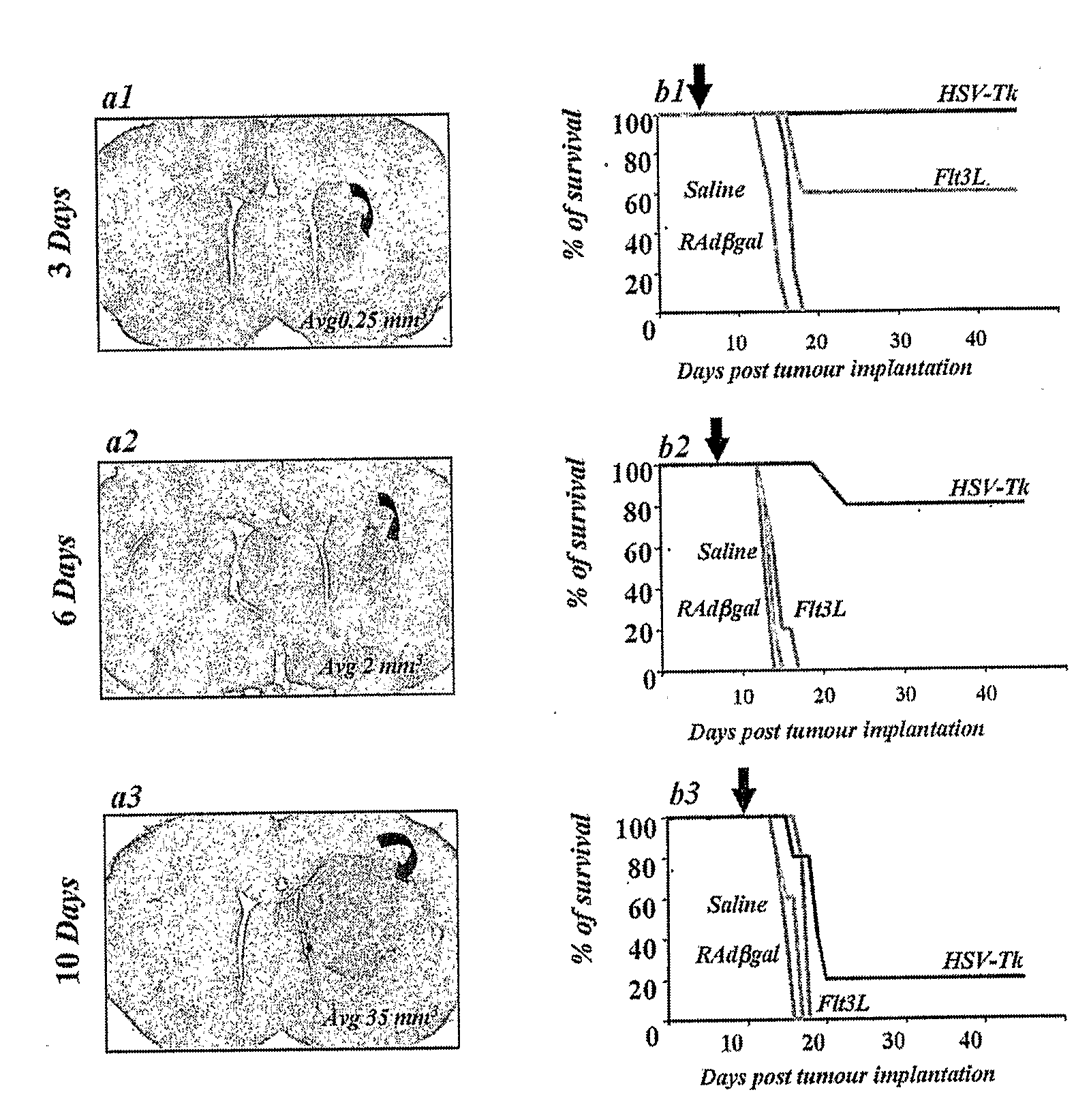
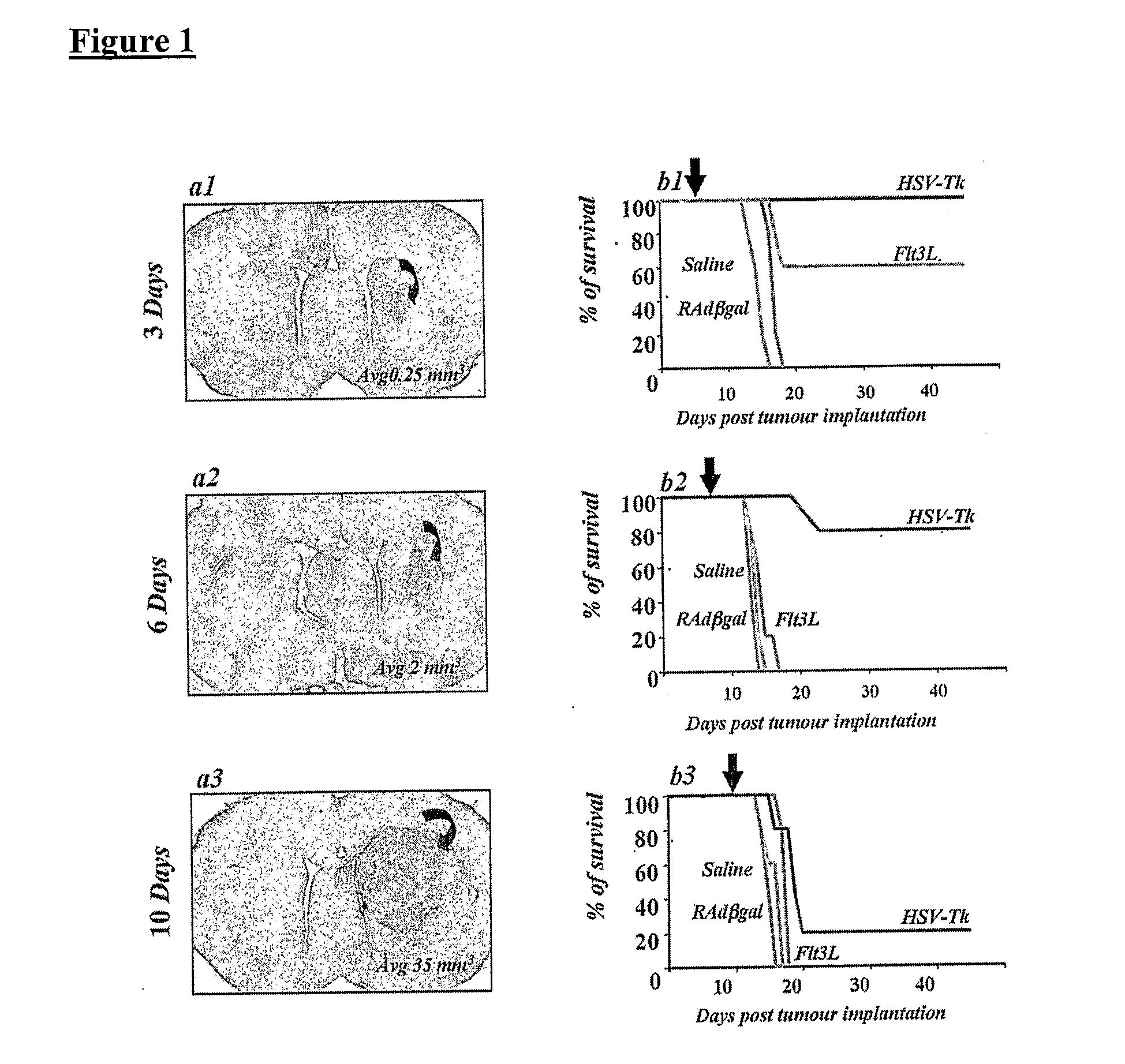
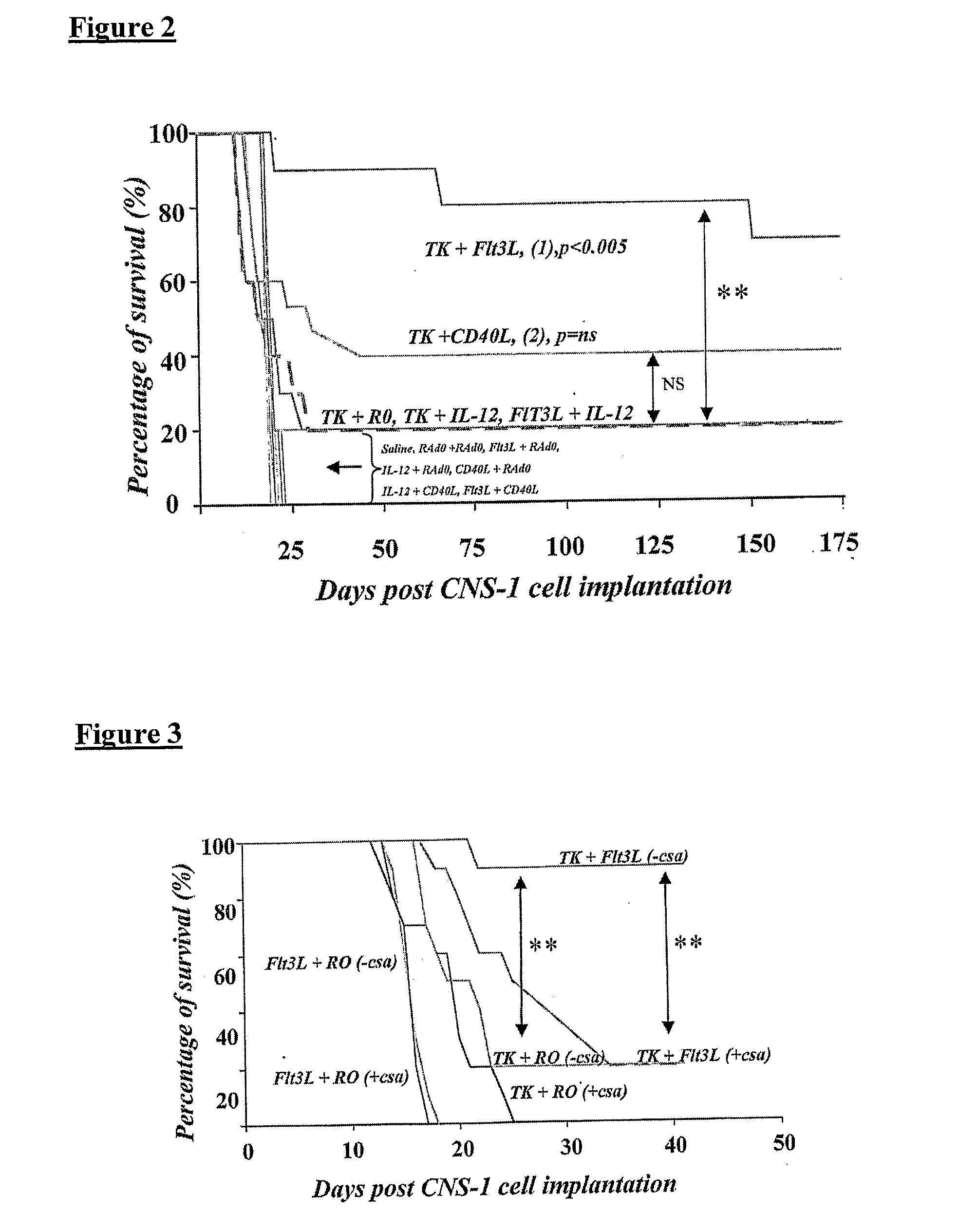
Described herein are compositions and methods for the treatment of cancer, and particularly brain cancer (e.g., glioma) in a mammal. In various embodiments of the invention, a combined therapeutic approach including TK with systemic ganciclovir administration and Flt3L are used in connection with gene therapeutic techniques or direct peptide injection for the aforementioned indications. Kits useful in practicing the inventive method are also disclosed, as are animal models useful for studying brain cancer.
Owner:CEDARS SINAI MEDICAL CENT
Ganciclovir freeze-dry preparation for injection and preparation method thereof
ActiveCN101711746AGuarantee product qualityGood inclusion effectPowder deliveryPharmaceutical product form changeMedicineFreeze-drying
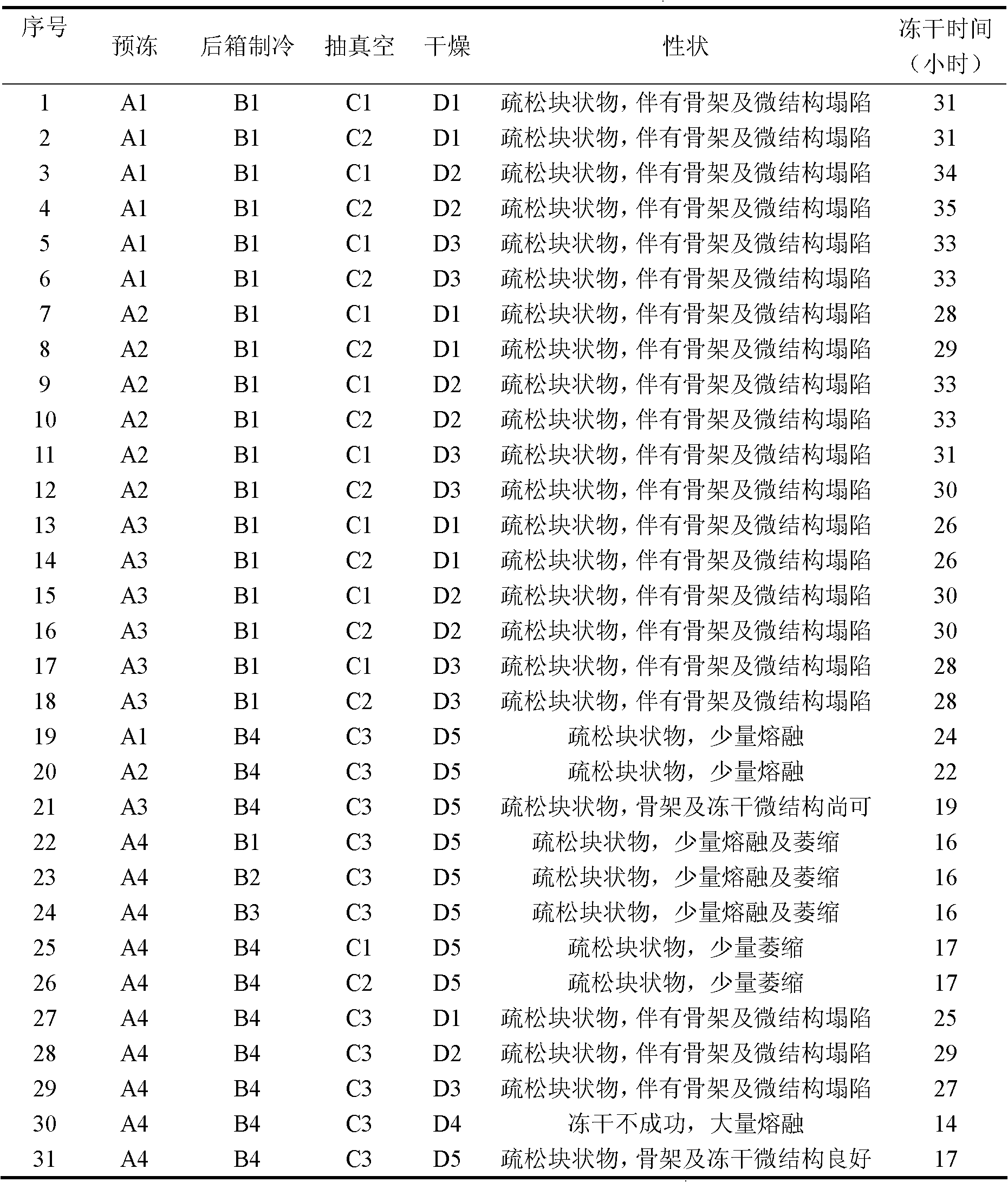
The invention relates to a Ganciclovir freeze-dry preparation for injection, comprising 50-250 parts of Ganciclovir and 100-200 parts of hydroxypropyl-beta-cyclodextrin. The invention also relates to a preparation method of the Ganciclovir freeze-dry preparation for injection. In the preparation process of the Ganciclovir freeze-dry preparation, Ganciclovir freeze-dry preparation can be dissolved into solvent when the pH value is almost neutral to improve the compliance for clinical application; freeze-dry time is shortened to be 22-26 hours, thus improving product quality, saving energy and lowering consumption.
Owner:MAANSHAN BBCA PHARMA
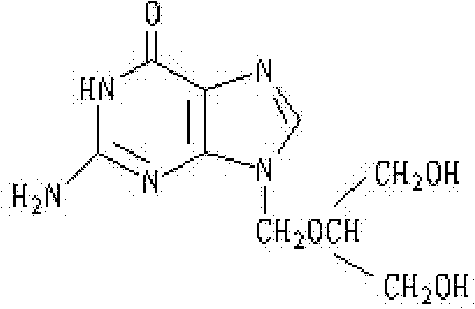
Method for preparing ganciclovir
The invention relates to a method for preparing ganciclovir. The method comprises the following steps of: a, adding paraformaldehyde into 1,3-dichloro-2-propanol [2], reacting under the action of a catalyst to obtain hemiformal [3], and reacting with an acetic anhydride to obtain 1,3-dichloro-2-acetoxylmethoxylpropane [4]; b, making 1,3-dichloro-2-acetoxylmethoxylpropane [4] react with absolute postassium acetate or anhydrous sodium acetate in an organic solvent medium in the presence of a tetraalkyl ammonium bromide catalyst with 1-20 carbon atoms and an acetic anhydride serving as a dehydrating agent to obtain 1,3-diacetoxy-2-acetoxymethoxyl propane [5]; c, performing a condensation reaction on 1,3-diacetoxy-2-acetoxymethoxyl propane [5] and 2,9-diacetyl guanine [6] in an organic solvent medium in the presence of a catalyst and an acetic anhydride serving as a dehydrating agent to obtain triacetyl ganciclovir [7]; and d, hydrolyzing the triacetyl ganciclovir [7] to obtain ganciclovir [1]. The method has the advantages of easy and controllable preparation process, high utilization ratios of raw materials, low cost and high yield of a prepared ganciclovir product.
Owner:HUBEI GEDIAN HUMANWELL PHARMACEUTICAL CO LTD
Multifunctional microemlusion gel preparation and preparation process thereof
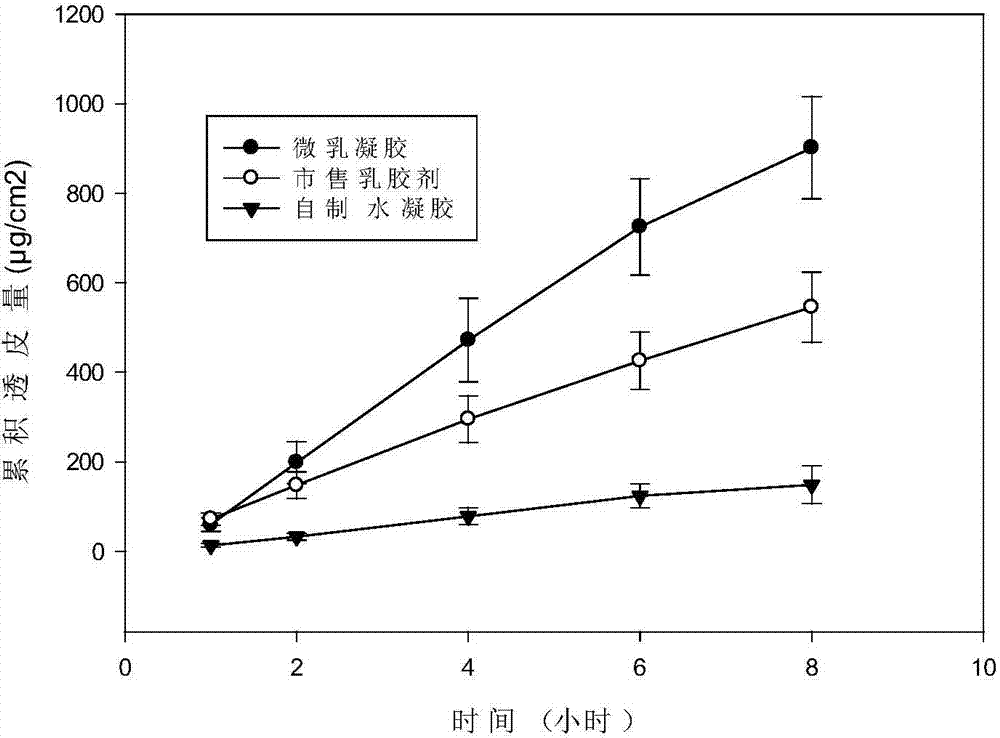
InactiveCN103655459AImprove skin penetrationImprove performanceAntimycoticsAntipyreticIndometacinActive agent
The invention discloses a multifunctional microemlusion gel preparation and a preparation process thereof, and belongs to the technical field of medicines. The preparation mainly comprises bulk pharmaceutical chemicals (such as non-steroidal anti-inflammatory drugs-diclofenac sodium, ibuprofen, indometacin, antifungal drugs-ornidazole, antiviral drugs-ganciclovir, hormone drugs-dexamethasone, local anesthesia drugs-lidocaine and irritants-menthol), a cationic polymer and a microemlusion, can further comprise gel or a thickener, and can be used for transdermal drug delivery and local drug delivery. The preparation process is simple, convenient, good in stability and pollution-free. Compared with existing cream and gel, the preparation has the advantages that a novel action mechanism is adopted, the accumulative penetration amount of unit area of drugs is remarkably increased, a certain slow-release effect is achieved, and the drug delivery frequency and the drug delivery amount can be reduced; a chemical penetration enhancer and a conventional preservative are not added, a certain bacterial inhibition effect is achieved, the skin irritation is avoided, and the use safety of the drugs is improved.
Owner:CHINA PHARM UNIV
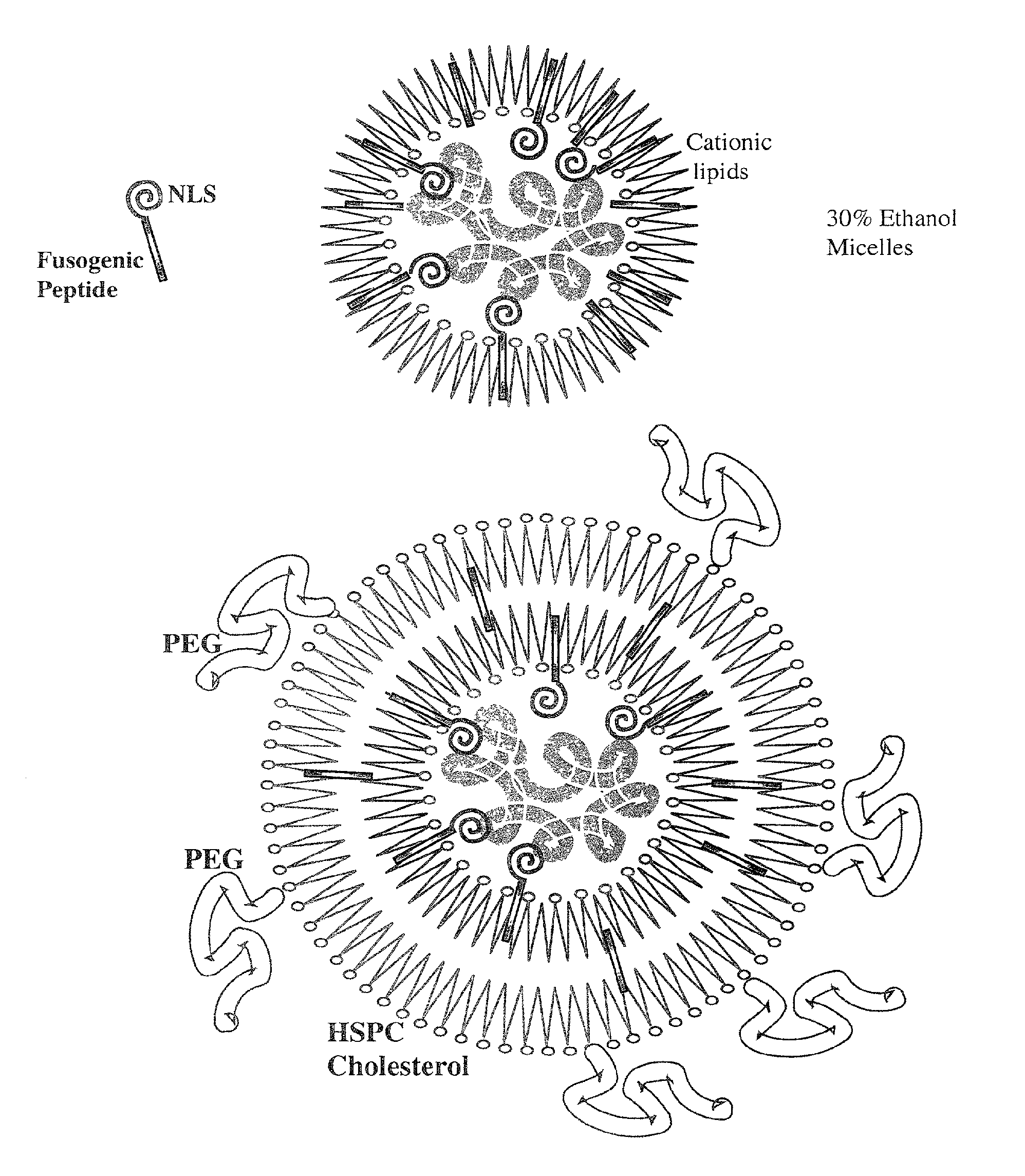
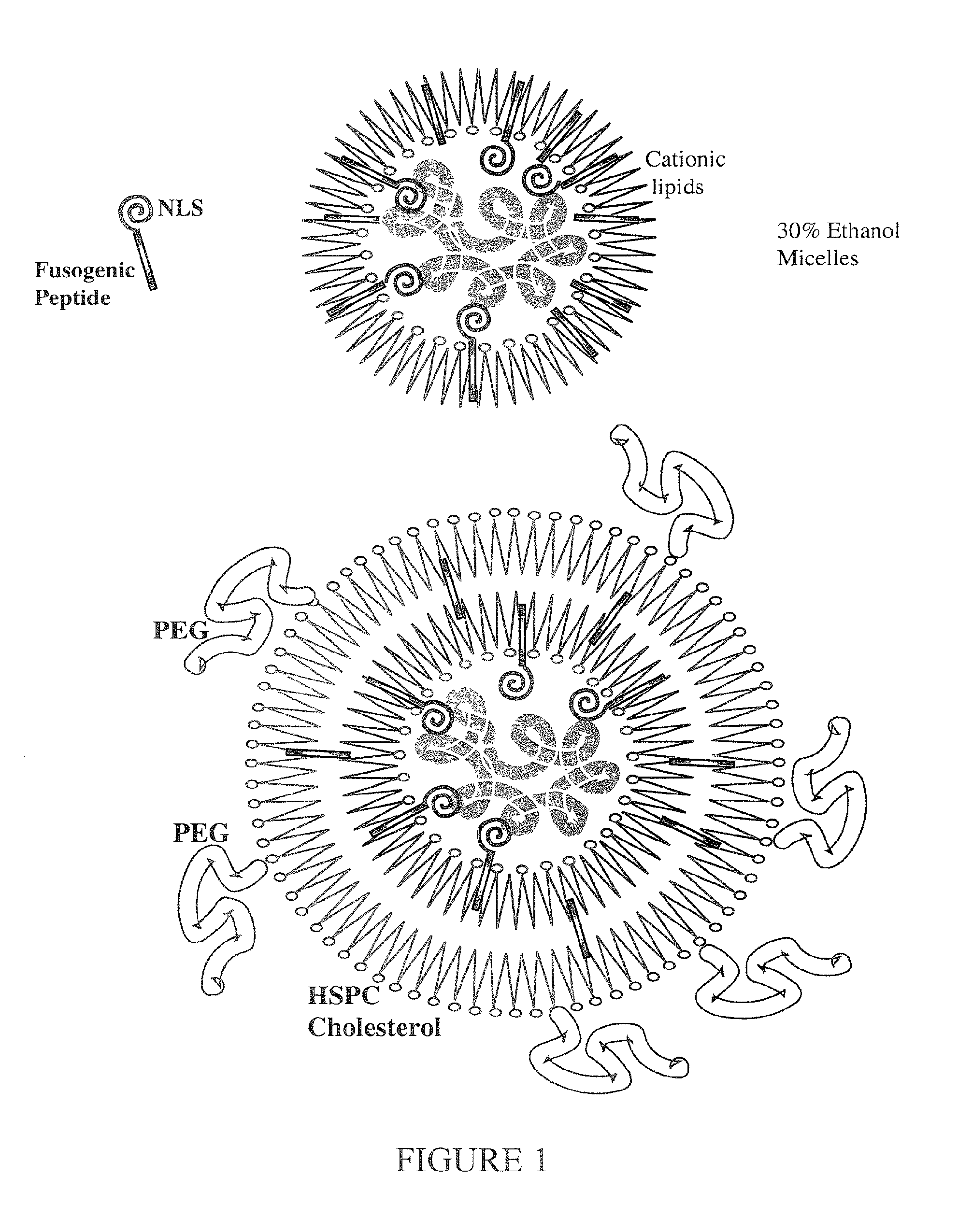
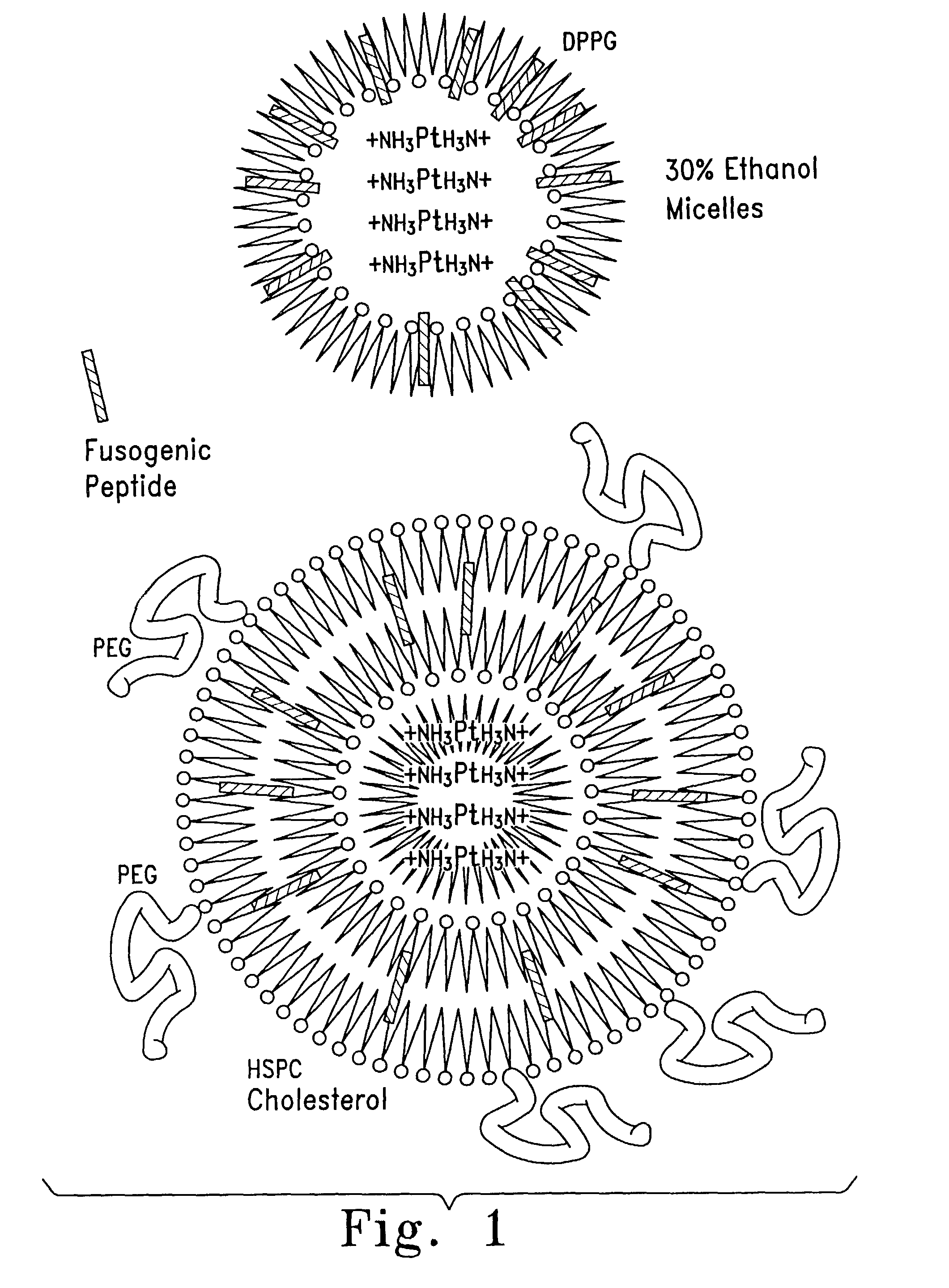
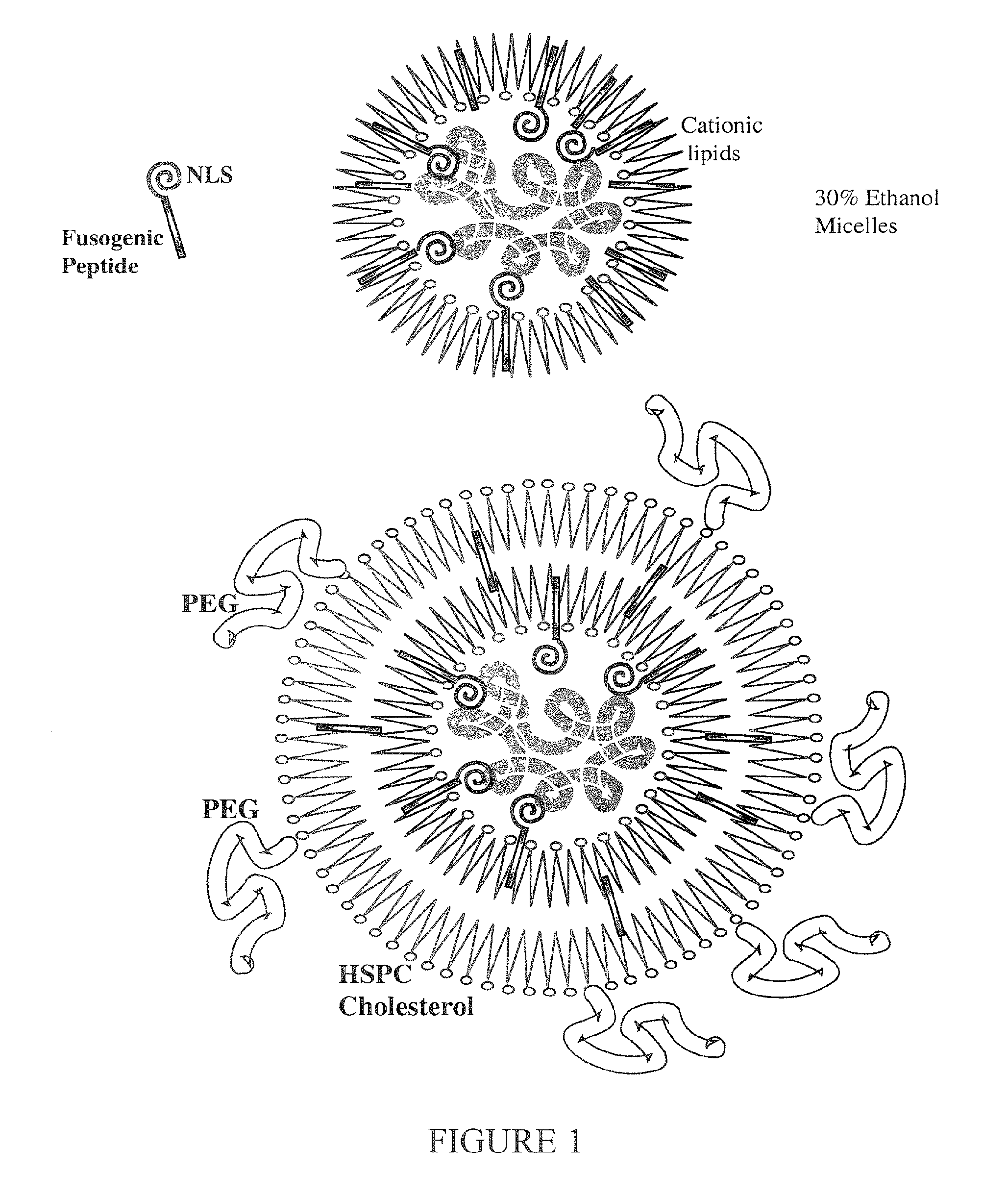
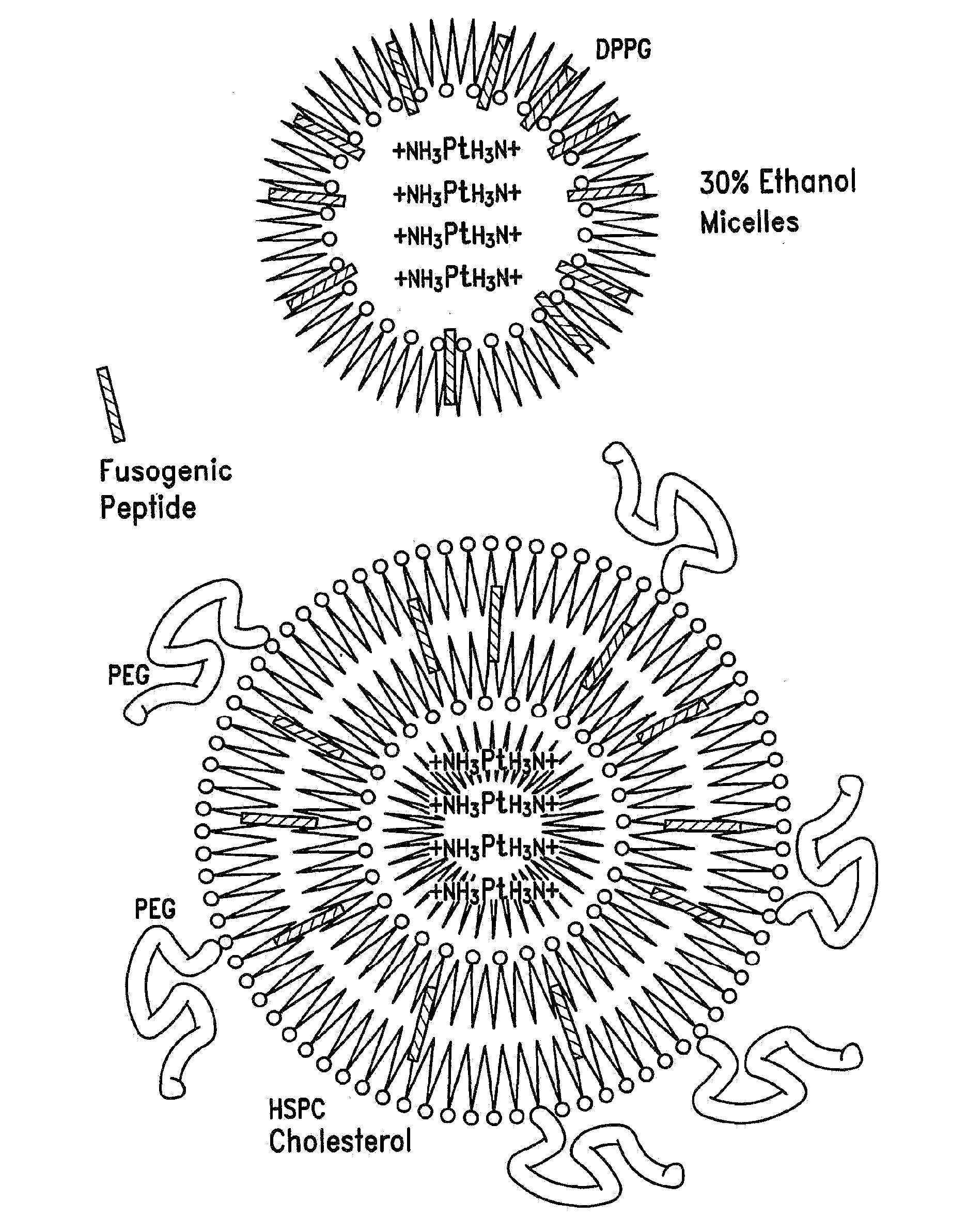
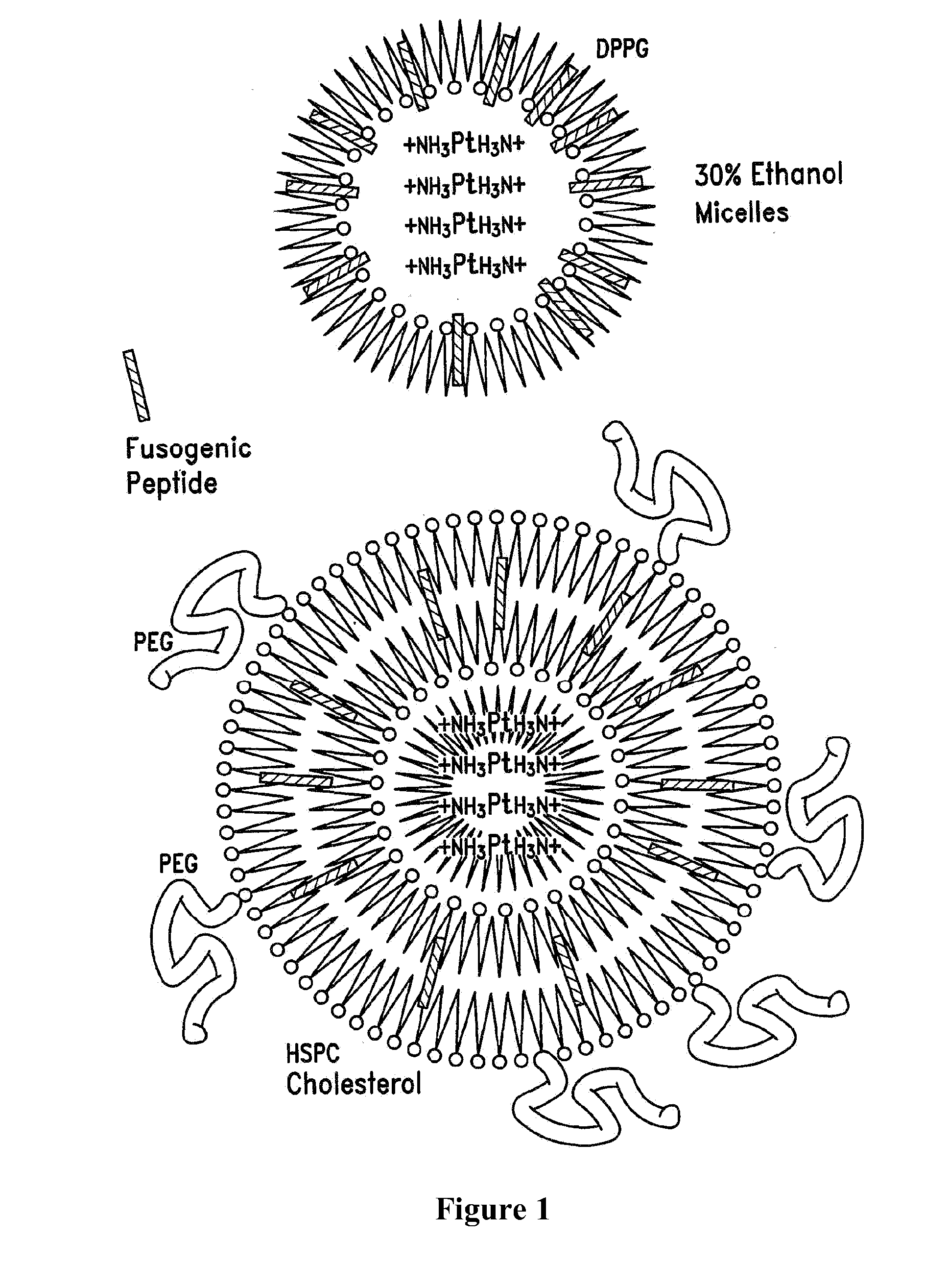
Encapsulation of Plasmid DNA (Lipogenes) and Therapeutic Agents with Nuclear Localization Signal/Fusogenic Peptide Conjugates into Targeted Liposome Complexes
InactiveUS20120183596A1Increase the entranceHigh yieldOrganic active ingredientsPeptide/protein ingredientsHuman tumorNucleotide
A method is disclosed for encapsulating plasmids, oligonucleotides or negatively-charged drugs into liposomes having a different lipid composition between their inner and outer membrane bilayers and able to reach primary tumors and their metastases after intravenous injection to animals and humans. The formulation method includes complex formation between DNA with cationic lipid molecules and fusogenic / NLS peptide conjugates composed of a hydrophobic chain of about 10-20 amino acids and also containing four or more histidine residues or NLS at their one end. The encapsulated molecules display therapeutic efficacy in eradicating a variety of solid human tumors including but not limited to breast carcinoma and prostate carcinoma. Combination of the plasmids, oligonucleotides or negatively-charged drugs with other anti-neoplastic drugs (the positively-charged cis-platin, doxorubicin) encapsulated into liposomes are of therapeutic value. Also of therapeutic value in cancer eradication are combinations of encapsulated the plasmids, oligonucleotides or negatively-charged drugs with HSV-tk plus encapsulated ganciclovir.
Owner:REGULON
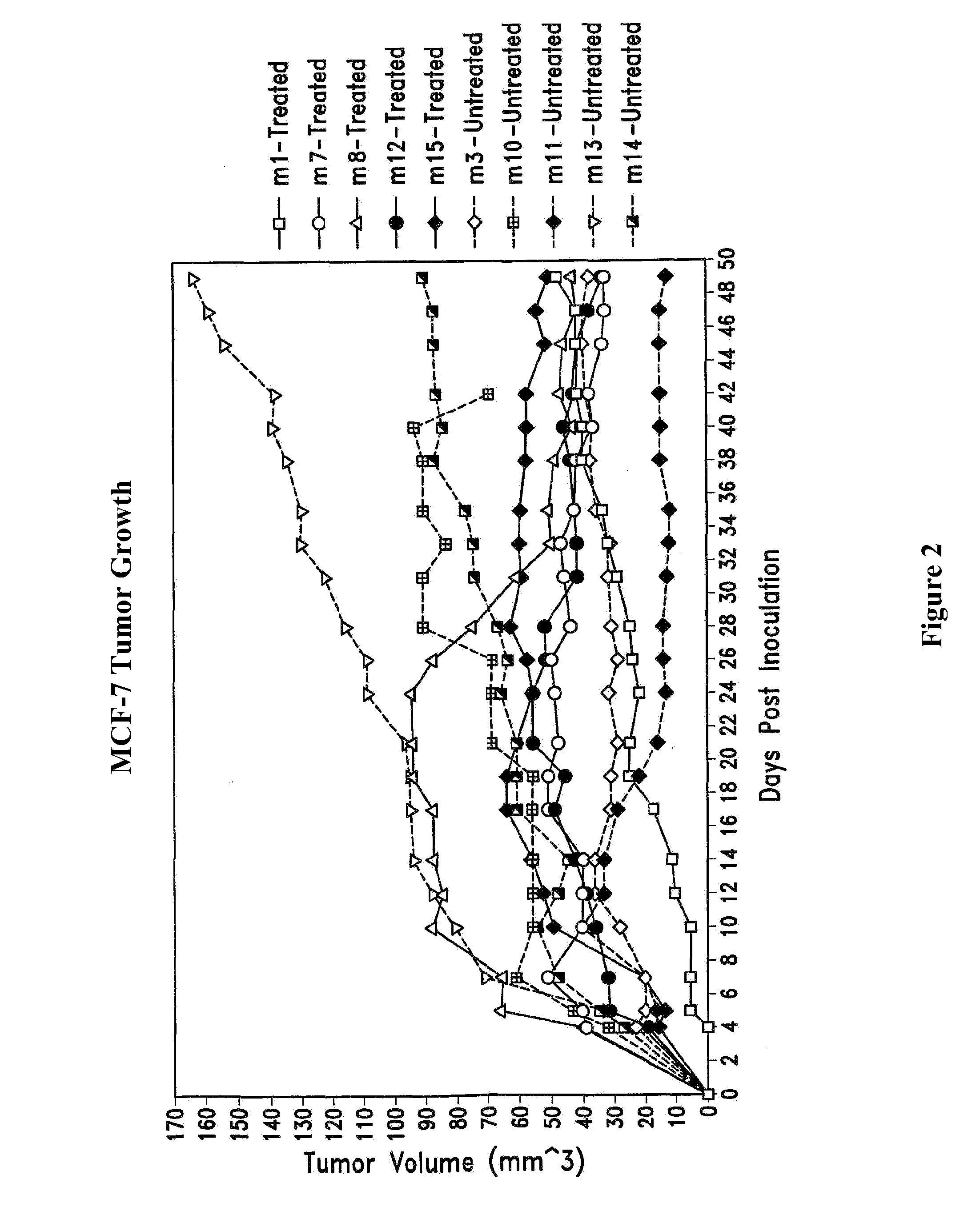
Therapy for human cancers using cisplatin and other drugs or genes encapsulated into liposomes
InactiveUS7393478B2Increase the entranceHigh yieldHeavy metal active ingredientsMicroencapsulation basedAngiostatinLymphatic Spread
A method for encapsulating cisplatin and other positively-charged drugs into liposomes having a different lipid composition between their inner and outer membrane bilayers is disclosed. The liposomes are able to reach primary tumors and their metastases after intravenous injection to animals and humans. The encapsulated cisplatin has a high therapeutic efficacy in eradicating a variety of solid human tumors including but not limited to breast carcinoma and prostate carcinoma. Combination of the encapsulated cisplatin with encapsulated doxorubicin or with other antineoplastic drugs are claimed to be of therapeutic value. Also of therapeutic value in cancer eradication are claimed to be combinations of encapsulated cisplatin with a number of anticancer genes including but not limited to p53, IL-2, IL-12, angiostatin, and oncostatin encapsulated into liposomes as well as combinations of encapsulated cisplatin with HSV-tk plus encapsulated ganciclovir.
Owner:REGULON
Anti-infective compositions, methods and systems for treating pathogen-induced disordered tissues
InactiveUS20060135464A1Stimulates rapid immunological attackExtraordinary therapeutic effectBiocidePharmaceutical delivery mechanismCidofovirMetabolite
Compositions, methods and systems for treating disordered epithelial tissues, such as is caused by pathogens and / or by toxins produced thereby. The invention relates to the use of an anti-infective and / or antimicrobial active agent in a carrier, with vigorous agitation of the disordered epithelial tissue for topical treatment thereof under such conditions sufficient to achieve clinically discernable improvement of the disordered epithelial tissue. The preferred anti-infective and / or antimicrobial active agent comprises a nucleoside, such as acyclovir, valcyclovir, penciclovir, famciclovir, ganciclovir, cidofovir, adefovir, and tenofovir, and derivatives, analogs, or metabolites thereof, or a mixture thereof, or 1-docosanol, optionally in combination with an organohalide. The inventive compositions and methods may employ the use of an applicator adapted for use in promoting the penetration of the treatment composition and / or the vigorous agitation of the disordered tissue.
Owner:QUADEX PHARMA
A kind of ganciclovir composition for injection and preparation method thereof
ActiveCN102274197AExcellent freeze-dried structureParticle size andPowder deliveryInorganic non-active ingredientsPorosityFreeze-drying
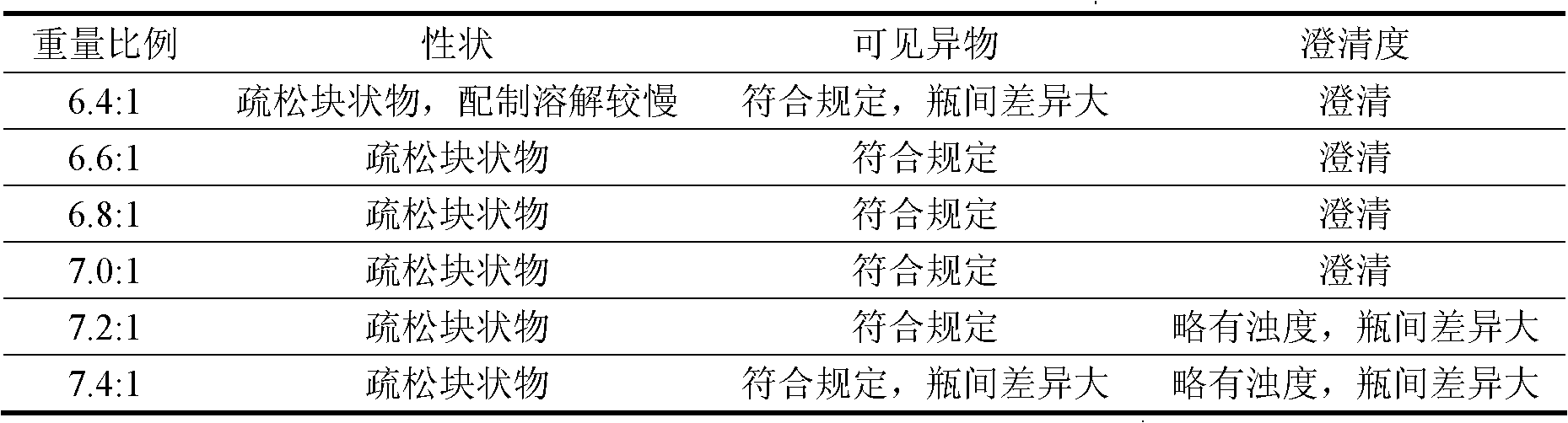
The invention relates to a ganciclovir composition for injection, which is freeze-dried powder composed of ganciclovir and sodium hydroxide, wherein the weight ratio of the ganciclovir to the sodium hydroxide is (6.6-7.0):1, the average particle size of the freeze-dried powder is 80-100nm, and the porosity is 94-98%. The preparation method comprises the following steps: 1) preparation: weighing ganciclovir and sodium hydroxide, putting the ganciclovir and sodium hydroxide into a preparation tank, adding water for injection, and stirring until the ganciclovir and sodium hydroxide are completely dissolved and evenly mixed; 2) sterile filtration and packaging; and 3) vacuum freeze drying. The invention has the advantages of simple formula, advanced technique, uniform quality and high stability, and has higher redissolution performance and clinical application safety.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Refining method for ganciclovir
ActiveCN102627643AReduce energy consumptionRefining cycle is shortOrganic chemistryGanciclovirFatty acid
The invention relates to the technical field of chemical refining, in particular to a refining method for ganciclovir. The refining method specifically comprises the following steps of: heating to dissolve the ganciclovir by using lower fatty acid aqueous solution; filtering off insoluble substances therein; cooling, crystallizing and suction-filtering filtrate; treating through inorganic alkali solution and then adjusting to be neutral, wherein the content of guanine impurity in the ganciclovir is reduced by a method of obtaining solid through crystallization. According to the method of reducing the content of the guanine impurity in the ganciclovir adopted in the refining method, the content of the guanine impurity can be reduced to be below 0.50 percent; and the method has the advantages of no need of repeating crystallization operation, short refining period, low energy consumption and high yield and is suitable for large-scale industrial production.
Owner:LIVZON GROUP CHANGZHOU KONY PHARMA
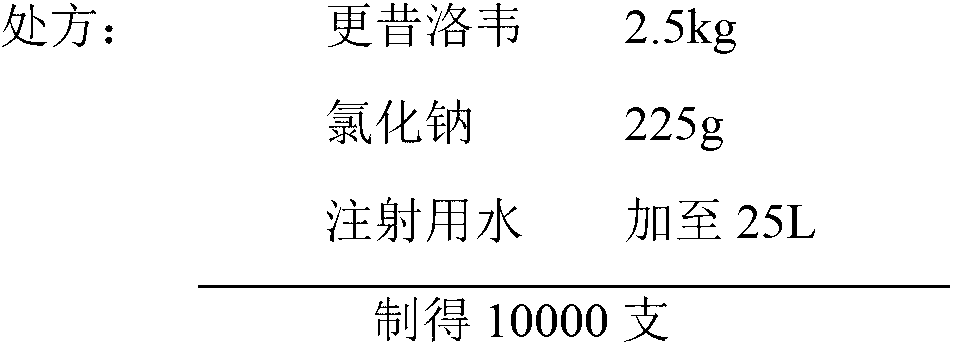
Ganciclovir for injection and preparation method thereof
ActiveCN103054819AAdvantages and Notable ImprovementsImprove yieldPowder deliveryInorganic non-active ingredientsMedicineFreeze-drying
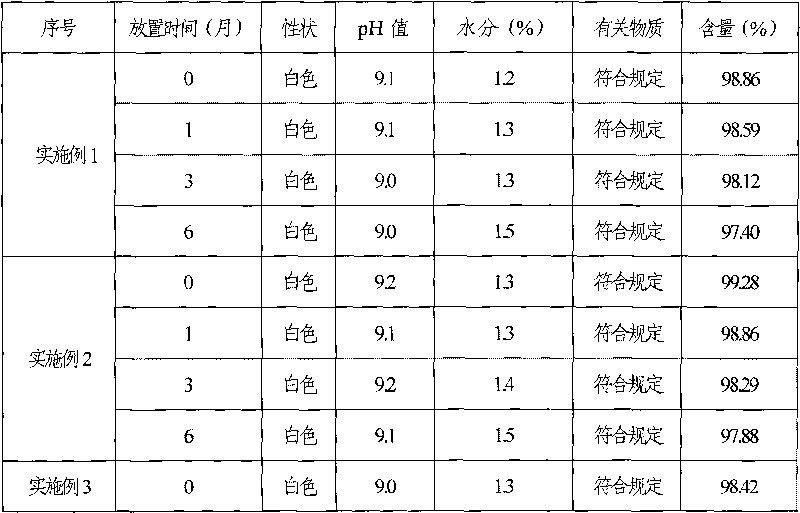
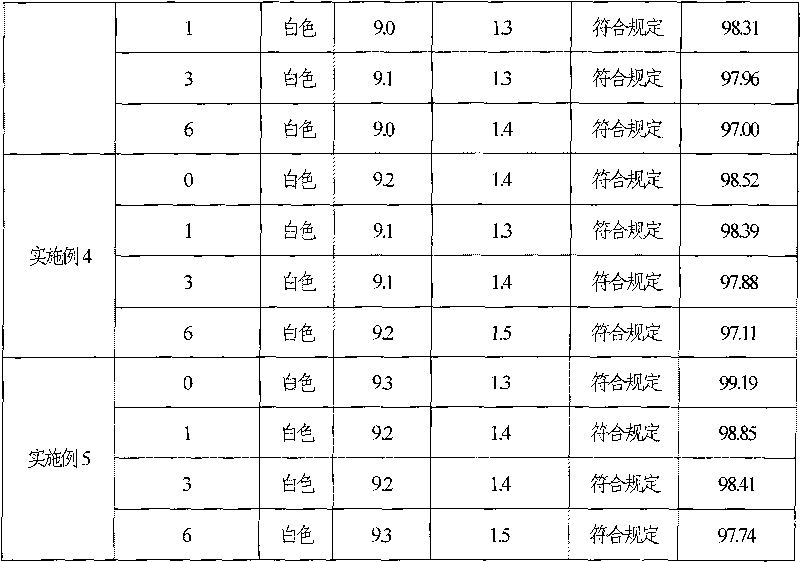
The invention discloses ganciclovir for injection and a preparation method thereof. The preparation is formed by regulating the pH of a solution containing ganciclovir and sodium chloride to 10.0-11.5 by adopting a pH regulator, and freezing and drying repetitively. The freeze-dried powder injection of the ganciclovir for injection has the advantages of high yield, good re-dissolubility, more stable quality and the like; change of related substances during the period of accelerated test is not obvious; and the ganciclovir for injection is safer for clinical application.
Owner:BEIJING WANPENGLANGGE PHARMA TECH
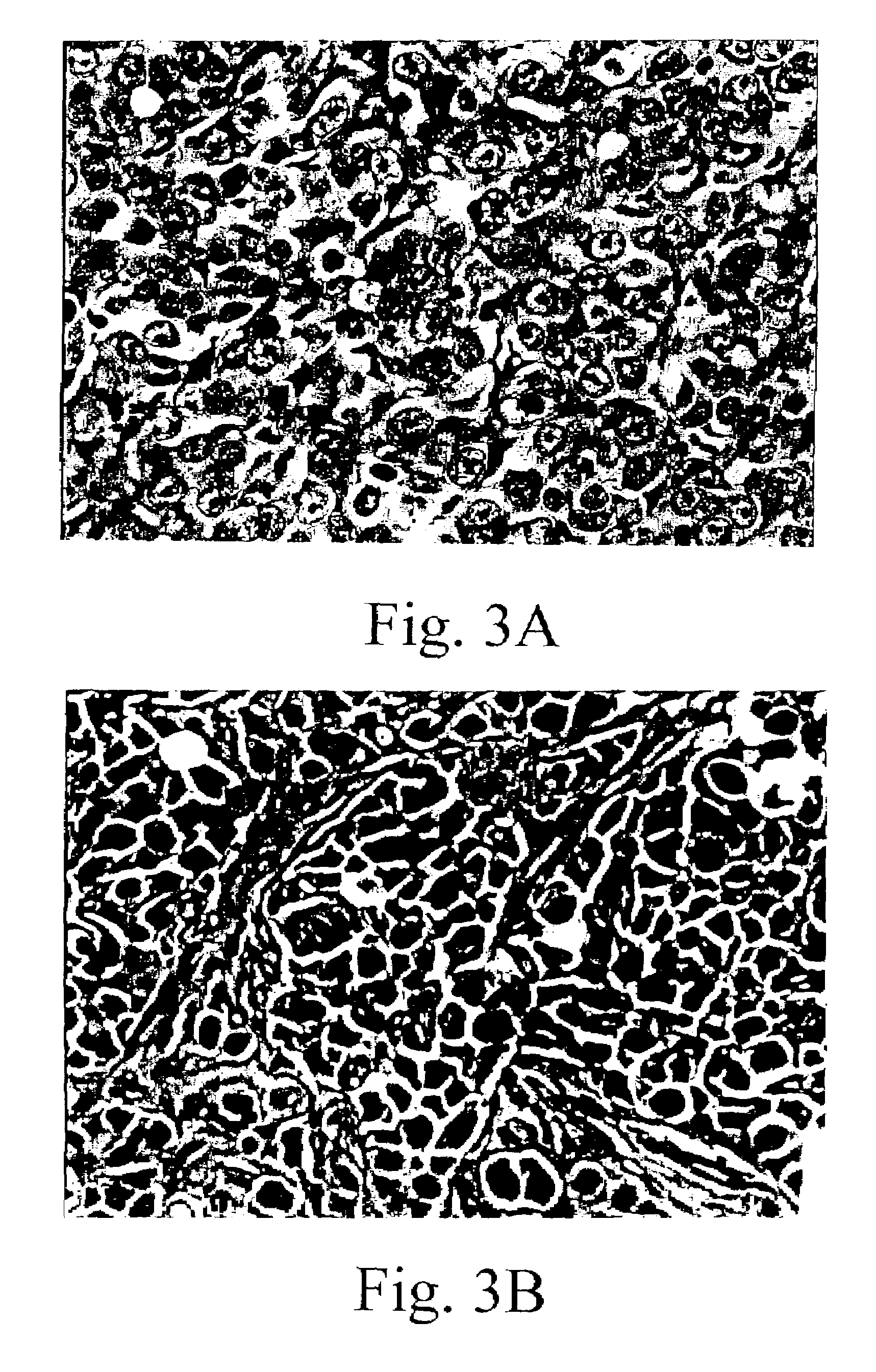
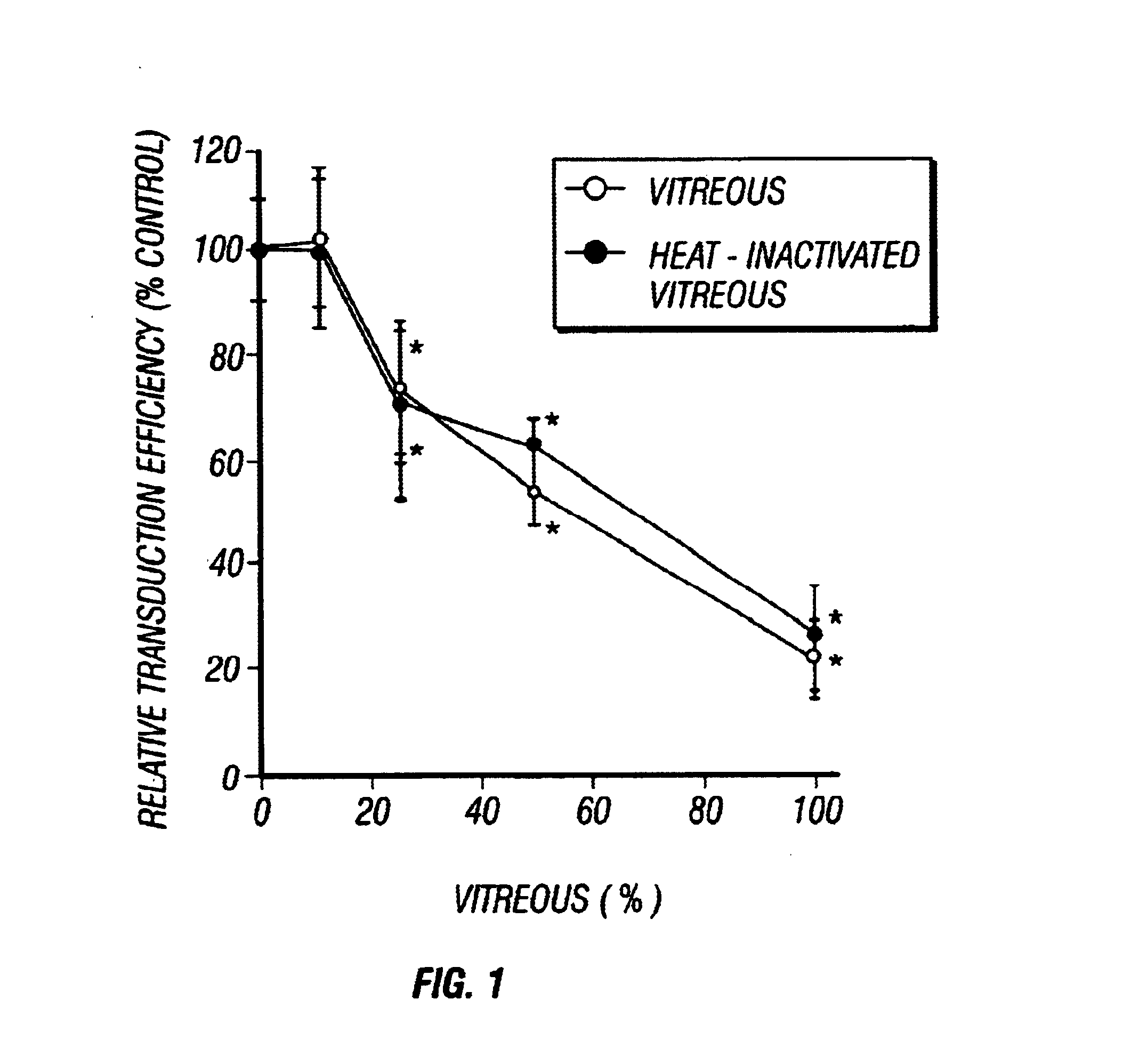
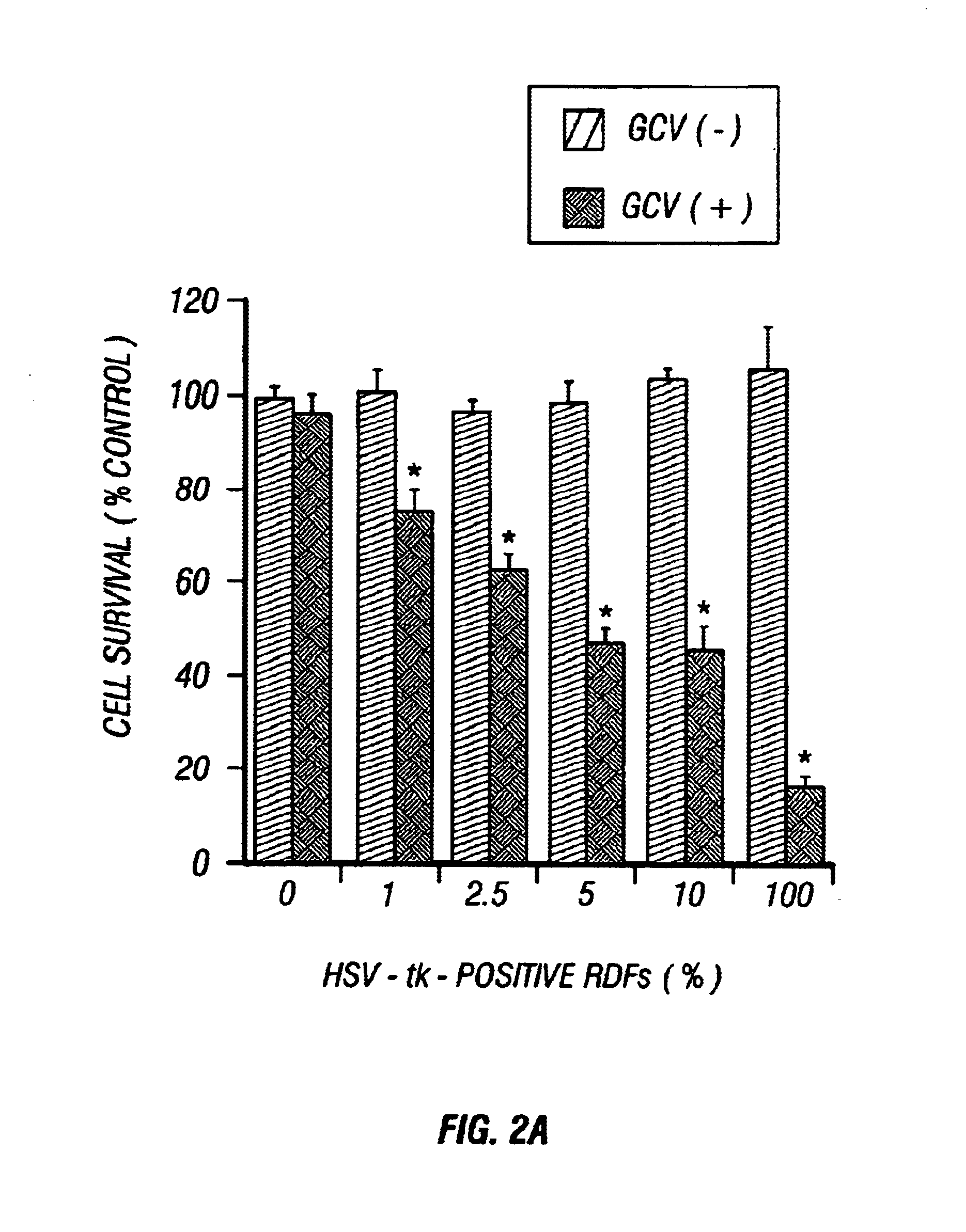
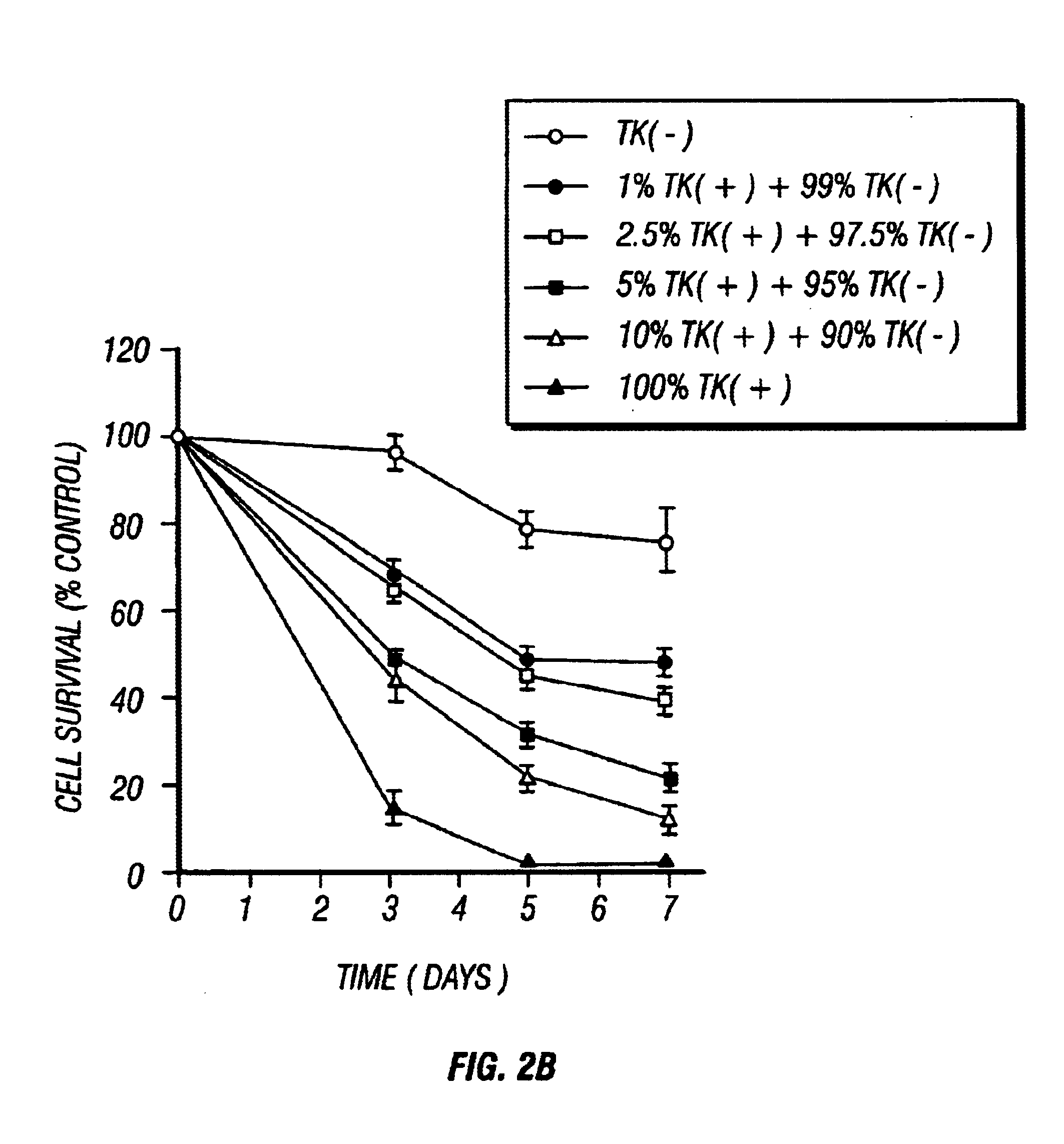
Gene Therapy for proliferative vitreoretinopathy
A method of treating ocular disorders (such as, for example, proliferative vitreoretinopathy or PVR) associated with replicating ocular cells by transfecting replicating ocular cells in vivo with a polynucleotide encoding an agent which is capable of providing for the inhibition, prevention, or destruction of the growth of the replicating ocular cells upon expression of the agent. The agent may be a viral thymidine kinase, and the polynucleotide encoding the agent may be contained in a retroviral vector. Once the replicating ocular cells are transduced with the retroviral vector, the patient is given a chemotherapeutic or interaction agent, such as ganciclovir, which kills the transfected replicating ocular cells.
Owner:UNIV OF SOUTHERN CALIFORNIA
Ganciclovir purification process
ActiveCN101851240AHigh purityThe number of process crystallization is lessOrganic chemistryAntiviralsActivated carbonOrganic solvent
The invention relates to a ganciclovir purification process, which specifically comprises the following steps: dissolving crude ganciclovir in alkaline solution, regulating pH, adding active carbon and conducting agitation and filtration; adding organic solvent in filtrate and filtering the obtained sediment; and dissolving filter cakes in deionized water, adding hydrochloric acid for neutralization, heating and dissolving the obtained sediment and then slowly decreasing temperature, and filtering and drying the produced crystals to obtain high-purity ganciclovir. Compared with the prior art, the invention has the advantages of simple operation, high yield, cost saving, reduced wastage in production, reduced environmental pollution and the like.
Owner:SHANGHAI YIWEI INDAL
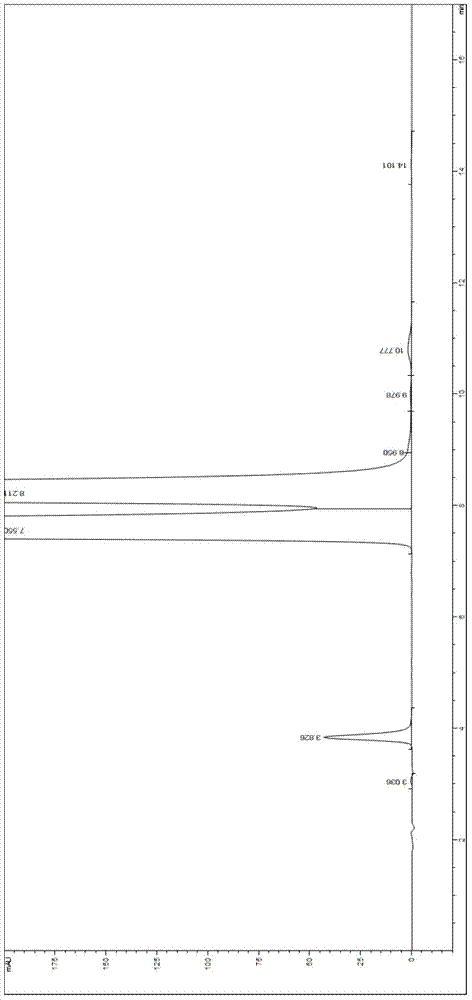
Valganciclovir hydrochloride impurity analytical detecting method
ActiveCN104749286ATroubleshooting Separation DifficultiesSimple and fast operationComponent separationValganciclovir HydrochlorideSilica gel
The invention belongs to the technical field of the analytical chemistry and in particular discloses a valganciclovir hydrochloride impurity HPLC (High Performance Liquid Chromatography) analytical detecting method, and more specifically, the invention relates to a method for analytically detecting guanine, ganciclovir, methoxymethylguanine, ganciclovir 1-N-methyl-valine, mono acetoxyl ganciclovir, mono chloro ganciclovir or a medicine or a preparation containing the above six impurities. A solution to be detected is filled in a high performance liquid chromatographic column taking phenylsilane bonded silica gel as a filling agent, a moving phase prepared by an amine-containing aqueous inorganic salt solution and a chromatographically pure organic solvent is adopted to wash and separate, and then ultraviolet analysis and detection is carried out. According to the method, ammonium acetate or ammonium formate with certain concentration is firstly added in the moving phase creatively, and difficult problems that the main peak and the impurity peak and the adjacent impurity peaks are separated difficultly are thoroughly solved, the operation is simple, the separation degree is high, the sensitivity is high, the accuracy is good, and no similar literature is reported through literature retrieval.
Owner:HUBEI LIYI PHARM TECH CO LTD +1
Refining method of ganciclovir
The invention relates to the technical field of chemical refining, particularly a refining method of ganciclovir, which comprises the following steps: heating to dissolve a ganciclovir crude product in a water and DMF (N,N-dimethylformamide) mixed solution, cooling to crystallize in a gradient cooling mode, and filtering the crystallized product to obtain the ganciclovir. The method for lowering the monochloro-ganciclovir impurity content in ganciclovir can lower the monochloro-ganciclovir impurity content to less than 0.10%; and the invention has the advantages of short refinement period, low energy consumption and high yield, and is suitable for large-scale industrial production.
Owner:LIVZON GROUP CHANGZHOU KONY PHARMA
Pharmaceutical composition containing ganciclovir compound, and preparation method thereof
InactiveCN102210686APromote dissolutionReduce the amount addedPowder deliveryInorganic non-active ingredientsInjection siteGanciclovir
The invention discloses a pharmaceutical composition containing a ganciclovir compound, and a preparation method thereof. The pharmaceutical composition is composed of ganciclovir, polysorbate 80, sodium hydroxide and dextran. The ganciclovir pharmaceutical composition overcomes the irritation of the existing ganciclovir lyophilized powder injections on an injection site, and improves the stability of the ganciclovir lyophilized powder injection and the compliance of patients.
Owner:罗诚
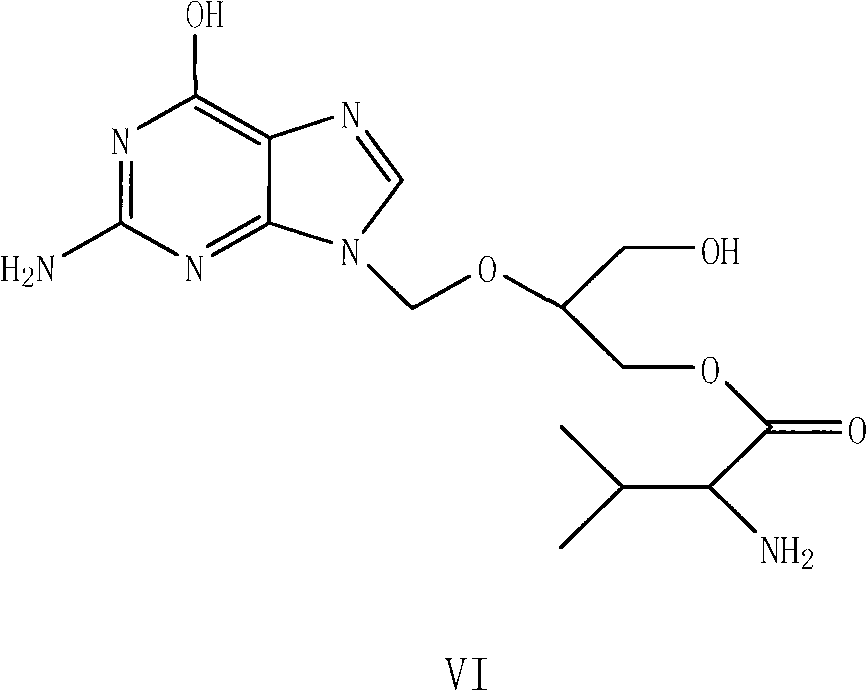
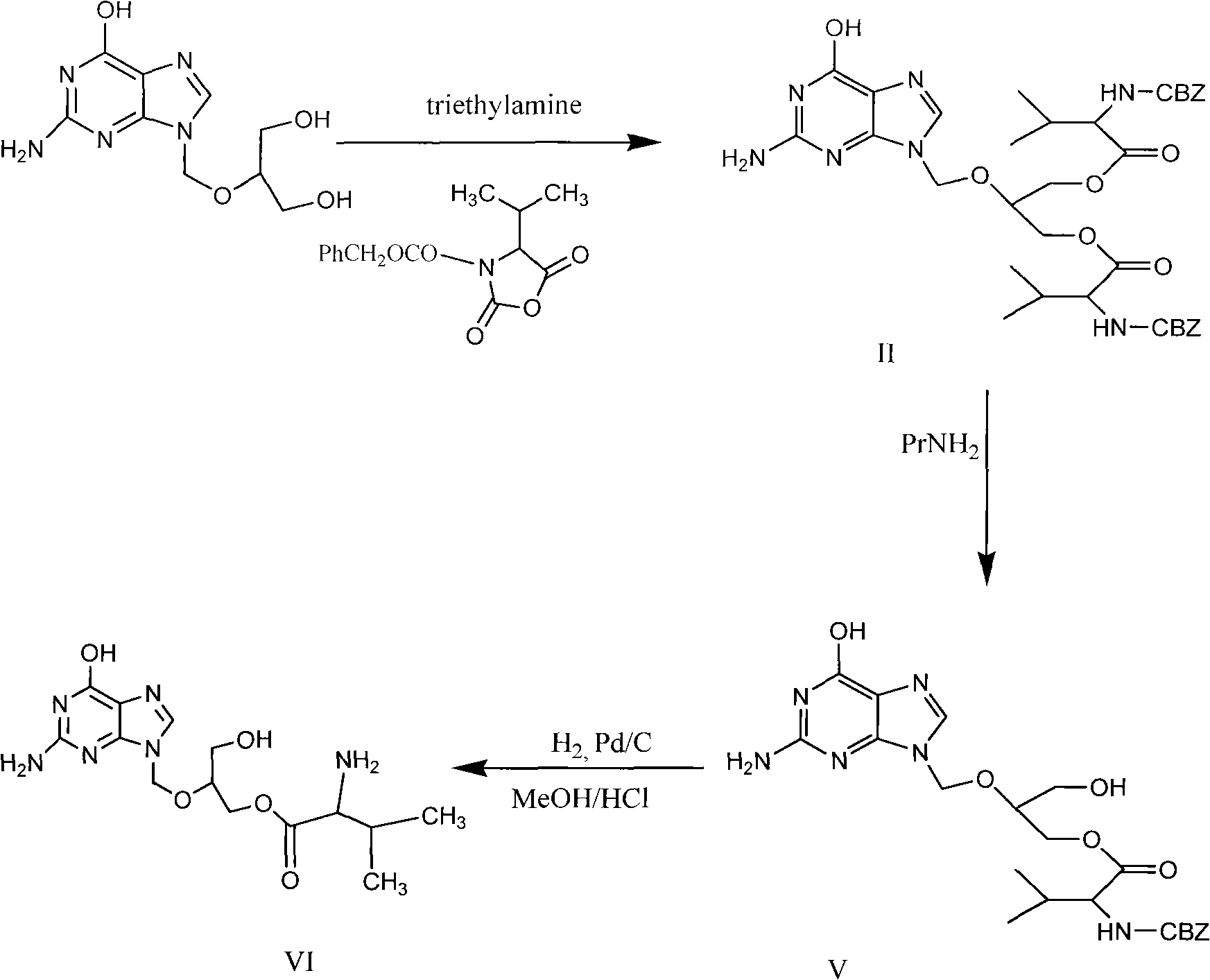
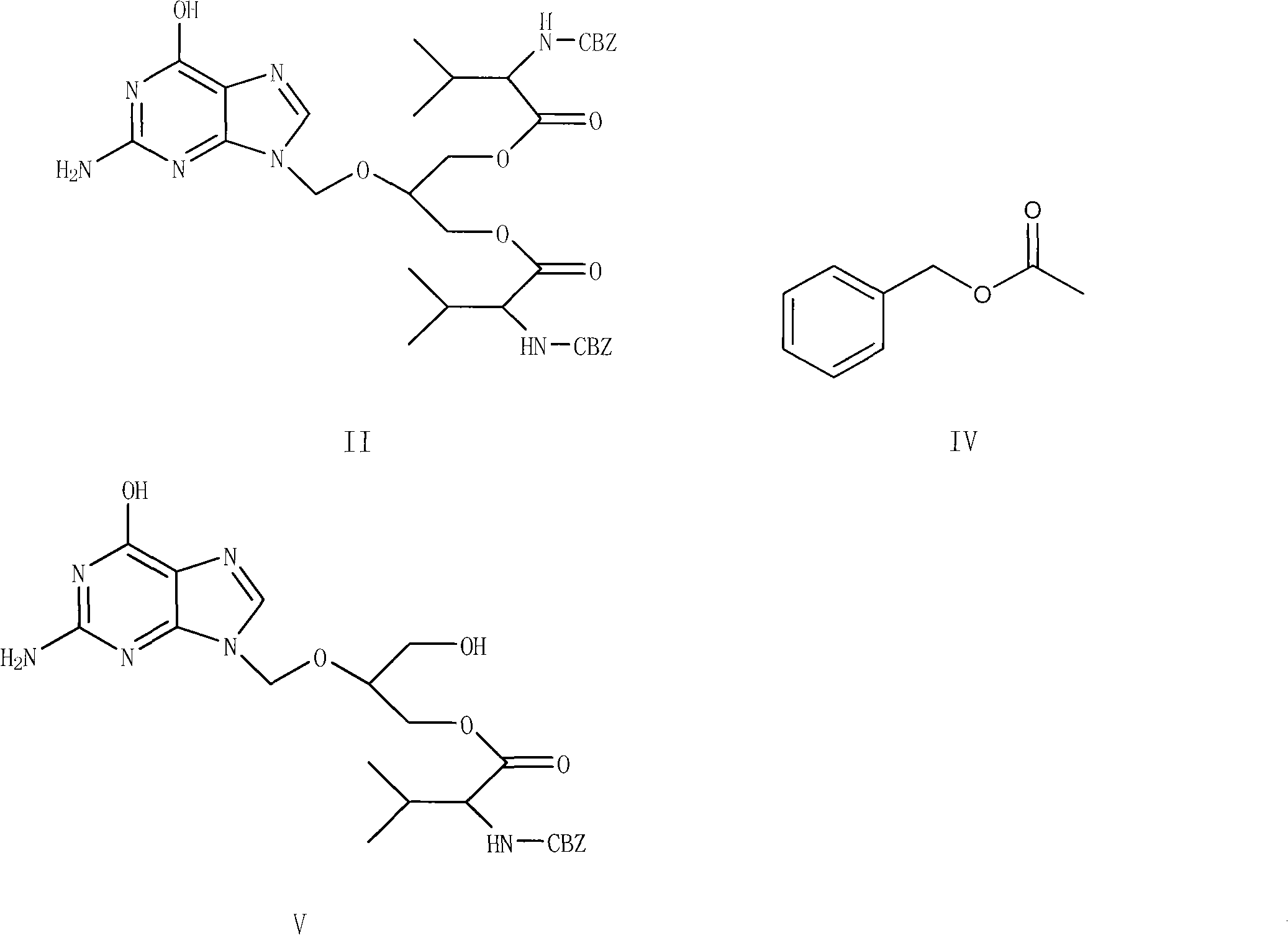
Preparation method for ganciclovir valine ester derivative
The invention discloses a preparation method for a ganciclovir valine ester derivative. The method comprises the following steps of: dissolving ganciclovir-CBZ-L-dual-valine ester shown as a formula II in a reaction solvent B at the temperature of 0 to 60 DEG C; adding a basic catalyst; keeping the temperature and reacting; tracking and monitoring the reaction solution until the reaction is finished; and obtaining a dissolving ganciclovir-CBZ-L-single-valine ester pure product by operations such as extracting, leaching, purifying and the like. The method has the advantages that: the route is simple and short; used auxiliary materials have no pollution to the environment basically; high-purity ganciclovir-CBZ-L-single-valine ester can be obtained; the molar yield of a refined product can reach over 50 percent and the content is easy to reach over 99.0 percent; complicated means such as column or column chromatography and the like are not needed; therefore, the method is very beneficialfor industrial production.
Owner:ZHEJIANG CHARIOTEER PHARMA
Liposome medicinal composition with tumor targeting, in-vivo tracing and treating functions and preparation method thereof
InactiveCN102429868AEffective targeted therapyNo side effectsOrganic active ingredientsGenetic material ingredientsSide effectTumor targeting
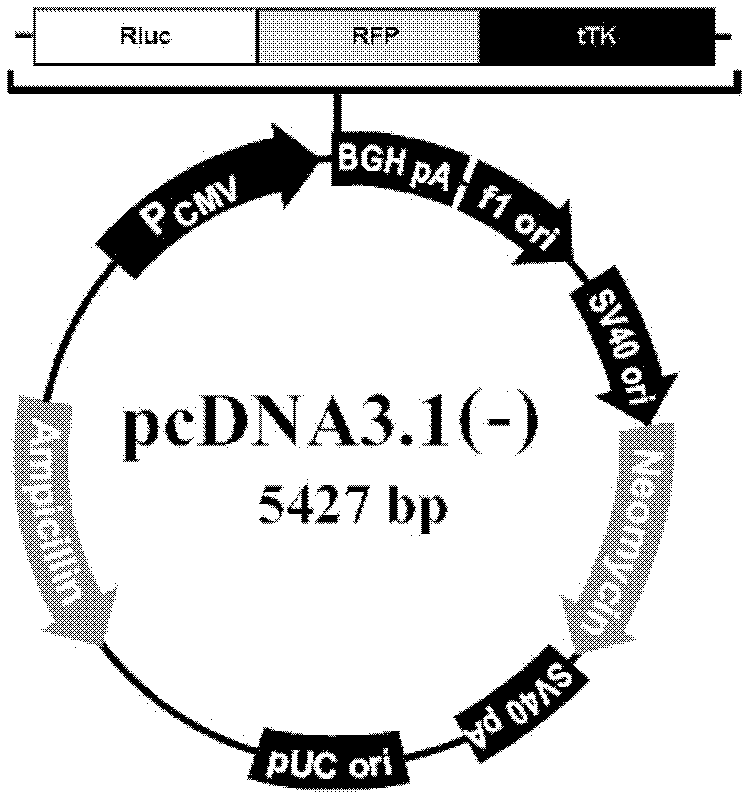
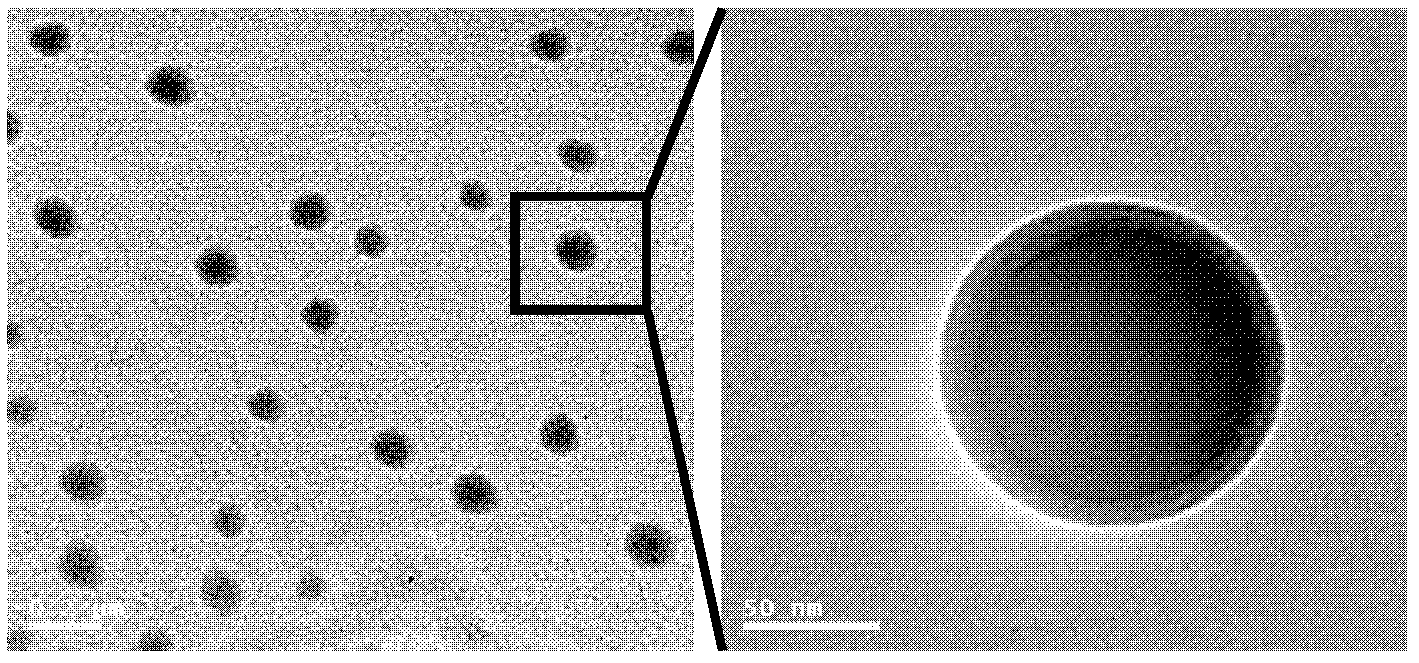
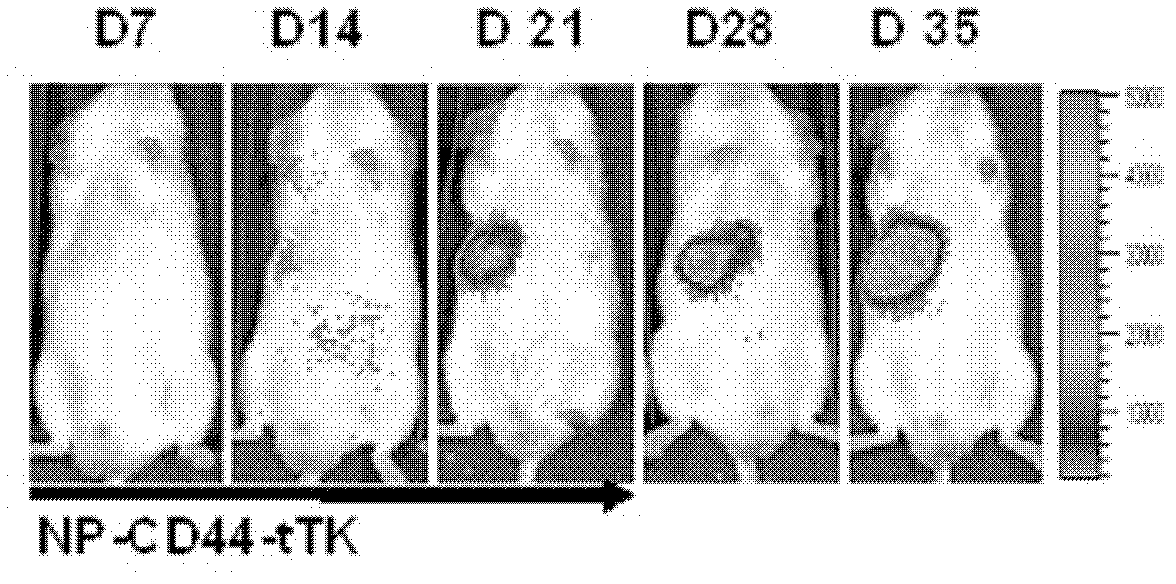
The invention discloses a liposome medicinal composition with tumor targeting, in-vivo tracing and treating functions and a preparation method thereof. The medicinal composition is a liposome targeting medicament which is resistant to CD44 antibody coupling, has a molecular imaging function simultaneously, and can be used for monitoring the in-vivo distribution of a medicament in real time in a living body state. In particular, plasmids containing three fusion genes, including renilla luciferase, red fluorescent proteins and suicide gene thymidine kinase, are coupled to CD44 antibody mediation-resistant immunoliposome, the specificity of liposome nanoparticles in a liver cancer in-situ model of an in-vivo targeting NOD / SCID (Non-Obese Diabetic / Severe Combined Immune-Deficiency) mouse is monitored by detecting a renilla luciferase signal with a living body imaging system, and apoptosis of liver cancer cells is induced by applying target thymidine kinase of ganciclovir; and moreover, a targeted liposome can be coated with adriamycin for inducing apoptosis of the liver cancer cells. The liposome medicinal composition provided by the invention does not have any toxic or side effect, has small damage and a good effect, and is suitable to be applied for a long time.
Owner:NANKAI UNIV
Stereochemically defined dipeptide esters of antiviral agents for enhanced ocular treatment
InactiveUS20090149482A1Enhanced enzymatic stabilitySufficient hydrophilicityBiocideSenses disorderDipeptideMedicine
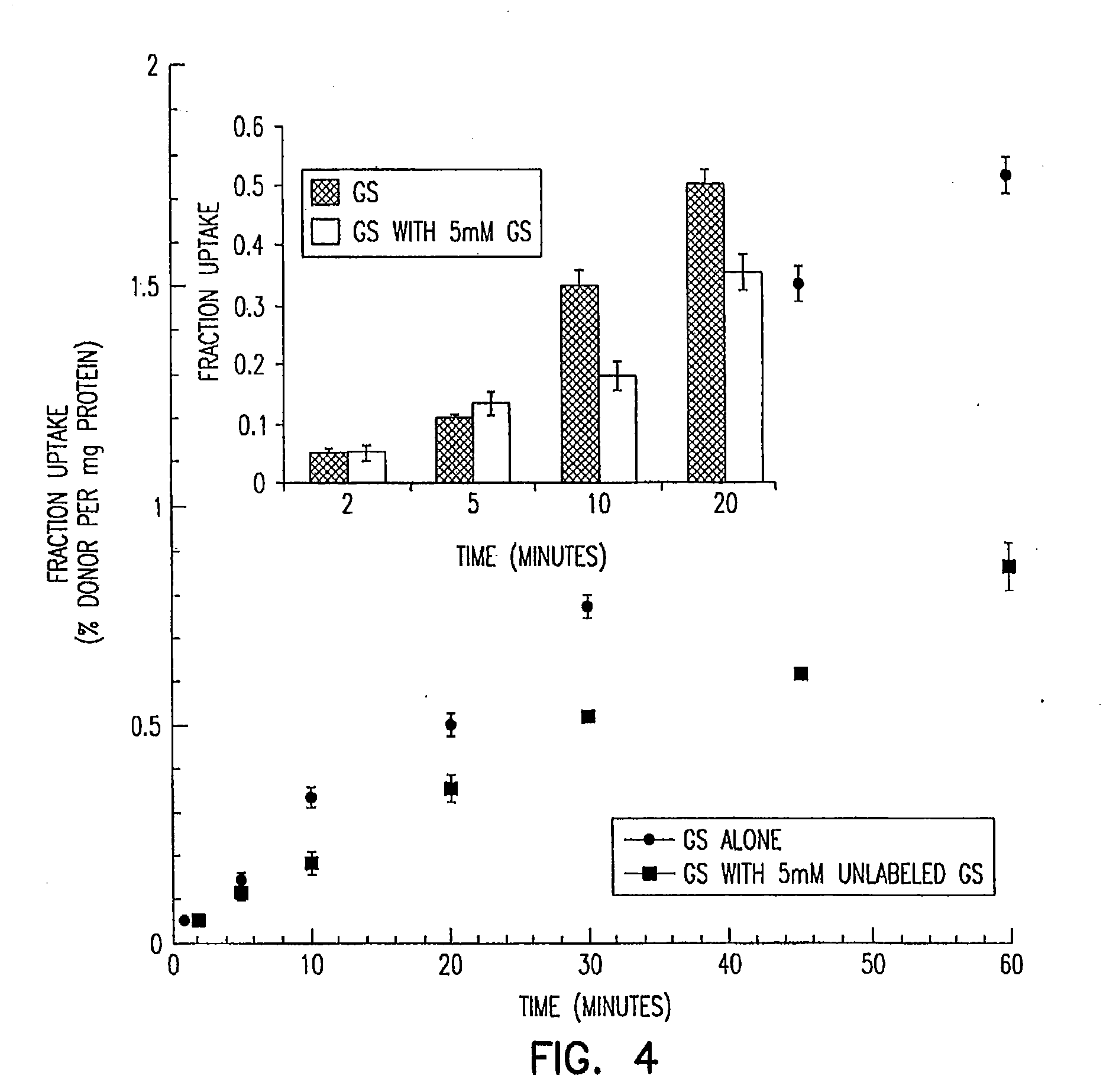
Stereochemically defined dipeptide esters of nucleoside-analogous antiviral agents including acyclovir and ganciclovir are provided. Certain of these stereochemically defined dipeptide esters are found to have unexpectedly enhanced delivery to and uptake by ocular tissues, crossing the blood-ocular barrier more effectively than other stereochemically defined dipeptide esters. For example, (L-Val)-(D-Val)-acyclovir was found to be taken up more effectively into corneal tissue than were underivatized acyclovir, monoesters (L-Val)-acyclovir or (D-Val)-acyclovir, or diester (L-Val)-(L-Val)-acyclovir.
Owner:UNIVERSITY OF MISSOURI
Process for the preparation of ganciclovir
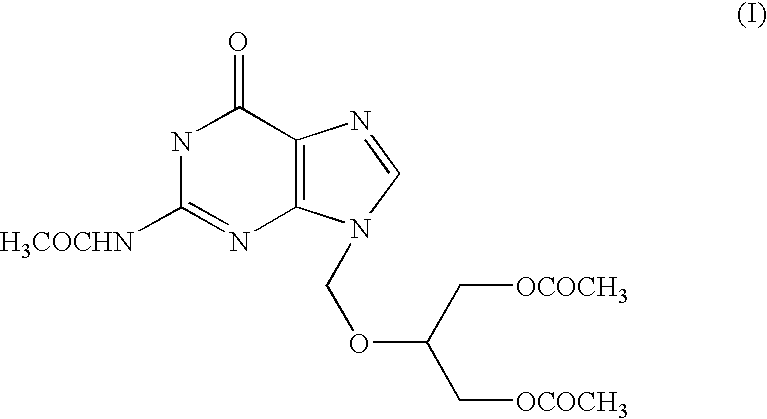
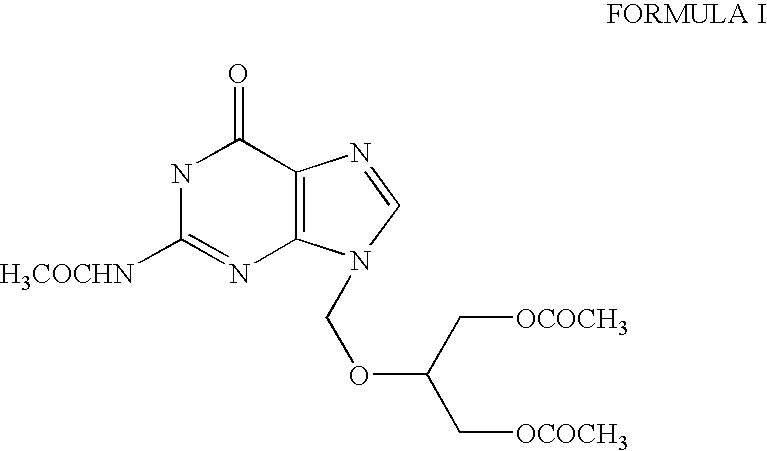
The present invention relates to a process for the preparation of N2-Acetyl-9-(1,3-diace-toxy-2-propoxymethyl) guanine, referred to here as N-9 alkylated isomer of structural formula I, and to the use of this compound as an intermediate for the preparation of antiviral compound, ganciclovir.
Owner:RANBAXY LAB LTD
Nano drug-carrying system, and preparation and application thereof
ActiveCN106822921AGood biocompatibilityLow cytotoxicityEnergy modified materialsGenetic material ingredientsPolyethylene glycolMesoporous silica
The invention relates to the technical field of medicine, and provides a nano drug-carrying system. The nano drug-carrying system comprises a magnetic-mesoporous silicon dioxide nanorod carrier, a pro-drug GCV (ganciclovir) carried on the carrier, and a graft copolymer PLL-g-PEG (poly-L-lysine graft polyethylene glycol) formed by PLL and PEG, wherein the PLL-g-PEG also carries a suicide gene TK. The magnetic-mesoporous silicon dioxide nanorods are used as the magnetic targeted carrier to carry the suicide gene TK / pro-drug GCV to enter cells, thereby implementing the time and space consistency of the suicide gene and pro-drug transfer, and further implementing accurate drug release. The magnetic-mesoporous silicon dioxide silicon dioxide nanorods and suicide gene / pro-drug treatment method are effectively combined, thereby enhancing the combined treatment effect on the liver cancer. The preparation method of the nano drug-carrying system is simple in technique and suitable for large-scale industrial production.
Owner:SUZHOU INST OF BIOMEDICAL ENG & TECH CHINESE ACADEMY OF SCI
Externally used drug for treating condyloma acuminata and genitalia herpes
InactiveCN101219156ALow priceLittle side effectsAntiviralsSexual disorderSide effectExternal application
The invention relates to a medicine for external application and for curing condyloma acuminatum and herpes progenitalis. The medicine is mixed by oleum fructus bruceae and clovirs antiviral drugs (such as acyclovir, ganciclovir, etc.) by a certain ratio, which are prepared into externally applicable dosage forms, such as liniment, ointment, etc. by a specific process with medicinally acceptable optional bases. The medicine for external application is characterized by low cost, good curative effect, high recovery rate, and low recurrence rate after recovery, as well as convenient use, safety, reliability and small side-effects during the therapeutic process.
Owner:BEIJING HOPE HUGE PHARM SCI
Method for determining human plasma antiviral drug concentration
InactiveCN101105478ASimple and fast operationEasy to operateComponent separationFluorescence/phosphorescenceAntiviral drugMedicine
The invention belongs to medical detection field, relates to an analysis detection method of drug in the body of a person, and specifically relates to the method that the densities of antiviral drugs in blood plasma of the person such as acyclovir, ganciclovir and penciclovir can be detected at the same time. The method in the invention is characterized in that pilot sample is pretreated; as acyclovir, ganciclovir and penciclovir have the character of strong fluorescence absorption, acyclovir, ganciclovir and penciclovir can be separated from each other in an acidity flowing phase chromatographic column and be detected by a fluorescence detector. The method in the invention has the advantages of little sample, simple, swift and sensitive pretreatment, short analysis period and low cost; furthermore, the invention doesn't need expensive equipment and reagent and is suitable for the detection of clinical conventional blood drug density of acyclovir, ganciclovir and penciclovir.
Owner:AFFILIATED HUSN HOSPITAL OF FUDAN UNIV
Gancilorvir dispersable tablet and its preparation
InactiveCN1634067AShort disintegration timeGood dispersionAntiviralsPill deliveryMagnesium stearateCross-linked polyethylene
The invention provides a Gancilorvir dispersable tablet and its preparation, wherein the dispersion tablet comprises 62.5 wt% of Ganciclovir, 10-25% of crystalline cellulose, or calcium hydrogen phosphate and / or starch, 1-10% of sodium carboxymethylstarch, or low substituted methylcellulose propylene glycol ether and / or cross bonding polyvinylpyrrolidone or crosslinked sodium carboxymethylcellulose, 0.1-1.4% of polyvinylpyrrolidone, or methyl hydroxypropylcellulose, 5-15% of lactose, 0.5-1.4% of magnesium stearate. The preparing process comprises sieving, mixing, granulating, drying, and tabletting.
Owner:HUAZHONG NORMAL UNIV
Therapy for human cancers using cisplatin and other drugs or genes encapsulated into liposomes
InactiveUS20090280164A1Heavy metal active ingredientsMicroencapsulation basedAngiostatinLymphatic Spread
A method for encapsulating cisplatin and other positively-charged drugs into liposomes having a different lipid composition between their inner and outer membrane bilayers is disclosed. The liposomes are able to reach primary tumors and their metastases after intravenous injection to animals and humans. The encapsulated cisplatin has a high therapeutic efficacy in eradicating a variety of solid human tumors including but not limited to breast carcinoma and prostate carcinoma. Combination of the encapsulated cisplatin with encapsulated doxorubicin or with other antineoplastic drugs are claimed to be of therapeutic value. Also of therapeutic value in cancer eradication are claimed to be combinations of encapsulated cisplatin with a number of anticancer genes including but not limited to p53, IL-2, IL-12, angiostatin, and oncostatin encapsulated into liposomes as well as combinations of encapsulated cisplatin with HSV-tk plus encapsulated ganciclovir.
Owner:REGULON INC
A treating method of a ganciclovir condensation compound isomer
The invention relates to the technical field of chemical compound recovery, and particularly relates to a method of converting a ganciclovir condensation compound isomer into a ganciclovir condensation compound. The method utilizes a characteristic that the ganciclovir condensation compound isomer can be converted into the ganciclovir condensation compound by structural transformation in a solution, and the ratio of the ganciclovir condensation compound isomer to the ganciclovir condensation compound in the solution is in a dynamic equilibrium relationship of about 35:65. By adoption of the technical scheme, the purity of the obtained ganciclovir condensation compound can be more than 98.0%, wherein the content of the ganciclovir condensation compound isomer is less than 0.5% and the yield is about 50%. The method effectively overcomes treatment problems of the ganciclovir condensation compound isomer, increases the total yield of the ganciclovir condensation compound, indirectly reduces the raw material cost of the ganciclovir condensation compound, reduces environment pollution and is suitable for popularization and application.
Owner:LIVZON GROUP CHANGZHOU KONY PHARMA
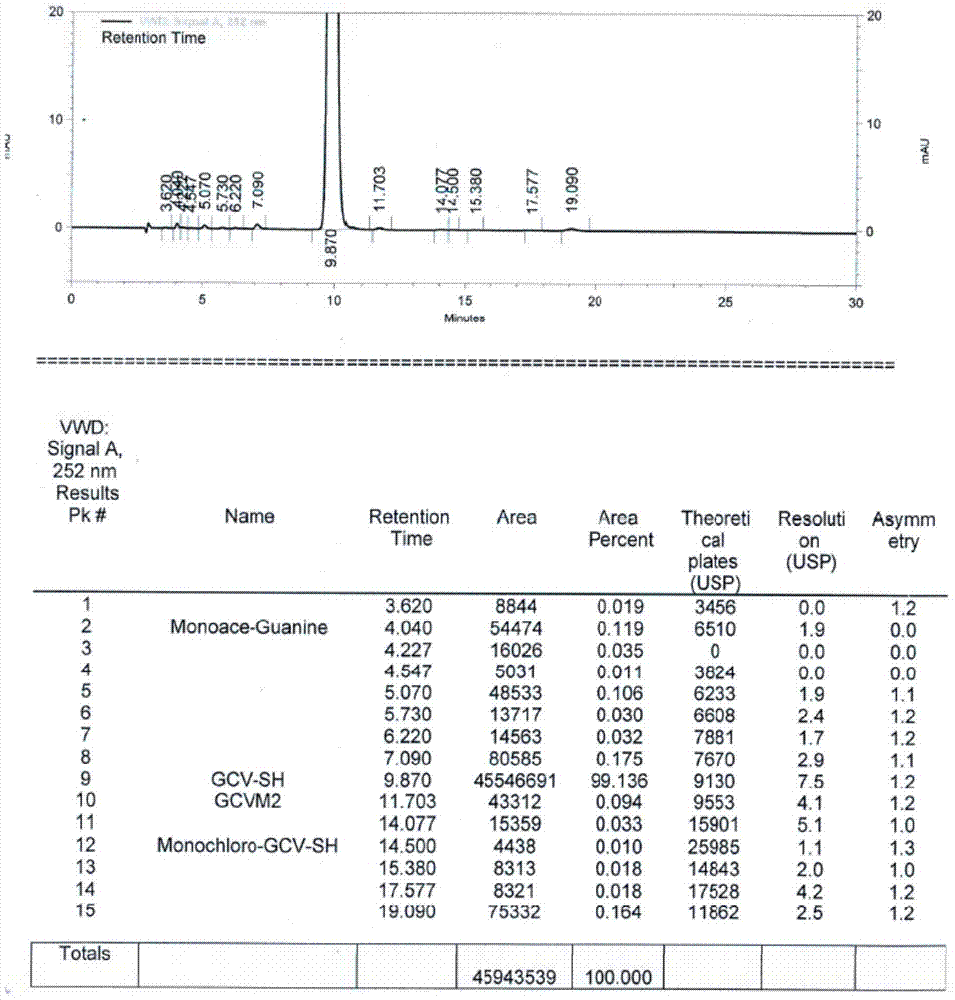
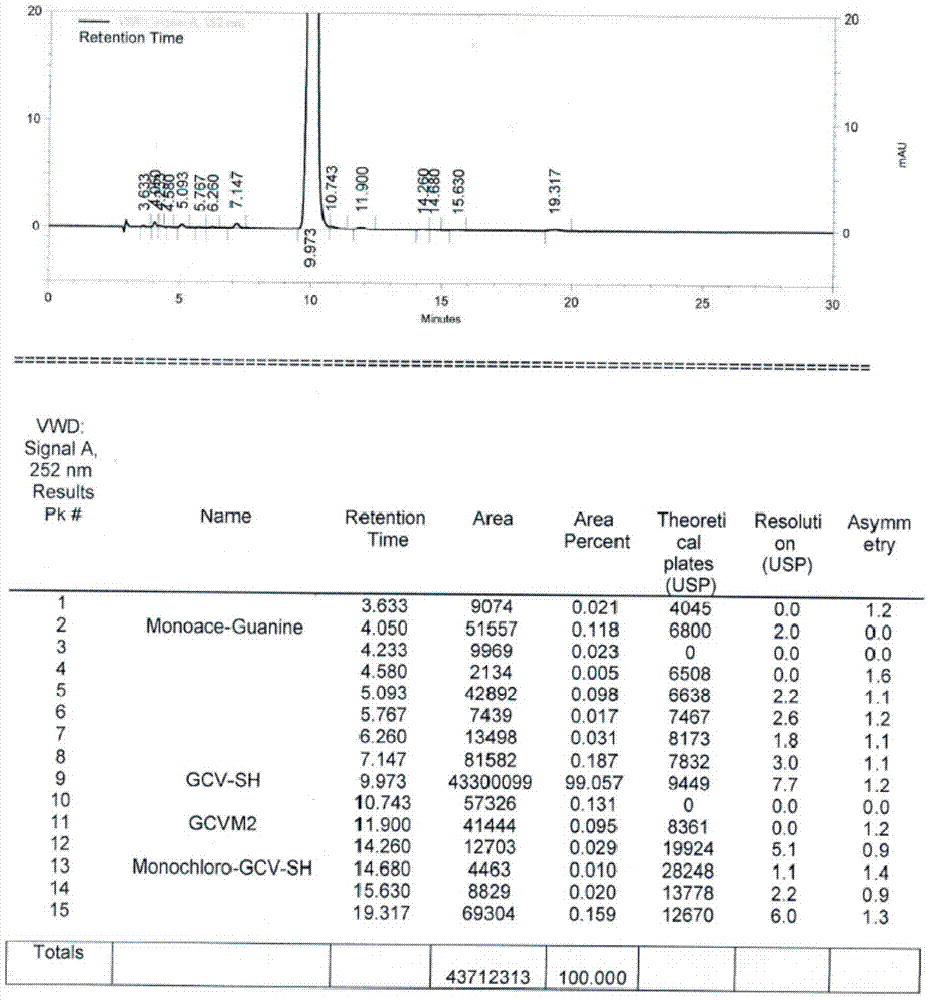
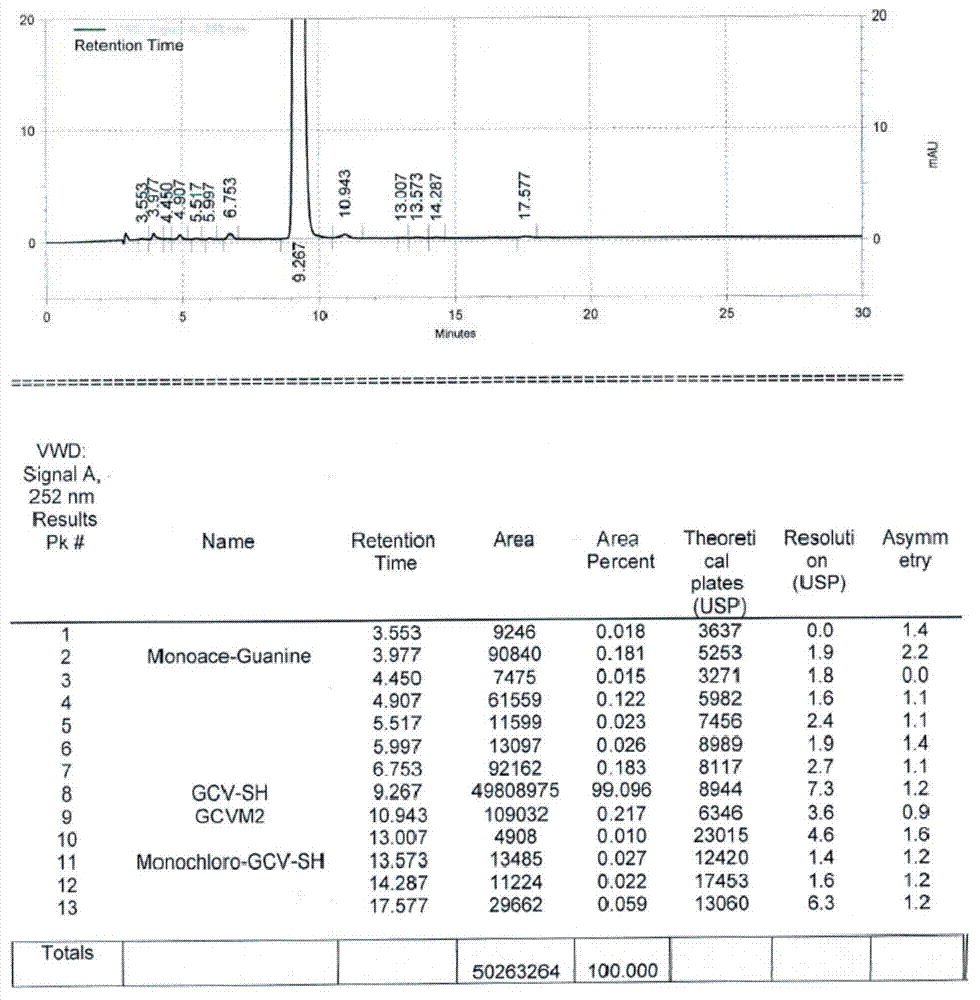
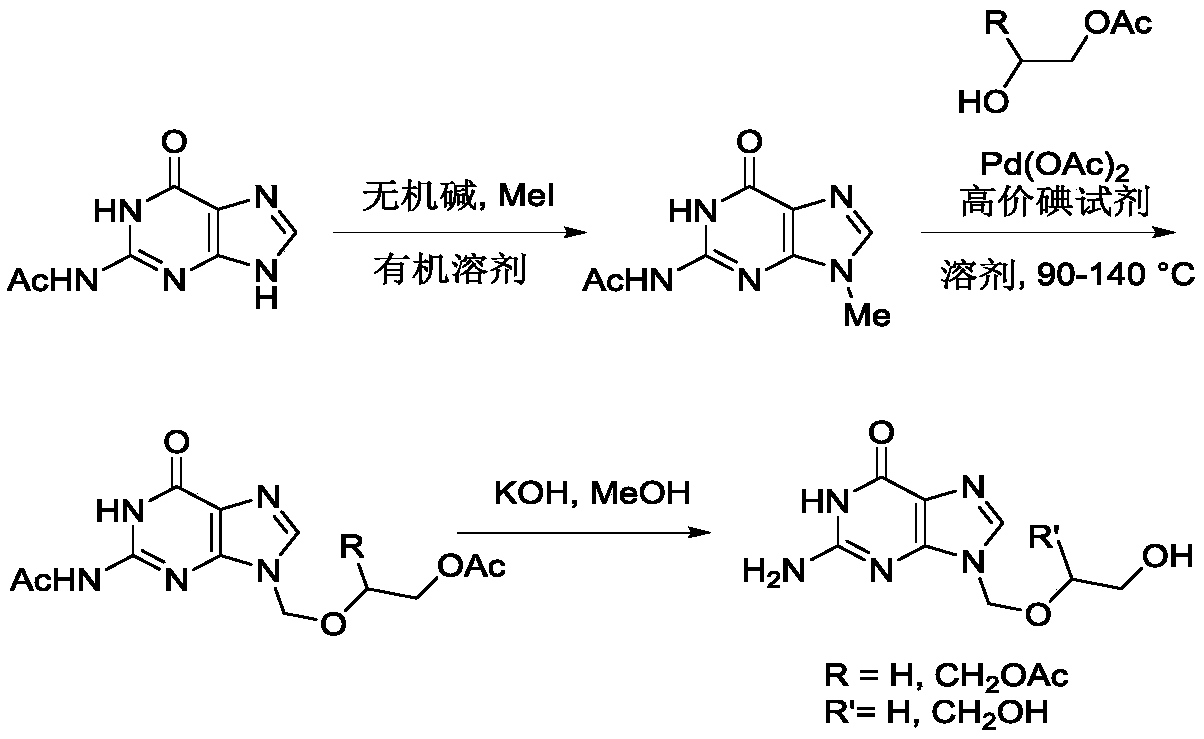
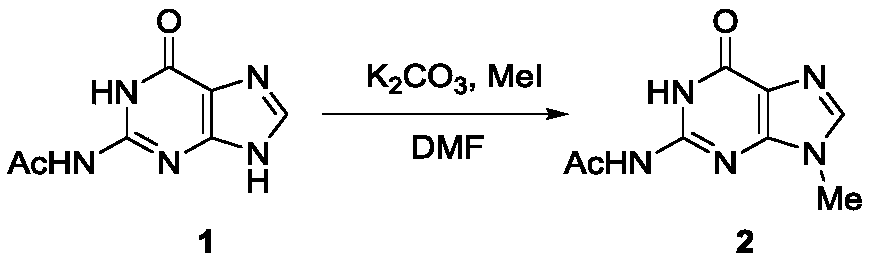
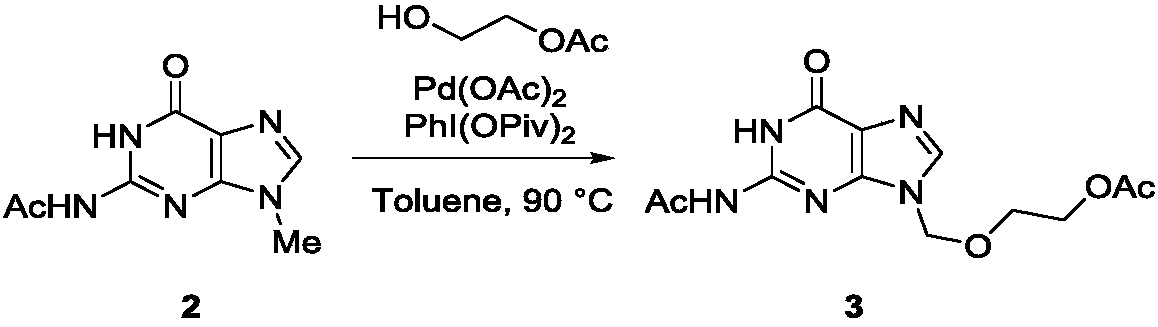
Method for synthesizing acyclovir and ganciclovir by carbon-hydrogen bond activation
The invention discloses a method for synthesizing acyclovir and ganciclovir by carbon-hydrogen bond activation and belongs to the field of organic synthesis. The method comprises that inexpensive guanine as a raw material undergoes methyl protection on 9th NH, a high-valent iodine reagent and monoacetyl-protected ethylene glycol or 1, 2-isopropylidene-protected glycerol are added into the raw material under catalysis of palladium acetate, the mixture undergo a heating reaction to produce acetyl-protected acyclovir or acetyl-protected ganciclovir, and the acetyl group is removed by an inorganicalkali alcohol solution so that acyclovir and ganciclovir are obtained. The method utilizes cheap and easily available raw materials, prevents use risk and corrosive reagents, has the advantages of short reaction route, simple operation, high atomic economy and high total product yield, provides a novel synthesis route of acyclovir and ganciclovir and has a potential application prospect.
Owner:XINYANG NORMAL UNIVERSITY
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com