Method for synthesizing acyclovir and ganciclovir by carbon-hydrogen bond activation
A technology of carbon-hydrogen bond activity and ganciclovir, which is applied in the field of synthesis of raw materials, can solve the problems of long side chain synthesis route and low total yield, and achieve the effect of cheap raw materials, short reaction route and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
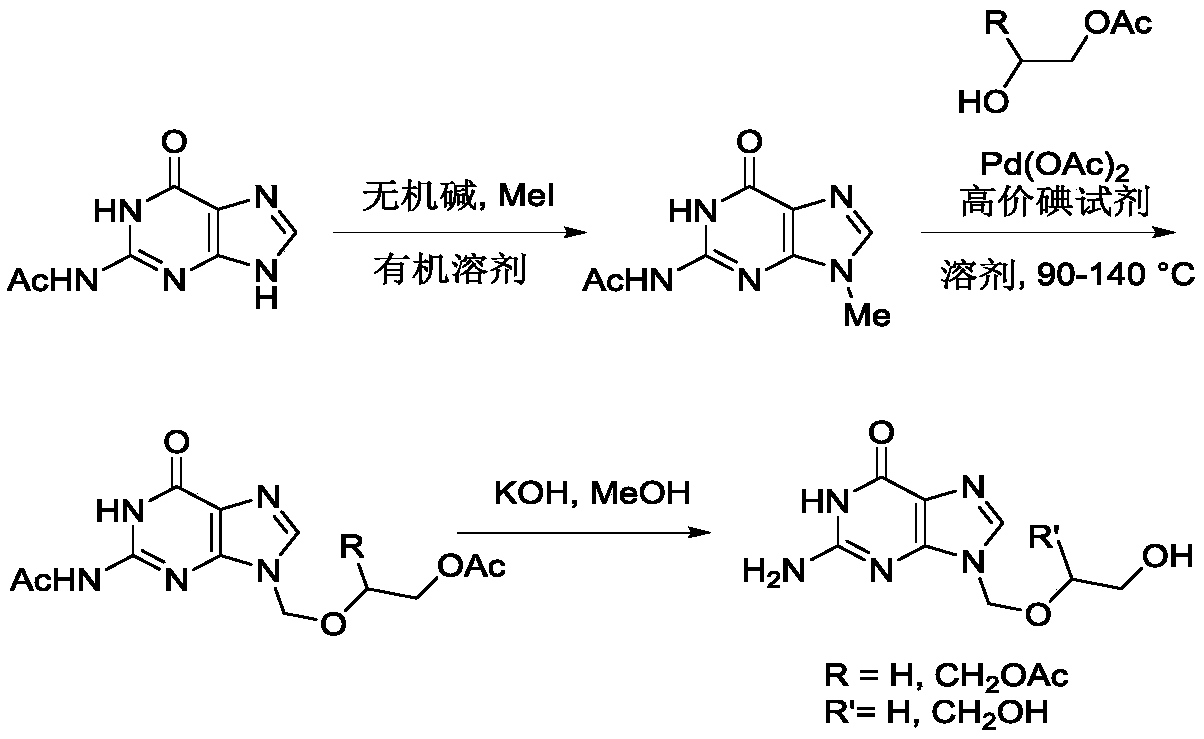
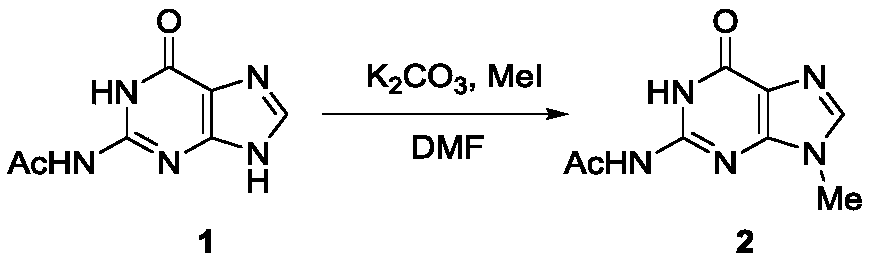
[0021] In the first step, the reaction formula is as follows:
[0022]
[0023] 5 mmoles of N2-acetylguanine (1) were dissolved in 15 mL of anhydrous DMF, 10 mmoles of potassium carbonate and 6 mmoles of methyl iodide were added, and the reaction was stirred at room temperature for 8 hours, and then 30 mL of ethyl acetate was added to fully Stir, transfer to a separatory funnel, wash twice with water and once with saturated brine to collect the organic phase, dry with anhydrous sodium sulfate, remove the solvent under reduced pressure, and purify the residue by silica gel column chromatography to obtain light yellow syrupy N9 -Methyl-N2-acetyl protected guanine (2), the product yield is 78%.
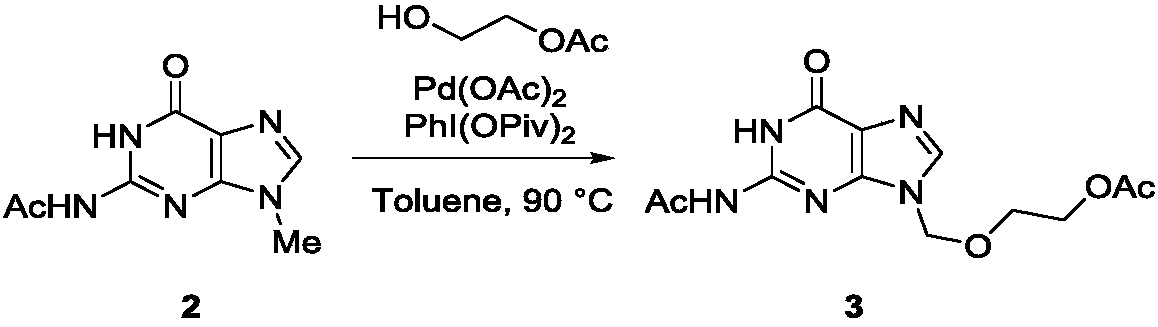
[0024] In the second step, the reaction formula is as follows:
[0025]
[0026] 5 mmoles of N9-methyl-N2-acetylguanine (2) were dissolved in 20mL of anhydrous toluene, 0.5 mmoles of palladium acetate were added, 6 mmoles of PhI(OPiv)2 and 6 mmoles of monoacetyl protected Ethylene glycol (HOC...
Embodiment 2
[0033] In the first step, the reaction formula is as follows:
[0034]
[0035] 5 mmoles of N2-acetylguanine (1) were dissolved in 15 mL of anhydrous DMF, 10 mmoles of potassium carbonate and 6 mmoles of methyl iodide were added, and the reaction was stirred at room temperature for 8 hours, and then 30 mL of ethyl acetate was added to fully Stir, transfer to a separatory funnel, wash twice with water and once with saturated brine to collect the organic phase, dry with anhydrous sodium sulfate, remove the solvent under reduced pressure, and purify the residue by silica gel column chromatography to obtain light yellow syrupy N9 -Methyl-N2-acetyl-protected guanine (2), the product yield is 78%.
[0036] In the second step, the reaction formula is as follows:
[0037]
[0038] 5 mmoles of N9-methyl-N2-acetylguanine (2) are dissolved in 20mL of anhydrous toluene, 0.5 mmoles of palladium acetate are added, 6 mmoles of PhI(OPiv)2 and 6 mmoles of 1,3-acetyl are added Glycerol, the temperat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com